Abstract
SARS-CoV-2 is a novel strain, causing a global pandemic since the end of 2019. The majority of patients showed nonspecific symptoms such as fever, dry cough, and fatigue. Most patients have a good prognosis while some with severe conditions could rapidly progress to acute respiratory distress syndrome, septic shock, metabolic acidosis, coagulation dysfunction, and even die. The exacerbation of the patient's condition may be due to a cytokine storm in the body. Effective targeted therapies including antiviral and immunization are urgently needed. Although many clinical trials are already underway and the majority of patients have received antiviral therapy based on medication experience with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), and preliminary results from some clinical trials, there are no antiviral drugs proven to be effective currently. We summarize the current therapeutic medicines used in the clinic, hope to be able to provide some implications for clinical medication.
Keywords: COVID-19, Therapy, Antiviral, Immunization
1. Introduction
In late December 2019, an outbreak of SARS-like pneumonia caused by a novel coronavirus occurred in the Chinese city of Wuhan, which was officially named as COVID-19 (coronavirus diseases 2019) by the World Health Organization (WHO) later and the novel coronavirus was designated SARS-COV-2, spreading nationwide and across the world. On March 11th, 2020, WHO assessed that COVID-19 could be considered as a pandemic. So far, the quantity of COVID-19 diagnosed worldwide is 132,758, and the death toll is 5420 as of March 13th, 2020 [1].
The majority of patients infected with SARS-CoV-2 showed nonspecific symptoms, fever, dry cough, fatigue, along with pneumonia demonstrated on chest CT scan imaging, and have a mild condition with a case fatality rate (CFR) around 2 % [2]. However, among elderly patients and patients with chronic underlying diseases such as hypertension, diabetes, there is a higher rate of transmission to the intensive care unit (ICU) and mortality [3].
Given SARS-CoV-2 is a novel strain, causing a global pandemic, effective targeted therapies are urgently needed. However, no proven effective antiviral or immunomodulatory therapies exist for the treatment of COVID-19 [4]. Most patients have received numerous potentially targeted therapies as described so far based on medication experience with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), which mostly belong to existing antiviral agents approved or in development for treating infections caused by human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV) and influenza, and a section of patients have participated in ongoing uncontrolled clinical trials.
In this review, we summarize the current therapeutic medicines used in the real world according to possible mechanisms based on Chinese clinical experience, hoping to provide some implications for clinical medication.
2. Antiviral therapies
2.1. Remdesivir
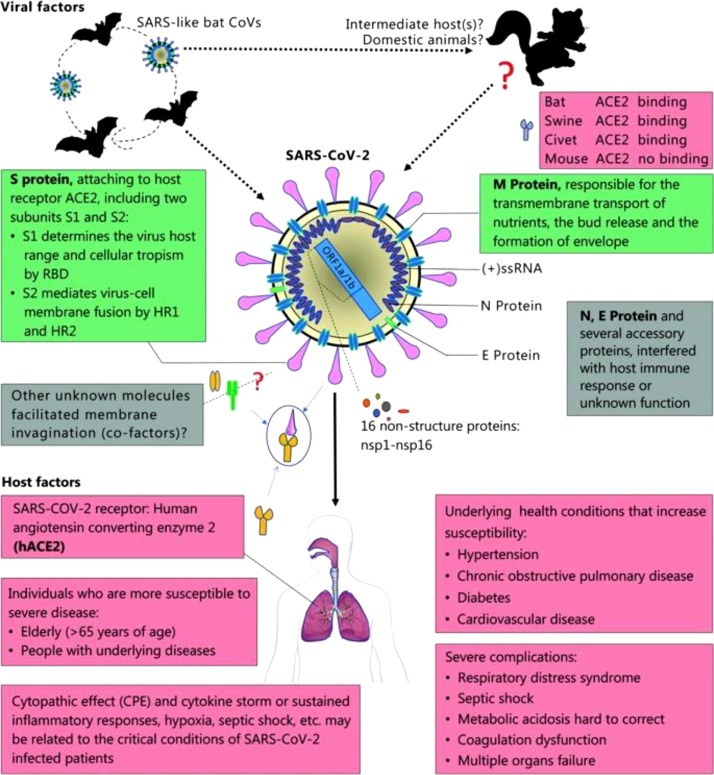
Remdesivir is a nucleoside analog effective against a variety of viruses, including MERS-CoV and SARS-CoV in vitro and animal models [5,6], and Phase III clinical trial for inhibiting Ebola virus are ongoing [7]. Research has revealed that in vitro, remdesivir has the ability to inhibit SARS-COV-2 recently [8]. The first COVID-19 case in the US has improved condition and declined the viral load after treated with remdesivir on illness Day 7, suggesting that remdesivir has the potential to treat COVID-19. Recently, the results of a trial of remdesivir in the treatment of patients with severe COVID-19 under sympathetic medication were published. The data have shown that 68 % of severe patients have relieved symptoms after using remdesivir and the mortality of those patients is 13 %, which is noteworthy though these findings need to be confirmed in the ongoing randomized, placebo-controlled trials of remdesivir therapy for COVID-19 [9]. Remdesivir is an investigational agent not commercially available, safety and efficacy have not been established in COVID-19 patients. It is available as part of several ongoing clinical trials for adult and nonpregnant patients, while individual compassionate use requests are limited to pregnant women or pediatric patients <18 years of age with confirmed COVID-19 and severe disease. Although there are no clear contraindications, in the population included in clinical trials (NCT04257656, NCT04252664, NCT04292899), people with a creatinine clearance below 30 mL per minute and serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) more than five times the upper limit of the normal range were excluded. Therefore, patients with liver and kidney function impairment should be used with caution (Fig. 1 ).
Fig. 1.
Viral and host factors that influence the pathogenesis of SARS-CoV-2. Bats are the reservoir of a wide variety of coronaviruses, including severe acute respiratory syndrome coronavirus (SARS-CoV) -like viruses. SARS-CoV-2 may originate from bats or unknown intermediate hosts and cross the species barrier into humans. Virus-host interactions affect viral entry and replication. Upper panel: Viral factor. SARS-CoV-2 is an enveloped positive single-stranded RNA (ssRNA) coronavirus. Two-thirds of viral RNA, mainly located in the first open reading frame (ORF 1a/b), encodes 16 non-structure proteins (NSPs). The rest part of the virus genome encodes four essential structural proteins, including spike (S) glycoprotein, small envelope (E) protein, matrix (M) protein, and nucleocapsid (N) protein, and also several accessory proteins. S glycoprotein of SARS-CoV-2 binds to host cell receptors, angiotensin-converting enzyme 2 (ACE2), that is a critical step for virus entry. The possible molecules facilitated membrane invagination for SARS-CoV-2 endocytosis are still unclear. Other virus proteins may contribute to pathogenesis. Host factors (Lower panel) can also influence susceptibility to infection and disease progression. The elderly and people with underlying disease are susceptible to SARS-CoV-2 and tend to develop into critical conditions. RBD, receptor-binding domain; HR1, heptad repeats 1; HR2, heptad repeats 2.
The pictures and notes are from Guo et al. [83].
2.2. Chloroquine and hydroxychloroquine
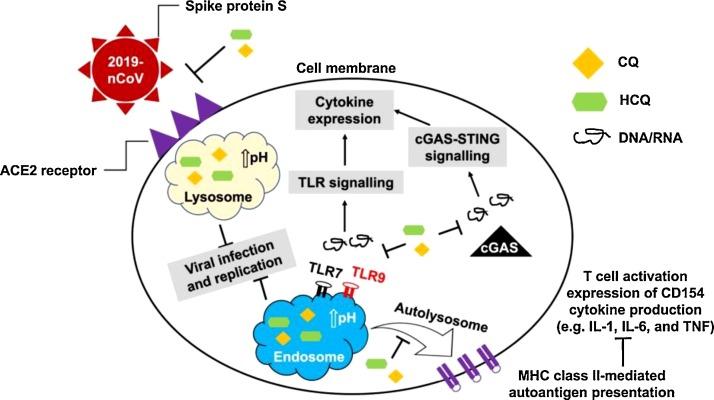
Chloroquine is a drug widely used in the treatment of malaria and autoimmune diseases [10], reported to having broad-spectrum antiviral potential in2006 [11]. In a recent study [8], chloroquine was found to inhibit SARS-COV-2 at low-micromolar (EC50 = 1.13 μM in Vero E6 cells), which can be clinically achievable based on experience with rheumatoid arthritis [12]. In addition, time-of-addition assay [8] demonstrated that chloroquine functioned at both entry and post-entry stages of the 2019-nCoV infection in Vero E6 cells. The antiviral activity of chloroquine may be due to the following three ways (Fig. 2 ): 1. reduce the ACE2 receptor terminal glycosylation on the surface of cells thus interfere with the binding of SARS-COV-2 to the ACE2 receptor [13]; 2. increase the pH of endosomes and lysosomes to prevent the fusion process of the virus with host cells and subsequent virus replication [14,15]; 3. impede pro-inflammatory signaling activation and production of cytokines (e.g., IL-1, IL-6, and TNF) by inhibiting lysosomal activity in antigen-presenting cells (APCs), interrupting binding between toll-like receptors (TLR7 and TLR9) and their RNA/DNA ligands, and interfering with the interaction between cytosolic DNA and the nucleic acid sensor cyclic GMP-AMP synthase (cGAS). The press released from the State Council of China indicated that chloroquine phosphate had demonstrated marked efficacy in preventing the deterioration of COVID-19 related pneumonia in multicenter clinical trials conducted in China on February 17, 2020 [16]. The latest guideline [17] recommended for adults 18–65 years old (500 mg each time, 2 times/day lasts 7days for patients who weigh more than 50 kg, 500 mg each time, 2 times/day lasts 2 days, and 500 mg each time, 1 time/day lasts 5 days for patients who weigh less than 50 kg), banned for people with underlying heart disease due to its cardiotoxicity. There are currently more than 20 clinical trials of chloroquine registered in the China Clinical Trial Registry.
Fig. 2.
A graphical illustration of the antiviral mechanisms of chloroquine (CQ) and hydroxychloroquine (HCQ).
The picture is come from Zhou et al. [84].
Hydroxychloroquine is an analogue of chloroquine with similar therapeutic effects and significantly reduced toxic side effects [18]. Besides, in vitro, hydroxychloroquine exhibited better anti-SARS-CoV-2 activity than chloroquine [19]. Comprehensive treatment and management of coronavirus disease 2019: an expert consensus statement from Shanghai [20] recommended hydroxychloroquine in an attempt of antiviral treatment; however, no dosage recommendations were made. Peking University Third Hospital established a physiology-based pharmacokinetic PBPK model based on the data of the hydroxychloroquine and chloroquine in vitro to simulate the distribution concentration of the drug in the lung, whole blood, and plasma after different dosing regimens. According to the results of the PBPK model, it is recommended that the oral loading dose of hydroxychloroquine 400 mg bid on the first day and the maintenance dose of 200 mg bid continue to be treated for four days. On the fifth day, the lung titer can reach more than three times the oral chloroquine phosphate (500 mg twice daily) [19]. This dose was subsequently confirmed in the first randomized controlled clinical trial (ChiCTR2000029559) of hydroxychloroquine for COVID-19, which enroll 62 patients with COVID-19 (31 received HCQ (400 mg/d, 200 mg bid), another 31 entered the control group), have found that after 5 days, the clinical recovery time of the hydroxychloroquine group was significantly shortened, and the symptoms of fever and cough were relieved faster (p < 0.05). 81 % of patients in the hydroxychloroquine group had significantly improved pneumonia, compared with 55 % in the control group (p < 0.05). All patients who progressed to critical illness were in the control group. This study affirmed the therapeutic effect of hydroxychloroquine [21].
Recently, in a clinical trial [22] involving 36 patients with COVID-19 shown the combination of hydroxychloroquine and azithromycin has a full and rapid viral clearance, the effects of which is better than hydroxychloroquine alone. The mechanism by which azithromycin enhances the efficacy of COVID-19 is unclear, but azithromycin has previously been shown to prevent severe respiratory infections in patients infected with the virus [23]. However, Jean et al. [24] have not observed similar results in 11 COVID-19 patients who have received the combination therapy of hydroxychloroquine and azithromycin. Given the trial is not an RCT, the number of cases is small, and the control group is from other clinical centers. An expanded clinical trial with good quality control is needed to confirm. In addition, the simultaneous use of hydroxychloroquine and azithromycin can lead to a prolonged QT interval and increase the risk of tip torsion arrhythmia, so careful evaluation is required.
Due to the cardiac and retinal toxicity, acute lethal risk of chloroquine and hydroxychloroquine, when chloroquine or hydroxychloroquine is used, electrocardiogram monitoring should be performed, and attention should be paid to changes in patients' vision and neurological symptoms.
2.3. Favipiravir
Favipiravir (T-705) belongs to nucleoside analogs like ribavirin, approved for the treatment of novel or re-emerging pandemic influenza in China and Japan, effectively against multiple viruses like influenza, Ebola, yellow fever, chikungunya, norovirus, and enterovirus [25]. Favipiravir is teratogenic and is strictly prohibited during pregnancy and lactation [26,27]. Wang et al. reported its activity against 2019-nCoV (EC50 = 61.88 μM in Vero E6 cells) [8], and subsequently, more than 10 clinical trials of favipiravir for the treatment of COVID-19 has been registered. The preliminary results from one of the clinical trials (ChiCTR2000029600) showed that group treated with favipiravir (35 cases) has shorter median time to virus clearance (4 days vs. 11 days) high improvement rates of chest image (91.43 % vs. 62.22 %) than control group treated with lopinavir and ritonavir (45 cases) with significant differences between the two groups, and adverse reactions were lower than that in the control group. The Zhongnan Hospital of Wuhan University initiated a multicenter, randomized, open, and positive, parallel-controlled clinical study of favipiravir in the treatment of COVID-19 (ChiCTR2000030254), with 240 patients enrolled and clinical treatment observations completed. The results showed that the experimental group (treated with favipilavir, 120 cases) is significantly better than the control group (treated with arbidol, 120 cases) in the treatment of COVID-19. In terms of secondary indicators, the clinical recovery rate of day 7 was significantly better in the experimental group than in the control group, which were 71.43 % and 55.86 %, respectively, in terms of secondary indicators, the time from randomization to fever reduction (2.5 days vs. 4.2 days), cough relief (4.57 days vs. 5.98 days) and the rate of auxiliary oxygen therapy or noninvasive mechanical ventilation (8.16 % vs. 17.12 %) during the trial in the experimental group was significantly better than the control group. In view of the good safety of favipiravir, and the drugs are available, experts have been formally recommended to be included in the guideline as soon as possible [28].
2.4. Arbidol
Arbidol has been licensed in Russia and China against upper respiratory tract infections caused by influenza A and B viruses. It is contraindicated in patients allergic to this product. Antiviral mechanism of arbidol is by inhibiting hemagglutininase, a protease on the surface of the influenza virus that binds to sialic acid receptors on human cells and then enters the cells through endocytosis to prevent the influenza virus from infecting human cells. Besides, Abidol can induce interferon production and exert a broad-spectrum antiviral effect [29]. A recent study has revealed that arbidol can inhibit SARS-CoV-2 infection at a concentration of 10–30 μM effectively in vitro [30]. Deng et al. [31] found patients taking arbidol and LPV/r (n = 16) had increased SARS-CoV-2 negative rate in 7-days and 14-days, and improved chest CT scan compared to oral LPV/r (n = 17) only. However, no effects of LPV/r or arbidol was found to improve symptoms or shorten the clearance time of nucleic acid in respiratory specimens, while there are more gastrointestinal adverse reactions comparing with the placebo control group in a clinical trial at Shanghai Public Health Clinical Center [32]. These contradictory results may be due to small sizes and inconsistent timing of medications, missing the best therapeutic window in part of patients. A clinical study of arbidol using for post-exposure prophylaxis of SARS-CoV-2 in a high-risk population, including medical staff, is in progress (ChiCTR2000029592), may providing us whether there are effects of arbidol on prevention and early treatment. The latest guide recommends that the course of treatment for arbidol should not exceed 10 days (200 mg/each time, 3 times/day).
2.5. Lopinavir/ritonavir
Lopinavir is approved by the Food and Drug Administration (FDA) to treat HIV infection as a protease inhibitor, with ritonavir as a booster by inhibiting cytochrome P450 to increase blood concentrations [33]. Lopinavir/ritonavir (LPV/r) is contraindicated for allergy to this product, severe liver dysfunction, and drugs that are highly dependent on CYP3A clearance and elevated plasma concentrations are associated with acute or life-threatening reactions. A non-randomized clinical trial showed that SARS patients treated with ribavirin combined with LPV/r had a lower risk of ARDS and death than ribavirin monotherapy [34]. A Korean COVID-19 patient has been found reduced viral loads and improved clinical symptoms when being treated with lopinavir/ritonavir [35], while it was not clear whether it was an effect of an antiviral drug or natural course, or both. LPV/r has been recommended for the treatment of COVID-19 in the latest guideline (400 mg/100 mg/each time, 2 times/day) based on experience in SARS-CoV and MERS-CoV. Nausea, vomiting, fat metabolism disorders, and elevated blood sugar are common adverse reactions to LPV/r. Elderly patients with chronic diseases such as type 2 diabetes and hyperlipidemia need close monitoring of blood glucose and blood lipids. However, with the increase in the number of clinical applications, most clinicians believe that it is not effective, and gastrointestinal adverse reactions also occur frequently [36], NEJM has published an article on March 18, 2020, reporting a randomized controlled open-label clinical trial involving 199 patients with severe COVID-19 in the China-Japan Friendship Hospital (ChiCTR2000029308), which proves that LPV/r has no significant effect on the treatment of COVID-19 [37]. That might confirm antiviral drugs themselves are challenging to reverse the lung injury caused by a cytokine storm.
2.6. Ribavirin
Ribavirin, a nucleoside analog, may through multiple mechanisms of action, including lethal mutagenesis, specific or nonspecific chain termination, and inhibition of nucleotide biosynthesis to eliminate RNA viruses [38]. It has been approved for the treatment of respiratory syncytial virus (RSV) by FDA [39], and contraindicated to autoimmune hepatitis, hemoglobin disease, impaired kidney function (creatinine clearance below 50 mL per minute), pregnant females or males with pregnant partners, and allergies to it [40]. Due to its broad-spectrum antiviral effect, it was widely used in the early stages of the SARS epidemic. However, the role ribavirin play is controversial; on the one hand, severe side effects such as hypoxia, anemia, and decreased hemoglobin levels are related to the use of high-dose ribavirin [41], the other hand contradictory results from clinical observation experiments [[42], [43], [44], [45], [46], [47], [48]]. In cell culture, ribavirin has an inhibitory effect on SARA-CoV-2, but requires higher concentrations. Ribavirin is not recommended for antiviral treatment in Diagnosis and Treatment Protocol for novel Coronavirus disease from Military Medical Team Supporting Wuhan(Trial Version 2), but has been included in the Guidelines for the Prevention, Diagnosis, and Treatment of Novel Coronavirus-induced Pneumonia (Trial Version 7), which recommend low-dose ribavirin (500 mg each time, 2–3 times/day) combined with lopinavir/ritonavir or interferon instead of monotherapy. A trial comparing effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus pneumonia is in progress (ChiCTR2000029387). The antiviral effect of ribavirin in the combination of multiple drugs is not clear, and clinical trials need to be confirmed (Table 1 ).
Table 1.
Targeted therapeutics against COVID-19 in the Guidelines (version 7).
| Medicine | Method of adminstration | Dosage | Course of treatment |
|---|---|---|---|
| Ribavirin | Intravenous infusion | 500 mg each time, 2–3 times/day in combination with IFN-α or lopinavir/ritonavir | No more than 10 days |
| Lopinavir/ritonavir | Oral | 200 mg/50 mg/capsule, 2 capsules each time, 2 times/day | No more than 10 days |
| Abidol | Oral | 200 mg each time, 3 times/day | No more than 10 days |
| Chloroquine phosphate | Oral | 500 mg each time, 2 times/day for patients who weigh more than 50 kg 500 mg each time, 2 times/day lasts 2 days, and 500 mg each time, 1 times/day lasts 5 days for patients who weigh less than 50 kg | 7 days |
| IFN-α | Vapor inhalation | 5 million U or equivalent dose each time, 2 times/day | N/A |
| Corticosteroids | Intravenous infusion | Methylprednisolone 1−2 mg/kg/day or equivalent dose | 3−5 days |
| Tocilizumab | Intravenous infusion | 4−8 mg/kg each time | No more than 2 times |
3. Immunization therapies
3.1. Interferon
The antiviral interferon system plays a critical role in the innate immune defense in human beings. IFNs are cytokines produced by cells when meeting viruses [49]. At present, IFNs are approved for indications such as hepatitis B, hepatitis C, and various malignancies by the FDA. In patients infected with SARS-CoV and MERS-CoV, the IFNs production is suppressed [50,51], Which is confirmed to be responsible for activating pro-inflammatory monocyte macrophages and cytokines in the lungs of the SARS mouse model [52]. Effect of IFN monotherapy or combined with ribavirin was observed in vitro and animal models if given early in the disease [[53], [54], [55], [56]]. However, in clinical trials, combination therapy with IFN did not show good curative effect and prognosis [43,[57], [58], [59]], which might occur due to medication delay and multiple organ damage caused by subcutaneous medications [57]. Guidelines [17] for the Prevention, Diagnosis, and Treatment of Novel Coronavirus-induced Pneumonia (Trial Version 7) recommend interferon inhalation as early as possible, which ensuring higher levels of the drug in lung tissue and longer drug circulation time than subcutaneous injection. It should be noted that nebulized interferon is contraindicated in patients with spontaneous pneumothorax and pulmonary bullae.
3.2. Corticosteroids
At present, the corticosteroid use is controversial in COVID-19. On the one hand, corticosteroids suppress lung inflammation and cytokine storms, which is essential for COVID-19 patients, especially severe and critical patients. On the other hand, increased secondary infection rate and delayed virus clearance can be found in SARS and influenza pneumonia patients using corticosteroids [[60], [61], [62]]. A small observational study of patients with COVID-19 showed potential benefits from low-dose corticosteroid treatment in critically ill patients, despite no significant improvement in overall survival [63]. Ni et al. [64] retrospectively analyzed the clinical data of 72 patients with COVID-19, and found that there was no statistically significant difference in the time of viral nucleic acid negation and lung image recovery between the low-medium dose glucocorticoids (methylprednisolone 0.75−1.5 mg/kg/d) treatment group and the non-glucocorticoid control group, glucocorticoid immunosuppressive therapy does not prolong virus clearance time in patients with COVID-19, suggesting that hormone therapy may be helpful to severe patients. A study reviewed the clinical data of 112 cases in Wuhan Red Cross Hospital with the treatment of low dose methylprednisolone when mild and common cases became severe and critical, and found that the optimal window period for low dose methylprednisolone treatment of COVID-19 is within 48 h after switching from mild condition to severe or critical. The early administration of low dose methylprednisolone within the window period for these patients could significantly reduce the critical illness rate and mortality rate [65]. The expert consensus statement in China [66] recommends low-to-moderate corticosteroid (≤0.5−1 mg/kg per day methylprednisolone or equivalent) should be used carefully in critically ill patients with COVID-19, and the duration should be short (≤7 days).
3.3. Tocilizumab
Tocilizumab is a recombinant humanized antibody that specifically binds the interleukin-6 (IL-6) receptor to inhibit the activity of the IL-6, licensed for adult rheumatoid arthritis and cytokine release syndrome [67]. IL-6 has long been considered as one of the key cytokines that induce cytokine storms. Among patients with COVID-19, IL-6 was significantly higher in severe and dead patients than in mild and discharged patients [3,68]. For the reasons above, the First Affiliated Hospital of University of science and technology of China initiated a multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of COVID-19 (ChiCTR2000029765), in the first phase of the clinical study, 14 patients with COVID-19 pneumonia (maximum age of 82 years, 9 of whom were severe and 2 of who were critically ill) had diffuse lesions in both lungs before treatment, of which 11 had a persistent fever. After treatment with the new treatment regimen of tocilizumab combined conventional therapy, the temperature of 11 patients with fever fell to normal within 24 h; 4 patients had better absorption of CT lesions in the lungs. Xu et al. [69] also found similar results in observational studies. Therefore, for patients with severe conditions caused by a cytokine storm, tocilizumab is a drug worthy of clinical research. There have been five clinical trials in addition to that mentioned above on trastuzumab in progress. For patients with extensive infiltrating lesions of the lungs and severe patients with elevated IL-6, tocilizumab treatment can be tried (the recommended dose is 400 mg, and the cumulative dose is up to 2 times), and medical staff should pay attention to allergic reactions and exclude active infections such as tuberculosis [17].
4. Traditional Chinese medicine
In the absence of particularly effective medicines from Western medicine, Chinese medicine has played a unique advantage in improving immunity and improving symptoms. In China, more than 90 % of people use traditional Chinese medicine for treatment [70]. Chinese medicine physicians summarized the law and experience of traditional Chinese medicine in the treatment of viral infectious diseases, deeply explored ancient classic recipes, combined with clinical practice, thus selecting a group of prescription drugs represented by the "three drugs and three prescriptions" with obvious curative effects: Jinhua qinggan Granules, Lianhua qingwen Capsules, Xuebijing Injection, QingfeiPaidu Decoction, Huashibaidufang, and Xuanfeibaidufang. Jinhua Qinggan Granule, and Lianhua Qingwen Capsule are used in no confirmed suspected cases, the former is mainly used for Patients with headache as the main symptom and mild fever symptoms, while the latter is used for patients with severe fever and dry stool. Qingfei Paidu Decoction is a general prescription for mild, severe, and critical COVID-19 patients [71]. In the treatment of severe and critically ill patients, Xuebijing injection may be a very important therapeutic drug. Xuebijing injection has a curative effect on the inflammatory storm and coagulation dysfunction. Early clinical studies have proved that Xuebijing has a certain effect in severe pneumonia [72]. Some observational studies [[73], [74], [75]] suggested that Traditional Chinese medicine can improve patients' symptoms, inflammation, and promote the absorption of lung infections. However, there is a lack of reliable RCT researches in COVID-19.
5. Antiviral therapy in combination
Based on the currently reported retrospective studies on COVID-19 [64,[76], [77], [78], [79], [80], [81]], we found that most people received two or more antiviral therapy in combination. Because it depends on the patient's liver and kidney function, whether there is complications and adverse reactions, there are various combinations, the most common combinations are the following: interferon (IFN) + LPV/r, IFN + arbidol, oseltamivir + arbiodol, IFN + LPV/r + arbidol. A multicenter, prospective study of 237 patients showed the triple combination antiviral therapy of IFN, LPV/r, and arbidol showed shorter viral shedding time and hospitalization time compared with the dual combination antiviral therapy of IFN and arbidol, and the earlier the time to initiate triple antiviral treatment, the shorter the time of virus shedding [82].
In addition, some studies have suggested that troponin is elevated in some severe and critically ill patients [4]. The most likely cause is myocardial damage caused by inflammatory storms. Cardioprotective drugs such as coenzyme Q10, trimetazidine hydrochloride, and creatine phosphate, and vitamin C could be used in severe and critical patients to improve cardiac energy metabolism [58]. In elderly patients, the hematopoietic function of liver, kidney, heart, and bone marrow declines with age, and there are more underlying complications and drug treatments. It is necessary to pay attention to the interaction between antiviral drugs and treatment drugs for basic diseases, and close monitoring and individualized treatment is required.
In conclusion, there are no antiviral drugs proven to be effective currently. Antiviral drugs should be used carefully to reduce viral replication in the early stages of disease based on the dynamic results of clinical trials, while it is not recommended to use antiviral drugs for the prevention of COVID-19. Since the antiviral effects of LPV/r and arbidol are not apparent, and gastrointestinal adverse reactions frequently occur, the therapeutic effect of the above two drugs on COVID-19 still needs further evaluation. Based on the current clinical trial results and clinical medication experience, we recommend favipiravir and interferon for patients with mild to moderate conditions. Given the good results of the clinical trial of remdesivir are focused on severe patients currently, we recommend it for severe patients. Chloroquine is another antiviral drug that has the potential to have an effect on severe patients due to its immunosuppressive effect in addition to the antiviral effect. In critically ill patients, low-to-moderate corticosteroid and tocilizumab should be used to combat cytokine storms and reduce mortality with caution.
Declaration of Competing Interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81570332) and Key Medical Subjects of Jiangsu Province (ZDRCA2016019).
References
- 1.Coronavirus disease 2019 (COVID-19) Situation Report – 53.
- 2.T. Novel Coronavirus Pneumonia Emergency Response Epidemiology The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020 doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 5.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulangu S., Dodd L.E., Davey R.T., Jr., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A., Ali R., Coulibaly S., Levine A.C., Grais R., Diaz J., Lane H.C., Muyembe-Tamfum J.J., Group P.W., Sivahera B., Camara M., Kojan R., Walker R., Dighero-Kemp B., Cao H., Mukumbayi P., Mbala-Kingebeni P., Ahuka S., Albert S., Bonnett T., Crozier I., Duvenhage M., Proffitt C., Teitelbaum M., Moench T., Aboulhab J., Barrett K., Cahill K., Cone K., Eckes R., Hensley L., Herpin B., Higgs E., Ledgerwood J., Pierson J., Smolskis M., Sow Y., Tierney J., Sivapalasingam S., Holman W., Gettinger N., Vallee D., Nordwall J., Team P.C.S., Randomized A. Controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6(2):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackenzie A.H. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am. J. Med. 1983;75(1A):40–45. doi: 10.1016/0002-9343(83)91269-x. [DOI] [PubMed] [Google Scholar]
- 13.Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrezenmeier E., Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017;5(1) doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2020. Audio Transcript of the News Briefing Held by the State Council of China on February 17, 2020, the National Health Commission of the People’s Republic of China.http://www.nhc.gov.cn/xcs/yqfkdt/202002/f12a62d10c2a4 8c6895cedf2faea6e1f.shtml (Accessed February 18, 2020). (in Chinese) [Google Scholar]
- 17.Guidelines for the Prevention, Diagnosis, and Treatment of Pneumonia Caused by COVID-19 issued by the National Health Commission of the People’s Republic of China (in Chinese).
- 18.Biot C., Daher W., Chavain N., Fandeur T., Khalife J., Dive D., De Clercq E. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J. Med. Chem. 2006;49(9):2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- 19.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanghai New Coronary Virus Disease Clinical Treatment Expert Group Shanghai 2019 coronary virus disease comprehensive treatment expert consensus. Chin. J. Infect. Dis. Committee. 2020:E016. [Google Scholar]
- 21.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 2020.03.22.20040758. [Google Scholar]
- 22.Gautreta Philippe, Lagiera Jean-Christophe, Parolaa Philippe. 2020. Hydroxychloroquine and Azithromycin As a Treatment of COVID-19 Results of an Openlabel Non Randomized Clinical Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamura K., Ichikado K., Takaki M., Eguchi Y., Anan K., Suga M. Adjunctive therapy with azithromycin for moderate and severe acute respiratory distress syndrome: a retrospective, propensity score-matching analysis of prospectively collected data at a single center. Int. J. Antimicrob. Agents. 2018;51(6):918–924. doi: 10.1016/j.ijantimicag.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Molina J.M., Delaugerre C., Goff J.L., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Médecine et Maladies Infectieuses. 2020 doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Clercq E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem. Asian J. 2019;14(22):3962–3968. doi: 10.1002/asia.201900841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Fapiravir medication instructions: https://www.info.pmda.go.jp/go/pack/625004XF1022_2_02/.
- 28.National Health Commission of the People’s Republic of China. [DOI] [PMC free article] [PubMed]
- 29.Blaising J., Polyak S.J., Pecheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.News: Abidol and darunavir can effectively inhibit coronavirus http://www.sd.chinanews.com/2/2020/0205/70145.html (Accessed February 21, 2020). (in Chinese).
- 31.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019:a retrospective cohort study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jun Chen, Yun Ling, Xiuhong Xi, Ping Liu, Feng Li, Tao Li, Zhiyin Shang, Mei Wang, Yinzhong Shen, Hongzhou Lu. Lopina virtonavir and Abidol for the treatment of new coronavirus pneumonia. Chin. J. Infect. Dis. 2020:E008. [Google Scholar]
- 33.Su B., Wang Y., Zhou R., Jiang T., Zhang H., Li Z., Liu A., Shao Y., Hua W., Zhang T., Wu H., He S., Dai L., Sun L. Efficacy and tolerability of Lopinavir/Ritonavir- and Efavirenz-based initial antiretroviral therapy in HIV-1-Infected patients in a tertiary care hospital in Beijing, China. Front. Pharmacol. 2019;10:1472. doi: 10.3389/fphar.2019.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y., H.U.S.S. Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.W., Kang Y.M., Lee B., Park S.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of Lopinavir/Ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020;35(6):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youning Liu. Thinking about new drugs for treating coronavirus infection. Chin. J. Tuberculosis Respir. Dis. 2020;3:E017. [Google Scholar]
- 37.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S., Kojan S., Al Jeraisy M., Deeb A.M., Assiri A.M., Al-Hameed F., AlSaedi A., Mandourah Y., Almekhlafi G.A., Sherbeeni N.M., Elzein F.E., Memon J., Taha Y., Almotairi A., Maghrabi K.A., Qushmaq I., Al Bshabshe A., Kharaba A., Shalhoub S., Jose J., Fowler R.A., Hayden F.G., Hussein M.A., the MIRACLE trial group Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29(3):695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Station W. Merck Sharp & Dohme Corp. (per FDA); 2015. Product Information: REBETOL(R) Oral Capsules, Oral Solution, Ribavirin Oral Capsules, Oral Solution. [Google Scholar]
- 41.Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.Y. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnard D.L., Day C.W., Bailey K., Heiner M., Montgomery R., Lauridsen L., Winslow S., Hoopes J., Li J.K., Lee J., Carson D.A., Cottam H.B., Sidwell R.W. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res. 2006;71(1):53–63. doi: 10.1016/j.antiviral.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng V.C., Lau S.K., Woo P.C., Yuen K.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20(4):660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 45.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., Walmsley S.L., Mazzulli T., Avendano M., Derkach P., Ephtimios I.E., Kitai I., Mederski B.D., Shadowitz S.B., Gold W.L., Hawryluck L.A., Rea E., Chenkin J.S., Cescon D.W., Poutanen S.M., Detsky A.S. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 46.Peiris J.S. Severe acute respiratory syndrome (SARS) J. Clin. Virol. 2003;28(3):245–247. doi: 10.1016/j.jcv.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.So L.K., Lau A.C., Yam L.Y., Cheung T.M., Poon E., Yung R.W., Yuen K.Y. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361(9369):1615–1617. doi: 10.1016/S0140-6736(03)13265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong S.S., Yuen K.Y. The management of coronavirus infections with particular reference to SARS. J. Antimicrob. Chemother. 2008;62(3):437–441. doi: 10.1093/jac/dkn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.tenOever B.R. The evolution of antiviral defense systems. Cell Host Microbe. 2016;19(2):142–149. doi: 10.1016/j.chom.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Zielecki F., Weber M., Eickmann M., Spiegelberg L., Zaki A.M., Matrosovich M., Becker S., Weber F. Human cell tropism and innate immune system interactions of human respiratory coronavirus EMC compared to those of severe acute respiratory syndrome coronavirus. J. Virol. 2013;87(9):5300–5304. doi: 10.1128/JVI.03496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou J., Chu H., Li C., Wong B.H., Cheng Z.S., Poon V.K., Sun T., Lau C.C., Wong K.K., Chan J.Y., Chan J.F., To K.K., Chan K.H., Zheng B.J., Yuen K.Y. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Dis. 2014;209(9):1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I Interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Channappanavar R., Lu L., Xia S., Du L., Meyerholz D.K., Perlman S., Jiang S. Protective effect of intranasal regimens containing peptidic middle east respiratory syndrome coronavirus fusion inhibitor against MERS-CoV infection. J. Infect. Dis. 2015;212(12):1894–1903. doi: 10.1093/infdis/jiv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362(9380):293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel beta coronavirus replication by a combination of interferon-alpha2b and ribavirin. Sci. Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan J.F., Chan K.H., Kao R.Y., To K.K., Zheng B.J., Li C.P., Li P.T., Dai J., Mok F.K., Chen H., Hayden F.G., Yuen K.Y. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J. Infect. 2013;67(6):606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shalhoub S., Farahat F., Al-Jiffri A., Simhairi R., Shamma O., Siddiqi N., Mushtaq A. IFN-alpha2a or IFN-beta1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J. Antimicrob. Chemother. 2015;70(7):2129–2132. doi: 10.1093/jac/dkv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., Almakhlafi G.A., Albarrak M.M., Memish Z.A., Albarrak A.M. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14(11):1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng V.C., Chan J.F., To K.K., Yuen K.Y. Clinical management and infection control of SARS: lessons learned. Antiviral Res. 2013;100(2):407–419. doi: 10.1016/j.antiviral.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee N., Allen Chan K.C., Hui D.S., Ng E.K., Wu A., Chiu R.W., Wong V.W., Chan P.K., Wong K.T., Wong E., Cockram C.S., Tam J.S., Sung J.J., Lo Y.M. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit. Care. 2019;23(1):99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou W., Liu Y., Tian D., Wang C., Wang S., Cheng J., Hu M., Fang M., Gao Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct. Target. Ther. 2020;5:18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qin Ni, Cheng Ding, Yongtao Li, Hong Zhao, Jun Liu, Xuan Zhang, Yanfei Chen, Yongzheng Guo, Liang Yu, Hongzhen Ju, Jingjing Tao, Ping Yi, Guanjing Lang, Junwei Su, Ding Shi, Wenrui Wu, Xiaoxin Wu, Ling Yu, Jifang Sheng, Kaijin Xu. Retrospective analysis of low-dose glucocorticoids on virus clearance in patients with new coronavirus pneumonia. Chin. J. Clin. Infect. Dis. 2020:E009. [Google Scholar]
- 65.Wei Li, Debing Liu. Methylprednisolone sodium succinate effective window period for the treatment of new coronavirus pneumonia. J. Wuhan Univ. (Med. Ed.) 2020:1–5. [Google Scholar]
- 66.Jianping Zhao, Yi Hu, Ronghui Du, Zhenshun Cheng, Yang Jin, Min Zhou, Jing Zhang, Jieming Qu, Bin Cao. Recommendations for the use of new coronavirus pneumonia glucocorticoids. Chin. J. Tuberculosis Respir. 2020:E007. [Google Scholar]
- 67.Hashizume M., Tan S.L., Takano J., Ohsawa K., Hasada I., Hanasaki A., Ito I., Mihara M., Nishida K. Tocilizumab, a humanized anti-IL-6R antibody, as an emerging therapeutic option for rheumatoid arthritis: molecular and cellular mechanistic insights. Int. Rev. Immunol. 2015;34(3):265–279. doi: 10.3109/08830185.2014.938325. [DOI] [PubMed] [Google Scholar]
- 68.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu X., Han Mingfeng, Li Tiantian, Sun Wei, Wang Dongsheng, Fu Binqing, Zhou Yonggang, Zheng Xiaohu, Yang Yun, Li Xiuyong, Zhang Xiaohua, Pan Aijun, Wei Haiming. 2020. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. [ChinaXiv:202003.00026] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.National Administration of Traditional Chinese Medicine. http://www.satcm.gov.cn/xinxifabu/meitibaodao/2020-04-07/14511.html.
- 71.Yizhu Wang, Fang Liu, Xianglin Zhang. Application of oral Chinese patent medicines in the diagnosis and treatment of new coronavirus pneumonia, evaluation and analysis of drug use in. Chin. Hosp. 2020;20(3) 257-261+267. [Google Scholar]
- 72.Song Y., Yao C., Yao Y., Han H., Zhao X., Yu K., Liu L., Xu Y., Liu Z., Zhou Q., Wang Y., Ma Z., Zheng Y., Wu D., Tang Z., Zhang M., Pan S., Chai Y., Song Y., Zhang J., Pan L., Liu Y., Yu H., Yu X., Zhang H., Wang X., Du Z., Wan X., Tang Y., Tian Y., Zhu Y., Wang H., Yan X., Liu Z., Zhang B., Zhong N., Shang H., Bai C. XueBiJing injection versus placebo for critically ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit. Care Med. 2019;47(9):e735–e743. doi: 10.1097/CCM.0000000000003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Can Duan, Wenguang Xia, Chanjuan Zheng, Guobing Sun, Zhengliang Li, Qinglin Li, Ping Li, Heling Zhang, Fengwen Yang, Boli Zhang, Qingquan Liu. Jinhua Qinggan Granules in clinical observation of new coronavirus infection pneumonia. J. Tradit. Chin. Med. 2020:1–5. [Google Scholar]
- 74.Kaitao Yao, Mingyu Liu, Xin Li, Jihan Huang, Hongbin Cai. Retrospective clinical analysis of traditional Chinese medicine Lianhua Qingwen in the treatment of new coronavirus pneumonia. Chin. J. Exp. Pharmacol. 2020:1–7. [Google Scholar]
- 75.Dezhong Cheng, Wenju Wang, Yi Li, Xiaodong Wu, Biao Zhou, Qiyong Song. 51 cases of new coronavirus pneumonia patients with Chinese medicine Lianhua Qingwen: analysis of multicenter retrospective study. Tianjin Chin. Med. 2020:1–6. [Google Scholar]
- 76.Chen, Jianzhong Liu, Sai Xia. Clinical analysis and discussion of 9 cases of new coronavirus pneumonia. Mod. Med. Health. 2020 [Google Scholar]
- 77.Fang Cheng, Qiang Li, Fang Zeng, Dongyuan Wang, Yong Han, Yongning Lv, Yu Zhang. Analysis and suggestion on the medication status of 290 patients with novel coronavirus pneumonia in Fangtang Hospital. Chin. J. Hosp. Pharm. 2020:1–4. [Google Scholar]
- 78.Li. Zhang Shuxiang, Zhou Pan, Ningxia Hui Autonomous Region New Coronavirus Pneumonia Clinical Analysis of 34 Patients (2020).
- 79.Wu, Shi Chen, Zhiming Li. 2020. Clinical Analysis of 109 Cases of New Coronavirus Pneumonia. [Google Scholar]
- 80.Liang, Rui Zhao, Yanrong Lin. 2020. Clinical characteristics of 28 patients with new coronavirus pneumonia. [Google Scholar]
- 81.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., Li S.B., Wang H.Y., Zhang S., Gao H.N., Sheng J.F., Cai H.L., Qiu Y.Q., Li L.J. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Runan Wei, Nanhong Zheng, Xiangao Jiang, Chunlian Ma, Xiaowei Xu, Shourong Liu, Yongping Chen, Kaijin Xu, Hainv Gao, Jiansheng Zhu, Qiang Shu, Jifang Sheng, Xiaoqiang Zhang, Minghui Li, Yan Zhang, Mengjie Ma, Xuan Zhang, Shibo Li, Qiujing Wang, Lingjun Ying, Yongjun Zhang, Yunzhen Shi, Lingyan Fan, Wanjun Yu, Huaying Wang, Dandan Sun, Xiaodong Wang, Qichan Shi, Yinghu Chen, Xinsheng Xie, Yunqing Chen, Weihong Wang, Zhaowei Tong, Lingling Tang, Mengfei Zhu, Lingjian Zhang, Lanjuan Li. Zhejiang New Coronavirus Pneumonia Prevention and Control and Clinical Treatment System Establishment and research group, a multi-center, prospective study of early Abidor + lopinavir / ritonavir + recombinant interferon α-2b combined antiviral therapy in patients with novel coronavirus pneumonia in Zhejiang Province. Chin. Clin. Infect. Dis. J. 2020:E010. [Google Scholar]
- 83.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou D., Dai S.M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]