Abstract
During virus infection, host toll-like receptors (TLRs) can recognize different pathogen-associated molecular patterns and trigger the innate immune response. TLR7/8 can identify the single-stranded RNA (ssRNA) of the virus. This study aimed to search ssRNA sequences recognized by TLR7/8 from the SARS-CoV-2, SARS-CoV, and MERS-CoV whole genomes by a bioinformatic technique. The immunoinformatic approach showed that the SARS-CoV-2 genome has more ssRNA fragments that could be recognized by TLR7/8 than the SARS-CoV genome. These findings suggest innate immune hyperactivation by SARS-CoV-2. This activity is possibly able to provoke a robust proinflammatory response via TLR7/8 recognition and cause acute lung injury.
Keywords: SARS-CoV-2, SARS, MERS, TLR7, TLR8
The novel coronavirus (SARS-CoV-2) represents a public health emergency of international concern [1]. As of 10th March 2020, the death toll from the novel coronavirus stood at 4,012, with more than 113,702 confirmed cases in China, as well as cases in 109 other countries [2]. Coronaviruses are single, positive-sense RNA viruses belonging to the family Coronaviridae, which includes Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV) [3].
Viral interactions with the host immune system play a central role in the outcome of infection. In the initial phase, recognition of evolutionarily conserved microbial structures, known as pathogen-associated molecular patterns (PAMPs), is an essential function of the innate immune system [4]. Germ line-encoded pattern recognition receptors (PRRs) are proteins expressed by a variety of cells and are responsible for sensing the presence of PAMPs. Sensing of PAMPs by PRRs markedly upregulates the transcription of genes involved in inflammatory responses [4].
Toll-like receptors (TLRs) belong to a conserved family of innate immune recognition receptors acting as the primary sensors of specific PAMPs expressed by numerous pathogens [5]. The human TLR family comprises 11 members, of which TLR-3, -7, and -8 are essential in recognition of structural components of RNA viruses [[6], [7], [8]]. TLR7 senses single-stranded RNA (ssRNA) oligonucleotides containing guanosine- and uridine-rich sequences from RNA viruses [6]. Recognition occurs in the endosomes of plasmacytoid dendritic cells (DCs) and B cells. TLR8 is phylogenetically and functionally closely related to TLR7 and recognizes ssRNA. It is preferentially expressed in myeloid DCs and monocytes [7].
In this study, we searched ssRNA fragments in the whole genome from SARS-CoV, MERS-CoV, and SARS-CoV-2 (from different geographical origins mainly from Germany [SARS-CoV-2/Germany] and Wuhan [SARS-CoV-2/Wuhan]) by a bioinformatics scanning technique to reveal important TLR7/8 recognition sites in the whole genome of these.
1. Materials and methods
1.1. Bioinformatics analysis of ssRNA sequences
All genomic sequences were collected on 31 January 2020 from GenBank or Gisaid [9]. Those of SARS coronavirus NC_004718.3; Middle East respiratory syndrome coronavirus NC_019843.3; BetaCoV/Wuhan/IPBCAMS-WH-01/2019 EPI_ISL_402123, and BetaCoV/Germany/BavPat1/2020 EPI_ISL_406862 were employed. SequenceSearcher software (http://athena.bioc.uvic.ca/tools/SequenceSearcher) [10] was used to perform motif ssRNA searches for the whole-genome sequences of SARS-CoV, MERS-CoV, SARS-CoV-2/Wuhan, and SARS-CoV-2/Germany.
2. Results
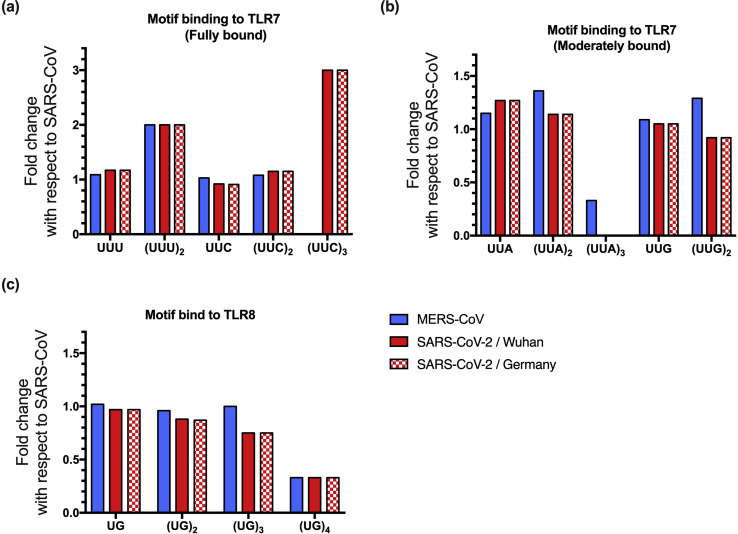
Crystallographic, biophysical, and cellular data have established ssRNA sequence preferences for TLR7. The UU(U/C) motif fully binds to TLR7, followed by the UU(G/A) motif (moderate binding) [11]. Therefore, we investigated the number of UUU, UUC, UUG, and UUA ssRNA fragments in the whole genome sequences of SARS-CoV-2/Wuhan, SARS-CoV-2/Germany, SARS-CoV and MERS-CoV. The four virus genomes analyzed showed similar amounts of U and G nucleotides, and the full lengths of each genome are comparable (Table 1 ). The SARS-CoV-2/Wuhan genome presented 708 UUU ssRNA fragments, which represents 17.4% more fragments than the SARS-CoV genome (1.17-fold change) and 8.1% more than the MERS-CoV genome (1.08-fold change). No difference was found in the number of UUU ssRNA fragments between the SARS-CoV-2/Wuhan genome and the SARS-CoV-2/Germany genome isolated on January 28, 2020 in Germany (only two additional sequences) (Table 1 and Fig. 1 ).
Table 1.
Analysis of the number of ssRNA fragments from the coronavirus whole genome recognized by TLR7/8.
| Genome | SARS-CoV | MERS-CoV | SARS-CoV-2/Wuhan | SARS-CoV-2/Germany |
|---|---|---|---|---|
| Reference Sequence: | NC_004718.3 | NC_019843.3 | EPI_ISL_402123 | EPI_ISL_406862 |
| U % (bp) | 30.73 (9143) | 32.94 (9799) | 32.24 (9593) | 32.16 (9567) |
| A % (bp) | 28.51 (8481) | 26.55 (7900) | 30.08% (8949) | 29.89 (8893) |
| C% (bp) | 19.97 (5940) | 20.56 (6116) | 18.46 (5492) | 18.39 (5471) |
| G % (bp) | 20.80 (6187) | 21.19 (6304) | 19.71 (5865) | 19.67 (5851) |
| Total nucleotides (bp) | 29,751 | 30,119 | 29,899 | 29,782 |
| The number of oligonucleotide fragments recognized by TLR7 | ||||
| Motif fully bound | ||||
| UUU | 603 | 655 | 708 | 706 |
| (UUU)2 | 2 | 4 | 4 | 4 |
| UUC | 563 | 581 | 518 | 513 |
| (UUC)2 | 13 | 14 | 15 | 15 |
| (UUC)3 | 1 | 0 | 3 | 3 |
| Motif moderately bound | ||||
| UUA | 689 | 791 | 876 | 873 |
| (UUA)2 | 22 | 30 | 25 | 25 |
| (UUA)3 | 3 | 1 | 0 | 0 |
| aUUG | 776 | 846 | 817 | 817 |
| (UUG)2 | 24 | 31 | 22 | 22 |
| (UUG)3 | 0 | 1 | 0 | 0 |
| The number of oligonucleotide fragments recognized by TLR8∗ | ||||
| UG | 2664 | 2704 | 2589 | 2586 |
| (UG)2 | 199 | 191 | 175 | 174 |
| (UG)3 | 16 | 16 | 12 | 12 |
| (UG)4 | 3 | 1 | 1 | 1 |
| (UG)5 | 1 | 0 | 0 | 0 |
UUG motifs are also recognized by TLR8.
Fig. 1.
Comparison of the number of ssRNA fragments from coronavirus genomes recognized by TLR7/8. The number of oligonucleotide fragments found in the SARS-CoV-2, MERS-CoV and 2020-nCoV genomes with respect to the SARS-CoV genome are shown by fold change. (A) Oligonucleotide fragments fully bound to TLR7, (B) oligonucleotide fragments moderately bound to TLR7, and (C) oligonucleotide fragments bound to TLR8. UUG motifs are also recognized by TLR8.
Interestingly, (UUU)2 repeated motifs were more abundant in the SARS-CoV-2/Wuhan and SARS-CoV-2/Germany genomes (n = 4) than in the SARS-CoV genome (n = 2) and equally represented in the MERS-CoV genome (Table 1 and Fig. 1).
The SARS-CoV-2/Wuhan genome presented 518 UUC ssRNA fragments, which was less than the number in the SARS-CoV genome (n = 563). However, (UUC)2 repeated motifs were 13.6% more abundant in the SARS-CoV-2/Wuhan genome than in the SARS-CoV genome (1.15-fold change) (Table 1 and Fig. 1).
Regarding UUA/G ssRNA fragments, show a lower binding affinity than the other motifs to TLR7, we found 27.1% (UUA; 1.27-fold change) and 5.3% (UUG; 1.05-fold change) more ssRNA fragments in the SARS-CoV-2 genome than in the SARS-CoV genome. No notable differences were found in the number of these ssRNA fragments between the SARS-CoV-2/Wuhan and SARS-Cov-2/Germany genomes (Table 1 and Fig. 1).
A study published by Tanji Hiromi et al. [12] revealed that in the crystallographic structure of TLR8–ORN06 (UUG6UU), only two or three nucleotides interact in the second active site of TLR8, namely, UG or UUG oligonucleotides [12]. Therefore, we investigated the number of UG ssRNA fragments in the whole-genome sequences of SARS-CoV-2, SARS-CoV and MERS-CoV. The number of (UG)n ssRNA motifs was always lower in the SARS-CoV-2/Wuhan genome than in the SARS-CoV genome. However, the number of UUG ssRNA fragments was higher in the SARS-CoV-2/Wuhan genome than in the SARS-CoV genome, as previously shown in the TLR7 analysis (Table 1 and Fig. 1).
Therefore, it can be speculated that the SARS-CoV-2 genome contains more ssRNA fragments with the possibility of interacting with TLR7/8 than the SARS-CoV genome. Accordingly, SARS-CoV-2 has many more chances than SARS-CoV to contact the host innate immune system, and SARS-CoV-2 has the similar probability than MERS-CoV to interact with TLR7/8.
3. Discussion
The SARS-CoV-2, SARS-CoV, and MERS-CoV infections show several similarities regarding the clinical presentations, which can vary from asymptomatic infection to severe disease [13]. Additionally, a cytokine-storm has been observed in the rapid course of SARS [14,15] and MERS [16,17], and in the serum of SARS-CoV-2-infected patients, proinflammatory cytokines are upregulated [18]. It was inferred that an overactive innate immune response could contribute to virus-induced immune pathology resulting in acute lung injury in the infected patients. Therefore, in this study, based on the essential structural feature of the PAMP-PRR complex, specifically the interaction of TLR7/TLR8 with ssRNA [11,12], we searched ssRNA fragments with a pathogenic molecular pattern from the SARS-CoV-2, MERS-CoV and SARS-CoV whole genomes.
Our bioinformatic analysis showed that the SARS-CoV-2 genome contains a large number of fragments that could be recognized by TLR7/8, and it even contains more fragments than the SARS-CoV genome. This result suggests the ability to induce a rapid type I interferon response [7]. The production of type I IFNs (primarily alpha IFN [IFN-α] and IFN-β) plays a central role in the induction of antiviral responses. Type I IFNs influence protein synthesis, growth regulation, and apoptosis and enhance the maturation of DCs, the cytotoxicity of natural killer cells, and the differentiation of virus-specific cytotoxic T lymphocytes [19]. However, innate immune hyperactivation could be involved in the dysregulation of a series of proinflammatory cytokines during viral infection [20].
The recognition of ssRNA fragments by TLR7/8 could depend on the virus replication manner, so that a more robust proinflammatory response should occur after a latent period, except for the host immune cell taking up viral particles. This effect is feasible because a number of immune cells display distinct susceptibility to MERS-CoV [17] and SARS-CoV [21].
Interestingly, in addition to its expression in immunological cells, TLR7/8 is expressed predominantly in the lung, bronchus, breast, rectum, smooth muscle tissue, cerebral cortex, and kidney [22]. Although TLR7 and TLR8 are phylogenetically and structurally related, TLR7- and TLR8-specific agonists trigger different cytokine induction profiles. TLR7-specific agonists generally induce IFN-regulated cytokines, but TLR8-specific agonists lead primarily to the production of proinflammatory cytokines [23]. These factors may provide SARS-CoV-2 with a shortcut to trigger an innate immune response through TLR7/8 and ultimately contribute to the development of immune pathology within the lungs.
Most likely, additional pathogenic molecular patterns can be recognized by other innate immune receptors, such as SARS-CoV spike protein, which was observed to be a TLR2 ligand [24], and damage-associated molecular patterns (DAMPs) that are released in response to tissue damage from cells killed by viruses [25]. All of these factors also probably contribute to the cytokine storm. The interaction between viral PAMPs and PRRs in immune cells plays an essential survival role in the response to viral infections but may be simultaneously responsible for tissue injury associated with severe virus-induced inflammation.
For positive-sense RNA viruses such as coronavirus, it is known that viral genomic ssRNA is recognized by either the endosomal RNA receptors (TLR7/TLR8), and the cytosolic RNA sensors, such as retinoid-inducible gene-1 (RIG1), melanoma differentiation-associated gene 5 (MDA5), laboratory of genetics and physiology 2 (LGP2), and cytoplasmic protein kinase R (PKR) [26]. Nevertheless, the consensus definition of cytosolic RNA sensor ligands remains controversial, and the crystal structures with their RNA sequence-specific are not available. For this reason, the cytosolic sensors were not included in the bioinformatic analysis.
In conclusion, the SARS-CoV-2 genome contains more ssRNA fragments that could be recognized by TLR7/8 than the SARS-CoV genome and similar ssRNA fragments than the MERS-CoV genome; possibly making it able to provoke a robust proinflammatory response via TLR7/8 recognition and cause acute lung injury, leading to death. These bioinformatic findings suggest that SARS-CoV-2 plays a crucial role in the overactive innate immune response.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World Health Organization; 2020. Coronavirus disease (COVID-2019) situation report – 50. [Google Scholar]
- 3.Masters P.S. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medzhitov R., Janeway C., Jr. Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 5.Imler J.L., Hoffmann J.A. Toll receptors in innate immunity. Trends Cell Biol. 2001;11:304–311. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- 6.Diebold S.S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 7.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 9.Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data - from vision to reality. Euro Surveill. 2017;22 doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marass F., Upton C. Sequence Searcher: a Java tool to perform regular expression and fuzzy searches of multiple DNA and protein sequences. BMC Res Notes. 2009;2:14. doi: 10.1186/1756-0500-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z., Ohto U., Shibata T., Taoka M., Yamauchi Y., Sato R. Structural analyses of toll-like receptor 7 reveal detailed RNA sequence specificity and recognition mechanism of agonistic ligands. Cell Rep. 2018;25:3371–33781 e5. doi: 10.1016/j.celrep.2018.11.081. [DOI] [PubMed] [Google Scholar]
- 12.Tanji H., Ohto U., Shibata T., Taoka M., Yamauchi Y., Isobe T. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat Struct Mol Biol. 2015;22:109–115. doi: 10.1038/nsmb.2943. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92:491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu H., Zhou J., Wong B.H., Li C., Cheng Z.S., Lin X. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;454–455:197–205. doi: 10.1016/j.virol.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou J., Chu H., Li C., Wong B.H., Cheng Z.S., Poon V.K. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J Infect Dis. 2014;209:1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesev E.V., LeDesma R.A., Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol. 2019;4:914–924. doi: 10.1038/s41564-019-0421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 21.Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papatheodorou I., Fonseca N.A., Keays M., Tang Y.A., Barrera E., Bazant W. Expression Atlas: gene and protein expression across multiple studies and organisms. Nucleic Acids Res. 2018;46:D246–D251. doi: 10.1093/nar/gkx1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorden K.B., Gorski K.S., Gibson S.J., Kedl R.M., Kieper W.C., Qiu X. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 24.Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vabret N., Blander J.M. Sensing microbial RNA in the cytosol. Front Immunol. 2013;4:468. doi: 10.3389/fimmu.2013.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]