Abstract
Background
As a common metabolic disorder, osteoporosis is characterized by decreasing bone mass density and increased possibility of fragility fracture. The incidence of senile osteoporosis increases year by year. There is no gold standard of treatment for osteoporosis. Tomatidine is the aglycone derivative of tomatine, having the ability to treat various diseases, including osteoporosis. However, the mechanism by which tomatidine improves osteoporosis has not been fully elucidated. Tomatidine is a potential and promising drug for osteoporosis.
Material/Methods
In this study, the KEGG pathways that tomatidine-targeted genes enriched in were obtained using bioinformatics methods. The KEGG pathways involved in osteoporosis that were also associated with tomatidine-targeted genes were selected. After analysis of these pathways, essential genes that may be involved in this biological process were identified and validated experimentally.
Results
We found 110 osteoporosis related KEGG pathways and 76 tomatidine-targeted genes-related KEGG pathways were obtained. 39 shared KEGG pathways were identified. The top 5 pathways were: pathway of chronic myeloid leukemia, pathway of B cell receptor signaling, pathway in cancer, bladder cancer pathway, and progesterone-mediated oocyte maturation pathway. MAPK1, MAP2K1, MAPK3, RAF1 were involved in all the 5 pathways. The p53 signaling pathway and the MAPK signaling pathway were involved in the 5 KEGG pathways. In vitro experiments showed that downregulating p53 expression could be potentially protective for osteoporosis.
Conclusions
Tomatidine can improve osteoporosis, and one of the mechanisms of its action is achieved by modulating p53. Tomatidine may be a promising drug for osteoporosis.
MeSH Keywords: Genes, p53; Osteoporosis; Therapeutics
Background
Osteoporosis is a metabolic disease that can cause pain and fragility fractures. It is characterized by bone mass loss and microarchitectural destruction. The incidence of osteoporotic fractures of women in their 50s is 50% [1]. There are 1 500 000 osteoporotic fractures each year in the USA [2]. Osteoporosis has become a serious worldwide health problem [3]. In recent years, progress has been made in the study of bone metabolism regulation and the pathogenesis of osteoporosis. The diagnosis and treatment methods for osteoporosis have also been rapidly developing. Further study of the osteoporosis pathogenesis and the action mechanism of osteoporosis drugs will promote the prevention and treatment for osteoporosis.
Tomatidine is a natural compound extracted from blue tomatoes [4]. It has a variety of biological activities and can protect tomato plants from bacteria, fungi, viruses, and certain insects during growth. Tomatidine may play a role in mitigating osteoporosis by inhibiting osteoclastogenesis and reducing estrogen deficiency-induced bone mass loss [5]. Nevertheless, related studies have been limited, and the mechanism remains unclear.
Bioinformatics is a method of synthesized analysis of biological information. With this method, we can make scientific hypotheses and predictions, find target genes, or perform significant data statistics. In some studies, the bioinformatics method has been applied to perform osteoporosis-related research, and the results are inspiring [6,7].
In this study, the shared KEGG pathways of osteoporosis and tomatidine-targeted genes were identified using bioinformatics methods. And then, the essential genes and signaling pathways were identified after the analysis of the top 5 shared KEGG pathways. Finally, the bioinformatics findings were validated by in vitro experiments.
Material and Methods
Tomatidine-targeted genes prediction
The target genes of tomatidine were obtained with Search Tool for Interacting Chemicals (STITCH). STITCH is a bioinformatic tool for retrieval and prediction of interactions among genes, chemicals, and proteins. The interaction network of tomatidine and its target genes was calculated with the STITCH online tool [8]. The calculation settings were as follows: 3 shells with the maximum number of interactions 10 for each shell; medium confidence score 0.4. Then the information of tomatidine-targeted genes was imported into Gephi software, and the weighted interaction network was constructed.
Genes enrichment analysis
Cytoscape 3.7.2 was used to construct the protein–protein interaction (PPI) network [9]. The centrality degree of each gene was obtained. The information of the genes was visualized by Circlize [10]. DAVID, (short for Database for Annotation, Visualization and Integrated Discovery [11,12]) was used for enrichment analysis. The symbols of all the tomatine-targeted genes were imported into DAVID. Enrichment results were visualized by GOplot [13].
Retrieval of the pathways involved in osteoporosis and calculation of shared pathways
MiRWalk is an online bioinformatics atlas tool [14]. In this study, the KEGG pathways involved in osteoporosis were retrieved with miRWalk. Then the intersection of tomatidine-targeted genes-related KEGG pathways (P≤0.05) and osteoporosis related KEGG pathways were obtained in Draw Venn Diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/). The top 5 shared KEGG pathways with the smallest P values were selected. The tomatidine-targeted genes-related part of the KEGG pathways was established with the Pathway Builder Tool 20 (www.proteinlounge.com).
Blood collection
From July 2017 to June 2019, peripheral blood samples from patients in Tongji Hospital, Tongji University School of Medicine (6 healthy volunteers, 6 postmenopausal osteoporosis patients) were collected for determination of mRNA levels. The patient portion of the research were approved by the Committees of Clinical Ethics in Tongji Hospital, Tongji University School of Medicine.
Cell culture and transfection
Human mesenchymal stem cells (hMSCs) were donated from Tongji Hospital, Tongji University School of Medicine. We cultured the cells in F12 media (Gibco; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum and 1% penicillin/streptomycin (all Gibco; Thermo Fisher Scientific, Inc.). Lipofectamine® 3000 was used to transfect hMSCs with small interfering (si)RNA oligos. P53 siRNAs (Guangzhou RiboBio Co., Ltd.) were transfected (50 nM).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Extraction of total RNA from cell and tissue samples was performed with TRIzol reagent from Thermo Fisher Scientific, Inc. The ReverTra Ace qPCR RT Master Mix of Toyobo Life Science was used to transcribe the RNA to cDNA. The RT reaction protocol: 42°C, 15 minutes; then 98°C, 5 minutes. The thermocycling protocol: 95°C, 30 seconds; 95°C, 5 seconds, 40 cycles; then 60°C, 30 seconds. The relative miRNA expression was normalized to those of the internal control (GAPDH) and were calculated using 2−ΔΔCq method. All procedures were conducted 3 times and the primer sequences are as followed:
-
Bcl-2, forward, GATAACGGAGGCTGGGATGC,
reverse, TCACTTGTGGCCCAGATAGG;
-
Bax, forward, CCCTTTTGCTTCAGGGTTTC,
reverse, GAGACACTCGCTCAGCTTCTTG;
-
cyclin D1, forward, TTGCCCTCTGTGCCACAGAT,
reverse, TCAGGTTCAGGCCTTGCACT;
-
cyclin D3, forward, CTGGCCATGAACTACCTGGA,
reverse, CCAGCAAATCATGTGCAATC;
-
ALP, forward, GCTCTGGAAAGTCCTTCAAAGC,
reverse, TCTTCTTCCCTGGACACTGCC;
-
OCN, forward, TCACACTCCTCGCCCTATTG,
reverse, CTCCTGAAAGCCGATGTGGT;
-
Runx2, forward, CTACTATGGCACTTCGTCAGGAT,
reverse, ATCAGCGTCAACACCATCATT;
-
GAPDH, forward, CCGTTGAATTTGCCGTGA,
reverse, TGATGACCCTTTTGGCTCCC.
Western blotting
We used phosphate-buffered saline (PBS) to rinse hMSCs 3 times. Radio immunoprecipitation assay (RIPA) lysis buffer (Aspen Pharmacare Holdings Ltd.; cat. no. AS1004) was used to extract the total proteins from cells. Cell lysates (1×104) were subjected to 10% SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) followed by the determination of protein concentration by the bicinchoninic acid (BCA) method. The proteins (50 μg) were then transferred onto a 10% SDS-polyvinylidene difluoride (PVDF) membrane; 5% milk solution was used for blocking at room temperature for 2 hours. A chemiluminescence detection system (Canon, Inc.; cat. no. LiDE110) was then used to visualize proteins based on the provided instructions. Antibodies: anti-Runt-related transcription factor 2 (Runx2; 1: 500; Abcam; cat. no. ab23981), anti-alkaline phosphatase (ALP; 1: 1,000; Abcam; cat. no. ab95462), anti-GAPDH (1: 10,000; Abcam; cat no. ab37168), anti-osteocalcin (OCN; 1: 500; Abcam; cat. no. ab93876). All experiments were conducted in triplicate.
Results
Tomatidine-targeted genes prediction
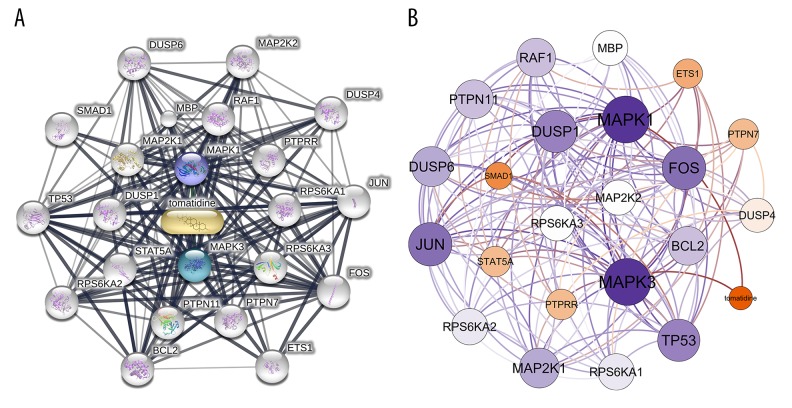
In total 22 genes were obtained from STITCH. The first shell (chemical-protein) included MAPK1 and MAPK3. The second shell (protein-protein) included RAF1, PTPRR, RPS6KA1, RPS6KA3, PTPN7, PTPN11, STAT5A, DUSP1, MAP2K1, and MBP. The third shell (protein-protein) included MAP2K2, DUSP4, JUN, FOS, ETS1, BCL2, RPS6KA2, TP53, SMAD1, and DUSP6. The interaction network constructed by STITCH is shown in Figure 1A. Next, the visualization of the network based on interaction weights was constructed, indicating MAPK3, MAPK1, JUN, TP53, FOS, and DUSP1 had the highest weights (Figure 1B).
Figure 1.
The interaction networks of tomatidine-targeted genes. (A) Interaction network constructed by STITCH. First shell (chemical-protein): MAPK1, MAPK3. Second shell (protein-protein): RAF1, PTPRR, RPS6KA1, RPS6KA3, PTPN7, PTPN11, STAT5A, DUSP1, MAP2K1, MBP. Third shell (protein-protein): MAP2K2, DUSP4, JUN, FOS, ETS1, BCL2, RPS6KA2, TP53, SMAD1, DUSP6. (B) Weighted interaction network constructed by Gephi, indicating MAPK3, MAPK1, JUN, TP53, FOS, and DUSP1 had the highest weights.
Identification of the KEGG pathways of tomatidine-targeted genes and osteoporosis
There were 77 tomatidine-targeted genes-related KEGG pathways obtained with DAVID enrichment analysis. There were 76 KEGG pathways with P value <0.05 selected. There were 110 osteoporosis related KEGG pathways retrieved after searching and calculation in miRWalk.
Identification of shared KEGG pathways
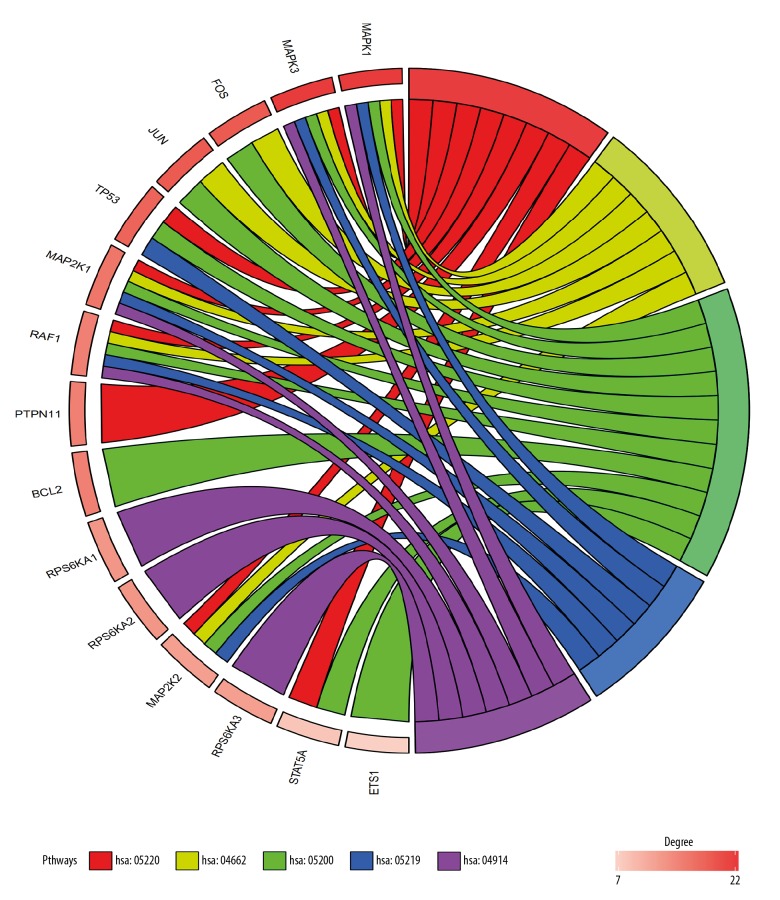
With Draw Venn Diagram, 39 shared KEGG pathways of tomatidine-targeted genes and osteoporosis were identified (Figure 2). As shown in Table 1, the top 5 KEGG pathways were pathway of chronic myeloid leukemia, B cell receptor signaling pathway, pathway in cancer, bladder cancer pathway and progesterone-mediated oocyte maturation pathway. MAPK1, MAP2K1, MAPK3, and RAF1 were involved in all 5 pathways. Therefore, they were considered as the hub genes (Figure 3). The information on chromosomal positions and connectivity of tomatidine-targeted genes is shown in Figure 4. MAPK1, MAP2K1, MAPK3, and RAF1 are located in chr22, chr15, chr16, and chr3, respectively.
Figure 2.

Identification of shared KEGG pathways of tomatidine-targeted genes and osteoporosis. There were 110 osteoporosis related KEGG pathways and 76 tomatidine-targeted genes-related KEGG pathways. There were 39 shared KEGG pathways identified with Draw Venn Diagram.
Table 1.
Top 5 KEGG pathway and involved genes.
| Term | KEGG pathway | Icariin-target genes | P-value |
|---|---|---|---|
| hsa05220 | Chronic myeloid leukemia | MAPK1, MAP2K1, MAP2K2, STAT5A, MAPK3, TP53, RAF1, PTPN11 | 7.1E-10 |
| hsa04662 | B cell receptor signaling pathway | MAPK1, FOS, MAP2K1, MAP2K2, JUN, MAPK3, RAF1 | 2.8E-8 |
| hsa05200 | Pathways in cancer | MAPK1, FOS, MAP2K1, ETS1, MAP2K2, STAT5A, JUN, BCL2, MAPK3, TP53, RAF1 | 3.7E-8 |
| hsa05219 | Bladder cancer | MAPK1, MAP2K1, MAP2K2, MAPK3, TP53, RAF1 | 8.5E-8 |
| hsa04914 | Progesterone-mediated oocyte maturation | MAPK1, RPS6KA3, RPS6KA1, MAP2K1, RPS6KA2, MAPK3, RAF1 | 1.2E-7 |
Figure 3.
Outcomes of gene enrichment analysis. MAPK1, MAP2K1, MAPK3, and RAF1 were involved in all 5 pathways. The top 3 genes of centrality degree were MAPK1, MAPK3, and FOS. hsa05220: pathway of chronic myeloid leukemia, hsa04662: B cell receptor signaling pathway, hsa05200: pathway in cancer, hsa05219: bladder cancer pathway, hsa04914: progesterone-mediated oocyte maturation pathway.
Figure 4.
Circular visualization of chromosomal positions and connectivity of tomatidine-targeted genes. The names of the genes are shown in the inner circle. For the heatmap, different colors represent different values of centrality degree. The outer circle represents chromosomes and lines coming from each gene point to their specific chromosomal locations. The 4 hub genes MAPK1, MAP2K1, MAPK3, RAF1 are highlighted in blue. They are located in chr22, chr15, chr16, and chr3, respectively.
Retrieval of the KEGG pathways related to tomatidine-targeted genes
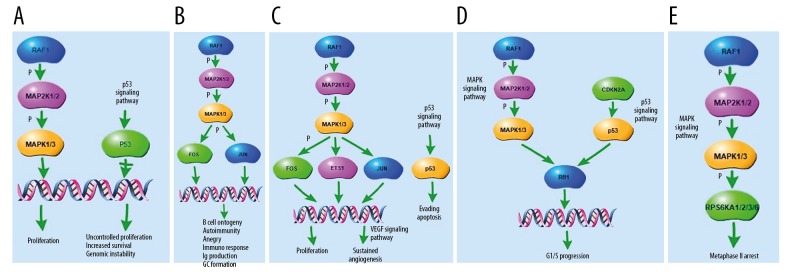
The tomatidine-targeted related parts of the top 5 shared KEGG pathways are shown in Figure 5. They were associated with proliferation, increased survival, genomic instability, B cell ontogeny, autoimmunity, anergy, immuno response, Ig production, GC formation, sustained angiogenesis, evading apoptosis, G1/S progression, and metaphase II arrest. They may play the biological action through p53 and MAPK signaling pathways.
Figure 5.
Tomatidine-targeted genes-related part of the top five shared KEGG pathways. (A) Tomatidine-targeted genes-related part of the chronic myeloid leukemia pathway. p53 signaling pathway was involved in the process, resulting in proliferation, increased survival, and genomic instability. (B) Tomatidine-targeted genes-related part of the B cell receptor signaling pathway, resulting in B cell ontogeny, autoimmunity, anergy, immuno response, Ig production, and GC formation. (C) Tomatidine-targeted genes-related part of the pathway in cancer. p53 signaling pathway was involved in the process, resulting in proliferation, sustained angiogenesis and evading apoptosis. (D) Tomatidine-targeted genes-related part of the bladder cancer pathway. p53 and MAPK signaling pathways are involved in the process, resulting in G1/S progression. (E) Tomatidine-targeted genes-related part of the progesterone-mediated oocyte maturation pathway. MAPK signaling pathway was involved in the process, resulting in metaphase II arrest.
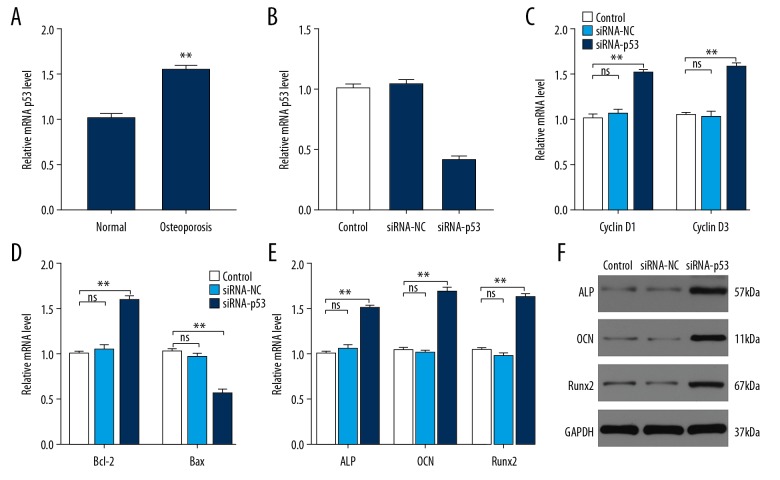
The p53 expression was enriched in the serum of osteoporosis patients and the downregulation of p53 partly reversed the impaired outcome of bone mineral density
The p53 expression between healthy volunteers and osteoporosis patients was measured by qRT-PCR and western blotting analysis and it was significantly increased in osteoporosis patients (Figure 6A). In hMSC cell culture, the expression of p53 was statistically decreased in the siRNA-p53 group, indicating p53 siRNA successfully knock-downed the p53 expression (Figure 6B). As expected, the expression level of cyclin D1/3 and Bcl-2 was increased in the siRNA-p53 group and Bax was decreased in the siRNA-p53 group (Figure 6C, 6D). The expression of osteogenesis-related gene ALP, OCN and Runx2 were significantly increased by siRNA-p53 treatment compared to the control and siRNA-NC (Figure 6E, 6F). Thus, downregulating p53 could potentially protect from osteoporosis in vitro.
Figure 6.
p53 is enriched in the serum of osteoporosis patients and the downregulation of p53 can partly reverse the impaired outcome of bone mineral density. (A) The relative mRNA level of p53 in normal people and osteoporosis patients (n=6 per group). (B) The relative mRNA expression level of p53 in control, siRNA-NC, and siRNA-p53 groups. (C) The effects of siRNA-p53 treatment on the proliferation-related genes Cyclin D1 and Cyclin D3 were assessed using qRT-PCR. (D) The effects of siRNA-p53 treatment on the apoptosis-related genes Bcl-2 and Bax were assessed using qRT-PCR. (E, F) The expressions of osteogenesis-related gene ALP, OCN and Runx2 were measured in control, siRNA-NC, and siRNA-p53 groups using qRT-PCR and western blotting. * P<0.05; ** P<0.01; *** P<0.001.
Discussion
Osteoporosis is a metabolic bone disease [15]. Multiple causes can cause it. Osteoporosis is characterized by decreasing bone mass and degradation of the microstructure, resulting in an increased possibility of fragility fractures [2]. During the development of osteoporosis, the calcium flows from bone to blood, resulting in demineralization and increased interspace of the inside bone. The speed of this process depends on the balance of osteoblasts and osteoclasts [16].
There are some classic osteoporosis drugs currently in use, most of which are drugs that inhibit osteoclast activity, such as calcitonin, bisphosphonates, estrogen, etc. [17]. There is a lack of drugs that stimulate osteoblast activity, or both stimulate bone formation and inhibit osteoclastogenesis [17]. Therefore, while there is in-depth research and discovery of synthetic chemical drugs, more attention should be paid to the development and study of natural plant compounds, finding new drugs for osteoporosis from natural plants.
Tomatine is a natural compound extracted from blue tomatoes. It has a variety of biological activities and can protect tomato plants from bacteria, fungi, viruses, and certain insects during growth [18,19]. Tomatidine may play a role in mitigating osteoporosis by inhibiting osteoclastogenesis and reducing estrogen deficiency-induced bone mass loss [5]. However, related studies have been limited and the mechanism has not been fully elucidated. In this study, 22 tomatidine-targeted genes were identified. Our study indicated that MAPK3, MAPK1, JUN, TP53, FOS, and DUSP1 had the highest weights in the interaction network. We found that there were 39 shared KEGG pathways of tomatidine-targeted genes and osteoporosis. The top 5 KEGG pathways were pathway of chronic myeloid leukemia, pathway of B cell receptor signaling, pathway in cancer, bladder cancer pathway, and progesterone-mediated oocyte maturation pathway. MAPK1, MAP2K1, MAPK3, and RAF1 were involved in all 5 pathways and they were considered as the hub genes. They may play a role in these KEGG pathways through some signaling pathways. With the signaling pathway simulation analysis, this study found p53 and MAPK signaling pathways were involved in the tomatidine-targeted genes part of these 5 KEGG pathways (Figure 5). In this study, the expression level of cyclin D1/3 and Bcl-2 was increased in the siRNA-p53 group. The expression of osteogenesis-related gene ALP, OCN, and Runx2 were significantly increased by siRNA-p53 treatment. Therefore, tomatidine may promote osteoblast proliferation by inhibiting the expression of p53. As is known, most of the existing osteoporosis drugs inhibit osteoclast activity. Therefore, tomatidine may become a promising drug for osteoporosis.
TP53 belongs to tumor suppressors [20]. Mutations of this gene occur in more than 50% of all malignancies [20]. p53 is a transcription factor that modulates the initiation of the cell cycle [20]. p53 protein can receive multiple signals and respond. Whether or not to start cell division is determined by this protein. If cells are irreversibly damaged, p53 will participate in the initiation process, leading to apoptosis [21]. p53-deficient cells are out of control and continue to divide even under inappropriate conditions. p53 gene slows or monitors the cell division [22]. It was revealed that Bre played a role in osteoblast differentiation by regulating p53 [23]. It was also found that p53 is required for dexamethasone-induced osteoblasts deaths [24]. In this study, p53 signaling pathway was found to be involved in the tomatidine-targeted genes part of the top 5 shared KEGG pathways (Figure 5). Tomatidine may achieve prevention of osteoporosis through these pathways. p53 was found to be enriched in the serum of osteoporosis patients; the downregulation of p53 can partly reverse the impaired outcome of bone mineral density.
There were some limitations of this study. Firstly, many anti-osteoporotic drugs have side effects. In this study, we only studied the anti-apoptotic effect of tomatidine through the p53 signaling pathway but did not study the side effects related mechanisms. Secondly, there are many subtypes of osteoporosis, the most common of which is postmenopausal osteoporosis. We did not perform a subtype study or discussion. The further direction of our research will be the systematic study of tomatidine in the treatment of osteoporosis and comparative study of its efficacy with other drugs.
Conclusions
In conclusion, using the method of bioinformatic analysis, we found that tomatidine may have the function of anti-osteoporosis through pathway of chronic myeloid leukemia, pathway of B cell receptor signaling, pathway in cancer, bladder cancer pathway, and progesterone-mediated oocyte maturation pathway. Through in vitro experiments, we found that tomatidine may play a biological role of mitigating apoptosis by inhibiting the expression of p53. Tomatidine may become a promising drug for osteoporosis.
Acknowledgments
The authors would like to thank all the blood donors from Shanghai Tongji Hospital, Tongji University School of Medicine, China.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the grants from the national natural science foundation of China (grant no. 81472144 and 31600754)
References
- 1.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–67. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374(3):254–62. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 3.Vijayakumar R, Busselberg D. Osteoporosis: An under-recognized public health problem. Journal of Local and Global Health Science. 2016;2(1):1–13. [Google Scholar]
- 4.Mitchell G, Lafrance M, Boulanger S, et al. Tomatidine acts in synergy with aminoglycoside antibiotics against multi-resistant Staphylococcus aureus and prevents virulence gene expression. J Antimicrob Chemother. 2012;67(3):559–68. doi: 10.1093/jac/dkr510. [DOI] [PubMed] [Google Scholar]
- 5.Hu B, Sun X, Yang Y, et al. Tomatidine suppresses osteoclastogenesis and mitigates estrogen deficiency-induced bone mass loss by modulating TRAF6-mediated signaling. FASEB J. 2019;33(2):2574–86. doi: 10.1096/fj.201800920R. [DOI] [PubMed] [Google Scholar]
- 6.Yang C, Ren J, Li B, et al. Identification of gene biomarkers in patients with postmenopausal osteoporosis. Mol Med Rep. 2019;19(2):1065–73. doi: 10.3892/mmr.2018.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong R, Ren S, Chen M, et al. Bioinformatics analysis reveals the altered gene expression of patients with postmenopausal osteoporosis using Liuweidihuang pills treatment. Biomed Res Int. 2019;2019 doi: 10.1155/2019/1907906. 1907906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szklarczyk D, Santos A, von MC, et al. STITCH 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res. 2016;44(D1):D380–84. doi: 10.1093/nar/gkv1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shannon P, Markiel A, Ozier O, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu Z, Gu L, Eils R, et al. Circlize Implements and enhances circular visualization in R. Bioinformatics. 2014;30(19):2811–12. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 11.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 13.Walter W, Sánchez-Cabo F, Ricote M. GOplot: An R package for visually combining expression data with functional analysis. Bioinformatics. 2015;31(17):2912–14. doi: 10.1093/bioinformatics/btv300. [DOI] [PubMed] [Google Scholar]
- 14.Dweep H, Gretz N. miRWalk2.0: A comprehensive atlas of microRNA-target interactions. Nat Methods. 2015;12(8):697. doi: 10.1038/nmeth.3485. [DOI] [PubMed] [Google Scholar]
- 15.McClung MR, O’Donoghue ML, Papapoulos SE, et al. Odanacatib for the treatment of postmenopausal osteoporosis: Results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study. Lancet Diabetes Endocrinol. 2019;7(12):899–911. doi: 10.1016/S2213-8587(19)30346-8. [DOI] [PubMed] [Google Scholar]
- 16.Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908–23. doi: 10.1016/S2213-8587(17)30184-5. [DOI] [PubMed] [Google Scholar]
- 17.Liu GF, Wang ZQ, Liu L, et al. A network meta-analysis on the short-term efficacy and adverse events of different anti-osteoporosis drugs for the treatment of postmenopausal osteoporosis. J Cell Biochem. 2018;119(6):4469–81. doi: 10.1002/jcb.26550. [DOI] [PubMed] [Google Scholar]
- 18.Jeon S, Kim MM. Tomatidine inhibits cell invasion through the negative modulation of gelatinase and inactivation of p38 and ERK. Chem Biol Interact. 2019;313:108826. doi: 10.1016/j.cbi.2019.108826. [DOI] [PubMed] [Google Scholar]
- 19.Diosa-Toro M, Troost B, van de Pol D, et al. Tomatidine, a novel antiviral compound towards dengue virus. Antiviral Res. 2019;161:90–99. doi: 10.1016/j.antiviral.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Sabapathy K, Lane DP. Understanding p53 functions through p53 antibodies. J Mol Cell Biol. 2019;11(4):317–29. doi: 10.1093/jmcb/mjz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafner A, Bulyk ML, Jambhekar A, Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20(4):199–210. doi: 10.1038/s41580-019-0110-x. [DOI] [PubMed] [Google Scholar]
- 22.Senturk E, Manfredi JJ. p53 and cell cycle effects after DNA damage. Methods Mol Biol. 2013;962:49–61. doi: 10.1007/978-1-62703-236-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin F, Wang Y, Wang X, et al. Bre enhances osteoblastic differentiation by promoting the Mdm2-mediated degradation of p53. Stem Cells. 2017;35(7):1760–72. doi: 10.1002/stem.2620. [DOI] [PubMed] [Google Scholar]
- 24.Zhen YF, Wang GD, Zhu LQ, et al. P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts. J Cell Physiol. 2014;229(10):1475–83. doi: 10.1002/jcp.24589. [DOI] [PubMed] [Google Scholar]