Abstract
Background
Westernization and etiologic heterogeneity may play a role in the rising breast cancer incidence in Asian American (AA) women. We report breast cancer incidence in Asian-origin populations.
Methods
Using a specialized Surveillance, Epidemiology, and End Results-9 Plus API Database (1990–2014), we analyzed breast cancer incidence overall, by estrogen receptor (ER) status, and age group among non-Hispanic white (NHW) and AA women. We used age-period-cohort models to assess time trends and quantify heterogeneity by ER status, race and ethnicity, and age.
Results
Overall, breast cancer incidence increased for most AA ethnicities (Filipina: estimated annual percentage change [EAPC] = 0.96%/year, 95% confidence interval [CI] = 0.61% to 1.32%; South Asian: EAPC = 1.68%/year, 95% CI = 0.24% to 3.13%; Chinese: EAPC = 0.65%/year, 95% CI = 0.03% to 1.27%; Korean: EAPC = 2.55%/year, 95% CI = 0.13% to 5.02%; and Vietnamese women: EAPC = 0.88%/year, 95% CI = 0.37% to 1.38%); rates did not change for NHW (EAPC = -0.2%/year, 95% CI = -0.73% to 0.33%) or Japanese women (EAPC = 0.22%/year, 95% CI = -1.26% to 1.72%). For most AA ethnicities, ER-positive rates statistically significantly increased, whereas ER-negative rates statistically significantly decreased. Among older women, ER-positive rates were stable for NHW and Japanese women. ER-negative rates decreased fastest in NHW and Japanese women among both age groups.
Conclusions
Increasing ER-positive incidence is driving an increase overall for most AA women despite declining ER-negative incidence. The similar trends in NHW and Japanese women (vs other AA ethnic groups) highlight the need to better understand the influences of westernization and other etiologic factors on breast cancer incidence patterns in AA women. Heterogeneous trends among AA ethnicities underscore the importance of disaggregating AA data and studying how breast cancer differentially affects the growing populations of diverse AA ethnic groups.
Breast cancer incidence is lower among Asian American (AA) women compared with non-Hispanic white (NHW) women; however, incidence rates among AAs have been rising continuously (1,2). For example, rates among Asian women in California increased steadily over the past 15 years, whereas incidence stabilized among NHW women (3). Additionally, between 2000 and 2015, the Asian population in the United States grew by 72%, from 11.9 million to 20.4 million people—the fastest growth rate of any major racial or ethnic group (4). High population growth rate and rising breast cancer incidence among Asian women indicate the need to better understand breast cancer epidemiology in this population, which could inform prevention methods to reduce the breast cancer burden in the United States.
Research suggests that westernization—a process whereby foreign-born groups acculturate to American lifestyles (eg, changes to diet, reproductive practices, and environmental exposures)—has an important influence on breast cancer risk among AA women (5). This risk may vary among ethnic groups. Gomez et al. (6) found that breast cancer incidence in California was higher among American-born Chinese and Filipina women compared with foreign-born Chinese and Filipina women in California; however, incidence was similar among American-born Japanese women compared with foreign-born Japanese women, who immigrated at earlier ages in life and are also rather westernized (6).
When racial and ethnic groups are analyzed separately, we can elucidate trends and glean insights that are frequently masked in combined data (7,8). Such disaggregation benefits analyses of AAs, a diverse group that includes more than 19 different origin groups with unique histories, cultures, languages, and other characteristics (4). Indeed, previous research suggests that breast cancer incidence among AA women in California and nationally is heterogeneous among ethnic groups (6,9).
Here, we have extended previous work disaggregating AA ethnicities (9) to better understand breast cancer incidence patterns and heterogeneity. Specifically, we analyzed incidence of invasive female breast cancers among AA ethnic groups using data from the Surveillance Epidemiology and End Results (SEER) Program. We applied age-period-cohort models to investigate, for the first time, AA ethnic group-specific secular trends by age, birth cohort patterns, and estrogen receptor (ER) status. Examining trends by ER status is particularly important within the context of this study. It is becoming inherently clear that there is notable etiologic heterogeneity in risk factor associations by ER status, particularly for age at first birth and body mass index (10,11). For example, younger age at first birth has been associated with increased risk of ER-negative but decreased risk of ER-positive breast cancer. A more complex relationship exists between obesity and breast cancer risk by ER status, menopausal status, and race and ethnicity (12,13). Careful interpretation of secular trends in breast cancer incidence can shed light on whether the observed patterns are consistent with the hypothesis that westernization is contributing to the rising incidence of breast cancer among AA women.
Methods
Study Data
Invasive breast cancer incidence data were obtained from the SEER Program database (3,14) for diagnosis years from 1990 through 2014. This database includes registries in the SEER 9 database (San Francisco–Oakland Standard Metropolitan Statistical Area, Connecticut, Detroit [Metropolitan], Hawaii, Iowa, New Mexico, Seattle [Puget Sound], Utah, and Atlanta [Metropolitan]) and the remainder of California and New Jersey. For our study, we included Filipina, Chinese, Japanese, South Asian (Asian Indian/Pakistani), Korean, Vietnamese, and NHW women (as a comparison group) ages 30–84 years. Asian ethnicity was assigned using the North American Association of Central Cancer Registries Asian and Pacific Islander Identification Algorithm (NAPIIA) (15). This algorithm uses information on birthplace and surname to assign Asian ethnicity if not already specified. However, 11% of the AA cancer cases were classified as “other Asian; Asian, not otherwise specified” and were not included in a specific AA category in this study. We used data for ages 30–84 years because of the relative rarity of very early onset breast cancers (ages 0 to 29 years) and because SEER does not have disaggregated population data for women ages 85 years and older. Data from the six AA ethnicities noted above were combined to form the “All Asian Americans” group.
The data were organized into eleven 5-year age groups (30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, 80–84 years) and five 5-year periods by year of diagnosis (1990–1994, 1995–1999, 2000–2004, 2005–2009, 2010–2014). We used a previously established imputation method (16) to assign cases with unknown ER status either an ER-positive or ER-negative value according to the observed proportions of existing ER-positive or ER-negative cases diagnosed in the same 5-year age and 5-year period group. This method is based on standard imputation procedures and is consistent with the approach presented by Howlader et al. (17). We imputed ER status for about 20% of NHW cases and about 13% for all AA cases and each ethnicity included.
Statistical Analysis
We calculated age-standardized incidence rates per 100 000 woman-years using the 2000 US standard population and estimated corresponding annual percent changes (EAPCs) in the age-standardized rates over time. We also applied age-period-cohort models (18) to calculate local drifts (19), which estimate the EAPCs specific for each age group. Data were stratified by race and ethnicity, ER status, and premenopausal (defined as ages 30–49 years) and postmenopausal (defined as ages 50–84 years) age groups. These analyses were performed using MATLAB (version 2018a, MathWorks Inc., Natwick, MA) using the age-standardized rates over time (18).
Heterogeneity in average local drifts between ethnic and age groups was assessed using age-period-cohort models and corresponding Wald tests (20). Specifically, we compared local drifts for 5-year age groups and the combination of age groups corresponding to premenopausal (four 5-year age groups 30–49 years) and postmenopausal (seven 5-year age groups 50–84 years) age categories. In our analyses, we focused on two primary contrasts: differences between all AA ethnicities combined vs NHW women and differences between the six AA ethnic groups. The test for heterogeneity in average local drifts between race and ethnic groups was adjusted for age group, whereas the heterogeneity test between age groups was adjusted for race and ethnicity. All Wald tests and confidence intervals were computed using an alpha level of 0.05, unless otherwise specified. All tests were two-sided.
Results
The analysis included 764 538 invasive breast cancer cases among NHW women and 62 121 cases among AA women ages 30–84 years and diagnosed from 1990 through 2014. Numbers of cases for the AA ethnicities from largest to smallest were Filipina (n = 20 513), Chinese (n = 14 274), Japanese (n = 12 850), South Asian (n = 5657), Korean (n = 4650), and Vietnamese (n = 4177). Among AAs, there were a total of 48 700 ER-positive cases and 13 421 ER-negative cases (Table 1). About 19% of NHW cases were younger than age 50 years at diagnosis, as were a similar percentage (20%) of Japanese cases. For the other AA ethnicities, a higher percentage of cases were under age 50 years: Filipina (30%), Chinese (34%), South Asians (38%), Vietnamese (41%), and Korean (41%). Supplementary Table 1 (available online) lists cases by registry, race and ethnicity (included in this analysis), and ER status for women aged 30 to 84 years.
Table 1.
Study counts by ER status, race and ethnicity, and premenopausal or postmenopausal age group, Specialized SEER 9 Plus API Database*
| Group | Overall |
ER-positive breast cancer |
ER-negative breast cancer |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Premenopausal-age group, 30–49 y |
Postmenopausal-age group, 50–84 y |
Premenopausal-age group, 30–49 y |
Postmenopausal-age group, 50–84 y |
|||||||
| Counts | Woman-Years | Counts | Woman-Years | Counts | Woman-Years | Counts | Woman-Years | Counts | Woman-Years | |
| Non-Hispanic white | 764 538 | 295 655 087 | 112 025 | 139 567 280 | 507 694 | 156 087 807 | 40 860 | 139 567 280 | 103 959 | 156 087 807 |
| All Asian Americans | 62 121 | 43 530 340 | 15 019 | 24 013 525 | 33 681 | 19 516 815 | 4717 | 24 013 525 | 8704 | 19 516 815 |
| Japanese | 12 850 | 6 175 845 | 2227 | 2 743 520 | 8347 | 3 432 325 | 535 | 2 743 520 | 1741 | 3 432 325 |
| Filipina | 20 513 | 11 880 329 | 4595 | 6 375 269 | 11 454 | 5 505 060 | 1551 | 6 375 269 | 2913 | 5 505 060 |
| South Asian | 5657 | 4 979 442 | 1573 | 3 324 678 | 2730 | 1 654 764 | 628 | 3 324 678 | 726 | 1 654 764 |
| Chinese | 14 274 | 11 546 698 | 3915 | 6 322 621 | 7314 | 5 224 077 | 1090 | 6 322 621 | 1955 | 5 224 077 |
| Korean | 4650 | 4 708 609 | 1427 | 2 714 287 | 1977 | 1 994 322 | 483 | 2 714 287 | 763 | 1 994 322 |
| Vietnamese | 4177 | 4 239 414 | 1275 | 2 533 154 | 1830 | 1 706 260 | 437 | 2 533 154 | 635 | 1 706 260 |
Counts for all Asian Americans combined and counts for each Asian ethnic group were separately imputed. Thus, the summed counts of Asian ethnic groups in a single column may be slightly different from the count for all Asian Americans combined. ER = estrogen receptor.
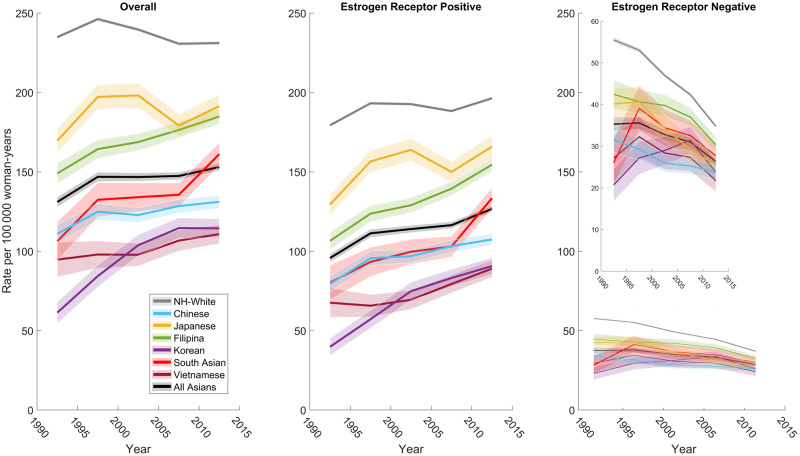
The overall breast cancer incidence rate in most AA ethnic groups increased statistically significantly in the United States from 1990 through 2014, whereas the rate did not change statistically significantly in NHW (EAPC = -0.2%/year, 95% confidence interval [CI] = -0.73% to 0.33%) and Japanese women (EAPC = 0.22%/year, 95% CI = -1.26% to 1.72%) (Table 2 and Figure 1A). The incidence rate increased statistically significantly over time among the remaining five AA ethnicities: Filipina (EAPC = 0.96%/year, 95% CI = 0.61% to 1.32%), South Asian (EAPC = 1.68%/year, 95% CI = 0.24% to 3.13%), Chinese (EAPC = 0.65%/year, 95% CI = 0.03% to 1.27%), Korean (EAPC = 2.55%/year, 95% CI = 0.13% to 5.02%), and Vietnamese (EAPC = 0.88%/year, 95% CI = 0.37% to 1.38%). Among these trends, Korean women had the greatest increase, whereas Chinese women had the smallest increase.
Figure 1.
Age-standardized incidence trends of female breast cancer among the six Asian American ethnicities and non-Hispanic white women by (A) all cases, (B) ER-positive breast cancer, and (C) ER-negative breast cancer. Specialized SEER 9 Plus API Database. ER = estrogen receptor; NH = non-Hispanic.
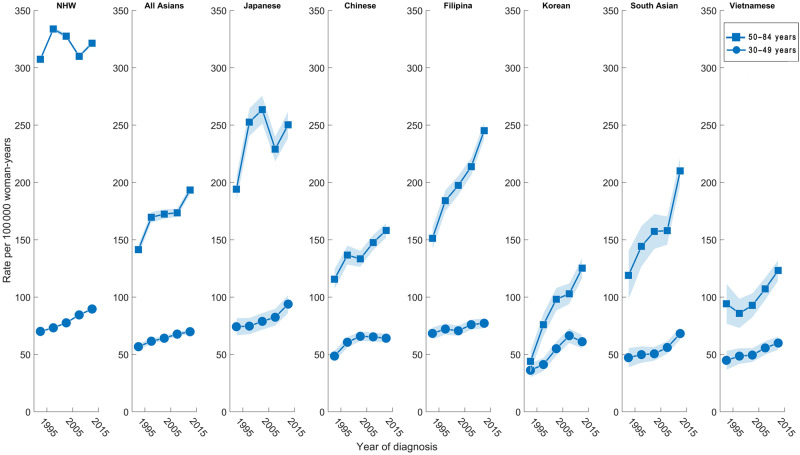
The EAPCs among AA ethnic groups also varied by ER and menopausal status. ER-positive breast cancer incidence in all age groups increased statistically significantly for all AA women combined (EAPC = 1.14%/year, 95% CI = 0.39% to 1.89%), whereas there was no statistically significant change for NHW women (EAPC = 0.30%/year, 95% CI = -0.29% to 0.89%; Table 2). The ER-positive EAPC increased statistically significantly for Filipina, South Asian, Chinese, Korean, and Vietnamese women, whereas it did not change statistically significantly for Japanese women (Table 2). NHW and Japanese women had the highest incidence of ER-positive breast tumors (Figure 1B). For ER-positive breast cancer among premenopausal-aged women, the EAPC increased statistically significantly for all groups except Chinese and Korean women. Premenopausal-aged NHW and Japanese women had the highest incidence of ER-positive tumors (Figure 2). Among postmenopausal-aged women, the EAPC for ER-positive tumors increased statistically significantly for all races and ethnicities except NHW and Japanese women (Table 2); although not increasing, these two groups had the highest incidence (Figure 2). ER-positive incidence increased more among premenopausal-aged vs postmenopausal-aged NHW and Japanese women; the opposite was true (eg, higher EAPC in postmenopausal-aged vs premenopausal-aged women) in the other AA ethnicities (Table 2). Among them, the EAPC for ER-positive breast cancer was greatest among postmenopausal-aged Korean women (EAPC = 3.91%/year, 95% CI = 1.15% to 6.75%).
Table 2.
Estimated annual percent change in the age-standardized rate per 100 000 woman-years (2000 US standard population) for female breast cancer among the six Asian American ethnicities and non-Hispanic white women by estrogen receptor status and age group, 1990–2014
| Group | Overall | ER-positive breast cancer |
ER-negative breast cancer |
||||
|---|---|---|---|---|---|---|---|
| All ages | All ages | Premenopausal age-group, 30–49 y | Postmenopausal age-group, 50–84 y | All ages | Premenopausal age-group, 30–49 y | Postmenopausal age-group, 50–84 y | |
| EAPC (95% CI) | EAPC (95% CI) | EAPC (95% CI) | EAPC (95% CI) | EAPC (95% CI) | EAPC (95% CI) | EAPC (95% CI) | |
| Non-Hispanic white | −0.20 (−0.73 to 0.33) | 0.30 (−0.29 to 0.89) | 1.28* (1.03 to 1.54) | 0.01 (−0.79 to 0.82) | −2.24* (−3.15 to -1.32) | −2.63* (−3.16 to -2.09) | −2.06* (−3.23 to -0.89) |
| All Asian Americans | 0.55 (−0.09 to 1.20) | 1.14* (0.39 to 1.89) | 0.98* (0.69 to 1.28) | 1.21* (0.19 to 2.24) | −1.52* (−2.56 to -0.46) | −2.55* (−3.28 to -1.82) | −0.98 (−2.35 to 0.42) |
| Japanese | 0.22 (−1.26 to 1.72) | 0.81 (−0.82 to 2.47) | 1.19* (0.39 to 2.00) | 0.64 (−1.75 to 3.09) | −2.44* (−3.70 to -1.17) | −3.76* (−4.74 to -2.78) | −1.85* (−3.34 to -0.33) |
| Filipina | 0.96* (0.61 to 1.32) | 1.71* (1.20 to 2.22) | 0.59* (0.13 to 1.06) | 2.18* (1.49 to 2.88) | −1.59* (−2.90 to -0.26) | −3.12* (−3.92 to -2.32) | −0.75 (−2.50 to 1.02) |
| South Asian | 1.68* (0.24 to 3.13) | 2.53* (0.76 to 4.33) | 2.05* (0.61 to 3.51) | 2.71* (0.66 to 4.79) | −1.06 (−4.22 to 2.22) | −1.27 (−3.79 to 1.31) | −0.99 (−4.78 to 2.95) |
| Chinese | 0.65* (0.03 to 1.27) | 1.21* (0.33 to 2.10) | 0.95 (−0.82 to 2.75) | 1.37* (0.55 to 2.20) | −1.40* (−1.94 to -0.86) | −3.11* (−3.55 to -2.67) | −0.46 (−1.17 to 0.26) |
| Korean | 2.55* (0.13 to 5.02) | 3.46* (1.20 to 5.78) | 2.78 (−0.01 to 5.65) | 3.91* (1.15 to 6.75) | 0.33 (−3.47 to 4.27) | −0.36 (−4.79 to 4.28) | 0.69 (−3.98 to 5.59) |
| Vietnamese | 0.88* (0.37 to 1.38) | 1.80* (0.70 to 2.90) | 1.50* (0.96 to 2.05) | 1.99* (0.45 to 3.54) | −1.65 (−3.94 to 0.70) | −2.10 (−4.64 to 0.50) | −1.46 (−4.65 to 1.85) |
Statistically significant change in the EAPC at the P < .05 level (two-sided test). CI = confidence interval; EAPC = estimated annual percentage change; ER = estrogen receptor.
Figure 2.
Age-standardized incidence trends of female breast cancer among the six Asian American ethnicities and non-Hispanic white women by premenopausal age group (ages 30–49 years) and postmenopausal age group (ages 50–84 years) for ER-positive breast cancers. ER = estrogen receptor; NHW = non-Hispanic white.
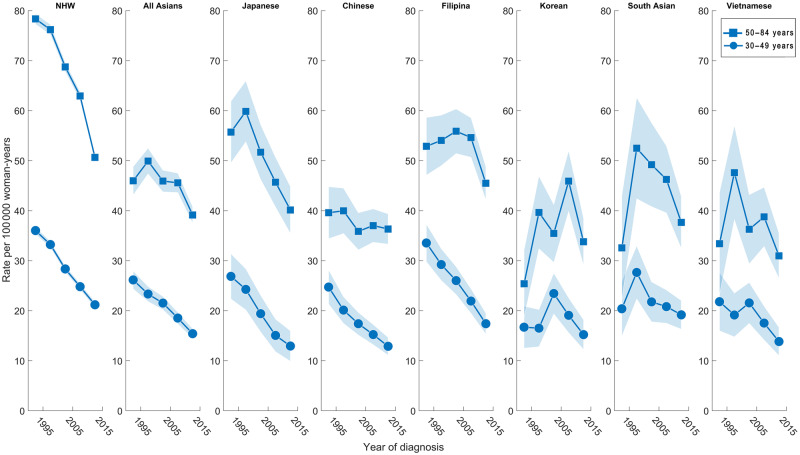
Overall, the EAPC of ER-negative breast cancer decreased less quickly in all AA women combined (-1.52%/year, 95% CI = −2.56% to −0.46%) compared with that of NHW women (−2.24%/year, 95% CI = −3.15% to −1.32%) (Table 2). Among AA ethnicities, the ER-negative EAPC decreased the most in Japanese women (−2.44%/year, 95% CI = −3.70% to −1.17%). The ER-negative EAPC also decreased statistically significantly among Filipina and Chinese women; trends among South Asian, Korean, and Vietnamese women were not statistically significant. For the ER-negative premenopausal age group, the EAPC decreased statistically significantly in the Japanese, Filipina, Chinese, and NHW groups and for all AAs combined; changes in other groups were not statistically significant. For the ER-negative postmenopausal age group, statistically significant EAPC decreases were found in the NHW and Japanese groups, which also had the highest incidence rates (Figure 3), whereas nonstatistically significant changes were found in the other groups.
Figure 3.
Age-standardized incidence trends of female breast cancer among the six Asian American ethnicities and non-Hispanic white women by premenopausal age group (ages 30–49 years) and postmenopausal age group (ages 50–84 years) for ER-negative breast cancers. ER = estrogen receptor; NHW = non-Hispanic white.
We used Wald tests to identify statistically significant trend heterogeneity in average local drifts within strata defined by age group and ethnicity. This approach is complimentary to the calculated EAPCs and, in some cases, is more conservative because it is impacted less by outliers. There was statistically significant heterogeneity in incidence trends between all AAs combined and NHW women among both pre- and postmenopausal age groups (Table 3). The average local drift among all AA women combined was statistically significantly different from that of NHW women, adjusted for age group (ER-positive P < .001 and ER-negative P < .001) (Table 3). The average local drift for breast cancer incidence was also statistically significantly heterogeneous by menopausal-age status, adjusted for race and ethnicity (ER-positive P = .008 and ER-negative P < .001). ER-positive breast cancer rates increased for both AA and NHW women, but compared with AA women, the increase was larger among NHW premenopausal-aged women and smaller among NHW postmenopausal-aged women. ER-negative rates decreased for both AA and NHW women, but among postmenopausal-aged women, the decrease was smaller in AA women compared with NHW women.
Table 3.
Average local drifts with 95% confidence intervals for female breast cancer among the six Asian American ethnicities, non-Hispanic white group, and the all Asian Americans group by ER status and age group, 1990–2014.
| Group | ER-positive breast cancer |
ER-negative breast cancer |
||
|---|---|---|---|---|
| Premenopausal-age group, 30–49 y | Postmenopausal-age group, 50–84 y | Premenopausal-age group, 30–49 y | Postmenopausal-age group, 50–84 y | |
| Average Local Drift (95% CI) | Average Local Drift (95% CI) | Average Local Drift (95% CI) | Average Local Drift (95% CI) | |
| Non-Hispanic white | 1.61 (1.24 to 1.98) | 0.08 (−0.14 to 0.30) | −2.22 (−2.56 to -1.89) | −1.94 (−2.12 to -1.75) |
| All Asian Americans | 1.06 (0.57 to 1.55) | 1.35 (1.07 to 1.64) | −2.35 (−2.75 to -1.95) | −0.70 (−1.00 to -0.39) |
| Phetage* | .008 | <.001 | ||
| Phetrace† | <.001 | <.001 | ||
| Japanese | 2.05 (1.56 to 2.55) | 0.84 (0.20 to 1.47) | −3.38 (−4.10 to -2.67) | −1.60 (−2.45 to -0.73) |
| Filipina | 0.55 (0.01 to 1.09) | 2.29 (0.77 to 3.82) | −2.95 (−3.65 to -2.25) | −0.09 (−2.0 to 1.8) |
| South Asian | 1.65 (0.42 to 2.89) | 2.76 (2.09 to 3.42) | −0.25 (−1.52 to 1.03) | 0.27 (−0.80 to 1.33) |
| Chinese | 1.19 (0.39 to 2.00) | 1.42 (0.53 to 2.31) | −3.25 (−4.14 to -2.35) | −0.89 (−2.42 to 0.64) |
| Korean | 2.83 (2.20 to 3.46) | 5.26 (4.61 to 5.91) | −0.57 (−1.75 to 0.61) | 2.11 (1.04 to 3.19) |
| Vietnamese | 0.43 (−0.29 to 1.15) | 1.64 (0.72 to 2.57) | −2.33 (−3.15 to -1.52) | −1.43 (−3.28 to 0.41) |
| Phetage* | <.001 | <.001 | ||
| Phetrace† | <.001 | <.001 | ||
Two-sided P value for heterogeneity in average local drifts between age groups (adjusted for race/ethnicity). CI = confidence interval; ER = estrogen receptor.
Two-sided P value for heterogeneity in average local drifts between race and ethnicity (adjusted for age group).
Trends were also statistically significantly heterogeneous among the AA ethnicities (Table 3). Local drifts for each race and ethnicity are presented in the Supplementary Figure 1 (available online). The average local drifts among the six AA ethnicities were statistically significantly heterogeneous when adjusted for age group (ER-positive P < .001 and ER-negative P < .001). The average local drift of premenopausal-aged AA women was statistically significantly heterogeneous from that of postmenopausal-aged AA women when adjusted for ethnicity (ER-positive P < .001 and ER-negative P < .001). ER-positive breast cancer rates increased for all AA ethnicities, but the rate increases varied. Decreases in ER-negative rates among AA ethnicities also varied, and some increases were noted among Korean and South Asian postmenopausal-aged women.
Discussion
We analyzed invasive female breast cancer incidence rates among six AA ethnic groups and NHW women from 1990 through 2014 using data from a specialized SEER 9 Plus API database. We found that overall breast cancer incidence rates for most AA ethnicities increased, whereas the overall rate did not statistically significantly change for Japanese women, similar to NHW women; the increases in overall incidence rates among most AA ethnicities are due to statistically significant increases in ER-positive breast cancer rates, whereas ER-negative rates are decreasing or comparatively stable; incidence trends are heterogeneous by AA ethnicity, overall and by menopausal age groups; and trends among NHW and Japanese women were most similar, which may be indicative of long-standing westernization effects on breast cancer in Japanese women compared with other AAs.
In this study, overall breast cancer incidence rates for most AA ethnicities increased, whereas the rates did not statistically significantly change for NHW and Japanese women. This finding is consistent with Gomez et al. (3), who found that breast cancer incidence in California increased for all AA groups studied except for Japanese women. Elevated rates and similar trends among NHW and Japanese women may be indicative of more established westernization among the Japanese ethnic group relative to the other Asian ethnic groups. The magnitude of age-standardized incidence rates for breast cancer cases overall and for ER-positive breast cancer of Japanese women were most like NHW women—these two groups had the highest rates out of all of the groups studied. These similarities may point to shared etiologic factors between Japanese and NHW women that could be an area for future research. Particular attention to rapid increases in breast cancer rates for Koreans, which may be multifactorial in origin (21–23), may also be warranted. Furthermore, incidence of ER-positive breast cancer increased statistically significantly in all AA women combined, whereas there was no significant change for NHW women; incidence of ER-negative breast cancer statistically significantly decreased less quickly in all AA women combined compared with NHW women. Understanding the etiology of rising ER-positive breast cancer rates and slow-to-decrease ER-negative breast cancer rates among most AAs could lead to important prevention and screening or early detection strategies.
Incidence rate trends were heterogeneous across AA ethnic groups and across age groups. Our analysis shows that disaggregated data for AA groups reveal heterogeneous trends that are masked when group data are aggregated into one Asian race category. Indeed, we found considerable and statistically significant variation in breast cancer incidence and in EAPCs among AA ethnicities by ER status and age and between the aggregated AA and NHW groups. For example, ER-positive breast cancer statistically significantly increased for Korean women by 3.46% per year, whereas there was no statistically significant change for Japanese women (EAPC = 0.81%/year). Similarly, an analysis of AA populations in California challenges the notion that breast cancer rates are uniformly low among AAs (6). We found that the average local drift of all premenopausal-aged AA women was statistically significantly heterogeneous from that of all postmenopausal-aged AA women. These findings support the view that incidence rates are heterogeneous by AA ethnic and age groups.
Migration history and other factors related to westernization may help explain these observed trends. Breast cancer incidence was high for both established AA ethnic groups and recently migrated groups in the United States. Incidence overall was highest in Japanese and Filipina women and lowest in Vietnamese women. South Asian women surpassed Chinese women in incidence despite the South Asian population generally having more recent migration to the United States after 1960 compared with the Chinese population, which has been arriving continuously to the United States since the 1820s (24). Filipinos have the longest history in the United States circa the 1800s, and Japanese individuals arrived in the 1880s. That current breast cancer incidence trends among Japanese women more closely follow NHW women than such trends among Filipina women may be due to more recent immigration waves of Filipinos to the United States compared with Japanese individuals. In 2015, 52% of Filipino Americans were foreign-born compared with 27% of Japanese Americans (25,26). Additionally, recent immigrants may be coming from regions in parts of Asia with rising incidence rates (27).
The heterogeneous breast cancer incidence trends found among AAs in the United States likely reflect the complexities of acculturation, screening factors, and breast cancer risk factors (23,28–36). The similarities between Japanese and NHW women in our study may be due to similar mammography behaviors, westernization correlated with long US residency, high socioeconomic status, high levels of health insurance coverage, and a usual source of medical care (23). In contrast to Japanese women, Vietnamese women in our study had the lowest breast cancer incidence rates. According to the US Census, the Vietnamese American population had the lowest English language proficiency rates, lower socioeconomic status, and lower educational attainment and were more likely to be employed in service jobs compared with the other AA ethnicities (30). These factors may be associated with screening behaviors. Vietnamese American cases also had one of the lowest median diagnostic ages, whereas, in contrast, Japanese Americans had a higher median age at diagnosis than the five other AA ethnicities in our study (25,26,37–40). We also observed that ER-positive breast cancer incidence rates increased faster in premenopausal-aged NHW and Japanese women, unlike all other AA ethnicities studied. This may be due in part to differences in screening behaviors because ER-positive breast cancer is two times more likely to be detected by mammography than ER-negative breast cancer (41,42). Lastly, potential anthropometric (43–45), behavioral (23,46–54), reproductive (55–61), and genetic (62,63) breast cancer risk factors may contribute to the observed findings in our study. Our findings are hypothesis-generating and highlight the need to uncover specific risk factors and mechanisms underlying the observed heterogeneous incidence patterns by menopausal age groups, ER status, and race and ethnicity to inform prevention and screening strategies among AA ethnicities as well as NHW women.
The strength of this study is its analysis of AA women by ethnicity, an analysis that was only possible through the comprehensive characterization of AA ethnicities captured by the specialized SEER 9 Plus API database. Limitations of the study are that the specialized SEER 9 Plus API data are not representative of the whole United States, ER status is missing, there lacks place of birth data, and there is potential ethnicity misclassification within the registry or based on the NAPIIA. Future research should investigate contributors to breast cancer incidence rates by ER and HER2 statuses among specific AA ethnicities, forecast rates among these groups, and analyze the relationship between factors related to acculturation and incidence. The incidence rate of ER-negative breast cancer, known for its early onset and poor prognosis (64), also did not decrease among all AA women as quickly as for NHW women; thus, enhanced preventive measures may be needed. The results of this study call attention to the need for cancer researchers to study how breast cancer differentially affects the growing populations of diverse AA ethnic groups.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Notes
The study sponsors had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author contributions: SLG, GLG, and PSR were responsible for the conduct and oversight of this project. AWT, BCDL, MY, GLG, and PSR were involved in the analytic concept and design. AWT and MY participated in the acquisition of the data. BCDL, PC, and PSR contributed to the statistical analyses. AWT, BCDL, GLG, and PSR participated in preparation of the manuscript. All authors participated in interpretation of the results and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
This research is supported by the Intramural Research Program, Cancer Research Training Award Fellowship Program, and the Cancer Prevention Fellowship Program of the National Cancer Institute at the National Institutes of Health. The authors acknowledge David Check in the National Cancer Institute Division of Cancer Epidemiology and Genetics Biostatistics Branch for graphical support.
All authors declare no competing financial interests.
Supplementary Material
References
- 1.American Cancer Society. Cancer Facts & Figures 2018 Atlanta, GA: American Cancer Society; 2018.
- 2. Lin CH, Yap YS, Lee KH, et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians versus the US population. J Natl Cancer Inst. 2019;111(12):1298–1306 doi:10.1093/jnci/djz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomez SL, Von Behren J, McKinley M, et al. Breast cancer in Asian Americans in California, 1988-2013: increasing incidence trends and recent data on breast cancer subtypes. Breast Cancer Res Treat. 2017;164(1):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopez G, Ruiz NG, Patten E. Key facts about Asian Americans, a diverse and growing population. Pew Research Center. https://www.pewresearch.org/fact-tank/2017/09/08/key-facts-about-asian-americans/. Published Septemeber 8, 2017. Accessed December 2017.
- 5. Ziegler RG, Hoover RN, Pike MC, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85(22):1819–1827. [DOI] [PubMed] [Google Scholar]
- 6. Gomez SL, Quach T, Horn-Ross PL, et al. Hidden breast cancer disparities in Asian women: disaggregating incidence rates by ethnicity and migrant status. Am J Public Health. 2010;100(suppl 1):S125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Medhanie GA, Fedewa SA, Adissu H, et al. Cancer incidence profile in sub-Saharan African-born blacks in the United States: similarities and differences with US-born non-Hispanic blacks. Cancer. 2017;123(16):3116–3124. [DOI] [PubMed] [Google Scholar]
- 8. Davis Lynn BC, Rosenberg PS, Anderson WF, et al. Black-White breast cancer incidence trends: effects of ethnicity. J Natl Cancer Inst. 2018;110(11):1270–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gomez SL, Noone AM, Lichtensztajn DY, et al. Cancer incidence trends among Asian American populations in the United States, 1990-2008. J Natl Cancer Inst. 2013;105(15):1096–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson KN, Schwab RB, Martinez ME.. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat. 2014;144(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amadou A, Ferrari P, Muwonge R, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14(8):665–678. [DOI] [PubMed] [Google Scholar]
- 13. Amadou A, Hainaut P, Romieu I.. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol. 2013;2013:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute Surveillance, Epidemiology, and End Results Program SEER 9, (1990-2014) National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. http://www.seer.cancer.gov. Published 2017. Accessed September 21, 2017.
- 15.North American Association of Central Cancer Registries. NAACCR Race and Ethnicity Work Group NAACCR Asian Pacific Islander Identification Algorithm [NAPIIA v1.2.1] Springfield, IL: North American Association of Central Cancer Registries; 2011.
- 16. Anderson WF, Katki HA, Rosenberg PS.. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103(18):1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howlader N, Noone AM, Yu M, et al. Use of imputed population-based cancer registry data as a method of accounting for missing information: application to estrogen receptor status for breast cancer. Am J Epidemiol. 2012;176(4):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenberg PS, Barker KA, Anderson WF.. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J Natl Cancer Inst. 2015;107(9):djv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anderson WF, Rabkin CS, Turner N, et al. The changing face of noncardia gastric cancer incidence among US non-Hispanic whites. J Natl Cancer Inst. 2018;110(6):608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenberg PS, Check DP, Anderson WF.. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sung HN, Rosenberg PS, Chen WQ, et al. Female breast cancer incidence among Asian and Western populations: more similar than expected. J Natl Cancer Inst. 2015;107(7):djv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Perry CS, Otero JC, Palmer JL, et al. Risk factors for breast cancer in East Asian women relative to women in the West. Asia-Pac J Clin Oncol. 2009;5(4):219–231. [Google Scholar]
- 23. McCracken M, Olsen M, Chen MS, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57(4):190–205. [DOI] [PubMed] [Google Scholar]
- 24. Zong J, Batalova J. Asian immigrants in the United States. Migration Policy Institute. https://www.migrationpolicy.org/article/asian-immigrants-united-states. Published January 6, 2016. Accessed December 2017.
- 25. Lopez G, Cilluffo A, Patten E. Filipinos in the U.S. fact sheet. Pew Research Center. https://www.pewsocialtrends.org/fact-sheet/asian-americans-filipinos-in-the-u-s/. Published September 8, 2017. Accessed December 2017.
- 26. Lopez G, Cilluffo A, Patten E.. Japanese in the U.S. fact sheet. Pew Research Center. https://www.pewsocialtrends.org/fact-sheet/asian-americans-japanese-in-the-u-s/. Published September 8, 2017. Accessed December 2017.
- 27. Morey BN, Gee GC, von Ehrenstein OS, et al. Higher breast cancer risk among immigrant Asian American women than among US-born Asian American women. Prev Chronic Dis. 2019;16:180221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harding C, Pompei F, Burmistrov D, et al. Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med. 2015;175(9):1483–1489. [DOI] [PubMed] [Google Scholar]
- 29.Center for Disease Control and Prevention. QuickStats: Percentage of U.S. Women Aged 50-74 Years Who Have Never Had a Mammogram, by Place of Birth and Length of Residence in the United States-National Health Interview Survey, 2013 and 2015 Atlanta, GA: Center for Disease Control and Prevention; 2017:309.
- 30. Reeves TJ, Bennett CE.. We the People: Asians in the United States, Census 2000 Special Reports; Suitland, MD: U.S. Census Bureau; 2004.
- 31. Misra R, Menon U, Vadaparampil ST, et al. Age- and sex-specific cancer prevention and screening practices among Asian Indian immigrants in the United States. J Investig Med. 2011;59(5):787–792. [DOI] [PubMed] [Google Scholar]
- 32. Menon U, Szalacha LA, Prabhughate A.. Breast and cervical cancer screening among South Asian immigrants in the United States. Cancer Nurs. 2012;35(4):278–287. [DOI] [PubMed] [Google Scholar]
- 33. Oh KM, Taylor KL, Jacobsen KH.. Breast cancer screening among Korean Americans: a systematic review. J Community Health. 2017;42(2):324–332. [DOI] [PubMed] [Google Scholar]
- 34. Choi KS, Lee S, Park EC, et al. Comparison of breast cancer screening rates between Korean women in America versus Korea. J Womens Health. 2010;19(6):1089–1096. [DOI] [PubMed] [Google Scholar]
- 35. Yao NL, Hillemeier MM.. Disparities in mammography rate among immigrant and native-born women in the US: progress and challenges. J Immigr Minor Health. 2014;16(4):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sabatino SA, White MC, Thompson TD, et al. Cancer screening test use-United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(17):464–468. [PMC free article] [PubMed] [Google Scholar]
- 37. Lopez G, Cilluffo A, Patten E. Indians in the U.S. fact sheet. Pew Research Center. https://www.pewsocialtrends.org/fact-sheet/asian-americans-indians-in-the-u-s/. Published September 8, 2017. Accessed December 2017.
- 38. Lopez G, Cilluffo A, Patten E. Chinese in the U.S. fact sheet. Pew Research Center. https://www.pewsocialtrends.org/fact-sheet/asian-americans-chinese-in-the-u-s/. Published September 8, 2017. Accessed December 2017.
- 39. Lopez G, Cilluffo A, Patten E. Koreans in the U.S. fact sheet. Pew Research Center. https://www.pewsocialtrends.org/fact-sheet/asian-americans-koreans-in-the-u-s/. Published September 8, 2017. Accessed December 2017.
- 40. Lopez G, Cilluffo A, Patten E. Vietnamese in the U.S. fact sheet. Pew Research Center. https://www.pewsocialtrends.org/fact-sheet/asian-americans-vietnamese-in-the-u-s-fact-sheet/. Published September 8, 2017. Accessed December 2017.
- 41. Munoz D, Near AM, van Ravesteyn NT, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014;106(11):dju289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen MJ, Wu WY, Yen AM, et al. Body mass index and breast cancer: analysis of a nation-wide population-based prospective cohort study on 1 393 985 Taiwanese women. Int J Obes. 2016;40(3):524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tseng M, Byrne C, Evers KA, et al. Acculturation and breast density in foreign-born, U.S. Chinese women. Cancer Epidemiol Biomark Prev. 2006;15(7):1301–1305. [DOI] [PubMed] [Google Scholar]
- 45. Bae JM, Kim EH.. Breast density and risk of breast cancer in Asian women: a meta-analysis of observational studies. J Prev Med Public Health. 2016;49(6):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malik A, Jeyaraj PA, Shankar A, et al. Passive smoking and breast cancer-a suspicious link. Asian Pac J Cancer Prev. 2015;16(14):5715–5719. [DOI] [PubMed] [Google Scholar]
- 47. Nitta J, Nojima M, Ohnishi H, et al. Weight gain and alcohol drinking associations with breast cancer risk in Japanese postmenopausal women-results from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev. 2016;17(3):1437–1443. [DOI] [PubMed] [Google Scholar]
- 48. Iwasaki M, Tsugane S.. Risk factors for breast cancer: epidemiological evidence from Japanese studies. Cancer Sci. 2011;102(9):1607–1614. [DOI] [PubMed] [Google Scholar]
- 49. Wu AH, Vigen C, Razavi P, et al. Alcohol and breast cancer risk among Asian-American women in Los Angeles County. Breast Cancer Res. 2012;14(6): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kruk J. Association between vegetable, fruit and carbohydrate intake and breast cancer risk in relation to physical activity. Asian Pac J Cancer Prev. 2014;15(11):4429–4436. [DOI] [PubMed] [Google Scholar]
- 51. Lee H, Wang Q, Yang F, et al. SULT1A1 Arg213His polymorphism, smoked meat, and breast cancer risk: a case-control study and meta-analysis. DNA Cell Biol. 2012;31(5):688–699. [DOI] [PubMed] [Google Scholar]
- 52. Xie Q, Chen ML, Qin Y, et al. Isoflavone consumption and risk of breast cancer: a dose-response meta-analysis of observational studies. Asia Pac J Clin Nutr. 2013;22(1):118–127. [DOI] [PubMed] [Google Scholar]
- 53. Woo HD, Park KS, Shin A, et al. Glycemic index and glycemic load dietary patterns and the associated risk of breast cancer: a case-control study. Asian Pac J Cancer Prev. 2013;14(9):5193–5198. [DOI] [PubMed] [Google Scholar]
- 54. Tam C, Li Q, Friedenreich C, et al. Lifetime physical activity in postmenopausal Caucasian and Chinese-Canadian women. Eur J Cancer Prev. 2014;23(2):90–95. [DOI] [PubMed] [Google Scholar]
- 55. Wu AH, Ziegler RG, Pike MC, et al. Menstrual and reproductive factors and risk of breast cancer in Asian-Americans. Br J Cancer. 1996;73(5):680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nichols HB, Trentham-Dietz A, Love RR, et al. Differences in breast cancer risk factors by tumor marker subtypes among premenopausal Vietnamese and Chinese women. Cancer Epidemiol Biomarkers Prev. 2005;14(1):41–47. [PubMed] [Google Scholar]
- 57. Thompson D. Postmenopausal women should still steer clear of HRT: task force. Medical Express website. Published 2017. https://medicalxpress.com/news/2017-12-postmenopausal-women-hrt-task.html. Accessed December 2017.
- 58. Kawai M, Kakugawa Y, Nishino Y, et al. Reproductive factors and breast cancer risk in relation to hormone receptor and menopausal status in Japanese women. Cancer Sci. 2012;103(10):1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin CH, Chen YC, Chiang CJ, et al. The emerging epidemic of estrogen-related cancers in young women in a developing Asian country. Int J Cancer. 2012;130(11):2629–2637. [DOI] [PubMed] [Google Scholar]
- 60. Li H, Sun XZ, Miller E, et al. BMI, reproductive factors, and breast cancer molecular subtypes: a case-control study and meta-analysis. J Epidemiol. 2017;27(4):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu JQ, Li YY, Ren JC, et al. Induced abortion and breast cancer: results from a population-based case control study in China. Asian Pac J Cancer Prev. 2014;15(8):3635–3640. [DOI] [PubMed] [Google Scholar]
- 62. Killelea BK, Chagpar AB, Horowitz NR, et al. Characteristics and treatment of human epidermal growth factor receptor 2 positive breast cancer: 43,485 cases from the National Cancer Database treated in 2010 and 2011. Am J Surg. 2017;213(2):426–432. [DOI] [PubMed] [Google Scholar]
- 63. Pathmanathan N, Geng JS, Li WC, et al. Human epidermal growth factor receptor 2 status of gastric cancer patients in Asia: results from a large, multicountry study. Asia-Pac J Clin Oncol. 2017;13(3):249–260. [DOI] [PubMed] [Google Scholar]
- 64. Munoz DF, Plevritis SK.. Estimating breast cancer survival by molecular subtype in the absence of screening and adjuvant treatment. Med Decis Mak. 2018;38(suppl 1):32s–43s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.