Abstract
Background
Hypertrophic scar results from an abnormal repair response to trauma in the skin and involves fibroblasts proliferation with increased collagen deposition. Transforming growth factor-β1 (TGF-β1) and TGF-β receptor type I (TGF-βR1) are involved in tissue repair and are increased by ubiquitin-specific protease 4 (USP4). This study aimed to investigate the effects of TGF-βR1 and USP4 in human tissue samples of hypertrophic scar and on cell proliferation and cell migration in primary fibroblast cultures in vitro.
Material/Methods
Skin excision tissue samples with adjacent normal skin were obtained from 15 patients with hypertrophic scar, which provided tissue sections and primary fibroblast culture for analysis. Immunohistochemistry detected the expression of USP4 and TGF-βR1 in tissue sections. MicroRNA (miRNAs) expression levels were measured by quantitative real-time polymerase chain reaction (qRT-PCR). Western blot was performed to measure protein expression levels. Cultured skin fibroblasts were investigated using immunofluorescence staining. Fibroblast proliferation, apoptosis, and migration were measured with the Cell Counting Kit-8 (CCK-8) assay, flow cytometry, and a wound-healing assay, respectively.
Results
The expression of USP4 and TGF-βR1 in hypertrophic scar were increased compared with normal skin. Fibroblasts cultured from hypertrophic scar tissue showed increased expression of of USP4 and TGF-βR1. Fibroblast transfection with USP4 short-interfering RNA (siRNA) resulted in reduced fibroblast proliferation and migration, and increased apoptosis. Downregulation of USP4 inhibited the expression of TGF-βR1 protein and increased the expression levels of Smad7 protein.
Conclusions
USP4 regulated the proliferation, migration, and apoptosis of hypertrophic scar fibroblasts by regulating the TGF-β1 signaling pathway.
MeSH Keywords: Cicatrix, Hypertrophic; Protease Inhibitors; Transforming Growth Factor beta
Background
Hypertrophic scar is a common condition that results from abnormal tissue repair in the skin that follows trauma from burns and surgery and is characterized by increased cell proliferation and increased collagen deposition [1,2]. Currently, the treatment of hypertrophic scar remains a significant challenge and requires surgical resection, laser treatment, and corticosteroid injection [3]. Abnormal proliferation and activation of fibroblasts are considered to be a major characteristic of hypertrophic scar [4–6]. However, the pathogenesis of hypertrophic scar remains to be determined.
Transforming growth factor β1 (TGF-β1) signaling plays a vital role in the formation of hypertrophic scar, and is initiated by the binding of TGF-β1 to TGF-β receptor type 1 (TGF-βR1) in the cell membrane to form a complex [7,8]. The binding of TGF-β1 to the TGF-β1 receptor complex on the cell surface initiates cellular functions associated with the activation of intracellular Smad signaling proteins [9]. Smads, particularly Smad7, induced by TGF-β1, plays an important role in negatively regulating the activation of the TGF-β1 signaling pathway [10].
As a member of the ubiquitin-specific protease (USP) family, ubiquitin-specific protease 4 (USP4) is involved in cell survival after DNA damage and is an important regulator of DNA double-strand break (DSB) terminal excision [11]. USP4 is overexpressed in malignant cells when compared with normal cells [12]. The growth of mouse embryonic fibroblasts with USP4-deficient was shown to be inhibited [13]. Also, USP4 de-ubiquitinated and then inhibited the activation of p53 and NF-κB [14]. Recently, USP4 was shown to be highly expressed in hepatocellular carcinoma, and de-ubiquitinated TGF-βR1, which increases epithelial-mesenchymal transition (EMT) induced by TGF-β1 signaling, has been shown to promote the invasion and metastasis of tumors, including breast cancer [15]. Also, USP4 mediates the activation of TGF-β1 and upregulates the expression of matrix metalloproteinase-9 (MMP-9), which mediates the migration and invasion of breast cancer cells [16]. However, the role of USP4 in the TGF-β1 signaling pathway in hypertrophic scar remains to be investigated. Therefore, this study aimed to investigate the effects of the TGF-β1 and USP4 in human tissue samples of hypertrophic scar and on cell proliferation and cell migration in vitro in primary fibroblast cultures.
Material and Methods
Ethical statement
This study was approved by the Ethics Committee of Yantai Yuhuangding Hospital (Approval number: YY2018042653). Written informed consent was obtained from all participants who participated in the study.
Skin tissue specimens
Fifteen skin specimens were obtained at surgery from patients with hypertrophic scar tissue, and normal adjacent skin tissues around the scar were included. The patients underwent surgery at Yantai Yuhuangding Hospital from April 2018 to March 2019. The scar tissue was red in color and firm and was seen as a nodular and raised area of skin. The scar and its surrounding normal skin tissue were divided into three. One set of tissue was fixed by 4% neutral buffered formalin, and paraffin-embedded for immunohistochemistry, one set was used for quantitative real-time polymerase chain reaction (qRT-PCR), and one was used for isolation and culture of fibroblasts. Inclusion criteria: patients did not use retinoic acid for one month. Patients were excluded from the study if they had infection or inflammation around the scar, and patients with severe hypertension or diabetes were also excluded.
Study groups
The differential expression of ubiquitin-specific protease 4 (USP4) and transforming growth factor-β receptor type 1 (TGF-βR1) in normal tissues and hypertrophic scar tissue were investigated. The tissues were divided into the normal skin (NS) group and hypertrophic scar (HS) group. The differential expression of USP4 and TGF-βR1, and Smad7 in normal skin fibroblasts and hypertrophic scar fibroblasts were studied in vitro. The cells were divided into the normal skin fibroblasts (NSFB) group and the hypertrophic scar tissue fibroblasts (HSFB) group. The effects of USP4 on the biological behavior of hypertrophic scar tissue fibroblasts were investigated by dividing the cells into three groups, the blank group (untreated control group), the silenced negative control (siNC) group (cells transfected with siNC), and the siUSP4 group (cells transfected with siUSP4).
Immunohistochemistry
Human hypertrophic scar tissues and normal skin tissues were fixed in 4% neutral buffered formalin. The specimens were embedded in paraffin wax and sectioned for immunohistochemistry. Sections were incubated with 3% H2O2 for 10 min at room temperature and then washed three times in distilled water. Antigen retrieval was performed using an autoclave. Tissue sections were incubated with primary antibodies that included rabbit anti-human USP4 (1: 100) (ab236987; Abcam, Cambridge, MA, USA) and TGF-βR1 (1: 100) (ab31013; Abcam, Cambridge, MA, USA) for 2 h at room temperature. The sections were then washed three times in PBS for 3 min, and then incubated in horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1: 200) (Solarbio, Beijing, China). After incubation for 30 min at room temperature, the cells were then washed three times in PBS for 5 min. After staining with the chromogen 3,3′-diaminobenzidine (DAB) (Solarbio, Beijing, China) for 2 min, the cells were counterstained with hematoxylin for 1 min, treated by hydrochloric acid ethanol for 2 s, washed with ammonia water for 10 s to remove the blue color, and the cells were rinsed in distilled water for 5 min. The expression and distribution of USP4 and TGF-βR1 in each section was detected using light microscopy and photographed.
Isolation and culture of hypertrophic scar fibroblasts and normal skin fibroblasts
Human hypertrophic scar tissues and normal skin tissues were sectioned, and 2 U/mL of dispase II neutral protease (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to separate the epidermis and dermis at 4°C overnight. Then, 300 μg/mL of collagenase type I (Sigma-Aldrich, St. Louis, MO, USA) was used to digest the dermal tissues. Fibroblasts were cultured in DMEM containing 10% fetal bovine serum (FBS) (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA).
Immunofluorescence staining
After the cultured fibroblasts reached 70–80% confluence, the cells were fixed in 4% formaldehyde for 30 min, then washed with PBS, and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA) for 10 min at room temperature. The cells were treated with 3% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA) for 30 min and incubated with primary mouse anti-vimentin antibody (ab8978; Abcam, Cambridge, MA, USA), diluted in PBS (1: 100), at 4°C overnight. After removing the primary antibody, the cells were washed by three times in PBS, and the secondary goat polyclonal anti-mouse IgG antibody was added, which was conjugated with fluorescein isothiocyanate (FITC) (1: 200) (ab150120; Abcam, Cambridge, MA, USA) and incubated at room temperature for 1 h. Then, the samples were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime Biotechnology, Inc., Shanghai, China).
Cell transfection
Cell transfection was performed using siUSP4 and siNC, which were transfected into hypertrophic scar fibroblasts using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). The USP4 siRNAs sequence was 5′-CCAUUUCUGCUUGGCUGUCUCCUUU-3, and the siNC sequence was 5′-GCGCGATAGCGCGAATATA-3′. Further studies were performed after transfection for 48 h.
Cell Counting Kit-8 (CCK-8) assay
Hypertrophic scar fibroblasts (5×107) were plated in a 96-well plate. PBS was added to the cells to a volume of 100 μL/well, and six wells were used for each group. After transfection for 48 h, the CCK-8 solution (10 μL) (Dojindo, Tokyo, Japan) was added to each well at days 3, 5, and 7 before cell transfection, to detect cell proliferation, and the cells were incubated at 37°C for 2 h. The absorbance was detected at a wavelength of 450 nm using a microplate reader (BioTek, Winooski, VT, USA). The mean value of the six wells was calculated.
Apoptosis assay
After transfection for 48 h, apoptosis of hypertrophic scar fibroblasts was detected by flow cytometry using a BD FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The cells were stained with 10 μL Annexin V-FITC (Thermo Fisher Scientific, Waltham, MA, USA) and 5 μL of propidium iodide (PI). The proportion of Annexin V/PI-stained cells was quantified by flow cytometry using software analysis (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot
The cells were washed in PBS 48 h after transfection. Total proteins of the cells were extracted, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked at room temperature for 1 h using PBST buffer containing 5% dried skimmed milk powder. The membranes were incubated overnight at 4°C with the following primary antibodies: USP4 (1: 500) (ab236987; Abcam, Cambridge, MA, USA); TGF-βR1 (1: 1000) (ab31013; Abcam, Cambridge, MA, USA); Smad7 (1: 200) (ab90086; Abcam, Cambridge, MA, USA); collagen I (1: 1000) (ab34710; Abcam, Cambridge, MA, USA); collagen III (1: 5000) (ab7778; Abcam, Cambridge, MA, USA); fibronectin (1: 1000) (ab2413; Abcam, Cambridge, MA, USA); and alpha-smooth muscle actin (α-SMA) (1: 1000) (ab5694; Abcam, Cambridge, MA, USA). The membranes were incubated with the secondary human HRP-conjugated antibody, anti-rabbit IgG (1: 5,000 dilution) at room temperature for 1 h. An antibody to GAPDH (1: 500) (ab8245; Abcam, Cambridge, MA, USA) was used as the internal control. Protein expression was detected using an electrochemiluminescence (ECL) microplate reader (Beyotime Biotechnology, Inc., Shanghai, China).
Wound-healing assay
When hypertrophic scar fibroblasts reached more than 90% confluence, the cells were scraped to create a defect on the bottom of the plate using the pipette tip. The cells were cultured in DMEM containing 1% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The width of the wound was calculated, and healing was detected by light microscopy and photographed immediately, and then at 12 h and 24 h after the test. The width of the wound was measured using Photoshop software. Data were shown as the relative cell migration rate.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs from normal skin tissues, hypertrophic scar tissues, and hypertrophic scar fibroblasts were obtained using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The cDNAs were synthesized from RNAs using a qRT-PCR kit (TaKaRa, Shiga, Japan). The qRT-PCR reaction was performed in CFX Connect™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA), and the PCR cycles were as follows: 40 cycles at 94°C for 5 s and at 60°C for 30 s.
The following primers were used:
USP4, forward: 5′-TACTAAACTGGTACGGCTGTGT-3′;
USP4, reverse: 5′-GGGATGTTGAATAGCTTCCGC-3′;
Collagen I, forward: 5′-AGGGCCAAGACGAAGACATC-3′;
Collagen I, reverse: 5′-GTCGGTGGGTGACTCTGAGC-3′;
Collagen III, forward: 5′-TGAAGGGCAGGGAACAACT-3′;
Collagen III, reverse: 5′-GGATGAAGCAGAGCGAGAAG-3′;
Fibronectin, forward: 5′-TGCGTTGGTTTGTACTTGTTA-3′;
Fibronectin, reverse: 5′-AAGTGTTCCCAGTGTACTTGTC-3′;
β-SMA, forward: 5′-CGTGGCTATTCCTTCGTTACTA-3′;
β-SMA, reverse: 5′-ATCAGGCAACTCGTAACTCTTC-3′.
The mRNAs expression levels were normalized to GAPDH. The GAPDH primers were GAPDH, forward: 5′-TGGATTTGGA CGCATTGGTC-3′, and GAPDH, reverse: 5′-TTTGCACTGGTAC GTGTTGAT-3′. The quantification of mRNAs was performed using the CFX manager protocol (Bio-Rad, Hercules, CA, USA). All qRT-PCR data were calculated and quantified by the 2−ΔΔCt method [17].
Statistical analysis
Data were presented as the mean±standard deviation (SD). Statistical analysis was performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Differences between two groups were determined by Student’s t-test. Differences between three or more groups were determined using one-way analysis of variance (ANOVA) followed by Tukey’s test. A P-value <0.05 was considered to be statistically significant.
Results
Differential expression of ubiquitin-specific protease 4 (USP4) and transforming growth factor-β receptor type 1 (TGF-βR1) in normal skin and hypertrophic scar tissue
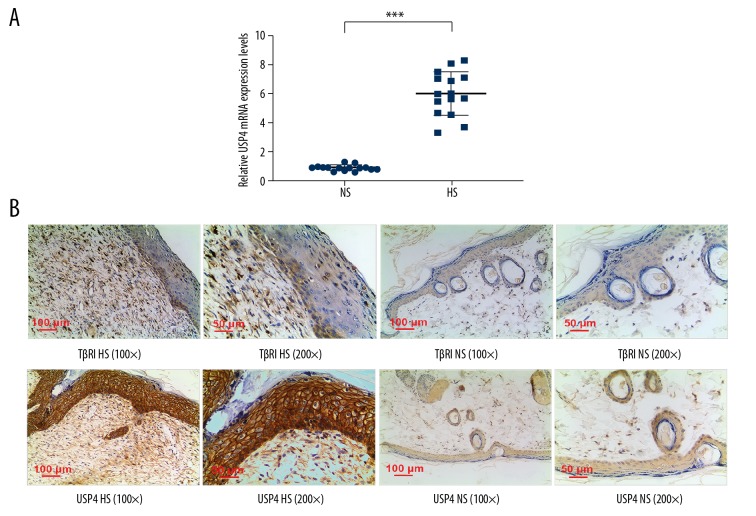
Quantitative real-time polymerase chain reaction (qRT-PCR) and immunohistochemistry were performed to detect the expression of USP4 and TGF-βR1from normal skin and hypertrophic scar tissues. The results of qRT-PCR showed that the USP4 expression levels were significantly increased in hypertrophic scar tissues compared with normal skin tissues (Figure 1A). The results of immunohistochemical staining showed that USP4 was positively expressed in basal epidermal cells of normal skin tissues, while reduced expression of USP4 and TGF-βR1 were observed in fibroblasts. However, USP4 showed high expression in basal epidermal cells of hypertrophic scars, and high USP4-positive and TGF-βR1-positive expression were found in fibroblast cell membranes and cytoplasm (Figure 1B).
Figure 1.
The expression of ubiquitin-specific protease 4 (USP4) and transforming growth factor-β receptor type 1 (TGF-βR1) in normal skin and hypertrophic scar tissue. (A) The results of quantitative real-time polymerase chain reaction (qRT-PCR) show that the expression level of USP4 in human hypertrophic scar tissues was significantly greater than that in normal tissues. *** p<0.001 vs. normal skin (NS). (B) The photomicrograph of the immunohistochemistry shows mild expression of USP4 and TGF-βR1 in the basal epidermal cells and fibroblasts of normal skin tissues. Positively stained cells for USP4 are present in the basal epidermal cells, fibroblast cell membranes and cytoplasm in hypertrophic scar tissue and positive staining for TGF-βR1 of fibroblast cell membranes and cytoplasm.
Differential expression of USP4, TGF-βR1, and Smad7 in normal skin fibroblasts and hypertrophic scar fibroblasts
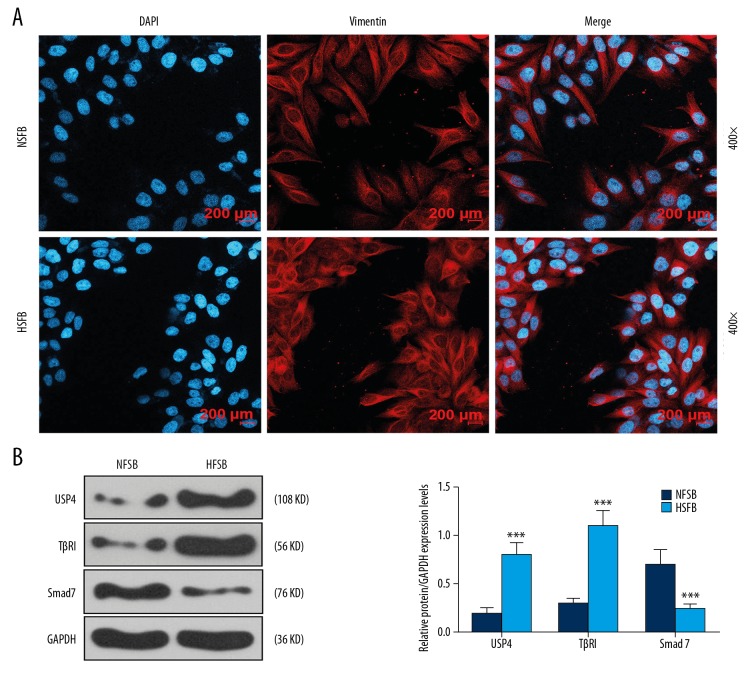
The cultured cells were analyzed by immunoassay, and blue fluorescence-labeled cell nuclei, and red fluorescence-labeled vimentin-positive cells were identified (Figure 2A). The protein expression of levels of USP4, TGF-βR1, and Smad7 from normal skin fibroblasts and hypertrophic scar fibroblasts measured by Western blot showed that USP4 and TGF-βR1 expression was upregulated in hypertrophic scar fibroblasts, and Smad7 expression was down-regulated in hypertrophic scar fibroblasts (Figure 2B).
Figure 2.
The expression of ubiquitin-specific protease 4 (USP4), transforming growth factor-β receptor type 1 (TGF-βR1), and Smad7 in normal skin fibroblasts and fibroblasts from hypertrophic scar fibroblasts cultured in vitro. (A) Immunofluorescence staining shows that the nuclei of cultured cells are stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue), and vimentin staining is positive. (B) Western blot shows that the expression of USP4 and TGF-βR1 were significantly increased in hypertrophic scar fibroblasts compared with normal skin fibroblasts. The expression of Smad7 in hypertrophic scar fibroblasts was significantly lower than in normal skin fibroblasts. *** p<0.001 vs. normal skin fibroblasts (NSFB).
The effects of low expression of USP4 on biological behaviors of hypertrophic scar fibroblasts
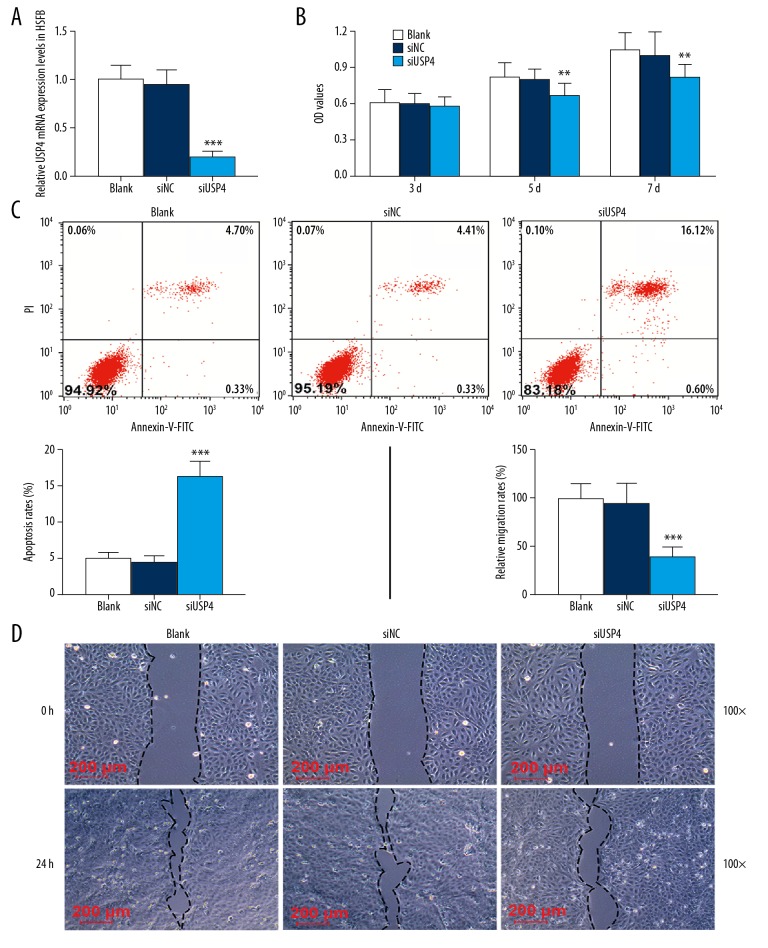
Construction of a low-expression USP4 vector was used to study the effects of low expression of USP4 on the biological behaviors of hypertrophic scar fibroblasts. The qRT-PCR results showed that USP4 was successfully transfected into hypertrophic scar fibroblasts (Figure 3A). By measuring the absorbance of hypertrophic scar fibroblasts at 450 nm in the Cell Counting Kit-8 (CCK-8) assay, the absorbance in each group increased with time, but the absorbance of cells transfected with siUSP4 at the same time on the fifth and seventh day were significantly less than those transfected with siNC (Figure 3B). Flow cytometry was performed to detect the apoptosis of hypertrophic scar fibroblasts and showed that cells transfected with siUSP4 had a higher apoptotic rate than normal cultured cells or those transfected with siNC (Figure 3C). Also, the in vitro wound-healing assay for cell migration showed that low expression of USP4 reduced cell migration. After 24 h, in the wound-healing assay, the width of the cell scrape of the siUSP4 group was significantly wider than that of the siNC group (Figure 3D).
Figure 3.
The effects of ubiquitin-specific protease 4 (USP4) on cell proliferation, apoptosis, and migration of hypertrophic scar fibroblasts cultured in vitro. (A) The transfection rate of USP4 in hypertrophic scar fibroblasts measured by quantitative real-time polymerase chain reaction (qRT-PCR). After transfection with siUSP4, the mRNA level of USP4 was significantly inhibited. (B) The Cell Counting Kit-8 (CCK-8) assay was performed to detect the activity of hypertrophic scar fibroblasts in vitro. The results show that the viability of cells transfected with siUSP4 is significantly reduced. (C) Flow cytometry for cell apoptosis shows that after transfection with siUSP4, the cell apoptosis rate is significantly increased. (D) The wound-healing assay detected cell migration. The results show that 24 h after the test, the width of the cell defect in the cells transfected with siUSP4 was significantly greater than that of cells in siNC group. ** p<0.01, *** p<0.001 vs. siNC.
The effects of USP4 on the collagen I, collagen III, fibronectin, α-SMA and TGF-β/Smad7 pathway
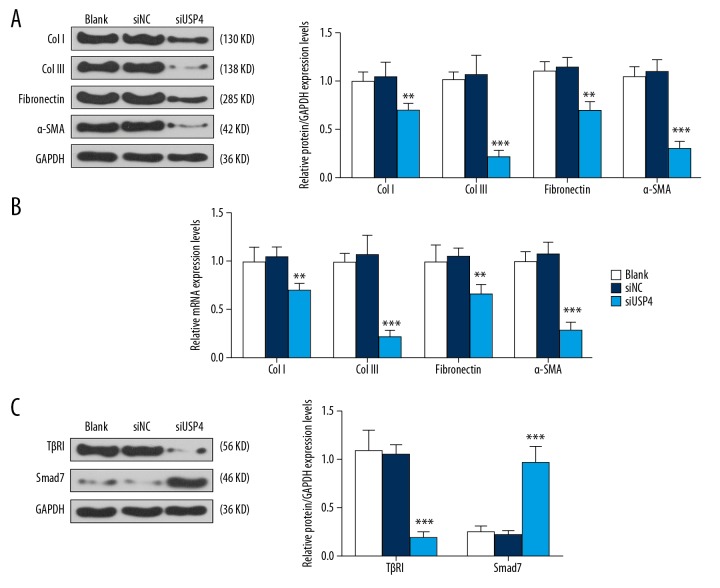
The expression of extracellular matrix (ECM) factors, including collagen I, collagen III, fibronectin, and α-SMA were detected by qRT-PCR and Western blot, respectively. The results showed that the expression of collagen I, collagen III, fibronectin, and α-SMA in the siUSP4 group were significantly lower than in the siNC group (Figure 4A, 4B). Also, the expression of the TGF-β/Smad7 pathway proteins was determined by Western blot and showed that when compared with hypertrophic scar fibroblasts in the control and siNC group, the expression level of TGF-βR1 protein was down-regulated, while Smad7 protein expression was upregulated in the cells transfected with siUSP4 (Figure 4C).
Figure 4.
The effects of ubiquitin-specific protease 4 (USP4) on the extracellular matrix (ECM), α-smooth muscle actin (α-SMA), and on the transforming growth factor-β (TGF-β) and Smad7 pathway. (A) ECM markers, including collagen I, collagen III, fibronectin, and α-SMA protein were detected by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot. Down-regulation of USP4 resulted in low expression of collagen I, collagen III, fibronectin, and α-SMA in hypertrophic scar fibroblasts. (B) The qRT-PCR assay shows that the down-regulation of USP4 reduced the expression of collagen I, collagen III, fibronectin, and α-SMA mRNAs in hypertrophic scar fibroblasts. (C) Western blot was performed to investigate the TGF-βR1/Smad7 pathway and showed that siUSP4 down-regulated TGF-βR1 and upregulated Smad7 in hypertrophic scar fibroblasts. ** p<0.01, *** p<0.001 vs. siNC.
Discussion
Wound healing, or tissue repair, is a complex and dynamic process that includes acute and chronic inflammation, organization, healing, and scar formation [18]. Hypertrophic scars, which are characterized by excessive collagen deposition in the subcutaneous and dermal tissue [19], can occur after abnormal wound healing. The risk of development of hypertrophic scar is increased with the severity of trauma, such as the depth and degree of the injury [20,21]. This study investigated the effects of transforming growth factor-β receptor type I (TGF-βRI) and ubiquitin-specific protease 4 (USP4) in human tissue samples of hypertrophic scar and on cell proliferation and cell migration in primary fibroblast cultures. The findings showed that USP4 was highly expressed in hypertrophic scar tissues and hypertrophic scar fibroblasts. By inhibiting the expression of USP4, down-regulation of USP4 inhibited the proliferation, migration, and apoptosis of hypertrophic scar fibroblasts through the TGF-β/Smad7 signaling pathway.
Transforming growth factor-β (TGF-β) promotes the synthesis of extracellular matrix (ECM) components, including collagen [22], promotes fibroblast proliferation, and induces fibroblast differentiation during scar formation, and participates in scar formation by regulating fibrosis and keratinocyte proliferation [23]. Also, as one of the negative feedback products of the TGF-β signaling pathway [24], Smad7 can regulate multiple responses induced by the TGF-β ligand [25]. The findings from the present study showed that USP4 and TGF-βR1 were upregulated, while Smad7 was down-regulated in hypertrophic scar fibroblasts. As shown in previous studies [26], USP4 is an effective inducer of TGF-β signal transduction, which interacts directly with TGF-βR1 and acts as a de-ubiquitinase enzyme to maintain the level of TGF-βR1 on the plasma membrane. The hypothesis that drove this study was that USP4 might also mediate the TGF-β signal transduction pathway in hypertrophic scar fibroblasts. According to a previous study by Iyengar [27], Akt promotes USP4 to enter the cell membrane and cytoplasm to phosphorylate USP4 and regulate its subcellular localization, and Akt phosphorylation of USP4 occurs by combining with other de-ubiquitinating enzymes (DUBs) to de-ubiquitinate the TGF-β receptor [27]. This finding suggests that USP4 may be a key factor in the interaction between the TGF-β signaling pathway and the AKT signaling pathway.
In the present study, hypertrophic scar fibroblasts were isolated and cultured from hypertrophic scar tissues and underwent USP4 siRNA transfection to investigate the role of USP4 in hypertrophic scar fibroblasts. The Cell Counting Kit-8 (CCK-8) assay results showed that the proliferation of hypertrophic scar fibroblasts transfected with siUSP4 was significantly inhibited on the fifth and seventh day, indicating that down-regulation of USP4 could suppress the proliferation of hypertrophic scar fibroblasts. Also, the cell migration of hypertrophic scar fibroblasts transfected with USP4 siRNA was inhibited, while the apoptosis rate was increased. These findings support that USP4 can bind to TGF-βR1 during hypertrophic scar formation and inhibit ubiquitination of TGF-βR1 by inhibiting the Smad7-mediated signaling pathway, to maintain high levels of TGF-βR1and stimulate TGF-β1 in the skin. Therefore, interfering with or inhibiting USP4 at the gene or protein level may block the TGF-β1/Smad7 signaling pathway and inhibit the formation of hypertrophic scar.
The mechanism of hypertrophic scar formation is associated with abnormal cell proliferation, the transformation of fibroblasts in response to damage, stimulation of cytokines, including TGF-β, and upregulation of genes such as α-SMA, which are involved in the extracellular matrix (ECM), collagen, and fibronectin production [28]. Also, α-SMA is the key factor involved in tissue contraction [29], and myofibroblasts contractility is involved in the pathogenesis of hypertrophic scar, resulting in irreversible tissue contracture and the release of TGF-β1, to promote the activity of myofibroblasts [30]. In the present study, the down-regulation of USP4 induced reduced expression of collagen I, collagen III, fibronectin, and α-SMA mRNAs in hypertrophic scar fibroblasts. These results suggest that USP4 may regulate the ECM in hypertrophic scar fibroblasts by regulating the TGF-β1/Smad7 signaling pathway. Also, detection of the expression levels of TGF-βR1and Smad7 proteins showed that the down-regulation of USP4 inhibited the expression of TGF-βR1 and promoted the expression of Smad7.
Conclusions
This study aimed to investigate the effects of transforming growth factor-β receptor type I (TGF-βR1) and ubiquitin-specific protease 4 (USP4) in human tissue samples of hypertrophic scar and normal skin, and on cell proliferation and cell migration in primary fibroblast cultures in vitro. The findings showed that the expression of TGF-βR1 and USP4 were increased in hypertrophic scar tissues and fibroblasts, and that down-regulating USP4 inhibited hypertrophic scar fibroblast proliferation and migration and induced cell apoptosis in vitro. The effects of USP4 were mediated through the TGF-β/Smad7 signaling pathway. Therefore, blocking USP4 might be a future approach for the prevention and treatment of hypertrophic scar. The findings from this preliminary study support the need for further studies on the role of USP4 and the molecular mechanisms involved in hypertrophic scar formation.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Shandong Key Research and Development Plan Project (No. 2018GSF118101)
References
- 1.Weng W, He S, Song H, et al. Aligned carbon nanotubes reduce hypertrophic scar via regulating cell behavior. ACS Nano. 2018;12(8):7601–12. doi: 10.1021/acsnano.7b07439. [DOI] [PubMed] [Google Scholar]
- 2.Matiasek J, Kienzl P, Unger LW, et al. An intra-individual surgical wound comparison shows that octenidine-based hydrogel wound dressing ameliorates scar appearance following abdominoplasty. Int Wound J. 2018;15(6):914–20. doi: 10.1111/iwj.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Toro D, Dedhia R, Tollefson TT. Advances in scar management: prevention and management of hypertrophic scars and keloids. Curr Opin Otolaryngol Head Neck Surg. 2016;24(4):322–29. doi: 10.1097/MOO.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 4.Sato C, Yamamoto Y, Funayama E, et al. Conditioned medium obtained from amnion-derived mesenchymal stem cell culture prevents activation of keloid fibroblasts. Plast Reconstr Surg. 2018;141(2):390–98. doi: 10.1097/PRS.0000000000004068. [DOI] [PubMed] [Google Scholar]
- 5.Tu L, Huang Q, Fu S, Liu D. Aberrantly expressed long noncoding RNAs in hypertrophic scar fibroblasts in vitro: A microarray study. Int J Mol Med. 2018;41(4):1917–30. doi: 10.3892/ijmm.2018.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng J, Shi Y, Gao Z, et al. Inhibition of pathological phenotype of hypertrophic scar fibroblasts via coculture with adipose-derived stem cells. Tissue Eng Part A. 2018;24(5–6):382–93. doi: 10.1089/ten.TEA.2016.0550. [DOI] [PubMed] [Google Scholar]
- 7.Hu H, Yang S, Zheng J, Mao G. Structure-based derivation of peptide inhibitors to target TGF-beta1 receptor for the suppression of hypertrophic scarring fibroblast activation. Chem Biol Drug Des. 2017;90(3):345–51. doi: 10.1111/cbdd.12954. [DOI] [PubMed] [Google Scholar]
- 8.Tang PM, Zhang YY, Lan HY. LncRNAs in TGF-beta-driven tissue fibrosis. Noncoding RNA. 2018;4(4) doi: 10.3390/ncrna4040026. pii: E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Z, Xia W, Fisher GJ, et al. YAP/TAZ regulates TGF-beta/Smad3 signaling by induction of Smad7 via AP-1 in human skin dermal fibroblasts. Cell Commun Signal. 2018;16(1):18. doi: 10.1186/s12964-018-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CM, He CH, Park JW, et al. Chitinase 1 regulates pulmonary fibrosis by modulating TGF-beta/SMAD7 pathway via TGFBRAP1 and FOXO3. Life Sci Alliance. 2019;2(3) doi: 10.26508/lsa.201900350. pii: e201900350. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Liu C, Liu C, Liu H, et al. Increased expression of ubiquitin-specific protease 4 participates in neuronal apoptosis after intracerebral hemorrhage in adult rats. Cell Mol Neurobiol. 2017;37(3):427–35. doi: 10.1007/s10571-016-0375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehic M, de Sa VK, Hebestreit S, et al. The deubiquitinating enzymes USP4 and USP17 target hyaluronan synthase 2 and differentially affect its function. Oncogenesis. 2017;6(6):e348. doi: 10.1038/oncsis.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Wang S, Liu W. EMT-related long non-coding RNA in hepatocellular carcinoma: A study with TCGA database. Biochem Biophys Res Commun. 2018;503(3):1530–36. doi: 10.1016/j.bbrc.2018.07.075. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Hao Q, Luo J, et al. USP4 inhibits p53 and NF-kappaB through deubiquitinating and stabilizing HDAC2. Oncogene. 2016;35(22):2902–12. doi: 10.1038/onc.2015.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu C, Liu Y, Mei Y, et al. Ubiquitin-specific protease 4 promotes metastasis of hepatocellular carcinoma by increasing TGF-β signaling-induced epithelial-mesenchymal transition. Aging (Albany NY) 2018;10(10):2783. doi: 10.18632/aging.101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao J, Ma Y, Shen J, Yao H. TGF-β promotes invasion and angiogenesis of oral squamous cell carcinoma SCC9 cells by upregulation of slug signal. Int J Clin Exp Pathol. 2017;10(5):5325–33. [Google Scholar]
- 17.Yun SI, Kim KK. Ubiquitin-specific protease 4 (USP4) suppresses myoblast differentiation by down regulating MyoD activity in a catalytic-independent manner. Cell Signal. 2017;35:48–60. doi: 10.1016/j.cellsig.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Yates CC, Rodrigues M, Nuschke A, et al. Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res Ther. 2017;8(1):193. doi: 10.1186/s13287-017-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J, Lin Q, Shao Y, et al. BMP7 suppresses excessive scar formation by activating the BMP7/Smad1/5/8 signaling pathway. Mol Med Rep. 2017;16(2):1957–63. doi: 10.3892/mmr.2017.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limandjaja GC, van den Broek LJ, Breetveld M, et al. Characterization of in vitro reconstructed human normotrophic, hypertrophic, and keloid scar models. Tissue Eng Part C Methods. 2018;24(4):242–53. doi: 10.1089/ten.TEC.2017.0464. [DOI] [PubMed] [Google Scholar]
- 21.Guo L, Xu K, Yan H, et al. MicroRNA expression signature and the therapeutic effect of the microRNA21 antagomir in hypertrophic scarring. Mol Med Rep. 2017;15(3):1211–21. doi: 10.3892/mmr.2017.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perumal N, Perumal M, Halagowder D, Sivasithamparam N. Morin attenuates diethylnitrosamine-induced rat liver fibrosis and hepatic stellate cell activation by co-ordinated regulation of Hippo/Yap and TGF-beta1/Smad signaling. Biochimie. 2017;140:10–19. doi: 10.1016/j.biochi.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Moon J, Yoon JY, Yang JH, et al. Atrophic acne scar: A process from altered metabolism of elastic fibres and collagen fibres based on transforming growth factor-beta1 signalling. Br J Dermatol. 2019;181(6):1226–37. doi: 10.1111/bjd.17851. [DOI] [PubMed] [Google Scholar]
- 24.Yang L, Du X, Liu L, et al. miR-1306 mediates the feedback regulation of the TGF-beta/SMAD signaling pathway in granulosa cells. Cells. 2019;8(4) doi: 10.3390/cells8040298. pii: E298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, Gu S, Li W, et al. Smad7 enables STAT3 activation and promotes pluripotency independent of TGF-beta signaling. Proc Natl Acad Sci USA. 2017;114(38):10113–18. doi: 10.1073/pnas.1705755114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Zhou F, van Dinther M, Ten Dijke P. Determining TGF-beta receptor levels in the cell membrane. Methods Mol Biol. 2016;1344:35–47. doi: 10.1007/978-1-4939-2966-5_2. [DOI] [PubMed] [Google Scholar]
- 27.Iyengar PV. Regulation of ubiquitin enzymes in the TGF-beta pathway. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040877. pii: E877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu X, Khalil H, Kanisicak O, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128(5):2127–43. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinde AV, Humeres C, Frangogiannis NG. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta Mol Basis Dis. 2017;1863(1):298–309. doi: 10.1016/j.bbadis.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang X, Chai B, Duan R, et al. Inhibition of FKBP10 attenuates hypertrophic scarring through suppressing fibroblast activity and extracellular matrix deposition. J Invest Dermatol. 2017;137(11):2326–35. doi: 10.1016/j.jid.2017.06.029. [DOI] [PubMed] [Google Scholar]