Significance Statement
Gdf15, which encodes a signaling factor activated by oxidative stress, DNA damage, and proinflammatory cytokines, is upregulated in the human and mouse kidney within a few hours of ischemia-reperfusion injury. Using novel mouse strains, the authors mapped cellular sites of Gdf15 expression in normal and injured kidney and examined Gdf15’s role in ischemia-reperfusion injury. They showed that Gdf15 is expressed within hypoxic regions of the kidney and is predominantly activated within tubular epithelial cells at injury repair sites; loss of Gdf15 exacerbated injury, enhancing the inflammatory response. In an analysis of clinical data, they demonstrated that single nucleotide polymorphisms linked to lower circulating GDF15 levels associate with an increased incidence of biopsy-proven acute rejection. These findings point to modulating GDF15 levels in patients receiving kidney transplant as a possible therapeutic strategy.
Keywords: GDF15, ischemia-reperfusion, kidney transplantation, acute kidney injury, acute allograft rejection
Abstract
Background
Gdf15 encodes a TGF-β superfamily member that is rapidly activated in response to stress in multiple organ systems, including the kidney. However, there has been a lack of information about Gdf15 activity and effects in normal kidney and in AKI.
Methods
We used genome editing to generate a Gdf15nuGFP-CE mouse line, removing Gdf15 at the targeted allele, and enabling direct visualization and genetic modification of Gdf15-expressing cells. We extensively mapped Gdf15 expression in the normal kidney and following bilateral ischemia-reperfusion injury, and quantified and compared renal responses to ischemia-reperfusion injury in the presence and absence of GDF15. In addition, we analyzed single nucleotide polymorphism association data for GDF15 for associations with patient kidney transplant outcomes.
Results
Gdf15 is normally expressed within aquaporin 1–positive cells of the S3 segment of the proximal tubule, aquaporin 1–negative cells of the thin descending limb of the loop of Henle, and principal cells of the collecting system. Gdf15 is rapidly upregulated within a few hours of bilateral ischemia-reperfusion injury at these sites and new sites of proximal tubule injury. Deficiency of Gdf15 exacerbated acute tubular injury and enhanced inflammatory responses. Analysis of clinical transplantation data linked low circulating levels of GDF15 to an increased incidence of biopsy-proven acute rejection.
Conclusions
Gdf15 contributes to an early acting, renoprotective injury response, modifying immune cell actions. The data support further investigation in clinical model systems of the potential benefit from GDF15 administration in situations in which some level of tubular injury is inevitable, such as following a kidney transplant.
AKI is associated with significant morbidity and mortality, with mortality rates up to 50%–70% in critically ill patients with AKI.1–3 Even mild AKI is associated with a significant increased risk in in-hospital mortality.4 Renal ischemia-reperfusion injury (IRI) frequently triggers proximal tubular damage leading to the clinical syndrome of AKI in varied settings including kidney transplantation,5 cardiothoracic6 and aortic surgery,7 and sepsis.8 Currently, no therapies exist to prevent or treat established AKI. Identifying potential therapeutic targets is an imperative to enable the development of strategies to prevent or ameliorate AKI and its sequelae in high-risk patients and those initiating AKI.
In a previous report, we coupled translating ribosome affinity purification (TRAP) methodology to a bilateral renal IRI model to characterize early translational profiles in distinct cell lineages of the kidney.9 These studies identified Gdf15, which encodes a divergent member of the TGFβ superfamily of cell signaling factors,10,11 as a rapidly induced target of IRI, activated to high levels within a few hours of vascular release. Analysis of biopsy specimens from human kidney transplants also showed the strong induction of Gdf15, within a similar time frame, after establishment of the renal vascular circuit,12 and in kidney transplants with delayed graft function.13 Thus, transcriptional activation of Gdf15 is a conserved response to ischemia-invoked renal stress and tubular damage. Interestingly, circulating GDF15 levels have been associated with an increased risk of CKD progression14 and elevated Gdf15 expression is evident in our group’s study of a long-term murine IRI model that replicates the transition from an AKI to the onset of CKD.15
Gdf15, also known as macrophage inhibitory cytokine 1 (MIC-1),10 placental TGF β (PTGFβ),16,17 and nonsteroidal anti-inflammatory drug–activated gene 1 (NAG1),17 was first identified as an autocrine regulatory molecule associated with macrophage activation.10 Gdf15 is known to be induced in macrophages by proinflammatory cytokines including TNFα, IL1β, and IL6, but not by IFNγ or LPSs.10 A variety of stressors including inflammation,18–20 oxidative stress,21 DNA damage,19,20,22–24 and integrated stress response25 also activate Gdf15 expression. Further, NF-κB (NF κ-light-chain-enhancer of activated B cells)-invoked Gdf15 expression has been linked to the suppression of macrophage surveillance in pancreatic tumors.26
Functional studies in several organ systems have given ambiguous insights into the actions of Gdf15. GDF15 is reported to exhibit a cardio-protective effect in mouse models of myocardial IRI.27,28 However, no clear role was observed for endogenous Gdf15 after ischemic liver,29,30 cerebral,31 and kidney injury.29 The disparate outcomes may reflect actual differences between organ systems or experimental differences among these studies in injury-invoked responses. Although Gdf15 was first identified in 1997,10 a GDF15 receptor was only reported in 2017.32–36 The receptor termed GFRAL acts in the brainstem to regulate appetite-associated body weight.32–36 Whether GFRAL or a different receptor acts to mediate GDF15 actions in stress-related responses is not clear.
Here, we validate new mouse strains that will be of broad utility to the study of Gdf15- and Gdf15-transcribing cell types, in normal and injured mouse kidneys. The genetic tools encoded by these strains have facilitated a characterization of normal and AKI-associated Gdf15 activity. Functional studies demonstrate that Gdf15 deficiency enhanced renal tubular injury and inflammation post-IRI. Further, genetic association studies suggest that lower circulating GDF15 levels link to an elevated risk of rejection in patients receiving kidney transplant.
Methods
Gene Targeting
The ES cell clone for Gdf15lacZ was obtained from the Knock-Out Mouse Project (KOMP) Repository (www.komp.org).37 The vectors for Gdf15 targeting (pL1L2_GT1_LF2A_nEGFPO_T2A_CreERT_puro) and dual-recombinase (pDIRE(iCRE&Flpo)) were generated in collaboration with scientists in the European Conditional Mouse Mutagenesis consortium at the Sanger Institute (www.knockoutmouse.org/about/eucommtools). The ES targeting procedure was performed according to a previously published protocol.38 Correct targeting was confirmed by 5′ and 3′ PCR with primers: 5′ forward: atcggaaatctgacgcaatc; 5′ reverse: gttgtgccggatcttgaagt; 3′ forward: ggcattatttaaagttaggcgcg; 3′ reverse: gcttgcacatccattccttc.
Animal Experiments
Mouse husbandry, handling, and surgical procedures were performed according to the guidelines issued by the Institutional Animal Care and Use Committee (IACUC) at the University of Southern California with protocol number 11911. Mouse line Gt(ROSA)26Sortm14(CAG-tdTomato)Hze was obtained from the Jackson Laboratory (#007914). Gdf15nuGFP-CE mouse line maintained on a C57BL/6 background has been submitted to the Jackson Laboratory (#034497). Warm renal IRI was performed on 10–12-week-old (25–28 g) male mice. The IRI procedure was the same as described in the published protocol15 except that both renal pedicles were clamped for 11.5, 15 and 19 minutes.
Serum and Urine Analysis
Serum creatinine levels were measured by the UT Southwestern Medical Center O’Brien Center for Kidney Disease Research (Dallas, TX) using capillary electrophoresis (PA800 Plus Pharmaceutical Analysis System; Beckman Coulter). BUN was assayed at the George M. O’Brien Kidney Center at Yale University using the BUN Procedure No. 0580 for the quantitative colorimetric assay with the readout performed on a Stanbio Excel analyzer. Urine albumin was assayed using the Mouse Albumin ELISA Quantitation Set (Cat. No. E90–134; Bethyl Laboratories) on a BIO-RAD microplate reader.
qPCR
Total RNA was prepared from whole kidney tissues using RNeasy mini kit (Qiagen). cDNA was synthesized with Superscript Vilo kit (Thermo Fisher Scientific). qPCR was done with SYBR green on Applied Biosystems 7500 fast real-time PCR system machine according to the manufacturer’s protocol. The relative mRNA expression of all genes was calculated by the 2−ΔΔCt method using Gapdh as reference. qPCR primers were following the online resource: https://pga.mgh.harvard.edu/primerbank/.
Immunofluorescence
Frozen sections were prepared and stained as described in the published protocol.15 The following antibodies were used in this study, recognizing Havcr1 (#AF1817, Kidney injury molecule–1 [KIM-1], goat, 1:1000; R&D Systems), Acta2 (α-SMA) (C6198, smooth muscle actin–Cy3, mouse monoclonal; Sigma), Cd3 (#ab16669, rabbit; Abcam), Cd4 (#550280, rat; BD Pharmingen), Ptprc (#AF114, CD45, goat; R&D Systems), Adgre1 (14–4801, F4/80, rat; eBiosciences), Ptprc (#557390, CD45R (B220), rat; BD Pharmingen), Calb1 (Calbindin-D-28K) (C9848, mouse; Sigma), LTL lectin-FITC conjugate (#FL-1321; Vector Laboratories), Aqp1 (#sc-20810, rabbit; Santa Cruz), Aqp1 (#sc-25287, mouse; Santa Cruz), GFP (#AB16901, chicken; Chemicon), Slc5a2 (#ab85626, rabbit; Abcam), Car4 (#AF2414, goat; R&D), Aqp2 (#sc-9882, goat; Santa Cruz), Atp6v1b1 (ab192612; Abcam), and EDU (#900584; Sigma), and were i.p. injected at 40 mg per kg body wt 3 hours before kidney collection and analyzed with the Click-iT Plus EdU Alexa Fluor 647 Imaging Kit (#C10640; Thermo Fisher Scientific). Fluorescent images were acquired on a Zeiss Axio Scan Z1 slide scanner and Zeiss LSM780 and Leica SP8 confocal microscopes.
Genetic Polymorphism Association
The generation and analysis of human sequence datasets has been described previously.39 In short, kidney transplant donors and recipients were genotyped using a transplant-specific gene array (Axiom Tx GWAS array; Affymetrix, CA). Haplotypes were phased using SHAPEIT and imputed with IMPUTE2 using 1000 Genomes Project phase 3 and Genome of the Netherlands v5 data as reference panels.40–43 SNP2HLA was used for the imputation of four-digit HLA types on the basis of the Type 1 Diabetes Genetics Consortium reference panel and HLAMatchmaker was used to compute HLA eplet mismatch.44,45 The study was approved by the institutional review boards of the Medical University of Vienna and the Institute for Clinical and Experimental Medicine in Prague, respectively (EK267/2011, G 05–04–03 and A 13–02–01 [83/13]). Hardy–Weinberg equilibrium of variants was checked by chi-squared test with continuity correction.
The association of rs749451 and rs888663 with the clinical outcome of biopsy-confirmed acute rejection/T cell–mediated rejection (BCAR/TCR) within the first year after transplantation was assessed in first-time deceased-donor kidney transplant recipients who reached primary graft function after transplantation. Odds ratios (ORs) were used to measure the association between specific genotypes and occurrence of BCAR/TCR. Kaplan–Meier analysis and Cox proportional hazard modeling were used to assess the association of genotypes with the time to the first BCAR/TCR occurrence. Log-rank test was used to test differences between survival curves. The Cox proportional hazard model was adjusted for clinical risk factors (HLA eplet mismatch, recipient and donor sex, and donor age).
Statistical Analyses
Quantification values for immunostaining were represented as histograms and mean±SEM. The significance of difference among groups was examined using unpaired two-tailed t test. A P value of <0.05 was considered significant. All analyses were on the basis of three independent repeats of experiments with 3–6 biologic replicates.
Study Approval
All surgical procedures and all mouse handling and husbandry were performed according to guidelines issued by the IACUC at the University of Southern California, and were performed on approval of each institution’s IACUC.
Results
Gdf15 Upregulation after Ischemic AKI
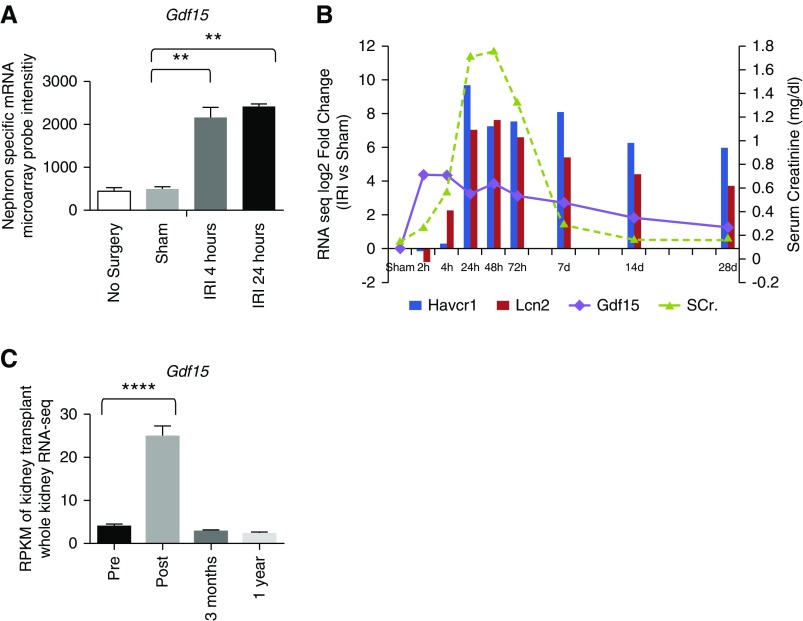
In earlier work, we identified cell type–specific responses in acute ischemic AKI by performing TRAP within distinct cell lineages of the mammalian kidney 24 hours post-IRI.9 From a more extended TRAP analysis examining the nephron in Six2TGC/+;Gt(ROSA)26Sortm9(EGFP/Rpl10a)Amc/+ mice 4 hours post–vascular release by microarray profiling of the nephron response,9 Gdf15 emerged as a target-of-interest, strongly activated as early as 4 hours post-IRI (Figure 1A). A follow-up whole-kidney RNA-seq transcriptome analysis15 showed a spike of Gdf15 mRNA expression within the first few hours (2–4 hours) post-IRI, preceding a significant elevation of serum creatinine levels and before the activation of Havcr1 and Lcn2, two genes explored as early diagnostic markers of AKI (Figure 1B).15 Gdf15 levels tracked with the normalization of creatinine levels by 14 days post-IRI, but remained elevated in this severe damage model, where repair is only partially effective (Figure 1B).15 In addition, RNA-seq analysis on protocol biopsy specimens obtained from 42 kidney transplant recipients showed elevated levels of Gdf15 transcripts within 1.5 hours post-transplantation (Figure 1C).
Figure 1.
Expression of Gdf15 transcripts was upregulated post ischemia in mouse and human. (A) Microarray intensity of Gdf15 on the basis of the published nephron-specific mRNA profile post-IRI.9 (B) The log2 fold change (IRI versus Sham) of Gdf15 on the basis of the RPKM of the published whole-kidney RNA-seq.15 (C) RPKM value of human kidney transplant on the basis of the published RNA-seq.12 **P<0.01; ****P<0.0001. SCr., serum creatinine.
Localization of Gdf15 Expression in the Mouse Kidney
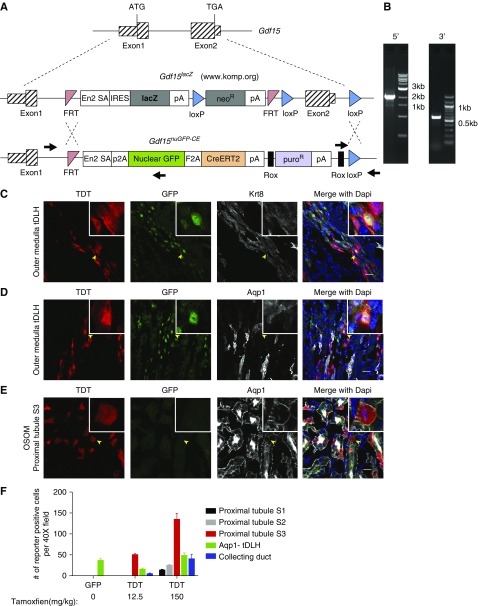
To examine in detail the cellular distribution and fate of Gdf15-expressing cells, we obtained embryo stem cells (ESCs) from KOMP (www.komp.org) in which homologous recombination was used to introduce a lacZ gene cassette into the Gdf15 locus (Gdf15lacZ). Insertion of the cassette blocks production of endogenous GDF15 from the targeted allele and is predicted to result in a lacZ expression mirroring the normal activity of Gdf15 (Figure 2A).37 Recombination-mediated cassette exchange38 was performed on Gdf15lacZ/+ ES cells, replacing lacZ sequences with a nuclearGFP-F2A-CREERT2-encoding expression cassette resulting in a Gdf15nuGFP-CE allele (Figure 2B). Expression of this cassette is predicted to enable visualization (nuclear GFP, “nuGFP” in the allele designation) and conditional genetic modification (tamoxifen-dependent CRE recombination; CRE::ERT2, “CE” in the allele designation) in Gdf15-expressing cell types. Importantly, the inbred C57BL6 background of targeted ESCs enhances phenotypic reproducibility and facilitates comparative analysis with relevant studies such as a transcriptional analysis of the AKI-CKD transition which was carried out in the C57BL6 mouse strain.15
Figure 2.
Nuclear GFP and TDT reporter activities reflected the endogenous expression of Gdf15 in Gdf15nuGFP-CE mouse line. (A) Schematic diagram of Gdf15 knock-in allele. (B) PCR verification of the targeted allele using primer pairs indicated by black arrows in (A). Confocal immunofluorescence showed nuclear GFP and TDT in Krt8+, Aqp1− tDLH (C and D); and TDT but not GFP in Aqp1+ proximal tubule S3 segments in the OSOM (E). (F) Quantification of GFP- and TDT-positive cells in different nephron segments per ×40 field of confocal microscopy. Scale bar in (C–E), 20 μm.
To examine the cellular distribution of Gdf15 expression, Gdf15nuGFP-CE mice were crossed to a TDT reporter mouse line Gt(ROSA)26Sortm14(CAG-tdTomato)Hze, hereafter referred to as R26TDT. Gdf15 locus–derived GFP and TDT activity faithfully recapitulated Gdf15 expression analysis by in situ hybridization46 and RNA-seq analysis of dissected nephron segments.47 In the uninjured adult kidney, nuclear GFP localized predominantly in Krt8/18+, Aqp1− cells of the thin descending limb of the loop of Henle (tDLH) connected to nephrons with cortically positioned glomeruli (Figure 2, C and D, Supplemental Figure 1, A and B). Most Aqp1+ and Lotus Tetragonolobus Lectin (LTL)–bound cells of adjacent S3 segments of the proximal tubule in the outer stripe of outer medulla (OSOM) showed no GFP activity (Figure 2, C and D, Supplemental Figure 1, A and B).
CRE-recombination can be a more sensitive indicator of locus activity than locus-driven GFP reporters. To examine CRE activity, a single intraperitoneal injection of a high dose of tamoxifen (150 mg/kg body wt) was given to adult Gdf15nuGFP-CE/+; R26TDT/+ mice and TDT+ cells, indicative of Gdf15-driven CE activity, were scored 48 hours later. TDT and GFP colabeled cells were observed in the tDLH (Figure 2, C and D, Supplemental Figure 1, A–D) but also in several GFP− cell types of the nephron and collecting system: (1) Aqp1+/LTL+ S3 cells of the proximal tubule (Figure 2E); (2) Aqp2+ principal cells in medullary collecting ducts (Supplemental Figure 1, D and E); (3) Aqp2−/Krt8/18+ cells of the thin loop of Henle in the inner stripe of outer medulla (ISOM; Supplemental Figure 1E); (4) Slc5a2+ cells of S1 region of the proximal tubule (Supplemental Figure 1F); and (5) Aqp1+ tDLH cells in the OSOM (Supplemental Figure 1G). Thus, CRE activity likely reveals lower levels of Gdf15 activity outside of the tDLH. Consistent with this view, when the tamoxifen dosage was markedly decreased to 12.5 mg/kg body wt, TDT activity was predominantly restricted to the S3 region of the proximal tubule and the tDLH, more similar to the nuGFP reporter (Supplemental Figure 2, A–D). Without tamoxifen, around 0.2% of proximal tubular cells in S3 segments were TDT+ only in the Gdf15nuGFP-CE/nuGFP-CE homozygous kidneys, suggesting minimal leakiness (data not shown). Figure 2F summarizes the GFP and TDT reporter activity in multiple nephron segments, quantifying the distribution of each cell type in particular target cell populations.
Gdf15 Upregulation in Defined Renal Compartments after IRI
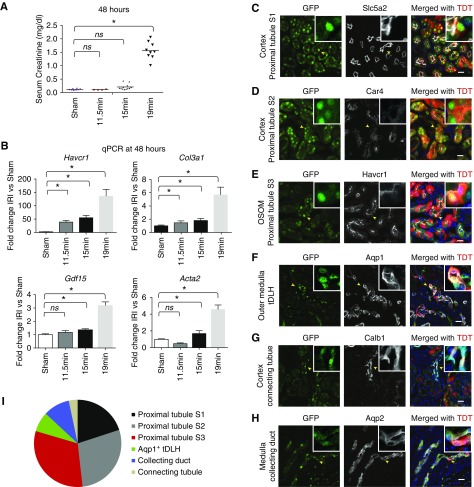
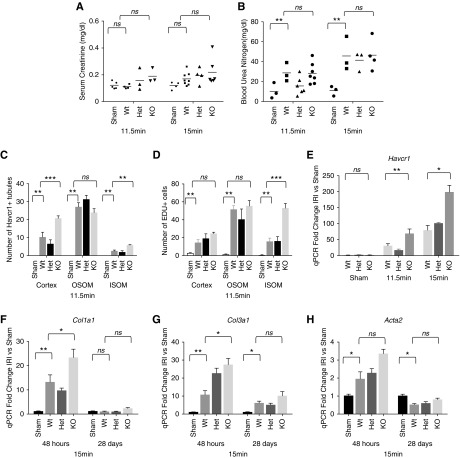
Before examining the postischemia response with Gdf15nuGFP-CE mice, we first calibrated IRI responses in 10–12-week old male C57BL/6J mice. Three cohorts were subjected to different periods of ischemia (11.5, 15, or 19 minutes) and serum creatinine levels were measured 48 hours after vascular release (Figure 3A). Whereas all time points led to significant elevation of Havcr1 and Col3a1 mRNA levels 48 hours postreperfusion, consistent with renal injury and an early fibrotic response, only the 19-minute IRI resulted in a robust and significant elevation in serum creatinine levels (Figure 3B). The 15- and 19-minute IRI also resulted in a significant elevation of Gdf15 and Acta2 expression that was not observed in 11.5-minute IRI kidney analysis, and which was more marked in the 19-minute IRI sample (Figure 3B). Hereafter, we refer to the characterized injury evoked by different periods of ischemia as mild (11.5 minutes), moderate (15.0 minutes), and severe (19.0 minutes).
Figure 3.
Gdf15 was upregulated in renal epithelial tubules at 48 hours postischemia. (A) Serum creatinine level at 48 hours post sham and 11.5-, 15-, and 19-minute ischemia. *P<0.05. NS, not significantly changed. (B) Quantitative PCR showing fold changes of Havcr1, Gdf15, Col3a1, and Acta2 after IRI compared with sham. Confocal immunofluorescence showed nuclear GFP and tamoxifen-induced TDT in (C) Slc5a2+ cortical proximal tubule S1; (D) Car4+ cortical proximal tubule S2; (E) Havcr1+ proximal tubule S3 in the OSOM; (F) Aqp1+ tDLH in the outer medulla; (G) Calb1+ cortical connecting tubule; and (H) Aqp2+ collecting duct. (I) Pie chart displays the distribution of number of GFP+ cells per ×40 field of confocal microscopy among different nephron segments. Scale bar in (A–F), 20 μm.
Compound heterozygous Gdf15nuGFP-CE/+; R26TDT/+ male mice were subjected to severe ischemia (19 minutes) and a single low dose of tamoxifen (12.5 mg per kg body wt) administered shortly after surgery. Analysis 48 hours post injury of kidneys showed nuclear GFP and TDT reporter activity in multiple cell types within renal tubular segments identified by coanalysis with antibodies recognizing key cell markers including: (1) Slc5a2+ in the S1 segment of the proximal tubule; (2) Car4+ cells in the S2 segment of the proximal tubule; (3) Havcr1+ cells in the S3 segment of the proximal tubule in the OSOM; (4) Aqp1+ cells in the outer medullary tDLH; (5) Calb1+ (Calbindin D28K) cells in the connecting tubules (CNT) in the cortex; and (6) Aqp2+ principal cells in the medullary collecting ducts (Figure 3, C–H). Taken together, these results showed a marked upregulation of Gdf15 expression in proximal tubule segments, tDLH, and distal domains of the nephron, and principal cells of the collecting duct.
Fate of Gdf15-Expressing Cells within Ischemic Stressed Segments of the Nephron
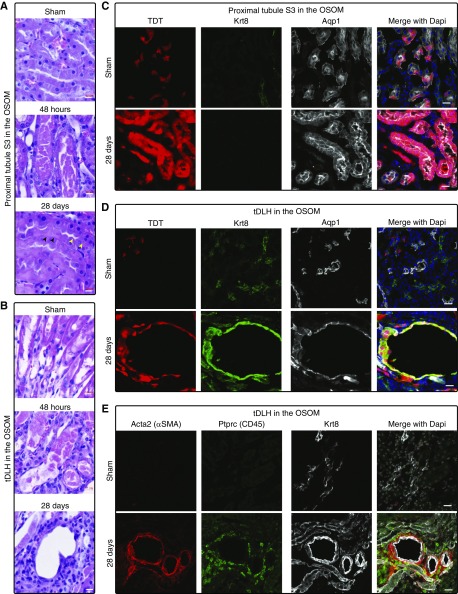
To examine the fate of cells activating Gdf15, we performed a 15-minute bilateral IRI on adult Gdf15nuGFP-CE/+; R26TDT/+ mice to invoke a moderate injury then injected tamoxifen at 48 hours (12.5 mg/kg) post vascular release, then visualized TDT+ cells in kidneys 28 days post-IRI.
Histologic analysis showed extensive sloughing off of cells in the S3 region of the PT and tDLH cells 2 days after IRI (Figure 4, A and B, Supplemental Figure 3A). However, by 28 days post-IRI, much of the epithelium of the S3 region was histologically normal, although some cells contained two nuclei (yellow arrows in Figure 4A), and others exhibited an abnormally light staining of nuclei and cytoplasm (black arrows in Figure 4A). Compared with the sparse contribution of TDT+ cells to the Aqp1+ S3 segment of the proximal tubule after sham-surgery, there was a marked increase in the percentage of TDT+ cell clusters in S3 segments 28 days post-IRI (Figure 4C). Further, many of these TDT+ cells were Aqp1+/Krt8− indicative of repair and recovery of the S3 segment. In contrast, in focal regions at the proximal end of the Apq1+ tDLH in the outer medulla, the lumen of the nephron was markedly enlarged (Figure 4, B–E, Supplemental Figure 3). Here, TDT+ cyst-like tubular structures showed ectopic Krt8+, a hallmark of maladaptive repair.15 These regions were surrounded by clusters of Acta2+ myofibroblasts and exhibited a marked infiltration of Ptprc (CD45)-positive immune cells (Figure 4E). Collectively, these findings indicate that activation of Gdf15 occurred within cells that participated substantially in normal repair in some nephron segments as well as in segments where compromised epithelial repair was associated with inflammation and renal fibrosis.
Figure 4.
Proximal tubule S3 and tDLH in the OSOM marked by Gdf15 reporter showed distinct cell fates after IRI. (A and B) H&E staining on kidneys collected at 48 hours and 28 days after sham and 15-minute ischemia. (A) Proximal tubule S3; (B) tDLH. (C–E) Confocal immunofluorescence on kidneys collected at 28 days post sham and IRI. Tamoxifen was injected at 48 hours after IRI. Gdf15-expressing cells represented by tamoxifen-dependent TDT in the proximal tubule S3 (C); and Aqp1+ tDLH cells costained with Krt8 (green) (D) that were surrounded by Acta2 (αSMA)+ and Ptprc (CD45)+ cells (E). Scale bar in (A and B), 10 μm; in (C–E), 20 μm.
GDF15 Deficiency Aggravated Acute Tubular Injury and Enhanced Inflammation Postischemia
To explore the role of Gdf15, we examined the effects of mild (11.5 minutes) and moderate (15 minutes) IRI on Gdf15nuGFP-CE/nuGFP-CE (KO) kidneys 48 hours post-IRI. BUN but not serum creatinine levels were significantly elevated in wild-type mice 48 hours after initiation of both mild and moderate IRI (Figure 5, A and B). However, quantitative PCR confirmed an enhanced injury in Gdf15 KO kidneys after mild IRI and immunostaining suggested increased injury within cortical PT regions (Figure 5, C–E, Supplemental Figure 4). However, examining mild IRI–invoked cell proliferation by EDU labeling of S-phase nuclei 45 and 48 hours post-IRI showed a striking increase in EDU+ cells in the medullary region of KO kidneys (Figure 5D, Supplemental Figure 4). DNA replication was observed in Aqp1+, Havcr1+ proximal tubule cells; Pdgfrb+ interstitial cells; Ptprc (CD45)+ immune cells (data not shown); and Aqp2+ principal cells in the epithelium of the medullary collecting duct (Supplemental Figure 5). Thus, loss of Gdf15 activity results in distinct cellular responses within several different regions and cell populations within the kidney 2 days after an initiating mild ischemic episode.
Figure 5.
GDF15 deficiency aggravated acute tubular injury. IRI in wild-type and GDF15-deficient mice. Creatinine (A) and BUN (B) were measured with serum samples collected at 48 hours post 11.5- and 15-minute ischemia. Quantification of number of Havcr1+ (C) tubules and EDU+ cells (D) per ×40 field of confocal microscopy at 48 hours post 11.5-minute ischemia. (E) qPCR for Havcr1 with whole-kidney samples at 48 hours post 11.5- and 15-minute ischemia. (F–H) qPCR for Col1a1, Col3a1, and Acta2 at 48 hours and 28 days post 15-minute ischemia. *P<0.05; **P<0.01; ***P<0.001. NS, not significantly changed. Het, heterozygous; KO, knockout; Wt, wild type.
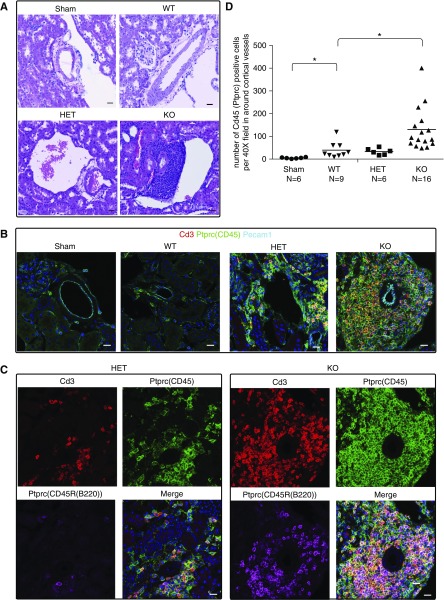
Moderate IRI (15 minutes) in Gdf15 KO kidneys significantly increased Havcr1, Col1a1, Col3a1, and Acta2 levels at 48 hours although no long-lasting difference could be scored by qPCR analysis 4 weeks post-IRI (Figure 5, E–H). However, a marked increase in lymphocyte aggregation was observed around blood vessels in five of 16 (31.3%) KO mutant mice (Figure 6, Supplemental Figure 6). These aggregates contained Cd3+ T (Figure 6, B–D) and Ptprc (CD45R)+ B cells suggestive of tertiary lymphoid organs arising in kidney transplants (Figure 6, B and C)48 that are also observed in the transition from acute injury to chronic disease in mouse studies.15,49,50 Together these data suggest that Gdf15 has an early renal protective response which in the longer term suppresses inflammation.
Figure 6.
GDF15 deficiency enhanced adaptive immunity. Kidneys from wild-type, heterozygous, and homozygous mutant mice were collected 28 days after sham and 15-minute ischemia. (A) H&E staining. (B–C) Confocal immunofluorescence showed merged (B) and separate channels (C) in the aggregates near the cortical vessels. (D) Quantification of number of Ptprc (CD45)+ cells per ×40 field of confocal microscopy in the aggregates. *P<0.05. Scale bar in (A–D), 20 μm. HET, heterozygous; KO, knockout; WT, wild type.
Association between GDF15-Related Single Nucleotide Polymorphisms and Acute Rejection Episodes after Kidney Transplantation
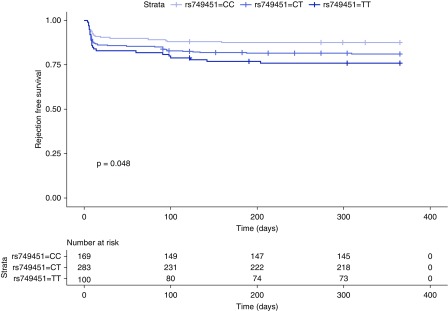
To investigate the clinical relevance of our experimental findings, we examined genetic polymorphisms in a cohort of 522 recipients who received a first deceased-donor kidney transplant from two transplant centers in Vienna and Prague. Among the single nucleotide polymorphisms previously associated with the circulating concentration of GDF15,51 we selected rs749451 and rs888663. Considering the low linkage disequilibrium between the two single nucleotide polymorphisms (0.43), we performed two independent analyses in which we stratified the study population on the basis of their genotype into GDF15 high and low expressers and asserted the differences in BCAR/TCR between the groups. Because of the low minor allele frequency (20%) of rs888663, and because only 23 recipients with the GG genotype were available for analysis, we thus focused on rs749451. The clinical characteristics of the groups were similar, except for the number of recipients who had a BCAR/TCR within the first year, and are presented in Table 1. The genotypes in the study population did not violate the Hardy–Weinberg equilibrium (rs749451: P=0.36; rs888663: P=0.86). In both analyses, homozygous carriers of the allele previously linked to a lower GDF15 concentration in plasma (rs749451: T; rs888663: G) displayed a significantly higher incidence of biopsy-proven acute rejection episodes in the first year after transplantation. Recipients with the rs888663 G/G genotype exhibited 35% and those with the T/T genotype exhibited 16% of BCAR/TCR (OR, 2.85; 95% confidence interval [95% CI], 1.15 to 7.03; P=0.023). Likewise, recipients with the rs749451 T/T genotype exhibited 24% and with the C/C genotype 12% of BCAR/TCR (OR, 2.23; 95% CI, 1.16 to 4.25; P=0.016). Kaplan–Meier analysis revealed that this effect was dose-dependent (P=0.048; Figure 7). A Cox model adjusted for HLA eplet mismatch, recipient and donor sex, and donor age found an 80% (hazard ratio 95% CI, 1.06 to 3.08; P=0.030) increased risk for BCAR/TCR in recipients carrying one or two copies of the T allele. A higher immune reactivity in association with lower GDF15 levels is consistent with the more pronounced lymphocytic infiltrates observed after kidney injury in the mouse model.
Table 1.
Clinical covariables by genotypes of rs749451
| Characteristic | rs749451 | ||
|---|---|---|---|
| CC | CT | TT | |
| Number of recipients | 169 | 283 | 100 |
| Recipient age, yr | 54.25 (46.08–62.12) | 57 (46.00–64.15) | 56.76 (41.37–63.86) |
| Recipient (female) | 62 (37%) | 99 (35%) | 42 (42%) |
| Donor age, yr | 51 (41.00–61.00) | 53 (43.00–61.00) | 52 (37.00–60.00) |
| Donor (female) | 64 (38%) | 102 (36%) | 42 (42%) |
| Follow up, yr | 5.67 (2.99–7.25) | 5.17 (2.67–7.25) | 5.67 (2.81–7.92) |
| HLA serotype mismatch | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| HLA eplet mismatcha | 41 (31.75–56.00) | 42 (32.00–54.00) | 38 (30.00–53.25) |
| Highest PRA% | 4 (0.00–20.00) | 4 (0.00–21.00) | 4 (0.00–21.75) |
| TCR within first yr | 21 (12%) | 53 (19%) | 24 (24%) |
Patient characteristics are described by median and first and third quartile for continuous variables or by frequency and percentage for binary variables. PRA%, panel-reactive antibodies.
Available for 477 donor-recipient pairs.
Figure 7.
GDF15-related single nucleotide polymorphisms are associated with acute rejection episodes after kidney transplantation. Kaplan–Meier plot of BCAR stratified for rs749451 genotypes. The P value was derived from the log-rank test.
Discussion
The normal kidney is constantly under metabolite-, toxin-, and hypoxia-invoked stresses.52–55 Our genetic studies highlight Gdf15 as a stress response gene. Using a newly generated, sensitive recombination-mediated reporter, we identified several normal domains of Gdf15 expression. The outer medullary region is relatively hypoxic52,56 and the kidney region most sensitive to IRI-invoked cell death.57 Reporter activity indicated the strongest Gdf15 activity in Apq1− tDLH cells in this region associated with short loop cortical nephrons emerging later in kidney development58,59 and in the S3 segment of the proximal tubule in the OSOM.52,56 These findings are in good agreement with predicted expression in “KidneyCellExplorer” (https://cello.shinyapps.io/kidneycellexplorer/), a single-cell RNA-seq–based molecular anatomic atlas of cell types in the adult mouse kidney (Supplemental Figure 7).60 Anaerobic glycolysis may result in the decrease of cellular pH in the hypoxic medullary region52 and Gdf15 expression was detected in principal cells in this region.
Surprisingly, even a moderate ischemic episode, which does not significantly affect serum creatinine levels 48 hours postinjury, results in substantial induction of Gdf15 in multiple segments of renal tubular epithelium. Gene expression analysis of kidneys with delayed graft function confirmed a similar induction in the human kidney.12,13 Acidosis had been shown to induce Gdf15 in the Aqp2+ collecting duct epithelium leading to the proliferation and expansion of the acid-secreting cellular pool.61 This is in line with the increased Gdf15 reporter activity in the principal cells of the collecting ducts and cortical connecting tubules postischemia.
Analysis of IRI in mouse kidneys in Gdf15 KOs supports a renal-protective function for GDF15 attenuating fibrotic and inflammatory responses. The appearance of multiple foci of lymphocyte aggregates in kidneys several weeks after a moderate IRI episode resembles aggregates observed in human kidneys from patients with lupus nephritis62 and tertiary lymphoid organs in human kidney allografts and mouse kidneys transitioning to CKD.48,50 Interestingly, the mouse studies may have clinical relevance. Kidney transplantation begins with IRI. The initial response to injury is important in modulating the subsequent immune response, although how is not clear.49 An association between GDF15-linked genetic polymorphisms influencing GDF15 serum levels in acute transplant rejection supports further study of the potential immunomodulatory action of GDF15.63–68
We attempted to directly test whether systemic delivery of recombinant GDF15 might suppress IRI-invoked injury responses. However, mice showed a dramatic weight loss before IRI precluding further study. Recent studies have demonstrated that GDF15 binding to a hypothalamic localized GFRAL triggers a receptor pathway leading to a rapid and marked reduction in food intake and body weight.32–36 This raises the possibility that an upregulation of GDF15 after kidney injury might contribute to weight loss and cachexia after AKI and in the progression to CKD given the link between circulating GDF15 and CKD.14,69,70
In terms of GDF15’s mechanism of action, we have failed to detect mRNA for Gfral according to published renal microarray9 or whole-organ15 or single-cell60 RNA-seq datasets, raising the question as to whether IRI-induced GDF15 functions similarly as a hormone or through alternative receptors outside of the brain. GDF15 has been reported to inhibit integrin activation and mouse neutrophil recruitment through the TGF β receptor I (TGF-βRI) (activin receptor–like kinase 5 [ALK-5]) and TGF-β receptor II (TGF-βRII) heterodimer,71 although a later in vitro receptor screen indicated only a low-affinity interaction with GDF15.32–36
Although the exact target of GDF15’s signaling function in modulating renal response to IRI remains to be determined, the observed changes in the immune cell compartment support an immunomodulatory action consistent with studies in other organ systems. In the mouse heart, GDF15 deficiency resulted in an enhanced inflammatory cell infiltration in both transient27 and prolonged28 ischemia models, although only the former showed an enlarged infarct in the absence of GDF15. In the liver, GDF15 deficiency had no reported effect on acute injury invoked by a single high dose of CCl430 but exhibited an enhanced inflammatory response in chronic liver fibrosis resulting from repeated, low-dose administration of CCl4 over 3 weeks.72 Furthermore, the recent report of a cardioprotective role for Gdf15 in sepsis supports a role for GDF15 in promoting survival during acute inflammation rather than direct pathogen control.73
The nuclear GFP and CRE-ERT2 reporter mouse line driven by Gdf15 provides a valuable sensitive genetic tool that will aid in further elucidation of Gdf15 pathway actions in the kidney. Further, the strain will facilitate broader analysis of stress-related events in the kidney and potentially other organs where Gdf15 activation is invoked early in the response to ischemic injury,28,29,31 idiopathic pulmonary fibrosis,74 and septic AKI.75
Disclosures
None.
Funding
This research was supported by funds from the California Institute of Regenerative Medicine (53-5178-7980) to Dr. A.P. McMahon. Dr. Cippà was supported by the Swiss National Science Foundation, grant number 167773. National Institutes of Health grants to Velocigene at Regeneron Inc. (U01HG004085) and the CHORI, Sanger Institute, and UC Davis Consortium (U01HG004080) funded the generation of gene-targeted embryonic stem cells for 8500 genes in the Knockout Mouse Project and archived and distributed by the Knockout Mouse Project Repository at University of California Davis and Children's Hospital Oakland Research Institute (U42RR024244).
Supplementary Material
Acknowledgments
We thank Kari Koppitch in the McMahon laboratory for help with RNA section in situ.
Dr. Liu and Dr. A.P. McMahon contributed to conception and experimental design. Dr. Liu and Dr. Kumar performed ischemia-reperfusion injury surgeries. Dr. Heinzel, Dr. Oberbauer, and Dr. Cippà performed single nucleotide polymorphism analysis. Dr. Liu, Dr. Kumar, Dr. Heinzel, Mr. Gao, Ms. Guo, Mr. Alvarado, Dr. Cippà, Dr. Krautzberger, Ms. J. McMahon, Dr. Oberbauer, and Dr. A.P. McMahon performed data acquisition or data analysis. Dr. Liu and Dr. A.P. McMahon prepared the manuscript incorporating comments from all authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019090876/-/DCSupplemental.
Supplemental Figure 1. Gdf15 reporter expression after high dose of tamoxifen.
Supplemental Figure 2. Gdf15 reporter expression after low dose of tamoxifen.
Supplemental Figure 3. De novo activation of Gdf15 in Aqp1+ of tDLH in the outer stripe of outer medulla after ischemia.
Supplemental Figure 4. GDF15 deficiency aggravated acute tubular injury post mild ischemia.
Supplemental Figure 5. Prominent proliferation in the medulla in the GDF15-deficient kidneys 48 hours post 11.5-minute ischemia.
Supplemental Figure 6. Inflammatory cell infiltration in the kidneys 28 days post 15-minute IRI.
Supplemental Figure 7. Expression of Gdf15 in adult mouse scRNA-seq datasets.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy EM, Viscoli CM, Horwitz RI: The effect of acute renal failure on mortality. A cohort analysis. JAMA 275: 1489–1494, 1996. [PubMed] [Google Scholar]
- 4.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR: The prognostic importance of a small acute decrement in kidney function in hospitalized patients: A systematic review and meta-analysis. Am J Kidney Dis 50: 712–720, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Aydin Z, van Zonneveld AJ, de Fijter JW, Rabelink TJ: New horizons in prevention and treatment of ischaemic injury to kidney transplants. Nephrol Dial Transplant 22: 342–346, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT: Renal dysfunction after myocardial revascularization: Risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 128: 194–203, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Kazmers A, Jacobs L, Perkins A: The impact of complications after vascular surgery in Veterans Affairs Medical Centers. J Surg Res 67: 62–66, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Prowle JR, Bellomo R: Sepsis-associated acute kidney injury: Macrohemodynamic and microhemodynamic alterations in the renal circulation. Semin Nephrol 35: 64–74, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Krautzberger AM, Sui SH, Hofmann OM, Chen Y, Baetscher M, et al.: Cell-specific translational profiling in acute kidney injury. J Clin Invest 124: 1242–1254, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al.: MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A 94: 11514–11519, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strelau J, Böttner M, Lingor P, Suter-Crazzolara C, Galter D, Jaszai J, et al.: GDF-15/MIC-1 a novel member of the TGF-beta superfamily. J Neural Transm Suppl 60: 273–276, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Cippà PE, Sun B, Liu J, Chen L, Naesens M, McMahon AP: Transcriptional trajectories of human kidney injury progression. JCI Insight 3: 123151, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damman J, Bloks VW, Daha MR, van der Most PJ, Sanjabi B, van der Vlies P, et al.: Hypoxia and complement-and-coagulation pathways in the deceased organ donor as the major target for intervention to improve renal allograft outcome. Transplantation 99: 1293–1300, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Nair V, Robinson-Cohen C, Smith MR, Bellovich KA, Bhat ZY, Bobadilla M, et al.: Growth differentiation factor-15 and risk of CKD progression. J Am Soc Nephrol 28: 2233–2240, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, et al.: Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight 2: 94716, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan M, Wang Y, Guan K, Sun Y: PTGF-β, a type β transforming growth factor (TGF-β) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-β signaling pathway. Proc Natl Acad Sci U S A 97: 109–114, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KS, Baek SJ, Flake GP, Loftin CD, Calvo BF, Eling TE: Expression and regulation of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) in human and mouse tissue. Gastroenterology 122: 1388–1398, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Brown DA, Moore J, Johnen H, Smeets TJ, Bauskin AR, Kuffner T, et al.: Serum macrophage inhibitory cytokine 1 in rheumatoid arthritis: A potential marker of erosive joint destruction. Arthritis Rheum 56: 753–764, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Karan D, Holzbeierlein J, Thrasher JB: Macrophage inhibitory cytokine-1: Possible bridge molecule of inflammation and prostate cancer. Cancer Res 69: 2–5, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, et al.: Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res 61: 5974–5978, 2001. [PubMed] [Google Scholar]
- 21.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al.: Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res 318: 325–333, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, et al.: Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A 100: 3410–3415, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung PK, Woolcock B, Adomat H, Sutcliffe M, Bainbridge TC, Jones EC, et al.: Protein profiling of microdissected prostate tissue links growth differentiation factor 15 to prostate carcinogenesis. Cancer Res 64: 5929–5933, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal MK, Hastak K, Jackson MW, Breit SN, Stark GR, Agarwal ML: Macrophage inhibitory cytokine 1 mediates a p53-dependent protective arrest in S phase in response to starvation for DNA precursors. Proc Natl Acad Sci U S A 103: 16278–16283, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, et al. : GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab 29: 707–718.e8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratnam NM, Peterson JM, Talbert EE, Ladner KJ, Rajasekera PV, Schmidt CR, et al.: NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J Clin Invest 127: 3796–3809, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, et al.: The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 98: 351–360, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al.: GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med 17: 581–588, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Zimmers TA, Jin X, Hsiao EC, McGrath SA, Esquela AF, Koniaris LG: Growth differentiation factor-15/macrophage inhibitory cytokine-1 induction after kidney and lung injury. Shock 23: 543–548, 2005. [PubMed] [Google Scholar]
- 30.Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, Lee SJ: Characterization of growth-differentiation factor 15, a transforming growth factor β superfamily member induced following liver injury. Mol Cell Biol 20: 3742–3751, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindowski K, von Bohlen und Halbach O, Strelau J, Ridder DA, Herrmann O, Schober A, et al.: Regulation of GDF-15, a distant TGF-β superfamily member, in a mouse model of cerebral ischemia. Cell Tissue Res 343: 399–409, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al.: GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 23: 1150–1157, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjær SB, et al.: GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 23: 1158–1166, 2017. [DOI] [PubMed] [Google Scholar]
- 34.Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al.: The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 23: 1215–1219, 2017. [DOI] [PubMed] [Google Scholar]
- 35.Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, et al. : Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550: 255–259, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Cimino I, Coll AP, Yeo GSH: GDF15 and energy balance: Homing in on a mechanism. Nat Med 23: 1119–1120, 2017. [DOI] [PubMed] [Google Scholar]
- 37.White JK, Gerdin AK, Karp NA, Ryder E, Buljan M, Bussell JN, et al.Sanger Institute Mouse Genetics Project ;: Genome-wide generation and systematic phenotyping of knockout mice reveals new roles for many genes. Cell 154: 452–464, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osterwalder M, Galli A, Rosen B, Skarnes WC, Zeller R, Lopez-Rios J: Dual RMCE for efficient re-engineering of mouse mutant alleles. Nat Methods 7: 893–895, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li YR, van Setten J, Verma SS, Lu Y, Holmes MV, Gao H, et al.: Concept and design of a genome-wide association genotyping array tailored for transplantation-specific studies. Genome Med 7: 90, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howie BN, Donnelly P, Marchini J: A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5: e1000529, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delaneau O, Marchini J, Zagury JF: A linear complexity phasing method for thousands of genomes. Nat Methods 9: 179–181, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al.1000 Genomes Project Consortium ;: A global reference for human genetic variation. Nature 526: 68–74, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genome of the Netherlands Consortium : Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat Genet 46: 818–825, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Jia X, Han B, Onengut-Gumuscu S, Chen WM, Concannon PJ, Rich SS, et al.: Imputing amino acid polymorphisms in human leukocyte antigens. PLoS One 8: e64683, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duquesnoy RJ, Askar M: HLAMatchmaker: A molecularly based algorithm for histocompatibility determination. V. Eplet matching for HLA-DR, HLA-DQ, and HLA-DP. Hum Immunol 68: 12–25, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Böttner M, Suter-Crazzolara C, Schober A, Unsicker K: Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res 297: 103–110, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonker M, Wubben JA, ’t Hart BA, Haanstra KG: Lymphoid-like structures with distinct B cell areas in kidney allografts are not predictive for graft rejection. A non-human primate study. Inflammation 38: 2191–2202, 2015. [DOI] [PubMed] [Google Scholar]
- 49.Cippà PE, Liu J, Sun B, Kumar S, Naesens M, McMahon AP: A late B lymphocyte action in dysfunctional tissue repair following kidney injury and transplantation. Nat Commun 10: 1157, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato Y, Mii A, Hamazaki Y, Fujita H, Nakata H, Masuda K, et al.: Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight 1: e87680, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho JE, Mahajan A, Chen MH, Larson MG, McCabe EL, Ghorbani A, et al.: Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem 58: 1582–1591, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neuhofer W, Beck FX: Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Gabert BJ, Kültz D: Osmoprotective proteome adjustments in mouse kidney papilla. Biochim Biophys Acta 1814: 435–448, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Perez A, Burg MB: Importance of organic osmolytes for osmoregulation by renal medullary cells. Hypertension 16: 595–602, 1990. [DOI] [PubMed] [Google Scholar]
- 55.Graham LA, Aman A, Campbell DD, Augley J, Graham D, McBride MW, et al.: Salt stress in the renal tubules is linked to TAL-specific expression of uromodulin and an upregulation of heat shock genes. Physiol Genomics 50: 964–972, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epstein FH: Oxygen and renal metabolism. Kidney Int 51: 381–385, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Bonventre JV, Yang L: Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhai XY, Fenton RA, Andreasen A, Thomsen JS, Christensen EI: Aquaporin-1 is not expressed in descending thin limbs of short-loop nephrons. J Am Soc Nephrol 18: 2937–2944, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Dantzler WH, Layton AT, Layton HE, Pannabecker TL: Urine-concentrating mechanism in the inner medulla: Function of the thin limbs of the loops of Henle. Clin J Am Soc Nephrol 9: 1781–1789, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ransick A, Lindström NO, Liu J, Zhu Q, Guo JJ, Alvarado GF, et al.: Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell 51: 399–413.e7, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duong Van Huyen JP, Cheval L, Bloch-Faure M, Belair MF, Heudes D, Bruneval P, et al.: GDF15 triggers homeostatic proliferation of acid-secreting collecting duct cells. J Am Soc Nephrol 19: 1965–1974, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, Hsieh C, et al.: In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J Immunol 186: 1849–1860, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salahudeen AK, Haider N, May W: Cold ischemia and the reduced long-term survival of cadaveric renal allografts. Kidney Int 65: 713–718, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Snoeijs MG, Vink H, Voesten N, Christiaans MH, Daemen JW, Peppelenbosch AG, et al.: Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol Renal Physiol 299: F1134–F1140, 2010. [DOI] [PubMed] [Google Scholar]
- 65.Cavaillé-Coll M, Bala S, Velidedeoglu E, Hernandez A, Archdeacon P, Gonzalez G, et al.: Summary of FDA workshop on ischemia reperfusion injury in kidney transplantation. Am J Transplant 13: 1134–1148, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Schröppel B, Legendre C: Delayed kidney graft function: From mechanism to translation. Kidney Int 86: 251–258, 2014. [DOI] [PubMed] [Google Scholar]
- 67.Lodhi SA, Meier-Kriesche HU: Kidney allograft survival: The long and short of it. Nephrol Dial Transplant 26: 15–17, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Salvadori M, Rosso G, Bertoni E: Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J Transplant 5: 52–67, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breit SN, Carrero JJ, Tsai VW, Yagoutifam N, Luo W, Kuffner T, et al.: Macrophage inhibitory cytokine-1 (MIC-1/GDF15) and mortality in end-stage renal disease. Nephrol Dial Transplant 27: 70–75, 2012. [DOI] [PubMed] [Google Scholar]
- 70.Lajer M, Jorsal A, Tarnow L, Parving HH, Rossing P: Plasma growth differentiation factor-15 independently predicts all-cause and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care 33: 1567–1572, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Artz A, Butz S, Vestweber D: GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-βRII heterodimer. Blood 128: 529–541, 2016. [DOI] [PubMed] [Google Scholar]
- 72.Chung HK, Kim JT, Kim HW, Kwon M, Kim SY, Shong M, et al.: GDF15 deficiency exacerbates chronic alcohol- and carbon tetrachloride-induced liver injury. Sci Rep 7: 17238, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, et al. : GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell 178: 1231–1244.e11, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Jiang M, Nouraie M, Roth MG, Tabib T, Winters S, et al.: GDF15 is an epithelial-derived biomarker of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 317: L510–L521, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abulizi P, Loganathan N, Zhao D, Mele T, Zhang Y, Zwiep T, et al.: Growth differentiation factor-15 deficiency augments inflammatory response and exacerbates septic heart and renal injury induced by lipopolysaccharide. Sci Rep 7: 1037, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.