1. Introduction
As of 20 April, almost 1.7 million people globally have been diagnosed with Corona Virus Disease 2019 (COVID-19), a pandemic that has evolved from the emergence of a new coronavirus strain, acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in China. More than 170,000 deaths have been reported, while there are certainly many more cases of milder disease that have not been diagnosed and officially confirmed due to limited testing capacity in most countries. The pandemic is a global emergency due to the rapid transmission of the disease and the potential to overwhelm the healthcare systems, and is expected to have considerable economic and health impacts. Contributing factors and their possible role in the relatively high infection, death rates between countries and origin have recently been studied [1,2]. This new outbreak has been additionally evaluated for current knowledge on coronaviruses based on a short history to epidemiology, pathogenesis, clinical manifestation of the disease, as well as treatment and prevention strategies [3]. The search for potential protective and therapeutic antiviral strategies is of particular and urgent concern [4].
While in most cases, especially in young people without any comorbidities, the disease is expected to be relatively mild, there is a substantial proportion of patients who develop complications and need intensive care-unit support and mechanical ventilation. In one case series of 1099 patients in China [5], 6.1 % of cases suffered from the primary composite end-point of admission to an intensive care unit, use of mechanical ventilation, or death. Patients with severe disease typically present with dyspnea and hypoxemia shortly after disease initiation, and may quickly progress to respiratory failure, acute respiratory distress syndrome (ARDS) and multi-organ failure [6]. Predictors of adverse outcomes include elevated levels of inflammatory markers and pro-inflammatory cytokines. A study of 150 COVID-19 cases reported that elevated levels of C-reactive protein (CRP), ferritin and IL-6 were associated with death [7]. IL-6, an important pro-inflammatory cytokine, was elevated in fatal cases of COVID-19 in another study of 191 patients [8]. Another study of 452 patients reported that those with severe disease showed lymphocytopenia, neutrophilia, low levels of monocytes, eosinophils and basophils, and elevated levels of infection-related biomarkers and inflammatory cytokines [9]. Pathological examination of a case in China revealed bilateral diffuse alveolar damage, desquamation of pneumocytes, hyaline membrane formation and interstitial mononuclear inflammatory infiltrates [10]. Flow cytometry of peripheral blood revealed reduced levels of CD4+ and CD8 + T cells, which however were hyper-activated, and elevated concentration of pro-inflammatory CCR6+ Th17 in CD4 + T cells. Such findings are hallmarks of ARDS and resemble features observed in SARS and Middle Eastern Respiratory Syndrome [11,12]. Systemic vasculitis was also observed [10]. Therefore, it seems that immune dysregulation may be implicated in the pathophysiology of severe COVID-19.
2. Cytokine storm
While for decades common thinking suggested that every immune response to antigenic invasion was always beneficial in averting potential damage, studies in the 1980s identified that immune cells produce proteins with pleiotropic properties, having the potential to be either beneficial or harmful [13]. The proteins, called cytokines, were found to cause clinical manifestations similar to sepsis such as hemodynamic instability, fever, and localized inflammation [14,15]. Cytokines are important in mediating both immune cell recruitment and complex intracellular signaling control mechanisms that characterize inflammation and infection control. They are expressed by numerous cells, including macrophages, monocytes, B cells and T cells, promote differentiation of T-helper cells and stimulate CD4+ cells [16]. While activation of the immune system is important in fighting pathogens, dysregulation of cytokine production may lead to uncontrolled effects that can ultimately be detrimental to health [16,17].
Cytokine storm (also called macrophage activation syndrome) is a systemic inflammatory response that can be triggered by a variety of factors such as infections and drugs [18]. It represents a failure of the inflammatory response to return to homeostasis. The resulting unregulated immune activity can potentially lead to catastrophic tissue damage. The term first appeared in 1993 in an article relevant to graft-versus-host disease [19]. Subsequently, cytokine storm was a phenomenon recognized in both viral and bacterial infections. It has been particularly studied in viral infections such as cytomegalovirus pneumonitis, influenza virus and SARS-CoV [[20], [21], [22], [23]]. Bermejo-Martin et al. [21] recruited both inpatients and outpatients during the first wave of the pandemic flu in 2009 (nvH1N1) and examined the effects of immune host responses to the evolution of mild or severe disease by measuring serum levels of several chemokines and cytokines. They found a dramatic increase of mediators that stimulate Th-1 and Th-17 responses (which are responsible for attacking intracellular pathogens and clearing pathogens during host defence reactions) among severe hospitalized patients compared to milder cases of nvH1N1 infection. The cytokine storm can result in acute lung injury and further progress to ARDS. This is characterized by local infiltration of inflammatory cells, increased vascular permeability and systemic spillover of inflammatory mediators that can cause systemic sepsis-like symptoms [23]. While focus on cytokine storm detection relies mostly on measuring cytokines in the systemic circulation, it has been suggested that measuring systemic inflammatory mediators may underestimate the extent of the immunological cascade that takes place locally in deep tissues such as the respiratory tract [23]. Considering the above, controlling the inflammatory response may be an effective way of preventing collateral damage caused by the excessive activation of the immune system to clear pathogens.
3. Cholinergic anti-inflammatory pathway
Since the early 2000s, the cholinergic nervous system has been identified as an important pathway that modifies and controls the inflammatory response. Surgical dissection of the vagus nerve in mice led to enhanced TNF production and excessive response to endotoxin administration, while vagus nerve electrical stimulation inhibits the synthesis of TNF and prevents the acute inflammatory response [[24], [25], [26]]. Several animal experimental models inducing pro-inflammatory cytokines, such as sepsis, ischemia-reperfusion and pancreatitis have shown that vagus stimulation improves outcomes. This effect is mediated by the nicotinic acetylcholine receptor (nAChR) α7 subunit on macrophages [27]. Mice deficient of the α7 subunit exhibited increased endotoxin-induced TNF production, and electrical vagus innervation failed to reduce serum TNF levels [27]. B-lymphocytes also express α7 nAChRs. Macrophages appear to be very sensitive to acetylcholine, which suggests that any source of acetylcholine, even from non-neuronal sources such as epithelial and endothelial cells, could also modulate the activity of adjacent macrophages [25]. Besides TNF, other pro-inflammatory cytokines are inhibited by acetylcholine, such as high mobility group B1 (HMGB1), IL-1, and IL-6 [28].
Modulation of inflammatory and immune response by the central nervous system (CNS) through the vagus nerve is based on bi-directional communication between the immune and nervous systems. Afferent vagus nerve fibers, located in nucleus tractus solitarius, provide sensory input to the CNS about the inflammatory status that can result in the transmission of efferent signals, originating from the dorsal motor nucleus, to control the inflammatory response [29]. Such a response is rapid and localized, unlike the diffusible anti-inflammatory network, which is slow, distributed, non-integrated and dependent on concentration gradients [25].
4. Nicotine, nicotinic cholinergic system and COVID-19
Smoking is known to increase the risk for respiratory infection susceptibility and severity [30,31]. Considering that COVID-19 was declared by the World Health Organization as a pandemic, a substantial disease burden would be expected among the estimated 1.1 billion smokers, especially in countries with high smoking prevalence. Therefore, there were understandable concerns about this population subgroup [32]. Additionally, smoking-related disease conditions such as cardiovascular disease and COPD are also established risk factors for adverse outcomes in COVID-19 [33]. China was the first country to be affected by the pandemic and has a high smoking prevalence. In 2018, the population smoking prevalence was 26.6 % with a much higher prevalence in men (50.5 %) than in women (2.1 %) [34]. Therefore, a high smoking prevalence among patients with COVID-19 would be expected, even if smoking did not adversely affect disease susceptibility and severity.
On 23 March, a preliminary analysis by some members of our group examined data from 5 case series of hospitalized COVID-19 patients from China, and calculated a smoking prevalence of 10.2 % (95 % CI: 8.7–11.8 %) while the estimated expected prevalence was 31.3 % (95 % CI: 8.7–11.8 %) [35]. The analysis was further expanded on 3 April by examining 13 Chinese studies and 5960 hospitalized COVID-19 patients, with a pooled smoking prevalence of 6.5 % (95 % CI: 4.9–8.2 %) [36]. On that date, we presented for the first time a hypothesis about the potential beneficial effects of nicotine, which was subsequently expanded [37]. While there were limitations in the study analysis, mainly due to the inability to adjust for confounding factors, the findings of low smoking prevalence among hospitalized COVID-19 patients in China were consistent across all studies and in agreement with case series from USA [38,39]. The original hypothesis was based on the anti-inflammatory properties of nicotine through the cholinergic anti-inflammatory system, acknowledging that the disease appeared to involve a dysregulation of the immune response to viral invasion.
It is obviously inappropriate to suggest that anyone should initiate smoking or continue to smoke, due to the well-established smoking-related morbidities and the large number of toxic chemicals in cigarette smoke. Furthermore, it is unlikely that any other compound in tobacco cigarette smoke, besides nicotine, would be implicated to the potential benefits observed in smokers. Moreover, due to the adverse effects of smoking and the fact that many smokers would suffer from co-morbidities (such as cardiovascular disease, COPD etc.), it is expected that the potential benefits of nicotine would be masked by the adverse effects of smoking.
Nicotine is a cholinergic agonist. Therefore, it is an important inhibitor of pro-inflammatory cytokines acting through the cholinergic anti-inflammatory pathway via α7-nAChRs. Nicotine inhibits TNF, IL-1, IL-6 and HMGB1 while it does not inhibit anti-inflammatory cytokines such as IL-10 [28]. In vivo animal models have found nicotine to be protective against lipopolysaccharide-induced ARDS by reducing leukocyte infiltration and pro-inflammatory mediators in bronchoalveolar lavage fluid [40]. Such effects are relevant to COVID-19 since cytokine storm appears to be the hallmark in severe cases [41,42]. Several pro-inflammatory cytokines, such as IL-1β, IL-2, IL-6, IL-17, IL-8, TNF and CCL2 are elevated in COVID-19 patients [43]. Treatment with anti-IL-6 anti-TNF medications has been proposed and clinical trials are already underway [44,45]. However, it may be more effective to inhibit several instead of selectively one cytokine, while some cytokine inhibitors are associated with elevated risk of opportunistic infections [46]. Also, it is possible that measuring blood levels of inflammatory cytokines does not accurately reflect the extent of the immune imbalance that exists locally in the lungs. In any case, the cholinergic anti-inflammatory system could provide better control and modulation of the cytokine response compared to blocking a single agent, and nicotine could effectively contribute to maintaining a balanced immune response against viral infection. Therefore, it is possible that the clinical manifestations of cytokine storm in COVID-19 patients are the result of dysfunction of the cholinergic anti-inflammatory pathway.
SARS-CoV-2 is known to use the angiotensin converting enzyme 2 (ACE2) as a receptor for cell entry [47]. ACE2 has well-established vasodilatory, anti-inflammatory and antioxidant properties. Studies on smoking and ACE2 have reported contradictory findings. Studies published before the COVID-19 pandemic reported that smoking and nicotine down-regulate ACE2 [48,49]. However, more recent studies suggest that they up-regulate ACE2 [[50], [51], [52]]. There is currently no evidence to suggest that up-regulation of ACE2 is associated with increased COVID-19 susceptibility or severity. In fact, up-regulation of ACE2 appears to be protective against tissue damage caused by SARS-CoV-2. ACE2 has been found to protect mice from developing ARDS [[53], [54], [55]]. Data from SARS experimental studies suggest that continuous SARS-CoV-2 infection and replication induces immediate down-regulation of ACE2 that may be implicated in organ damage and disease severity [56]. Further support for the beneficial role of ACE2 comes from data that estrogens appear to up-regulate ACE2 while children and younger adults have higher ACE2 levels compared to older people [57,58]. At the same time, women, children and young people have milder COVID-19 symptoms. If accurate and verified, the recently-observed ACE2 up-regulation in smokers is probably induced as a defence mechanism to counteract the effects of angiotensin II. There is probably a dynamic balance between ACE and ACE2, which is continuously changing, depending on stressors and stimuli. Thus, there is uncertainty on whether nicotine affects COVID-19 progression through the renin-angiotensin-aldosterone axis and there is no known interaction between ACE2 and nAChR receptors.
Importantly, ACE2 is expressed in several regions in the brain. The regions where vagal afferent fibers terminate and vagal efferent fibers originate exhibit ACE2 expression [59,60]. Neuroinvasion is a common feature of coronaviruses [61]. Anosmia and ageusia have been reported by COVID-19 patients [62]. SARS-CoV-2 may enter the CNS either through the blood stream or via the olfactory nerve across the cribriform plate [63,64]. A case series of 214 patients reported that 36.4 % had neurological manifestations [65]. Thus, it is possible that the virus might infect the terminal areas of vagus afferent fibers or the origin of vagus efferent fiber causing down-regulation of ACE2 and resulting in local inflammation that could disrupt the cholinergic anti-inflammatory pathway and dysregulate the inflammatory response. Nicotine could have protective properties against possible brain inflammation caused by SARS-CoV-2, mediated through α7-AChRs [66].
A noteworthy parameter relative to anosmia and ageusia observed among COVID-19 patients is that these are characteristic and prodromal non-motor manifestations of Parkinson’s disease [67,68]. While ageusia has not been extensively studied, olfactory disturbance is a very common feature, observed in up to 95 % of Parkinson’s disease patients [68], and may appear several years before the onset of motor symptoms. There is no olfactory improvement with dopamine agonists [69,70]. Unlike the general population where smoking is associated with impaired olfactory function, smokers with Parkinson’s disease experience less decline in olfactory sense compared to non-smokers, suggesting a protective effect of smoking [71]. This maybe explained by the fact olfactory loss has been linked to impairment of cholinergic transmission [72] while nicotine improved the olfactory impairment in a mouse model of Parkinson’s disease [73]. The olfactory bulb has a rich network of nAChRs, but α7 nAChRs may also be expressed on the axon terminals of the olfactory receptor neurons [74]. While this may suggest facilitated brain infection through anterograde transport along the olfactory nerve, it is possible that olfactory receptor neurons may act as first-line viral sensors and initiate a rapid immune response [75]. This would explain the mild symptoms in COVID-19 patients with olfactory loss. In any case, anosmia may represent another sign of dysfunction of the nicotinic cholinergic system in COVID-19.
A prominent feature of COVID-19 is coagulopathy that results in thromboembolic complications. Venous thromboembolism was reported in 25 % of patients who were not under thromboprophylaxis, and was associated with higher mortality rate [76]. Abnormal coagulation parameters were also associated with poor survival [77]. Although venous thromboembolism is a well-known complication of any serious infection, additional mechanisms such as endothelial damage, increased vascular permeability and microvascular occlusion may be implicated in COVID-19 [78]. It is important to note that platelets express functional α7-AChRs [79] while hematopoietic α7 nAChR deficiency increases inflammation and platelet activity [80]. Recently, acetylcholine was found to be an endogenous inhibitor of platelet activation [81]. Therefore, dysfunction of the nicotinic cholinergic system could be implicated in the thrombotic and vascular complications of COVID-19.
5. COVID-19 could be a disease of the nicotinic cholinergic system
The observation of a low prevalence of hospitalized COVID-19 patients in China led to the development of a hypothesis that nicotine could have protective effects by enhancing the cholinergic anti-inflammatory pathway [36]. As more studies presented the clinical manifestations, laboratory findings and disease progression in COVID-19 patients, it became apparent that the nicotinic cholinergic system could explain most (if not all) of the disease characteristics. It would be unlikely for a single “defence system” to ameliorate all the diverse and complex manifestations of COVID-19, unless that “defence mechanism” was the target of the viral host. Could that be possible?
SARS-CoV-2 appears to have originated from a bat coronavirus. Ji et al. [82] carried out comprehensive sequence analysis in conjunction with relative synonymous codon usage bias and reported that the virus may have been a recombinant virus between the bat coronavirus and an unknown-origin coronavirus [83]. One possible intermediate host could have been a snake. Taking into consideration that snake venom toxins are competitive antagonists of acetylcholine on α7-nACh receptor with high affinity, we decided to explore the hypothesis that SARS-CoV-2 may have acquired sequences by any of the potential, and not defined yet, intermediates through genomic recombination. We compared the protein sequences between SARS-CoV-2 and snake venom neurotoxins. We were able to identify regions with four or five amino acids identity between the coronavirus and several neurotoxin molecules (e.g. SARS-CoV-2 compared with Muscarinic toxin like protein, Fig. 1A; SARS-CoV-2 and Cobrotoxin - Naja siamensis, Fig. 1B).
Fig. 1.
BLAST-P alignment of the SARS-CoV-2 protein against Muscarinic toxin like protein (A) and Cobratoxin (Naja siamensis) (B) indicating regions with relatively high identity.
Therefore, we hypothesize that these, or other, sequences on the SARS-CoV-2 proteins, being similar to the active sites of a neurotoxin, can result in binding to nAChRs and may adversely affect their function by preventing the action of acetylcholine.
6. Nicotine as a potential treatment for COVID-19
Nicotine could act as a competitive agonist for the nAChRs that could restore the compromised function of the nicotinic cholinergic system. This may be feasible through repurposing already approved (for other indications) pharmaceutical nicotine products such as nicotine patches for use by non-smokers, or even by using these products as already indicated (i.e. as smoking substitutes) among current smokers. These products are available over-the-counter in most countries. They have been administered therapeutically in non-smokers for neurological conditions and inflammatory bowel disease for larger periods than would be needed for COVID-19 [[83], [84], [85]]. No abuse liability was observed in non-smokers despite being administerd for several weeks [84,85]. Besides gums and patches, nicotine can be administered though inhalation, with the use of a nebulizer or other aerosol systems, if necessary. Nicotine administration could be added on top of antiviral or other therapeutic options for COVID-19. By restoring and re-activating the cholinergic anti-inflammatory pathway, a more universal suppression of the cytokine storm could probably be achieved compared to administering inhibitors of a single cytokine. The potential need to provide pharmaceutical nicotine products to smokers and users of other nicotine products who experience abrupt nicotine cessation when hospitalized for COVID-19 or aim to follow medical advice to quit smoking, should also be examined. If the hypothesis about the beneficial effects of nicotine is valid, smokers who quit nicotine use when hospitalized will be deprived from these benefits. In France, the Addiction Prevention Network (RESPADD) officially recommends the use of nicotine replacement therapies for smokers when hospitalized for any illness [86]. Clinical trials will dictate future approaches and the role of nicotine in COVID-19, while further experimental studies should examine the affinity of the virus to nAChRs.
7. Conclusions
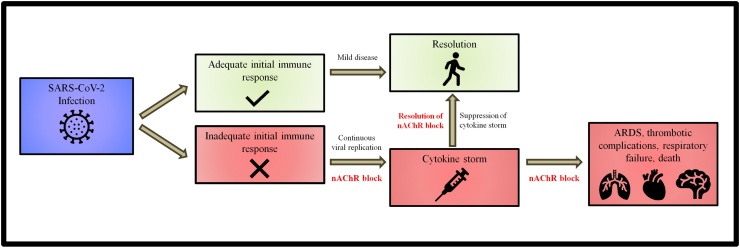
In conclusion, we noticed that most of the clinical characteristics of severe COVID-19 could be explained by dysregulation of the cholinergic anti-inflammatory pathway. The observation that patients eventually develop cytokine storm which results in rapid clinical deterioration, led to the development of a hypothesis about the series of events associated with adverse outcomes in COVID-19 (Fig. 2).
Fig. 2.
Progression of COVID-19 after SARS-CoV-2 infection.
Once someone is infected with SARS-CoV-2, the immune system is mobilized. As the virus replicates, cell and viral debris or virions may interact with the nAChRs blocking the action of the cholinergic anti-inflammatory pathway. If the initial immune response is not enough to combat the viral invasion at an early stage, the extensive and prolonged replication of the virus will eventually disrupt the cholinergic anti-inflammatory pathway seriously compromising its ability to control and regulate the immune response. The uncontrolled action of pro-inflammatory cytokines will result in the development of cytokine storm, with acute lung injury leading to ARDS, coagulation disturbances and multiorgan failure. Based on this hypothesis, COVID-19 appears to eventually become a disease of the nicotinic cholinergic system. Nicotine could maintain or restore the function of the cholinergic anti-inflammatory system and thus control the release and activity of pro-inflammatory cytokines. This could prevent or suppress the cytokine storm. This hypothesis needs to be examined in the laboratory and the clinical setting.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Funding
No funding was provided for this study.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgement
None.
References
- 1.Goumenou M., Sarigiannis D., Tsatsakis A., Anesti O., Docea A.O., Petrakis D., Tsoukalas D., Kostoff R., Rakitskii V., Spandidos D.A., Aschner M., Calina D. COVID 19 in Northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (Review) Mol. Med. Rep. 2020;0.0(1899) doi: 10.3892/mmr.2020.11079. 0-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goumenou M., Spandidos D.A., Tsatsakis A. "[Editorial] Possibility of transmission through dogs being a contributing factor to the extreme Covid 19 outbreak in North Italy. Mol. Med. Rep. 2020;21.6:2293–2295. doi: 10.3892/mmr.2020.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docea A.O., Tsatsakis A., Albulescu D., Cristea O., Zlatian O., Vinceti M., Moschos S.A., Tsoukalas D., Goumenou M., Drakoulis N., Dumanov J.M., Tutelyan V.A., Onischenko G.G., Aschner M., Spandidos D.A., Calina D. A new threat from an old enemy: Re emergence of coronavirus (Review) Int. J. Mol. Med. 2020;45.6:1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S., Svistunov A.A., Petrakis D., Spandidos D.A., Aaseth J., Tsatsakis A., Tinkov A.A. Zinc and respiratory tract infections: perspectives for COVID 19 (Review) Int. J. Mol. Med. 2020;0.0(1899) doi: 10.3892/ijmm.2020.4575. 0-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.L.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y. Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;3(March) doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;12(March) doi: 10.1093/cid/ciaa248.3. pii: ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(April(4)):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng D.L., Al Hosani F., Keating M.K., Gerber S.I., Jones T.L., Metcalfe M.G., Tong S., Tao Y., Alami N.N., Haynes L.M., Mutei M.A., Abdel-Wareth L., Uyeki T.M., Swerdlow D.L., Barakat M., Zaki S.R. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of middle east respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am. J. Pathol. 2016;186(March(3)):652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Y., Wang H., Shen H., Li Z., Geng J., Han H., Cai J., Li X., Kang W., Weng D., Lu Y., Wu D., He L., Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J. Pathol. 2003;200(July(3)):282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracey K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007;117(February(2)):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracey K.J., Beutler B., Lowry S.F., Merryweather J., Wolpe S., Milsark I.W., Hariri R.J., Fahey T.J., 3rd, Zentella A., Albert J.D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234(October (4775)):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello C.A., Cannon J.G., Wolff S.M., Bernheim H.A., Beutler B., Cerami A., Figari I.S., Palladino M.A., Jr, O’Connor J.V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J. Exp. Med. 1986;163(June (6)):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta. 2014;1843(November(11)):2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello C.A. Historical insights into cytokines. Eur. J. Immunol. 2007;37(November (Suppl 1)):S34–45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018;6(June (1)):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrara J.L., Abhyankar S., Gilliland D.G. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant. Proc. 1993;25(February(1 Pt 2)):1216–1217. [PubMed] [Google Scholar]
- 20.Barry S.M., Johnson M.A., Janossy G. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplant. 2000;26(September(6)):591–597. doi: 10.1038/sj.bmt.1702562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T., Rello J., Almansa R., Ramírez P., Martin-Loeches I., Varillas D., Gallegos M.C., Serón C., Micheloud D., Gomez J.M., Tenorio-Abreu A., Ramos M.J., Molina M.L., Huidobro S., Sanchez E., Gordón M., Fernández V., Del Castillo A., Marcos M.A., Villanueva B., López C.J., Rodríguez-Domínguez M., Galan J.C., Cantón R., Lietor A., Rojo S., Eiros J.M., Hinojosa C., Gonzalez I., Torner N., Banner D., Leon A., Cuesta P., Rowe T., Kelvin D.J. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care. 2009;13(6):R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005;75(February(2)):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76(March(1)):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(May (6785)):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 25.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 26.Blalock J.E. Harnessing a neural-immune circuit to control inflammation and shock. J. Exp. Med. 2002;195:F25–8. doi: 10.1084/jem.20020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C.J., Tracey K.J. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(January (6921)):384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 28.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov. 2005;4(August(8)):673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 29.Pavlov V.A., Wang H., Czura C.J., Friedman S.G., Tracey K.J. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9(May-August(5-8)):125–134. Review. [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S., Tyrrell D.A., Russell M.A., Jarvis M.J., Smith A.P. Smoking, alcohol consumption, and susceptibility to the common cold. Am. J. Public Health. 1993;83(September(9)):1277–1283. doi: 10.2105/ajph.83.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millett E.R., De Stavola B.L., Quint J.K., Smeeth L., Thomas S.L. Risk factors for hospital admission in the 28 days following a community-acquired pneumonia diagnosis in older adults, and their contribution to increasing hospitalisation rates over time: a cohort study. BMJ Open. 2015;5(December (12)) doi: 10.1136/bmjopen-2015-008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berlin I., Thomas D., Le Faou A.L., Cornuz J. COVID-19 and smoking. Nicotine Tob. Res. 2020;3(April) doi: 10.1093/ntr/ntaa059. pii: ntaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;24(February) doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization (WHO) 2018. Global Adult Tobacco Survey (GATS). Fact Sheet China. Available at: https://www.who.int/docs/default-source/wpro---documents/countries/china/2018-gats-china-factsheet-cn-en.pdf?sfvrsn=3f4e2da9_2 (accessed on March 30, 2020) [Google Scholar]
- 35.Farsalinos K., Barbouni A., Niaura R. Qeios ID: Z69O8A.2; 2020. Smoking, Vaping and Hospitalization for COVID-19. [DOI] [Google Scholar]
- 36.Farsalinos K., Barbouni A., Niaura R. Qeios ID: Z69O8A.11; 2020. Smoking, Vaping and Hospitalization for COVID-19. [DOI] [Google Scholar]
- 37.Farsalinos K., Barbouni A., Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID-19 patients in China: could nicotine be a therapeutic option? Intern. Emerg. Med. 2020 doi: 10.1007/s11739-020-02355-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — united States, February 12–march 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020 doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richardson S., Hirsch J.S., Narasimhan M.N., Crawford J.M., McGinn T., Davidson K.W. The northwell COVID-19 research consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mabley J., Gordon S., Pacher P. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation. 2011;34(4):231–237. doi: 10.1007/s10753-010-9228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(March (10229)):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020;3(April) doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020 doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Georgiev T. Coronavirus disease 2019 (COVID-19) and anti-rheumatic drugs. Rheumatol. Int. 2020;30(March) doi: 10.1007/s00296-020-04570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutherford A.I., Subessinghe S., Hyrich K.L., Galloway J.B. Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann. Rheum. Dis. 2018;77:905–910. doi: 10.1136/annrheumdis-2017-212825. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(April(4)):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oakes J.M., Fuchs R.M., Gardner J.D., Lazartigues E., Yue X. Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315(5):R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yue X., Basting T.M., Flanagan T.W., Xu J., Lobell T.D., Gilpin N.W., Gardner J.D., Lazartigues E. Nicotine downregulates the compensatory angiotensin-converting enzyme 2/Angiotensin type 2 receptor of the renin–Angiotensin system. Ann. Am. Thorac. Soc. 2018;15(April(Suppl 2)):S126–S127. doi: 10.1513/AnnalsATS.201706-464MG. [DOI] [Google Scholar]
- 50.Cai G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. Preprints. 2020 doi: 10.20944/preprints202002.0051.v3. 2020020051 (prepublication) [DOI] [Google Scholar]
- 51.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., Dorscheid D.R., Sin D.D. ACE-2 Expression in the Small Airway Epithelia of Smokers and COPD Patients: Implications for COVID-19. medRxiv 2020.03.18.20038455. doi. 10.1101/2020.03.18.20038455. (prepublication). [DOI] [PMC free article] [PubMed]
- 52.Blake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting Enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19).) J. Clin. Med. 2020;9(3):841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hung Y.H., Hsieh W.Y., Hsieh J.S., Liu F.C., Tsai C.H., Lu L.C., Huang C.Y., Wu C.L., Lin C.S. Alternative roles of STAT3 and MAPK signaling pathways in the MMPs activation and progression of lung injury induced by cigarette smoke exposure in ACE2 knockout mice. Int. J. Biol. Sci. 2016;12:454–465. doi: 10.7150/ijbs.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imai Y., Kuba K., Penninger J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008;93(May(5)):543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(July (7047)):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-Aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cristiani L., Mancino E., Matera L., Nenna R., Pierangeli A., Scagnolari C., Midulla F. Will children reveal their secret? The coronavirus dilemma. Eur. Respir. J. 2020;2(April) doi: 10.1183/13993003.00749-2020. pii: 2000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciaglia E., Vecchione C., Puca A.A. COVID-19 infection and the predictive ACE2 soluble levels: the favourable protection of children and women. Front. Pediatr. 2020 doi: 10.3389/fped.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia H., Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr. Hypertens. Rep. 2010;12(June(3)):170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(January(1)):R373–81. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Desforges M., Le Coupanec A., Dubeau P. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S., Galli M. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin. Infect. Dis. 2020;26(March) doi: 10.1093/cid/ciaa330. pii: ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Manji H., Carr A.S., Brownlee W.J., Lunn M.P. Neurology in the time of covid-19. BMJ. 2020 doi: 10.1136/jnnp-2020-323414. doi.0.1136/jnnp-2020-323414. [DOI] [PubMed] [Google Scholar]
- 64.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(April (7)):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 65.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;10(April) doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bencherif M., Lippiello P.M., Lucas R., Marrero M.B. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell. Mol. Life Sci. 2011;68(March(6)):931–949. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oppo V., Melis M., Melis M., Tomassini Barbarossa I., Cossu G. Smelling and tasting" parkinson’s disease: using senses to improve the knowledge of the disease. Front. Aging Neurosci. 2020;25(February (12)):43. doi: 10.3389/fnagi.2020.00043. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haehner A., Hummel T., Reichmann H. Olfactory loss in Parkinson’s disease. Parkinsons Dis. 2011;2011 doi: 10.4061/2011/450939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doty R.L., Deems D.A., Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(August(8)):1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 70.Müller A., Müngersdorf M., Reichmann H., Strehle G., Hummel T. Olfactory function in Parkinsonian syndromes. J. Clin. Neurosci. 2002;9(September(5)):521–524. doi: 10.1054/jocn.2001.1071. [DOI] [PubMed] [Google Scholar]
- 71.Sharer J.D., Leon-Sarmiento F.E., Morley J.F., Weintraub D., Doty R.L. Olfactory dysfunction in Parkinson’s disease: positive effect of cigarette smoking. Mov. Disord. 2015;30:859–862. doi: 10.1002/mds.26126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bohnen N.I., Müller M.L., Kotagal V., Koeppe R.A., Kilbourn M.A., Albin R.L., Frey K.A. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson’s disease. Brain. 2010;133(June(Pt 6)):1747–1754. doi: 10.1093/brain/awq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J., Lv D.J., Li L.X., Wang Y.L., Qi D., Chen J., Mao C.J., Wang F., Liu Y., Hu L.F., Liu C.F. Nicotine improved the olfactory impairment in MPTP-induced mouse model of Parkinson’s disease. Neurotoxicology. 2019;73(July):175–182. doi: 10.1016/j.neuro.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 74.D’Souza R.D., Vijayaraghavan S. Paying attention to smell: cholinergic signaling in the olfactory bulb. Front. Synaptic Neurosci. 2014;6(September (21)) doi: 10.3389/fnsyn.2014.00021. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Butowt R., Bilinska K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem. Neurosci. 2020;13(April) doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 76.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang N., Li D., Wang X., Sun Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br. J. Haematol. 2020;18(April) doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schedel A., Thornton S., Schloss P., Klüter H., Bugert P. Human platelets express functional alpha7-nicotinic acetylcholine receptors. Arterioscler. Thromb. Vasc. Biol. 2011;31(April(4)):928–934. doi: 10.1161/ATVBAHA.110.218297. [DOI] [PubMed] [Google Scholar]
- 80.Kooijman S., Meurs I., van der Stoep M., Habets K.L., Lammers B., Berbée J.F., Havekes L.M., van Eck M., Romijn J.A., Korporaal S.J., Rensen P.C. Hematopoietic α7 nicotinic acetylcholine receptor deficiency increases inflammation and platelet activation status, but does not aggravate atherosclerosis. J. Thromb. Haemost. 2015;13(January(1)):126–135. doi: 10.1111/jth.12765. [DOI] [PubMed] [Google Scholar]
- 81.Bennett J.A., Ture S.K., Schmidt R.A., Mastrangelo M.A., Cameron S.J., Terry L.E., Yule D.I., Morrell C.N., Lowenstein C.J. Acetylcholine inhibits platelet activation. J. Pharmacol. Exp. Ther. 2019;369(May(2)):182–187. doi: 10.1124/jpet.118.253583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92(April(4)):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pullan R.D., Rhodes J., Ganesh S., Mani V., Morris J.S., Williams G.T., Newcombe R.G., Russell M.A., Feyerabend C., Thomas G.A., Sawe U. Transdermal nicotine for active ulcerative colitis. N. Engl. J. Med. 1994;330(March (12)):811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 84.Villafane G., Cesaro P., Rialland A., Baloul S., Azimi S., Bourdet C., Le Houezec J., Macquin-Mavier I., Maison P. Chronic high dose transdermal nicotine in Parkinson’s disease: an open trial. Eur. J. Neurol. 2007;14(December(12)) doi: 10.1111/j.1468-1331.2007.01949.x. 1313-6. Epub 2007 Oct 17. [DOI] [PubMed] [Google Scholar]
- 85.Newhouse P., Kellar K., Aisen P., White H., Wesnes K., Coderre E., Pfaff A., Wilkins H., Howard D., Levin E.D. Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology. 2012;78(January (2)):91–101. doi: 10.1212/WNL.0b013e31823efcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.RESPADD . 2020. Prendre en charge les fumeurs dans les lieux de santé. Un livret d’aide à la pratique pour les professionnels. Available at: https://www.respadd.org/wp-content/uploads/2019/09/Livret-Prise-en-charge-LSST-09-2019-BAT3.pdf (accessed on April 18, 2020) [Google Scholar]