Abstract
Prevention is essential for avoiding the complications of muscle hematomas (pseudotumors, compartment syndromes and peripheral nerve lesions) in hemophilic patients. This is achieved through early diagnosis of muscle hematomas and proper long-term hematological treatment until they have resolved (confirmed by image studies). Ultrasound-guided percutaneous drainage could be beneficial in terms of achieving better and faster symptom relief. When suspecting a hemophilic pseudotumor, biopsy will help us confirm the diagnosis and rule out true tumors (chondrosarcoma, liposarcoma, synovial sarcoma) that sometimes mimic hemophilic pseudotumor. Surgical removal of hemophilic pseudotumor is the best solution. As alternatives, there are curettage and filling with cancellous bone and radiotherapy (when surgery is contraindicated). Preoperative arterial embolization (ideally 2 weeks before surgery) helps control intraoperative bleeding during surgery for giant pelvic pseudotumors.
Key Words: Hemophilia, Muscle hematomas, Prevention, Pseudotumors, Treatment
Introduction
The clinical severity of hemophilia is commonly related to the plasma level of factor VIII or factor IX. Patients are classified as having mild, moderate or severe hemophilia depending on the level of the deficient factor, which can be >5% of normal in mild cases and <1% of normal in severe hemophilia. This is reflected in the frequency and causes of bleeding. Whereas a patient with mild hemophilia will bleed rarely, usually only after significant trauma or surgery, those with severe hemophilia may have several episodes per month, and typically bleed spontaneously as a result of minimal trauma or activities of daily living (1, 2).
The main problem for patients with hemophilia is that they suffer from hemorrhages in the musculoskeletal system, both joints (hemarthrosis) and muscles. These represent approximately 80% of all hemorrhages and usually begin in childhood. At the joint level, they usually occur mainly in the knees, ankles and elbows; at the muscular level, they can appear in any muscle group of the body (although muscle hematomas are less frequent than hemarthroses) (1, 2).
The most accepted treatment for muscle hematomas in patients with congenital coagulopathies is intravenous infusion of the deficient factor until achieving total spontaneous resorption of the hematoma (confirmed by imaging studies – ultrasonography-US, computed tomography-CT or magnetic resonance imaging-MRI). Ultrasound-guided percutaneous drainage could be beneficial in terms of achieving better and faster symptom relief. Ultrasound guided hematoma evacuation is a safe procedure. However, the proportion of unsuccessful drainages and hematoma recurrence is substantial (13%). Such a rate of unsuccessful evacuation is due to excessive density and/or viscosity of the content. Ideally, hematoma drainage must be performed before 3 to 5 days since the beginning of the muscular bleed (3). However, when treatment is insufficient, some muscle hematomas can cause serious complications such as acute compartment syndrome (ACS), pseudotumor and peripheral nerve compression (1-9).
Patients with congenital coagulopathies may suffer from ACS due to a hematoma in some inextensible compartment of their anatomy. It is a rare problem but can be very serious. Typically, the ACS of the volar aspect of the forearm (Volkmann’s syndrome) causes a retraction of the volar musculature that causes a great deformity and permanent disability of the hand. It is essential to carry out the urgent opening of the affected compartment (fasciotomy) as soon as its existence is suspected (5, 7).
ACS is characterised by an increase in intramuscular pressure in an anatomical compartment. This causes a decrease in capillary perfusion that threatens tissue survival (5, 7). If the pressure increase lasts a few hours, there will be necrosis of the muscle and nerve tissue which will cause a contracture of the affected limb and a permanent loss of function. Therefore, early diagnosis and treatment is essential.
The diagnosis should be based on the clinical examination (intense pain when the affected muscles are stretched) and objective measurement of the limb perfusion pressure (diastolic blood pressure minus intramuscular pressure) in the affected compartment. To achieve a reliable diagnosis, the patient should be evaluated for 1-2 hours. In children and in patients with loss of consciousness, a diagnosis cannot be made based on the level of pain, so it will be essential to measure the pressure of the compartment. If the mean perfusion pressure of the limb measured during a 12-hour period (monitored every 1-2 hours) is less than 30 mmHg, fasciotomy should be performed.
Unfortunately, fasciotomy is not exempt from potential complications. These include the need for further surgery because of a delay in the healing of the surgical wound, the need for a skin graft, pain, esthetic problems, nerve injury, permanent muscle weakness and chronic venous insufficiency.
In hemophilia, the first step in the treatment of an ACS should be adequate substitution of the deficient coagulation factor (intravenous infusion). In some patients with congenital coagulopathies, it is possible to halt disease progression by means of appropriate hematological treatment, leaving surgery (fasciotomy) reserved for those cases in which, despite said hematological treatment, the ACS does not remit (5, 7).
Peripheral nerve lesions are infrequent in patients with congenital coagulopathies. The most common injury is to the femoral nerve (due to an iliopsoas muscle hematoma) (6). A case of entrapment of the peroneal nerve secondary to a muscle hematoma, and a combined nerve injury (severe in the median nerve, mild in the ulnar nerve) in a young hemophilic patient secondary to a hematoma on the forearm has also been described (8, 9). Hematological treatment is sometimes enough. Otherwise, surgical neurolysis of the affected nerve must be performed. Surgery would only be indicated if the problem is not resolved within 24 hours.
In this article, the literature on the management of pseudotumors in patients with hemophilia will be reviewed.
Hemophilic pseudotumors: Definition and epidemiology
A pseudotumor is a recurrent chronic muscle hematoma, which in patients with hemophilia ends up developing the form of a tumor [Figure 1]. It is essential to remember that when there is a suspicion of a pseudotumor in a patient with congenital coagulopathy, we must always rule out the existence of a true tumor, which can be achieved through exhaustive study of the case (diagnostic imaging) and ultimately by performing a biopsy (always according to tumour criteria and under adequate hemostatic control). Ideally, this type of injury should be treated in specialized centres with expertise in bleeding disorder management in collaboration with a “Bone Tumor Unit”.
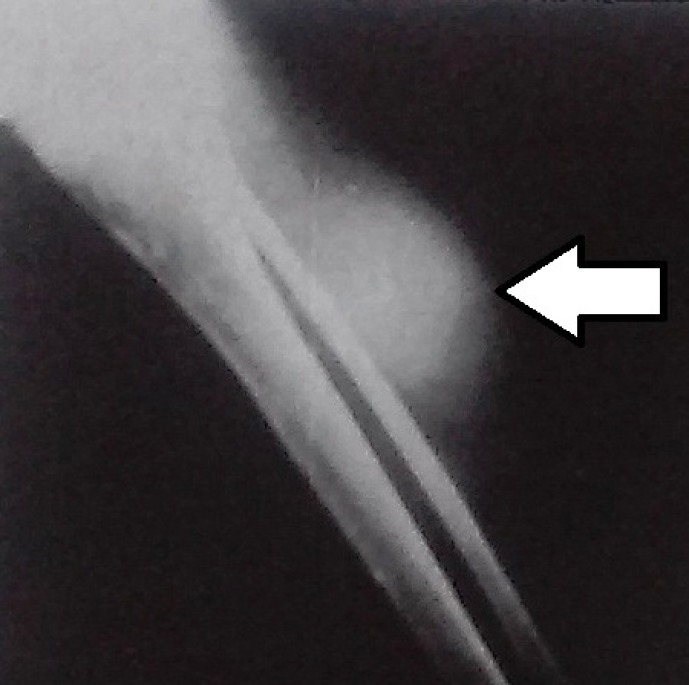
Figure 1.
Radiograph of a pseudotumor of the tibia (arrow) secondary to a recurrent muscle hematoma in the area
When pseudotumors are large, they can compress neighboring anatomical structures. The most frequent is the pelvic pseudotumor, secondary to a hematoma of the iliopsoas muscle [Figures 2; 3] (4).
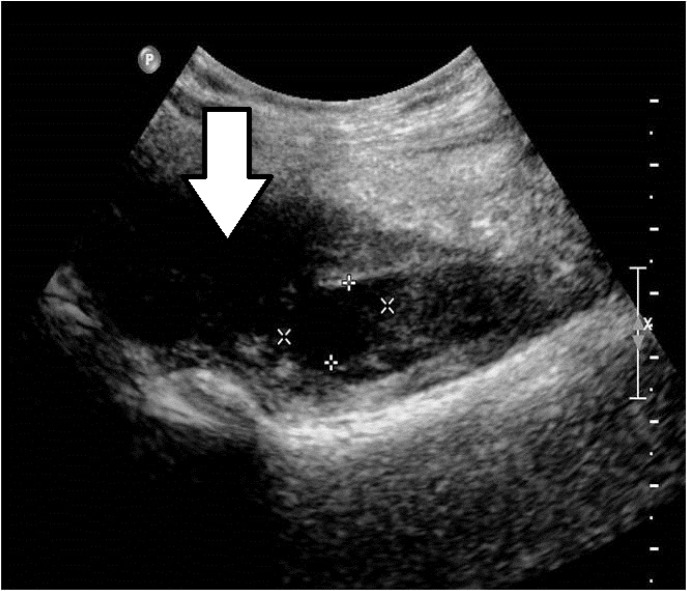
Figure 2.
Ultrasound image of an iliopsoas muscle hematoma (arrow) in a patient with hemophilia
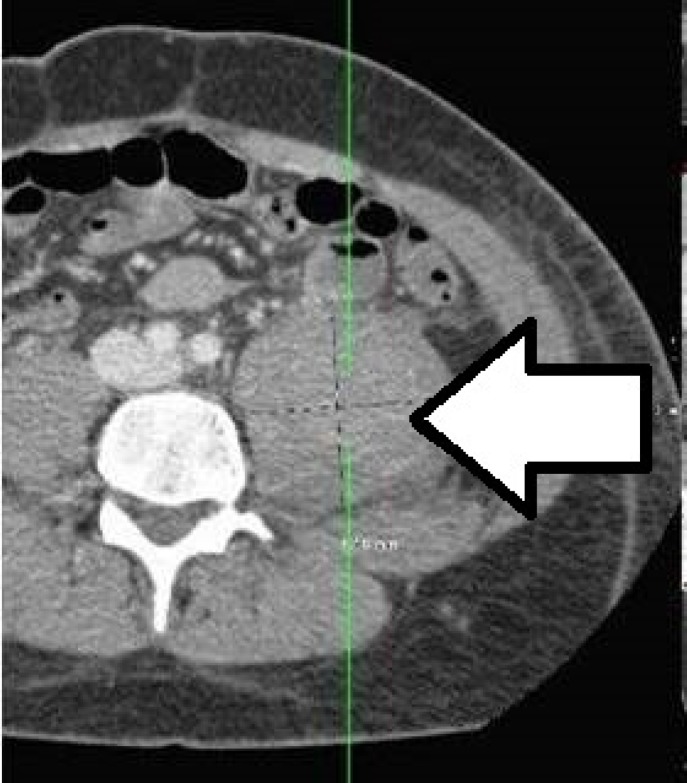
Figure 3.
CT scan of an iliopsoas muscle hematoma in a hemophilic patient
Another form of pseudotumor is a bone cyst, which is considered secondary to bleeding within a bone cavity. These lesions can be controlled by curettage and filling with bone graft or other similar material, provided that the bone cortices are intact [Figure 4].
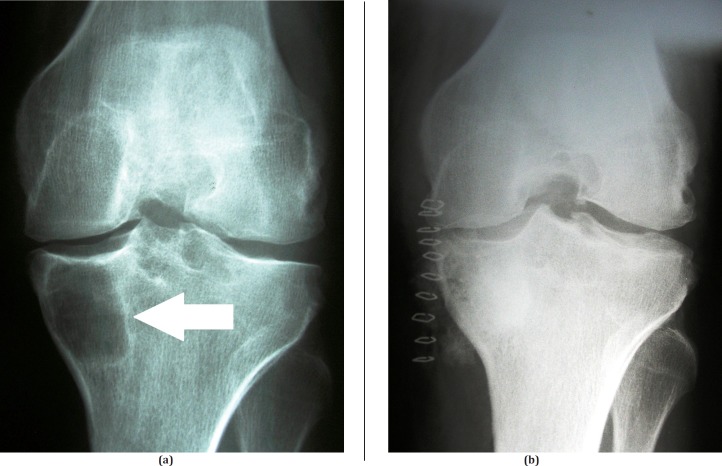
Figure 4.
Pseudotumor (bone cyst) of the proximal tibia successfully treated by curettage and filling with cancellous bone: (a) preoperative anteroposterior radiograph of the cyst (arrow); (b) postoperative anteroposterior radiograph
In one study, 49 cystic lesions were analyzed in 37 hemophilic patients (10). Their average age was 23 years and the mean follow-up was 10 years. The locations of the lesions were as follows: 24 (49%) in the tibia, seven (14.4%) in the ulna, six (12.2%) in the talus (12.2%), five (10.2%) in the humerus, five in the femur (10.2%) and two (4%) in distal radius. After aspirating the cystic content, the cavity was filled with impacted coral hydroxyapatite. In 48 of the 49 cases bone structure was restored, with only one patient needing a second intervention to resolve the problem.
Treatment of hemophilic pseudotumors
The best treatment for pseudotumor in patients with congenital coagulopathies is prevention, that is, proper long-term hematological treatment of muscle hematomas, until total resolution (reabsorption). Imaging tests (US, CT scan, MRI) are fundamental for monitoring the evolution of muscle hematomas in patients with congenital coagulopathies. In most cases the best solution is radical surgical excision. Preoperative arterial embolization (ideally 2 weeks before surgery) may be helpful in giant pelvic pseudotumors (11-13).
It has been observed that with primary hematological prophylaxis, the rate of pseudotumor has diminished considerably, being an indirect measure of the quality of a country’s hematological prophylaxis. It is essential to avoid muscle hematomas and diagnose them early. An untreated hematoma will tend to destroy adjacent soft tissues, erode the surrounding bone, and produce neurological damage (such as femoral nerve injury in hematomas of the iliopsoas muscle). It must be remembered that a pain in the right iliac fossa in a patient with congenital coagulopathy may be due to an iliopsoas hematoma and not to an acute appendicitis.
The combination of hematological treatment and radiotherapy can be used in pseudotumors located in the distal parts of the extremities, especially when there is a contraindication for surgery. Radiation should be done in small doses of 2 Gy or less until a total dose of 6-23.5 Gy is reached, which is the recommended radiation dosage (13).
Surgical treatment of pseudotumors should be performed in centers specialized in congenital coagulopathies and by a multidisciplinary team (hematologists, orthopedic surgeons, etc.). The main postoperative complications are infection, fistulation, pathological fractures (which may sometimes require amputation of the affected extremity) and even the death of the patient.
Table 1 summarises the most important publications in the literature on hemophilic pseudotumors (10, 14-54).
Table 1.
Main data on hemophilic pseudotumors in the literature
| AUTHORS | YEAR | FINDINGS |
|---|---|---|
| Hampton et al (16) | 1990 | These authors reported a patient with mild hemophilia who developed a pelvic pseudotumor that was successfully treated with surgery with no evidence of recurrence five years after operation. |
| Marisavljević et al (17) |
1991 | This article dealt with the successful extirpation of a femoral pseudotumor in a 53-year-old patient with severe hemophilia A and an inhibitor to factor VIII. A pseudotumor measuring 15 cm in diameter was extirpated. |
| Heim et al (18) | 1997 | A 13-year-old boy with severe hemophilia presented with gross swelling of his foot and infrapatellar area. X-rays revealed two separate pseudotumors. The patient underwent a transfemoral amputation. |
| Maliekel et al (19) | 1997 | In this report, the authors described an elderly woman who required surgical excision of a large hemophilic pseudotumor adjacent to the left gluteal muscle. The pseudotumor was surgically removed. During an 18-month follow-up period there has been no recurrence of bleeding or of the pseudotumor. |
| Ishiguro et al (20) | 1998 | This report presented three surgical cases of pseudotumors involved in a pathological fracture in the extremities. All cases showed a favourable postoperative course. |
| Heeg et al (21) | 1998 | The authors reported the case of a patient with a hemophilic pseudotumor of the ilium who developed chronic fistulation, 6 months after en-bloc resection. During the initial resection, the large defect in the iliac wing was filled with bone cement, which may have contributed to formation of the fistula. A second surgical procedure consisted of excision of the fistula and bone cement and the dead space was obliterated by bringing the gluteus medius muscle into the defect. The fistula recurred, however. Re-excision of the fistula and obliteration of the dead space by a pedicled rectus abdominis muscle flap resulted in eradication of the fistula. These authors emphasized the importance of obliterating the dead space that results from large pseudotumor resection. The use of bone cement was not advocated. They concluded that if a fistula does occur, a pedicled rectus abdominis muscle flap may be considered. |
| Sevilla et al (22) | 1999 | The authors presented a case of hemophilic pseudotumor of the iliac and caecum with cutaneous fistulas, with a septic process of endogenous origin. It was treated with surgical resection after performing arterial embolization to reduce the pseudotumor’s vascularization, thereby reducing its size and the risk of bleeding complications during surgery. |
| Raj et al (23) | 1999 | The authors reported a case of a hemophilic pseudotumor in the bony nasal pyramid, and believed this case was also unique on account of it having occurred in a patient with mild hemophilia. |
| Heaton et al (24) | 2000 | Two cases of iliopsoas hemophilic pseudotumors were presented by these authors. In one patient, a fistula developed between a pseudotumor and the large bowel. This resulted in an abscess involving the pseudotumor and adjacent tissues. It resolved after 5 years of therapy involving percutaneous drainage and closure of the fistula. The second patient had a massive pseudotumor that had obstructed both ureters. Later he suffered fatal mixed Gram negative septicaemia probably related to erosion into the colon. |
| Sagarra et al (25) | 2000 | Surgical or percutaneous treatment and refilling with fibrin sealant was shown to be successful in a 19-year-old male with severe hemophilia B. The pseudotumor, in the upper pad of the left leg, was filled with hydroxyapatite after surgery. The authors suggested that the use of hydroxyapatite is a new and useful option in the surgical treatment of hemophilic pseudotumor. |
| Bellinazzo et al (26) | 2000 | The authors reported 4 pseudotumors of the ilium in hemophilia treated by means of exeresis and transposition of the omentum in the residual cavity The long follow-up of these four patients suggested that this procedure was feasible and curative; local bleeding, infection and fistulation did not recur and the patients remained ambulant with the aid of appropriate devices. |
| Kale et al (27) | 2001 | The authors presented imaging findings of a histopathologically proven mandibular hemophilic pseudotumor. |
| Wexler et al (28) | 2001 | The authors reported a case of a proximal pseudotumor occurring in a 36-year-old patient with mild von Willebrand disease who made a good recovery with conservative management. |
| Gupta et al (29) | 2001 | A case of pseudotumor of the paranasal sinuses occurring in a patient with hemophilia A was reported by these authors. There was a favorable response to combined treatment with radiation therapy and factor VIII replacement. |
| O’Connell et al (30) | 2002 | The authors documented the first successful report of the surgical resection of a massive pseudotumor in a patient with high responding FVIII inhibitors. |
| Stevenson and Keast (31) | 2002 | The authors described a case of epistaxis due to a mass in the maxillary antrum that when biopsied had the histological appearance of a hemophilic pseudotumor. The epistaxis was eventually controlled by external beam radiotherapy to the pseudotumor. |
| Eby et al (32) | 2002 | The authors reported a 41-year-old patient with type 3 von Willebrand disease who underwent incomplete resection of a large retroperitoneal pseudocyst in 1995 and presented with a recurrent, extensive right abdominal and flank mass and signs and symptoms of large bowel obstruction. He required emergency partial colectomy for bowel ischaemia and removal of his right kidney, which was hydronephrotic due to prolonged ureteral obstruction by the pseudocyst. Following repeat partial resection of the pseudotumor, he developed persistent bleeding into the operative site despite aggressive administration of von Willebrand factor-rich factor VIII concentrates, resulting in retroperitoneal hematomas and abscesses, which resolved after 13 months of percutaneous drainage, extended supplementation of von Willebrand factor and antibiotic therapy. |
| Keller et al (33) | 2002 | The authors reported on a 45-year-old man with hemophilia A and high inhibitor titres who developed an extensive hemophilic pseudotumor with progressive destruction of the right ilium over a 12-year period. |
| Takedani et al (34) | 2004 | The authors described a patient with hemophilia A and factor VIII inhibitor who underwent surgical excision of a large pseudotumor in the left femoral region. The pseudotumor was surgically removed. |
| Libby and White (35) | 2004 | The authors present the fourth case describing intracranial pseudotumor in hemophilia. |
| O’Dowd et al (36) | 2006 | The authors presented an extremely unusual presentation where a large hemophilic pseudotumor of the pelvis extended to impinge the adjacent colon, resulting in large bowel obstruction. |
| Valentino et al (14) | 2006 | The successful removal of the giant pseudotumor described in this case report was achieved by a multidisciplinary team and demonstrated that this approach, albeit associated with significant risk, may be curative. |
| Ahuja et al (37] | 2007 | The authors described the management of a young boy with mild hemophilia A and a massive iliac pseudotumor with a multi-modality approach involving factor replacement, radiation therapy, embolization and surgery. The patient was initially treated with recombinant factor VIII and radiation therapy. Because of inadequate response and worsening of bony erosion, the patient had a preoperative embolization followed by surgical excision. The surgical procedure was associated with minimal blood loss and the patient had a relatively smooth postoperative course with no physical morbidity. |
| Rey et al (38) | 2007 | The authors reported three cases of pseudotumor of the mandible in young patients with mild hemophilia A. |
| Al Saadi et al (39) | 2008 | The authors presented the case of a 20-year-old male patient with a six-month history of left leg weakness, limited movement and muscle wasting. He was diagnosed clinically as having a psoas muscle rhabdomyosarcoma. During a CT-guided Tru-cut biopsy, he developed a serious and life-threatening bleed from a retroperitoneal muscular hematoma. The patient underwent laparotomy prior to his final diagnosis of an iliopsoas pseudotumor. |
| D’Young (40) | 2009 | Two cases were described where physiotherapy treatment was applied to large masses at the shoulder and femur respectively, where therapy commenced within the first 6 months following onset. These were presented relative to a case that was managed over a much longer period without early physiotherapy input, and the relative outcomes are examined. While both the early physiotherapy-managed cases showed a complete resolution at follow-up examination, the more established chronic pseudotumor required surgical excision, with significant residual muscle contractility, length and strength issues noted on clinical and MRI review. |
| Toepfer et al (41) | 2008 | The authors presented the case of a 59-year-old male patient suffering from a hemophilic pseudotumor of the right distal femur. After verification of the diagnosis by means of an open biopsy, final surgery with curettage and plombage with bone cement was performed. |
| Shi et al (42) | 2009 | The authors presented imaging findings of a histopathologically proven hemophilic pseudotumor involving the left mandibular region in a 9-year-old Chinese boy. |
| Bernstaedt et al (43) | 2009 | The authors reported on two patients with hemophilic pseudotumors who did not respond to intensive factor replacement therapy. Therefore, the pseudotumors had to be removed surgically in both cases. |
| Petratos et al (44) | 2009 | The authors reported a case of a large pseudotumor in the right talus of an 11-year-old boy with severe hemophilia A. The described intraosseous lesion was treated with surgical curettage and autologous bone grafting. Twenty months after surgery, computed tomography scan showed no signs of recurrence. Forty months after surgery, radiological studies confirmed satisfactory incorporation of the graft and no evidence of bone growth disturbance. At the same time, he was able to fully participate in his daily activities, presenting painless and almost full range of motion of his right ankle joint. |
| Yoshitake et al (45) | 2011 | A hemophilic pseudotumor was identified in the mandible of a 5-year-old male with severe hemophilia A. The patient initially experienced painless swelling of the mandible. CT revealed a marked enlargement of the lower right mandibular border, which was associated with a low-density area, and irregular absorption of the lingual cortex bone. A malignant tumour was suspected and a biopsy was performed after the administration of coagulation factor (Factor VII). A histopathologic diagnosis of hemophilic pseudotumor was made and the patient subsequently underwent surgical treatment. A cavity was created in the multilocular bone cyst and surgical curettage and irrigation were performed with the same haemorrhagic control as in the biopsy procedure. The multilocular cyst was contained within a hematoma and was surrounded by thin granular tissue. Three years after surgery, no abnormal signs were detected by radiography during follow-up examinations. |
| Liu et al (46) | 2011 | An HIV infected hemophilia patient with a huge inflammatory pseudotumor was severely ill. Right hip joint amputation was performed with perioperative infusion of coagulation factor VIII and highly active antiretroviral therapy (HAART). Pathology studies revealed inflammatory cell infiltration, formation of folliculus lymphaticus, muscular fibre breakage, fibrous tissue hyperplasia, and necrosis in both soft tissue and bone were seen in an inflammatory pseudotumor. The vital signs remained stable during the operation and patient’s overall health improved significantly ten months after operation. |
| Yang et al (47) | 2012 | The authors reported three pediatric patients with histology-proven hemophilic pseudotumors arising from the maxillary bone. All three patients underwent CT and/or MRI. Combined with six previously reported cases in the literature, the imaging features were comprehensively analyzed. |
| Panotopoulos et al (48) |
2012 | The authors reported six patients with hemophilic pseudotumors. The mean age at surgery was 45.9 (range, 40-61) years. The iliac bone was affected in three patients (one right, two left), the right tibia (distal diaphysis) in one, the right thigh in two and the right ulna (proximal part) in one patient. One patient had two pseudotumors. At the latest follow-up after 8.4 (range, 4-24) years, normal healing with no recurrence was observed. |
| Serban et al (49) |
2012 | The authors presented a 14-year follow-up of a patient with severe hemophilia A, treated sporadically with fresh plasma, cryoprecipitate and factor concentrates, who developed a giant iliopsoas pseudotumor. Since he was an infant with on-demand therapy with fresh frozen plasma, cryoprecipitate and low doses of factor concentrates, he presented many spontaneous bleeds, developing multiple disabling arthropathies. At the age of 14 years, an iliopsoas hematoma occurred, which relapsed several times, developing an iliopsoas pseudotumor. After 5 years, Klebsiella sepsis was diagnosed. A CT scan revealed a fistula between the pseudotumor and the gut. The sepsis improved on antibiotics, but over a period of 10 months, 5 episodes of hematemesis and melena, followed by one episode of macroscopic haematuria occurred; two months later he developed an inguino-crural mass which fistulised through the abdominal wall. After 108 hospitalization days and consumption of 104840 IU of factor VIII, he left the clinic in good condition. One year later, the temporary colostomy with anus stoma was closed. After almost 10 years, follow-up revealed a favourable outcome. The patient was well, active within his family and profession. |
| Lim et al (50) | 2014 | The authors described the clinical features and management of hemophilic pseudotumor by retrospectively reviewing the medical records of hemophilia patients with a diagnosis of pseudotumor seen at their Hemophilia Center from 1981 to 2011. They identified 12 pseudotumors in 11 patients over a 30-year period. Six patients had known inhibitors or a history of inhibitor. An etiological antecedent leading to the development of pseudotumor was reported in nine cases. The location of the pseudotumor was confined to soft tissue (n = 3) and bone (n = 8). Six of the 12 pseudotumors (50%) were not diagnosed at the time of initial presentation, with a delay ranging from 6 weeks to 6 years. In eight cases, surgical intervention (surgical drainage, n = 2; excision, n = 4; limb amputation, n = 2) was the initial treatment, with complete resolution in six cases. Conservative management with close monitoring occurred in three cases, with one case subsequently requiring surgical resection. |
| Low et al (51) | 2014 | The authors reported a case of a 43-year-old man with hemophilia A who presented with a gradually enlarging left thigh mass for 8 months. There were no constitutional symptoms. Plain radiograph showed an expansile lytic lesion with ‘soap-bubble’ appearance arising from the left femoral diaphysis. On MRI, it appeared as a non-enhancing, multilobulated lesion expanding the medullary and subperiosteal spaces. The mass exhibited a concentric ring sign with heterogeneous intermediate signal intensity in the core lesion, reflective of chronic hematoma with blood degradation products of different stages. A diagnosis of hemophilic pseudotumor was made. Hypercalcemia, however, raised a diagnostic dilemma as bone malignancy needed to be considered. An open excisional biopsy and subsequent amputation confirmed the diagnosis of osseous hemophilic pseudotumor. Nuclear medicine study later revealed a concurrent parathyroid adenoma. |
| Kamal et al (52) | 2014 | The authors reported on a 30-year-old hemophilic man with a pelvic pseudotumor compressing adjacent structures causing pain and swelling and destruction of surrounding soft tissues and bones. He underwent evacuation of the pseudotumor, acetabular reconstruction using the Harrington procedure, and total hip arthroplasty. |
| Purkait et al (53) |
2014 | This report described an 11-year-old boy with mild factor IX deficiency (17 % of normal factor IX activity), who developed a pseudotumor of the femur. |
| Caviglia et al (10) |
2015 | The aim of this study was to show the treatment of 10 pseudotumous in 7 patients with inhibitors. Eight were bone pseudotumors and two were soft tissue pseudotumors. Only one patient responded to conservative treatment. Surgery was performed on the others six patients, since their pseudotumors did not shrink to less than half their original size. |
| Dutt et al (54) | 2015 | In this case report, the authors described the natural history, clinical course and successful surgical management of a patient with hemophilia who presented with a massive pseudotumor. |
| Kwon et al (55) | 2016 | The authors reported a case of hemophilic pseudotumor in two parts of the maxilla. Contrast CT revealed an expansive and thinly corticated lesion with fluid attenuation at the left anterior maxilla which appeared to be a postoperative maxillary cyst, ameloblastoma or odontogenic cyst. In addition, the thickened left palatal process of the maxilla appeared to be fibrous dysplasia or an intraosseous vascular malformation. |
| Kumar et al (56) | 2018 | The authors reported a rare case of mandibular pseudotumor in a patient with moderate hemophilia and Glanzmann’s thrombasthenia, treated successfully with decompression of the hematoma. Following decompression, serial radiographs revealed spontaneous bone regeneration at the site of the lesion. At 2 year follow-up, the mandible had no residual lesion. |
True tumors that mimic hemophilic pseudotumors
Sometimes we can find true tumours that mimic hemophilic pseudotumors, such as chondrosarcoma, liposarcoma, and synovial sarcoma (55, 56). A series of six cases in which surgery was performed under the diagnosis of pseudotumor has been published, confirming that they were actually malignant primary neoplasms (57).
Other authors have published the case of a hemophilic patient who suffered a pathological fracture of the tibia. This fracture was presumably due to a pseudotumor but later confirmed to be due to non-Hodgkin’s lymphoma (58). The patient unfortunately died. The first known case of a giant gluteal neurofibroma in a 22-year-old hemophilic patient has also been published, which was resolved with satisfactory results by surgical removal (59).
The prevention of pseudotumors is fundamental. This is achieved through accurate diagnosis of muscle hematomas and proper long-term hematological treatment until their resolution (confirmed by imaging studies). Ultrasound-guided percutaneous evacuation could be beneficial in terms of achieving better and faster symptom relief. Biopsy will help us confirm the diagnosis and rule out true tumours that sometimes mimic hemophilic pseudotumors. Radical surgical removal of hemophilic pseudotumors is the best solution. Alternatives include curettage and filling with cancellous bone and radiotherapy (when surgery is contraindicated). Preoperative arterial embolization (ideally 2 weeks before surgery) will help control intra-operative bleeding during surgical removal of a giant pelvic pseudotumor.
The main limitation of the literature on hemophilic pseudotumors is that all publications have a low degree of evidence (case reports, case series, expert opinion, etc). However, it cannot be forgotten that this is a very infrequent and potentially serious injury.
References
- 1.Rodriguez-Merchan EC. The role of orthopaedic surgery in haemophilia: current rationale, indications and results. EFORT Open Rev. 2019;4(5):165–73. doi: 10.1302/2058-5241.4.180090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Merchan EC. Musculo-skeletal manifestations of haemophilia. Blood Rev. 2016;30(5):401–9. doi: 10.1016/j.blre.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 3.De la Corte-Rodriguez H, Rodriguez-Merchan EC. Treatment of muscle haematomas in haemophiliacs with special emphasis on percutaneous drainage. Blood Coagul Fibrinolysis. 2014;25(8):787–94. doi: 10.1097/MBC.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Merchan EC. The haemophilic pseudotumour. Haemophilia. 2002;8(1):12–6. doi: 10.1046/j.1365-2516.2002.00577.x. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Merchan EC. Acute compartment syndrome in haemophilia. Blood Coagul Fibrinolysis. 2013;24(7):677–82. doi: 10.1097/MBC.0b013e3283631e1a. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson J, Goddard N. Compartment syndrome in patients with haemophilia. J Orthop. 2015;12(4):237–41. doi: 10.1016/j.jor.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Large DF, Ludlam CA, Macnicol MF. Common peroneal nerve entrapment in a hemophiliac. Clin Orthop Relat Res. 1983;181:165–6. [PubMed] [Google Scholar]
- 8.Kaymak B, Ozçakar L, Cetin A, Erol K, Birsin Ozçakar Z. Concomitant compression of median and ulnar nerves in a hemophiliac patient: a case report. Joint Bone Spine. 2002;69(6):611–3. doi: 10.1016/s1297-319x(02)00460-8. [DOI] [PubMed] [Google Scholar]
- 9.Caviglia H, Candela M, Landro ME, Douglas Price AL, Neme D, Galatro GA. Haemophilia pseudotumours in patients with inhibitors. Haemophilia. 2015;21(5):681–5. doi: 10.1111/hae.12632. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Merchan EC, Jimenez-Yuste V. The role of selective angiographic embolization of the musculo-skeletal system in haemophilia. Haemophilia. 2009;15(4):864–8. doi: 10.1111/j.1365-2516.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 11.Espandar R, Heidari P, Rodriguez-Merchan EC. Management of haemophilic pseudotumours with special emphasis on radiotherapy and arterial embolization. Haemophilia. 2009;15(2):448–57. doi: 10.1111/j.1365-2516.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Merchan EC. Haemophilic cysts (pseudotumours) Haemophilia. 2002;8(3):393–401. doi: 10.1046/j.1365-2516.2002.00609.x. [DOI] [PubMed] [Google Scholar]
- 13.Hampton KK, Grant PJ, Johnston D, Prentice CR. Pelvic haemophilic pseudotumour occurring in a patient with mild haemophilia: a brief report. Blood Coagul Fibrinolysis. 1990;1(6):747–8. [PubMed] [Google Scholar]
- 14.Marisavljević D, Glisić M, Elezović I, Popović A, Rolović Z. Successfull extirpation of femoral pseudotumour in a patient with severe haemophilia A and an inhibitor to factor VIII. Srp Arh Celok Lek. 1991;119(11-12):338–42. [PubMed] [Google Scholar]
- 15.Heim M, Horoszowski H, Schulman S, Varon D, Barzilai A, Engelberg S, et al. Multifocal pseudotumour in a single limb. Haemophilia. 1997;3(1):50–3. doi: 10.1046/j.1365-2516.1997.00072.x. [DOI] [PubMed] [Google Scholar]
- 16.Maliekel K, Rana N, Green D. Recombinant factor VIIa in the management of a pseudotumour in acquired haemophilia. Haemophilia. 1997;3(1):54–8. doi: 10.1046/j.1365-2516.1997.00075.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishiguro N, Iwahori Y, Kato T, Ito T, Kojima T, Takamatsu J, et al. The surgical treatment of a haemophilic pseudotumour in an extremity: a report of three cases with pathological fractures. Haemophilia. 1998;4(2):126–31. doi: 10.1046/j.1365-2516.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- 18.Heeg M, Smit WM, van der Meer J, van Horn JR. Excision of a haemophilic pseudotumour of the ilium, complicated by fistulation. Haemophilia. 1998;4(2):132–5. doi: 10.1046/j.1365-2516.1998.00165.x. [DOI] [PubMed] [Google Scholar]
- 19.Sevilla J, Alvarez MT, Hernández D, Canales M, De Bustos JG, Magallón M, et al. Therapeutic embolization and surgical excision of haemophilic pseudotumour. Haemophilia. 1999;5(5):360–3. doi: 10.1046/j.1365-2516.1999.00330.x. [DOI] [PubMed] [Google Scholar]
- 20.Raj P, Wilde JT, Oliff J, Drake-Lee AB. Nasal haemophilic pseudotumour. J Laryngol Otol. 1999;113(10):924–7. doi: 10.1017/s002221510014561x. [DOI] [PubMed] [Google Scholar]
- 21.Heaton DC, Robertson RW, Rothwell AG. Iliopsoas haemophiliac pseudotumours with bowel fistulation. Haemophilia. 2000;6(1):41–3. doi: 10.1046/j.1365-2516.2000.00349.x. [DOI] [PubMed] [Google Scholar]
- 22.Sagarra M, Lucas M, De la Torre E, Almagro D, González R, García T, et al. Successful surgical treatment of haemophilic pseudotumour, filling the defect with hydroxyapatite. Haemophilia. 2000;6(1):55–6. doi: 10.1046/j.1365-2516.2000.00344.x. [DOI] [PubMed] [Google Scholar]
- 23.Bellinazzo P, Silvello L, Caimi TM, Mostarda G, Decataldo F, Baudo F. Long-term evaluation of a novel surgical approach to the pseudotumour of the ilium in haemophilia: exeresis and transposition of the omentum in the residual cavity. Haemophilia. 2000;6(6):702–4. doi: 10.1046/j.1365-2516.2000.00441.x. [DOI] [PubMed] [Google Scholar]
- 24.Kale HA, Rathod KR, Prasad SR, Madiwale CM, Sheth RJ. Mandibular haemophilic pseudotumour containing a fluid-fluid level. Br J Radiol. 2001;74(878):186–8. doi: 10.1259/bjr.74.878.740186. [DOI] [PubMed] [Google Scholar]
- 25.Wexler S, Edgar M, Thomas A, Learmonth I, Scott G. Pseudotumour in von Willebrand disease. Haemophilia. 2001;7(6):592–4. doi: 10.1046/j.1365-2516.2001.00552.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Mohapatra BB, Ghai S, Seith A, Kashyap R, Sharma R, et al. Haemophilic pseudotumour of the paranasal sinuses: management with radiotherapy and factor replacement therapy. Haemophilia. 2001;7(6):595–9. doi: 10.1046/j.1365-2516.2001.00566.x. [DOI] [PubMed] [Google Scholar]
- 27.O’Connell N, Chen J, Byrne M, O’Shea E, Smyth H, Smith OP. Massive pseudotumour resection with recombinant factor VIIa (NovoSeven) cover. Br J Haematol. 2002;116(3):645–8. doi: 10.1046/j.0007-1048.2001.03297.x. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson DS, Keast AT. An unusual cause of epistaxis: a haemophilic pseudotumour in a non-haemophiliac, arising in a paranasal sinus. J Laryngol Otol. 2002;116(4):294–5. doi: 10.1258/0022215021910573. [DOI] [PubMed] [Google Scholar]
- 29.Eby CS, Caracioni AA, Badar S, Joist JH. Massive retroperitoneal pseudotumour in a patient with type 3 von Willebrand disease. Haemophilia. 2002;8(2):136–41. doi: 10.1046/j.1365-2516.2002.00588.x. [DOI] [PubMed] [Google Scholar]
- 30.Keller A, Terrier F, Schneider PA, Bianchi S, Howarth N, De Moerloose P. Pelvic haemophilic pseudotumour: management of a patient with high level of inhibitors. Skeletal Radiol. 2002;31(9):550–3. doi: 10.1007/s00256-002-0518-8. [DOI] [PubMed] [Google Scholar]
- 31.Takedani H, Mikami S, Kawasaki N, Abe Y, Arai M, Naka H, et al. Excision of pseudotumour in a patient with haemophilia A and inhibitor managed with recombinant factor VIIa. Haemophilia. 2004;10(2):179–82. doi: 10.1111/j.1365-2516.2004.00784.x. [DOI] [PubMed] [Google Scholar]
- 32.Libby EN, White GC. Cranial pseudotumour in haemophilia. Haemophilia. 2004;10(2):186–8. doi: 10.1046/j.1365-2516.2003.00848.x. [DOI] [PubMed] [Google Scholar]
- 33.O’Dowd M, Geoghegan T, Munk PL, McAuley G, Torreggiani WC. Haemophilic pseudotumour presenting with large bowel obstruction. Australas Radiol. 2006;50(4):386–8. doi: 10.1111/j.1440-1673.2006.01607.x. [DOI] [PubMed] [Google Scholar]
- 34.Ahuja SP, Sidonio R Jr, Raj AB, Bertolone SJ, Silverman C, Antekeier DP, et al. Successful combination therapy of a proximal haemophilic pseudotumour with surgery, radiation and embolization in a child with mild haemophilia A. Haemophilia. 2007;13(2):209–12. doi: 10.1111/j.1365-2516.2006.01425.x. [DOI] [PubMed] [Google Scholar]
- 35.Rey EA, Puia S, Bianco RP, Pinto MT. Haemophilic pseudotumour of the mandible: report of three cases. Int J Oral Maxillofac Surg. 2007;36(6):552–5. doi: 10.1016/j.ijom.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Al Saadi AS, Al Wadan AH, El Hamarneh SA, Emad ME. Life-threatening biopsy of an iliopsoas pseudotumour in a patient with haemophilia: a case report. J Med Case Rep. 2008;2:135. doi: 10.1186/1752-1947-2-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Young AI. Conservative physiotherapeutic management of chronic haematomata and haemophilic pseudotumours: case study and comparison to historical management. Haemophilia. 2009;15(1):253–60. doi: 10.1111/j.1365-2516.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 38.Toepfer A, Diehl P, Gradinger R, Rudert M. Haemophilic pseudotumour of the distal femur - a case report and characterisation of this entity. Z Orthop Unfall. 2008;146(5):651–4. doi: 10.1055/s-2008-1038837. [DOI] [PubMed] [Google Scholar]
- 39.Shi H, Wang S, Wang P, Yu Q. Haemophilic pseudotumour of the mandible. Dentomaxillofac Radiol. 2009;38(3):182–4. doi: 10.1259/dmfr/28176102. [DOI] [PubMed] [Google Scholar]
- 40.Bernstaedt M, Kunze C, Brandt J, Hasenmueller S, Oldenburg J, Koerholz D, et al. Haemophiliac pseudotumour: two case reports of patients with moderate haemophilia A. Klin Padiatr. 2009;221(3):172–3. doi: 10.1055/s-0029-1220704. [DOI] [PubMed] [Google Scholar]
- 41.Petratos DV, Pergantou H, Matsinos GS, Anastasopoulos JN, Platokouki H. Intraosseous pseudotumour of the talus in a child with severe haemophilia. J Pediatr Orthop B. 2009;18(6):357–61. doi: 10.1097/BPB.0b013e32832f07d4. [DOI] [PubMed] [Google Scholar]
- 42.Yoshitake Y, Nakayama H, Takamune Y, Yasunaga M, Hiraki A, Shinohara M. Haemophilic pseudotumour of the mandible in a 5-year-old patient. Int J Oral Maxillofac Surg. 2011;40(1):120–3. doi: 10.1016/j.ijom.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Liu B, Liu L, Feng Y, Li L. A case report on the surgical treatment of the huge inflammatory pseudotumor in the AIDS patient with hemophilic. Case Rep Pathol. 2011;2011:798649. doi: 10.1155/2011/798649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang BT, Wang YZ, Wang XY, Wang ZC. Imaging features of paediatric haemophilic pseudotumour of the maxillary bone: report of three cases and review of the literature. Br J Radiol. 2012;85(1016):1107–11. doi: 10.1259/bjr/12938443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panotopoulos J, Ay C, Trieb K, Funovics PT, Stockhammer V, Lang S, et al. Surgical treatment of the haemophilic pseudotumour: a single centre experience. Int Orthop. 2012;36(10):2157–62. doi: 10.1007/s00264-012-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serban M, Mihailov D, Savescu D, Bordos D, Talpos Niculescu S, Jinca C, et al. Long-term outcome of an unusual haemophilic pseudotumour. Hamostaseologie. 2012;32(Suppl 1):S43–4. [PubMed] [Google Scholar]
- 47.Lim MY, Nielsen B, Ma A, Key NS. Clinical features and management of haemophilic pseudotumours: a single US centre experience over a 30-year period. Haemophilia. 2014;20(1):e58–62. doi: 10.1111/hae.12295. [DOI] [PubMed] [Google Scholar]
- 48.Low SF, Sridharan R, Ngiu CS, Haflah NH. Osseous haemophilic pseudotumour and concurrent primary hyperparathyroidism: a diagnostic conundrum. BMJ Case Rep. 2014;2014:bcr2013203282. doi: 10.1136/bcr-2013-203282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamal AF, Sukrisman L, Dilogo IH, Priyamurti H, Qomaruzzaman MN. Pelvic haemophilic pseudotumour: a case report. J Orthop Surg (Hong Kong) 2014;22(2):263–8. doi: 10.1177/230949901402200232. [DOI] [PubMed] [Google Scholar]
- 50.Purkait R, Mukherji A, Dolai TK, Bhadra R. Intraosseous pseudotumour in a child with mild hemophilia B: report of a rare case and brief review of literature. Indian J Hematol Blood Transfus. 2014;30(Suppl 1):366–8. doi: 10.1007/s12288-014-0414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutt K, Agarwal PN, Singh R, Tomar VS. Haemophilic pseudotumour: surgical management of a rare case. Indian J Surg. 2015;77(1):62–4. doi: 10.1007/s12262-013-1016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwon AY, Huh KH, Yi WJ, Symkhampha K, Heo MS, Lee SS, et al. Haemophilic pseudotumour in two parts of the maxilla: case report. Dentomaxillofac Radiol. 2016;45(6):20150440. doi: 10.1259/dmfr.20150440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar S, Charllu AP, Chacko R, Porinchu J. Spontaneous bone regeneration in a large haemophilic pseudotumour of mandible. BMJ Case Rep. 2018;2018:bcr–2018-226088. doi: 10.1136/bcr-2018-226088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez Merchan EC. The haemophilic pseudotumour. Int Orthop. 1995;19(4):255–60. doi: 10.1007/BF00185235. [DOI] [PubMed] [Google Scholar]
- 55.Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212. doi: 10.1155/SRCM/2006/27212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen DJ, Goddard NJ, Mann HA, Rodriguez-Merchan EC. Primary malignancies mistaken for pseudotumours in haemophilic patients. Haemophilia. 2007;13(4):383–6. doi: 10.1111/j.1365-2516.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Merchan EC, Gomez-Cardero P. Pathological fracture of a true tumour mimicking a haemophilic pseudotumor. Haemophilia. 2005;11(2):188–90. doi: 10.1111/j.1365-2516.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Delgado J, Rodriguez-Merchan EC, Martinez-Mendez JR. First giant gluteal neurofibroma reported in the literature in a person with haemophilia and its high risk of massive bleeding to death. Haemophilia. 2005;11(4):419–21. doi: 10.1111/j.1365-2516.2005.01105.x. [DOI] [PubMed] [Google Scholar]