Abstract
Medicinal plants are promising candidates for Alzheimer’s disease (AD) research but there is lack of systematic algorithms and procedures to guide their selection and evaluation. Herein, we developed an AD Neuroprotective Potential Algorithm (NPA) by evaluating twenty-three standardized and chemically characterized Ayurvedic medicinal plant extracts in a panel of bioassays targeting oxidative stress, carbonyl stress, protein glycation, amyloid beta (Aβ) fibrillation, acetylcholinesterase (AChE) inhibition, and neuroinflammation. The twenty-three herbal extracts were initially evaluated for: 1) total polyphenol content (Folin-Ciocalteu assay), 2) free radical scavenging capacity (DPPH assay), 3) ferric reducing antioxidant power (FRAP assay), 4) reactive carbonyl species scavenging capacity (methylglyoxal trapping assay), 5) anti-glycative effects (BSA-fructose, and BSA-methylglyoxal assays) and, 6) anti-Aβ fibrillation effects (thioflavin-T assay). Based on assigned index scores from the initial screening, twelve extracts with a cumulative NPA score ≥ 40 were selected for further evaluation for their: 1) inhibitory effects on AChE activity, 2) in vitro anti-inflammatory effects on murine BV-2 microglial cells (Griess assay measuring levels of lipopolysaccharide-induced nitric oxide species), and 3) in vivo neuroprotective effects on Caenorhabditis elegans post induction of Aβ1-42 induced neurotoxicity and paralysis. Among these, four extracts had a cumulative NPA score ≥ 60 including Phyllanthus emblica (amla; Indian gooseberry), Mucuna pruriens (velvet bean), Punica granatum (pomegranate) and Curcuma longa (turmeric; curcumin). These extracts also showed protective effects on H2O2 induced cytotoxicity in differentiated cholinergic human neuronal SH-SY5Y and murine BV-2 microglial cells and reduced tau protein levels in the SH-SY5Y neuronal cells. While published animal data support the neuroprotective effects of several of these Ayurvedic medicinal plant extracts, some remain unexplored for their anti-AD potential. Therefore, the NPA may be utilized, in part, as a strategy to help guide the selection of promising medicinal plant candidates for future AD-based research using animal models.
Keywords: Antioxidant, Alzheimer’s disease (AD), Glycation, Beta amyloid (Aβ), Neuroinflammation, Caenorhabditis elegans
Graphical abstract
1. Introduction
Alzheimer’s disease (AD) is a common neurodegenerative disorder characterized by the progression of cognitive decline leading to severe dementia (Buckner et al., 2005). The accumulation of senile plaques and neurofibrillary tangles in cerebral cortex and hippocampus are two major pathological hallmarks of AD (Ittner and Götz, 2011). However, due to the complexity of the disease, the precise factors which trigger the development of AD remains unknown (Alzheimer’s Association, 2015). Moreover, given the unclear etiology of AD, current therapeutic approaches focus mainly on symptom management but no treatment is available to alter or reverse the course of the disease (Alzheimer’s Association, 2015; Citron, 2010). Although the pathogenesis of AD is still under investigation, increasing evidence suggest that AD is a multifactorial disease which develops as a result of several risk contributors instead of a single cause alone (Norton et al., 2014; Reitz and Mayeux, 2014).
Oxidative stress and the production of reactive oxygen species (ROS) have been implicated in the pathogenesis of AD and are believed to be leading causative factors for neuronal cell dysfunction and cell death (Lin and Beal, 2006; Smith et al., 2000). It has been demonstrated that the products of protein oxidation and lipid peroxidation are elevated in AD patients (Christen, 2000). In addition, in the AD brain, the activities of antioxidant enzymes are altered, accompanied with a decline in the expression of these antioxidant enzymes (Christen, 2000; Smith et al., 2000). Given the established links between oxidative stress and AD, antioxidants, including those from natural products, are extensively studied for their neuroprotective abilities and constitute dietary intervention strategies for AD prevention and treatment (Alzheimer’s Association, 2015; Choi et al., 2012; Praticò, 2008).
Apart from oxidative stress, carbonyl stress and the formation of advanced glycation end-products (AGEs) resulting from protein glycation are also believed to be vital contributors to AD (Srikanth et al., 2011; Vicente Miranda and Outeiro, 2010). Glycation is one type of post-translational modification of proteins, resulting in the formation of AGEs both intracellularly and extracellularly. Glycation and AGEs formation are associated with AD due to several reasons. First, AGEs bind to the transmembrane receptor, RAGE (receptor for AGEs), upregulate RAGE expression, and activate RAGE-mediated neuronal dysfunction and neuron damages (Srikanth et al., 2011). Second, RAGE mediates the transportation of beta amyloid (Aβ) across the blood brain barrier (BBB) (Donahue et al., 2006). Therefore, the activation of RAGE by AGE can cause Aβ accumulation in the brain. Third, during the course of glycation and AGE formation, ROS and reactive carbonyl species (RCS) are generated as by-products which, in turn, promote AGE formation and cause neurotoxicity (Ahmed et al., 2005; Münch et al., 2012; Picklo et al., 2002). Consequently, all of the factors involved in this positive feedback loop including AGEs, RCS, and ROS are considered to be promising targets for AD prevention and treatment.
Another common target for AD therapy is the Aβ peptide which consists of 40 to 42 amino acids and is generated from the cleavage of the Aβ precursor protein. Aβ is the major component of senile plaques and neurofibrillary tangles, two pathological hallmarks of AD (Buckner et al., 2005; Palop and Mucke, 2010). In AD patients, elevated Aβ levels were observed in both cerebrospinal fluid and blood (Mawuenyega et al., 2010). In addition, certain forms of Aβ, including fibrillated Aβ and glycated Aβ (Aβ-AGEs), have been shown to be neurotoxic (Butterfield, 2002; Koo et al., 1999; Li et al., 2013). Fibrillated Aβ can induce neurotoxicity by enhancing neuronal oxidative stress and neuroinflammation (Butterfield, 2002; Koo et al., 1999). Aβ-AGEs can induce intracellular oxidative stress and inflammation by activating RAGE and upregulating RAGE expression in neuronal cells (Li et al., 2013). Therefore, considerable research efforts have been directed to finding inhibitors which may prevent or reverse the formation of Aβ fibrils and Aβ-AGEs. For example, aminoguanidine (AG), a synthetic glycation inhibitor, can reduce glycated Aβ formation, attenuate RAGE upregulation, and restore the cognitive deficit in AD animal models (Li et al., 2013). However, AG failed in human clinical trials due to severe side effects (Thornalley, 2003) leading to the search for non-toxic alternatives including medicinal plants and their derived natural products and botanical extracts (Solanki et al., 2016; Venigalla et al., 2016).
In addition to oxidative stress, glycation, and Aβ formation, neuroinflammation is another pivotal factor implicated in the development of neurodegenerative diseases with increased inflammation observed in AD (Eikelenboom et al., 2002). In addition, inflammatory stress leads to the activation of microglia cells, the immune cells in the central nervous system, which release nitric oxide species (NOS) including nitrates and nitrites. These NOS are neurotoxic and cause massive neuronal death further exacerbating neurodegenerative diseases (Eikelenboom et al., 2002; Eikelenboom et al., 2006).
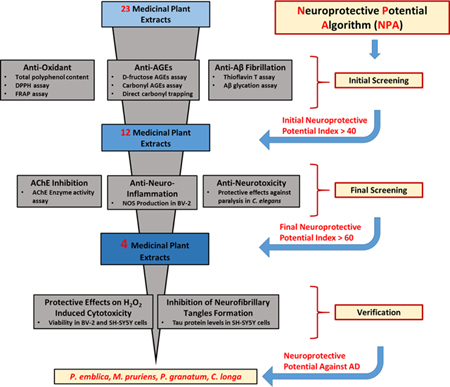
For centuries, traditional systems of medicines such as Ayurveda [from India, a country which has one of the lowest incidences of AD worldwide (Chandra et al., 2001; Vas et al., 2001)] and traditional Chinese medicine (TCM) (Steele et al., 2013), have used medicinal plants to treat several ailments including neurodegenerative diseases. While neurochemical/biological studies have been conducted on some these traditional medicinal plants, there is a lack of systematic procedures and algorithms to help guide the selection and evaluation of the most promising candidates for further AD-based research using animal models. This is urgently needed given the large variety of medicinal plant species (and combinations thereof) used worldwide in the traditional systems of medicines of various cultures. Furthermore, although medicinal plants are consumed as foods, herbs, spices, beverages, and botanical extracts, their underlying mechanisms of neuroprotective effects remain unclear. Therefore, given all of the aforementioned factors, herein, we utilized a panel of bioassays including total polyphenol contents, antioxidant capacities, anti-glycation effects, carbonyl scavenging abilities, anti-Aβ fibrillation, acetylcholinesterase (AChE) inhibition, and anti-neuroinflammatory activities to develop a Neuroprotective Potential Algorithm (NPA) to aid in the evaluation and selection of promising medicinal plant candidates for future AD based research using pre-clinical animal models (see Figure 1). We selected twenty-three commercially available and chemically characterized medicinal plant extracts (see Table 1), commonly consumed as foods (in India, and elsewhere), and used in Ayurveda, to develop the NPA.
Figure 1.
Neuroprotective Potential Algorithm (NPA) for selecting and evaluating medicinal plants as potential candidates for Alzheimer’s disease based research.
Table 1.
Medicinal plants and their total polyphenol contents and antioxidant activity (DPPH and FRAP assays)
| Species | Common name | Traditional use | Plant part | Total polyphenol content%a | DPPH activityb | FRAP activityc |
|---|---|---|---|---|---|---|
| Azadirachta indica | Neem | antidiabetic | Leaves | 12.9 ± 0.4 | 781.3 ± 49.0 | 203.8 ± 9.7 |
| Bacopa monnieri | Waterhyssop | enhance memory | Whole herb | 3.4 ± 1.9 | n.a. | 105.6 ± 5.8 |
| Boswellia serrata | Indian olibanum | joint health | Gum resin | 2.4 ± 0.1 | n.a. | 11.8 ± 1.8 |
| Elettaria cardamomum | Cardamom | digestive disorders | Fruit | 2.9 ± 0.7 | n.a. | n.a. |
| Centella asiatica | Gotu kola | antidiabetic | Whole herb | 27.0 ± 1.6 | 263.1 ± 25.4 | 224.2 ± 10.9 |
| Cinnamomum cassia | Cinnamon | antidiabetic | Bark | 22.4 ± 1.0 | 68.0 ± 10.3 | 324.2 ± 21.1 |
| Curcuma longa | Turmeric, Curcumin | anti-inflammatory | Rhizomes | 31.5 ± 1.8 | 111.4 ± 25.2 | 407.6 ± 12.8 |
| Foeniculum vulgare | Fennel | anti-inflammatory | Seeds | 24.0 ± 1.9 | 544.0 ± 14.6 | 179.3 ± 6.2 |
| Gymnema sylvestre | Gymnema | antidiabetic | Leaves | 21.0 ± 1.2 | 468.0 ± 9.4 | 253.4 ± 8.0 |
| Mangifera indica | Mango | clearing digestion | Leaves | 27.9 ± 2.8 | 60.6 ± 5.7 | 695.5 ± 21.7 |
| Moringa oleifera | Moringa | joint health | Fruit | 19.9 ± 2.5 | 758.9 ± 23.8 | 123.6 ± 7.1 |
| Mucuna pruriens | Velvet bean | neurodegenerative | Seeds | 37.0 ± 2.4 | 22.4 ± 1.8 | 2269.2 ± 61.3 |
| Ocimum tenuiflorum | Holy basil | spice | Leaves | 37.1 ± 1.7 | 72.5 ± 4.2 | 754.5 ± 16.8 |
| Phyllanthus emblica | Amla | anti-inflammatory | Juice | 38.9 ± 2.3 | 11.1 ± 1.7 | 2405.7 ± 5.9 |
| Pterocarpus marsupium | Indian Kino | antidiabetic | Bark | 20.6 ± 1.4 | 73.9 ± 11.0 | 774.9 ± 17.3 |
| Punica granatum | Pomegranate | antidiabetic | Fruit Peel | 41.2 ± 0.7 | 13.7 ± 0.7 | 2032.9 ±57.5 |
| Salacia reticulata | Salacia | antidiabetic | Roots | 22.9 ± 1.4 | 791.5 ± 6.1 | 400.9 ± 13.3 |
| Sesamum indicum | Sesame | spice | Seeds | 1.0 ± 0.1 | n.a. | 64.9 ±0.5 |
| Syzygium cumini | Jamun, Black plum | antidiabetic | Fruit pulp | 21.3 ± 3.2 | 129.9 ± 2.7 | 502.0 ± 4.5 |
| Tamarindus indica | Indian date | fever | Fruit | 3.6 ± 1.6 | 614.8 ± 12.7 | 75.2 ± 2.7 |
| Terminalia arjuna | Arjuna | heart disease | Bark | 24.6 ± 0.9 | 84.3 ± 19.2 | 652.2 ± 19.9 |
| Tinospora cordifolia | Guduchi | anticancer | Stem | 8.8 ± 0.5 | 571.1 ± 212.9 | 241.6 ± 2.7 |
| Withania somnifera | Ashwagandha, Indian ginseng | anti-ulcer | Roots | 25.7 ± 0.4 | 279.6 ± 20.1 | 166.1 ± 3.1 |
| BHT* | – | – | – | – | 493.6 ± 8.7 | – |
(w/w % as of gallic acid equivalents)
value = IC50 (μg/mL), n.a. = not active (IC50 >2000 μg/mL)
n.d. = not detected
value = ascorbic acid equivalents/mg dry plant material, n.a. = not active
Positive control: Butylated hydroxytoluene, BHT
2. Material and methods
2.1. Chemicals
The herbal extracts are botanically authenticated and chemically standardized GRAS (generally regarded as safe) extracts which are commercially available for human consumption and sourced from a single reputable natural products supplier, namely, Verdure Sciences (Noblesville, IN, USA), to ensure access to validated and consistent samples. The Latin binomial and common names, as well as the traditional uses, of the twenty-three medicinal plant species are provided in Table 1 and additional details (lot numbers, solvent to extract ratio/yields, etc.) are provided in the Supplementary Content. Each extract was dissolved in DMSO (20.0 mg/mL) and diluted to test concentrations (10–2000 μg/mL) with 0.1 M phosphate buffer (pH=7.2). The following chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA): butylated hydroxytoluene (BHT), bovine serum albumin (BSA), methylglyoxal (MGO), aminoguanidine hydrochloride (AG), gallic acid, resveratrol (RESV), galanthamine, 1,2-phenylenediamine (PD), 2,3-dimethylquinoxaline (DQ), 2,4,6-tripyridyl-s-triazine, iron (III) chloride hexahydrate, L-ascorbic acid, HPLC-grade methanol and trifluoroacetic acid (TFA), thioflavin T agent (ThT), Tris-HCl buffer (pH 8.0), acetylcholinesterase (AChE; from electric eel), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), and acetylthiocholine iodide (ATCI). Human beta amyloid 1-42 (Aβ1-42) peptide (Catalog #: AS-2027) was purchased from Anaspec (San Jose, CA, USA).
2.2. Antioxidant assays
2.2.1. Free radical scavenging activity (DPPH assay)
The extracts were evaluated for their free radical scavenging capacity by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay as previously reported by our laboratory (Ma et al., 2016). Briefly, 100 μL serial dilutions (10–2000 μg/mL) of each test sample were mixed with 100 μL of a 0.2 mM DPPH solution in a 96-well plate. After incubation at room temperature for 30 min, sample absorbance was read (at 517 nm) using a microplate reader (SpectraMax M2, Molecular Devices Corp., Sunnyvale, CA, USA).
2.2.2. Ferric reducing antioxidant power (FRAP assay)
The ferric reducing antioxidant power (FRAP) of the extracts were measured following published methods (Maksimović et al., 2005; Tsai et al., 2002). The FRAP reagent contained 100 mL acetate buffer (3 mM, pH 3.6), 10 mL 2,4,6-tripyridyl-s-triazine (10 mM) in 40 mM hydrochloric acid, and 10 mL FeCl3·6H2O (20 mM). Briefly, 1.5 mL of freshly prepared FRAP reagent was mixed with 50 μL of each extract (final concentration of 100 μg/mL) and the absorbance was measured at 593 nm after 5 minutes using an UltroSpec 2100 instrument (Biochrom Ltd, Cambridge, UK). L-ascorbic acid (0.1 – 3 mM) was used as the positive control and to generate a calibration curve. The FRAP capacity of each extract was expressed as ascorbic acid equivalents (AAE)/mg where AAE is defined as follows: the reducing power of 1 mg dry plant material is equivalent to the reducing power of 1 nM of L-ascorbic acid (Maksimović et al., 2005). All data were the average of three individual experiments.
2.3. Total polyphenol content
The extracts were quantified for total phenolic content using the Folin–Ciocalteu method and expressed as gallic acid equivalents (GAEs) as previously reported by our laboratory (Jean-Gilles et al., 2012). Briefly, each sample (5 mg) was dissolved in 500 μL of 50% aqueous methanol, and 100 μL of each sample was incubated with 50 μL of the Folin–Ciocalteu reagent for 5 min at room temperature. Then 150 μL of a 20% sodium carbonate (Na2CO3) solution and 250 μL of distilled water were added to each sample and the resulting solution was vortexed. The reaction mixtures were further incubated for 30 min at 40 °C and then immediately cooled to room temperature. The standard (i.e. gallic acid) was prepared in parallel with the samples. Each sample was centrifuged and 200 μL of supernatant was added to a 96-well plate and absorbance read at 756 nm on a Spectramax plate reader (SpectraMax M2, Molecular Devices Corp., Sunnyvale, CA, USA).
2.4. BSA-fructose assay
The extracts were evaluated for their anti-glycation effects using the BSA-fructose assay based on previously reported methods by our laboratory (Liu et al., 2014; Ma et al., 2015). Briefly, 100 μg/mL of each extract was added to the glycation reaction mixture containing 10 mg/mL BSA and 100 mM D-fructose and incubated at 37 °C for 21 days. The intrinsic fluorescence of each sample was measured at excitation and emission wavelengths of 360 nm and 435 nm, respectively. The synthetic anti-gycating agent, aminoguanidine (AG), at an equivalent concentration of 100 μg/mL, served as the positive control.
2.5. BSA-MGO assay
The extracts were evaluated for their inhibitory effects on carbonyl species induced AGEs formation using a reaction model containing BSA (10 mg/mL), MGO (5 mM), and sample (100 μg/mL) as previously reported by our laboratory (Sun et al., 2016). Each sample was measured for intrinsic fluorescence after 7 days incubation at 37 °C with excitation and emission wavelengths of 360 nm and 435 nm, respectively. The aforementioned wavelengths were found optimal for the detection of MGO derived AGEs. The positive control, AG, was evaluated at an equivalent concentration of 100 μg/mL.
2.6. MGO trapping assay
The extracts were measured for their trapping capacity of RCS as previously reported by our laboratory (Liu et al., 2014). Briefly, each reaction solution contained MGO (5 mM), and test sample (100 μg/mL), or the positive control, AG (1000 μg/mL), and the mixtures were incubated at 37 °C for 4 hours. Afterwards, the derivatization reagent, PD (20 mM), and internal standard, DQ (5 mM), in 0.1 M phosphate buffer, pH 7.2 were added to the reaction mixture, and the remaining MGO was quantified by HPLC-DAD. The percentage decrease of MGO was calculated using the following equation: MGO decrease % = [1 - (MGO amounts in solution with tested sample/MGO amounts in control solution)] x 100%.
2.7. Thioflavin T assay
The thioflavin T (ThT) assay was used to measure the inhibitory effects of the extracts on Aβ1-42 fibrillation as previously reported by our laboratory (Yuan et al., 2015). In each sample, the final concentrations of Aβ1-42 and test sample were adjusted to 50 μM and 100 μg/mL, respectively. Resveratrol (RESV; at 100 μg/mL) served as the positive control. To induce Aβ1-42 fibrillation, two individual protocols were followed. In the thermo induced fibrillation assay, Aβ1-42 solutions at a concentration of 50 μM were incubated at 37 °C for 72 hours with or without the herbal extracts. In the MGO-induced fibrillation assay, 1 mM MGO was added to the reaction mixture and all samples were incubated at 37 °C for 72 hours. Before and after incubation, 100 μL of each sample was added to an equal volume of ThT solution (50 μM) and fluorescence was measured on a plate reader (SpectraMax M2, Molecular Devices Corp., Sunnyvale, CA, USA) at excitation and emission wavelengths of 450 nm and 490 nm, respectively.
2.8. Aβ1-42-AGEs assay
In this assay, each extract was evaluated for its inhibitory effect against Aβ1-42-AGE formation induced by methylglyoxal (MGO), a reactive carbonyl species. Briefly, 50 μM Aβ1-42 solution was mixed with 1 mM MGO to produce Aβ1-42-AGEs following published methods (Li et al., 2013). Treatments included either 100 μg/mL of each extract or 100 μg/mL of the positive control, AG. After 72-hour incubation at 37 °C, the Aβ1-42-AGEs level was measured by intrinsic fluorescence using a plate reader (SpectraMax M2, Molecular Devices Corp., Sunnyvale, CA, USA) at excitation and emission wavelengths of 360 and 435 nm, respectively.
2.9. Acetylcholinesterase (AChE) inhibition assay
The AChE inhibitory activities of the herbal extracts were evaluated using a published spectrophotometric method with slight modifications (Ellman et al., 1961). Briefly, a reaction mixture consisting of 100 μL of 100 mM Tris-HCl buffer (pH 8.0), 20 μL AChE enzyme solution (0.5 U/mL) and 20 μL of test samples (concentration ranging from 25 – 300 μg/mL in 100 mM Tris-HCl buffer containing 50% methanol) was co-incubated in the dark at 37 °C for 20 minutes in a 96-well plate. Next, 40 μL of 0.75 mM DTNB and 20 μL of 1.5 mM ATCI were added to the reaction mixture and incubated at room temperature for 5 min in the dark. Then the absorbance was measured at a wavelength of 405 nm using a plate reader (SpectraMax M2, Molecular Devices Corp., Sunnyvale, CA, USA). A 50% methanol in 100 mM Tris-HCl buffer served as the blank control and galanthamine, a known AChE inhibitor, served as the positive control. Each sample (at each concentration) was also tested without enzyme as background for the inherent color present in each sample. Inhibition of AChE was calculated as: % Inhibition = [1-(Asample - Abackground )/Ablank] × 100.
2.10. Cell culture conditions
Murine microglial BV-2 cells were a kind gift from by Dr. Grace Y. Sun (University of Missouri at Columbia, MO, USA) and human neuronal SH-SY5Y cells were obtained from American Type Culture Collection (ATCC, VA, USA). The cells were maintained using high glucose (4.5 g/L) DMEM/F12 supplemented with 10% heat inactivated FBS, 1% P/S (100 U/ml penicillin, 100 μg/mL streptomycin (Life Technologies, Gaithersburg, MD, USA) and incubated in 5% CO2 at 37 °C. All test samples were dissolved in DMSO to yield a 10 mg/mL stock solution and further diluted with media to yield final solutions with DMSO < 0.1%.
2.11. Cell viability
Cellular viability was assessed using the Cell Titer Glo 2.0 (CTG 2.0) one step assay (Promega). Briefly, the murine BV-2 microglial cells were seeded at 100,000 cells/mL in a standard white walled clear bottom 96-well plate. After a 24-hour incubation period, CTG 2.0 was added in and mixed for 2 minutes on an orbital shaker prior to luminescence measurement on a plate reader (SpectraMax M2, Molecular Devices Corp., Sunnyvale, CA, USA).
2.12. Quantification of nitric oxide species (NOS) by the Griess assay
The murine BV-2 microglial cells, in 24-well plates (n=4) at 85% confluency (105 cells/well, were serum-starved for 4 hours prior to the treatments. The cells were then incubated with test samples (10 μg/mL) or the positive control, resveratrol (RESV; 5 μg/mL) for 1 hour after which inflammation was induced by lipopolysaccharide (LPS). Cells were incubated for 23 hours and the culture media were collected and centrifuged. The supernatants were measured for total nitric oxide species (NOS) by the Griess assay kit (Promega, Fitchburg, WI, USA) as previously reported by our laboratory (Nahar et al, 2014).
2.13. In vivo Caenorhabditis elegans assay
The in vivo neuroprotective effects of the extracts were evaluated using a Caenorhabditis elegans assay as previously reported by our group with minor modifications (Yuan et al., 2015). Transgenic Caenorhabditis elegans (CL4176) were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA). This strain expresses a heat-induced human Aβ1-42 gene in the muscle tissues and prolonged expression of the gene leads to paralysis and death. The stock culture was maintained at 18 °C on Nematode Growth Medium (NGM) plates (1.7% Agar, 0.3% NaCl, 0.25% peptone, 1 mM CaCl2, 1 mM MgSO4, 5 mg/L cholesterol, and 2.5 mM KPO4). NGM plates were seeded with 50 μL OP50 Escherichia coli and allowed to incubate overnight at 37 °C. Before seeding of the plates, the E. coli cultures were treated with the herbal extracts or vehicle control (0.05% DMSO). Once the age of the worms were synchronized to reach the L3 stage, they were placed in a 25 °C incubator for 20 hours to induce the expression of the human Aβ1-42 gene. After 20 hours the worms were counted every 2 hours for a total of 30 hours of incubation time. Dead worms were characterized by lack of pharyngeal pumping or movement and >100 worms were used for each treatment. Kaplan-Meier curves and curve comparisons statistics were generated using GraphPad Prism software.
2.14. Inhibition of cytotoxicity of murine BV-2 microglia and differentiated human SH-SY5Y neuronal cells induced by hydrogen peroxide (H2O2)
Cellular viability was assessed using the Cell Titer Glo 2.0 (CTG 2.0) one step assay as previously reported by our laboratory (Ma et al., 2016). Briefly, murine BV-2 microglia and differentiated human SH-SY5Y neuronal cells were seeded at 100,000 cells/mL to yield 80–85% confluency in a standard white walled clear bottom 96-well plate. The BV-2 cells were exposed to the herbal extracts and resveratrol (RESV; used as the positive control) for 1 hour prior to exposure to 100 μM of H2O2 for 6 hours. The SH-SY5Y cells were incubated with the extracts and RESV for 24 hours at 37°C prior to exposure to 100 μM of H2O2 for 6 hours. Following incubation, CTG 2.0 was added in a 1:1 ratio directly to existing media and mixed for 2 minutes on an orbital shaker prior to luminescence measurement (SpectraMax M2, Molecular Devices Corp., Sunnyvale, CA, USA).
2.15. Quantification of total tau protein levels in differentiated human SH-SY5Y neuronal cells
The protein content collected from the cell culture media of differentiated SH-SY5Y neuronal cells were analyzed for total tau protein levels by using an enzyme linked immunosorbent assay (ELISA) (Thermo Fisher, Grand Island, NY, USA). The ELISA was performed as per the manufacturer’s protocol. The SH-SY5Y cells were initially plated on 100 mm dishes at a concentration of 100,000 cells/mL. Cells were then differentiated for 7 days using retinoic acid (10 μM) prior to treatment with test samples and resveratrol (RESV; used as the positive control). Once differentiated, the SH-SY5Y cells were exposed to vehicle control (DMSO) or the herbal extracts for 24 hours. After pre-treatment, the cells were then exposed to H2O2 (100 μM) for 6 hours, after which the cell culture media was collected, centrifuged at 10,000 rpm for 5 minutes and assayed for total tau protein levels.
2.16. Statistical Analysis
Unless otherwise indicated, all assays were performed in triplicate and all data were expressed as the mean ± standard deviation (n=3). Significance was analyzed by one-way factorial ANOVA with Tukey–Kramer post hoc comparisons. A value of p<0.05 were considered as significant. In the BSA-fructose, BSA-MGO, and ThT assays, the inhibition rate (% inhibition) was defined using the following equation: % inhibition = [1-(fluorescence intensity of solution with treatment/fluorescence intensity of control solution)] x 100%.
Using a similar approach as previously reported (Seeram et al., 2008), an index score from each individual assay was determined by assigning an index value of 100 to the best score for each test, and then scores for the other samples were calculated using the following formula: [(sample score/best score) × 100]. The average of the chemical/biochemical assays for each extract was then taken for the initial neuroprotective potential index (Table 2). Similarly, the final neuroprotective potential index was determined by the average of the test scores from the in vitro (in BV-2 cells), AChE inhibitory, and in vivo C. elegans assays using the aforementioned formula (Table 4). Figure 1 shows the algorithm used to derive the overall index scores for the medicinal plant extracts.
Table 2.
Initial screening for neuroprotective potential index based on total polyphenol content, antioxidant, anti-glycation, and anti-Aβ fibrillation activities
| Ranking | Plant species | Total phenolic content | DPPH | FRAP | MGO trapping | Anti-AGEsa | Anti-AGEsb | Anti Aβ-AGEs | Anti-Aβ Agg.c | Anti-Aβ Agg.d | Neuro-protective potential index |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P. granatum | 100 | 99 | 85 | 100 | 100 | 66 | 93 | 79 | 97 | 91 |
| 2 | P. emblica | 95 | 100 | 100 | 90 | 94 | 100 | 99 | 53 | 76 | 90 |
| 3 | M. pruriens | 90 | 95 | 94 | 85 | 61 | 39 | 63 | 43 | 63 | 70 |
| 4 | C. longa | 77 | 52 | 17 | 99 | 41 | 63 | 71 | 37 | 75 | 59 |
| 5 | C. asiatica | 66 | 67 | 9 | 23 | 57 | 52 | 74 | 99 | 63 | 57 |
| 6 | P. marsupium | 50 | 59 | 32 | 3 | 60 | 50 | 76 | 62 | 83 | 53 |
| 7 | C. cassia | 55 | 87 | 13 | 12 | 43 | 42 | 56 | 45 | 57 | 46 |
| 8 | O. tenuiflorum | 90 | 63 | 31 | 45 | 72 | 50 | 50 | 0 | 1 | 45 |
| 9 | T. arjuna | 60 | 56 | 27 | 22 | 47 | 47 | 100 | 25 | 8 | 44 |
| 10 | B. monnieri | 8 | 3 | 4 | 45 | 35 | 37 | 49 | 100 | 100 | 42 |
| 11 | M. oleifera | 49 | 32 | 5 | 64 | 83 | 40 | 7 | 49 | 43 | 41 |
| 12 | S. reticulata | 56 | 22 | 17 | 11 | 42 | 30 | 90 | 41 | 64 | 41 |
| 13 | S. cumini | 52 | 53 | 21 | 0 | 21 | 18 | 91 | 30 | 26 | 35 |
| 14 | F. vulgare | 59 | 35 | 7 | 40 | 63 | 52 | 40 | 0 | 0 | 33 |
| 15 | T. indica | 9 | 41 | 3 | 43 | 48 | 42 | 92 | 11 | 0 | 32 |
| 16 | W. somnifera | 63 | 36 | 7 | 10 | 38 | 41 | 81 | 7 | 0 | 31 |
| 17 | T. cordifolia | 21 | 38 | 10 | 16 | 56 | 48 | 89 | 0 | 0 | 31 |
| 18 | M. indica | 68 | 68 | 29 | 11 | 42 | 44 | 0 | 19 | 0 | 31 |
| 19 | G. sylvestre | 51 | 43 | 11 | 13 | 26 | 23 | 38 | 28 | 8 | 27 |
| 20 | A. indica | 31 | 21 | 8 | 0 | 26 | 23 | 79 | 13 | 32 | 26 |
| 21 | S. indicum | 2 | 1 | 3 | 25 | 47 | 40 | 46 | 30 | 17 | 23 |
| 22 | B. serrata | 6 | 7 | 0 | 14 | 19 | 18 | 34 | 60 | 46 | 23 |
| 23 | E.cardamomum | 7 | 2 | 0 | 12 | 48 | 34 | 40 | 0 | 0 | 16 |
BSA AGEs formation induced by D-fructose
BSA AGEs formation induced by methylglyoxal (MGO)
thermo induced Aβ fibrillation (37 °C)
Methylglyoxal (MGO) induced Aβ fibrillation
Table 4.
Final screening for medicinal plant extracts for neuroprotective potential index based on the AChE inhibitory activity assay, in vitro anti-neuro-inflammatory effects in murine BV-2 microglial cells, and in vivo neuroprotective effects against Aβ1-42 induced neurotoxicity and paralysis in C. elegans
| Ranking | Plant species | AChE inhibitory activity assay | Neuroinflammation assay | in vivo C. elegans assay | Neuroprotective potential index |
|---|---|---|---|---|---|
| 1 | P. emblica | 90 | 80 | 100 | 90 |
| 2 | M. pruriens | 100 | 49 | 96 | 82 |
| 3 | P. granatum | 73 | 71 | 96 | 80 |
| 4 | C. longa | 23 | 82 | 82 | 62 |
| 5 | P. marsupium | 0 | 100 | 60 | 53 |
| 6 | C. asiatica | 0 | 88 | 70 | 53 |
| 7 | C. cassia | 0 | 94 | 42 | 45 |
| 8 | B. monnieri | 0 | 64 | 38 | 34 |
| 9 | O. tenuiflorum | 0 | 27 | 35 | 21 |
| 10 | M. oleifera | 0 | 19 | 42 | 20 |
| 11 | S. reticulata | 8 | 0 | 14 | 7 |
| 12 | T. arjuna | 12 | 0 | 10 | 7 |
3. Results and discussion
3.1. Total polyphenol content and antioxidant capacities
The herbal extracts were first evaluated for their total polyphenol contents and antioxidant activities (DPPH and FRAP assays) since several natural plant antioxidants, for example, resveratrol (RESV; from grapes) and curcumin (from the Indian turmeric Curcuma longa spice) have been reported to show promising neuroprotective effects against AD (Li et al., 2012; Lim et al., 2001). As shown in Table 1, the twenty-three medicinal plant extracts were evaluated for their phenolic content by the Folin-Ciocalteu method based on gallic acid equivalents (GAEs). The total polyphenol contents ranged from 1.0 to 41.2% (w/w, based GAEs) and this wide range was attributed to the diverse types (hydrophilic vs. lipophilic) of chemical constituents found in these various extracts. As expected, among the extracts, two well-known polyphenol-rich plants, Punica granatum (41.2%) and Phyllanthus emblica (38.9%) showed the highest polyphenol contents followed by Ocimum tenuiflorum (37.1%), Mucuna pruriens (37.0%) and C. longa (31.5%). Similarly, as expected, the extracts with highest polyphenol contents also showed the strongest antioxidant activities in both the DPPH and FRAP assays (see Table 1). For example, the most potent antioxidant extracts in the DPPH assay were P. emblica, P. granatum and M. pruriens, which had IC50 values of 11.1, 13.7 and 22.4 μg/mL, respectively, followed by Cinnamomum cassia (IC50 68.9 μg/mL), O. tenuiflorum (IC50 72.5 μg/mL), Pterocarpus marsupium (IC50 73.9 μg/mL) and Terminalia arjuna (IC50 84.3 μg/mL). All of these extracts showed superior antioxidant activity compared to the positive control, BHT (butylated hydroxytoluene), a synthetic commercial antioxidant (IC50 493.6 μg/mL). The antioxidant data obtained from the FRAP assay were in agreement with the antioxidant data obtained from the DPPH assay. For example, at a concentration of 100 μg/mL, P. emblica showed the highest FRAP capacity (2405.7 AAE/mg), followed by M. pruriens (2269.2 AAE/mg) and P. granatum (2032.9 AAE/mg). Other herbal extracts which showed moderate FRAP capacity were P. marsupium (774.9 AAE/mg), O. tenuiflorum (754.5 AAE/mg), Mangifera indica (695.5 AAE/mg), T. arjuna (652.2 AAE/mg), and Syzygium cumini (502.0 AAE/mg).
3.2. Anti-glycation and reactive carbonyl species (RCS) scavenging capacities
Next, the extracts were evaluated for their anti-glycative abilities and reactive carbonyl species (RCS) scavenging activities using three individual assays. First, the BSA-fructose assay was used to evaluate the inhibitory effects of the extracts on total AGEs formation, Second, the BSA-methylglyoxal (BSA-MGO) assay was used to evaluate the inhibitory effects of the extracts on RCS induced AGE formation, typically seen at the middle stage of glycation (Wu and Yen, 2005). Third, the extracts were evaluated for their direct scavenging capacity on methylglyoxal (MGO) using the MGO-trapping assay.
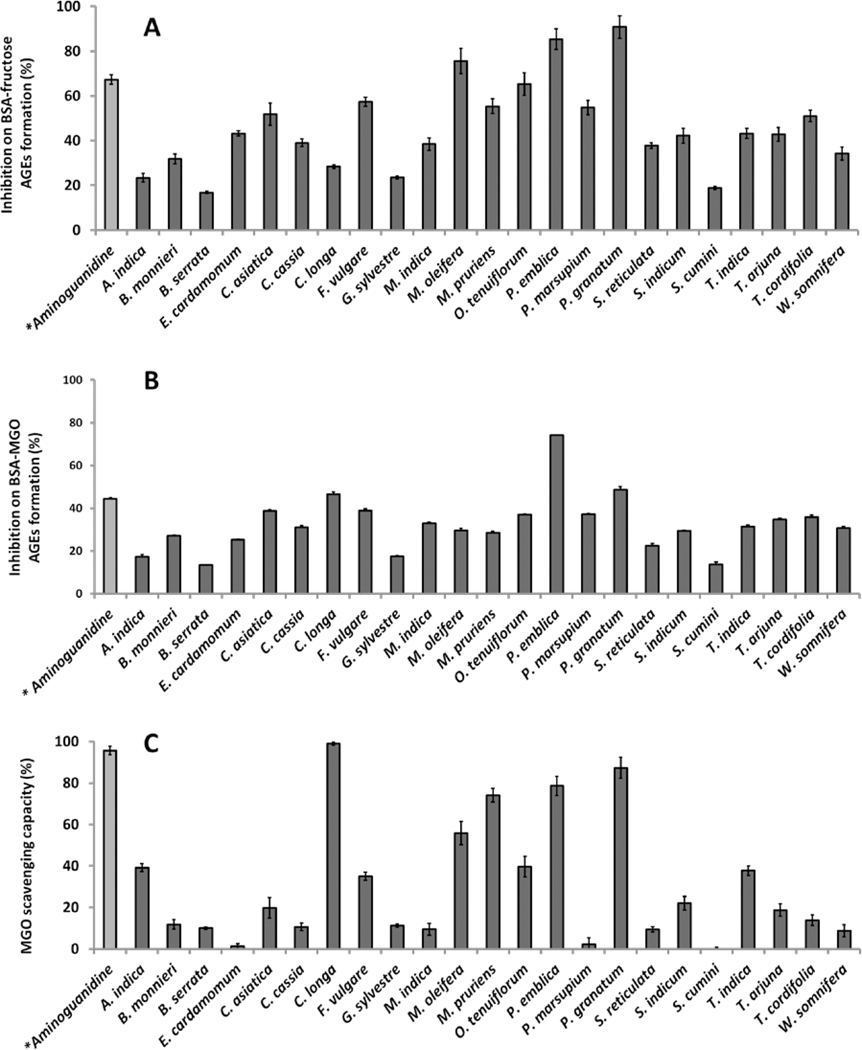
As shown in Figure 2A, all twenty-three extracts (100 μg/mL) showed anti-AGEs effects which ranged from 15.1 to 90.8% in the BSA-fructose assay. Among all of the extracts, P. granatum showed the highest inhibition of 90.8%, which was significantly higher than the positive control, AG (67.2%), a synthetic anti-glycative agent. Extracts of P. emblica (85.3%) and Moringa oleifera (75.6%) also showed anti-AGE effects superior to AG. Additionally, several other extracts showed more than 50% inhibitory effects including O. tenuiflorum (65.3%), Foeniculum vulgare (57.4%), M. pruriens (55.3%), P. marsupium (54.8%), Centella asiatica (51.8%) and Tinospora cordifolia (51.0%).
Figure 2.
Inhibitory effects of medicinal plant extracts (100 μg/mL) on D-fructose induced BSA AGEs formation (A), MGO induced BSA AGEs formation (B) and MGO scavenging capacity (C). Aminoguanidine (at 100 μg/mL) served as the positive control.
We then evaluated these herbal extracts for their inhibitory effects on MGO induced AGEs formation. As shown in Figure 2B, P. emblica and P. granatum extracts were the most potent inhibitors of AGE formation (i.e. 74.1% and 48.7% respectively), superior to AG at an equivalent concentration of 100 μg/mL (44.4%). These extracts were followed by C. longa (46.4%), P. vulgare (38.8%), C. asiatica (38.8%), P. marsupium (37.2%), O. tenuiflorum (36.9%), T. cordifolia (35.7%), and T. arjuna (34.7%) which were comparable to the positive control, AG (44.4%).
In addition to the anti-glycative effects of the extracts, their MGO scavenging capacity may also contribute to their potential neuroprotective effects. Therefore, the MGO trapping ability of the extracts were evaluated as shown in Figure 2C. At a concentration of 100 μg/mL, extracts of C. longa, P. granatum, and P. emblica scavenged MGO by 99.0, 87.3, and 78.6%, respectively. This trend was similar to the BSA-MGO assay wherein these extracts also showed the highest inhibition levels on MGO induced glycation. The extracts of M. oleifera, O. tenuiflorum, Bacopa monnieri, Tamarindus indica and F. vulgare also showed strong scavenging activities yielding MGO quenching levels ranging from 55.9 to 35.0%. Notably, the positive control, AG, a known MGO scavenging agent, showed 95.7% MGO trapping ability but at a much higher concentration of 1000 μg/mL.
In summary, the anti-glycation activities of the most potent AGE inhibitors were P. granatum, P. emblica, and C. longa which were attributed to their strong antioxidant and MGO scavenging activities. The anti-AGEs effects of P. granatum is in agreement with our previously reported study (Liu et al., 2014).
3.3. Inhibitory effects on Aβ1-42 fibrillation and Aβ1-42–AGEs formation
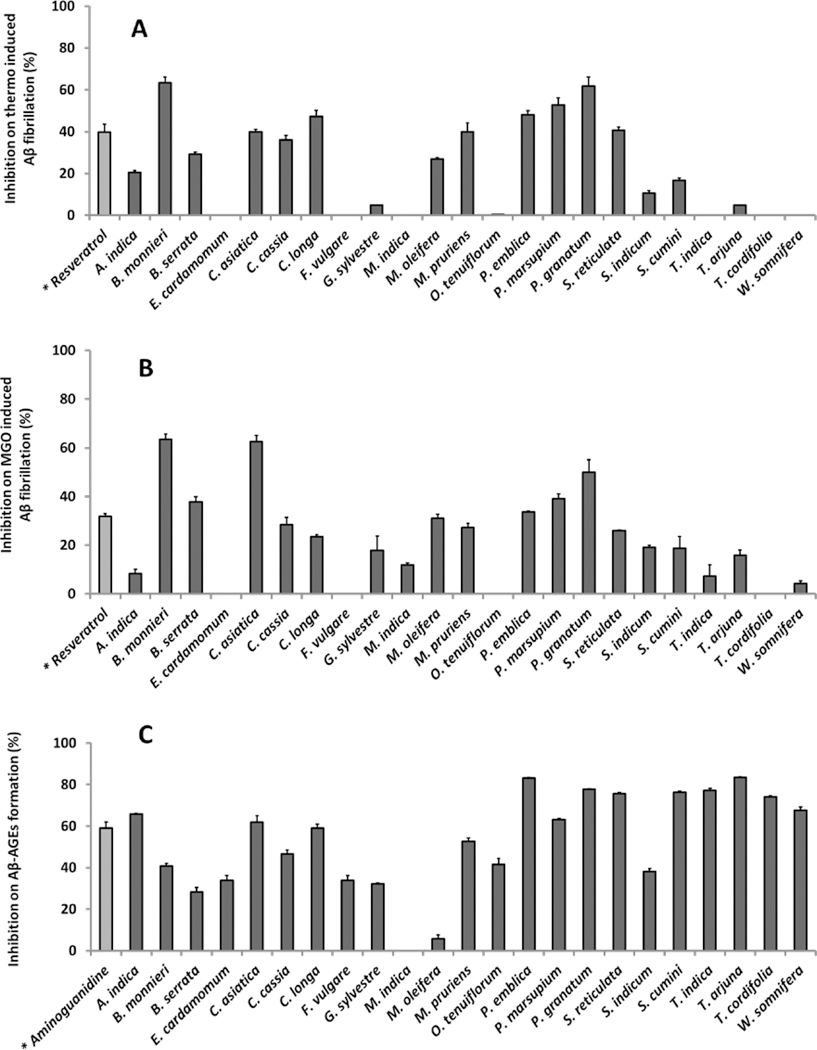
Numerous studies have shown that certain forms of Aβ, such as fibrillated Aβ and glycated Aβ (Aβ-AGEs) are more toxic than its native counterpart (Butterfield, 2002; Koo et al., 1999; Li et al., 2013). Therefore, we evaluated the twenty-three medicinal plant extracts for their inhibitory effects on the formation of fibrillated Aβ and glycated Aβ, two common targets for AD treatment.
The ThT binding assay was used to evaluate the fibrillation level of human Aβ1-42. In the thermo-induced fibrillation assay, Aβ1-42 fibril formation was confirmed by a significant increase in ThT fluorescence after 72 hours incubation at 37 °C. The fluorescence levels of Aβ treated with the extracts were then compared with the control and their inhibition levels on Aβ fibrillation are shown in Figure 3A. Upon treatment with the extracts, the lowest fibrillation level was seen in Aβ solution treated with B. monnieri (63.3% inhibition), followed by P. granatum (61.7% inhibition), P. marsupium (53.7% inhibition), P. emblica (47.9% inhibition) then C. longa (47.2% inhibition). The inhibition levels of these extracts were all significantly higher than positive control resveratrol (RESV, 31.8% inhibition), a natural phenolic compound known for its anti-amyloidosis and neuroprotective effects (Feng et al., 2009; Li et al., 2012).
Figure 3.
Inhibitory effects of medicinal plant extracts (100 μg/mL) on thermo-induced Aβ fibrillation (A), MGO induced Aβ fibrillation (B) and MGO induced Aβ-AGEs formation (C). Resveratrol at 100 μg/mL served as the positive control for Aβ fibrillation studies (A and B) while aminoguanidine at 100 μg/mL served as the positive control for Aβ-AGEs study (C).
Next, the herbal extracts were evaluated for their inhibitory abilities on MGO-induced Aβ1-42 fibrillation using the ThT binding assay. Studies have shown that reactive carbonyl species (RCS) such as MGO can induce Aβ fibrillation and aggregation (Chen et al., 2007; Chen et al., 2006). Consequently, carbonyl stress conditions with elevated RCS levels play an important role in the progression of neurodegenerative diseases including AD. Therefore, the inhibitory effects of the extracts against MGO-induced Aβ fibril assembly may also result in neuroprotective activities. As shown in Figure 3B, the highest inhibitory effect was observed in samples treated with B. monnieri (63.5%) and C. asiatica (62.6%), with effects significantly higher than the positive control, RESV (32.1%). In addition, extracts of P. granatum (49.9%), P. marsupium (39.1%), Boswellia serrata (37.9%) and P. emblica (33.7%) also showed inhibitory effects against Aβ1-42 fibrillation superior to the positive control, RESV (32.1%).
Besides fibrillated Aβ, glycated Aβ (Aβ-AGEs) produced from the modification of Aβ by sugars and sugar metabolites also exacerbates the neurotoxicity of native Aβ (Li et al., 2013). Therefore, we next evaluated the inhibitory effects of the herbal extracts on the formation of glycated Aβ (Aβ-AGEs). As shown in Figure 3C, the positive control, AG, at a concentration of 100 μg/mL, reduced glycated Aβ formation by 58.9%. Among the extracts, T. arjuna showed the highest inhibition (83.8%) followed by P. emblica (83.2%), and P. granatum (77.7%). Several other extracts also showed inhibition levels superior to the positive control, AG, including T. indica (77.1%), S. cumini (76.2%), and Salacia reticulata (75.5%) extracts.
Based on the data obtained from these six bioassays, namely, total polyphenol content, anti-oxidant capacity (DPPH and FRAP assays), anti-glycation effects, MGO trapping activity, Aβ fibrillation inhibition, and Aβ-AGEs inhibition, index scores were generated for each extract as previously reported (Seeram et al., 2008) and these results are summarized in Table 2. Extracts of P. granatum and P. emblica showed the highest score (91 and 90, respectively), followed by M. pruriens (70), C. longa (59) and C. asiatica (57). The medicinal plant extracts with a neuroprotective potential index score ≥ 40, namely, the top twelve candidates, were selected for further evaluations for their neuroprotective potential using in vitro (BV-2 cells), AChE inhibitory, and in vivo (Caenorhabditis elegans) bioassays.
3.4. Inhibition of acetylcholinesterase (AChE) enzyme activity
Inhibitors of the acetylcholinesterase (AChE) enzyme are currently used as treatments for mild to moderate AD in human subjects (Birks et al., 2000) and this assay is widely used to screen for candidates with anti-AD potential (Birks, 2006). Therefore, the medicinal plant extracts (see Table 2) which had a neuroprotective potential index score ≥ 40 (from the initial set of bioassays described in Sec. 3.1–3.3) were next evaluated for their inhibitory effects on the AChE enzyme. As shown in Table 3, the top four candidates namely, M. pruriens, P. emblica, P. granatum, and C. longa showed AChE inhibitory effects of 48.1, 43.1, 35.1, and 11.3% at 100 μg/mL, respectively, with IC50 values ranging from 91.3 to 304.6 μg/mL. In addition, among the twelve herbal extracts, T. arjuna also showed inhibitory effects against the AChE enzyme with an IC50 value of 313.6 μg/mL (Table 3).
Table 3.
Inhibitory effects on AChE activity of selected medicinal herbal extracts (top 12 based on initial screening)
| Plant Species | AChE inhibition (%) at 100 μg/mL | AChE IC50 (μg/mL) |
|---|---|---|
| P. granatum | 35.06 ± 2.93 | 304.62 ± 1.27 |
| P. emblica | 43.11 ± 1.72 | 163.92 ± 3.55 |
| M. pruriens | 48.11 ± 2.78 | 91.35 ± 2.80 |
| C. longa | 11.31 ± 1.81 | – b |
| C. asiatica | n.a. a | – |
| P. marsupium | n.a. | – |
| C. cassia | n.a. | – |
| B. monnieri | n.a. | – |
| O. tenuiflorum | n.a. | – |
| M. oleifera | n.a. | – |
| T. arjuna | 12.88 ± 2.58 | 313.58 ± 6.21 |
| S. reticulata | 7.64 ± 1.43 | – |
| galanthamine c | 68.50 ± 1.10 | 5.37 ± 3.79 |
n.a. = not active; shown as mean values ± SD (n ≥ 3).
, > 600 μg/mL.
Used as positive control at 12 μg/mL.
3.5. Inhibition of lipopolysaccharide (LPS)-induced nitric oxide species (NOS) production in murine BV-2 microglial cells
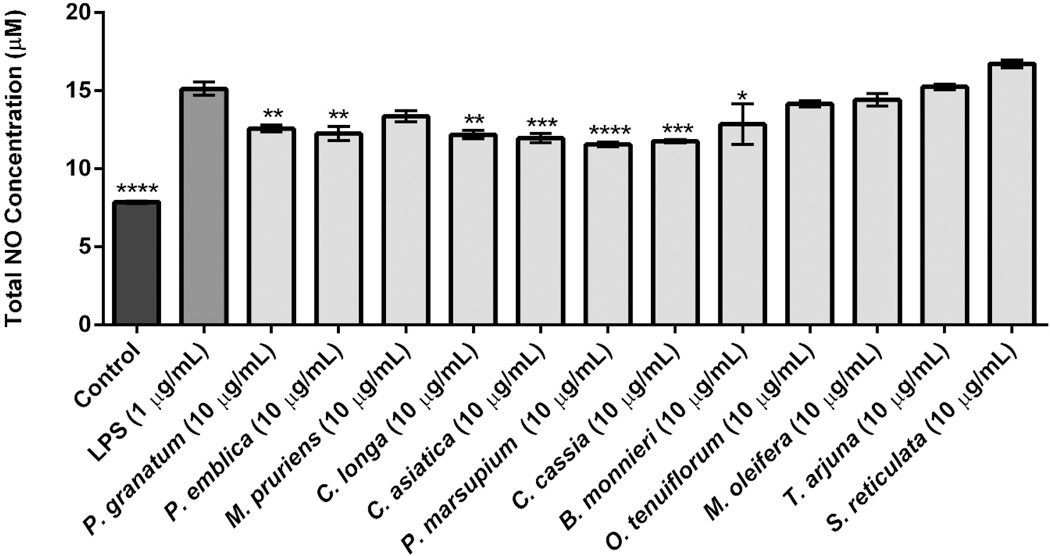
The medicinal plant extracts (see Table 2) which had a neuroprotective potential index score ≥ 40 (from the initial set of bioassays described in Sec. 3.1–3.3) were next evaluated for their effects on LPS-induced neuroinflammation in an established in vitro murine BV-2 microglia cell model. As shown in Figure 4, seven of these extracts inhibited NOS levels at 10 μg/mL. The most active extracts were P. marsupium and C. cassia, which reduced NOS production by 23.6 and 22.2%, respectively. Also, C. asiatica, C. longa, P. emblica, P. granatum, and B. monnieri suppressed NOS production by 20.8, 19.4, 19.0, 16.7 and 15.0%, respectively. The extracts of M. pruriens, O. tenuiflorum, and M. oleifera did not significantly reduce NOS production while T. arjuna and S. reticulata extracts increased the levels of NOS. The positive control, RESV (10 μg/mL), showed a 38.6% reduction of NOS production in the LPS-stimulated BV2 cells.
Figure 4.
Anti-neuro-inflammatory effects of medicinal plant extracts on murine BV-2 cells microglial cells by measuring NOS production induced by LPS. BV-2 cells were treated with each extracts (10 μg/mL) for 1 h followed by exposure to LPS (1 μg/mL) for 24 h. The cell culture media were used to assay the amount of NOS production by the Griess assay. Data are presented as means ± SDs of three independent experiments.
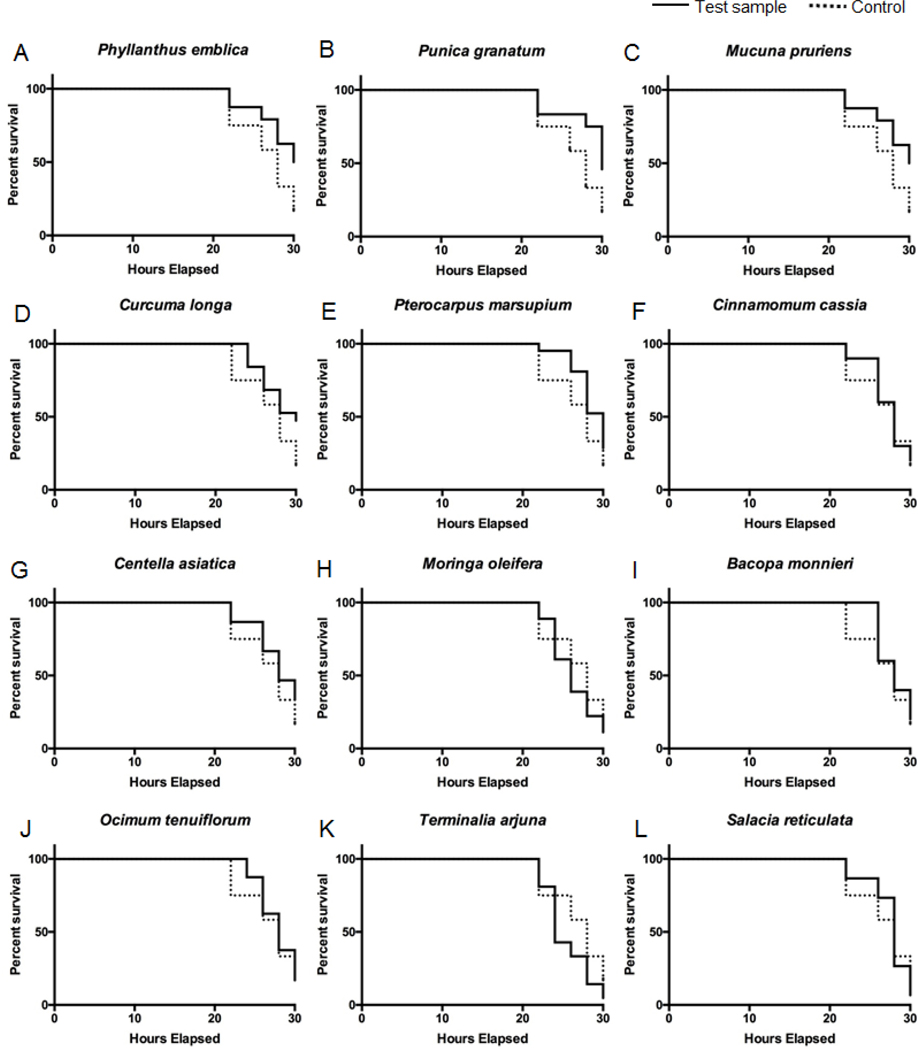
3.6. Reduction on Aβ1-42 induced neurotoxicity and paralysis in Caenorhabditis elegans
Next, the twelve extracts (which had a neuroprotective potential index score ≥ 40 as described above) were evaluated using an in vivo model using a transgenic C. elegans strain (CL4176) as previously reported by our group (Yuan et al., 2015). The CL4176 strain has a mutation of human Aβ1-42 expression in the muscle of the worms and the deposition of Aβ1-42 leads to neurotoxicity and paralysis. As shown in Figure 5, the heat shock induced Aβ1-42 neurotoxicity decreased the survival rate of C. elegans to 15.3% at 30 hours. However, among the extracts, P. emblica, P. granatum, M. pruriens, and C. longa significantly increased the survival rate of C. elegans to 47.8, 45.8, 44.1, and 39.1%, respectively. Several of the other extracts including P. marsupium, C. cassia, M. oleifera, B. monnieri, and O. tenuiflorum increased the survival rate of C. elegans from 33.3 to 16.7% while S. reticulata and T. arjuna did not show any neuroprotective effects with a survival rate of C. elegans of 6.7 and 4.8%, respectively. Based on the results from the C. elegans lifespan assay, P. emblica, P. granatum, M. pruriens, and C. longa were ranked among the top extracts which is in agreement with the ranking obtained from the in vitro BV-2 cell assay, wherein C. longa, P. emblica, P. granatum, and M. pruriens were also the most active extracts.
Figure 5.
Protective effects of medicinal plant extracts against neurotoxicity and paralysis in Caenorhabditis elegans in vivo. Mobility curves of transgenic (CL4176) C. elegans 30 hours post Aβ1-42 induction of muscular paralysis at 25 °C. Kaplan-Meier mobility plots of C. elegans worms fed on twelve extracts [Control (NGM), in dot line; (A-L) test samples: (NGM + 10 μg/mL test sample), in solid line].
3.7. Evaluation of herbal extracts against H2O2 induced cytotoxicity in murine BV2 microglia and human neuronal SH-SY5Y cells
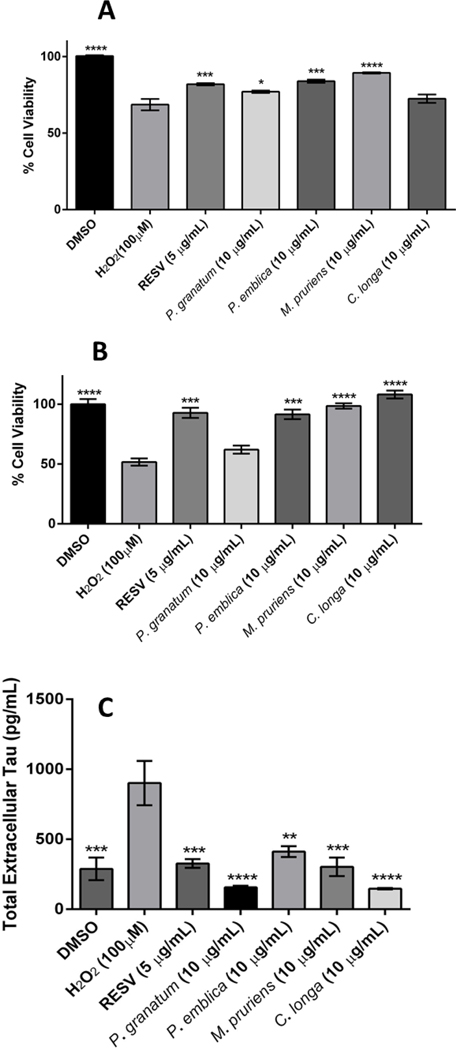
It is well established that oxidative stress is linked with the pathology of several neurodegenerative diseases including AD (Butterfield, 2002; Christen, 2000). Therefore, based on the bioassays described in Sec. 3.4–3.6, a final neuroprotective potential index score was calculated (see Table 4) and the extracts which had an index score ≥ 60, namely, P. granatum, P. emblica, M. pruriens and C. longa, were selected for further evaluation of their neuroprotective effects against H2O2 induced cytotoxicity in murine BV-2 microglia and differentiated human SH-SY5Y neuronal cells. As shown in Figure 6A, the cell viability of the H2O2-treated murine microglial BV-2 cells decreased by 31.7% as compared to the control group. When the H2O2-exposed cells were subsequently treated with the herbal extracts (concentration of 10 μg/mL), M. pruriens, P. emblica, P. granatum, and C. longa increased cell viability by 30.2, 22.3, 12.3, and 5.6%, respectively, as compared to the cells exposed to H2O2 alone. Similarly, as shown in Figure 6B, the cellular viability of H2O2-treated human neuronal SH-SY5Y cells decreased by 48.2% as compared to the control group. When the H2O2-exposed cells were subsequently treated with the herbal extracts (each at a concentration of 10 μg/mL), C. longa, M. pruriens, and P. emblica significantly increased the cell viability by 109.1, 90.6, and 76.9%, respectively. Resveratrol (RESV), which served as the positive control, also increased cell viability of H2O2-treated murine BV-2 microglia (19.5%) and differentiated human SH-SY5Y neuronal (79.3%) cells (Figure 6).
Figure 6.
Protective effects of medicinal plant extracts (10 μg/mL) on H2O2 induced cytotoxicity in murine BV-2 microglia (A) and differentiated human SH-SY5Y neuronal cells (B). Cellular viability was assessed using Cell Titer Glo 2.0 (CTG 2.0) one step assay. Inhibitory effects of medicinal plant extracts (10 μg/mL) on tau protein levels in differentiated SH-SY5Y neurons (C). All data are presented as mean ± SDs of three independent experiments.
3.8. Evaluation of herbal extracts on tau protein production in differentiated human SH-SY5Y neuronal cells
Studies have shown that in neurodegenerative models, including AD, neurons undergoing cell stress release tau proteins which on hyperphosphorylation form neurofibrillary tangles which can ultimately lead to cell death (Gomez-Ramos et al., 2006). Therefore, as described above (see Section 3.7), the extracts which had an index score ≥ 60, namely, P. granatum, P. emblica, M. pruriens and C. longa, were selected for further evaluation of their inhibitory effects on tau protein production in human neuronal SH-SY5Y cells. As shown in Figure 6C, extracts of C. longa, P. granatum, M. pruriens, and P. emblica reduced extracellular tau protein levels by 83.7, 82.6, 66.4, and 52.4%, respectively.
4. Conclusion
In summary, using bioassays with established links between AD and oxidative stress, carbonyl stress, glycation, Aβ fibrillation, Aβ-AGE formation, AChE inhibition, and neuroinflammation, an AD Neuroprotective Potential Algorithm (NPA) was developed as part of a strategy to help guide the selection and evaluation of medicinal plant candidates for future AD based research. From the current study, four extracts identified with a cumulative neuroprotective potential index score ≥ 60 were Phyllanthus emblica (amla; Indian gooseberry), Mucuna pruriens (velvet bean), Punica granatum (pomegranate) and Curcuma longa (curcumin; turmeric). Interestingly, published animal data support the neuroprotective effects of two of these extracts namely, P. granatum (Yuan et al., 2015; Ahmed et al., 2014) and C. longa (Ringman et al., 2005) against AD. While the other two extracts remain underexplored for their anti-AD potential, P. emblica has been shown to improve memory deficits in mice (Ashwlayan and Singh, 2011) and M. pruriens has been shown to have protective effects against Parkinson’s disease in human subjects (Katzenschlager et al., 2004).
There are several limitations to the NPA developed herein, primarily, due to the fact that botanical extracts are complicated mixtures containing multiple ‘multi-targeting’ phytochemicals which undergo complex metabolic processes in vivo (including metabolism by the liver enzymes and gut microflora) which influence their bioavailability, metabolism, excretion, and generation of ‘further’ bioactive metabolites (Wang et al., 2015; Yuan et al., 2015; Wang et al., 2014). For example, our group has recently reported that the gut microbial metabolites produced from the colonic microflora metabolism of the natural ellagitannins present in the pomegranate (P. granatum) fruit are potentially brain absorbable and may contribute to the anti-AD effects reported for this natural product (Yuan et al., 2015). Therefore, a major limitation of the NPA developed here is the lack of critical in vivo data (accumulated from animal models) which would account for the aforementioned important physiological considerations (Singh et al., 2008; Ebrahimi and Hermann, 2012). Other limitations of the NPA include utilizing additional AD-targeted bioassays and its validation with an increased sample size of medicinal plant extracts and their combinations thereof. Nevertheless, given all of these limitations, the NPA may be utilized in consideration with published literature data (when available) of animal and human studies, traditional and ethnomedicinal use, etc., as part of a more comprehensive research strategy to guide the selection of promising medicinal plant candidates for future AD based research using in vivo models.
Supplementary Material
Acknowledgments
HM was supported by the Omar Magnate Family Foundation Scholarship. The herbal extracts were kindly provided by Verdure Sciences (Noblesville, IN, USA) courtesy of Mr. Ajay Patel. Spectrophotometric data were acquired from instruments in the RI-INBRE core facility located at the University of Rhode Island (Kingston, RI, USA) supported by grant # 5P20GM103430-13 from the National Institute of General Medical Sciences of the National Institutes of Health.
Abbreviations
- AD
Alzheimer’s disease
- ROS
reactive oxygen n species
- AGEs
advanced glycation end-products
- RAGE
receptor for advanced glycation end-products
- Aβ
beta amyloid
- BBB
blood brain barrier
- RCS
reactive carbonyl species
- APP
Aβ precursor protein
- AG
aminoguanidine
- FRAP
ferric reducing antioxidant power
- AChE
acetylcholinesterase
- CNS
central nervous system
- NOS
nitric oxide species
- TCM
traditional Chinese medicine
- NPA
Neuroprotective Potential Algorithm
- BHT
Butylated hydroxytoluene
- BSA
bovine serum albumin
- MGO
methylglyoxal
- RESV
resveratrol
- PD
1,2-phenylenediamine
- DQ
2,3-dimethylquinoxaline
- TFA
trifluoroacetic acid
- ThT
thioflavin T
- Aβ1-42
beta amyloid 1–42
- DMSO
dimethyl sulfoxide
- GAEs
gallic acid equivalents
- DMEM/F12
Dulbecco’s modified eagle medium: nutrient mixture F-12
- FBS
fetal bovine serum
- CTG 2.0
CellTiter-Glo 2.0
- LPS
lipopolysaccharide
- NGM
nematode growth medium
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- Ahmed AH, Subaiea GM, Eid A, Li L, Seeram NP and Zawia NH, 2014. Pomegranate extract modulates processing of amyloid-β precursor protein in an aged Alzheimer’s disease animal model. Curr. Alzheimer Res. 11, 834–843. [PubMed] [Google Scholar]
- Ahmed N, Ahmed U, Thornalley PJ, Hager K, Fleischer G, Münch G, 2005. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 92, 255–263. [DOI] [PubMed] [Google Scholar]
- Ashwlayan VD, Singh R 2011. Reversal effect of Phyllanthus emblica (Euphorbiaceae) Rasayana on memory deficits in mice. Int. J. Appl. Pharm, 3, 10–15. [Google Scholar]
- Alzheimer’s Association, 2015. 2015 Alzheimer’s disease facts and figures. Alzheimers & Dement. 11, 332–384. [DOI] [PubMed] [Google Scholar]
- Birks J, 2006. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane. Database Syst. Rev. 25, CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J, Melzer D, Beppu H, 2000. Donepezil for mild and moderate Alzheimer’s disease. Cochrane. Database Syst. Rev. 4, CD001190. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA, 2005. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 25, 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, 2002. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 36, 1307–1313. [DOI] [PubMed] [Google Scholar]
- Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M, 2001. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology 57, 985–989. [DOI] [PubMed] [Google Scholar]
- Chen K, Kazachkov M, Yu PH, 2007. Effect of aldehydes derived from oxidative deamination and oxidative stress on beta-amyloid aggregation; pathological implications to Alzheimer’s disease. J. Neural. Transm. (Vienna) 114, 835–839. [DOI] [PubMed] [Google Scholar]
- Chen K, Maley J, Yu PH, 2006. Potential implications of endogenous aldehydes in β‐amyloid misfolding, oligomerization and fibrillogenesis. J. Neurochem. 99, 1413–1424. [DOI] [PubMed] [Google Scholar]
- Choi D-Y, Lee Y-J, Hong JT, Lee H-J, 2012. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 87, 144–153. [DOI] [PubMed] [Google Scholar]
- Christen Y, 2000. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 71, 621s–629s. [DOI] [PubMed] [Google Scholar]
- Citron M, 2010. Alzheimer’s disease: strategies for disease modification. Nat. Rev. Drug Discov. 9, 387–398. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran M, Holcomb LA, Hitt AR, Tharakan B, Porter JW, Young KA, Manyam BV 2009. Centella asiatica extract selectively decreases amyloid β levels in hippocampus of Alzheimer’s disease animal model. Phytother. Res, 23, 14–19. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, Silverberg GD, Miller MC, Tavares R, Yang W, Wu Q, Sabo E, Hovanesian V, Stopa EG, 2006. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 112, 405–415. [DOI] [PubMed] [Google Scholar]
- Ebrahimi A and Schluesener H, 2012. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res. Rev. 11, 329–345. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V and Featherstone RM, 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7, 88–95. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Bate C, Van Gool W, Hoozemans J, Rozemuller J, Veerhuis R, Williams A, 2002. Neuroinflammation in Alzheimer’s disease and prion disease. Glia 40, 232–239. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Veerhuis R, Scheper W, Rozemuller A, Van Gool W, Hoozemans J, 2006. The significance of neuroinflammation in understanding Alzheimer’s disease. J. Neural Transm. 113, 1685–1695. [DOI] [PubMed] [Google Scholar]
- Feng Y, Wang X-P, Yang S-G, Wang Y-J, Zhang X, Du X-T, Sun X-X, Zhao M, Huang L, Liu R-T, 2009. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 30, 986–995. [DOI] [PubMed] [Google Scholar]
- Gómez-Ramos A, Díaz-Hernández M, Cuadros R, Hernández F and Avila J, 2006. Extracellular tau is toxic to neuronal cells. FEBS Lett. 580, 4842–4850. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Götz J, 2011. Amyloid-β and tau--a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 12, 65–72. [DOI] [PubMed] [Google Scholar]
- Jean-Gilles D, Li L, Ma H, Yuan T, Chichester CO, Seeram NP, 2012. Anti-inflammatory effects of polyphenolic-enriched red raspberry extract in an antigen-induced arthritis rat model. J. Agric. Food Chem. 60, 5755–5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenschlager R, Evans A, Manson A, Patsalos PN, Ratnaraj N, Watt H, 2004. Mucuna pruriens in Parkinson’s disease: a double blind clinical and pharmacological study. J. Neurol. Neurosurg. Psychiatry. 75,1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaraju J, Madhunapantula SV, Chinni S, Khatwal RB, Dubala A, Nataraj SKM, & Basavan D 2014. Dipeptidyl peptidase-4 inhibition by Pterocarpus marsupium and Eugenia jambolana ameliorates streptozotocin induced Alzheimer’s disease. Behav. Brain Res, 267, 55–65. [DOI] [PubMed] [Google Scholar]
- Li F, Gong Q, Dong H, Shi J, 2012. Resveratrol, a neuroprotective supplement for Alzheimer’s disease. Curr. Pharm. Des. 18, 27–33. [DOI] [PubMed] [Google Scholar]
- Li X-H, Du L-L, Cheng X-S, Jiang X, Zhang Y, Lv B-L, Liu R, Wang J-Z, Zhou X-W, 2013. Glycation exacerbates the neuronal toxicity of β-amyloid. Cell Death Dis. 4, e673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM, 2001. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 21, 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF, 2006. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. [DOI] [PubMed] [Google Scholar]
- Liu W, Ma H, Frost L, Yuan T, Dain JA, Seeram NP, 2014. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food & Funct. 5, 2996–3004. [DOI] [PubMed] [Google Scholar]
- Ma H, DaSilva NA, Liu W, Nahar PP, Wei Z, Liu Y, Pham PT, Crews R, Vattem DA, Slitt AL Shaikh ZA, Seeram NP 2016. Effects of a standardized phenolic-enriched maple syrup extract on β-amyloid aggregation, neuroinflammation in microglial and neuronal cells, and β-amyloid induced neurotoxicity in Caenorhabditis elegans. Neurochem. Res. doi: 10.1007/s11064-016-1998-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Liu W, Frost L, Kirschenbaum LJ, Dain JA, Seeram NP, 2016. Glucitol-core containing gallotannins inhibit the formation of advanced glycation end-products mediated by their antioxidant potential. Food & Funct. DOI: 10.1039/C6FO00169F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Liu W, Frost L, Wang L, Kong L, Dain JA, Seeram NP, 2015. The hydrolyzable gallotannin, penta-O-galloyl-β-D-glucopyranoside, inhibits the formation of advanced glycation endproducts by protecting protein structure. Mol. Biosyst. 11, 1338–1347. [DOI] [PubMed] [Google Scholar]
- Maksimović Z, Malenčić Đ and Kovačević N, 2005. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 96, 873–877. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ, 2010. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch G, Westcott B, Menini T, Gugliucci A, 2012. Advanced glycation endproducts and their pathogenic roles in neurological disorders. Amino Acids 42, 1221–1236. [DOI] [PubMed] [Google Scholar]
- Nahar PP, Driscoll MV, Li L, Slitt AL and Seeram NP, 2014. Phenolic mediated anti-inflammatory properties of a maple syrup extract in RAW 264.7 murine macrophages. J. Funct. Foods, 6,126–136. [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C, 2014. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 13, 788–794. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Mucke L, 2010. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat. Neurosci. 13, 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picklo MJ, Montine TJ, Amarnath V, Neely MD, 2002. Carbonyl toxicology and Alzheimer’s disease. Toxicol. Appl. Pharmacol. 184, 187–197. [DOI] [PubMed] [Google Scholar]
- Praticò D, 2008. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Ann. NY Acad. Sci. 1147, 70–78. [DOI] [PubMed] [Google Scholar]
- Reitz C, Mayeux R, 2014. Alzheimer’s disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 88, 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, & Cummings JL 2005. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alz. Res. 2, 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D, 2008. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 56, 1415–1422. [DOI] [PubMed] [Google Scholar]
- Singh M, Arseneault M, Sanderson T, Murthy V and Ramassamy C, 2008. Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 56, 4855–4873. [DOI] [PubMed] [Google Scholar]
- Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G, 2000. Oxidative stress in Alzheimer’s disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1502, 139–144. [DOI] [PubMed] [Google Scholar]
- Solanki I, Parihar P, Parihar MS, 2016. Neurodegenerative diseases: From available treatments to prospective herbal therapy. Neurochem. Int. 95, 100–108. [DOI] [PubMed] [Google Scholar]
- Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Münch G, 2011. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol. Aging 32, 763–777. [DOI] [PubMed] [Google Scholar]
- Steele ML, Truong J, Govindaraghavan S, Ooi L, Sucher NJ, Münch G, 2013. Cytoprotective properties of traditional Chinese medicinal herbal extracts in hydrogen peroxide challenged human U373 astroglia cells. Neurochem. Int. 62, 522–529. [DOI] [PubMed] [Google Scholar]
- Sun J, Liu W, Ma H, Marais JP, Khoo C, Dain JA, Rowley DC, Seeram NP, 2016. Effect of cranberry (Vaccinium macrocarpon) oligosaccharides on the formation of advanced glycation end-products. J. Berry Res, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley PJ, 2003. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 419, 31–40. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Van der Giessen R, Lees AJ.Koo EH, Lansbury PT, Kelly JW, 1999. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc. Natl. Acad. Sci. USA 96, 9989–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PJ, McIntosh J, Pearce P, Camden B and Jordan BR, 2002. Anthocyanin and antioxidant capacity in Roselle (Hibiscus sabdariffa L.) extract. Food Res. Int. 35, 351–356. [Google Scholar]
- Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, Sachdeva S, 2001. Prevalence of dementia in an urban Indian population. Int. Psychogeriatr. 13, 439–450. [DOI] [PubMed] [Google Scholar]
- Venigalla M, Sonego S, Gyengesi E, Sharman MJ, Münch G, 2016. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 95, 63–74. [DOI] [PubMed] [Google Scholar]
- Vicente Miranda H, Outeiro TF, 2010. The sour side of neurodegenerative disorders: the effects of protein glycation. J. Pathol. 221, 13–25. [DOI] [PubMed] [Google Scholar]
- Wang D, Ho L, Faith J, Ono K, Janle EM, Lachcik PJ, Cooper BR, Jannasch AH, D’Arcy BR, Williams BA and Ferruzzi MG, 2015. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 59,1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Bi W, Cheng A, Freire D, Vempati P, Zhao W, Gong B, Janle EM, Chen TY, Ferruzzi MG and Schmeidler J, 2014. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 6, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-H, Yen G-C, 2005. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 53, 3167–3173. [DOI] [PubMed] [Google Scholar]
- Yuan T, Ma H, Liu W, Niesen DB, Shah N, Crews R, Rose KN, Vattem DA, Seeram NP, 2015. Pomegranate’s neuroprotective effects against Alzheimer’s disease are mediated by urolithins, its ellagitannin-gut microbial derived metabolites. ACS Chem. Neurosci. 7, 26–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.