Abstract
Glycan-mediated interactions are essential in many biological processes and regulate a wide variety of cellular functions. However, characterizing these interactions is difficult because glycan biosynthesis is not template-driven and because carbohydrate recognition events are usually low-affinity and transient. Photocrosslinking carbohydrate probes can form a covalent bond with molecules in close proximity upon UV irradiation, and are capable of capturing interactions between glycans and glycan-binding proteins in situ. Because of these advantages, multiple photocrosslinking carbohydrate probes have been designed and applied to study the biological functions of glycans. This review will discuss recent advances in the development of novel photocrosslinking functional groups and the design of photocrosslinking probes to detect interactions mediated by glycolipids, peptidoglycan, and multivalent carbohydrate ligands. These probes have demonstrated the potential to address some of the major challenges in the study of glycan-mediated interactions in both model systems and in more complex biological settings.
Graphical Abstract.
Schematic representation of photocrosslinking probes and their applications in the study of glycan-mediated interactions.
Introduction
Glycosylation is a common posttranslational modification (PTM) that is characterized by the addition and extension of carbohydrates on proteins and lipids [1]. Glycosylation is essential in many biological processes, including regulation of protein folding and trafficking, protein-ligand interactions, signal transduction, cell-cell interactions and cell-matrix interactions [1,2]. Deficiencies in glycosylation pathways can lead to more than a hundred human diseases [3], and dysregulation of glycosylation has been associated with multiple human diseases, including diabetes, neurodegenerative diseases, and cancers [4–6]. Glycans are also involved in numerous host-pathogen interactions, such as the adhesion of pathogens to host cells and recognition of host receptors by some bacterial toxins [1,2].
Many glycan functions are achieved through direct interactions between glycans and glycan-binding proteins (GBPs) [1,2]. However, characterization of these interactions is challenging. Unlike proteins and nucleic acids, the “non-template” nature of glycan biosynthesis makes it almost impossible to predict the glycan structures by simply examining gene expression [1]. Glycosylation is also a highly dynamic and context-dependent process, varying in different species and tissues and in response to various environmental stimuli [1]. In addition, glycan-mediated interactions are usually low-affinity [7], which makes them difficult to study using traditional methods for analyzing protein-protein interactions (PPIs) like co-immunoprecipitation and affinity chromatography. Adding to this complexity is the compartment-specificity of some carbohydrate-dependent interactions, as interactions may be restricted by the localization of some glycans and GBPs in specific organelles or microdomains [7,8].
To address these challenges, photoactivable crosslinkers (photocrosslinkers) have been applied in characterizing glycan-protein interactions [9,10]. Photocrosslinkers can be activated upon UV irradiation and form a covalent bond to nearby molecules, making them capable of capturing low-affinity and transient interactions covalently [11]. This allows for harsh washing during purification processes, reducing co-elution of nonspecific proteins. Furthermore, some photocrosslinking probes can be used in live cells and capture the interactions in situ, leading to the identification of more physiologically relevant interactions [8]. Therefore, these photocrosslinkers have been used to study the interactions of proteins with a wide variety of ligands, including small molecules, lipids, glycans and proteins [9–12].
Here, we will review recent advances in the development of novel photocrosslinking groups, the design of photocrosslinking carbohydrate probes, and the application of these probes in the study of glycan-protein interactions.
Novel photocrosslinking functional groups for carbohydrates
The most commonly used photocrosslinkers are benzophenone (BP), diazirine (DAz) and aryl azide (AAz) (Figure 1a). All three photocrosslinkers can be activated by UV irradiation to generate highly reactive intermediates, which form covalent bonds to biomolecules in close proximity [11]. Although BP, AAz and DAz have been widely used to study the interactions of proteins with various ligands, limitations in the application of these photocrosslinkers still exist. For example, although these photocrosslinkers are generally considered to be non-selective, they still demonstrate some extent of preference towards different amino acids [13–15]. Other concerns include the non-specific binding through hydrophobic interactions and the possibility of the photocrosslinking groups to sterically interfere with protein binding, especially when photocrosslinkers with bulky aryl groups (BP, AAz and aryl DAz) are used [16]. Furthermore, AAz and DAz can only be irreversibly activated by UV irradiation and may undergo photolysis and rearrangement after UV irradiation, leading to the reduced efficiency of photoaffinity labeling (PAL) [11,16–18]. Therefore, novel photocrosslinkers with improved properties and different mechanisms of crosslinking have the potential to enhance study of protein-ligand interactions.
Figure 1. Structures of biocompatible photocrosslinkers.
(a) Structures of benzophenone (BP), diazirine (DAz), aryl azide (AAz) and the formation of their corresponding intermediates after UV irradiation. (b) Structure of thienyl-substituted α-ketoamide and the formation of intermediate after UV irradiation. (c) Structures of tetrazole-based photocrosslinkers.
Recently, thienyl-substituted α-ketoamide (Figure 1b) has been employed for PAL for the first time. Despite their known photoactivity, α-ketoamides have not been used for PAL because of their instability under physiological conditions [17]. By screening for optimal photochemical properties of α-ketoamide with different α-substituents, Sodeoka and coworkers successfully identified thienyl-substituted α-ketoamide as a photocrosslinker that is potentially useful in biological settings [17]. Thienyl-substituted α-ketoamide displays reasonable stability in the aqueous environment and a suitable half-life under UV irradiation for PAL. To demonstrate the utility of the photocrosslinker, the authors tested its ability to crosslink a carbohydrate to a lectin (a GBP that binds to specific monosaccharides or glycan structures). Indeed, mannose-conjugated thienyl-substituted α-ketoamide was crosslinked successfully to ConA, a mannose-binding lectin. Crosslinking was inhibited by α-D-methyl mannose, confirming its specificity. In the presence of nontarget proteins or HeLa cell lysates, the thienyl-substituted α-ketoamide probe showed higher specificity and efficiency compared to BP, AAz and aryl DAz probes, possibly because it is less hydrophobic. Although more detailed studies on the photochemical properties of thienyl-substituted α-ketoamide are needed before further applications, current data suggest that thienyl-substituted α-ketoamide may be an alternative for BP and AAz, especially when nonspecific crosslinking is a concern.
Another category of novel photocrosslinking groups are based on tetrazole. In addition to its established application in photoclick chemistry [19], tetrazoles (Figure 1c) were recently reported to possess photocrosslinking activity [20,21]. These tetrazole-based photocrosslinkers have been applied to crosslink small molecules [20–22] and proteins to their binding partners [23,24]. Tetrazole-based photocrosslinkers can be crosslinked to biological nucleophiles and can be optimized for additional functions. For example, Yao and coworkers designed diaryl tetrazole photocrosslinkers that include fluorophores. These photocrosslinkers demonstrate increased fluorescence after being crosslinked to proteins, which therefore enables no-wash imaging [21]. Although tetrazole-based photocrosslinking groups have not yet been used to crosslink carbohydrates to proteins, they have potential applications in glycobiology such as cellular imaging of glycan-protein interactions.
Photoaffinity probes for glycolipids
Glycolipids are composed of a glycan head and lipid tails. As with glycoproteins, a variety of glycan structures can be identified on glycolipids, ranging from a single monosaccharide to more complex glycans such as the blood group antigens [1]. Glycolipids are also involved in multiple biological processes, such as regulation of signal transduction, intercellular interactions, and pathogen invasion [1]. However, study of glycolipids is challenging because of their small size and amphiphilic nature. Moreover, the glycan heads and the lipid tails of the glycolipids both vary among different cell types [1,25], making it critical to capture glycolipid-mediated interactions in situ. Therefore, multi-functional glycolipid probes have been designed to characterize these interactions. These probes can be functionalized on the glycan and/or the lipid part [25,26]. Strategies for the synthesis of glycolipid probes have been reviewed recently [25]. In this review we will focus only on the design and applications of photocrosslinking glycolipid probes in recent years (Figure 2a).
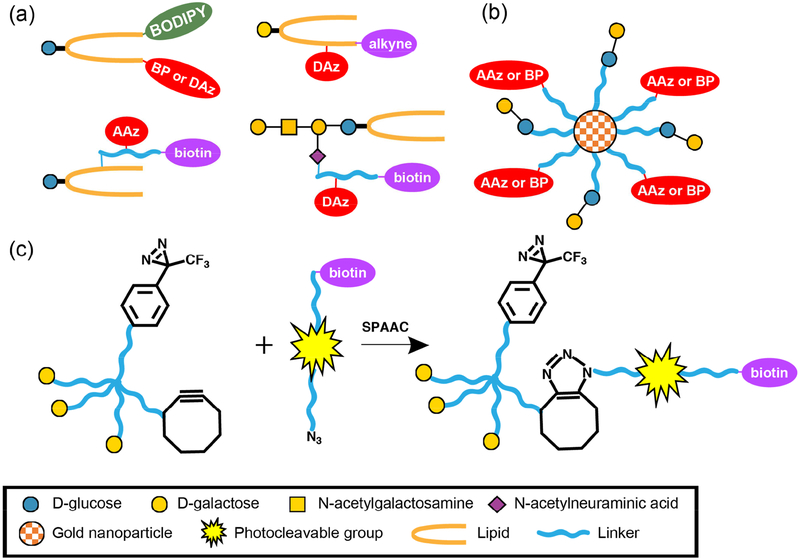
Figure 2. Design of recent glycolipid photocrosslinking probes and multivalent carbohydrate photocrosslinkers.
(a) Design of representative glycolipid photocrosslinking probes from recent studies. Photocrosslinking groups, purification tags, and fluorophores can be incorporated into a single probe on lipid tails and glycan heads. (b) Design of multivalent carbohydrate photocrosslinkers based on gold nanoparticles (AuNPs). (c) Design of a multivalent carbohydrate photocrosslinker and a photocleavable biotin purification tag. The multivalent photocrosslinker can be modified covalently with the photocleavable biotin purification tag through strain-promoted azide-alkyne cycloaddition (SPAAC).
The design of the photocrosslinking glycolipid probes usually includes both a photocrosslinking group, as well as an additional functional group(s) that allows for tracking of the probes. For example, Mizuno and coworkers designed two photocrosslinking glycolipid probes with DAz or BP for photocrosslinking and a fluorescent BODIPY group for imaging, each located at the end of a lipid chain [27]. Functional groups that enable isolation of the crosslinked complexes have also been used in photocrosslinking glycolipid probes because they facilitate identification of the crosslinked GBPs [28–30]. Strategies that have been used to synthesize and study other non-glycosylated lipids may also be applied for glycolipids [25,31]. For example, Schultz and coworkers designed several trifunctional lipid probes that contain a photocrosslinking group, a caging group, and a bio-orthogonal alkyne group [32]. The addition of a photocleavable caging group allows for rapid release of the bioactive lipids by a pulse of light, which reduces undesired metabolism of lipids before the crosslinking and increases the accuracy in studies of lipid-protein interactions. On the other hand, crosslinking of the trifunctional lipid probes to proteins prevents further diffusion or loss of the probes during the fixation and washing, therefore benefits microscopic studies on localization and transport of the lipids. Furthermore, the rapid uncaging processes enable time-dependent studies [31,32]. Because of these advantages, trifunctional glycolipid probes may be designed and become useful in glycobiology.
Bifunctional photocrosslinking glycolipid probes have been applied successfully to solve biological questions. For example, Lei and coworkers designed a bifunctional glycolipid probe that mimics the dilinolenoyl fatty acid ester of monogalactosyldiacylglycerol (dilinolenoyl MGDG) [30]. Dilinolenoyl MGDG is a plant glycolipid that demonstrates anti-inflammatory activity in human peripheral blood neutrophils through unidentified mechanisms [30]. A fatty acid with a DAz and an alkyne group was used to replace one of the fatty acid chains of dilinolenoyl MGDG. The glycolipid probe was then crosslinked to human chondrocytes, and the alkyne group was modified with a biotin-azide using the copper (I) catalyzed azide-alkyne cycloaddition (CuAAC). The proteins crosslinked to the probes were isolated and analyzed by proteomics, leading to the identification of toll-like receptor 4 (TLR4) and another protein as candidate receptors. From the two candidates, TLR4 was further confirmed as a functional receptor for dilinolenoy MGDG using a variety of biochemical methods. This study provides an example of how photocrosslinking glycolipid probes can be used with complementary methods to identify and validate the binding partners of glycolipids from complex biological systems.
In addition to applications in receptor identification, photocrosslinking glycolipids have also been used to stabilize noncovalent interactions mediated by glycolipids. Porcelli and coworkers used analogs of α-galactosylceramide (α-GC) with a BP group on a fatty acid chain with various lengths to crosslink α-GC analogs to CD1d, a protein that presents lipid antigens to invariant natural killer T cells (iNKT cells) [33]. These analogs can be crosslinked to both purified recombinant CD1d and CD1d expressed on antigen-presenting cells, leading to the formation of stable covalent conjugates. Although lower activity was observed for α-GC analogs with shorter lipid chains bound non-covalently to CD1d, crosslinking improved their potency and extended the duration of activity in vivo. Therefore, photocrosslinking α-GC analogs with shorter lipid chains may be useful in immunotherapies, because they are expected to retain activity in stimulating iNKT cells while reducing the side effects due to dissociation of the α-GC analogs. These findings suggest that carbohydrate photocrosslinkers can potentially be useful in antigen presentation and aid in the development of glycan-based immunotherapies.
Multivalent photocrosslinking probes for carbohydrates
The interactions between individual glycans and GBPs are usually low-affinity. However, GBPs are often composed of multiple lectin domains that can bind to glycans, resulting in increased avidity through multivalent recognition [34,35]. Numerous multivalent glycan-based probes have been designed based on various scaffolds, including nanoparticles, small organic molecules, polymers, dendrimers, liposomes, and peptides [35–37]. Despite the improved affinity by increasing valency of binding, co-elution of nonspecific proteins still causes a major challenge in the identification of the real targets. To address this challenge, multivalent carbohydrate probes functionalized by photocrosslinkers have been designed [38–43]. Among them, a polymer-based multivalent photocrosslinking carbohydrate probe was used successfully to identify novel GBPs that recognize fucose-α(1–2)-galactose from rat brain lysates [40]. Here we highlight recent progress in the design and use of multivalent photocrosslinking probes for the study of glycan-mediated interactions.
Gold nanoparticles (AuNPs) have been used as a scaffold in the design of many multivalent carbohydrate probes. AuNPs can be easily and covalently modified with multiple functional groups, which allows for facile optimization. Therefore, AuNPs have been used to study glycan-biomolecule interactions and also demonstrated potential therapeutic applications, such as targeted drug delivery and detection of pathogens [35,44]. Recently, Okada and coworkers designed some multivalent carbohydrate probes that include photocrosslinking groups (Figure 2b) and are based on a AuNP scaffold [42]. The covalent crosslinking of GBPs to the probes allowed for the harsh washes with guanidine hydrochloride, largely reducing nonspecific interactions. As a proof of principle, lactose- and BP-modified AuNPs were used to purify PNA, a galactose-recognizing lectin, from a mixture of PNA and HeLa cell lysates. They successfully eluted PNA from AuNPs and observed minimal co-elution of nonspecific proteins.
Another recent study on multivalent carbohydrate photocrosslinkers used a small organic molecule as the scaffold. Lin and coworkers designed a photo-cleavable biotin tag to be used with a photocrosslinking multivalent carbohydrate probes (Figure 2c) [43]. This study took advantage of their previous observation that enhanced crosslinking of GBPs can be achieved using a photocrosslinking probe with an aryl DAz and a trivalent galactose unit compared to a single galactose [41]. In a recent study, the trivalent galactose unit, an aryl DAz, and a cyclooctyne were combined in a single probe. To enable the facile release of crosslinked GBPs, a photo-cleavable biotin-azide tag was designed. After the multivalent carbohydrate probe was crosslinked to GBPs, this biotin-azide tag was conjugated to the multivalent probe through the strain-promoted azide-alkyne cycloaddition (SPAAC). This cleavable probe allowed for the facile release of target proteins by UV irradiation, and also reduced the co-elution of nonspecific proteins bound to the beads. The probe and the photo-cleavable tag were then used to successfully purify RCA120, a lectin that recognizes β-galactose, from the mixture of RCA120 and mouse brain lysates.
These multivalent carbohydrate photocrosslinking probes take advantage of both multivalent interactions and photocrosslinking, which greatly enhances their ability in capturing low-affinity glycan-protein interactions. Although many of these probes have so far been used only to crosslink GBPs with known specificity, these probe molecules as well as the general design principles can potentially be used in future studies to identify novel GBPs.
Applications of carbohydrate photocrosslinkers
In the development of novel carbohydrate photocrosslinkers, the first step is usually to crosslink these probes to GBPs with known specificity. After optimization, these photocrosslinkers may be applied in more physiologically relevant systems to solve biological questions [9,10]. Here we will review a few recent examples to demonstrate the application of carbohydrate photocrosslinkers.
Work from our lab and others [8,45–48] has shown that some photocrosslinking carbohydrate probes can be metabolically incorporated into cellular glycans (Figure 3a and 3b). These probes are usually analogs of natural carbohydrates so that they can enter the glycan biosynthesis pathways and become incorporated into cellular glycans. To promote the cellular uptake, these analogs are sometimes ester derivatized [46,47]. For example, photocrosslinking carbohydrate analogs of sialic acid or its precursor N-acetylmannosamine (ManNAc) have been developed for metabolic labeling [8,45,46,48–51]. These analogs can be metabolized by cells and be incorporated into cell surface glycans on both proteins and lipids. These analogs have been used to study interactions between cell surface glycoconjugates with GBPs, including CD22 [8,52] and cholera toxin B subunit [53–56]. In addition to photocrosslinking analogs, Chen and coworkers developed a bifunctional sialic acid analog functionalized by a DAz and an alkyne group that can be incorporated into cell surface glycans (Figure 3a) [48]. This bifunctional probe enables both crosslinking and purification, and is therefore a potentially powerful reagent for the discovery and characterization of glycan-mediated interactions.
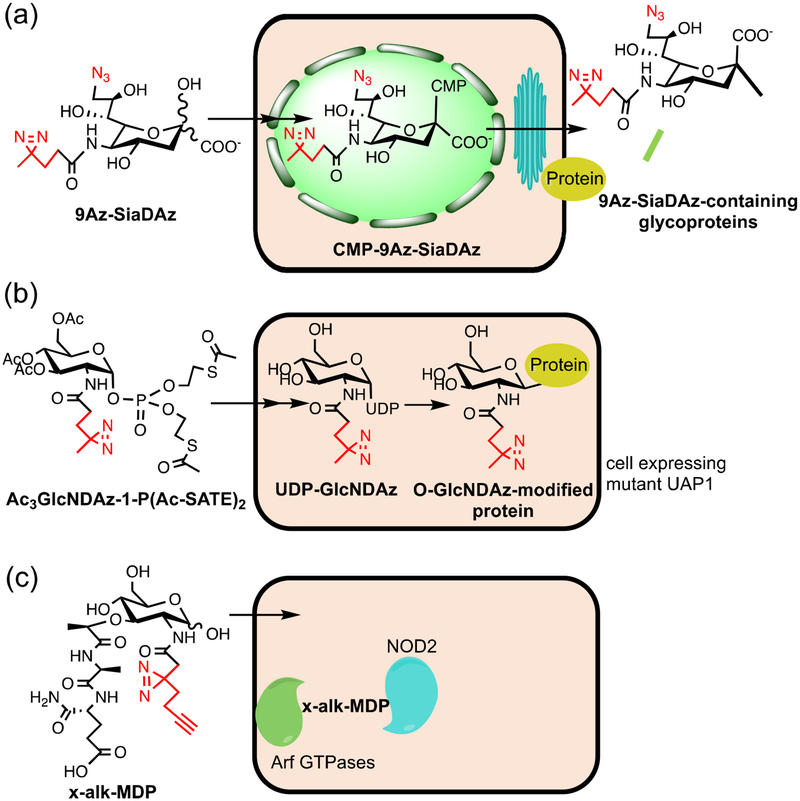
Figure 3. Structures of photocrosslinking carbohydrate probes and their applications in cells.
(a) Structure of 9Az-SiaDAz and its metabolism in cells. After entering the cells, 9Az-SiaDAz is metabolized to generate functionalized CMP-sialic acid (CMP-9Az-SiaDAz), which is used by sialyltransferases inside the Golgi, resulting in 9Az-SiaDAz incorporation into cell surface glycans. (b) Structure of GlcNDAz precursor and its metabolism in cells. GlcNDAz precursor can enter cells, be deprotected, and be metabolized by genetically-modified cells to generate functionalized UDP-GlcNAc (UDP-GlcNDAz). UDP-GlcNDAz is then used by OGT to modify proteins. (c) Structures of a photocrosslinking analog of peptidoglycan fragment muramyl-dipeptide (MDP), which crosslinks to and promotes interactions between NOD2 and Arf GTPases.
Another photocrosslinking analog that mimics N-acetylglucosamine (GlcNAc) has been used to study O-linked GlcNAc (O-GlcNAc), a single sugar PTM on the serine or threonine residues on numerous cytoplasmic and nuclear proteins [1]. O-GlcNAcylation has been reported to regulate multiple cellular processes, such as nutrient- and stress-responses, signal transduction, gene transcription, and chromatin modification. Furthermore, dysregulation of O-GlcNAcylation is associated with a number of diseases, including neurodegenerative diseases, diabetes, and cancer [57–60]. Despite the large number of proteins identified to be O-GlcNAcylated [61], the exact functions of O-GlcNAcylation on many proteins remain unknown. To study the interactions mediated by O-GlcNAc, Kohler and coworkers developed a photocrosslinking analog of GlcNAc functionalized by a DAz group (GlcNDAz; Figure 3b) [47]. The precursor of GlcNDAz can be metabolized by genetically-modified mammalian cells to generate DAz-functionalized UDP-GlcNAc, a nucleotide sugar that is the donor for various GlcNAc transferases. The functionalized UDP-GlcNAc can then be used by O-GlcNAc transferase (OGT) to modify proteins.
Recently, Boyce and co-workers used GlcNDAz to study the interactions regulated by O-GlcNAc on several components of the coat protein complex II (COP II), which mediates the cargo trafficking from the endoplasmic reticulum (ER) [62]. They first mapped the O-GlcNAc modification sites and then used GlcNDAz to successfully crosslink several COP II components to known or unknown protein binding partners, suggesting that O-GlcNAc is directly involved in these interactions. To identify the functional O-GlcNAc modification sites on SEC23A, an essential component of COP II, serine or threonine residues identified as potential O-GlcNAc modification sites were individually mutated to alanine. Five mutants demonstrated weakened or eliminated crosslinking of SEC23A, among which the S184A mutant was identified to impair the trafficking of collagen in human chondrosarcoma cells. In other studies, GlcNDAz was used to study the functions of vimentin O-GlcNAcylation [63] and the recognition of O-GlcNAcylated proteins by “reader” proteins [64]. Because of the short half-life of the carbene formed from DAz, GlcNDAz are expected to only capture biomolecules in close proximity. Therefore, this crosslinking approach is primed to capture binding interactions where O-GlcNAc is near the interaction interface, including interactions that may be directly mediated by O-GlcNAc. This is an advantage to placing the crosslinker directly on the O-GlcNAc modification, as compared to other chemical crosslinking approaches, which may capture PPIs without distinguishing whether the interactions are mediated by O-GlcNAc.
Photocrosslinking carbohydrate probes have also been constructed for non-mammalian glycans. For example, Grimes and coworkers have been working to metabolically incorporate analogs of N-acetyl muramic acid (MurNAc) into peptidoglycan in live bacteria [65]. Recently, they successfully synthesized analogs of UDP-MurNAc modified with different functional groups, including DAz, using chemoenzymatic methods [66]. These photocrosslinking analogs may be applied in future co-culture assays to study interactions mediated by bacterial peptidoglycan. Another tool to study bacterial peptidoglycan is the photocrosslinking probes that mimic bacterial peptidoglycan fragments designed by Hang and coworkers. These probes were synthesized chemically and used to study the interactions of peptidoglycan fragments with receptors in mammalian cells [67]. Among these probes, x-alk-MDP (Figure 3c) is a photocrosslinking analog of muramyl-dipeptide (MDP), one of the peptidoglycan fragments. The ability of MDP to bind to and activate nucleotide-binding oligomerization domain-containing protein 2 (NOD2) has been shown previously [68–71], but the direct binding had not been demonstrated in cells. Using x-alk-MDP, they successfully captured the interaction of MDP with NOD2. Surprisingly, Arf GTPases were also identified as novel binding partners of MDP by proteomic analysis of the crosslinked complexes and they were shown to form a complex with NOD2 and MDP. X-alk-MDP was also used to crosslink the Arf6 and NOD2 mutants, which revealed that a Crohn’s disease mutant of NOD2 abrogated the formation of MDP:Arf6:NOD2 complex.
These studies provide examples of how photocrosslinking carbohydrate probes can be applied to pinpoint the binding interactions in which glycans engage within complex physiological systems. We anticipate that more photocrosslinking carbohydrate probes will be developed to identify physiologically-significant glycan-dependent phenomena in cellular settings.
Conclusions and perspectives
Glycans are essential biomolecules and mediate many important biological interactions. Although the illumination of molecular mechanisms of glycan functions has accelerated in recent years, there is still much to be uncovered in glycobiology [2]. However, glycan-mediated interactions are usually challenging to study using traditional methods. Therefore, efforts have been made on development and applications of chemical biology methods, including the employment of photocrosslinking carbohydrate probes to address the challenges in glycobiology.
While this review focuses on photocrosslinkers, it is worthwhile to note that chemical crosslinkers that are not photoactive can also be applied to covalently capture carbohydrate binding interactions [72,73]. Photoactivable and chemical crosslinkers have complementary advantages. Photocrosslinkers are generally considered to be noninvasive and can be activated at any time by UV-irradiation. Incorporation of photocrosslinkers into biomolecules eliminates the necessity for the crosslinkers to diffuse to the interacting sites and potentially allows for time-dependent studies [11]. Photocrosslinkers are also generally considered to be less selective than chemical crosslinkers, because many chemical crosslinkers require specific amino acid(s) to achieve crosslinking. Nevertheless, chemical crosslinkers may demonstrate higher crosslinking efficiency [73]. Furthermore, some chemical crosslinkers can be added externally, eliminating the necessity to incorporate the crosslinkers into the biomolecules. This makes these crosslinkers easy to use and also minimizes the possibility of disturbing interactions by introducing unnatural functional groups. In addition to photoactivable and chemical crosslinkers, mechanism-based probes have also been designed to covalently label glycosyltransferases and glycosidases [74]. Unlike photocrosslinking carbohydrate probes, these mechanism-based probes should display higher selectivity towards glycosyltransferases and glycosidases compared to other GBPs. Therefore, they are powerful tools for enzyme characterization.
Most studies discussed here used photocrosslinking carbohydrate probes to identify GBPs from complex biological systems. However, photocrosslinkers have also been used to stabilize an interaction covalently to prevent dissociation of the glycan-protein complexes [33]. Photocrosslinkers have been used in concert with mass spectrometry (MS) to identify substrate binding pockets in enzymes involved in glycan biosynthesis [10]. Additionally, MS analysis of a glycan-protein binding interaction via crosslinking (albeit not photoactivated crosslinking) has provided information that is complementary to that obtained by NMR spectroscopy [75]. Indeed, PAL is now being applied successfully to locate the crosslinking sites for proteins crosslinked to small molecules [20,76,77], other proteins [78] and glycolipids [33]. Taken together, these studies predict future applications of PAL in characterizing the molecular details of glycan recognition events.
Because of the advantages demonstrated by photocrosslinkers in studying glycan-protein interactions, we anticipate that novel photocrosslinking carbohydrate probes will continue to be designed and developed. Furthermore, new methods to incorporate the photocrosslinking probes into biomolecules chemically or metabolically will facilitate the use of such reagents. The next generation of probes may address some of the limitations on current ones. For example, the application of bifunctional carbohydrate analogs that can be metabolically incorporated into cellular glycans, such as the 9Az-SiaDAz (Figure 3a) [48], may allow the ready isolation of the crosslinked glycan-protein complexes. In addition, such bifunctional probes can potentially be coupled with other probes through click chemistry, such as photo-cleavable biotin tags [43] for optimal purification and fluorescent tags for imaging. Combined with other chemical biology methods, current and emerging photocrosslinking carbohydrate probes will greatly assist our understanding of how glycans function in physiological and pathological contexts.
Acknowledgements
We thank Dr. Daniela Carroll, Dr. Amberlyn Wands, Dr. Nageswari Yarravarapu and Atossa Ghorashi for helpful discussions. We acknowledge financial support from a McKnight Fellowship (Department of Biochemistry, UT Southwestern Medical Center) to HW, the National Institutes of Health (R21DK112733), and the Welch Foundation (I-1686).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors have no competing interests.
References
- 1.Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, et al. : Essentials of glycobiology. Cold Spring Harbor (NY); 2017. [PubMed] [Google Scholar]
- 2.Varki A: Biological roles of glycans. Glycobiology 2017, 27:3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng BG, Freeze HH: Perspectives on Glycosylation and Its Congenital Disorders. Trends Genet 2018, 34:466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinho SS, Reis CA: Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer 2015, 15:540–555. [DOI] [PubMed] [Google Scholar]
- 5.Abou-Abbass H, Abou-El-Hassan H, Bahmad H, Zibara K, Zebian A, Youssef R, Ismail J, Zhu R, Zhou S, Dong X, et al. : Glycosylation and other PTMs alterations in neurodegenerative diseases: Current status and future role in neurotrauma. Electrophoresis 2016, 37:1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reily C, Stewart TJ, Renfrow MB, Novak J: Glycosylation in health and disease. Nat Rev Nephrol 2019, 15:346–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulson JC, Blixt O, Collins BE: Sweet spots in functional glycomics. Nat Chem Biol 2006, 2:238–248. [DOI] [PubMed] [Google Scholar]
- 8.Han S, Collins BE, Bengtson P, Paulson JC: Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat Chem Biol 2005, 1:93–97. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai K: Photoaffinity Probes for Identification of Carbohydrate-Binding Proteins. Asian Journal of Organic Chemistry 2015, 4:116–126. [Google Scholar]
- 10.Yu SH, Wands AM, Kohler JJ: Photoaffinity probes for studying carbohydrate biology. J Carbohydr Chem 2012, 31:325–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preston GW, Wilson AJ: Photo-induced covalent cross-linking for the analysis of biomolecular interactions. Chem Soc Rev 2013, 42:3289–3301. [DOI] [PubMed] [Google Scholar]
- 12.Murale DP, Hong SC, Haque MM, Lee JS: Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein-protein interactions (PPIs). Proteome Sci 2016, 15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deseke E, Nakatani Y, Ourisson G: Intrinsic Reactivities of Amino Acids towards Photoalkylation with Benzophenone − A Study Preliminary to Photolabelling of the Transmembrane Protein Glycophorin A. European Journal of Organic Chemistry 1998, 1998:243–251. [Google Scholar]
- 14.Wiegand M, Lindhorst TK: Synthesis of photoactive alpha-mannosides and mannosyl peptides and their evaluation for lectin labeling. European Journal of Organic Chemistry 2006:4841–4851. [Google Scholar]
- 15.Ziemianowicz DS, Bomgarden R, Etienne C, Schriemer DC: Amino Acid Insertion Frequencies Arising from Photoproducts Generated Using Aliphatic Diazirines. J Am Soc Mass Spectrom 2017, 28:2011–2021. [DOI] [PubMed] [Google Scholar]
- 16.Sakurai K, Ozawa S, Yamada R, Yasui T, Mizuno S: Comparison of the reactivity of carbohydrate photoaffinity probes with different photoreactive groups. Chembiochem 2014, 15:1399–1403. [DOI] [PubMed] [Google Scholar]
- 17.Ota E, Usui K, Oonuma K, Koshino H, Nishiyama S, Hirai G, Sodeoka M: Thienyl-Substituted alpha-Ketoamide: A Less Hydrophobic Reactive Group for Photo-Affinity Labeling. ACS Chem Biol 2018, 13:876–880. [DOI] [PubMed] [Google Scholar]; •• This paper reports tha application of alpha-ketoamide for photocrosslinking in biological systems. This photocrosslinker can potentially be applied as an alternative for benzophenone and aryl azide when nonspecific binding from hydrophobic interactions is a concern.
- 18.Iacobucci C, Gotze M, Piotrowski C, Arlt C, Rehkamp A, Ihling C, Hage C, Sinz A: Carboxyl-Photo-Reactive MS-Cleavable Cross-Linkers: Unveiling a Hidden Aspect of Diazirine-Based Reagents. Anal Chem 2018, 90:2805–2809. [DOI] [PubMed] [Google Scholar]
- 19.Ramil CP, Lin Q: Photoclick chemistry: a fluorogenic light-triggered in vivo ligation reaction. Curr Opin Chem Biol 2014, 21:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herner A, Marjanovic J, Lewandowski TM, Marin V, Patterson M, Miesbauer L, Ready D, Williams J, Vasudevan A, Lin Q: 2-Aryl-5-carboxytetrazole as a New Photoaffinity Label for Drug Target Identification. J Am Chem Soc 2016, 138:14609–14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Qian L, Li L, Bernhammer JC, Huynh HV, Lee JS, Yao SQ: Tetrazole Photoclick Chemistry: Reinvestigating Its Suitability as a Bioorthogonal Reaction and Potential Applications. Angew Chem Int Ed Engl 2016, 55:2002–2006. [DOI] [PubMed] [Google Scholar]
- 22.Cheng K, Lee JS, Hao P, Yao SQ, Ding K, Li Z: Tetrazole-Based Probes for Integrated Phenotypic Screening, Affinity-Based Proteome Profiling, and Sensitive Detection of a Cancer Biomarker. Angew Chem Int Ed Engl 2017, 56:15044–15048. [DOI] [PubMed] [Google Scholar]
- 23.Tian Y, Jacinto MP, Zeng Y, Yu Z, Qu J, Liu WR, Lin Q: Genetically Encoded 2-Aryl-5-carboxytetrazoles for Site-Selective Protein Photo-Cross-Linking. J Am Chem Soc 2017, 139:6078–6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Y, Lin Q: Genetic encoding of 2-aryl-5-carboxytetrazole-based protein photo-cross-linkers. Chem Commun (Camb) 2018, 54:4449–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter CD, Guo T, Daskhan G, Richards MR, Cairo CW: Synthetic Strategies for Modified Glycosphingolipids and Their Design as Probes. Chem Rev 2018, 118:8188–8241. [DOI] [PubMed] [Google Scholar]
- 26.Stocker BL, Timmer MS: Chemical tools for studying the biological function of glycolipids. Chembiochem 2013, 14:1164–1184. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai K, Yamaguchi T, Mizuno S: Design and synthesis of fluorescent glycolipid photoaffinity probes and their photoreactivity. Bioorg Med Chem Lett 2016, 26:5110–5115. [DOI] [PubMed] [Google Scholar]
- 28.Budani M, Mylvaganam M, Binnington B, Lingwood C: Synthesis of a novel photoactivatable glucosylceramide cross-linker. J Lipid Res 2016, 57:1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komura N, Yamazaki A, Imamura A, Ishida H, Kiso M, Ando H: Syntheses of bifunctional photoaffinity ganglioside probes for studying raft-associated interactions. Trends in Carbohydrate Research 2017, 9:1–26. [Google Scholar]
- 30.Liu X, Dong T, Zhou Y, Huang N, Lei X: Exploring the Binding Proteins of Glycolipids with Bifunctional Chemical Probes. Angew Chem Int Ed Engl 2016, 55:14330–14334. [DOI] [PubMed] [Google Scholar]; • A photocrosslinking analog of a plant glycolipid with anti-inflammatory activity was used to identify its receptor. This study provides an example of how photocrosslinking glycolipid probes can be used in combination with other methods in receptor identification.
- 31.Laguerre A, Schultz C: Novel lipid tools and probes for biological investigations. Curr Opin Cell Biol 2018, 53:97–104. [DOI] [PubMed] [Google Scholar]
- 32.Hoglinger D, Nadler A, Haberkant P, Kirkpatrick J, Schifferer M, Stein F, Hauke S, Porter FD, Schultz C: Trifunctional lipid probes for comprehensive studies of single lipid species in living cells. Proc Natl Acad Sci U S A 2017, 114:1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veerapen N, Kharkwal SS, Jervis P, Bhowruth V, Besra AK, North SJ, Haslam SM, Dell A, Hobrath J, Quaid PJ, et al. : Photoactivable Glycolipid Antigens Generate Stable Conjugates with CD1d for Invariant Natural Killer T Cell Activation. Bioconjug Chem 2018, 29:3161–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A photocrosslinking probe that mimics α-galactosylceramide was crosslinked to CD1d and the crosslinking complex was evaluated for its ability to activate iNKT cells. Results from this study indicate that photocrosslinking glycolipid probes can be potentially applied in antigen presentation and glycan- based immunotherapies.
- 34.Collins BE, Paulson JC: Cell surface biology mediated by low affinity multivalent protein-glycan interactions. Curr Opin Chem Biol 2004, 8:617–625. [DOI] [PubMed] [Google Scholar]
- 35.Adak AK, Lin HJ, Lin CC: Multivalent glycosylated nanoparticles for studying carbohydrate-protein interactions. Org Biomol Chem 2014, 12:5563–5573. [DOI] [PubMed] [Google Scholar]
- 36.Muller C, Despras G, Lindhorst TK: Organizing multivalency in carbohydrate recognition. Chem Soc Rev 2016, 45:3275–3302. [DOI] [PubMed] [Google Scholar]
- 37.Delbianco M, Bharate P, Varela-Aramburu S, Seeberger PH: Carbohydrates in Supramolecular Chemistry. Chem Rev 2016, 116:1693–1752. [DOI] [PubMed] [Google Scholar]
- 38.Lauc G, Lee RT, Dumiae J, Lee YC: Photoaffinity glycoprobes-a new tool for the identification of lectins. Glycobiology 2000, 10:357–364. [DOI] [PubMed] [Google Scholar]
- 39.Lee MR, Jung DW, Williams D, Shin I: Efficient solid-phase synthesis of trifunctional probes and their application to the detection of carbohydrate-binding proteins. Org Lett 2005, 7:5477–5480. [DOI] [PubMed] [Google Scholar]
- 40.Wibowo A, Peters EC, Hsieh-Wilson LC: Photoactivatable glycopolymers for the proteome-wide identification of fucose-alpha(1–2)-galactose binding proteins. J Am Chem Soc 2014, 136:9528–9531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang TC, Lai CH, Chien CW, Liang CF, Adak AK, Chuang YJ, Chen YJ, Lin CC: Synthesis and evaluation of a photoactive probe with a multivalent carbohydrate for capturing carbohydrate-lectin interactions. Bioconjug Chem 2013, 24:1895–1906. [DOI] [PubMed] [Google Scholar]
- 42.Sakurai K, Hatai Y, Okada A: Gold nanoparticle-based multivalent carbohydrate probes: selective photoaffinity labeling of carbohydrate-binding proteins. Chem Sci 2016, 7:702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A gold nanoparticle was used as a scaffold for multivalent carbohydrate photocrosslinkers. This scaffold has the potential to be readily functionalized with more complex glycans and applied in identification of glycan-binding proteins.
- 43.Chang TC, Adak AK, Lin TW, Li PJ, Chen YJ, Lai CH, Liang CF, Chen YJ, Lin CC: A photo-cleavable biotin affinity tag for the facile release of a photo-crosslinked carbohydrate-binding protein. Bioorg Med Chem 2016, 24:1216–1224. [DOI] [PubMed] [Google Scholar]
- 44.Compostella F, Pitirollo O, Silvestri A, Polito L: Glyco-gold nanoparticles: synthesis and applications. Beilstein J Org Chem 2017, 13:1008–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luchansky SJ, Goon S, Bertozzi CR: Expanding the diversity of unnatural cell-surface sialic acids. Chembiochem 2004, 5:371–374. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Kohler JJ: Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J Am Chem Soc 2008, 130:3278–3279. [DOI] [PubMed] [Google Scholar]
- 47.Yu SH, Boyce M, Wands AM, Bond MR, Bertozzi CR, Kohler JJ: Metabolic labeling enables selective photocrosslinking of O-GlcNAc-modified proteins to their binding partners. Proc Natl Acad Sci U S A 2012, 109:4834–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feng L, Hong S, Rong J, You Q, Dai P, Huang R, Tan Y, Hong W, Xie C, Zhao J, et al. :Bifunctional unnatural sialic acids for dual metabolic labeling of cell-surface sialylated glycans. J Am Chem Soc 2013, 135:9244–9247. [DOI] [PubMed] [Google Scholar]
- 49.Cheng B, Xie R, Dong L, Chen X: Metabolic Remodeling of Cell-Surface Sialic Acids:Principles, Applications, and Recent Advances. Chembiochem 2016, 17:11–27. [DOI] [PubMed] [Google Scholar]
- 50.Bond MR, Zhang H, Vu PD, Kohler JJ: Photocrosslinking of glycoconjugates using metabolically incorporated diazirine-containing sugars. Nat Protoc 2009, 4:1044–1063. [DOI] [PubMed] [Google Scholar]
- 51.Bond MR, Zhang H, Kim J, Yu SH, Yang F, Patrie SM, Kohler JJ: Metabolism of diazirine-modified N-acetylmannosamine analogues to photo-cross-linking sialosides. Bioconjug Chem 2011, 22:1811–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramya TN, Weerapana E, Liao L, Zeng Y, Tateno H, Liao L, Yates JR 3rd, Cravatt BF, Paulson JC: In situ trans ligands of CD22 identified by glycan-protein photocross-linking-enabled proteomics. Mol Cell Proteomics 2010, 9:1339–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bond MR, Whitman CM, Kohler JJ: Metabolically incorporated photocrosslinking sialic acid covalently captures a ganglioside-protein complex. Mol Biosyst 2010, 6:1796–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wands AM, Fujita A, McCombs JE, Cervin J, Dedic B, Rodriguez AC, Nischan N, Bond MR, Mettlen M, Trudgian DC, et al. : Fucosylation and protein glycosylation create functional receptors for cholera toxin. Elife 2015, 4:e09545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cervin J, Wands AM, Casselbrant A, Wu H, Krishnamurthy S, Cvjetkovic A, Estelius J, Dedic B, Sethi A, Wallom KL, et al. : GM1 ganglioside-independent intoxication by Cholera toxin. PLoS Pathog 2018, 14:e1006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sethi A, Wands AM, Mettlen M, Krishnamurthy S, Wu H, Kohler JJ: Cell type and receptor identity regulate cholera toxin subunit B (CTB) internalization. Interface Focus 2019, 9:20180076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slawson C, Hart GW: O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer 2011, 11:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bond MR, Hanover JA: O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev Nutr 2013, 33:205–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X, Qian K: Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol 2017, 18:452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hart GW: Nutrient regulation of signaling and transcription. J Biol Chem 2019, 294:2211–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma J, Hart GW: O-GlcNAc profiling: from proteins to proteomes. Clin Proteomics 2014, 11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cox NJ, Unlu G, Bisnett BJ, Meister TR, Condon BM, Luo PM, Smith TJ, Hanna M, Chhetri A, Soderblom EJ, et al. : Dynamic Glycosylation Governs the Vertebrate COPII Protein Trafficking Pathway. Biochemistry 2018, 57:91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study employed a previously reported carbohydrate photocrosslinker that can metabolically label glycoproteins on the O-GlcNAc modification sites. This photocrosslinker was combined with other genetic and biochemical methods to reveal the site-dependent function of O-GlcNAcylation on COP II components.
- 63.Tarbet HJ, Dolat L, Smith TJ, Condon BM, O’Brien ET 3rd, Valdivia RH, Boyce M: Site-specific glycosylation regulates the form and function of the intermediate filament cytoskeleton. Elife 2018, 7:e31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toleman CA, Schumacher MA, Yu SH, Zeng W, Cox NJ, Smith TJ, Soderblom EJ, Wands AM, Kohler JJ, Boyce M: Structural basis of O-GlcNAc recognition by mammalian 14-3-3 proteins. Proc Natl Acad Sci U S A 2018, 115:5956–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang H, DeMeester KE, Hou CW, Parent MA, Caplan JL, Grimes CL: Metabolic labelling of the carbohydrate core in bacterial peptidoglycan and its applications. Nat Commun 2017, 8:15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeMeester KE, Liang H, Jensen MR, Jones ZS, D’Ambrosio EA, Scinto SL, Zhou J, Grimes CL: Synthesis of Functionalized N-Acetyl Muramic Acids To Probe Bacterial Cell Wall Recycling and Biosynthesis. J Am Chem Soc 2018, 140:9458–9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang YC, Westcott NP, Griffin ME, Hang HC: Peptidoglycan Metabolite Photoaffinity Reporters Reveal Direct Binding to Intracellular Pattern Recognition Receptors and Arf GTPases. ACS Chem Biol 2019, 14:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this study, photocrosslinking probes that mimic fragments of bacterial peptidoglycan were designed and used to identify receptors of these fragments in mammalian cells. The receptors identified confirmed and expanded prior knowledge about binding partners of these peptidoglycan fragments.
- 68.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ: Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003, 278:8869–8872. [DOI] [PubMed] [Google Scholar]
- 69.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, et al. : Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem 2003, 278:5509–5512. [DOI] [PubMed] [Google Scholar]
- 70.Mo J, Boyle JP, Howard CB, Monie TP, Davis BK, Duncan JA: Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP. J Biol Chem 2012, 287:23057–23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grimes CL, Ariyananda Lde Z, Melnyk JE, O’Shea EK: The innate immune protein Nod2 binds directly to MDP, a bacterial cell wall fragment. J Am Chem Soc 2012, 134:13535–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blaum BS, Deakin JA, Johansson CM, Herbert AP, Barlow PN, Lyon M, Uhrin D: Lysine and arginine side chains in glycosaminoglycan-protein complexes investigated by NMR, cross-linking, and mass spectrometry: a case study of the factor H-heparin interaction. J Am Chem Soc 2010, 132:6374–6381. [DOI] [PubMed] [Google Scholar]
- 73.Ueda M, Manabe Y, Otsuka Y, Kanzawa N: Cassia obtusifolia MetE as a cytosolic target for potassium isolespedezate, a leaf-opening factor of Cassia plants: target exploration by a compact molecular-probe strategy. Chem Asian J 2011, 6:3286–3297. [DOI] [PubMed] [Google Scholar]
- 74.Xu Y, Uddin N, Wagner GK: Covalent Probes for Carbohydrate-Active Enzymes: From Glycosidases to Glycosyltransferases. Methods Enzymol 2018, 598:237–265. [DOI] [PubMed] [Google Scholar]
- 75.Blaum BS, Deakin JA, Johansson CM, Herbert AP, Barlow PN, Lyon M, Uhrín D: Lysine and Arginine Side Chains in Glycosaminoglycan–Protein Complexes InvesCgated by NMR, Cross-Linking, and Mass Spectrometry: A Case Study of the Factor H–Heparin Interaction. J Am Chem Soc 2010, 132:6374–6381. [DOI] [PubMed] [Google Scholar]
- 76.Parker CG, Galmozzi A, Wang Y, Correia BE, Sasaki K, Joslyn CM, Kim AS, Cavallaro CL, Lawrence RM, Johnson SR, et al. : Ligand and Target Discovery by Fragment-Based Screening in Human Cells. Cell 2017, 168:527–541.e529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao J, Mfuh A, Amako Y, Woo CM: Small Molecule Interactome Mapping by Photoaffinity Labeling Reveals Binding Site Hotspots for the NSAIDs. J Am Chem Soc 2018, 140:4259–4268. [DOI] [PubMed] [Google Scholar]
- 78.Freinkman E, Chng SS, Kahne D: The complex that inserts lipopolysaccharide into the bacterial outer membrane forms a two-protein plug-and-barrel. Proc Natl Acad Sci U S A 2011, 108:2486–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]