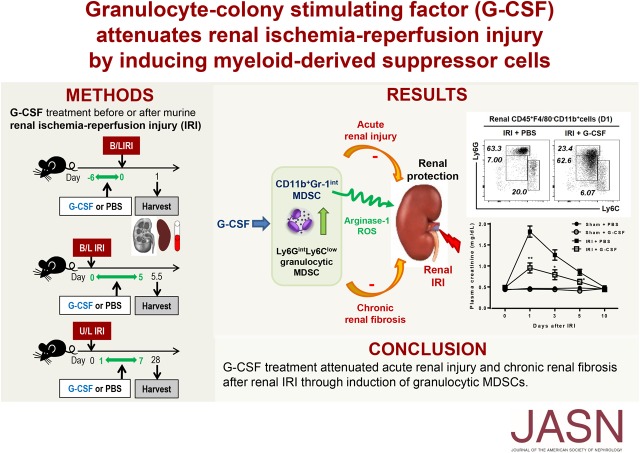
Significance Statement
Myeloid-derived suppressor cells are innate suppressors that play an immunoregulatory role in autoimmunity, transplantation, and antitumor immunity; however, their effects on renal ischemia-reperfusion injury remain unclear. The authors showed that granulocyte colony-stimulating factor (G-CSF) increased renal infiltration of myeloid-derived suppressor cells after ischemia-reperfusion injury. When given before ischemia-reperfusion, G-CSF subsequently attenuated acute tissue injury, renal apoptosis, and renal inflammation; when given after ischemia-reperfusion, G-CSF facilitated renal recovery and attenuated renal fibrosis. They also showed that granulocytic myeloid-derived suppressor cells played a role in the beneficial effects induced by G-CSF via arginase-1 and reactive oxygen species. These findings elucidate protective roles of G-CSF–induced myeloid-derived suppressor cells against ischemia-reperfusion injury and indicate that human studies investigating the therapeutic potential of myeloid-derived suppressor cells and G-CSF in renal ischemia-reperfusion injury are warranted.
Keywords: Myeloid-derived suppressor cells, acute kidney injury, Ischemia-reperfusion injury, Granulocyte-colony stimulating factor
Visual Abstract
Abstract
Background
Granulocyte colony-stimulating factor (G-CSF) can increase populations of myeloid-derived suppressor cells, innate immune suppressors that play an immunoregulatory role in antitumor immunity. However, the roles of myeloid-derived suppressor cells and G-CSF in renal ischemia-reperfusion injury remain unclear.
Methods
We used mouse models of ischemia-reperfusion injury to investigate whether G-CSF can attenuate renal injury by increasing infiltration of myeloid-derived suppressor cells into kidney tissue.
Results
G-CSF treatment before ischemia-reperfusion injury subsequently attenuated acute renal dysfunction, tissue injury, and tubular apoptosis. Additionally, G-CSF treatment suppressed renal infiltration of macrophages and T cells as well as renal levels of IL-6, MCP-1, IL-12, TNF-α, and IFN-γ, but it increased levels of IL-10, arginase-1, and reactive oxygen species. Moreover, administering G-CSF after ischemia-reperfusion injury improved the recovery of renal function and attenuated renal fibrosis on day 28. G-CSF treatment increased renal infiltration of myeloid-derived suppressor cells (F4/80−CD11b+Gr-1int), especially the granulocytic myeloid-derived suppressor cell population (CD11b+Ly6GintLy6Clow); splenic F4/80−CD11b+Gr-1+ cells sorted from G-CSF–treated mice displayed higher levels of arginase-1, IL-10, and reactive oxygen species relative to those from control mice. Furthermore, these splenic cells effectively suppressed in vitro T cell activation mainly through arginase-1 and reactive oxygen species, and their adoptive transfer attenuated renal injury. Combined treatment with anti–Gr-1 and G-CSF showed better renoprotective effects than G-CSF alone, whereas preferential depletion of myeloid-derived suppressor cells by pep-G3 or gemcitabine abrogated the beneficial effects of G-CSF against renal injury.
Conclusions
G-CSF induced renal myeloid-derived suppressor cells, thereby attenuating acute renal injury and chronic renal fibrosis after ischemia-reperfusion injury. These results suggest therapeutic potential of myeloid-derived suppressor cells and G-CSF in renal ischemia-reperfusion injury.
Renal ischemia-reperfusion injury (IRI) is an acute inflammatory disease, which involves both immune effector cells and immunosuppressive cells in its pathogenesis and recovery.1 Regulatory T cells (Tregs), well known adaptive suppressors, suppress acute injury and facilitate recovery after renal IRI.2,3 Furthermore, convenient therapy with IL-2/anti-IL complexes ameliorates renal IRI by inducing Tregs.4
Myeloid-derived suppressor cells (MDSCs) are innate suppressors that suppress antitumor immunity and thereby, contribute to tumor progression.5–7 Recent reports indicated that MDSCs suppress autoimmune disease and transplant rejection as well as Tregs.5,7–10 Additionally, MDSCs play an important role in glucocorticoid-mediated amelioration of FSGS.11 Immature myeloid cells in bone marrow quickly differentiate into mature granulocytes, macrophages, and dendritic cells under healthy conditions, whereas they can be differentiated into MDSCs under pathologic conditions, such as infection, cancer, and trauma.5 Murine CD11b+Gr-1+ MDSCs are classified as granulocytic MDSCs (CD11b+Ly6G+Ly6Clow) and monocytic MDSCs (CD11b+Ly6G−Ly6Chigh) that differ from mature neutrophils, monocytes, and macrophages. Although both subsets use arginase-1 (Arg1) for suppressive action, granulocytic and monocytic MDSCs use reactive oxygen species (ROS) and nitric oxide (NO), respectively, to suppress T cells.5 Human MDSCs are characterized as CD11b+CD33+HLA-DR− and exhibit immunosuppressive functions, with human CD15+ and CD14+ MDSCs corresponding to human granulocytic and monocytic MDSCs, respectively.7,9
Among regulatory myeloid cells, M2 macrophages are involved in recovery after renal IRI in contrast to M1 macrophages, which contribute to acute injury after renal IRI.12,13 A recent report demonstrated increased renal infiltration of CD11b+Gr-1+ MDSCs after renal IRI; however, neither the effects of MDSCs on renal function and tissue injury nor the related mechanisms were studied.14 Therefore, roles of MDSCs remain uncertain in renal IRI, where innate immunity plays important roles.
Granulocyte colony-stimulating factor (G-CSF) is widely used to treat neutropenia in the clinic, and it is capable of inducing the expansion of murine and human MDSCs.5,15,16 Additionally, G-CSF treatment prolongs murine skin graft survival and human islet graft survival by inducing MDSC expansion.15,17 In this study, we investigated whether G-CSF can attenuate renal IRI by increasing MDSC infiltration into renal tissue.
Methods
Animals and IRI Models
Male 6- to 7-week-old C57BL/6J (B6) mice were purchased from KOATECH (Pyeongtaek, Korea), and 7- to 8-week-old (7.10±0.01 weeks, mean±SEM) mice were used in all experiments. Renal IRI was induced by clamping the bilateral renal pedicles for 27 minutes or the unilateral renal pedicle for 40 minutes as previously described.4,18 Levels of plasma creatinine and BUN were measured using QuantiChrom creatinine and urea assay kits, respectively (BioAssay Systems, Hayward, CA).19 Recombinant human G-CSF (Grasin; Kyowa Kirin, Korea) was subcutaneously administrated at a dose of 10 μg for 7 consecutive days prior to bilateral IRI or at a dose of 14 μg for 5 consecutive days after bilateral IRI on the basis of a previous study and dose-response experiments (Supplemental Figure 1).15 Additionally, G-CSF was administered at a dose of 10 μg for 7 consecutive days after unilateral IRI. Anti–Gr-1 antibody (200 μg, RB6–8C5; BioXcell, West Lebanon, NH) was intraperitoneally administered to mice 1 day before IRI.11,20 Pep-G3, a peptibody that selectively depletes MDSCs (days −5, −3, and −1, 100 μg; provided by Larry Kwak, City of Hope National Medical Center),21 or gemcitabine (days −4 and −1, 100 mg/kg; Sigma-Aldrich, St. Louis, MO)22,23 was intraperitoneally administered to mice before IRI. All experiments were conducted according to a protocol approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital (IACUC 17–0222-S1A1).
Isolation and Adoptive Transfer of MDSCs
Splenic F4/80−CD11b+Gr-1+ MDSCs, F4/80−CD11b+Ly6GhighLy6Clow mature neutrophils, F4/80−CD11b+Ly6GintLyClow granulocytic MDSCs, and F4/80−CD11b+Ly6G−LyChigh monocytic MDSCs were isolated with a purity of 99% by sorting using an FACSAria II system (BD Biosciences, San Diego, CA). The purified MDSCs (2×106) were adoptively transferred to mice via the tail vein 1 day before IRI. Sorted splenic F4/80−CD11b+Gr-1high and F4/80−CD11b+Gr-1low cells were smeared on the slides by cytospin centrifugation, and their morphologies were assessed by Wright–Giemsa staining (BioVision Inc, San Francisco, CA).
In Vitro Suppression Assay
Splenic T cells were isolated by a Pan T-cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) and labeled with 5,6-carboxyfluorescein diacetate succinimidyl ester (Thermo Fisher Scientific, Waltham, MA) or CellTracker Violet (Thermo Fisher Scientific). The labeled T cells (2×105 per well) were mixed with splenic F4/80−CD11b+Gr-1+ MDSCs at a ratio of 2:1 and stimulated for 3 days by plate-bound anti-CD3 and anti-CD28 (2 μg/ml; Thermo Fisher Scientific). For inhibitor experiments, cocultured cells were incubated with N-acetyl-l-cysteine (NAC; 0.5 mM; Sigma-Aldrich) as an ROS inhibitor, NG-monomethyl-l-arginine (L-NMMA; 0.1 mM; Sigma-Aldrich) as an inducible nitric oxide synthase (iNOS) inhibitor, or Norvaline (20 mM; Sigma-Aldrich) as an Arg1 inhibitor. T cell proliferation was analyzed by flow cytometry, and results were expressed as division index.24 Proportion and proliferation of Tregs after coculture of T cells and MDSCs were also measured.
Flow Cytometric Analyses
Kidneys and spleens were harvested after perfusion, and single-cell suspensions were prepared by passing tissues through a 70-μm cell strainer (BD Biosciences). Kidneys were disrupted mechanically using a Stomacher 80 Biomaster (Sewart, Worthing, United Kingdom), and renal leukocytes were isolated using a Percoll gradient (GE Healthcare Bio-Sciences, Uppsala, Sweden).25 Isolated cells were stained with antibodies against CD45 (30-F11), Gr-1 (RB6–8C5), Ly6G (1A8), Ly6C (AL-21; BD Biosciences), F4/80 (BM8), CD11b (M1/70), CD19 (1D3), CD244 (eBio244F4), CD25 (PC61), forkhead box P3 (FJK-16S; Thermo Fisher Scientific), CD3 (145–2C11), CD4 (RM4–5; BioLegend, San Diego, CA), and G-CSF receptor (R&D Systems, Minneapolis, MN). 7-Aminoactinomycin D (BD Biosciences) was added to stain dead cells. Flow cytometric analysis was performed using an FACS Canto II flow cytometer (BD Biosciences), and the gating strategy is summarized in Supplemental Figure 2.
Measurement of Intracellular ROS and Renal Hydroxyproline Content
Intracellular ROS generation was measured using a dichlorofluorescein diacetate (DCF-DA)–based flow cytometric assay.26 Renal leukocytes were incubated for 20 minutes with a DCF-DA (5 µm; Thermo Fisher Scientific), followed by stimulation with phorbol 12-myristate 13-acetate (50 ng/ml; Sigma-Aldrich) for 2 hours and measurement of the intracellular dichlorofluorescein of Gr-1+ cells by flow cytometry. Sorted splenic F4/80−CD11b+Gr-1+ cells were also incubated with DCF-DA for measurement of ROS. The hydroxyproline content in the kidney was measured by the Hydroxyproline Colorimetric Assay Kit (BioVision Inc) and expressed as hydroxyproline (micrograms) content per kidney weight (milligrams).
ELISA
Expression levels of IL-6, monocyte chemoattractant protein-1, IL-12p70, TNF-α, IFN-γ, and IL-10 in kidney tissues and culture supernatant from suppression assays were measured using an ELISA kit (BioLegend). Renal tissue was homogenized with radioimmunoprecipitation assay lysis buffer containing a protease inhibitor cocktail (Roche, Basel, Switzerland), and the supernatant was used for cytokine measurement. Cytokine levels were normalized to protein quantities from renal tissues.
Real-Time PCR
Renal tissue was homogenized with the Trizol reagent (Thermo Fisher Scientific), and RNA was reverse transcribed into cDNA using Superscript II reverse transcription. Each reaction mixture included 2× SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) and 10 pmol/μl corresponding primers (Supplemental Table 1). Analysis of real-time PCR was performed using a QuantStudio (v.3.o; Thermo Fisher Scientific). mRNA levels of C-type lectin domain family 7 member A encoding Dectin-1, iNos, Cd206, Arg1, Il10, collagen type IV (ColIV), fibronectin, α-smooth muscle actin (αSma), tnfa, myeloperioxidase (Mpo), Gcsf, and G-CSF receptor were normalized to that of glyceraldehyde 3-phosphate dehydrogenase.
Western Blot
Renal tissue lysates were stained with anti-COLIV, antifibronectin, anti–α-SMA, and anti–β-actin antibodies (Abcam, Cambridge, United Kingdom) at a 1:1000 dilution. Signals developed using the ECL Plus detection system (Amersham Pharmacia Biotech, Piscataway, NJ) were detected with the ImageQuant LAS 4000 system (GE Healthcare Bio-Sciences).
Histologic Analyses
For morphologic assessment, kidney sections (4 μm) were stained with periodic acid–Schiff. Tubular injury score was assessed in periodic acid–Schiff-stained sections on the basis of morphologic variables, including tubular dilation, tubular cell necrosis, cast formation, and tubular brush borders, and it was graded from 1 to 4 by assessing the affected percentage of tubules (0: normal; 1: 1%–25%; 2: 26%–50%; 3: 50%–75%; and 4: 75%–100%) on at least four randomly selected high-power fields in each section as previously described.27 Terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (Roche Diagnostics, Risch-Rotkreuz, Switzerland) staining was used to assess apoptosis of renal tubular epithelia cells. Renal fibrosis was assessed on day 28 after IRI using Masson trichrome staining. The ImageJ plugin (https://imagej.nih. gov) was used for cell counting, and positive cells were measured by counting four high-power fields (200×) per section. All histologic analyses were performed by two independent researchers blinded to the treatment group.
Statistical Analyses
We verified the normal distribution of data using the Shapiro–Wilk normality test. When the normality is certified, the data were presented as mean with the SEM. The two-group comparison was performed using t test. Comparison among more than three groups was performed using ANOVA test and Tukey post hoc analysis. When the data were not normally distributed, a nonparametric method, such as the Mann–Whitney test or the Kruskal–Wallis test, was used, and the data were presented as median with interquartile range. P=0.05 was considered statistically significant. All analyses were performed using GraphPad Prism (v. 7.0; GraphPad Software, La Jolla, CA).
Results
G-CSF Treatment Prior to IRI Attenuates AKI
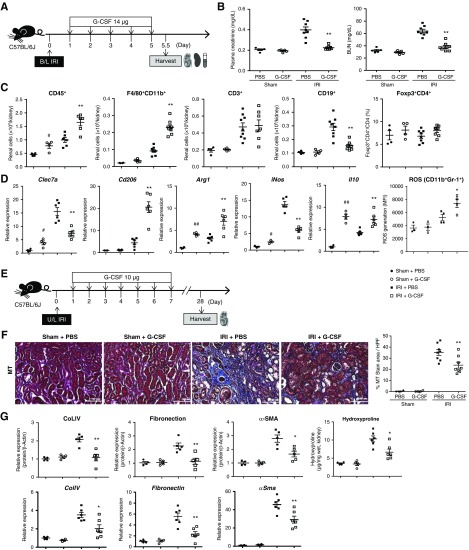
IRI upregulated renal mRNA levels of G-CSF and G-CSF receptor (Supplemental Figure 3, A and B), with these levels subsequently decreasing at 5 and 10 days after IRI, respectively. CD11b+Gr-1int MDSCs showed higher expression of G-CSF receptor than CD11b+Gr-1high mature neutrophils and CD11b−Gr-1− cells (Supplemental Figure 3C). Administration of G-CSF prior to IRI (G-CSF/IRI group) resulted in significantly lower plasma creatinine and BUN levels from days 1 to 5 relative to those in the group (PBS/IRI group) administered PBS (Figure 1, A and B). Additionally, G-CSF treatment decreased tissue injury scores and renal tubular apoptosis on day 1 after IRI (Figure 1C) as well as decreased renal infiltration of leukocytes, including F4/80+CD11b+ macrophages, CD3+ T cells, and CD19+ B cells (Figure 1D). Although renal infiltration of CD4+forkhead box P3+ Tregs increased in the G-CSF/sham group relative to that in the PBS/sham group, there was no difference in the renal Tregs between the G-CSF/IRI and PBS/IRI groups (Figure 1D). Moreover, renal levels of proinflammatory cytokines, such as IL-6, monocyte chemoattractant protein-1, IL-12, TNF-α, and IFN-γ, were lower, whereas that of IL-10 was higher in the G-CSF/IRI group compared with the PBS/IRI group (Figure 1E). Renal expression of Dectin-1 was lower in the G-CSF/IRI group than in the PBS/IRI group, whereas that of CD206 was higher in the G-CSF/IRI group (Figure 1F). Suppression of renal tissue injury and inflammation in the G-CSF/IRI group was maintained on days 3 and 5 (Supplemental Figures 4 and 5). Furthermore, G-CSF treatment increased renal expression of Arg1 and IL-10 irrespective of the presence of IRI, whereas it decreased iNOS expression after IRI (Figure 1F). Additionally, ROS production in renal CD11b+Gr-1+ cells increased following G-CSF treatment (Figure 1G). These results indicated that G-CSF treatment prior to IRI attenuated acute renal injury and renal inflammation.
Figure 1.
G-CSF treatment prior to bilateral IRI attenuates AKI. (A) G-CSF or PBS was administered to B6 mice prior to IRI or sham operation. Kidneys and the spleens were harvested on day 1, and blood was extracted on days 1, 3, 5, and 10 after IRI. (B) Changes in levels of plasma creatinine and BUN after IRI. n=3–8 per group at each time point. (C) Renal tissue injury scores and renal tubular apoptosis (terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling [TUNEL]) were measured on day 1 after IRI. Magnification, ×200. (D) Absolute numbers of CD45+ leukocytes, F4/80+CD11b+ macrophages, CD3+ T cells, and CD19+ B cells that infiltrated into the kidneys. Proportions of renal CD4+forkhead box P3 (Foxp3)+ Tregs among CD4+ T cells. (E) Renal levels of IL-6, monocyte chemoattractant protein-1 (MCP-1), IL-12p70, TNF-α, IFN-γ, and IL-10. (F) mRNA expression of C-type lectin domain family 7 member A (Clec7a; encoding Dectin-1), Cd206, Arg1, Il10, and iNos in renal tissues normalized to glyceraldehyde 3-phosphate dehydrogenase expression. (G) ROS production (DCF-DA) in renal CD11b+Gr-1+ leukocytes on day 1 after IRI or sham operation. Lines and whiskers in dot plots indicate (B–F) the mean and SEM, respectively, or (G) the median and interquartile range, respectively. B/L, bilateral; DCF, dichlorofluorescein; HPF, high-power field; MFI, mean fluorescence intensity. *P=0.05 for PBS/IRI group versus G-CSF/IRI group; **P=0.01 for PBS/IRI group versus G-CSF/IRI group; #P=0.05 for PBS/sham group versus G-CSF/sham group (t test in [B–F]; Mann–Whitney test in [G]); ##P=0.01 for PBS/sham group versus G-CSF/sham group (t test in [B–F]; Mann–Whitney test in [G]).
G-CSF Treatment following IRI Facilitates Renal Recovery and Attenuates Renal Fibrosis
We then investigated the effect of G-CSF treatment following IRI on renal IRI (Figure 2A). Levels of plasma creatinine and BUN were significantly lower in the G-CSF/IRI group relative to those in the PBS/IRI group on day 5 (Figure 2B). G-CSF treatment following IRI increased renal infiltration of F4/80+CD11b+ macrophages and revealed a trend of increasing Tregs on day 5 (P=0.08) (Figure 2C). Renal expression of Dectin-1 and iNOS was lower in the G-CSF/IRI group than that in the PBS/IRI group, whereas that of CD206 was higher in the G-CSF/IRI group (Figure 2D). Additionally, G-CSF treatment increased renal expression of Arg1 and IL-10 after IRI (Figure 2D), and ROS production in renal CD11b+Gr-1+ cells also increased in the G-CSF/IRI group (Figure 2D).
Figure 2.
G-CSF treatment following IRI facilitates renal recovery and attenuates renal fibrosis. (A) G-CSF or PBS was administered to B6 mice after bilateral IRI or sham operation, and kidneys and spleens were harvested along with blood sampling on day 5.5 after IRI. (B) Levels of plasma creatinine and BUN on day 5.5 after IRI. (C) Absolute numbers of CD45+ leukocytes, F4/80+CD11b+ macrophages, CD3+ T cells, and CD19+ B cells that infiltrated the kidneys. Proportions of renal CD4+forkhead box P3 (Foxp3)+ Tregs among CD4+ T cells. (D) mRNA expression of C-type lectin domain family 7 member A (Clec7a; encoding Dectin-1), Cd206, Arg1, Il10, and iNos in renal tissues normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression and ROS production (DCF-DA) in renal CD11b+Gr-1+ leukocytes. (E) G-CSF or PBS was administered to B6 mice after unilateral IRI or sham operation, and kidneys were harvested on day 28 after IRI. (F) Renal fibrosis was measured by Masson trichrome (MT) staining. Magnification, ×200. (G) Renal deposition of COLIV, fibronectin, and α-SMA was measured by western blot and normalized to β-actin levels. mRNA expression of ColIV, fibronectin, and αSma in renal tissues was normalized to Gapdh expression. Renal hydoxyproline contents were also measured. Lines and whiskers in dot plots indicate the mean and SEM, respectively. B/L, bilateral; HPF, high-power field; U/L, unilateral. *P=0.05 for PBS/IRI group versus G-CSF/IRI group; **P=0.01 for PBS/IRI group versus G-CSF/IRI group; #P=0.05 for PBS/sham group versus G-CSF/sham group (t test); ##P=0.01 for PBS/sham group versus G-CSF/sham group (t test).
Assessment of long-term renal fibrosis revealed significant decreases in the degree of renal fibrosis in the G-CSF/IRI group compared with that observed in the PBS/IRI group (Figure 2, E and F). Moreover, renal mRNA and protein levels of COLIV, fibronectin, and α-SMA were decreased following G-CSF treatment (Figure 2G, Supplemental Figure 6). Renal hydroxyproline contents were also decreased by G-CSF treatment (Figure 2G). These findings suggest that G-CSF treatment following IRI facilitated renal recovery and attenuated renal fibrosis.
G-CSF Treatment Increases Renal Infiltration of MDSCs
We found no difference in renal infiltration of F4/80−CD11b+Gr-1+ cells between the PBS/IRI and G-CSF/IRI groups (Figure 3A). When we divided CD11b+Gr-1+ cells into CD11b+Gr-1int MDSCs and CD11b+Gr-1high mature neutrophils, G-CSF treatment increased renal infiltration of CD11b+Gr-1int MDSCs and decreased that of CD11b+Gr-1high mature neutrophils after IRI (Figure 3A). Additionally, we found that G-CSF treatment prior to IRI increased CD11b+Gr-1int MDSCs in the bone marrow, peripheral blood, and spleen (Supplemental Figure 7). When granulocytic and monocytic MDSCs were defined as CD11b+Ly6GintLyClow and CD11b+Ly6G−Ly6Chigh, respectively, we observed that G-CSF treatment increased renal infiltration of CD11b+Ly6GintLyClow granulocytic MDSCs and decreased that of CD11b+Ly6G−Ly6Chigh monocytic MDSCs and CD11b+Ly6GhighLy6Clow mature neutrophils after IRI (Figure 3B). However, G-CSF–induced MDSC expansion was not observed on day 3 or 5 (Supplemental Figures 4 and 5). Next, analysis of the effect of G-CSF treatment following IRI on MDSCs revealed that post-IRI G-CSF treatment also increased renal infiltration of CD11b+Gr-1+ and CD11b+Gr-1int MDSCs (Figure 3C). Furthermore, renal infiltration of CD11b+Ly6GintLyClow granulocytic MDSCs on day 5.5 after IRI increased along with no change in the renal infiltration of CD11b+Ly6G−Ly6Chigh monocytic MDSCs (Figure 3D). These data demonstrated that G-CSF treatment increased renal infiltration of CD11b+Gr-1int MDSCs and especially, CD11b+Ly6GintLyClow granulocytic MDSCs.
Figure 3.
G-CSF treatment increases renal infiltration of MDSCs. (A and B) Absolute numbers of renal MDSCs on day 1 after IRI or sham operation, where G-CSF or PBS was administered prior to IRI or sham operation. (A) Renal CD45+F4/80− cells were categorized as CD11b+Gr-1+ cells, CD11b+Gr-1int MDSCs, or CD11b+Gr-1high mature neutrophils according to flow cytometric staining patterns for CD11b and Gr-1. (B) Renal CD45+F4/80−CD11b+ cells were categorized as Ly6GintLyClow granulocytic MDSCs, Ly6GhighLy6Clow mature neutrophils, or Ly6G−Ly6Chigh monocytic MDSCs. (C and D) Absolute numbers of renal MDSCs on day 5.5 after IRI or sham operation, where G-CSF or PBS was administered following IRI or sham operation. Renal (C) CD45+F4/80− cells and (D) CD45+F4/80−CD11b+ cells were categorized similar to (A and B), respectively. Lines and whiskers in dot plots indicate the mean and SEM, respectively. **P=0.01 for PBS/IRI group versus G-CSF/IRI group (t test).
G-CSF–Induced MDSCs Suppress In Vitro T Cell Activation
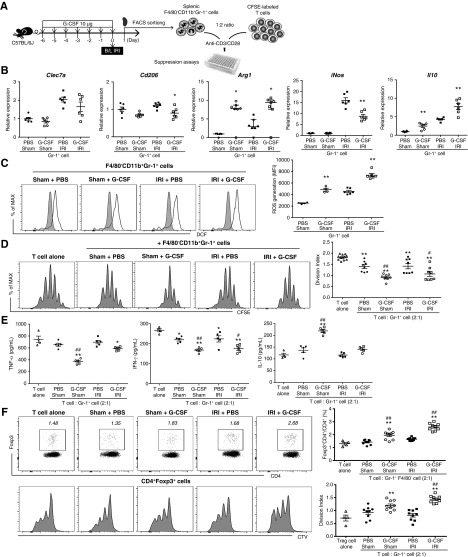
Next, we investigated whether G-CSF–induced Gr-1+ cells are true MDSCs capable of suppressing T cell activation. At 1 day after G-CSF treatment and IRI, we sorted splenic F4/80−CD11b+Gr-1+ cells and added them to 5,6-carboxyfluorescein diacetate succinimidyl ester–labeled T cells stimulated by plate-bound anti-CD3 and anti-CD28 (Figure 4A). Sorted Gr-1+ cells in the G-CSF/IRI group expressed higher levels of Arg1 and IL-10 than those in the sham/IRI group, whereas they expressed lower levels of iNOS (Figure 4B). Additionally, ROS production in isolated Gr-1+ cells was higher in the G-CSF/sham group than in the PBS/sham group, and it was highest in the G-CSF/IRI group (Figure 4C). In suppression assays, Gr-1+ cells induced by G-CSF in the presence or absence of IRI successfully suppressed T cell proliferation (Figure 4D) and secretion of TNF-α and IFN-γ, whereas they induced IL-10 secretion (Figure 4E). G-CSF–induced MDSCs also increased proportions and proliferation of Tregs by coculture (Figure 4F).
Figure 4.
G-CSF–induced MDSCs suppress in vitro T cell activation. (A) G-CSF or PBS was administered prior to IRI or sham operation. Splenic F4/80−CD11b+Gr-1+ cells were sorted on day 1 after IRI or sham operation and cocultured with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled T cells stimulated by plate-bound anti-CD3/CD28. (B) mRNA expression of C-type lectin domain family 7 member A (Clec7a; encoding Dectin-1), Cd206, Arg1, Il10, and iNos in splenic F4/80−CD11b+Gr-1+ cells was normalized to glyceraldehyde 3-phosphate dehydrogenase expression. (C) ROS production in splenic CD11b+Gr-1+ cells was measured by DCF-DA. *P=0.05 for PBS/sham group versus G-CSF/sham group or PBS/IRI group versus G-CSF/IRI group in (B and C); t test in all except Arg1; Mann–Whitney test for Arg1; **P=0.01 for PBS/sham group versus G-CSF/sham group or PBS/IRI group versus G-CSF/IRI group in (B and C); t test in all except Arg1; Mann–Whitney test for Arg1. (D) T cell proliferation measured by CFSE staining was expressed as division index. (E) Levels of TNF-α, IFN-γ, and IL-10 in coculture supernatant. (F) Proportions of Tregs (forkhead box P3 [Foxp3]+CD4+/CD4+; percentage) were measured by flow cytometric analysis, and Treg proliferation measured by CellTrace Violet (CTV) staining was expressed as division index. *P=0.05 compared with T cell alone group; **P=0.01 compared with T cell alone group; #P=0.05 in comparison between PBS/sham group versus G-CSF/sham group or PBS/IRI group versus G-CSF/IRI group (ANOVA test, Tukey post hoc analysis) in (D–F); ##P=0.01 in comparison between PBS/sham group versus G-CSF/sham group or PBS/IRI group versus G-CSF/IRI group (ANOVA test, Tukey post hoc analysis) in (D–F). Lines and whiskers in dot plots indicate the mean and SEM, respectively, or the median and interquartile range (Arg1), respectively. B/L, bilateral; DCF, dichlorofluorescein; MFI, mean fluorescence intensity.
When we divided Gr-1+ cells into Gr-1high and Gr-1int subsets, Gr-1high cells include more mature neutrophils with segmented nuclei than Gr-1int cells. Immature myeloid cells with banded or ring-shaped nuclei were dominant in the Gr-1int cells (Supplemental Figure 8A). Gr-1int cells expressed higher levels of CD244 than Gr-1high cells (Supplemental Figure 8B). Additionally, Gr-1int cells exhibited lower expression of TNF-α and higher expression of MPO, Arg1, and IL-10 than Gr-1high cells (Supplemental Figure 8C). Comparison of the in vitro suppressive activity of Gr-1high and Gr-1int cells revealed that Gr-1int cells effectively suppressed T cell proliferation, whereas Gr-1high cells failed to suppress this activity (Supplemental Figure 8D). These data suggested that Gr-1int cells represented the main G-CSF–induced MDSC population and that Gr-1high cells represented proinflammatory mature neutrophils.
Adoptive Transfer of G-CSF–Induced MDSCs Attenuates Renal IRI
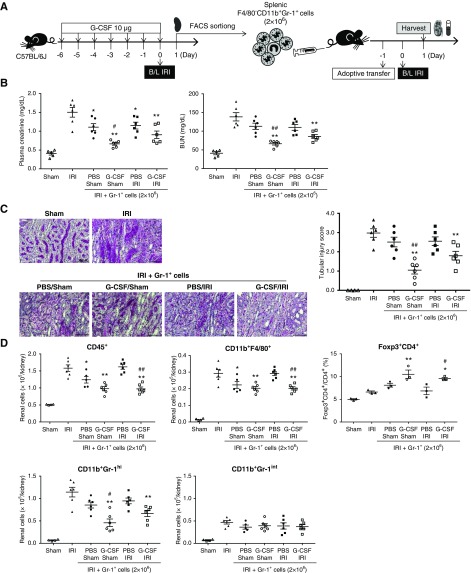
We then sorted splenic F4/80−CD11b+Gr-1+ cells from mice that were treated with G-CSF or PBS in the presence or absence of IRI (Figure 5A). Adoptive transfer of these cells 1 day prior to IRI significantly improved renal functions on day 1 after IRI (Figure 5B). Furthermore, transfer of Gr-1+ cells induced by G-CSF led to the best renal function (Figure 5B) and simultaneously improved renal tissue injury (Figure 5C). Additionally, these cells significantly suppressed renal infiltration of F4/80+CD11b+ macrophages and CD11b+Gr-1high neutrophils 1 day after IRI (Figure 5D). Although there was no increase in renal CD11b+Gr-1int MDSCs, MDSC transfer increased renal infiltration of Tregs (Figure 5D). These data identified G-CSF–induced Gr-1+ cells as functional MDSCs capable of attenuating renal IRI. Furthermore, adoptive transfer of G-CSF–induced Ly6GintLyClow granulocytic and Ly6G−LyChigh monocytic MDSCs improved renal functions and reduced renal infiltration of inflammatory cells, whereas transfer of Ly6GhighLy6Clow neutrophils did not (Supplemental Figure 9). Therefore, G-CSF–induced Ly6GintLyClow and Ly6G−LyChigh cells represent functional granulocytic and monocytic MDSCs, respectively.
Figure 5.
Adoptive transfer of G-CSF–induced MDSCs prior to IRI attenuates renal IRI. (A) G-CSF or PBS was administered prior to IRI or sham operation, and splenic F4/80−CD11b+Gr-1+ cells were sorted on day 1 after IRI or sham operation. Sorted cells were then adoptively transferred to mice 1 day prior to IRI, and the kidneys were harvested along with blood sampling on day 1 after IRI. (B) Levels of plasma creatinine and BUN on day 1. (C) Renal tissue injury scores were measured on day 1 after IRI. Magnification, ×200. (D). Absolute numbers of CD45+ leukocytes, F4/80+CD11b+ macrophages, CD11b+Gr-1high neutrophils, and CD11b+Gr-1int MDSCs that infiltrated the kidneys. Proportions of renal CD4+forkhead box P3 (Foxp3)+ Tregs among CD4+ T cells. Line and whiskers in dot plots indicate the mean and SEM, respectively. B/L, bilateral. *P=0.05 compared with T cell alone group; **P=0.01 compared with T cell alone group; #P=0.05 in comparison between PBS/sham group versus G-CSF/sham group or PBS/IRI group versus G-CSF/IRI group (ANOVA test, Tukey post hoc analysis); ##P=0.01 in comparison between PBS/sham group versus G-CSF/sham group or PBS/IRI group versus G-CSF/IRI group (ANOVA test, Tukey post hoc analysis).
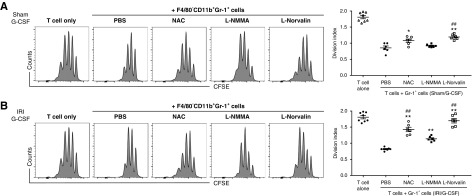
G-CSF–Induced MDSCs Suppressed T Cell Proliferation Mainly through ROS and Arg1
To elucidate the suppressive mechanisms associated with G-CSF–induced Gr-1+ MDSCs against T cell proliferation, we added NAC, L-NMMA, or L-Norvaline to cocultures of T cells and MDSCs as an inhibitor of ROS, iNOS, or Arg1, respectively. Use of F4/80−CD11b+Gr-1+ MDSCs induced by G-CSF in the absence of IRI revealed that NAC and L-Norvaline attenuated their suppressive activity, whereas L-NMMA did not (Figure 6A). Additionally, both NAC and L-Norvaline abrogated the suppressive activity of Gr-1+ MDSCs induced by G-CSF in the presence of IRI. Moreover, the inhibitory effects of NAC and L-Norvaline on MDSC-mediated suppression were greater than those of L-NMMA (Figure 6B). These results suggested that the primary mechanisms associated with the suppressive effects of G-CSF–induced MDSCs involved ROS and Arg1 rather than iNOS.
Figure 6.
G-CSF–induced MDSCs suppress T cell proliferation mainly through ROS and Arg1. Splenic F4/80−CD11b+Gr-1+ MDSCs prepared from the (A) G-CSF/sham group or the (B) G-CSF/IRI group were cocultured with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled T cells. NAC, L-NMMA, L-Norvaline, or PBS was added to the coculture of T cells and MDSCs, and T cell proliferation was measured by CFSE staining and expressed as division index. Lines and whiskers in dot plots indicate the mean and SEM, respectively. *P=0.05 compared with the PBS group; **P=0.01 compared with the PBS group; ##P=0.01 compared with the L-NMMA group (ANOVA test, Tukey post hoc analysis).
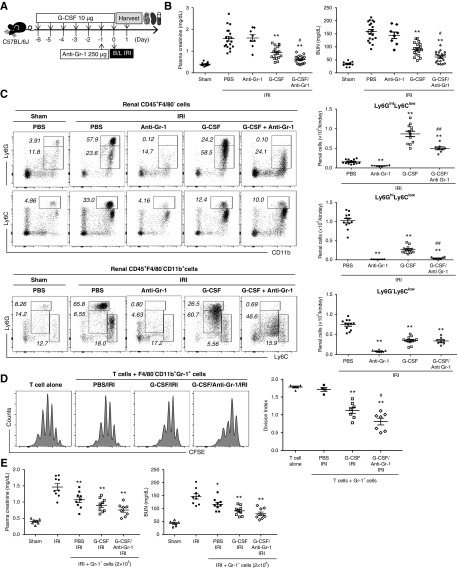
Combined Treatment with G-CSF and Anti–Gr-1 Antibody Synergistically Attenuates Renal IRI
Next, we investigated the combined effects of G-CSF and anti–Gr-1 on IRI (Figure 7A). Anti–Gr-1 treatment alone did not improve renal function after IRI; however, addition of anti–Gr-1 to G-CSF led to improved renal function relative to that observed following G-CSF treatment alone, with combination therapy with G-CSF and anti–Gr-1 almost completely ameliorating renal dysfunction after IRI (Figure 7B). G-CSF treatment decreased CD11b+Ly6GhighLy6Clow mature neutrophils after IRI compared with the PBS control; however, they still remain in the kidney. By contrast, addition of anti–Gr-1 to G-CSF depleted CD11b+Ly6GhighLy6Clow mature neutrophils with the remaining CD11b+Ly6GintLyClow granulocytic MDSCs in the kidney after IRI (Figure 7C). Combination treatment of anti–Gr-1 antibodies with G-CSF induced relative dominance of renal MDSCs over mature neutrophils compared with G-CSF treatment alone. When F4/80−CD11b+Gr-1+ cells were sorted from mice and added to T cells, Gr-1+ cells in the G-CSF/anti–Gr-1/IRI group suppressed in vitro T cell proliferation more effectively than Gr-1+ cells in the G-CSF/IRI group (Figure 7D). Moreover, adoptive transfer of Gr-1+ cells from the G-CSF/anti–Gr-1/IRI group at 1 day prior to IRI improved renal function on day 1 after IRI; however, their renoprotective effect was not significantly better than that of Gr-1+ cells from the G-CSF/IRI group (Figure 7E). These findings suggested that combined treatment with G-CSF and anti–Gr-1 increased renal infiltration of granulocytic MDSCs with depletion of mature neutrophils.
Figure 7.
Combined treatment with G-CSF and the anti–Gr-1 antibody synergistically attenuates renal IRI. (A) G-CSF with or without anti–Gr-1 was administered prior to IRI, and kidneys were harvested along with blood sampling on day 1 after IRI. (B) Levels of plasma creatinine and BUN on day 1. (C) Proportions of CD11b+Ly6GhighLy6Clow mature neutrophils, CD11b+Ly6GintLyClow granulocytic MDSCs, and CD11b+Ly6G−LyChigh monocytic MDSCs among renal CD45+F4/80−CD11b+ cells. (D) F4/80−CD11b+Gr-1+ cells sorted from the PBS/IRI group, the G-CSF/IRI group, or the G-CSF/anti–Gr-1/IRI group were assessed for their suppressive activity against T cell proliferation. In vitro T cell proliferation was measured by 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) and expressed as division index. (E) F4/80−CD11b+Gr-1+ cells sorted from the PBS/IRI group, the G-CSF/IRI group, or the G-CSF/anti–Gr-1/IRI group were adoptively transferred to mice at 1 day prior to IRI. Levels of plasma creatinine and BUN 1 day after IRI. Lines and whiskers in dot plots indicate the mean and SEM, respectively. B/L, bilateral. *P=0.05 compared with the PBS/IRI group; **P=0.01 compared with the PBS/IRI group; #P=0.05 between the G-CSF/IRI group and the G-CSF/anti–Gr-1/IRI group (ANOVA test, Tukey post hoc analysis); ##P=0.01 between the G-CSF/IRI group and the G-CSF/anti–Gr-1/IRI group (ANOVA test, Tukey post hoc analysis).
Preferential Depletion of MDSCs by Pep-G3 or Gemcitabine Abrogated Beneficial Effects of G-CSF on Renal IRI
When pep-G3 along with G-CSF was administered prior to IRI (Figure 8A), pep-G3 treatment abrogated the beneficial effects of G-CSF on renal IRI (Figure 8B). Pep-G3 depleted both Ly6GintLyClow granulocytic MDSCs and Ly6G−LyChigh monocytic MDSCs with minimal effect on Ly6GhighLy6Clow mature neutrophils in kidney after IRI (Figure 8C). Gemcitabine also abrogated renoprotective effects of G-CSF against renal IRI by depleting MDSCs, especially granulocytic MDSCs (Supplemental Figure 10). These results supported that renal MDSC induction is the main mechanism of G-CSF–mediated renoprotective effects against renal IRI.
Figure 8.
Preferential depletion of MDSCs by pep-G3 abrogated beneficial effects of G-CSF on renal IRI. (A) G-CSF with or without pep-G3 was administered prior to IRI, and kidneys were harvested along with blood sampling on day 1 after IRI. (B) Levels of plasma creatinine and BUN on day 1. (C) Proportions of CD11b+Ly6GhighLy6Clow mature neutrophils, CD11b+Ly6GintLyClow granulocytic MDSCs, and CD11b+Ly6G−LyChigh monocytic MDSCs among renal CD45+F4/80−CD11b+ cells. Lines and whiskers in dot plots indicate the mean and SEM, respectively. B/L, bilateral. *P=0.05 compared with the PBS/IRI group; **P=0.01 compared with the PBS/IRI group; ##P=0.01 between the G-CSF/IRI group and the G-CSF/pep-G3/IRI group (ANOVA test and Tukey post hoc analysis).
Discussion
This study showed that G-CSF treatment increased MDSC infiltration, especially granulocytic MDSCs, into renal tissue after IRI and that G-CSF treatment prior to IRI attenuated acute tissue injury, renal apoptosis, and renal inflammation after IRI. Additionally, G-CSF treatment following IRI facilitated renal recovery and attenuated renal fibrosis. Moreover, combined treatment with G-CSF and the anti–Gr-1 antibody synergistically attenuated renal IRI by increasing renal infiltration of MDSCs and minimizing infiltration of mature neutrophils. Furthermore, we successfully demonstrated the suppressive activity of G-CSF–induced Gr-1+ MDSCs against in vitro T cell activation and in vivo renal IRI, suggesting that these cells were indeed immunosuppressive MDSCs. Our results suggested that granulocytic MDSCs played a major role through ROS and Arg1 in G-CSF–induced beneficial effects on renal IRI.
We selected CD11b+Gr-1+ MDSCs, CD11b+Ly6GintLy6Clow granulocytic MDSCs, and CD11b+Ly6G−Ly6Chigh monocytic MDSCs from the F4/80− population to maximize exclusion of M2 macrophage populations. However, there remains an overlap between monocytic MDSCs and other myeloid suppressors, including M2 macrophages, and exclusion of the F4/80− population might also remove some M2-like monocytic MDSCs.28 In this study, results showing that G-CSF treatment and subsequent induction of renal MDSCs decreased renal F4/80+CD11b+ cells on day 1 and increased renal F4/80+CD11b+ cells on day 5.5 were indicative of decreased infiltration of proinflammatory M1 macrophages and expression of Dectin-1 and iNOS during the acute injury phase and increased infiltration of anti-inflammatory M2 macrophages and expression of CD206 during the recovery phase in the kidneys.
It is difficult to distinguish granulocytic MDSCs from mature neutrophils on the basis of cell surface markers without functional activity.29–31 However, granulocytic MDSCs express higher levels of CD244 and MPO, express lower levels of TNF-α, and exhibit higher activities of Arg1 and ROS than mature neutrophils.29,31 Morphologically, mature neutrophils show typical segmented nuclei, whereas granulocytic MDSCs include many immature myeloid cells with banded or ring-shaped nuclei.32 Furthermore, granulocytic MDSCs are capable of suppressing T cell activation in contrast to neutrophils.31 In this study, we found that sorted Gr-1int cells exhibited higher levels of CD244, MPO, Arg1, and IL-10; lower levels of TNF-α; and more banded or ring-shaped nuclei than sorted Gr-1high cells and successfully suppressed in vitro T cell proliferation in contrast to Gr-1high cells, which failed to suppress T cell proliferation. Furthermore, Ly6GintLyClow and Ly6G−LyChigh cells attenuated renal IRI, whereas Ly6GhighLy6Clow cells did not. Therefore, a majority of the CD11b+Ly6GhighLy6C− population seemed to represent mature neutrophils, whereas granulocytic MDSCs seemed dominant among the CD11b+Ly6GintLy6Clow population, despite some overlap.
Although anti–Gr-1 antibodies have been used to deplete MDSCs, their effects on preferential depletion of MDSCs are controversial.33–35 In this study, anti–Gr-1 antibodies depleted Ly6GhighLy6Clow mature neutrophils nearly completely, and they also reduced both Ly6GintLy6Clow granulocytic MDSCs and Ly6G−Ly6Chi monocytic MDSCs. The synergistic effects of G-CSF and the anti–Gr-1 antibody could be attributed to the depletion of proinflammatory mature neutrophils by anti–Gr-1 treatment despite a reduced number of anti-inflammatory granulocytic MDSCs: in other words, tipping balance to anti-inflammatory MDSCs over proinflammatory mature neutrophils in renal tissues. However, preferential depletion of MDSCs with relative saving of mature neutrophils by pep-G321 or gemcitabine22,23 abrogated renoprotective effects of G-CSF against renal IRI, confirming that renal MDSC expansion is the primary mechanism of G-CSF–induced renoprotective effects against renal IRI. One of obstacles to clinical application of combined treatment of G-CSF anti–Gr-1 antibody is the lack of known human homolog of murine Gr-1; however, human CD177 has been proposed as a potential homolog, and therefore, it is worthy of further investigation as a potential therapeutic target.36
MDSCs can suppress T cells via ROS, NO, or Arg1 and B cell accumulation through signal transducer and activator of transcription 3.8 Additionally, MDSCs can induce Tregs through IFN-γ, IL-10, or cytotoxic T lymphocyte–associated antigen-4 independent of NO and TGF-β.5 In this study, we found that G-CSF treatment increased levels of ROS, Arg1, and IL-10 and that G-CSF treatment showed a trend of increasing renal Tregs. Furthermore, G-CSF–induced MDSCs increased proportions and proliferation of Tregs in coculture, and adoptive transfer of MDSCs significantly increased renal Tregs after IRI. These data suggested that Tregs might partially contribute to G-CSF/MDSC-induced beneficial effects on IRI. Granulocytic MDSCs suppress T cell activation mainly through ROS and Arg1, whereas monocytic MDSCs suppress this activity mainly through NO and Arg1.5,37 In this study, in vitro inhibition experiments demonstrated that the suppressive effects of G-CSF–induced MDSCs were mainly dependent on ROS and Arg1 rather than NO, which was consistent with findings that G-CSF treatment mainly induced granulocytic MDSCs rather than monocytic MDSCs in IRI.
We used G-CSF as a simple and effective method of inducing MDSC expansion because G-CSF treatment is more convenient than MDSC therapy for clinical applications. A recent study reported that granulocyte macrophage colony-stimulating factor (GM-CSF) treatment promoted alternative activation of macrophages and contributed to recovery after IRI.38 Additionally, a pathway associated with macrophage colony-stimulating factor (M-CSF) contributed to recovery from AKI through induction of M2 macrophges.39 Although MDSCs share many phenotypes with M2 macrophages, they represent differently defined immunosuppressive populations, and we focused on MDSCs in this study. Previous studies indicated that G-CSF treatment showed better protective effects against cisplatin-induced AKI than M-CSF and that G-CSF induced MDSC expansion to a greater extent than GM-CSF.15,40 Another reason for choosing G-CSF rather than GM-CSF or M-CSF for MDSC induction was that it is more commonly used in clinics, and its safety is well established. Interestingly, G-CSF levels in patients with cancer have been proposed as a potential marker of early cancer progression and therapeutic response in parallel with a potential prognostic role of MDSCs in cancer progression and therapeutic response.6
This study has a few limitations. First, better cellular markers are required to distinguish MDSCs or MDSC subsets from mature neutrophils, M2 macrophages, and other regulatory myeloid cells because the current markers cannot avoid some overlap among these populations; therefore, further studies are warranted to address this issue. Second, we used human G-CSF instead of mouse G-CSF in mice due to its availability and future clinical application.15 Because of species incompatibility, we used human G-CSF at a much higher dose in mice than the usual clinical dose administered for neutropenia.15 Therefore, further studies are needed to investigate the optimal dose of human G-CSF necessary to induce human MDSCs and whether G-CSF treatment can attenuate renal IRI in humans.
Nevertheless, this study elucidated the protective roles of G-CSF–induced MDSCs against renal IRI and significantly contributes to this field by providing a basis for further studies investigating the roles of MDSCs and the clinical utility of G-CSF treatment in human renal IRI.
In conclusion, G-CSF induced MDSCs and thereby, attenuated acute renal injury and chronic renal fibrosis after IRI, suggesting the therapeutic potential of MDSCs and G-CSF for renal IRI.
Disclosures
Dr. Yan reports grants from the Ministry of Science and ICT, Republic of Korea, during the conduct of the study. Dr. Piao reports grants from the Ministry of Science and ICT, Republic of Korea, during the conduct of the study. Dr. Jang reports grants from the Ministry of Science and ICT, Republic of Korea, during the conduct of the study. Dr. Yang reports grants from the Ministry of Science and ICT, Republic of Korea, during the conduct of the study. All remaining authors have nothing to disclose.
Funding
This study was supported by a grant from the Ministry of Science and ICT grants (NRF-2015R1A2A2A01002706 and NRF-2018R1A2B3001179).
Supplementary Material
Acknowledgments
We thank Je-In Youn (Research Institute, ProGen Inc., Republic of Korea) and Myung-Gyu Kim (Korea University Medical College) for their critical review, and we also thank Kyohwa Kirin Korea Co., Ltd. and Larry Kwak for providing us human granulocyte colony-stimulating factor and pep-G3, respectively.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Coaxing Anti-Inflammatory Granulocytes to Prevent Ischemic Kidney Injury: A Fine Balance,” on pages 668–670.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019060601/-/DCSupplemental.
Supplemental Figure 1. Dose-response effects of granulocyte colony-stimulating factor on renal ischemia-reperfusion injury.
Supplemental Figure 2. Flow cytometric gating strategies for immune cells in the kidneys and spleen.
Supplemental Figure 3. Renal ischemia-reperfusion injury increases granulocyte colony-stimulating factor and granulocyte colony-stimulating factor receptor levels in the kidney.
Supplemental Figure 4. Effects of granulocyte colony-stimulating factor treatment prior to ischemia-reperfusion injury on renal ischemia-reperfusion injury on day 3.
Supplemental Figure 5. Effects of granulocyte colony-stimulating factor treatment prior to ischemia-reperfusion injury on renal ischemia-reperfusion injury on day 5.
Supplemental Figure 6. Granulocyte colony-stimulating factor treatment decreased renal fibrosis after renal ischemia-reperfusion injury.
Supplemental Figure 7. Granulocyte colony-stimulating factor treatment induces myeloid-derived suppressor cell expansion in the bone marrow, peripheral blood, and spleen.
Supplemental Figure 8. F4/80−CD11b+Gr-1int myeloid-derived suppressor cells suppress in vitro T cell activation, whereas F4/80−CD11b+Gr-1high mature neutrophils do not.
Supplemental Figure 9. Effect of transfer of granulocyte colony-stimulating factor–induced granulocytic myeloid-derived suppressor cells, mature neutrophils, or monocytic myeloid-derived suppressor cells on renal ischemia-reperfusion injury.
Supplemental Figure 10. Preferential depletion of myeloid-derived suppressor cells by gemcitabine abrogated beneficial effects of granulocyte colony-stimulating factor on renal ischemia-reperfusion injury.
Supplemental Table 1. Primer sets used for real-time RT-PCR.
References
- 1.Jang HR, Rabb H: Immune cells in experimental acute kidney injury. Nat Rev Nephrol 11: 88–101, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, et al.: Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, et al.: Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int 76: 717–729, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Kim MG, Koo TY, Yan JJ, Lee E, Han KH, Jeong JC, et al.: IL-2/anti-IL-2 complex attenuates renal ischemia-reperfusion injury through expansion of regulatory T cells. J Am Soc Nephrol 24: 1529–1536, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabrilovich DI, Nagaraj S: Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavakkoli M, Wilkins CR, Mones JV, Mauro MJ: A novel paradigm between leukocytosis, G-CSF secretion, neutrophil-to-lymphocyte ratio, myeloid-derived suppressor cells, and prognosis in non-small cell lung cancer. Front Oncol 9: 295, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budhwar S, Verma P, Verma R, Rai S, Singh K: The yin and yang of myeloid derived suppressor cells. Front Immunol 9: 2776, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knier B, Hiltensperger M, Sie C, Aly L, Lepennetier G, Engleitner T, et al.: Myeloid-derived suppressor cells control B cell accumulation in the central nervous system during autoimmunity. Nat Immunol 19: 1341–1351, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scalea JR, Lee YS, Davila E, Bromberg JS: Myeloid-derived suppressor cells and their potential application in transplantation. Transplantation 102: 359–367, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lees JR, Azimzadeh AM, Bromberg JS: Myeloid derived suppressor cells in transplantation. Curr Opin Immunol 23: 692–697, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Zhang T, Diao W, Jin F, Shi L, Meng J, et al.: Role of myeloid-derived suppressor cells in glucocorticoid-mediated amelioration of FSGS. J Am Soc Nephrol 26: 2183–2197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al.: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang MZ, Wang X, Wang Y, Niu A, Wang S, Zou C, et al.: IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int 91: 375–386, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pegues MA, McWilliams IL, Szalai AJ: C-reactive protein exacerbates renal ischemia-reperfusion injury: Are myeloid-derived suppressor cells to blame? Am J Physiol Renal Physiol 311: F176–F181, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adeegbe D, Serafini P, Bronte V, Zoso A, Ricordi C, Inverardi L: In vivo induction of myeloid suppressor cells and CD4(+)Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell Transplant 20: 941–954, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Wang K, Lv M, Chang YJ, Zhao XY, Zhao XS, Zhang YY, et al.: Early myeloid-derived suppressor cells (HLA-DR-/lowCD33+CD16-) expanded by granulocyte colony-stimulating factor prevent acute graft-versus-host disease (GVHD) in humanized mouse and might contribute to lower GVHD in patients post allo-HSCT. J Hematol Oncol 12: 31, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoso A, Serafini P, Lanzoni G, Peixoto E, Messinger S, Mantero A, et al.: G-CSF and exenatide might be associated with increased long-term survival of allogeneic pancreatic islet grafts. PLoS One 11: e0157245, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koo TY, Lee JG, Yan JJ, Jang JY, Ju KD, Han M, et al.: The P2X7 receptor antagonist, oxidized adenosine triphosphate, ameliorates renal ischemia-reperfusion injury by expansion of regulatory T cells. Kidney Int 92: 415–431, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Salih E, Igwebuike C, Mulhern R, Bonegio RG, Havasi A, et al.: Nucleophosmin phosphorylation as a diagnostic and therapeutic target for ischemic AKI. J Am Soc Nephrol 30: 50–62, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr KD, Sieve AN, Indramohan M, Break TJ, Lee S, Berg RE: Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur J Immunol 41: 2666–2676, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, et al.: Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 20: 676–681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al.: 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 70: 3052–3061, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Yi H, Guo C, Yu X, Zuo D, Wang XY: Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol 189: 4295–4304, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brender C, Tannahill GM, Jenkins BJ, Fletcher J, Columbus R, Saris CJ, et al.: Suppressor of cytokine signaling 3 regulates CD8 T-cell proliferation by inhibition of interleukins 6 and 27. Blood 110: 2528–2536, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Yan JJ, Lee JG, Jang JY, Koo TY, Ahn C, Yang J: IL-2/anti-IL-2 complexes ameliorate lupus nephritis by expansion of CD4+CD25+Foxp3+ regulatory T cells. Kidney Int 91: 603–615, 2017. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Liu L, Pazhanisamy SK, Li H, Meng A, Zhou D: Total body irradiation causes residual bone marrow injury by induction of persistent oxidative stress in murine hematopoietic stem cells. Free Radic Biol Med 48: 348–356, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK: Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21: 1231–1239, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Ochando JC, Chen SH: Myeloid-derived suppressor cells in transplantation and cancer. Immunol Res 54: 275–285, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zilio S, Serafini P: Neutrophils and granulocytic MDSC: The janus god of cancer immunotherapy. Vaccines (Basel) 4: E31, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Nefedova Y, Lei A, Gabrilovich D: Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin Immunol 35: 19–28, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI: Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 91: 167–181, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al.: Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Reports 10: 562–573, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Xing YF, Zhou YQ, Ma GW, Feng DY, Cai XR, Li X: Issues with anti-Gr1 antibody-mediated myeloid-derived suppressor cell depletion. Ann Rheum Dis 75: e49, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Wang XY, Yi H, Li J: Response to: ‘Issues with anti-Gr1 antibody-mediated myeloid-derived suppressor cell depletion’ by Xing et al. Ann Rheum Dis 75: e50, 2016. [DOI] [PubMed] [Google Scholar]
- 35.Ma C, Kapanadze T, Gamrekelashvili J, Manns MP, Korangy F, Greten TF: Anti-Gr-1 antibody depletion fails to eliminate hepatic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 92: 1199–1206, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hickey MJ: Has Ly6G finally found a job? Blood 120: 1352–1353, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al.: Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111: 4233–4244, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Huen SC, Huynh L, Marlier A, Lee Y, Moeckel GW, Cantley LG: GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol 26: 1334–1345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, et al.: CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwasaki M, Adachi Y, Minamino K, Suzuki Y, Zhang Y, Okigaki M, et al.: Mobilization of bone marrow cells by G-CSF rescues mice from cisplatin-induced renal failure, and M-CSF enhances the effects of G-CSF. J Am Soc Nephrol 16: 658–666, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.