Abstract
We propose a likely contribution to severe COVID-19 morbidity by extracellular DNA in neutrophil extracellular traps (NETs). Dornase alfa degrades extracellular DNA to reduce mucus rigidity and accumulation, and was associated with respiratory improvement in a first patient. Dornase alfa should be considered for clinical trials in treatment of severe COVID-19.
Keywords: acute respiratory distress syndrome, COVID-19, DNAse 1, dornase alfa, Neutrophil extracellular trap
Dornase alfa (Pulmozyme; Genentech, South San Francisco, CA, USA) is recombinant human deoxyribonuclease I, the only drug in its class that acts as a mucolytic by cleaving extracellular chromosomal DNA from neutrophil extracellular traps (NET) and other cell-free DNA. The drug's on-label clinical use is to reduce the viscosity and quantity of airway mucus in individuals with cystic fibrosis, thereby improving mucociliary clearance [1]. Dornase alfa is commonly used in individuals with cystic fibrosis, including those with severe complications requiring mechanical ventilation in intensive care units, and is compatible with co-administration of other routine drugs including antibiotics. Off-label use of dornase alfa includes reports treating acute respiratory distress syndrome (ARDS), where the drug can lead to mucus plug clearance and accelerated recovery in humans and mice [2,3]. A controlled clinical trial for treating ARDS with dornase alfa is currently underway [4]. In the Critical Care setting, rare and minor adverse effects associated with dornase alfa include voice alteration and rash [1].
The cellular and molecular mechanisms proposed for dornase alfa activity in severely distressed lungs of individuals with cystic fibrosis and many ARDS patients are as follows. Inflammation results in neutrophilia and neutrophil infiltration in the lungs, where these cells produce NETs, largely comprised of sticky, large chromosomal DNA that physically reinforces airway mucus viscosity and accumulation [5,6]. Thick mucus that clears poorly can lead to airway obstruction, bronchiectasis, lung injury, hypoxia and respiratory failure. Dornase alfa facilitates airway clearance by breaking up reinforcement of mucus by NETs, by far the greatest source of extracellular DNA in inflamed lungs [5,6].
Severe cases of coronavirus disease 2019 (COVID-19) have shown an inflammatory neutrophil and mucus-mediated airway exclusion pathway with striking similarities to that described above (Fig. 1). Unlike mild COVID-19, which is often associated with fever and upper-airway symptoms, individuals with severe COVID-19 often progress to an ARDS condition: hypoxaemic respiratory failure associated with neutrophilia and neutrophil infiltration in the lungs, thick mucus in bronchi, and bronchiectasis [[7], [8], [9], [10]]. Because lung neutrophilia in ARDS is generally known to involve high NET production [5], we feel it is rational to hypothesize that NETs contribute to severe pathology in COVID-19. Indeed, lung neutrophilia and NET production have been shown to contribute to the development of ARDS in other severe viral respiratory infections, including H1N1 influenza [11].
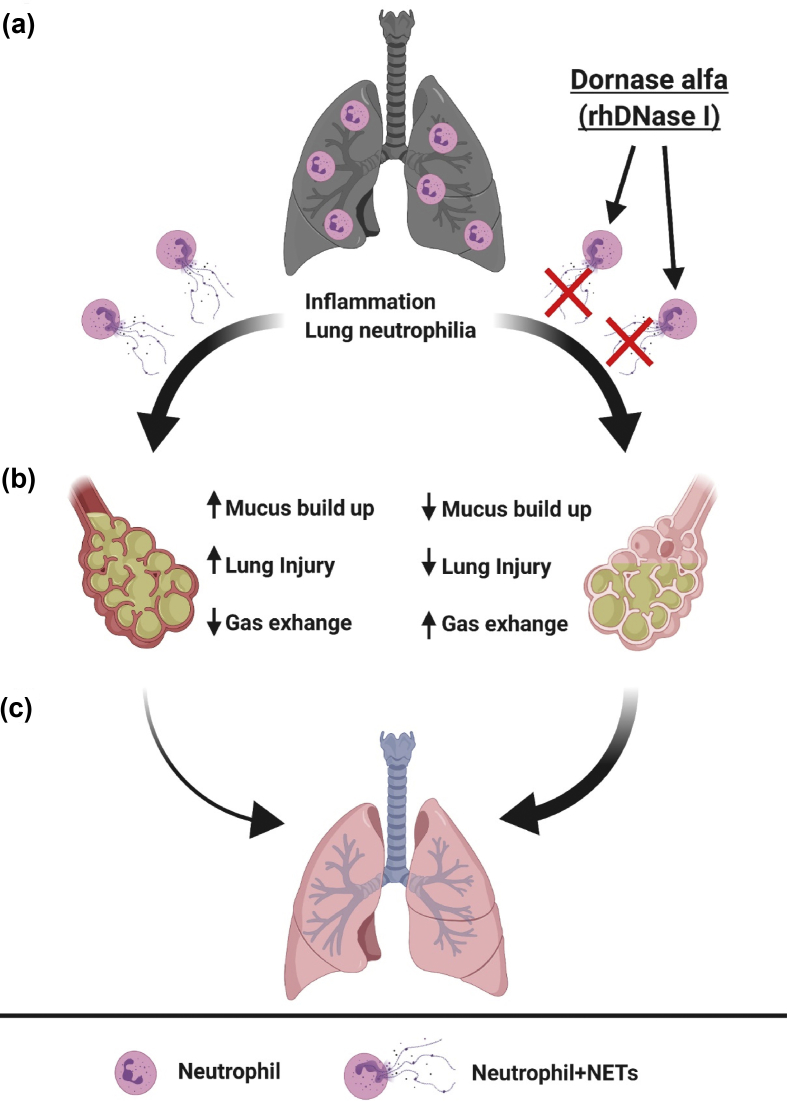
Fig. 1.
Model of how dornase alfa-sensitive neutrophil extracellular traps (NETs) from neutrophils may reinforce mucus accumulation, rigidity and airway occlusion in severe coronavirus disease 2019 (COVID-19). (a) Viral infection causes inflammation and respiratory distress (darkened lungs). Lung-infiltrating neutrophils produce NETs whose large quantities of chromosomal, extracellular DNA are susceptible to degradation by dornase alfa (recombinant human deoxyribonuclease I, right). (b) Close up view of alveoli. Without dornase alfa treatment (left), NETs reinforce the accumulation and rigidity of mucus that can increase lung injury and reduce oxygenation. Dornase alfa treatment (right) reduces NET-mediated reinforcement of mucus, making it less rigid (lighter yellow) and facilitating mucus clearance, so reducing lung injury and increasing gas exchange. (c) The rate at which recovery from severe COVID-19 occurs naturally (left, thin arrow) might be increased by dornase alfa treatment (right, thicker arrow). Figure was created with BioRender.
We postulate that nebulized dornase alfa may effectively treat a deleterious effect of NETs in the airways and so promote recovery in individuals with COVID-19-related ARDS (Fig. 1). Dornase alfa can be easily administered to mechanically ventilated patients and is well tolerated in intensive care unit settings. Anecdotally, an individual with COVID-19 who had been intubated for 5 days was given 3 days of nebulized dornase alfa (2.5 mg twice daily) with continued standard intensive care unit care. Improvements in oxygenation and lung compliance were observed comparing before with after the 3-day period (changes: arterial oxygen partial pressure (PaO2)/fractional inspired oxygen (FiO2) = P/F, 212 to 305; FiO2, 65% to 40%; positive end-expiratory pressure (PEEP), 20 to 14). Four additional days were followed by extubation and six more days in hospital before the patient was considered recovered and discharged home. At this juncture of a rapidly evolving pandemic associated with high mortality in severely ill individuals and the concepts discussed above, we suggest the consideration of including inhaled dornase alfa in clinical trials for severe COVID-19 associated with ARDS.
Funding
AE and AGS were supported by the University of Missouri Biomedical Innovation recruitment funds, and AGS was supported by NIH grant R01GM103841.
Author contributions
AE contributed to conceptualization, investigation and writing the original draft; ZMH contributed to investigation, writing, review and editing; and HVH and AGS contributed to writing, review, editing and supervision.
Conflicts of interest
The authors declare no conflicts of interest and no relation with Genentech, Inc.
Acknowledgements
We thank William Boyan (Pulmonary Critical Care, Providence Medical Group, Olympia, Washington) and Eva Carmona, Patricio Escalante and Tobias Peikert (Pulmonary & Critical Care, Mayo Clinic, Rochester, Minnesota) for helpful discussions.
Contributor Information
A.P. Earhart, Email: apey5x@mail.missouri.edu.
A.G. Schrum, Email: schruma@health.missouri.edu.
References
- 1.Yang C., Montgomery M. Dornase α for cystic fibrosis. Cochrane Database Syst Rev. 2018;9:CD001127. doi: 10.1002/14651858.CD001127.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris C., Mullan B. Use of dornase α in the management of ARDS. Anaesthesia. 2004;59:1249. doi: 10.1111/j.1365-2044.2004.04018.x. [DOI] [PubMed] [Google Scholar]
- 3.Riethmueller J., Borth-Bruhns T., Kumpf M., Vonthein R., Wiskirchen J., Stern M. Recombinant human deoxyribonuclease shortens ventilation time in young, mechanically ventilated children. Pediatr Pulmonol. 2006;41:61–66. doi: 10.1002/ppul.20298. [DOI] [PubMed] [Google Scholar]
- 4.Pottecher J., Noll E., Borel M., Audibert G., Gette S., Meyer C. Protocol for TRAUMADORNASE: a prospective, randomized, multicentre, double-blinded, placebo-controlled clinical trial of aerosolized dornase α to reduce the incidence of moderate-to-severe hypoxaemia in ventilated trauma patients. Trials. 2020;21:274. doi: 10.1186/s13063-020-4141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng O.Z., Palaniyar N. NET balancing: a problem in inflammatory lung diseases. Front Immunol. 2013;4:1. doi: 10.3389/fimmu.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Aleman S.R., Campos-Garcia L., Palma-Nicolas J.P., Hernandez-Bello R., Gonzalez G.M., Sanchez-Gonzalez A. Understanding the entanglement: neutrophil extracellular traps (NETs) in cystic fibrosis. Front Cell Infect Microbiol. 2017;7:104. doi: 10.3389/fcimb.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health . WHO; Geneva: 2020. Organization report of the WHO—China joint mission on coronavirus disease 2019 (COVID-19) [Google Scholar]
- 8.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020 doi: 10.1007/s00330-020-06801-0. epub ahead of print. PubMed PMID: 32193638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang G., Zhang J., Wang B., Zhu X., Wang Q., Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Resp Res. 2020;21(1):74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]