Abstract
Background:
Maternal obesity is associated with many adverse obstetric outcomes including cesarean delivery. It is unclear whether induction of labor can reduce these risks. Previous studies report conflicting results on the outcomes of elective induction of labor among obese women.
Objective:
To compare maternal and neonatal outcomes between obese women undergoing elective induction of labor and those undergoing expectant management at ≥39 weeks.
Study design:
This was a retrospective cohort study from the Consortium on Safe Labor of obese women (defined by pre-pregnancy body mass index (BMI) ≥ 30kg/m2) with singleton gestations at ≥39 weeks without medical co-morbidities from 2002 through 2008. Women scheduled for medically indicated induction of labor were excluded. The primary outcome of cesarean delivery was compared between obese women undergoing elective induction of labor and expectant management during 39th, 40th and 41st weeks using univariable and multivariable analyses, stratifying by parity.
Results:
In all, 7,298 nulliparous and 9,789 parous women were eligible for analysis. After controlling for potential confounders, elective induction of labor during 39th week in nulliparous and parous women was associated with lower odds of cesarean delivery (39.1% vs. 41.6%, adjusted OR 0.47, 95% CI 0.30– 0.74 for nulliparous and 5.5% vs. 10.1%, adjusted OR 0.34, 95% CI 0.20–0.61 for parous women) compared to expectant management. Elective induction of labor during 40th and 41st week was not associated with lower odds of cesarean delivery. In addition, macrosomia was reduced in nulliparous women undergoing elective induction of labor during the 40th week (12.1% vs. 18.5%, adjusted OR 0.56, 95% CI 0.35– 0.87) and in parous women undergoing elective induction of labor during 39th (11.6% vs. 17.6%, adjusted OR 0.50, 95% CI 0.38–0.66) and 40th weeks (16.4% vs. 22.2%, adjusted OR 0.53, 95% CI 0.36–0.78).
Conclusion:
Elective induction of labor at 39 weeks, when compared to expectant management, was associated with lower cesarean deliveries in obese nulliparous and parous women.
Précis:
Elective induction of labor during the 39th week among obese nulliparous and parous women, compared to expectant management, is associated with decreased cesarean delivery rate.
Introduction
The prevalence of obesity continues to increase1–2 and according to the 2011–2014 National Center for Health Statistics data it affects one in three women of reproductive age.3 Pre-pregnancy body mass index (BMI) over 29.9 kg/m2 was present in 24.8% of women giving birth in the United States in 2014 .2 Maternal obesity is associated with many adverse obstetric outcomes, including preeclampsia,4–5 , cesarean delivery6–8 and stillbirth9–12. It is unclear whether induction of labor can reduce some of these risks.
Previous literature examining outcomes of elective induction of labor in obese women is mixed. Two studies found that obese women have higher rates of failed induction as well as higher neonatal intensive care unit admission and composite neonatal morbidity.13–14 However, these studies either used a comparison group of normal-weight women13 or included only women with unfavorable cervices.14 Two larger studies comparing elective induction of labor in obese women to expectant management found that cesarean delivery, macrosomia, severe maternal morbidity, and neonatal morbidity were lower in the induction group.15 with no change in the odds of infant and neonatal mortality.15,16 Another recent analysis restricted only to morbidly obese women, defined as BMI >40 kg/m2, did not find an association between elective induction of labor after 37 weeks and cesarean delivery.17
The remainder of the published studies combined all obese women undergoing medical and elective inductions and found higher cesarean deliveries and postpartum complications, including hemorrhage, infection and incisional morbidity.17–19 The neonates of obese women who failed to achieve a vaginal delivery after induction of labor also had increased respiratory morbidity, antibiotic use and admission to the neonatal intensive care unit .20 However, it remains unclear whether elective induction of labor in obese women with no medical indication for induction leads to the same consequences.
Therefore, the objective of this analysis was to compare maternal and neonatal outcomes between obese women undergoing elective induction of labor versus expectant management at or after 390/7 weeks of gestation. Our primary outcome was the rate of cesarean delivery. We hypothesized that elective induction of labor at or after 390/7 weeks in this population would not be associated with an increased odds of cesarean delivery.
Materials and methods
This was a secondary analysis of data from the Consortium on Safe Labor (CSL), a retrospective cohort study of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).21 The CSL collected detailed information from electronic medical records in over two hundred and twenty thousand deliveries from 12 medical centers with 19 hospitals across 9 American Congress of Obstetricians and Gynecologists (ACOG) districts and was conducted between 2002 and 2008. CSL was a retrospective abstraction of electronic medical records (EMR) of women who were admitted for delivery. The EMR coded data into pre-specified fields that would allow for data to be abstracted and combined into a uniform dataset.The primary goal of the CSL was to characterize labor and delivery in a contemporary cohort of women and assess risks for cesarean delivery. In the present analysis, we included women from the CSL database who were obese (defined by pre-pregnancy BMI ≥ 30 kg/m2) with a singleton gestation and cephalic presentation at ≥ 39 weeks. Elective induction was identified by using the CSL variable “Ind_elect”, standing for elective induction.
In the CSL database there was no a-priori standardized definitions for variables. Thus, the meaning of a variable Ind_elect (e.g., elective induction) could vary from hospital to hospital. The elective induction variable could be a check box in some hospitals. During the recoding, induction for social or logistic reasons was coded as “elective”. However, if the indication for induction field was left blank, it was coded as “unknown”, not “elective”. The following exclusion criteria were applied: women with chronic medical illness requiring induction of labor during the 39th week (i.e. pre-gestational or diabetes mellitus requiring pharmacotherapy, chronic hypertension, renal or heart disease); women with a history of cesarean delivery; or women with acute obstetric indication for induction such as gestational hypertension, preeclampsia, non-reassuring antenatal surveillance, abnormal amniotic fluid, fetal growth restriction, placenta previa and placental abruption. However, if women developed these obstetric complications while being expectantly managed, they were still included in the expectant management group of this analysis, as these can be consequences of expectant management and we wanted to assess their frequency. In addition, women who presented in spontaneous labor or premature rupture of membranes were excluded from the induction of labor group at each subsequent week of gestational age.
Nulliparous and parous women who underwent elective induction of labor were stratified into three comparison groups based on the timing of their induction of labor: 390/7-393/7 weeks, 400/7-403/7 weeks, and 410/7-413/7 weeks. Gestational age was assigned according to the best obstetric estimate as recorded in the medical record. Women who underwent elective induction in each of these groups were compared with women who were managed expectantly after the same gestational age (i.e. >39 3/7 weeks, >40 3/7 weeks, >413/7weeks, respectively). This design was used in order to resemble the prospective choice the provider has of scheduling induction of labor during a given period of time at the start of a given week of gestation or continuing to manage the pregnancy expectantly from that time forward.
The following sociodemographic and clinical characteristics were abstracted: maternal age, race-ethnicity, pre-pregnancy BMI class, defined as class I obesity = BMI of 30.0–34.9 kg/m2, class II obesity = BMI of 35.0–39.9 kg/m2 and class III = BMI of ≥ 40 kg/m2, BMI on admission, parity, marital status, prior vaginal delivery, cigarette smoking, alcohol or illicit drug use, maternal depression, simplified Bishop score on admission using dilation, station and effacement (≤4 would indicate unfavorable score),22 neonatal birthweight and sex. Women undergoing scheduled repeat cesarean delivery for the indication of previous cesarean delivery were excluded, but women undergoing cesarean deliveries for other reasons were included in the analysis and indications for cesarean deliveries were abstracted from the medical records. The rationale behind including these women is to avoid skewed results as women with class III obesity may be scheduled for elective cesarean deliveries by their providers due to concerns for difficulties with fetal heart rate tracing in labor and ability to perform an emergent cesarean delivery safely. If these concerns are truly valid, then the results may be skewed if women with severe obesity are excluded from our analysis.
Maternal outcomes of elective induction of labor included cesarean or operative vaginal delivery, 3rd/4th degree or other major lacerations of the sulcus or vaginal wall, postpartum hemorrhage, blood transfusion, endometritis, wound infection, hysterectomy, intensive care unit admission and death. Neonatal outcomes included Apgar score <7 at 5 minutes, birth weight, macrosomia, defined as birth weight ≥ 4000g, small-for-gestational age neonate (defined as birth weight less than the 10th percentile for a given gestational age), large-for-gestational age neonate (defined as a birth weight equal or greater than the 90th percentile for a given gestational age), shoulder dystocia (defined as an application of additional obstetric maneuvers following failure of gentle downward traction on the fetal head to enable delivery of the fetal shoulders), neonatal intensive care unit admission, hypoxic ischemic encephalopathy and perinatal death (including antenatal, intrapartum or neonatal death) at each gestational age group and were compared to expectant management beyond each gestational age period.
In order to ensure that our results were not solely dependent upon our primary analytic approach, we performed additional analyses in which the inclusion criteria for the group of women managed expectantly were altered slightly. In one analysis, we changed the gestational age window for the expectant management group, starting at 390/7 instead of 394/7. In another analysis, we excluded women in the expectant management group if they underwent an elective primary cesarean delivery.
Univariable comparisons of maternal and neonatal characteristics and pregnancy outcomes, stratified by parity were performed using Student’s t test, Chi-square, Fisher exact test, and Wilcoxon rank-sum (Mann-Whitney U) test as appropriate. Additionally, multivariable logistic and linear (for the continuous outcome of birth weight) regressions were performed for the rates of cesarean deliveries and all dichotomous secondary outcomes. Potential confounding variables were entered into the regression equation if they were previously reported to be associated with mode of delivery. Odds ratios (OR) with 95% confidence intervals (CI) were estimated from the logistic regression equations. Institutional Review Board approval was obtained by all centers participating in the CSL NICHD original study and this analysis was exempt as the data was received in de-identified form from the NICHD website (https://dash.nichd.nih.gov/). All statistical analyses were performed with Stata version 14.2 (StataCorp College Station, TX).
Results
The number of deliveries occurring after 23 weeks and included in the CSL database from 2002 through 2008 was 228,438. After applying all exclusion criteria, 7,298 nulliparous and 9,789 parous women were eligible for analysis (Figure 1). The number of women undergoing elective induction of labor during each week was 2,810 at 39 weeks, 3,294 at 40 weeks and 1,433 at 41 weeks .
Figure 1. Flowchart describing exclusion criteria and study population.
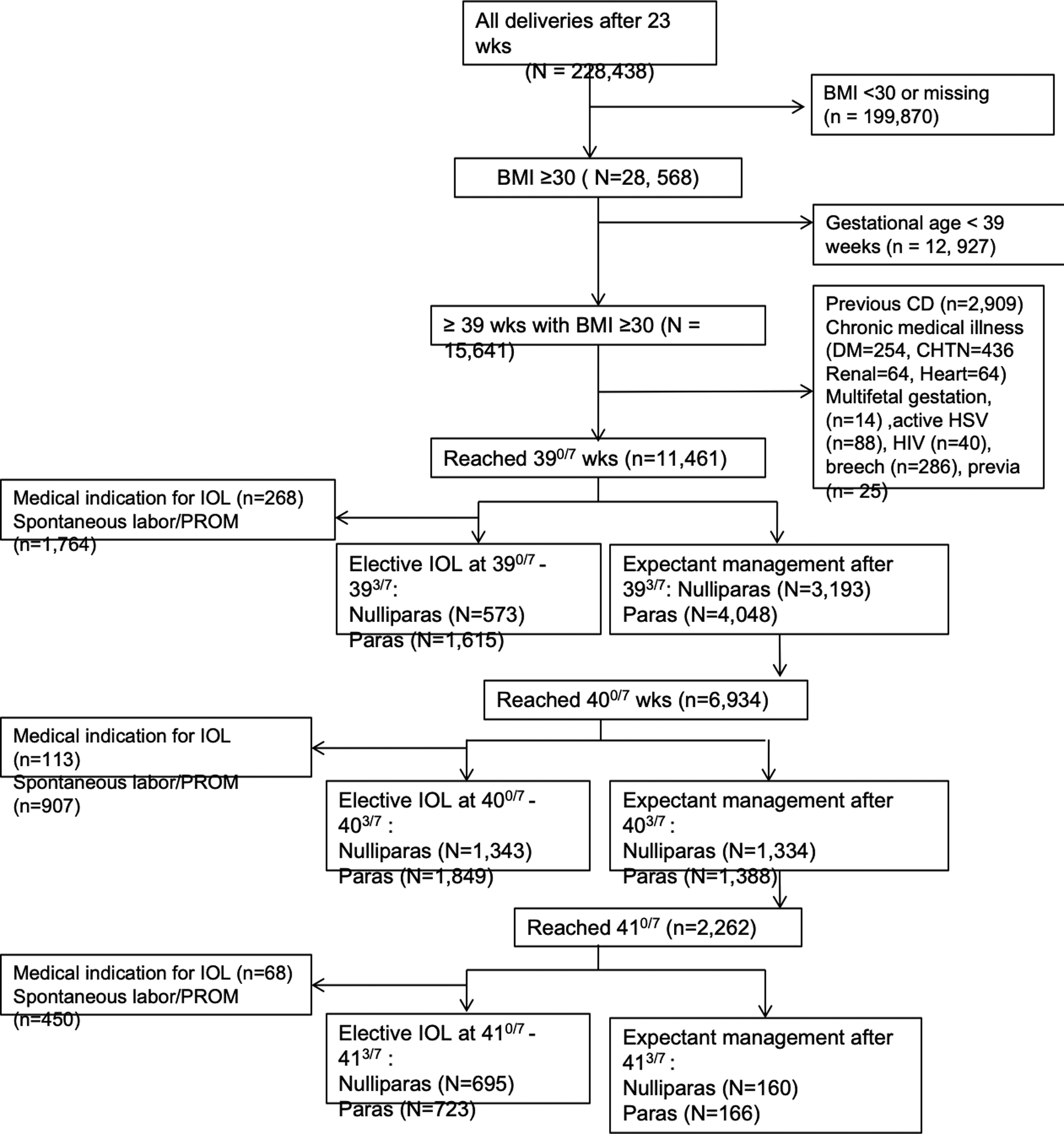
BMI, body mass index; CD, cesarean delivery; DM, diabetes mellitus; HTN, hypertension; HSV, herpes simple virus; IOL, induction of labor; PROM, premature rupture of membranes.
Maternal and neonatal characteristics of women included in the analysis are described by parity in Table 1 (nulliparous women) and Table 2 (parous women). Women undergoing elective induction of labor during 39th and 40th week of gestation were older, more likely to be of a non-Hispanic white race-ethnicity and more likely to be married. In addition, nulliparous women undergoing elective induction during 40th week were more likely to have class I obesity and have a more favorable simplified Bishop score. Similarly, among parous women, women undergoing elective induction during 39th and 40th weeks had a more favorable simplified Bishop score. Neonatal birthweight was higher among nulliparous and parous women managed expectantly during the 39th and 40th weeks, compared to women who were induced.
Table 1.
Characteristics of nulliparous obese women undergoing elective induction of labor compared to expectant management
| eIOL | EM | eIOL | EM | eIOL | EM | |
|---|---|---|---|---|---|---|
| 390/7 -393/7 | >393/7 | 400/7 -403/7 | >403/7 | 410/7 -413/7 | >413/7 | |
| Nulliparous women | (n=573) | (n=3,139) | (n=1,343) | (n=1,334) | (n=695) | (n=160) |
| Age (years) | 25.4 ± 5.6* | 24.6 ± 5.5 | 25.0 ± 5.6* | 24.3 ± 5.4 | 24.2 ± 5.4 | 23.7 ± 5.6 |
| Pre-pregnancy BMI class | ||||||
| Class I | 325 (56.7) | 1,873 (58.7) | 825 (61.4)* | 748 (56.1) | 397 (57.1) | 90 (56.3) |
| Class II | 152 (26.5) | 797 (25.0) | 322 (24.0)* | 340 (25.5) | 178 (25.6) | 40 (25.0) |
| Class III | 96 (16.8) | 523 (16.4) | 196 (14.6)* | 246 (18.4) | 120 (17.3) | 30 (18.8) |
| BMI on admission | 40.6 ± 5.5 | 40.5 ± 5.6 | 40.3 ± 5.3 | 40.7 ± 5.9 | 40.7 ± 5.9 | 40.7 ± 5.5 |
| Race | ||||||
| Non Hispanic White | 284 (49.6)* | 1,463 (46.6) | 664 (49.4)* | 569 (42.6) | 287 (41.3) | 57 (35.6) |
| Non Hispanic Black | 154 (26.9)* | 980 (31.2) | 349 (26.0)* | 463 (34.7) | 226 (32.5) | 62 (38.8) |
| Hispanic | 105 (18.3)* | 510 (16.3) | 229 (17.1)* | 201 (15.1) | 123 (17.7) | 28 (17.5) |
| Other | 17 (3.0)* | 113 (3.6) | 55 (4.1)* | 45 (3.4) | 28 (4.0) | 4 (2.6) |
| Missing | 13 ( 2.2)* | 73 (2.3) | 46 (3.4)* | 56 (4.2) | 31 (4.5) | 9 (5.5) |
| Marital Status | ||||||
| Married | 289 (50.4)* | 1,410 (44.2) | 654 (48.7)* | 537 (40.3) | 280 (40.3)* | 53 (33.1) |
| Divorced/Widowed | 7 (1.2)* | 18 (0.6) | 7 (0.5)* | 4 (0.3) | 0 (0.0)* | 2 (1.3) |
| Single | 270 (47.1)* | 1,682 (52.7) | 647 (48.2)* | 755 (56.6) | 397 (57.1)* | 99 (61.9) |
| Missing | 7 (1.2)* | 83 (2.6) | 35 (2.6)* | 38 (2.8) | 18 (2.6 )* | 6 (3.8) |
| Cigarette use during pregnancy | 37 (6.5)* | 257 (8.1) | 87 (6.5)* | 129 (9.7) | 63 (9.1) | 20 (12.5) |
| Alcohol use during Pregnancy | 5 (0.9) | 48 (1.5) | 17 (1.3) | 19 (1.4) | 9 (1.3) | 2 (1.3) |
| Illicit drug use during Pregnancy | 6 (1.2) | 66 (2.4) | 21 (1.9) | 27 (2.4) | 7 (1.2)* | 5 (4.2) |
| Simplified Bishop score ≤4 on admission† | 23/149 (15.4) | 135/875 (15.4) | 60/430 (14.0)* | 65/319 (20.4) | 40/188 (21.3) | 4/15 (26.7) |
| Indications for primary cesarean delivery | (n=224) | (n=1,329) | (n=532) | (n=603) | (n=330) | (n=70) |
| Elective | 3 (1.3) | 38 (2.8) | 20 (3.8) | 30 (5.0) | 21 (6.4) | 6 (8.6) |
| Non reassuring FHTs | 40 (17.9) | 318 (23.9) | 143 (26.8) | 151 (25.0) | 71 (21.5) | 16 (22.9) |
| Failure to progress | 151 (67.4) | 813 (61.2) | 292 (54.9) | 361 (59.9) | 204 (61.8) | 42 (60.0) |
| Chorioamnionitis | 1 (0.5) | 9 (0.7) | 6 (1.3) | 6 (1.0) | 2 (0.6) | 1 (1.4) |
| Unknown/missing | 29 (12.9) | 151 (11.4) | 71 (13.3) | 55 (9.1) | 32 (9.7) | 5 (7.1) |
| Male sex | 283 (49.4) | 1,575 (49.3) | 671 (50.0) | 647 (48.5) | 343 (49.4) | 74 (46.3) |
All data presented as mean standard deviation or N (%)
= p<0.05 for comparison of elective labor induction versus expectant management at the given gestational age
= The denominator excludes deliveries with missing data for this variable
eIOL, elective induction of labor; EM, expectant management; BMI, body mass index; FHT: fetal heart tones
Class I obesity =BMI of 30.0–34.9, Class II obesity=BMI of 35.0–39.9, Class III=BMI of ≥40
Table 2.
Characteristics of parous obese women undergoing elective induction of labor compared to expectant management
| eIOL | EM | eIOL | EM | eIOL | EM | |
|---|---|---|---|---|---|---|
| 390/7 -393/7 | >393/7 | 400/7 -403/7 | >403/7 | 410/7 -413/7 | >413/7 | |
| Parous women | (n=1,615) | (n=4,048) | (n=1,849) | (n=1,388) | (n=723) | (n=166) |
| Age (years) | 29.1 ± 5.0* | 28.4 ± 5.5 | 28.8 ± 5.5* | 28.0 ± 5.5 | 28.1 ± 5.6 | 28.5 ± 6.1 |
| Pre-pregnancy BMI class | ||||||
| Class I | 944 (58.5) | 2,456 (60.7) | 1114 (60.3) | 851 (61.3) | 463 (64.0) | 106 (63.9) |
| Class II | 430 (26.6) | 972 (24.0) | 467 (25.3) | 308 (22.2) | 156 (21.6) | 32 (19.3) |
| Class III | 241 (14.9) | 620 (15.3) | 268 (14.5) | 229 (16.5) | 104 (14.4) | 28 (16.9) |
| BMI on admission | 38.9 ± 5.3 | 39.3 ± 5.3 | 39.2 ± 5.3* | 39.6 ± 5.3 | 39.2 ± 5.1 | 39.9 ± 5.4 |
| Race | ||||||
| Non Hispanic White | 1,044 (64.6)* | 1,467 (36.2) | 718 (38.8)* | 422 (30.4) | 234 (32.4)* | 46 (27.7) |
| Non Hispanic Black | 264 (16.3)* | 1,370 (33.8) | 553 (29.9)* | 516 (37.2) | 244 (33.7)* | 62 (37.4) |
| Hispanic | 219 (13.6)* | 941 (23.2) | 428 (23.2)* | 356 (25.6) | 192 (26.6)* | 46 (27.7) |
| Other | 53 (3.3)* | 176 (4.3) | 102 (5.5)* | 59 (4.3) | 31 (4.3)* | 9 (5.4) |
| Missing | 35 (2.2)* | 94 (2.3) | 48 (2.6)* | 35 (2.5) | 22 (3.0)* | 3 (1.8) |
| Marital Status | ||||||
| Married | 1,182 (73.1)* | 2,052 (50.7) | 1,026 (55.5)* | 621 (40.7) | 335 (46.3) | 65 (39.2) |
| Divorced/Widowed | 46 (2.9)* | 101 (2.5) | 35 (1.9)* | 46 (3.3) | 23 (3.2) | 4 (2.4) |
| Single | 361 (22.4)* | 1,777 (43.9) | 728 (39.4)* | 674 (48.6) | 338 (46.8) | 88 (53.0) |
| Missing | 26 (1.6)* | 118 (2.9) | 60 (3.2)* | 47 (3.4) | 27 (3.7 ) | 9 (5.4) |
| Cigarette use during Pregnancy | 96 (6.0)* | 395 (9.8) | 171 (9.3) | 145 (10.5) | 76 (10.5) | 20 (12.1) |
| Alcohol use during Pregnancy | 33 (2.0) | 86 (2.1) | 29 (1.6) | 31 (2.2) | 13 (1.8)* | 8 (4.8) |
| Illicit drug use during Pregnancy | 16 (1.1)* | 94 (2.7) | 38 (2.5) | 31 (2.6) | 15 (2.5)* | 3 (2.2) |
| Simplified Bishop score ≤4 on admission† | 74/854 (8.7)* | 95/1,021 (9.3) | 41/530 (7.7)* | 37/258 (14.3) | 27/149 (18.1) | 1/16 (6.3) |
| Indications for primary cesarean delivery | (n=88) | (n=410) | (n=178) | (n=165) | (n=80) | (n=27) |
| Elective | 7 (7.9) | 61 (14.9) | 27 (15.2) | 25 (15.2) | 13 (16.2) | 4 (14.8) |
| Non reassuring FHTs | 32 (36.4) | 133 (32.4) | 40 (22.4) | 60 (36.3) | 27 (33.8) | 10 (37.0) |
| Failure to progress | 49 (55.7) | 215 (52.4) | 85 (47.8) | 66 (40.0) | 32 (40.0) | 10 (37.0) |
| Chorioamnionitis | 0 (0.0) | 1 (0.3) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown/missing | 0 (0.0) | 0 (0.0) | 25 (14.0) | 14 (8.5) | 8 (10.0) | 3 (11.2) |
| Male sex | 779 (48.2) | 2,014 (49.7) | 957 (51.7) | 664 (47.8) | 355 (49.1) | 74 (44.6) |
All data presented as mean standard deviation or N (%)
= p<0.05 for comparison of elective labor induction versus expectant management at the given gestational age
= The denominator excludes deliveries with missing data for this variable
eIOL, elective induction of labor; EM, expectant management; BMI, body mass index; FHT: fetal heart tones
Class I obesity =BMI of 30.0–34.9, Class II obesity=BMI of 35.0–39.9, Class III=BMI of ≥40
Maternal and neonatal outcomes by parity are described in Tables 3 and 4. The rate of the primary outcome, cesarean delivery, was lower among nulliparous women undergoing elective induction of labor during 400/7 - 403/7 weeks (39.6% versus 45.2%, p=0.003) compared to women managed expectantly beyond that time. The rate of cesarean delivery did not differ between nulliparous women undergoing induction of labor during 390/7 −393/7 (39.1% versus 41.6%, p=0.257) and 410/7 – 413/7 weeks (47.5% versus 43.8%, p=0.394) compared to women managed expectantly beyond that time. Among parous women, the rate of cesarean delivery was lower with elective induction of labor during 390/7 −393/7 (5.5% versus 10.1%, p<0.001) and 400/7 −403/7 weeks (9.6% versus 11.9%, p=0.039) and similar with induction during 410/7 – 413/7 weeks (11.1% versus 16.3%, p=0.063) compared to expectant management. In addition, nulliparous women undergoing elective induction of labor during the 40th week had higher 3rd and 4th degree perineal lacerations, and parous women undergoing elective induction during the 39th week had higher operative vaginal deliveries and blood transfusions (Tables 3 and 4). When evaluating neonatal outcomes, elective induction of labor during the 39th and 40th weeks had reduced occurrences of macrosomia both in nulliparous and parous women. Additional findings were reduction in neonatal intensive care unit admissions with induction of labor during the 40th week in nulliparous women and reduction in Apgar scores <7 at 5 minutes with elective induction during the 41st week in parous women. Antepartum stillbirths and neonatal deaths did not differ between the groups for any gestational age comparison.
Table 3.
Maternal and neonatal outcomes of nulliparous obese women undergoing elective induction of labor compared to expectant management
| eIOL | EM | eIOL | EM | eIOL | EM | |
|---|---|---|---|---|---|---|
| Pregnancy outcomes: | 390/7 -393/7 | >393/7 | 400/7 -403/7 | >403/7 | 410/7 -413/7 | >413/7 |
| Nulliparous women | (n=573) | (n=3,139) | (n=1,343) | (n=1,334) | (n=695) | (n=160) |
| Maternal outcomes | ||||||
| Cesarean delivery | 224 (39.1) | 1,329 (41.6) | 532 (39.6)* | 603 (45.2) | 330 (47.5) | 70 (43.8) |
| Operative vaginal delivery | 39 (6.8) | 256 (8.0) | 117 (8.7) | 96 (7.2) | 49 (7.1) | 9 (5.6) |
| 3rd/4th degree laceration | 16 (2.8) | 89 (2.8) | 51 (3.8)* | 22 (1.7) | 13 (1.9) | 4 (2.5) |
| Postpartum Hemorrhage | 18/416 (4.3) | 101/2,379 (4.3) | 40/944 (4.2) | 51/1,063 (4.8) | 21/530 (4.0) | 7/141 (5.0) |
| Endometritis† | 5/228 (2.2) | 25/1,351 (1.9) | 7/528 (1.3) | 13/597 (2.2) | 7/312 (2.2) | 2/69 (2.9) |
| Wound complications (infection/separation)† | 4/332 (1.2) | 32/1,848 (1.7) | 6/848 (0.7) | 18/3,629 (0.5) | 9/1,043 (0.9) | 0/218 (0.0) |
| Blood transfusion† | 14/369 (3.8) | 76/1,807 (4.2) | 32/739 (4.3) | 28/692 (4.1) | 12/323 (3.7) | 2/62 (3.2) |
| Hysterectomy | 0 (0.0) | 2 (0.1) | 1 (0.1) | 1 (0.1) | 0 (0.0) | 1 (0.6) |
| Pulmonary embolism | 0 (0.0) | 3 (0.1) | 1 (0.1) | 1 (0.1) | 1 (0.2) | 0 (0.0) |
| ICU admission† | 0/508 (0.0) | 4/2,578 (0.2) | 2 (0.2) | 2 (0.2) | 1 (0.2) | 0 (0.0) |
| Maternal death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neonatal outcomes | ||||||
| Birth weight | 3,385 ± 436* | 3,535 ± 448 | 3,514 ± 436* | 3,602 ± 449 | 3,616 ± 449 | 3,662 ± 478 |
| Shoulder dystocia | 11 (2.0) | 52 (1.8) | 17 (1.3) | 22 (1.9) | 8 (1.3) | 2 (1.7) |
| SGA | 64 (11.2) | 341 (10.7) | 150 (11.2) | 133 (10.0) | 75 (10.8) | 15 (9.4) |
| LGA | 59 (10.3) | 370 (11.6) | 143 (10.7) | 170 (12.8) | 83 (12.0) | 24 (15.0) |
| Macrosomia | 46 (8.0)* | 456 (14.3) | 162 (12.1)* | 247 (18.5) | 138 (19.9) | 36 (22.5) |
| Apgar<7 at 5 min | 3 (0.5) | 45 (1.4) | 13 (1.0) | 23 (1.7) | 10 (1.4) | 2 (1.3) |
| NICU admission | 43 (7.5) | 318 (10.0) | 115 (8.6)* | 148 (11.1) | 73 (10.5) | 20 (12.5) |
| HIE | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antepartum/intrapartum death | 2 (0.4) | 7 (0.2) | 5 (0.4) | 2 (0.2) | 0 (0.0) | 0 (0.0) |
| Neonatal death | 0 (0.0) | 1 (0.03) | 0 (0.0) | 1.(0.1) | 1 (0.3) | 0 (0.0) |
All data presented as mean standard deviation or N (%)
= p<0.05 for comparison of elective labor induction versus expectant management at the given gestational age
= The denominator excludes deliveries with missing data for this variable
eIOL, elective induction of labor; EM, expectant management; BMI, body mass index
SGA, small-for-gestational-age, LGA, large-for-gestational-age, NICU, neonatal intensive care unit, HIE, hypoxic ischemic encephalopathy, ICU, intensive care unit.
Table 4.
Maternal and neonatal outcomes of parous obese women undergoing elective induction of labor compared to expectant management
| Pregnancy outcomes: | eIOL 390/7 −393/7 |
EM >393/7 |
eIOL 400/7 −403/7 |
EM >403/7 |
eIOL 410/7 −413/7 |
EM >413/7 |
|---|---|---|---|---|---|---|
| Parous women | (n=1,615) | (n=4,048) | (n=1,849) | (n=1,388) | (n=723) | (n=166) |
| Maternal outcomes | ||||||
| Cesarean delivery | 88 (5.5)* | 410 (10.1) | 178 (9.6)* | 165 (11.9) | 80 (11.1) | 27 (16.3) |
| Operative vaginal delivery | 75 (4.6)* | 123 (3.0) | 57 (3.1) | 34 (2.5) | 14 (1.9) | 7 (4.2) |
| 3rd/4th degree laceration | 9 (0.6) | 20 (0.5) | 9 (0.5) | 8 (0.6) | 2 (0.3) | 1 (0.6) |
| Postpartum Hemorrhage† | 33/963 (3.4) | 84/3,118 (2.7) | 31/1,341 (2.3) | 37/1,144 (3.2) | 14/581 (2.4) | 3/150 (2.0) |
| Endometritis† | 0/370 (0.0) | 8/1,779 (0.5) | 4/738 (0.5) | 3/675 (0.4) | 0/342 (0.0)* | 1/71 (1.4) |
| Wound complications (infection/separation)† | 0/1,194 (0.0) | 8/2,246(0.4) | 5/959 (0.5) | 2/733 (0.3) | 2/353 (0.6) | 0/67 (0.0) |
| Blood transfusion† | 76/1,274 (6.0)* | 72/2,017 (3.6) | 29/921 (3.2) | 17/586 (2.9) | 3/261 (1.2) | 0 (0.0) |
| Hysterectomy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pulmonary embolism | 0 (0.0) | 2 (0.1) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ICU admission† | 3/1,510 (0.5) | 10/3,261 (0.3) | 4 (0.3) | 4 (0.4) | 2 (0.4) | 1 (0.6) |
| Maternal death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neonatal outcomes | ||||||
| Birthweight | 3,497 ± 423* | 3,589 ± 454 | 3,577 ± 444* | 3,657 ± 462 | 3,679 ± 465 | 3,717 ± 489 |
| Shoulder dystocia | 39 (2.5) | 88 (2.4) | 40 (2.3) | 28 (2.3) | 10 (1.6) | 1 (0.7) |
| SGA | 107 (6.6) | 323 (7.8) | 157 (8.5) | 118 (8.5) | 63 (8.7) | 14 (8.4) |
| LGA | 236 (14.7) | 609 (15.1) | 264 (14.3) | 221 (16.0) | 112 ( 15.5) | 25 (15.1) |
| Macrosomia | 187 (11.6)* | 714 (17.6) | 304 (16.4)* | 308 (22.2) | 175 (24.2) | 47 (28.3) |
| Apgar<7 at 5 min | 8 (0.5) | 30 (0.7) | 14 (0.8) | 13 (1.0) | 6 (0.8)* | 5 (3.0) |
| NICU admission | 89 (5.5) | 215 (5.3) | 97 (5.3) | 75 (5.4) | 36 (5.0) | 11 (6.6) |
| HIE | 0 (0.0) | 1 (0.02) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Antepartum/intrapartum death | 5 (0.3) | 7 (0.2) | 4 (0.2) | 1 (0.1) | 0 (0.0) | 0 (0.0) |
| Neonatal death | 0 (0.0) | 1 (0.03) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
= p<0.05 for comparison of elective labor induction versus expectant management at the given gestational age
= The denominator excludes deliveries with missing data for this variable
eIOL, elective induction of labor; EM, expectant management; BMI, body mass index
SGA, small-for-gestational-age, LGA, large-for-gestational-age, NICU, neonatal intensive care unit, HIE, hypoxic ischemic encephalopathy, ICU, intensive care unit.
In multivariable analysis, after controlling for potential confounders, including maternal age, race-ethnicity, marital status, pre-pregnancy BMI class, simplified Bishop score ≤4 and smoking, elective induction of labor at 390/7 - 393/7 weeks in nulliparous and parous women was found to be associated with a significantly lower odds of cesarean delivery compared to expectant management (OR 0.47, 95% CI 0.30–0.74 for nulliparous women; OR 0.34, 95% CI 0.20–0.61 for parous women) (Table 5). It was also found to be associated with lower rates of postpartum hemorrhage (OR 0.23, 95% CI 0.05 – 0.96). Although induction of labor at later gestational ages also was associated with lower odds of cesarean delivery, this difference did not reach statistical significance. In addition, induction of labor in nulliparous women at 400/7 - 403/7 weeks was associated with higher rates of 3rd and 4th degree lacerations (OR 2.9, 95% CI 1.14 – 7.42). Finally, elective induction of labor in multiparous women at 410/7 - 413/7 weeks was associated with lower rates of postpartum hemorrhage (OR 0.11, 95% CI 0.01 – 0.92). Regarding neonatal outcomes, elective induction of labor in nulliparous women during 390/7 - 393/7 weeks was associated with higher risk of shoulder dystocia (OR 3.86, 95% CI 1.24 – 11.97). In addition, elective induction of labor at 400/7 - 403/7 weeks remained independently associated with lower odds of macrosomia and elective induction of labor in parous women remained to be associated with lower rates of macrosomia during both 390/7 - 393/7 and 400/7 - 403/7 weeks and LGA during 390/7 - 393/7 weeks , compared to expectant management beyond that time (Table 5). Overall, elective induction led to a reduction in birth weight in nulliparous women induced at 400/7 - 403/7 weeks and in multiparous women induced at 390/7 - 393/7 and 400/7 - 403/7 weeks (Table 5). Results did not differ for the analyses that used different inclusion criteria to construct the expectant management group by either changing the gestational age window for the expectant management group to start at 390/7 instead of 393/7 or by excluding elective primary cesarean delivery from the expectant management group (data not shown).
Table 5.
Results of multivariable logistic regression for association of elective induction of labor with pregnancy outcomes compared to expectant management in nulliparous and parous women
| Nulliparous women | Parous women | |||
|---|---|---|---|---|
| Gestational age at eIOL, weeks | OR with 95% CI | aOR *with 95% CI | OR with 95% CI | aOR *with 95% CI |
| Maternal outcomes | ||||
| Cesarean delivery | ||||
| 390/7 - 393/7 | 0.90 (0.75 – 1.08) | 0.47 (0.30 – 0.74) | 0.51 (0.40 – 0.65) | 0.34 (0.20 – 0.61) |
| 400/7 - 403/7 | 0.79 (0.68 – 0.93) | 0.84 (0.61 – 1.16) | 0.79 (0.63 – 0.98) | 0.78 (0.42 – 1.46) |
| 410/7 - 413/7 | 1.16 (0.82 – 1.64) | 0.58 (0.15 – 2.21) | 0.64 (0.40 – 1.03) | 0.46 (0.06 – 3.20) |
| Operative vaginal delivery† | ||||
| 390/7 - 393/7 | 0.84 (0.59 – 1.18) | 0.83 (0.50 – 1.40) | 1.55 (1.16 – 2.08) | 1.29 (0.87 – 1.93) |
| 400/7 - 403/7 | 1.23 (0.93 – 1.63) | 1.08 (0.70 – 1.66) | 1.27 (0.82 – 1.95) | 0.78 (0.38 – 1.58) |
| 410/7 - 413/7 | 1.27 (0.61 – 2.65) | - | 0.45 (0.18 – 1.13) | - |
| Postpartum hemorrhage | ||||
| 390/7 - 393/7 | 1.52 (1.17 – 1.97) | 0.23 (0.05 – 0.96) | 1.22 (1.0 – 1.49) | 1.02 (0.55 – 1.87) |
| 400/7 - 403/7 | 0.96 (0.76 – 1.22) | 0.84 (0.44 – 1.61) | 1.19 (0.97 – 1.48) | 0.62 (0.25 – 1.53) |
| 410/7 - 413/7 | 0.94 (0.58 – 1.52) | 2.39 (0.24 – 24.08) | 0.98 (0.63 – 1.55) | 0.11 (0.01 – 0.92) |
| 3rd or 4th degree laceration† | ||||
| 390/7 - 393/7 | 1.00 (0.58 – 1.72) | 0.80 (0.31 – 2.11) | 1.13 (0.51 – 2.48) | 1.32 (0.31 – 5.65) |
| 400/7 - 403/7 | 2.35 (1.42 – 3.90) | 2.9 (1.14 – 7.42) | 0.84 (0.32 – 2.19) | - |
| 410/7 - 413/7 | 0.74 (0.24 – 2.31) | 0.73 (0.04 – 14.75) | 0.46 (0.04 – 5.08) | - |
| Endometritis | ||||
| 390/7 - 393/7 | 1.19 (0.45 – 3.14) | - | - | - |
| 400/7 - 403/7 | 0.60 (0.24 – 1.52) | - | 1.22 (0.27 – 5.47) | - |
| 410/7 - 413/7 | 0.77 (0.16 – 3.78) | - | - | - |
| Wound complications (infection/separation) | ||||
| 390/7 - 393/7 | 0.69 (0.24 – 1.97) | 0.89 (0.26 – 3.08) | - | - |
| 400/7 - 403/7 | 0.98 (0.45 – 2.14) | 0.63 (0.24 – 1.64) | 1.92 (0.37 – 9.90) | 0.71 (0.04 – 14.32) |
| 410/7 - 413/7 | - | - | - | - |
| Blood transfusion | ||||
| 390/7 - 393/7 | 0.89 (0.50 – 1.60) | 1.04 (0.54 – 2.02) | 1.71 (1.23 – 2.38) | 0.98 (0.68 – 1.42) |
| 400/7 - 403/7 | 1.07 (0.64 – 1.80) | 0.85 (0.46 – 1.58) | 1.09 (0.59 – 1.99) | 0.65 (0.32 – 1.31) |
| 410/7 - 413/7 | 1.16 (0.25 – 5.30) | 0.39 (0.03 – 4.52) | - | - |
| Hysterectomy | ||||
| 390/7 - 393/7 | - | - | - | - |
| 400/7 - 403/7 | 0.99 (0.06 – 15.89) | - | - | - |
| 410/7 - 413/7 | - | - | - | - |
| Pulmonary embolism | ||||
| 390/7 - 393/7 | - | - | - | - |
| 400/7 - 403/7 | 0.99 (0.06 – 15.89) | - | - | - |
| 410/7 - 413/7 | - | - | - | - |
| ICU admission | ||||
| 390/7 - 393/7 | - | - | 0.65 (0.18 – 2.35) | 1.16 (0.10 – 13.30) |
| 400/7 - 403/7 | 1.01 (0.14 – 7.20) | 0.31 (0.02 – 3.89) | 0.75 (0.18 – 3.00) | 0.60 (0.03 – 10.72) |
| 410/7 - 413/7 | - | - | 0.49 (0.04 – 5.44) | - |
| Maternal death | ||||
| 390/7 - 393/7 | - | - | - | - |
| 400/7 - 403/7 | - | - | - | - |
| 410/7 - 413/7 | - | - | - | - |
| Neonatal outcomes | ||||
| Birth weight | ||||
| 390/7 - 393/7 | −149.30 (−189.07 −109.54) | −116.00 (−193.79 – 38.22) | −91.85 (−117.62 - −66.08) | −141.05 (−180.81 - −101.29) |
| 400/7 - 403/7 | −87.82 (−121.54 - −54.08) | −154. 76 (−218.12 - −91.39) | −80.17 (−111.76 - −48.58) | −145.12 (−209.67 - −80.57) |
| 410/7 - 413/7 | −45.19 (−123.75 – 33.36) | −38.20 (−284.23 – 207.82) | −38.79 (−118.39 – 40.80) | - |
| Shoulder dystocia | ||||
| 390/7 - 393/7 | 1.11 (0.58 – 2.15) | 3.86 (1.24 – 11.97) | 1.04 (0.71 – 1.52) | 0.69 (0.38 – 1.28) |
| 400/7 - 403/7 | 0.72 (0.38 – 1.36) | 0.98 (0.23 – 4.24) | 1.01 (0.62 – 1.65) | 1.24 (0.46 – 3.34) |
| 410/7 - 413/7 | 0.76 (0.16 – 3.60) | - | 2.19 (0.28 – 17.31) | - |
| SGA | ||||
| 390/7 - 393/7 | 1.05 (0.79 – 1.39) | 1.12 (0.64 – 1.99) | 0.82 (0.65 – 1.03) | 1.17 (0.78 – 1.77) |
| 400/7 - 403/7 | 1.13 (0.88 – 1.45) | 1.40 (0.85 – 2.32) | 0.99 (0.78 – 1.28) | 1.61 (0.81 – 3.19) |
| 410/7 - 413/7 | 1.17 (0.65 – 2.10) | - | 1.04 (0.56 – 1.89) | 1.11 (0.08 – 15.24) |
| LGA | ||||
| 390/7 - 393/7 | 0.87 (0.65 – 1.17) | 1.46 (0.87 – 2.43) | 0.97 (0.82 – 1.14) | 0.93 (0.56 – 0.96) |
| 400/7 - 403/7 | 0.81 (0.64 – 1.03) | 0.71 (0.43 – 1.15) | 0.88 (0.72 – 1.07) | 0.76 (0.51 – 1.15) |
| 410/7 - 413/7 | 0.77 (0.47 – 1.26) | 1.41 (0.16 – 12.76) | 1.03 (0.64 – 1.65) | 0.46 (0.14 – 1.59) |
| Macrosomia | ||||
| 390/7 - 393/7 | 0.52 (0.38 – 0.72) | 0.97 (0.56 – 1.66) | 0.61 (0.51 – 0.73) | 0.50 (0.38 – 0.66) |
| 400/7 - 403/7 | 0.60 (0.48 – 0.75) | 0.56 (0.35 – 0.87) | 0.69 (0.58 – 0.82) | 0.53 (0.36 – 0.78) |
| 410/7 - 413/7 | 0.85 (0.56 – 1.29) | 0.49 (0.11 – 2.21) | 0.81 (0.55 – 1.18) | 0.47 (0.15 – 1.47) |
| Apgar<7 at 5 min | ||||
| 390/7 - 393/7 | 0.37 (0.11 – 1.19) | 1.54 (0.17 – 14.43) | 0.67 (0.31 – 1.46) | 1.43 (0.26 – 7.67) |
| 400/7 - 403/7 | 0.55 (0.28 – 1.10) | 3.73 (0.24 – 56.86) | 0.81 (0.38 – 1.73) | 0.29 (0.03 – 3.53) |
| 410/7 - 413/7 | 1.16 (0.25 – 5.33) | - | 0.27 (0.08 – 0.89) | - |
| NICU admission | ||||
| 390/7 - 393/7 | 0.73 (0.53 – 1.02) | 0.81 (0.41 – 1.62) | 1.04 (0.81 – 1.34) | 0.99 (0.63 – 1.56) |
| 400/7 - 403/7 | 0.75 (0.58 – 0.97) | 0.74 (0.44 – 1.25) | 0.96 (0.71 – 1.32) | 1.06 (0.49 – 2.28) |
| 410/7 - 413/7 | 0.82 (0.48 – 1.39) | 0.81 (0.08 – 7.87) | 0.74 (0.37 – 1.48) | 0.62 (0.06 – 6.70) |
| HIE | ||||
| 390/7 - 393/7 | - | - | - | - |
| 400/7 - 403/7 | - | - | - | - |
| 410/7 - 413/7 | - | - | - | - |
| Antepartum/intrapartum death | ||||
| 390/7 - 393/7 | 1.59 (0.33 – 7.69) | - | 1.79 (0.57 – 5.66) | - |
| 400/7 - 403/7 | 2.49 (0.48 – 12.85) | - | 3.01 (0.33 – 26.93) | - |
| 410/7 - 413/7 | - | - | - | - |
| Neonatal death | ||||
| 390/7 - 393/7 | - | - | - | - |
| 400/7 - 403/7 | - | - | - | - |
| 410/7 - 413/7 | - | - | - | - |
= Adjusted for maternal age, race/ethnicity, marital status, BMI class, cigarette smoking, simplified Bishop score ≤4
Regression model did not converge due to small numbers for operative vaginal delivery variable among nulliparous and parous women during 41st week and for 3rd and 4th degree laceration variable among parous women during 40th and 41st weeks.
eIOL, elective induction of labor; OR, odds ratio; CI, 95% confidence interval
Discussion
In this analysis, we found decreased odds of cesarean delivery among nulliparous and parous obese women undergoing elective induction of labor during the 39th week of gestation compared to expectant management. This finding did not persist when elective induction of labor was performed during the 40th or 41st weeks. There were higher 3rd and 4th degree lacerations and lower rates of postpartum hemorrhage with elective induction of labor at 400/7−3/7 weeks among nulliparous women. Among neonatal outcomes, the most significant benefit from elective induction of labor was a decrease in the occurrence of macrosomia and LGA..
Our findings are consistent with an analysis done by Lee et al15 of a large de-identified administrative California database. In that study, the authors examined obese women undergoing elective induction of labor starting at 37 weeks. They found that in nulliparous obese women, induction at 37 and 39 weeks was associated with a reduced risk of cesarean delivery, and in parous obese women, induction during each week starting at ≥ 37 weeks was associated with a reduced risk of cesarean delivery. That study used hospital discharge data from 2007 and did not have information about cervical exam on admission, or information regarding perinatal mortality. Another study by Wolfe et al14 retrospectively examined 60 nulliparous obese women with an unfavorable cervix undergoing elective induction of labor between 39 – 40.9 weeks and 410 obese women undergoing expectant management beyond 39 weeks. They found more cesarean deliveries in the elective induction of labor group. Their findings can be explained by the inclusion of nulliparous women with only unfavorable cervices, defined as simplified Bishop score <5 in the induction of labor group. The simplified Bishop score of women in the expectant management group was significantly higher and the differences were not adjusted for in the analysis of cesarean delivery outcomes.
We found that the association between elective induction of labor and cesarean delivery was stronger in multiparous compared to nulliparous women. Some observational studies suggest that cesarean deliveries in multiparous women having elective inductions is similar to that in women with a spontaneous onset of labor .23–26 Rates of primary cesarean delivery are known to be lower among multiparous women27 as most of them previously had a successful vaginal delivery (i.e. some had a previous cesarean delivery). In a retrospective study by Pickens et al, cesarean deliveries after elective induction of labor were also reduced more significantly among multiparous women16. This association deserves further investigation in future studies.
The most recent study that examined outcomes of elective induction of labor and used a control group of expectant management was limited to morbidly obese women with BMI of ≥ 40kg/m2, also from the Consortium on Safe Labor database.17 The analysis compared outcomes of elective induction of labor during early term (37–38 weeks) and full-term (39–40 weeks) to expectant management. They found similar occurrences of cesarean delivery in all morbidly obese women induced during the early term period and fewer cesarean deliveries among parous morbidly obese women who were induced at full-term, compared to expectant management. Similar to our study, many other maternal outcomes were too rare to detect significant difference between the groups in other studies.14–16 Notable exceptions include Lee et al who demonstrated reduced rate of postpartum hemorrhage among obese mulliparous women undergoing elective induction at 40 weeks that was similar to our finding of reduced postpartum hemorrhage in multiparous women with induction at 41 weeks and in nulliaprous women with induction at 39 weeks.15 Pickens et al as well demonstrated lower rates of postpartum hemorrhage among obese nulliparous and multiparous women undergoing elective induction at 39–40 weeks and higher rates of 3rd and 4th lacerations among obese multiparous women undergoing elective induction of labor at 40 weeks.16,28
Regarding neonatal outcomes, two of the studies described above had lower occurrences of macrosomia with elective induction of labor.15–16 We also found lower occurrences of macrosomia among nulliparous women induced during the 40th week, and among parous women induced during the 39th and 40th week. Given the known association between maternal obesity and abnormal fetal growth,29–30 it is plausible that an elective induction of labor can diminish these risks, however further study is indicated to support this hypothesis. Currently, suspected macrosomia is not an indication for induction of labor as it is unclear whether induction will lead to a reduction in a shoulder dystocia and brachial plexus injury among pregnancies complicated by macrosomia.31 Overall, our study demonstrated that elective induction of labor was associated with reduced birth weight for all women induced during the 40th week and for multiparous women induced at 39 weeks as well. This did not affect the risk for shoulder dystocia. In fact, the risk of shoulder dystocia was increased in nulliparous women undergoing elective induction at 39 weeks. This finding is not supported by studies published by Lee et al15 , Gibbs Pickens et al16 and Kawakita et al17 and requires investigation.
In recent years, there is a growing body of evidence that elective induction of labor is associated with decreased or at least similar odds of cesarean delivery and overall equivalent neonatal outcomes compared to expectant management.32–36 This has been shown in nulliparous women with and without a favorable cervix,32–33 in women of advanced maternal age34 and in women with a prior cesarean delivery.35 A recent multicenter randomized controlled trial reported that induction of labor in nulliparous low-risk women significantly reduced the risk of cesarean delivery (18.6% versus 22.2%, relative risk 0.84;95% CI 0.76 – 0.93).37 Prespecified subgroup analysis of that study according to maternal BMI did not alter these results.
Maternal obesity, regardless of its degree, is currently not a medical indication for induction of labor in otherwise healthy parturients. Nevertheless, maternal obesity increases the risk of stillbirth by 2.1–2.8 fold12 and the risk of neonatal death by 1.07–1.23 fold.10 Although there is no evidence showing an improvement in pregnancy outcomes with antepartum surveillance of obese women, some institutions, including our own, initiate weekly antepartum surveillance, at least in women with morbid obesity.38–39 Our analysis was not powered to show a decreased risk of perinatal death with elective induction. However, since it appears that elective induction of labor does not increase the risk of cesarean delivery or adverse neonatal outcomes, consideration may be given to pursuing induction of labor after the 39th week instead of initiating or continuing weekly antenatal testing.
Our study is not without limitations. First, although we attempted to control for the cervical exam between obese women undergoing elective induction of labor versus expectant management, the information on the simplified Bishop score was missing in a large number of women across all groups of gestational ages. It is possible that more women with a simplified Bishop score >4 underwent induction of labor. Moreover, only admission Bishop score was available for the analysis and we did not have the information regarding the Bishop score in the outpatient settings, at the time of the decision making regarding continuing expectant management or scheduling elective induction. Second, although this was a large cohort, the perinatal deaths were too small to assess whether induction of labor in obese women can reduce this devastating adverse birth outcome. In addition, we would like to acknowledge that the sample size in the 40th and 41st week groups was too small to assess most of maternal morbidity and some neonatal morbidity outcomeFinally, it is possible that with larger sample size during those weeks, the association between elective induction of labor and cesarean delivery would have reached statistical significance. Third, this was a retrospective analysis and it cannot conclude whether a policy of elective labor induction among obese women would result in the same outcomes. While we did perform adjustments for potential confounding factors, we cannot eliminate the potential concern for selection bias related to the managing physician rationale for the decision of induction of labor versus expectant management. The strengths of our study are that we included all classes of obesity. We also collected information on the indications for cesarean delivery, which were not reported in previous studies. This information is important when comparing rates of cesarean delivery in order to assess for information bias since providers may perform an elective cesarean delivery in obese women. We limited the gestational age of our cohort to ≥ 39 weeks, thus making the results more applicable to the contemporary obstetric recommendations and practices of elective labor induction. Lastly, we stratified the analysis by parity and chose the appropriate comparison group of expectant management and not spontaneous labor in a similar population.
In conclusion, we found that elective induction of labor during the 39th week in nulliparous and parous obese women was associated with decreased cesarean delivery. This association did not persist when elective induction of labor was conducted during the 40th and 41st weeks. We recommend incorporating our findings in the counseling and the decision-making process on timing of delivery in this obstetric population.
Acknowledgments
The data included in this paper were obtained from the Consortium on Safe Labor, which was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through Contract No. HHSN267200603425C. Institutions involved in the Consortium include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital , MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; The EMMES Corporation, Rockville MD (Data Coordinating Center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, Texas. The named authors alone are responsible for the views expressed in this manuscript, which does not necessarily represent the decisions or the stated policy of the NICHD.
Footnotes
Financial disclosures: None
The authors did not report any potential conflict of interest.
This study was an oral presentation at the 38th annual meeting of the Society for Maternal-Fetal Medicine, Dallas, Texas, Jan 31-Feb 3, 2018
References
- 1.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Preventive medicine. 2013;56:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prepregnancy Body Mass Index by Maternal Characteristics and State: Data From the Birth Certificate, 2014 [PubMed]
- 3.National health Statistics data- most recent web site acces on October 28th 2018, https://www.cdc.gov/nchs/data/hestat/obesity_adult_13_14/obesity_adult_13_14.htm [Google Scholar]
- 4.Bodnar LM, Catov JM, et al. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007; 18:234–239. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JL, Malone FD, Emig D, Ball RH, Nyberg DA, Comstock CH, et al. Obesity, obstetric complications and cesarean delivery rate--a population-based screening study. FASTER Research Consortium. Am J Obstet Gynecol. 2004;190:1091–7. [DOI] [PubMed] [Google Scholar]
- 6.Kominiarek MA, Vanveldhuisen P, Hibbard J, Landy H, Haberman S, Learman L, et al. The maternal body mass index: a strong association with delivery route. Am J Obstet Gynecol. 2010; 203: 264.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Dwyer V, O’Kelly S, Monaghan B, Rowan A, Farah N, Turner MJ. Maternal obesity and induction of labor. Acta Obstet Gynecol Scand. 2013; 92:1414–8. [DOI] [PubMed] [Google Scholar]
- 8.Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, et al. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007; 8:385–94. [DOI] [PubMed] [Google Scholar]
- 9.Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007; 197:223–8. [DOI] [PubMed] [Google Scholar]
- 10.Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis death: a systematic review and meta-analysis. JAMA. 2014; 311:1536–46. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009; 113:748–61. [DOI] [PubMed] [Google Scholar]
- 12.Yao R, Ananth CV, Park BY, Pereira L, Plante LA. Obesity and the risk of stillbirth: a population-based cohort study. Am J Obstet Gynecol. 2014; 210:457.e1–9. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe KB, Rossi RA, Warshak CR. The effect of maternal obesity on the rate of failed induction of labor. Am J Obstet Gynecol. 2011; 205:128.e1–7. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe H, Timofeev J, Tefera E, Desale S, Driggers RW. Risk of cesarean in obese nulliparous women with unfavorable cervix: elective induction vs expectant management at term. Am J Obstet Gynecol. 2014; 211:53.e1–5. [DOI] [PubMed] [Google Scholar]
- 15.Lee VR, Darney BG, Snowden JM, Main EK, Gilbert W, Chung J, et al. Term elective induction of labour and perinatal outcomes in obese women: retrospective cohort study. BJOG. 2016; 123:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbs Pickens CM, Kramer MR, Howards PP, Badell ML, Caughey AB, Hogue CJ. Term Elective Induction of Labor and Pregnancy Outcomes Among Obese Women and Their Offspring. Obstet Gynecol. 2018;131:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakita T, Iqbal SN, Huang CC, Reddy UM. Nonmedically indicated induction in morbidly obese women is not associated with an increased risk of cesarean delivery. Am J Obstet Gynecol. 2017;217:451.e1–451.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Dwyer V, O’Kelly S, Monaghan B, Rowan A, Farah N, Turner MJ. Maternal obesity and induction of labor. Acta Obstet Gynecol Scand. 2013; 92:1414–8. [DOI] [PubMed] [Google Scholar]
- 19.Robinson CJ, Hill EG, Alanis MC, Chang EY, Johnson DD, Almeida JS. Examining the effect of maternal obesity on outcome of labor induction in patients with preeclampsia. Hypertens Pregnancy. 2010; 29:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arrowsmith S, Wray S, Quenby S. Maternal obesity and labour complications following induction of labour in prolonged pregnancy. BJOG. 2011; 118:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010; 203:326.e1–326.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laughon SK, Zhang J, Troendle J, Sun L, Reddy UM. Using a simplified Bishop score to predict vaginal delivery. Obstet Gynecol 2011; 117: 805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dublin S, Lydon-Rochelle M, Kaplan RC, Watts DH, Critchlow CW. Maternal and neonatal outcomes after induction of labor without an identified indication. Am J Obstet Gynecol. 2000; 183: 986–994. [DOI] [PubMed] [Google Scholar]
- 24.Macer JA, Macer CL, Chan LC. Elective induction versus spontaneous labor: a retrospective study of complications and outcome. Am J Obstet Gynecol. 1992; 166: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 25.Smith LP, Nagourney BA, McLean FH, Usher RH. Hazards and benefits of elective induction of labor. Am J Obstet Gynecol. 1984;148: 579–585. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson M, Caughey AB, Stenson MH, et al. The active management of risk in multiparous pregnancy at term: association between a higher preventive labor induction rate and improved birth outcomes. Am J Obstet Gynecol .2009; 200: 250.e1–250.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbs Pickens CM, Kramer MR, Badell ML, Caughey AB, Hogue CJ. In Reply. Obstet Gynecol. 2018. June;131(6):1162. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2012. Natl Vital Stat Rep. 2013; 62:1–20. [PubMed] [Google Scholar]
- 29.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195: 1100–3. [DOI] [PubMed] [Google Scholar]
- 30.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Practice Bulletin No. 173: Fetal Macrosomia. Obstet Gynecol. 2016;128:e195–e209. [DOI] [PubMed] [Google Scholar]
- 31.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198:416.e1–6. [DOI] [PubMed] [Google Scholar]
- 32.Osmundson SS, Ou-Yang RJ, Grobman WA. Elective induction compared with expectant management in nulliparous women with a favorable cervix. Obstet Gynecol. 2010;116: 601–5. [DOI] [PubMed] [Google Scholar]
- 33.Osmundson SS, Ou-Yang RJ, Grobman WA. Elective induction compared with expectant management in nulliparous women with an unfavorable cervix. Obstet Gynecol. 2011; 117:583–7. [DOI] [PubMed] [Google Scholar]
- 34.Walker KF, Bugg GJ, Macpherson M, McCormick C, Grace N, Wildsmith C. Randomized Trial of Labor Induction in Women 35 Years of Age or Older. N Engl J Med. 2016;374:813–22 [DOI] [PubMed] [Google Scholar]
- 35.Palatnik A, Grobman WA. Induction of labor versus expectant management for women with a prior cesarean delivery. 1. Am J Obstet Gynecol. 2015;212:358.e1–6 [DOI] [PubMed] [Google Scholar]
- 36.Stock S, Ferguson E, Duffy A, Ford I, Chalmers J, Norman JE. Outcomes of elective induction of labour compared with expectant management: population based study. BMJ 2012; 344:e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grobman WA, Rice MM, Reddy UM, Tita ATN, Silver RM, Mallett G, et al. Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. N Engl J Med. 2018;379:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edelson PK, Bastek JA, Levine LD. Evaluating the Obstetrical Implications of Antenatal Testing for Women with Morbid Obesity: Maternal and Fetal Outcomes of Increased Surveillance. Am J Perinatol. 2016; 33:839–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown S, Wolfe MD, Coalson R, Myers OB, Rayburn WF. Maternal obesity and nonstress testing. Am J Perinatol. 2011; 28:723–8. [DOI] [PubMed] [Google Scholar]


