Abstract
Background
Exposure to PM2.5 (fine particulate matter ≤2.5 μm in aerodynamic diameter) in air increases the risk of lung injury and pulmonary fibrosis (PF). Extracellular vesicles (EVs) derived from adipose mesenchymal stem cells (ADSCs) have been identified as a potential treatment based on the proteins or RNAs delivery and immunomodulatory properties. Here, we assessed the protective effects and mechanisms of ADSCs-EVs on PM2.5-induced lung injury or PF.
Material/Methods
Rats (male, 6 weeks old) were exposed to PBS or PM2.5 (1.5 mg/kg/day) for 3 days a week for 4 weeks. ADSCs-EVs were extracted by ultracentrifugation. PBS and ADSCs-EVs were administrated through intratracheal instillation. After the end of exposure, the rats were anesthetized and killed. Lung tissues with different treatments were collected for Western blot analysis and HE, IHC, and IF staining analysis. Cells exposed to PM2.5 or “PM2.5+ADSCs-EVs” in vitro were also collected for further Western blotting, qRT-PCR, and IF staining evaluation.
Results
The results indicated that the initial response of lungs exposed to PM2.5 was lung injury with oxidative stress and inflammation. Long-term PM2.5 exposure resulted in obvious PF in rats. Treatment with ADSCs-EVs decreased PM2.5-induced apoptosis and necrosis in type II alveolar epithelial cells and alleviated lung injury and PF in rats. ADSCs-EVs suppressed reactive oxygen species (ROS) levels and inflammation induced by PM2.5. Furthermore, ADSCs-EVs inhibited TGF-βRI by transferring let-7d-5p and further mitigated PF.
Conclusions
Our results suggest that EVs derived from ADSCs can alleviate PM2.5-induced lung injury and PF.
MeSH Keywords: Lung Injury, MicroRNAs, Oxidative Stress, Particulate Matter, Pulmonary Fibrosis, Transforming Growth Factor beta1
Background
Pulmonary fibrosis (PF) is a serious interstitial lung disease characterized by distributive and progressive remodeling of the lung, with the formation of irreversible scars due to repetitive lung epithelial cell damage from harmful environmental factors and inherent genetic susceptibility [1]. Although previous studies indicated that PM2.5 (≤2.5 μm in aerodynamic diameter, PM2.5) is involved in the pathogenesis of various respiratory and cardiovascular diseases [2–4], whether PM2.5 can induce PF independently has not been well researched. A previous study indicated oxidative stress, inflammation, chemotaxis of monocytes/macrophages, epithelial mesenchymal transition (EMT), and abnormal activation or differentiation of lung fibroblasts are all involved in the development of PF [5]. Oxidative stress and inflammation are 2 major characteristics induced by PM2.5. Reactive oxygen species (ROS) produce oxidative stress and trigger the cell apoptosis and necrosis associated with PM2.5 exposure [6]. In addition, dysregulation of inflammatory mediators is closely associated with the progression of PM2.5-induced lung injury and PF [7–9]. TGF-β1 (transforming growth factor-β1) is also an extensively described pro-fibrosis factor that takes part in the onset and progression of PF by interacting with 2 specific receptors – type I receptor (TGF-βRI) and type II receptor (TGF-βRII) [4] – but the mechanisms underlying PM2.5-induced lung injury and PF are complicated and elusive. PF is an irreversible disease, for which treatment and prevention strategies are inadequate.
Extracellular vesicles (EVs, 20~2000 nm) are important mediators of intercellular communications [10–13] and are generally classified into exosomes, microvesicles (MVs), and apoptotic bodies according to their origin, size, and content [14]. Exosomes and MVs are together often called EVs; they contain plasma membrane proteins such as tetraspanin (CD9, CD63, and CD81), lipid raft proteins (flotillin and caveolin-1), RNAs, and cytoplasmic proteins [15]. EVs are considered to have cellular functions similar to their mother cells [14,16]. For instance, EVs originating from resting macrophages exert an anti-inflammatory effect, whereas those after LPS (lipopolysaccharide) stimulation have proinflammatory effects [17]. EVs are secreted by most cell types, including ADSCs (adipose mesenchymal stem cells), macrophages, dendritic cells, and epithelial cells. ADSCs are reported to be efficient mass producers of EVs [18] and these EVs are considered as potential therapeutic agents based on their antioxidative stress and anti-inflammation properties [19–21]. Therefore, we hypothesized that EVs released by ADSCs can mitigate PM2.5-induced lung injury and PF, and we performed relevant experiments to test this.
Material and Methods
Antibodies and reagents
Antibodies against rat Nrf2, TGF-β1, TGF-βRII, TGF-βRI, β-actin, TSG101, CD63, Alix, GM130, and ELISA kit for TNF-α were obtained from Abcam PLC (MA, USA). DMEM, F12 nutrient medium, fetal bovine serum (FBS) without exosome, Trizol-LS, exosomal protein extraction kit, and Annexin V-FITC Apoptosis Kit were purchased from Invitrogen (Carlsbad, USA). qRT-PCR primers, let-7d-5p mimics, miR-NC, and let-7d-5p inhibitor were from Sangon Biotech (Shanghai, China). DCFH-DA and PKH67 green fluorescent cell linker mini-kit were obtained from Sigma Chemical Company (St. Louis, USA). The kit for bicinchoninic acid (BCA) protein assay was purchased from Beyotime Biotechnology (Shanghai, China).
PM2.5 collection and analysis
PM2.5 samples were collected at Xinsi Road, Xi’an, China between January 2017 and December 2018. The collection method of PM2.5 in this study was based on a previous report [22]. The collected PM2.5 samples were filtrated by multi-layer sterile gauze. The filtrate was centrifuged at 4°C and 10 000 rpm for 20 min. The supernatant was oscillated by an ultrasonic oscillator and the particulate matter at the bottom was collected. Finally, the supernatant and collected particles were mixed and underwent vacuum freeze-drying. The samples were preserved at −80°C and diluted with PBS for later use.
PM2.5 morphology and size were assessed by scanning electron microscopy (SEM, Tokyo, Japan) according to the methods of a previous study [23].
Animals and cells culture
The animal experiments were performed on 6-week-old male Sprague-Dawley rats from the Experimental Animal Center of the Fourth Military Medical University (Xi’an, China). The animal experiments were approved by the Fourth Military Medical University Animal Care and Use Committee (approval no. 20190229).
Rat type II alveolar epithelial cells (ATII) were purchased from Shanghai Cell Institute Country Cell Bank (Shanghai, China) and placed in DMEM/F12 supplemented with 10% FBS and appropriate growth factors, at 37°C in an atmosphere of 5% CO2. ADSCs were primary cultured based on a previous report [24].
Extraction and identification of ADSCs-EVs
The EVs extraction process was performed according to previously described methods [25]. Transmission electron microscope (TEM) analysis of ADSCs-EVs was performed using the method of Li et al. [24]. The size and concentration of ADSCs-EVs were determined by Nanoparticle Tracking Analysis (Nanosight ns500, Malvern Instruments, UK). A size distribution plot was constructed, with the x-axis representing the particle diameter (nm) and the y-axis showing the particle percentage. Trizol-LS and an exosomal protein extraction kit were used to extract RNA and proteins from ADSCs-EVs, according to the manufacturer’s instructions. ADSCs-EVs were stored at −80°C and used for subsequent experiments. Western blotting was performed to determine the expression levels of TSG101, Alix, GM130, and CD63 in EVs and cell lysates.
Uptake of ADSCs-EVs
To determine whether ADSCs-EVs could be absorbed by cells, ADSCs-EVs (5×108) were suspended in 0.5 mL diluent C. Then, 4 μL PKH67 was added into 0.5 mL diluent C and incubated at room temperature for 4 min. Next, 2 mL PBS was added to prevent excessive dyeing, and centrifugation of labeled ADSCs-EVs was performed for 1 h to eliminate the residual dyes. The suspended ADSCs-EVs were precipitated in 200 μL PBS at 4°C in the dark. The labeled ADSCs-EVs were added to the recipient cells (in a confocal culture dish, 1×106 cells) and incubated at 37°C for 4 h in the dark. The cells were then washed 3 times with PBS. Then, 1 mL of fixative solution was added and the mixture was incubated in the dark for 10 min. Fixative fluid was removed, and the cells were washed 3 times with PBS for 2 min each time. Hoechst 33342 (500 μL, stain for the cell nucleus) was added and incubated for 5 min in the dark. Hoechst 33342 was removed and the cells were washed 3 times with PBS for 2 min each time. F-actin (100 μL; for staining of the cytoskeleton) was added and incubated in the dark for 30 min. The cells were then washed 3 times with PBS for 2 min each time. Finally, 1 mL of PBS was added into the confocal dish to prevent cell drying, and the dish was kept in the dark. Fluorescent photography of the control and experimental groups were performed by confocal microscopy (Olympus, Japan) the next day.
In vivo PM2.5 exposure and treatment of ADSCs-EVs
Rats were divided into 4 groups based on different treatments: “PBS” (none), “ADSCs-EVs+PBS”, “PM2.5+PBS”, or “PM2.5+ADSCs-EVs”, with 5 rats in each group. Rats were intratracheally instilled with 20 μL concentrated PM2.5 (1.5 mg/kg/day) solution (PBS) 3 days a week (Monday, Wednesday, and Friday) for 4 weeks. The exposure dose of PM2.5 was based on a previous study [26]. Treatments of PBS or ADSCs-EVs (2.5~2.8×1010 in 20 μL PBS) were administrated via intratracheal instillation at 1 h after PBS/PM2.5 exposure. The treatment dose of EVs was determined according to the study by Willis [27]. At 3, 6, 9, 12, and 24 h after single exposure or 24 h after the end of the 4-week exposure, the rats were anesthetized with pentobarbital sodium (40 mg/kg) and killed. Samples of lung tissue and bronchoalveolar lavage fluid (BALF) were collected for analysis according to a previously described method [6].
In vitro PM2.5 exposure and treatment of ADSCs-EVs
ATII cells (1×106) were exposed to PBS, ADSCs-EVs (1×109), PM2.5 (50 μg/mL), or PM2.5 (50 μg/mL) +ADSCs-EVs (1×109, 1 h after the cells were exposed to PM2.5). Six hours later, apoptosis (Annexin V-FITC) was determined by flow cytometry analysis (Becton Dickinson and Company, USA) using a previously described method [24]. Proteins or RNAs were extracted from cells with different treatments for Western blotting and qRT-PCR analysis.
Staining analysis
Hematoxylin-eosin (HE) staining and immunohistochemistry (IHC) staining analyses were performed as described by Li et al. [28]. Stained slides were assessed according to the staining intensity (strong: 3; moderate: 2; weak: 1; and negative: 0) and the abundance of positive cells (≤5%: 0; 6–25%: 1; 26–50%: 2; 51–75%: 3, and ≥76%: 4). A final score obtained from the intensity score multiplied by the extent score was used to identify various target expression levels.
Masson’s trichrome staining was performed as previously described [29]. The relative collagenous fiber area was determined by use of Image J software. Immunofluorescence staining was performed as previously reported [30]. ROS staining [31] and TEM assay [6] were performed as previously reported.
Luciferase activity assay
ATII cells were transfected with 50 pmol of miR-NC or let-7d-5p. The next day, the cells were transfected with 0.2 μg of the psiCHECK2 vector (Promega, Madison, USA) expressing the 3′ UTR of the rat TGF-βRI mRNA or the mutated 3′ UTR of the TGF-βRI mRNA (Quik Change II Site-Directed Mutagenesis Kit, Agilent, USA) with JETPEI (Polyplus transfection) according to the manufacturer’s instructions. After 24 h, the luciferase assay was performed using the Dual-Luciferase Reporter Assay System (Promega).
qRT-PCR analysis and Western blotting
The cells or EVs were resuspended in Trizol-LS and total RNA was extracted and reverse transcribed according to the manufacturer’s instructions. The sequence for rat let-7d-5p, miR-98-5p, and let-7i-5p are shown in Table 1.
Table 1.
Primer sets for qRT-PCR.
| miRNAs | Sequence (F) |
|---|---|
| let-7d-5p | CGCCAGAGGTAGTAGGTTGCATAGTT |
| miR-98-5p | CGCGCCTGAGGTAGTAAGTTGTATTGT |
| let-7i-5p | CGCTGAGGTAGTAGTTTGTGCTGTT |
For Western blot analysis, proteins were extracted from the cells via lysis buffer (with 1% PMSF and phosphatase inhibitor) on ice for 20 min. Samples were then centrifuged at 12 000 g and 4°C for 20 min, then the supernatant was collected for further assay. Samples containing 40 μg of proteins were loaded onto a 10% sodium dodecyl sulfate (SDS)-PAGE gel and electrophoretically transferred to polyvinylidene fluoride membranes. Then, primary antibodies were added and incubated overnight with the membrane at 4°C. After washing with TBST 3 times, membranes were incubated with Anti-rabbit Ig G secondary fluorescent antibody (CST, Beverly, USA) for 1 h in the dark. The membranes were then washed 3 times with TBST. Antibody-bound membranes were examined by using the Li-Cor Odyssey system (Li-Cor Biosciences).
Statistical analysis
Mean±standard deviation (SD) were used to express results. Each variable for differences among the different groups were tested by t test and one- or two-way analysis of variance (ANOVA). P<0.05 was considered to indicate statistical significance. All data shown are representative of experiments conducted at least 3 times.
Results
Rats exposed to PM2.5 developed lung injury and PF
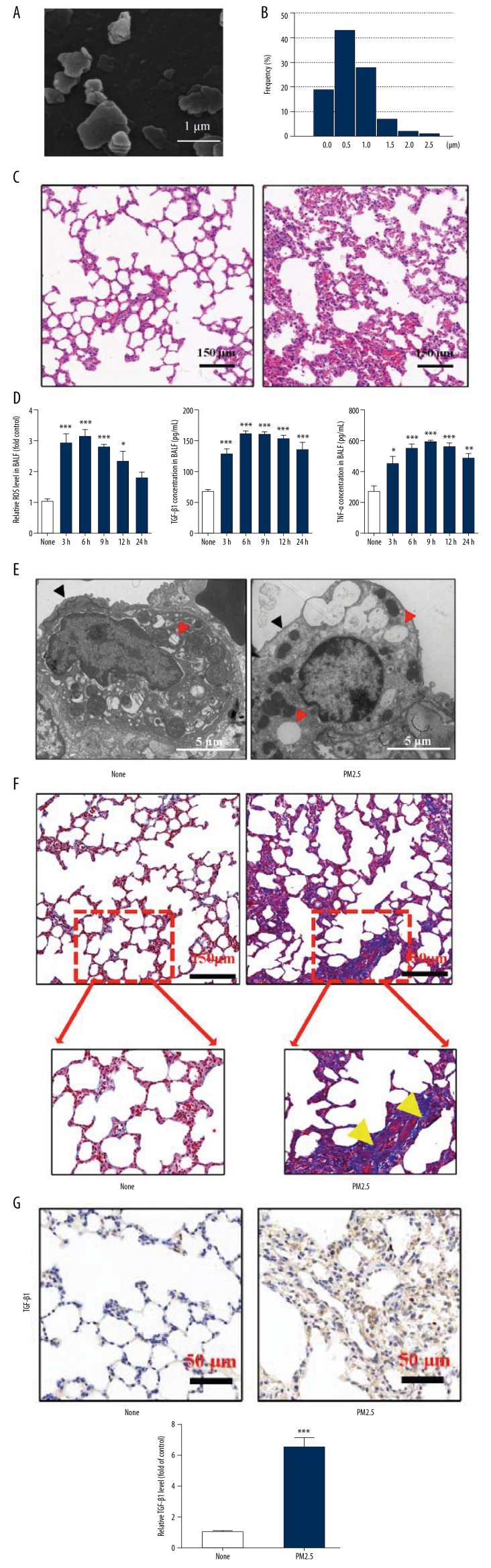
The morphology of PM2.5 is shown in Figure 1A and size analysis of PM2.5 is shown in Figure 1B. HE staining showed that 6 h after single exposure to PM2.5, the lungs developed acute pathological changes, including alveolar congestion, hemorrhage, edema, and alveolar destruction (Figure 1C), and exposure to PM2.5 elevated levels of ROS, TGF-β1, and inflammatory factor (TNF-α) in BALF (Figure 1D). TEM results showed that after exposure to PM2.5, the “villous-like” surfactant coating of normal type II alveolar epithelial cells was eliminated and the number of impaired lamellar bodies increased (Figure 1E), suggesting the functional impairment of type II alveolar epithelial cells. These findings indicate that single PM2.5 exposure can cause serious lung injury. Further, we observed that long-term (4 weeks) PM2.5 exposure led to obviously fibrotic changes in the lung, accompanied with an increase of TGF-β1 (Figure 1F, 1G).
Figure 1.
PM2.5 exposure induced lung injury and PF. (A) SEM image of PM2.5. (B) Particles size distribution of PM2.5. (C) HE staining of lungs at 6 h after single exposure to PBS (None) or PM2.5. (D) Relative ROS of cells, TGF-β1 and TNF-α concentrations in BALF at different time points (3, 6, 9, 12, and 24 h after PM2.5 single exposure) were detected. (E) TEM of type II alveolar epithelial cells 6 hours after rats were treated with PBS (None) or PM2.5. (F) Masson staining of lung tissues with treatments of PBS or PM2.5 for 4 weeks (Yellow arrows represented PF). (G) IHC staining of TGF-β1 in lungs after treatments of PBS or PM2.5 for 4 weeks. (* p<0.05; ** p<0.01; *** p<0.001).
ADSCs-EVs alleviated PM2.5-induced lung injury and PF in rats
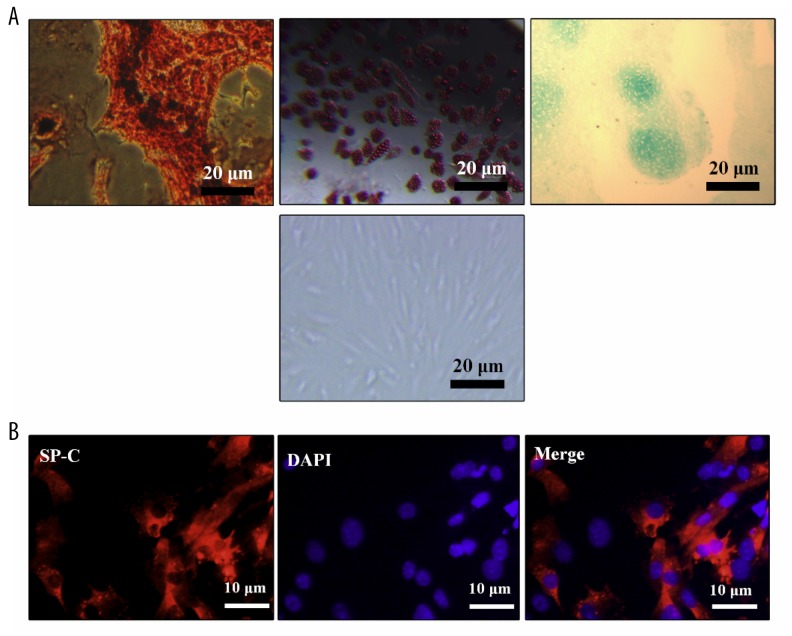
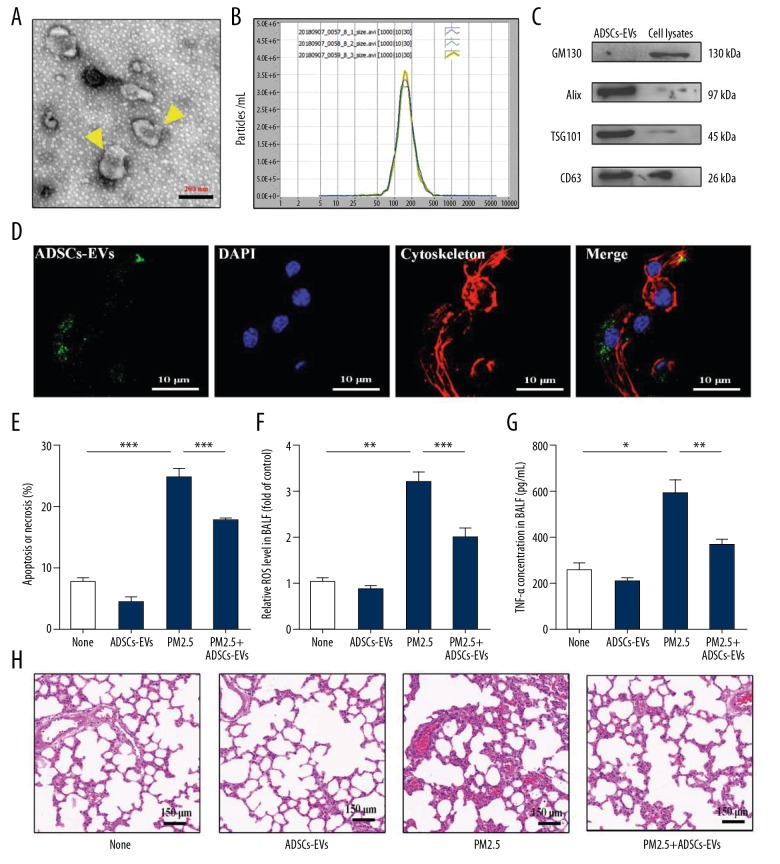
Rat ADSCs were identified through their multilineage differentiation ability (Figure 2A). ATII cells were identified through anti-SP-C immunofluorescence staining (Figure 2B). EVs derived from ADSCs were collected and analyzed. TEM (Figure 3A) and NTA (Figure 3B) analyses revealed a heterogeneous population of ADSCs-EVs, which had a typical diameter of 170 nm and exhibited distinct biconcave morphological feature. The expressions of GM130, Alix, TSG101, and CD63 of ADSCs-EVs were detected by Western blotting (Figure 3C).
Figure 2.
The identification of ADSCs and AT II cells. (A) Multilineage differentiation of ADSCs (upper left: osteoblast differentiation, upper middle: adipocyte differentiation, upper right: cartilage differentiation) and typical image of ADSCs (lower). (B) SP-C immunofluorescence staining for AT II cells.
Figure 3.
ADSCs-EVs alleviated lung injury induced by PM2.5. (A) TEM identification of ADSCs-EVs. (B) NTA analysis of ADSCs-EVs. (C) GM130, Alix, TSG101, and CD63 expressions in ADSCs-EVs and cell lysates were detected by Western blotting. (D) The uptake of ADSCs-EVs in ATII cells was detected by a confocal microscopy. (E) Apoptosis or necrosis of ATII cells with treatments of PBS (None), ADSCs-EVs, PM2.5, or PM2.5+ADSCs-EVs was detected by flow cytometry assay. (F) Six hours after the rats treated with PBS (None), ADSCs-EVs, PM2.5 or PM2.5+ADSCs-EVs, relative ROS level in BALF was measured. (G) Six hours after the rats treated with PBS (None), ADSCs-EVs, PM2.5, or PM2.5+ADSCs-EVs, TNF-α concentration in BALF was evaluated. (H) HE staining of lungs exposed to PBS (None), ADSCs-EVs, PM2.5 or PM2.5+ADSCs-EVs. (* p<0.05; ** p<0.01; *** p<0.001).
We performed in vitro experiments to determine the protective effects of ADSCs-EVs on PM2.5-exposed ATII cells. Using laser scanning confocal microscopy, we observed ADSCs-EVs were taken up by ATII cells (Figure 3D). Apoptosis assay indicated that PM2.5 exposure resulted in early and late apoptosis or necrosis in ATII cells. ADSCs-EVs treatment considerably decreased the apoptosis or necrosis (Figure 3E). Further, the protective effects of ADSCs-EVs on PM2.5-induced lung injury in vivo was evaluated after the rats were exposed to PM2.5, ADSCs-EVs, or PM2.5+ADSCs-EVs 1 time. We found that PM2.5 could induce high production of ROS in BALF, and this upregulation was suppressed by ADSCs-EVs treatment (Figure 3F). Similarly, inflammation factor (TNF-α) in BALF was also repressed by ADSCs-EVs (Figure 3G). HE staining results showed that the ADSCs-EVs reduced the levels of alveolar congestion, hemorrhage, edema, and alveolar destruction induce by PM2.5 (Figure 3H). Taken together, these results suggest that ADSCs-EVs could serve as efficient antioxidative and anti-inflammatory interventions and further protect rats from lung injury induced by PM2.5.
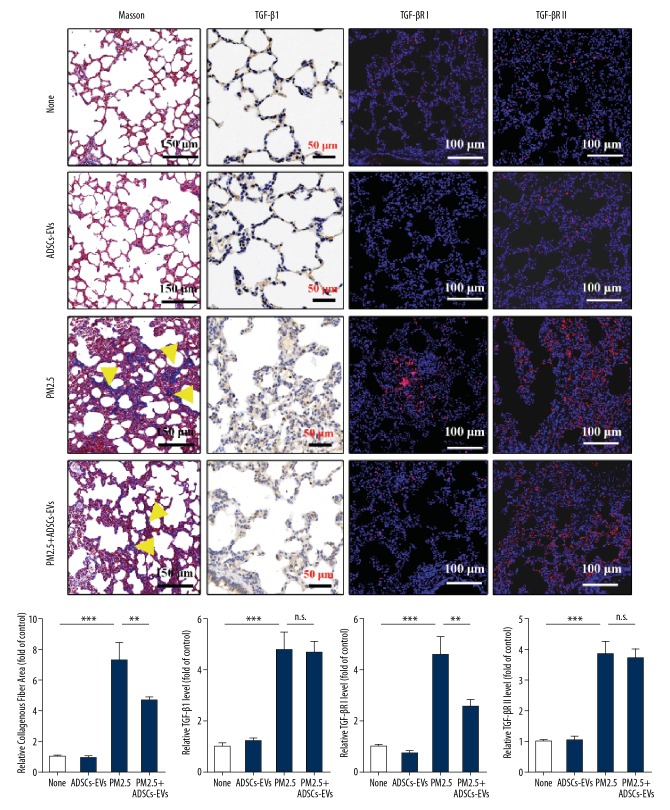
To investigate the effect of ADSCs-EVs on PF induced by PM2.5, we established long-term PM2.5 exposure models in rats and treated the rats with or without ADSCs-EVs. PF level was evaluated by Masson staining and quantified based on the collagenous fiber area. The results indicated that rats exposed to PM2.5 (4 weeks) showed more obvious PF compared with those exposed to PBS, but the PM2.5-exposed rats treated with ADSCs-EVs showed less PF (p<0.01) than those that were not treated (Figure 4). The levels of pro-fibrosis factors TGF-β1, TGF-βRI, and TGF-βRII were measured by IHC staining or IF staining. The results suggested that PM2.5 induced high expression of these cytokines. TGF-βRI was obviously repressed after ADSCs-EVs treatment (p<0.01), but this was not observed for TGF-β1 and TGF-βRII. Compared with the normal rats, the rats treated with ADSCs-EVs for 4 weeks showed no obvious alterations in lungs, which suggests that ADSCs-EVs had no obvious negative effects on the lungs.
Figure 4.
ADSCs-EVs alleviated PF induced by PM2.5. Masson staining, IHC staining for TGF-β1, IF staining for TGF-βRI and TGF-βRII in lungs exposed to PBS (None), ADSCs-EVs, PM2.5, or PM2.5+ADSCs-EVs for 4 weeks. (* p<0.05; ** p<0.01; *** p<0.001).
ADSCs-EVs suppressed PF by inhibition of TGF-βRI by transferring let-7d-5p
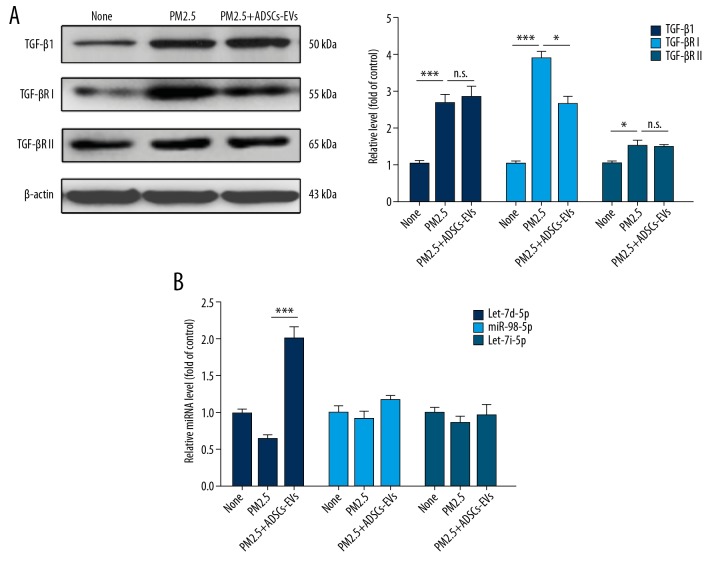
Previous results indicated that ADSCs-EVs mitigated PM2.5-induced PF. To further define the underlying mechanism of the anti-fibrosis effect of ADSCs-EVs, we then tested pro-fibrosis cytokines associated with the TGF-β1 pathway (TGF-βRI and TGF-βRII) in ATII cells exposed to different treatments in vitro by Western blot analysis (Figure 5A). Consistent with our in vivo experiment results, Western blot analysis indicted that TGF-βRI was remarkably downregulated in PM2.5-exposed ATII cells following treatment with ADSCs-EVs (p<0.05). Given that TGF-β1 induced PF by activation of Smad2/3 through combination with TGF-βRI and TGF-βRII [32], the regulation of TGF-βRI was critical in the relief of PF. Therefore, we mainly focused on the effect of ADSCs-EVs on the expression of TGF-βRI in recipient cells and the underlying mechanisms.
Figure 5.
ADSCs-EVs repressed TGF-βRI expression. (A) Western blotting analysis of TGF-β1, TGF-βRI, and TGF-βRII levels in ATII cells under different treatments. (B) The miRNA levels in ATII cells with different treatments were measured by qRT-PCR. (* p<0.05; ** p<0.01; *** p<0.001).
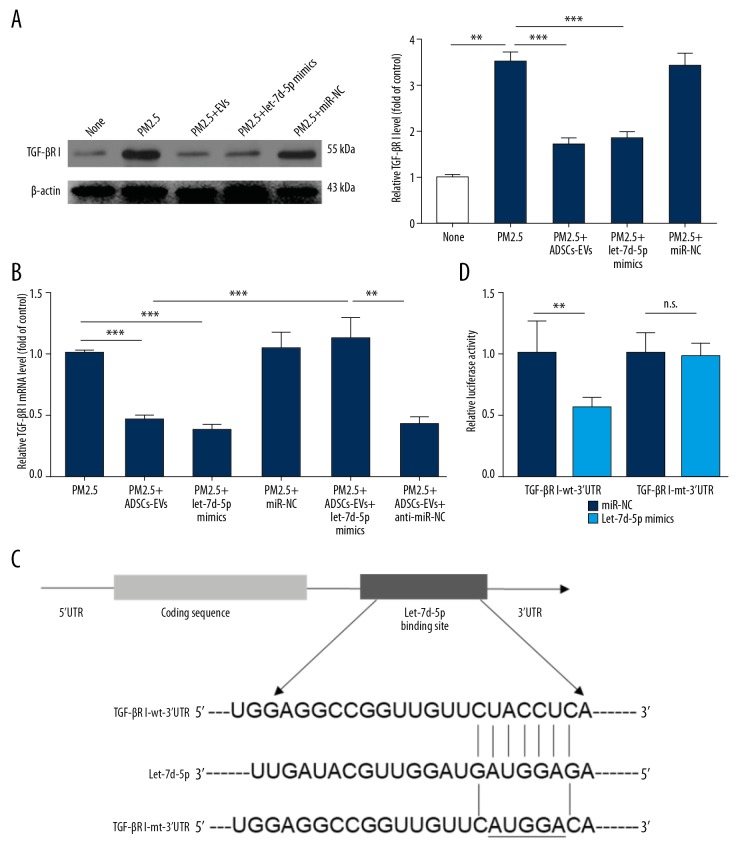
miRNAs are known to exert their regulatory effects on protein expression, which suggests that the exosomal delivery of miRNAs could be a novel mechanism for intercellular communication. We hypothesized that ADSCs-EVs repressed the expression of TGF-βRI by delivering upstream miRNAs into recipient cells. Therefore, we utilized 3 miRNA target-predicting algorithms (miRTarBase, miRDB and TargetScan) to identify the potential upstream targets of TGF-βRI, and 3 miRNAs were identified (let-7d-5p, miR-98-5p, and let-7i-5p). First, we measured levels of these miRNAs in ATII cells treated with PBS, PM2.5, or PM2.5+ADSCs-EVs. The results indicated that treatment of ADSCs-EVs significantly increased the let-7d-5p level in ATII cells (Figure 5B), but this effect of ADSCs-EVs was not observed with miR-98-5p and let-7i-5p. Western blot analysis (Figure 6A) suggested that PM2.5-induced high expression of TGF-βRI could be downregulated by ADSCs-EVs as well as by let-7d-5p mimics. qRT-PCR analysis further indicated that the upregulation of TGF-βRI mRNA induced by PM2.5 was suppressed by ADSCs-EVs and let-7d-5p mimics (Figure 6B). Consistently, let-7d-5p inhibitor rescued the reduction of TGF-βRI mRNA induced by ADSCs-EVs, which suggested that ADSCs-EVs-induced TGF-βRI downregulation was potentially mediated by let-7d-5p. Luciferase assay (Figure 6C, 6D) further confirmed that the reporter activity was significantly reduced after co-transfection with psiCHECK2-TGF-βRI-wt-3′UTR and let-7d-5p mimics (p<0.01), compared with cells co-transfected with psiCHECK2-TGF-βRI-mt-3′UTR and let-7d-5p mimics, suggesting that TGF-βRI is a direct target gene of let-7d-5p. Taken together, these observations suggest that ADSCs-EVs inhibited the expression of TGF-βRI by transferring let-7d-5p.
Figure 6.
let-7d-5p directly inhibited the expression of TGF-βRI. (A) The expression of TGF-βRI under different treatments was tested by Western blotting. (B) The expression of TGF-βRI mRNA under different treatments was tested by qRT-PCR. (C) Schematic representation of matching sequence between TGF-βRI 3′UTR mRNA and let-7d-5p. (D) Luciferase activity of ATII cells co-transfected with reporter plasmid (“TGF-βRI wt” or “TGF-βRI mt”) and let-7d-5p or miRNA-NC. (* p<0.05; ** p<0.01; *** p<0.001).
Discussion
We found that exposure to PM2.5 initially caused lung injury, including morphological alterations, oxidative stress, and inflammation, and then gradually led to PF. ADSCs-EVs protected lungs from the effects of PM2.5 exposure. Our findings show that ADSCs-EVs can mitigate PM2.5-induced PF via inhibiting TGF-βRI by transferring let-7d-5p.
The lungs are the major targets for most air-borne particles. Evidence indicates that an increase in the concentration of PM2.5 is related to elevated morbidities of pulmonary or cardiovascular diseases [6]. Excessive production of ROS and inflammatory cytokines are the key causes of PM2.5-induced cytotoxicity, which are the triggers of apoptosis, necrosis, lung injury, or pro-fibrosis changes [33]. It is still unclear which parts or factors of PM2.5 play key roles in inducing ROS and inflammation. Several studies have shown that carbonaceous materials, metals, and PAHs can all individually cause ROS and inflammation [34]. Our study shows that PM2.5 induces obvious oxidative stress and inflammation. The suppression of ROS or inflammation by ADSCs-EVs contributes to the viability of cells and relieves lung injury and PF. Similar findings were previously reported in kidney injury [35], and our study verifies these effects in the lungs.
Mesenchymal stem cells (MSCs) are multipotent, nonhematopoietic adult stem cells that contain bone marrow mesenchymal stem cells (BMSCs), umbilical cord mesenchymal stem cells (UMSCs), placenta mesenchymal stem cells (PMSCs), and ADSCs [36]. ADSCs have been widely used for EVs collection, mostly because they are readily available and relatively stable [18]. EVs derived from MSCs have been reported to be efficient carriers for proteins or RNAs delivery, especially miRNAs [36]. Some miRNAs, such as let-7c, miR-21, and miR-192, have been reported to be involved in fibrosis via regulation of the TGF-β signaling pathway [37]. TGF-β1 is extensively reported to take part in the onset and progression of PF [4,37]. All TGF-β isoforms bind to TGF-βRII as homodimers (their active form); TGF-βRII then recruits and activates TGF-βRI [38]. TGF-βRI kinase subsequently activates downstream signaling by phosphorylating Smad2 and Smad3, which oligomerize with Smad4 and then translocate to the nucleus to regulate pro-fibrosis gene expression [32]. TGF-βRI, also known as ALK5, can also activate a wide variety of Smad-independent pathways (known as noncanonical signaling) to modify cell function [37]. Our work is the first to demonstrate that ADSCs-derived EVs can suppress TGF-βRI induced by PM2.5 through transferring let-7d-5p into recipient cells, thus relieving pulmonary fibrosis. The enrichment of let-7 family miRNAs in EVs derived from MSCs has been previously reported, suggesting a new treatment based on EVs to attenuate renal fibrosis [39]. Thereby, the anti-fibrosis effect of MSCs-EVs has been validated in different organs.
Therapy based on MSCs-EVs has become an important research focus in many areas. For instance, Willis et al. suggested that EVs derived from MSCs can reduce the severity of experimental bronchopulmonary dysplasia and restore lung function via immunomodulatory effects on macrophages [27]. Li et al. reported that EVs from ADSCs could be used to promote wound healing in diabetic foot ulcers [24]. Based on our findings and relevant studies, we infer that the possible mechanisms underlying the therapeutic effects of EVs are as follows: EV-encapsulating proteins or RNAs are resistant to proteases or RNases, which gives EVs good stability in the circulation when traversing long distances within the body, under both physiological and pathological conditions [40,41]. Therefore, EVs are a consistent source of protein, mRNA, or miRNAs for targeted delivery and for use as a biomarker with low immunogenicity and antigenicity. MSCs can induce expression of anti-inflammatory-tolerant phenotypes of various cells and the immunomodulatory properties of MSCs are also observed in their EVs [42].
Conclusions
We find that EVs derived from ADSCs can exert protective effects against PM2.5-induced lung injury and PF, and also provides a strategy for the further application of EVs in relieving lung disease associated with environmental pollution.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by grants from the Shaanxi Key R&D Program (2017ZDL-SF-14-6) and the National Natural Science Foundation of China (81970076)
References
- 1.Wuyts WA, Agostini C, Antoniou KM, et al. The pathogenesis of pulmonary fibrosis: A moving target. Eur Respir J. 2013;41:1207–18. doi: 10.1183/09031936.00073012. [DOI] [PubMed] [Google Scholar]
- 2.Li N, Hao M, Phalen RF, et al. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109:250–65. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Hertz-Picciotto I, Baker RJ, Yap PS, et al. Early childhood lower respiratory illness and air pollution. Environ Health Perspect. 2007;115:1510–18. doi: 10.1289/ehp.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dysart MM, Galvis BR, Russell AG, Barker TH. Environmental particulate (PM2.5) augments stiffness-induced alveolar epithelial cell mechanoactivation of transforming growth factor beta. PLoS One. 2014;9:e106821. doi: 10.1371/journal.pone.0106821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair GB, Matela A, Kurbanov D, Raghu G. Newer developments in idiopathic pulmonary fibrosis in the era of anti-fibrotic medications. Expert Rev Respir Med. 2016;10:699–711. doi: 10.1080/17476348.2016.1177461. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Shen X, Tian G, et al. AMPKalpha2 deficiency exacerbates long-term PM2.5 exposure-induced lung injury and cardiac dysfunction. Free Radic Biol Med. 2018;121:202–14. doi: 10.1016/j.freeradbiomed.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 7.He M, Ichinose T, Yoshida S, et al. PM2.5-induced lung inflammation in mice: Differences of inflammatory response in macrophages and type II alveolar cells. J Appl Toxicol. 2017;37:1203–18. doi: 10.1002/jat.3482. [DOI] [PubMed] [Google Scholar]
- 8.Dagher Z, Garcon G, Gosset P, et al. Pro-inflammatory effects of Dunkerque city air pollution particulate matter 2.5 in human epithelial lung cells (L132) in culture. J Appl Toxicol. 2005;25:166–75. doi: 10.1002/jat.1050. [DOI] [PubMed] [Google Scholar]
- 9.Watterson TL, Sorensen J, Martin R, Coulombe RA., Jr Effects of PM2.5 collected from Cache Valley Utah on genes associated with the inflammatory response in human lung cells. J Toxicol Environ Health A. 2007;70:1731–44. doi: 10.1080/15287390701457746. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Liu K, Liu Y, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nat Immunol. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 11.Qin J, Xu Q. Functions and application of exosomes. Acta Pol Pharm. 2014;71:537–43. [PubMed] [Google Scholar]
- 12.Yoon YJ, Kim OY, Gho YS. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014;47:531–39. doi: 10.5483/BMBRep.2014.47.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Mahairaki V, Bai H, et al. Highly purified human extracellular vesicles produced by stem cells alleviate aging cellular phenotypes of senescent human cells. Stem Cells. 2019;37:779–90. doi: 10.1002/stem.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 15.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 16.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–8. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–44. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo RW, Lai RC, Zhang B, et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336–41. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Chang CL, Sung PH, Chen KH, et al. Adipose-derived mesenchymal stem cell-derived exosomes alleviate overwhelming systemic inflammatory reaction and organ damage and improve outcome in rat sepsis syndrome. Am J Transl Res. 2018;10:1053–70. [PMC free article] [PubMed] [Google Scholar]
- 20.Fareh M, Almairac F, Turchi L, et al. Cell-based therapy using miR-302-367 expressing cells represses glioblastoma growth. Cell Death Dis. 2017;8:e2713. doi: 10.1038/cddis.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinge CM. Non-coding RNAs in breast cancer: Intracellular and intercellular communication. Noncoding RNA. 2018;4(4) doi: 10.3390/ncrna4040040. pii: E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng X, Zhang F, Rui W, et al. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol In Vitro. 2013;27:1762–70. doi: 10.1016/j.tiv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Billet S, Garcon G, Dagher Z, et al. Ambient particulate matter (PM2.5): Physicochemical characterization and metabolic activation of the organic fraction in human lung epithelial cells (A549) Environ Res. 2007;105:212–23. doi: 10.1016/j.envres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Xie X, Lian W, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:29. doi: 10.1038/s12276-018-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bao L, You B, Shi S, et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene. 2018;37:2873–89. doi: 10.1038/s41388-018-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Qimuge A, Wang H, et al. IRE1alpha/XBP1s branch of UPR links HIF1alpha activation to mediate ANGII-dependent endothelial dysfunction under particulate matter (PM) 2.5 exposure. Sci Rep. 2017;7:13507. doi: 10.1038/s41598-017-13156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–16. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Li H, Liu S, et al. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol Immunol. 2018;99:134–44. doi: 10.1016/j.molimm.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Lin Z, Wang Y, et al. Nickle(II) ions exacerbate bleomycin-induced pulmonary inflammation and fibrosis by activating the ROS/Akt signaling pathway. Environ Sci Pollut Res Int. 2018;25:4406–18. doi: 10.1007/s11356-017-0525-x. [DOI] [PubMed] [Google Scholar]
- 30.Cheng X, He S, Yuan J, et al. Lipoxin A4 attenuates LPS-induced mouse acute lung injury via Nrf2-mediated E-cadherin expression in airway epithelial cells. Free Radi Biol Med. 2016;93:52–66. doi: 10.1016/j.freeradbiomed.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Zheng JQ, Zhang GR, Li J, Bi HW. Neutrophil elastase inhibitor suppresses oxidative stress in obese asthmatic rats by activating Keap1/Nrf2 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:361–69. doi: 10.26355/eurrev_201901_16784. [DOI] [PubMed] [Google Scholar]
- 32.Muthusamy BP, Budi EH, Katsuno Y, et al. ShcA protects against epithelial-mesenchymal transition through compartmentalized inhibition of TGF-beta-induced Smad activation. PLoS Biol. 2015;13:e1002325. doi: 10.1371/journal.pbio.1002325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurgueira SA, Lawrence J, Coull B, et al. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–55. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15:1–21. doi: 10.1080/10937404.2012.632359. [DOI] [PubMed] [Google Scholar]
- 35.Zhang G, Zou X, Huang Y, et al. Mesenchymal stromal cell-derived extracellular vesicles protect against acute kidney injury through anti-oxidation by enhancing Nrf2/ARE activation in rats. Kidney Blood Press Res. 2016;41:119–28. doi: 10.1159/000443413. [DOI] [PubMed] [Google Scholar]
- 36.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal stem cell-derived extracellular vesicles: Toward cell-free therapeutic applications. Mol Ther. 2015;23:812–23. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: The master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–38. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 38.Xu P, Liu J, Derynck R. Post-translational regulation of TGF-beta receptor and Smad signaling. FEBS Lett. 2012;586:1871–84. doi: 10.1016/j.febslet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh W, Sheng CT, Tan B, et al. Analysis of deep sequencing microRNA expression profile from human embryonic stem cells derived mesenchymal stem cells reveals possible role of let-7 microRNA family in downstream targeting of hepatic nuclear factor 4 alpha. BMC Genomics. 2010;11(Suppl 1):S6. doi: 10.1186/1471-2164-11-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521:167–75. doi: 10.1016/j.ijpharm.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 41.Yousefpour P, Chilkoti A. Co-opting biology to deliver drugs. Biotechnol Bioeng. 2014;111:1699–716. doi: 10.1002/bit.25307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]