Abstract
Background
Handwashing is important to reduce the spread and transmission of infectious disease. Ash, the residue from stoves and fires, is a material used for cleaning hands in settings where soap is not widely available.
Objectives
To assess the benefits and harms of hand cleaning with ash compared with hand cleaning using soap or other materials for reducing the spread of viral and bacterial infections.
Search methods
On 26 March 2020 we searched CENTRAL, MEDLINE, Embase, WHO Global Index Medicus, and the WHO International Clinical Trials Registry Platform.
Selection criteria
We included all types of studies, in any population, that examined hand cleaning with ash compared to hand cleaning with any other material.
Data collection and analysis
Two review authors independently screened titles and full texts, and one review author extracted outcome data and assessed risk of bias, which another review author double‐checked. We used the ROBINS‐I tool for observational studies, we used RoB 2.0 for three interventional studies, and we used GRADE to assess the certainty of the evidence. We planned to synthesise data with random‐effects meta‐analyses. Our prespecified outcome measures were overall mortality, number of cases of infections (as defined in the individual studies), severity of infectious disease, harms (as reported in the individual studies), and adherence.
Main results
We included 14 studies described in 19 records using eight different study designs, but only one randomised trial. The studies were primarily conducted in rural settings in low‐ and lower‐middle‐income countries. Six studies reported outcome data relevant to our review.
A retrospective case‐control study and a cohort study assessed diarrhoea in children under the age of five years and self‐reported reproductive tract symptoms in women, respectively. It was very uncertain whether the rate of hospital contacts for moderate‐to‐severe diarrhoea in children differed between households that cleaned hands using ash compared with households cleaning hands using soap (RR 0.97, 95% CI 0.84 to 1.11; very low‐certainty evidence). Similarly, it was very uncertain whether the rate of women experiencing symptoms of reproductive tract infection differed between women cleaning hands with ash compared with cleaning hands using soap (RR 0.48, 95% CI 0.12 to 1.86; very low‐certainty evidence) or when compared with handwashing with water only or not washing hands (RR 0.50, 95% CI 0.13 to 1.96; very low‐certainty evidence).
Four studies reported on bacteriological counts after hand wash. We rated all four studies at high risk of bias, and we did not synthesise data due to methodological heterogeneity and unclear outcome reporting.
Authors' conclusions
Based on the available evidence, the benefits and harms of hand cleaning with ash compared with soap or other materials for reducing the spread of viral or bacterial infections are uncertain.
Plain language summary
Does cleaning hands with ash stop or reduce the spread of viral and bacterial infections compared with soap or other materials?
Background
Some infectious diseases are spread by airborne droplets from coughs and sneezes, which can infect people who touch contaminated skin or surfaces. Washing hands with soap and water may prevent these diseases from spreading. People with no soap may use other materials like ash, mud, soil with or without water, or water alone, to clean their hands. Hand cleaning with ash (the solid remains from cooking stoves and fires) might work by rubbing away or inactivating the virus or bacteria. However, chemicals in the ash could also damage the skin.
If ash is an effective hand cleanser, it could reduce the spread of coronavirus (COVID‐19) and other infectious diseases in low‐income areas where soap is not widely available.
What did we want to find out?
We wanted to know whether people who use ash for hand cleaning are more or less likely to catch infectious diseases than people who use soap, water, mud or soil, or who do not clean their hands. We also wanted to know whether using ash causes unwanted effects, like sore hands or a rash.
Our methods
We looked for studies that examined hand cleaning with ash compared with soap, mud, soil, water only or no hand cleaning. To answer our questions, the studies could include adults and children and take place anywhere.
COVID‐19 is spreading rapidly, so we needed to answer this question quickly. This meant we shortened some steps of the normal Cochrane Review process. We could not find the full texts of five potentially relevant studies, or contact study authors for additional data. Although we searched several databases we may have missed some studies. We plan to include all relevant information in a future version of the review.
Results
We identified 14 studies that assessed ash for hand cleaning. Only one small study directly compared people chosen at random to use ash or soap or other materials (randomised studies produce the best evidence). The studies included people of all ages and mainly took place in low‐income, rural communities. Six studies provided information to help answer our question.
One study investigated children who had been to hospital with diarrhoea compared with children who had not. Study authors looked at the hand washing area in the children’s houses to see how they cleaned their hands. They found that families that used ash for hand cleaning made a similar number of hospital visits for children with diarrhoea as those families that used soap.
Another study investigated whether women with unusual vaginal itching or discharge were more likely to clean their hands with ash than women who had not experienced such symptoms. They found that women who used ash and water for hand cleaning were as likely to experience vaginal itching or discharge as those women who used soap.
Four studies measured bacteria on hands after using ash, soap, water, mud or no hand cleaning. We are uncertain about the effect of ash compared with other materials for hand cleaning on bacteria on people’s hands because the studies used unreliable methods and their results were unclear.
None of the studies provided information about the severity of infectious diseases, whether people used ash or another material consistently, the number of deaths, or unwanted effects due to hand cleaning with ash.
Certainty of the evidence
Our certainty (confidence) in the evidence was limited because we found few studies; those we did find had unreliable methods and different kinds of participants, and none of the studies we found reliably examined whether participants got infections.
Conclusion
We are uncertain whether hand cleaning with ash compared with hand cleaning with soap, water, mud, soil or no hand cleaning stops or reduces the spread of viral or bacterial infections. We do not know if hand cleaning with ash causes unwanted effects.
Search date
This review includes evidence published up to 26 March 2020.
Summary of findings
Background
Description of the condition
On 11 March 2020 the World Health Organization (WHO) declared COVID‐19 a global pandemic (WHO 2020a). The disease COVID‐19 is caused by the beta‐coronavirus SARS‐CoV‐2, which is transmitted via airborne droplets, contaminated surfaces, and through close contact with an infector (WHO 2020b). High virulence likely increases the spread of acute respiratory infection outbreaks (Jefferson 2006), such as the current COVID‐19 outbreak. There are currently no effective vaccines or approved treatments for COVID‐19. Reducing the spread of the infection is therefore an important health measure in response to the outbreak of COVID‐19 as well as for other outbreaks with infectious agents.
Description of the intervention
One of the most important measures to prevent the spread of pathogens, including bacteria and viruses that cause respiratory infections, is frequent and proper cleaning of the hands. According to a 2017 United Nations International Children's Emergency Fund (UNICEF) and WHO report, 1.6 billion people had limited access to clean water and soap facilities, and 1.4 billion had access to no facilities at all (WHO 2019, p9). In rural areas and low‐income countries, other materials than soap and water may be used for hand cleaning, including scrubbing with mud, soil or ashes with or without subsequent rinsing with water (Bloomfield 2009). In a case‐control study in Bangladesh, 11% of the surveyed participants only had access to ash to clean their hands (GEMS 2014). Ash is the waste product of burnt wood, coal, leaves and other biomaterials and may be polluted by toxins, metals, or contaminated by microbial pathogens (Bloomfield 2009). The WHO described ash as a cleaning and disinfecting agent when used with water to eliminate pathogens on hands and utensils in a publication from 2002 (Howard 2002, p. 65), and in their 2009 guideline on 'Hand Hygiene in Health Care', they mentioned that ash may be as effective as soap, with referral to the Hoque and Briend, 1991 study (Hoque 1991; WHO 2009, p. 78). UNICEF suggests using ash in the absence of soap to prevent the spread of coronavirus during the current COVID‐19 pandemic (UNICEF 2020).
How the intervention might work
It is hypothesised that hand cleaning with ash might reduce the quantity of virus and bacteria and thus the spreading of disease. This potential effect could be caused by the physical removal of pathogens through scrubbing, as well as the alkaline properties of ashes in reaction with water (GEMS 2014). If ash can effectively reduce the quantity of infectious agents, hand cleaning with ash might be an alternative option to hand cleaning with soap or other methods to interrupt or reduce spread of infectious diseases including, but not limited to, COVID‐19 in rural and low‐income areas where soap is not widely available. This must be balanced against the possibility that hand cleaning with ash may cause harms, if for example the ash used contains chemical or infectious pollutants or its use damages the skin.
Objectives
To assess the benefits and harms of hand cleaning with ash compared with hand cleaning with soap or other materials for reducing the spread of viral and bacterial infections.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include the following study designs:
Randomised, non‐randomised and cluster‐randomised trials
Prospective, retrospective and non‐concurrent cohort studies
Retrospective and prospective case‐control studies
Non‐comparative studies
Controlled before‐and‐after studies
Minimum study duration
We considered studies of any duration.
Types of participants
We included studies in any population without restrictions.
Types of interventions
Intervention
We included studies examining hand cleaning with ash, with or without water.
Comparators
We included all studies that compared hand cleaning with ash to another method of hand cleaning, for example, soap, mud, soil, or water only, or no hand wash.
Types of outcome measures
Primary outcomes
These were our prespecified outcomes:
Overall mortality
Number of cases of infections (we analysed cases as defined in the individual studies, e.g. according to serological tests or by clinical diagnosis)
Severity of infectious disease (we assessed the burden of the consequence, e.g. absence from work, use of primary care services or hospitalisation)
Harms (as reported in the individual studies, e.g. skin lesions)
Adherence
We included studies irrespective of their measured and reported outcomes.
Search methods for identification of studies
We adhered to the following methods prespecified in the protocol (Appendix 1). An information specialist (IK) designed and conducted all searches. The search strategy was independently peer reviewed.
Electronic searches
We searched:
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 3) in the Cochrane Library (searched 26 March 2020);
Ovid MEDLINE(R) ALL (1946 to 26 March 2020);
Embase.com (Elsevier) from inception to 26 March 2020;
WHO Global Index Medicus (https://search.bvsalud.org/gim/) from inception to 26 March 2020.
The search strategy can be seen in Appendix 2.
Searching other resources
We also searched the WHO International Clinical Trials Registry Platform (www.who.int/ictrp/en/; searched 26 March 2020) to identify ongoing or unpublished trials.
Data collection and analysis
We prespecified the following in the protocol.
Selection of studies
All titles and abstracts were independently screened for eligibility by two review authors (ASP‐M, KB or KM). The full‐text reports of potentially eligible records were also independently reviewed by two review authors. Any discrepancies were resolved by consensus. We included all types of records, including abstracts, conference proceedings and trial registry entries. We reported the reason for exclusion for all studies excluded after the full‐text review.
Inclusion of non‐English language studies
Abstracts and full‐text reports in all languages were included. If non‐English records were identified, we planned to obtain a translation of the full‐text report of the record to assess eligibility.
Software
We screened for eligibility using Covidence. Data extraction was done in Microsoft Excel. We carried out quantitative analyses using R and RStudio (R 2020; RStudio 2019). We made 'Summary of findings' (SoF) tables using GRADEpro (GRADEpro GDT)
Data extraction and management
One review author (ASP‐M, KB or KM) carried out data extraction using a pilot‐tested Microsoft Excel spreadsheet and a second review author checked it. We piloted the data extraction spreadsheet during the first phase of data extraction. We recorded the following:
Study design (publication year, period of study conduct, methods, location, groups)
Setting (type of epidemic outbreak, rurality)
Participant characteristics (sex, age, household, socioeconomic status, toilet facilities, disease type)
Intervention characteristics (washing opportunities, water source, type of ash)
Comparator characteristics (washing opportunities, water source, type of material)
Outcomes assessed
Numerical data for outcomes of interest
Assessment of risk of bias in included studies
We assessed randomised trials using the Cochrane Risk of Bias tool version 2.0 (Sterne 2019). We assessed observational studies using the Risk of Bias in Non‐randomized Studies – of Interventions (ROBINS‐I) tool (Sterne 2016). We did not use any algorithm to decide the overall risk of bias (ROBINS‐I), rather we based the assessment on the overall impression of the study.
Two review authors independently carried out all assessments. We resolved discrepancies by discussion with a third review author.
Contacting study authors
Due to the short timeframe of this rapid review, we did not contact study authors. We will contact the study authors and update the review if we acquire additional data.
Data management
None of the included studies reported any data that needed to be standardised. We did not encounter any discrepancies between different sources of data.
Measures of treatment effect
As per our protocol (Appendix 1), we had not prespecified methods for quantitative analysis and data synthesis as we anticipated including many diverse study designs. For three studies reporting dichotomous outcomes we calculated risk ratios (RRs). For one study reporting means and standard deviations we calculated mean differences (MDs). None of the other included studies reported data that could be analysed quantitatively.
Unit of analysis issues
We planned to handle unit of analysis issues according to methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We reported individual studies only and did not pool data across treatment groups.
Assessment of heterogeneity
We planned to assess heterogeneity by inspecting forest plots, I2 statistics (Higgins 2003), and by exploring potential sources of heterogeneity of study results by using subgroups based on, for example, region, type of ash, whether ash is used with or without water and the context of cleaning (e.g. after defecation or before eating). As we did not perform any meta‐analyses, we did not assess heterogeneity.
Assessment of reporting biases
We planned to assess reporting biases by inspecting funnel plots, but we were unable to do so due to a lack of studies. If study protocols were available, we checked the planned study outcomes against those in the report of the studies.
Data synthesis
If possible, we wanted to synthesise data by conducting meta‐analyses of results from RCTs and observational studies, separately. For studies that reported data that we could not meta‐analyse, we described the results narratively and reported the raw data in the full dataset.
Model
We planned to synthesise the results using a random‐effects model with inverse variance weighting (DerSimonian and Laird method; DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We prespecified the following subgroup analyses: geographic region; ash used with or without water; context (i.e. when the ash was used); type of infection; and type of study. As we did not perform any meta‐analyses this was not possible.
Sensitivity analysis
We planned to conduct sensitivity analyses excluding studies rated as high risk of bias. Any other type of sensitivity analysis would be decided during the review process depending on the available studies and outcome data. As we did not perform any meta‐analyses no sensitivity analyses were conducted.
Summary of findings and assessment of the certainty of the evidence
We applied the GRADE tool to studies that reported prespecified outcome data to assess our certainty in the evidence (Schünemann 2013). We summarised our findings in 'Summary of findings' tables.
Results
Description of studies
Results of the search
We identified 88 records through our searches of bibliographic databases and trials registries, and one record from handsearching the reference lists of the included studies. After removing duplicates, we screened 58 titles and abstracts, of which we excluded 23 records that were not relevant. We obtained full‐text reports of 30 records that we screened for eligibility. We were unable to retrieve the full‐text reports of the remaining five records; we requested the records from a research library, which was unable to process the request because of closed services due to the COVID‐19 outbreak. We excluded 11 full‐text reports and provided reasons for exclusions together with a diagram of the search and study selection process in a PRISMA flow‐chart (Moher 2009; Figure 1). We identified three qualitative studies that were not eligible for the review but provided information on barriers and motivation for using ash for cleaning hands (Afroz 2010; Blum 2017b; McMichael 2016). The results of these studies are summarised in Table 3.
1.
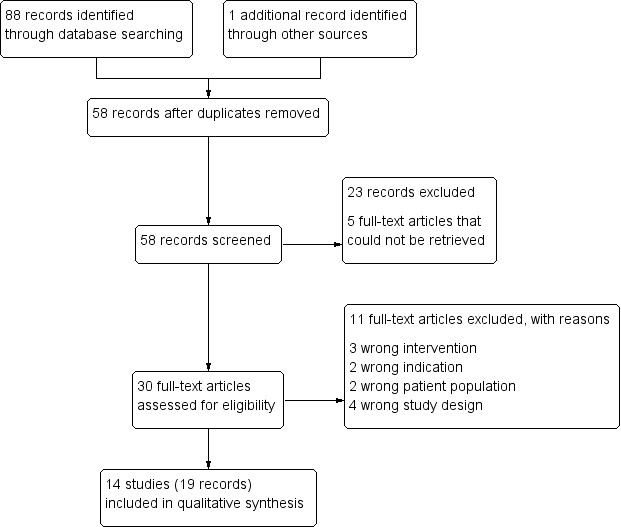
Study flow diagram
1. Studies with relevant qualitative information.
| Study ID | Country | Population | Design details | Main findings |
| Afroz 2010a | Bangladesh | Children and adults in rural villages | In‐depth interview with 25 adult males and females and pocket‐voting with 30 school children | Participants explained that "Ash is only used after defecation, when soap is not available" |
| Blum 2017a and Blum 2017ba | Democratic Republic of Congo | Camp for internally displaced persons | Informant interviews with 9 NGO officials, in‐depth interviews with 18 mothers of children < 5 years, and 4 group discussions with camp residents | "Handwashing using either soap or ash was observed to occur after 10% of latrine use events" and mother‐respondents and group discussion participants reported that "ash does not remove all dirty substances associated with illness or have cleansing capabilities, with many never previously using ash for handwashing." Other disadvantages cited were "that ash leaves a white substance on the hands, does not make the hands feel good, makes handwashing hardware dirty and less attractive, is not valued, and is only used for handwashing after using the toilet, but not during other events or for other purposes." |
| McMichael 2016a | Nepal | Men and women from rural Nepal | 13 focus group discussions, 29 in‐depth interviews, and 16 ‘most significant change’ drawings | Study does not report on barriers and motivation for cleaning hands with ash specifically, but for handwashing with ash or soap in general. |
| Nguyen 2015 | Malawi | Women from Mzimba and Salima Districts | Interviews with key informants (number not specified) | Participants disliked the smell of soap but found the smell of ash and mud acceptable. Additionally, ash was "available at no cost to the family because they practiced cooking with wood stoves" |
| Nizame 2015 | Bangladesh | Households with children under 5 years of age | In‐depth interviews with 15 female and 9 male adults | 22 of 24 informants from rural Bangladesh said that "ash was freely available from cooking stoves" and "because soap is expensive, they like to minimize the use of soap and use ash and soil to wash hands." Eleven informants "stated that soil or ash can clean hands as effectively as soap", and seven informants "perceived soap as a modern product that cleaned visible dirt and removed germs and bad odor from hands more effectively compared with other agents (soil, ash, water only)" |
aThese studies were not included in the review, but can be found in the Excluded studies.
Included studies
We included 14 studies described in 19 records (Anuradha 1999; Aziz 1990; Baker 2017; Edward 2019; GEMS 2014; Hoque 1991; Hoque 1995; Huda 2010; Jha 2006; Khin 1997; Nguyen 2015; Nizame 2015; Ravindra 2019; Zeitlin 1986). There were five non‐randomised trials, four non‐comparative studies, three prospective cohort studies, one retrospective case‐control studies, and one randomised trial. We have summarised the key study characteristics in Table 4.
2. Summary of included trials.
| Trial ID | Design | Country | Population | Intervention details | Research question |
| Aziz 1990 | Non‐randomised trial | Bangladesh | Village populations | No information provided | Effects of hygiene education |
| Anuradha 1999 | Prospective cohort study | India | Women with children between 1‐2 years of age | No information provided – seems likely that ash was used with water | Effects of different washing materials on bacteriological counts |
| Baker 2017 | Retrospective cohort study | India | Women and girls aged 14‐45 years | Ash was used with water. No information provided on source of ash | Effects of different washing materials on symptoms of reproductive tract infection |
| Edward 2019 | Non‐randomised trial | Cambodia, Guatemala, Kenya and Zambia | Women with childbirth within 2 previous years | No information provided | Effects of hygiene education |
| GEMS 2014 | Retrospective case‐control study | The Gambia, Kenya, Mali, Mozambique, Bangladesh, India, and Pakistan | Children > 5 years of age | Exposure was whether soap or ash was present at handwashing station – no additional information provided | Indicators of handwashing practices with the prevalence of diarrhoea |
| Hoque 1991 | Randomised trial | Bangladesh | Women living in slum quarters | Ash and mud were sterilised before use. Ash was used with water. | Effects of different washing materials on bacteriological counts |
| Hoque 1995 | Non‐randomised trial | Bangladesh | Rural women | Ash was used with 2 L of tube well water. No information on source of ash | Handwash practice and bacteriological counts |
| Huda 2010 | Prospective cohort study | Bangladesh | Rural households | No information provided | Effects of hygiene education |
| Jha 2006 | Non‐randomised trial | Nepal | Households | No information provided | Effects of hygiene education |
| Khin 1997 | Non‐randomised trial | Myanmar | Not reported | Ash used with water. No information provided on source of ash | Effects of different washing materials on bacteriological counts |
| Nguyen 2015 | Non‐comparative study | India | Menstruating women | No information provided | Hygiene practice and symptoms of reproductive tract infections |
| Nizame 2015 | Non‐comparative study | Bangladesh | Households with children < 5 | No information provided | Observation of handwash practices |
| Ravindra 2019 | Non‐comparative study | India | Households | No information provided | Sanitation and hygiene practices |
| Zeitlin 1986 | Non‐comparative study | Bangladesh | Crawling infants and mothers | No information provided | Effects of hygiene education |
Excluded studies
We excluded 11 full‐text articles (Afroz 2010; Bennett 1997; Blum 2017a; Hoffman 1997; Luby 2008; McMichael 2016; Min 1988; NCT01900912; Ngulube 2015; Russpatrick 2015; Yeboah‐Antwi 2017). Three had the wrong intervention, two had the wrong indication, two included the wrong patient population and four used an ineligible study design.
Risk of bias in included studies
We have summarised the 'Risk of bias' assessments in Figure 2 and Figure 3.
2.
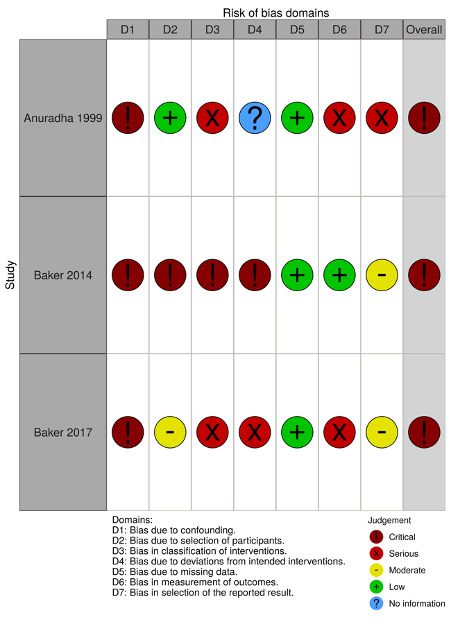
'Risk of bias' domains: ROBINS‐I
3.
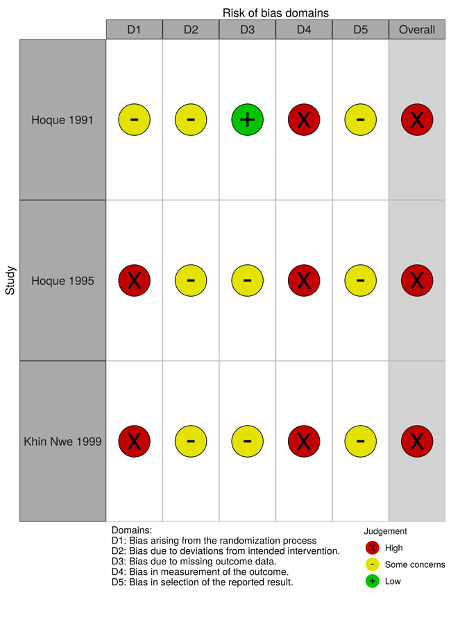
'Risk of bias' domains
We assessed the risk of bias in three observational studies (Anuradha 1999; Baker 2017; GEMS 2014) using the Risk of Bias in Non‐randomized Studies of Interventions (ROBINS‐I) tool (Appendix 3).
We applied the Cochrane RoB 2.0 tool to three studies that reported data on bacteriological counts (Hoque 1991; Hoque 1995; Khin 1997; Appendix 4).
Effects of interventions
Overall mortality
None of the included studies reported eligible outcome data for overall mortality.
Number of cases of infections
Clinically diagnosed cases of diarrhoea
None of the included studies reported on clinically diagnosed infections, whether by clinical examination alone or assisted by laboratory tests. One retrospective case‐control study investigated the hand‐cleaning practices in households in Bangladesh where children under the age of five had been diagnosed with moderate‐to‐severe diarrhoea during a hospital visit (GEMS 2014). Cases were diagnosed based on a clinical presentation without requirement of laboratory tests to determine whether the diarrhoea was caused by bacteria, virus, or something else; age, sex, and community‐matched control children without diarrhoea were randomly selected from a local database. Hand‐cleaning practice in the household of the child was assessed by the observed presence of soap, detergent, ash and water source at the handwashing area in the household. The study found that there was little to no difference in the rate of hospital contacts for moderate‐to‐severe diarrhoea in children under the age of five between households cleaning hands with ash compared with households cleaning hands with soap, but the confidence interval (CI) was relatively wide (RR 0.97, 95% CI 0.84 to 1.11; very low‐certainty evidence). A report of the same study that presented aggregated results across study sites in six additional countries grouped hand‐cleaning practice with soap or ash and therefore did not report quantitative data relevant to our review (GEMS 2014).
Self‐reported symptoms of reproductive tract infections
A cohort study investigated the association between hand‐cleaning practices and symptoms of reproductive tract infections in women and girls between the ages of 14 and 45 years in India (Baker 2017). The outcome was defined based on self‐reported symptoms of reproductive tract infection, and hand‐cleaning practice was assessed based on self‐report of the type of material that the included participants used to clean their hands. The study found that the risk ratio for women experiencing symptoms of reproductive tract infection between women cleaning hands at any time with ash and water and women cleaning hands with soap and water was 0.48 (95% CI 0.12 to 1.86; very low‐certainty evidence). The risk ratio for women experiencing symptoms of reproductive tract infection between women cleaning hands with ash compared with women cleaning hands with water only or not washing hands after defecation was 0.50 (95% CI 0.13 to 1.96; very low‐certainty evidence).
Contamination of hands with fecal coliform bacteria as a surrogate for infection
Four studies measured the presence of fecal coliform bacteria on hands through finger‐dip or swab methods (Anuradha 1999; Hoque 1991; Hoque 1995; Khin 1997). The studies had heterogeneous study designs and we did not synthesise their results. Hoque 1995 was a non‐randomised trial, where researchers measured the level of bacteria after post‐defecation handwashing in rural women in Bangladesh; Hoque 1991, a randomised trial, collected samples from women living in a slum in Bangladesh. The samples were not collected in relation to any specific activities. The women rotated between five different washing materials (water, soap, mud, ash, no handwash) over five days. Anuradha 1999 was a prospective cohort study, where the hands of Indian mothers were swabbed after handwash (soap, water, ash, no handwash) just before they fed their babies; and Khin 1997 was a non‐randomised trial of several different soaps and other materials including ash in an unknown population in Myanmar. The quantitative analyses were reported differently across the studies; Hoque 1991 reported on the presence of any colony‐forming units as a dichotomous outcome, whereas the three other studies reported the number of colony‐forming units. There were several uncertainties regarding the results. In Hoque 1995, the number of observations did not match the reported population of 60 women; in Khin 1997 it was not clear whether the unit of analysis was one hand, one participant, or one group. In Hoque 1991, they reported the numbers of contaminated hands after washing hands with different materials based on the presence of colony‐forming units; the risk of having contaminated hands was lower when using ash compared with soap (RR 0.75, 95 % CI 0.19 to 2.93; very low‐certainty evidence); the risk was lower when using ash compared with mud (RR 0.75, 95 % CI 0.19 to 2.93; very low‐certainty evidence); the risk was lower for the use of ash compared with water alone (RR 0.38, 95 % CI 0.12 to 1.21; very low‐certainty evidence), and for hand cleaning with ash compared with no hand wash (RR 0.25, 95 % CI 0.08 to 0.75; very low‐certainty evidence). In Anuradha 1999, the count of bacteria was higher on those using ash compared to soap (mean difference (MD) 158, 95% CI 43 to 273; very low‐certainty evidence), but lower than water alone (MD 435, 95% CI 34 to 837; very low‐certainty evidence), or those not washing hands at all (MD 904, 95% CI 709 to 1100; very low‐certainty evidence). The ash and mud used in this study were sterilised before use, which limits the external validity of the findings. In Hoque 1995, the numerical count of bacteria was lower when using ash for hand cleaning than soap and three types of soil (kitchen, latrine or wet), but the variance was not reported so we were unable to calculate mean differences of colony‐forming units. We were not able to interpret the values reported in Khin 1997. Considering the methodological heterogeneity, sparse reporting, and the fact that contamination of hands is a surrogate outcome it is not possible to draw conclusions about the effectiveness of hand cleaning with ash compared to other materials for reducing the spread of infections based on these studies. The raw data, including bacteriological counts, are available from the full dataset (see data sharing statement for details).
Severity of infectious disease
None of the included studies reported outcome data for the severity of infectious disease. One case‐control study investigated the hand‐cleaning practices in households in Bangladesh where children under the age of five had been diagnosed with moderate to severe diarrhoea during a hospital visit (GEMS 2014). The report did not provide data on the severity of diarrhoea, length of hospitalisation, or subsequent use of primary care facilities or other potential indicators of the severity of illness (GEMS 2014). Another case‐control study (Baker 2017), investigated the association between hand‐cleaning practices and symptoms of reproductive tract infections. They categorised participants as having reproductive tract infections based on self‐reported symptoms but did not provide information on indicators of the severity of potential infections.
Harms
None of the included studies reported on harms of using ash for cleaning hands.
Adherence
None of the included studies examined hand cleaning using ash as an intervention over a given period of time, thus none of the included studies reported eligible quantitative outcome data for adherence.
Several of the included studies reported on the prevalence of the use of ash for cleaning hands. Hoque 1991 reported that none of 20 women used ash for hand cleaning post‐defecation; Hoque 1995 reported that two of 95 women used ash to clean hands post‐defecation; Nizame 2015 reported that 45 of 349 post‐defecation hand cleans involved ash; Jha 2006 reported that 16 of 60 participants used ash for hand cleaning; Anuradha 1999 reported that eight of 40 women cleaned their hands with ash before feeding their children, and in Aziz 1990, which promoted hand wash with ash rather than mud, 85% of the households in the intervention village used ash compared to 2% before the intervention.
GRADE
We rated the certainty of the evidence as very low for all reported outcomes. We downgraded two levels due to high risk of bias and one level due to indirectness of the evidence.
Discussion
Summary of main results
The main findings of the review are summarised in Table 1 and Table 2.
Summary of findings 1. Hand cleaning with ash versus soap for reducing the spread of viral and bacterial infections.
| Hand cleaning with ash versus soap for reducing the spread of viral and bacterial infections | ||||||
|
Patient or population: people at risk of viral and bacterial infections Settings: any Intervention: ash Comparison: soap | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with soap | Risk with ash | |||||
| Death | No data on mortality were available | 0 studies | ||||
| Infectionsa | Not pooled | Not pooled | Not pooled | 1057 cases 3336 controls (2 observational studies) | ⊕⊝⊝⊝ Very lowb,c | The evidence is very uncertain about the effect of cleaning of hands with ash versus soap on infections |
| Severity of infections | No data on severity of infections were available | 0 studies | ‐ | |||
| Harms | No data on harms were available | 0 studies | ‐ | |||
| Adherence | No data on adherence were available | 0 studies | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWhile two studies (GEMS 2014; Baker 2017), measured cases of diarrhoea and symptoms of reproductive tract infections, we did not consider meta‐analysis appropriate. bWe downgraded by two levels due to risk of bias, as we rated both studies as being at critical risk of bias using the ROBINS‐I tool. cWe downgraded by one level due to indirectness, as neither of the studies looked at confirmed infections, rather the outcomes were symptoms of reproductive tract infections and cases of diarrhoea.
Summary of findings 2. Hand cleaning with ash versus water or no wash for reducing the spread of viral and bacterial infections.
| Hand cleaning with ash versus water or no wash for reducing the spread of viral and bacterial infections | ||||||
|
Patient or population: people at risk of viral and bacterial infections Settings: any Intervention: ash Comparison: water or no wash | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with water or no wash | Risk with ash | |||||
| Death | No data on mortality were available | 0 studies | ‐ | |||
| Infectionsa | 99 per 1000 | 50 per 1000 (13 to 195) | RR 0.50 (0.13 to 1.96) | 196 cases 1797 controls (1 observational study) | ⊕⊝⊝⊝ Very lowb,c | The evidence is very uncertain about the effect of cleaning of hands with ash versus water or no wash on infections. |
| Severity of infections | No data on severity of infections were available | 0 studies | ‐ | |||
| Harms | No data on harms were available | 0 studies | ‐ | |||
| Adherence | No data on adherence were available | 0 studies | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOne study (Baker 2017), measured symptoms of reproductive tract infections. bWe downgraded by two levels due to risk of bias, as we rated the included study as being at critical risk of bias using the ROBINS‐I tool. cWe downgraded by one level due to indirectness, as the study did not look at confirmed infections, rather the outcome was symptoms of reproductive tract infections.
It is very uncertain whether hand cleaning with ash compared with soap or any other material reduces the spread of viral or bacterial infections. There was very low‐certainty evidence from one observational study that the rate of moderate‐to‐severe diarrhoea in children did not differ between households that cleaned hands with ash compared with households that cleaned hands with soap. There was very low‐certainty evidence from one other observational study about the rate of women experiencing symptoms of reproductive tract infection between women cleaning hands with ash compared with women cleaning hands with soap; there was also very low‐certainty evidence from this study about the rate of women experiencing symptoms of reproductive tract infection between women cleaning hands with ash compared with women washing hands with water alone or not washing hands.
Four studies measured the presence of fecal coliform bacteria on hands of women after cleaning hands with different materials. As this is a surrogate outcome and the studies had important methodological limitations, these studies could not provide any reliable evidence regarding the effectiveness of ash compared with other materials for cleaning hands. We identified no studies looking at the presence of virus after cleaning of hands.
None of the included studies reported data on the severity of infectious disease, mortality or harms associated with cleaning hands with ash.
In summary, it is very uncertain whether cleaning hands with ash is beneficial or harmful for reducing the spread of viral or bacterial infections.
Overall completeness and applicability of evidence
Our search was comprehensive, considering the rapid review format; we searched multiple databases and, through a common platform, multiple trials registries, which included for example the Indian and Pan African Trials Registries. In addition, we screened the reference lists of included studies. In spite of this, we identified only a small body of evidence and we identified only one randomised trial. The included studies looking at infections did not specify whether these were caused by bacteria, virus, or something else. We identified four studies that examined the effect of cleaning of hands using ash on the number of fecal coliform bacteria, but this is a surrogate outcome and we are not aware of any studies examining the correlation between the presence of disease‐causing agents on hands and clinically relevant outcomes, such as infection rates. We identified no studies that examined the presence of virus after cleaning of hands using ash. None of the included studies considered harms associated with using ash for cleaning hands.
Certainty of the evidence
The overall certainty of the evidence was very low. We identified only one randomised trial, which was small and reported on a surrogate outcome. The two observational studies that reported relevant data did not allow for causal inference. The four included studies that assessed the presence of bacteria after handwash were small and poorly reported, and the presence of bacteria is a surrogate for reducing the spread of infections. We rated all studies with relevant quantitative data at either ‘critical’ (using ROBINS‐I; Appendix 3) or ‘high’ risk of bias (using the Cochrane RoB 2.0 tool; Appendix 4).
Potential biases in the review process
We conducted this rapid review over a short period of time, which may have led to several limitations. First, we were unable to identify full‐text reports for five potentially eligible studies. Upon inspecting the abstracts of these records, we judged that one of the five records (Ray 2009), could potentially hold information relevant to the review; however, this was a study looking at bacterial counts, a surrogate outcome, and as such it is unlikely that the inclusion of the study would change the overall conclusions of this review. We plan to include this information in a future update of the review, if eligible. Second, we did not have time to contact study authors to ask for additional data. Third, one review author extracted data and another checked them, instead of independent data extraction. Fourth, although we searched multiple databases, WHO’s trials registry platform, and handsearched reference lists of included reports, we cannot discount the possibility that there are relevant studies that our searches did not identify. Fifth, we did not prespecify our quantitative analyses, as we anticipated including a range of diverse study designs. Finally, we chose to include studies measuring the presence of bacteria on hands after cleaning although this is a surrogate outcome and it was not prespecified in our protocol. As such, this outcome should be interpreted with caution.
Agreements and disagreements with other studies or reviews
Previous systematic reviews have reported that hygiene measures, including hand wash, can prevent the spread of respiratory viruses (Jefferson 2011), and the promotion of hand wash reduces the number of diarrhoea episodes (Ejemot‐Nwadiaro 2015). To our knowledge, this is the first systematic review to assess the benefits and harms of using ash for cleaning hands.
Authors' conclusions
Implications for practice
We are uncertain whether cleaning hands with ash is effective compared to soap or other materials for reducing the spread of viral or bacterial infections. We are also uncertain whether using ash for cleaning hands is harmful. More research would provide clearer evidence for communities and healthcare workers.
Implications for research
Considering the scarcity and low quality of available evidence, our review points to an important gap in the evidence base. Randomised trials looking at clinically relevant outcomes such as bacterial or viral infections are needed before we can draw valid conclusions about the benefits and harms of using ash for cleaning hands.
What's new
| Date | Event | Description |
|---|---|---|
| 24 July 2020 | Amended | Republished to change the review type from Prototype to Rapid (no changes to content) |
History
Review first published: Issue 4, 2020
Acknowledgements
The EMD Editorial Service managed the editorial process for this review in collaboration with the Cochrane Infectious Diseases Group (CIDG).
The CIDG editorial base is funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the UK government’s official policies.
We thank Robin Featherstone for assistance in the planning of the review and Doug Salzwedel for peer‐review of the search strategy. We are grateful to Dr Sumanth Gandra and Dr. Foyeke Tolani, who kindly peer‐reviewed the draft of this review. Thanks also to Prof. Victor DiRita, Dr Wojkowska‐Mach and Prof. Mical Paul for the microbiology advice they provided to the editorial team.
Data sharing
The extracted data and analysis code are available on the Open Science Framework.
Appendices
Appendix 1. Protocol
Protocol information
Authors
Asger Sand Paludan‐Müller; Kim Boesen; Irma Klerings; Karsten Juhl Jørgensen; Klaus Munkholm
Contact
Klaus Munkholm Nordic Cochrane Centre E‐mail: km@cochrane.dk
Date protocol completed
27 March 2020
Background
Brief description of the condition/issue under consideration
On 11 March 2020, the World Health Organization (WHO) declared COVID‐19 a global pandemic (WHO 2020a). The disease COVID‐19 is caused by the beta‐coronavirus SARS‐CoV‐2, which is transmitted via airborne droplets, contaminated surfaces, and through close contact with an infector (WHO 2020b). A high viral load and high virulence likely increases the spread of acute respiratory infection outbreaks (Jefferson 2006), such as the current COVID‐19 outbreak. There are currently no effective vaccines or treatments for COVID‐19. Interrupting or reducing the spread of the infection is therefore an important health measure in response to the outbreak of COVID‐19 as well as for other outbreaks with infectious agents.
Description of the intervention
One of the most important measures to prevent the spread of pathogens, including bacteria and viruses that cause respiratory infections, is frequent and proper hand sanitisation. According to a 2017 UNICEF (United Nations Children's Fund) and WHO report, 1.6 billion people had limited access to clean water and soap facilities, and 1.4 billion had access to no facilities at all (WHO 2019, p. 9). In rural areas and low‐income countries, other remedies than soap and water may be used for hand cleaning, including scrubbing with mud, soil or ashes, with or without subsequent rinsing with water (Bloomfield 2009). In a case‐control study in Bangladesh, 11 % of the surveyed participants had access to ash only to clean their hands (Baker 2014). Ash is the waste product of burnt wood, coal, leaves and other biomaterials and may be polluted by toxic metals or contaminated by microbial pathogens (Bloomfield 2009). The WHO described ash as a cleaning and disinfecting agent when used with water to eliminate pathogens on hands and utensils in a publication from 2002 (Howard 2002, p. 65). UNICEF suggests using ash in the absence of soap to prevent the spread of coronavirus during the current COVID‐19 pandemic (UNICEF 2020).
How the intervention/test might work
It is hypothesised that hand cleaning with ash might reduce the quantity of virus and bacteria and thus the spread of disease. This potential effect could be caused by the physical removal of pathogens through scrubbing, as well as the alkaline properties of ash in reaction with water (Baker 2014). If ash can effectively reduce the quantity of infectious agents, this might be an alternative option to hand cleaning with soap or other methods to interrupt or reduce the spread of infectious diseases including, but not limited to COVID‐19 in rural and low‐income areas where soap is not widely available. This must be balanced against the possibility that hand cleaning with ash may cause harms, if for example the ash used contains chemical or infectious pollutants or its use damages the skin.
Objectives
To assess the benefits and harms of hand cleaning with ash compared with hand cleaning with soap or other materials for interrupting or reducing the spread of viral and bacterial infections.
Methods
Criteria for considering studies for this review
| Study and source eligibility | |
| Study design | We will include the following study designs.
|
| Minimum duration | We will consider studies of any duration |
| ‘PICO’ eligibility | |
| Population | We will include participants without restrictions |
| Intervention | Hand cleaning with ash, with or without water |
| Comparators | Hand cleaning with soap, mud, soil, water only, or any other material |
| Outcome(s) |
|
Search methods for identification of studies
| Search methods | |||||
| Expertise | An Information Specialist (IK), will design and conduct the searches and another Information Specialist will independently peer review them. | ||||
| Electronic databases (Few references in CENTRAL: we should search trials registry) |
Database ☒ MEDLINE ☒ CENTRAL ☒ Embase ☒ Other: WHO Global Index Medicus ☒ Clinical Trials Registry (WHO ICTRP) |
From: inception |
To: present | ||
| Other searches | ☒ Systematic review references ☒ Reference lists of included studies ☐ Grey literature (please specify) ☐ Citation tracking ☐ Data from the pharmaceutical industry ☐ Contact experts for references ☐ Other (please specify) |
||||
| Approach to ongoing and unpublished studies | ☒ Include ongoing studies ☐ Unpublished studies ☒ Studies in press ☐ Exclude all studies that are ongoing, unpublished, or in press |
We will search the WHO International Clinical Trials Registry Platform (ICTRP) for ongoing studies. Embase also includes conference abstracts, which might refer to ongoing studies. Both MEDLINE and Embase include 'ahead of print' references to journal articles |
|||
| Methods for screening search results | |||||
| Expertise | Screening will be performed by ASP‐M, KB or DM in Covidence | ||||
| Screening methods | Dual; second reviewer checks all excluded records Dual; second reviewer checks [X%] of excluded records Dual; independent screen and cross check |
Abstract ☐ ☐ ☒ |
Full text ☐ ☐ ☒ |
||
| Discrepancy resolution | ☒ Consensus and/or third reviewer ☐ Other (please specify) |
||||
| Excluded studies | All decisions taken during screening will be documented and outlined in the final report with a list of excluded studies | ||||
| Inclusion of abstracts and conference proceedings | ☐ Exclude all ☐ Include if clearly eligible and have usable data ☒ Include if clearly eligible regardless of usable data ☐ Include if eligibility is unclear and add to section in report |
||||
| Inclusion of non‐English language studies (we will include all languages) |
☒ Include abstracts and full texts [in Chinese/any language] ☐ Include full texts only [in Chinese only/ language] ☐ Exclude |
||||
| ☒ All potentially relevant abstracts will progress to full‐text screen ☐ [Single/dual] title/abstract screen by foreign‐language speaker(s) ☒ [Abstract/methods/full text] will be translated for abstract/full‐text screen ☐ Listed as non‐English language and not assessed further | |||||
Data collection and analysis
| Data extraction | ||
| Expertise | Experienced systematic reviewers from the Nordic Cochrane Centre (KM, KB and ASP) will perform data extraction. | |
| Software | We will extract data using data extraction forms in Microsoft Excel We have prepared a data extraction Excel spreadsheet and we will pilot it on the first 10 included studies. |
|
| Data to be extracted | We will extract the following data.
|
|
| Data extraction methods | ☐ Single, no second reviewer ☒ Dual; second reviewer checks all data ☐ Dual; second reviewer checks [add proportion] ☐ Dual; independent screen and cross check |
|
| Risk of bias tool | For randomised controlled trials we will use the Cochrane 'Risk of bias' tool version 2.0 (Sterne 2019), and for observational studies we will use the ROBINS‐I tool (Sterne 2016). If we include studies using other designs, we will try to identify relevant tools, or make our own qualitative assessments pertaining to the relevant study designs. | |
| Method of risk of bias assessment | ☐ Single, no second reviewer ☒ Dual; second reviewer checks all judgements ☐ Dual; second reviewer checks [add proportion] ☐ Dual; independent screen and cross check |
☒ All outcomes ☐ Primary only |
| Discrepancy resolution | ☒ Consensus and/or third reviewer ☐Other (please specify) |
|
| Contacting study authors | ☐ Authors will be contacted for missing information and data ☒ Authors will be contacted for missing outcome data only ☐ Authors will not be contacted We will contact study authors if necessary. We do not expect to receive replies within the specified time frame of the current review, but additional data can be included in a future update of the review. |
|
| Data management | ||
| Software | We will use Covidence for screening studies, Microsoft Excel for data extraction, and we will use R and RStudio for quantitative analyses (R 2020; RStudio 2019). | |
| Standardisation | If more than one study reports on the same continuous outcome using different measures, we will standardise to the same unit of measurement, such as the standardised mean difference (SMD). | |
| Resolving conflicts between sources | If there are discrepancies between data reported across multiple sources for the same study, we will make an ad hoc decision to decide which data to include. We do not expect this to occur in this review. | |
Data synthesis
| Measures of treatment effect | As we are including many different study designs and outcomes, we are not able to prespecify our methods of data synthesis. We will use the methods appropriate for the different measures and study designs. |
| Unit of analysis issues | We will handle issues in relation to unit of analysis issues in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). |
| Assessment of heterogeneity | ☒ Inspecting forest plots ☐ Statistical test (Chi2) for heterogeneity [specify P value] ☒ I2 statistic. We will interpret the I2 statistic (Higgins 2003), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019). ☒ Explore potential sources of the heterogeneity among study results by using subgroups based on country/region, type of ash, whether ash is used with or without water and the context of cleaning (e.g. after toilet use or before eating.) ☐ Sensitivity analysis by excluding outlying studies |
| Assessment of reporting biases | ☒ Funnel plots ☐ Test for funnel plot asymmetry (e.g. Begg, Egger test) ☐ Trim and fill technique |
| Data synthesis | If possible, we will synthesise data by conducting meta‐analyses of results from randomised controlled trials and observational studies separately. If we cannot synthesise data using meta‐analysis, we will describe the results narratively. We will combine analyses with different types of soaps but will not combine other types of comparators. |
| Model | ☐ Fixed‐effect meta‐analyses ☒ Random‐effects meta‐analyses (DerSimonian and Laird method) ☐ Other [please specify] |
| Subgroup analyses | The following subgroups will be explored: · Region · Ash used with or without water · Context · Type of infection · Type of study |
| Sensitivity analysis | ☒ Excluding studies at high risk of bias ☐ Excluding studies with dubious eligibility ☐ Alternative analysis methods ☐ Other We will justify any post hoc sensitivity analyses that arise during the review process in the final report. |
| GRADE approach | ☒ We will use GRADE for all outcomes and present results in a 'Summary of findings' table. |
Acknowledgements
We thank Robin Featherstone for assistance in the planning of the review and Doug Salzwedel for peer‐review of the search strategy.
Declarations of interest
Asger Sand Paludan‐Müller: no conflicts of interest to declare Kim Boesen: no conflicts of interest to declare Irma Klerings: no conflicts of interest to declare Karsten Juhl Jørgensen: no conflicts of interest to declare Klaus Munkholm: no conflicts of interest to declare
Appendix 2. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, issue 3) in the Cochrane Library (searched 26 March 2020)
| # | Search | Results |
| 1 | [mh ^"Hand Hygiene"] | 61 |
| 2 | [mh ^"Hand Disinfection"] | 372 |
| 3 | handwash*:ti,ab,kw | 431 |
| 4 | (hand? NEAR/2 (wash* or clean* or disinfect* or hygien*)):ti,ab,kw | 1334 |
| 5 | {or #1‐#4} | 1414 |
| 6 | (ash or ashes):ti,ab,kw | 638 |
| 7 | #5 and #6 in Trials | 9 |
Ovid MEDLINE(R) ALL 1946 to 26 March 2020
| # | Searches | Results |
| 1 | Hand Hygiene/ | 1255 |
| 2 | Hand Disinfection/ | 5514 |
| 3 | handwash*.mp. | 1997 |
| 4 | (hand? adj2 (wash* or clean* or disinfect* or hygien*)).mp. | 11293 |
| 5 | or/1‐4 | 12010 |
| 6 | (ash or ashes).mp. | 17614 |
| 7 | 5 and 6 | 24 |
Embase.com (Elsevier) from inception to 26 March 2020
| # | Searches | Results |
| 1 | hand washing'/de OR 'hand disinfection'/de | 13617 |
| 2 | handwash*:ti,ab,kw | 2494 |
| 3 | (hand$ NEAR/2 (wash* OR clean* OR disinfect* OR hygien*)):ti,ab,kw | 12598 |
| 4 | #1 OR #2 OR #3 | 19371 |
| 5 | 'ash'/exp | 12361 |
| 6 | ash:ti,ab,kw OR ashes:ti,ab,kw | 27121 |
| 7 | #5 OR #6 | 29130 |
| 8 | #4 AND #7 | 40 |
WHO Global Index Medicus (https://search.bvsalud.org/gim/) from inception to 26 March 2020
| # | Search | Results |
| 1 | (tw:(handwash* OR (hand* AND (clean* OR wash* OR disinfect* OR hygien*)))) AND (tw:(ash OR ashes)) | 14 |
WHO International Clinical Trials Registry Platform (ICTRP) 26 March, 2020
| # | Search | Results |
| 1 | ash AND hand* OR ashes AND hand* | 1 |
Appendix 3. Review authors' 'Risk of bias' assessments using ROBINS‐I
ROBINS‐I tool (Stage I): at protocol stage
Applicable to all three non‐randomised studies ((Anuradha 1999; Baker 2017; GEMS 2014) assessed using the Risk of Bias in Non‐randomized Studies of Interventions (ROBINS‐I) tool.
| Specify the review question | |
| Participants | People of all ages |
| Experimental intervention | Hand cleaning with ash, with or without water |
| Comparator | Hand cleaning with soap, mud, soil, water only, or any other material |
| Outcomes | Overall mortality Number of cases of infections (we will analyse cases as defined in the individual studies, e.g. according to serological tests or by clinical diagnosis) Severity of infectious disease (we will assess the burden of the consequence, e.g. absence from work, use of primary care services or hospitalisation) Harms (as reported in the individual studies, e.g. skin lesions) Adherence |
| List the confounding domains relevant to all or most studies | |
| Socioeconomic status; age; acute or chronic illness; toilet facilities; water source; occupation | |
| List co‐interventions that could be different between intervention groups and that could impact on outcomes | |
| Other hand washing means | |
ROBINS‐I tool (Stage II): at review stage
| Anuradha 1999 | |
| Design | Prospective cohort study |
| Participants | Mothers with children of 1‐2 years of age in rural India |
| Experimental intervention | Ash |
| Comparator | Soap, plain water and no wash |
| Is your aim for this study…? | To assess the effect of assignment to intervention |
| Outcome | Total bacterial count on hands before feeding the child |
| Numerical result being assessed | Ash versus soap, MD 158.02 (42.92 to 273.11) – calculated in R using meta::metacont, using data from Table 1 Ash versus water, MD −435.38 (−837.15 to −33.61) – calculated in R using meta::metacont, using data from Table 1 Ash versus no wash, MD −904.38 (−1099.97 to −708.79) – calculated in R using meta::metacont, using data from Table 1 |
| MD: mean difference | |
| Confounding | ||||
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?* | Is the confounding domain measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Socioeconomic status | High or low income group | No | Probably not – we only look at low income group, but there might be substantial variation within the group | |
| Age | None | No | NA | |
| Acute‐ or chronic illness | None | No | NA | |
| Toilet facilities | None | No | ||
| Water source | None | No | ||
| Occupation | None | No | ||
| N/A: not applicable | ||||
| Risk of bias assessment | ||
| Signalling questions | Description | Response options |
| Bias due to confounding | ||
| 1.1 Is there potential for confounding of the effect of intervention in this study? | Y | |
| 1.2. Was the analysis based on splitting participants’ follow up time according to intervention received? | N | |
| 1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? | NA | |
| Questions relating to baseline confounding only | ||
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding domains? | The authors did not adjust for confounding. | N |
| 1.5. If Y/PY to 1.4: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | NA | |
| 1.6. Did the authors control for any post‐intervention variables that could have been affected by the intervention? | N | |
| Questions relating to baseline and time‐varying confounding | ||
| 1.7. Did the authors use an appropriate analysis method that controlled for all the important confounding domains and for time‐varying confounding? | NA | |
| 1.8. If Y/PY to 1.7: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | NA | |
| Risk of bias judgement | Critical | |
| Optional: What is the predicted direction of bias due to confounding? | Unpredictable | |
| Bias in selection of participants into the study | ||
| 2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? If N/PN to 2.1: go to 2.4 |
Households were selected randomly Quote: "Twenty households belonging to low income group (LIG) and twenty households belonging to high income group (HIG) having 1‐2 year old children were randomly selected from the rural areas." |
PN |
| 2.2. If Y/PY to 2.1: Were the post‐intervention variables that influenced selection likely to be associated with intervention? 2.3 If Y/PY to 2.2: Were the post‐intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? |
NA NA |
|
| 2.4. Do start of follow‐up and start of intervention coincide for most participants? | Participants’ hands were swapped immediately after hand cleaning. | Y |
| 2.5. If Y/PY to 2.2 and 2.3, or N/PN to 2.4: Were adjustment techniques used that are likely to correct for the presence of selection biases? | NA | |
| Risk of bias judgement | Low | |
| Optional: What is the predicted direction of bias due to selection of participants into the study? | Unpredictable | |
| Bias in classification of interventions | ||
| 3.1 Were intervention groups clearly defined? | Only the material used is reported, all other characteristics of handwash such as water source, duration, thoroughness etc. could be different between groups. | N |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | Researchers observed the handwashing practice as it took place and classified according to this. | PY |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | It is possible that researchers could identify risk‐factors for high bacterial count in households when determining intervention status, but probably unlikely. | PN |
| Risk of bias judgement | Serious | |
| Optional: What is the predicted direction of bias due to classification of interventions? | Unpredictable | |
| Bias due to deviations from intended interventions | ||
| If your aim for this study is to assess the effect of assignment to intervention, answer questions 4.1 and 4.2 | ||
| 4.1. Were there deviations from the intended intervention beyond what would be expected in usual practice? | No information on deviations from intended practice was provided. | NI |
| Risk of bias judgement | NI | |
| Optional: What is the predicted direction of bias due to deviations from the intended interventions? | Unpredictable | |
| Bias due to missing data | ||
| 5.1 Were outcome data available for all, or nearly all, participants? | Data were available for all participants | Y |
| 5.2 Were participants excluded due to missing data on intervention status? | N | |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | N | |
| 5.4 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? | NA | |
| 5.5 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Is there evidence that results were robust to the presence of missing data? | NA | |
| Risk of bias judgement | Low | |
| Optional: What is the predicted direction of bias due to missing data? | Unpredictable | |
| Bias in measurement of outcomes | ||
| 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | It is possible that both the collection of samples and the subsequent analysis was influenced by knowledge of the intervention received | Y |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | Not described, but based on setting and context blinding is unlikely | PY |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | Y | |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | NI | |
| Risk of bias judgement | Serious | |
| Optional: What is the predicted direction of bias due to measurement of outcomes? | Unpredictable | |
| Bias in selection of the reported result | ||
| Is the reported effect estimate likely to be selected, on the basis of the results, from... | ||
| 7.1. ... multiple outcome measurements within the outcome domain? | We do not have access to a protocol | NI |
| 7.2 ... multiple analyses of the intervention‐outcome relationship? | NI | |
| 7.3 ... different subgroups? | NI | |
| Risk of bias judgement | Serious | |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Unpredictable | |
| Overall bias | ||
| Risk of bias judgement | Due to serious risk of bias in domain 1, 3, and 6 the overall risk of bias is judged to be "critical" | Critical |
| Optional: What is the overall predicted direction of bias for this outcome? | ||
| N/A: not applicable; NI: no information; PN: probably no; PY: probably yes; Y: yes | ||
| Baker 2017 | |
| Design | Retrospective case‐control study |
| Participants | Menstruating girls and women from two rural districts in India |
| Experimental intervention | Hand cleaning with ash/mud/soil and water |
| Comparator | Hand cleaning with soap/detergent and water OR water only OR no wash |
| Is your aim for this study…? | To assess the effect of assignment to intervention |
| Outcome | Self‐reported symptoms of reproductive tract infections |
| Numerical result being assessed | RR for RTI when using ash + water vs soap + water for handwashing at any time: RR 0.48 (95% CI 0.12 to 1.87) ‐ calculated using IPD available as supplementary data RR for RTI when using ash/mud/soil + water vs soap for handwashing after defecation: RR 1.12 (95% CI 0.90 to 1.42) ‐ calculated using IPD available as supplementary data RR for RTI when using 'other' vs soap for cleansing of body: RR 1.01 (95% CI 0.34 to 2.95) ‐ calculated using IPD available as supplementary data |
| IPD: individual participant data; RR: risk ratio;RTI: reproductive tract infection | |
| Confounding | ||||
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?* | Is the confounding domain measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Socioeconomic status | Possession of "Below poverty line"‐card, education, occupation | No | Maybe | Favour comparator |
| Water source | Bathing water source | No | Maybe – unclear whether participants would use this water for washing of hands | Unpredictable |
| Physical illness | None | No | No | Unpredictable |
| Toilet facilities | Sanitation access | No | Yes | Unpredictable |
| Risk of bias assessment | ||
| Signalling questions | Description | Response options |
| Bias due to confounding | ||
| 1.1 Is there potential for confounding of the effect of intervention in this study? | Yes there is potential for confounding of the effect of the intervention | Y |
| 1.2. Was the analysis based on splitting participants’ follow up time according to intervention received? | N | |
| 1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? | ||
| Questions relating to baseline confounding only | ||
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding domains? | N | |
| 1.5. If Y/PY to 1.4: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | N/A | |
| 1.6. Did the authors control for any post‐intervention variables that could have been affected by the intervention? | N/A | |
| Questions relating to baseline and time‐varying confounding | ||
| 1.7. Did the authors use an appropriate analysis method that controlled for all the important confounding domains and for time‐varying confounding? | N | |
| 1.8. If Y/PY to 1.7: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | ||
| Risk of bias judgement | Serious | |
| Optional: What is the predicted direction of bias due to confounding? | Most confounding variables would be expected to have a positive association with socioeconomic status, which would be expected to be associated with using soap. | Favours comparator |
| Bias in selection of participants into the study | ||
| 2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? If N/PN to 2.1: go to 2.4 |
Participants were randomly selected from eligible households. Eligibility criteria were not related to handwashing practice. | PN |
| 2.2. If Y/PY to 2.1: Were the post‐intervention variables that influenced selection likely to be associated with intervention? 2.3 If Y/PY to 2.2: Were the post‐intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? |
||
| 2.4. Do start of follow‐up and start of intervention coincide for most participants? | Handwashing practices would likely have been the same before follow‐up and could influence prognosis after. | PN |
| 2.5. If Y/PY to 2.2 and 2.3, or N/PN to 2.4: Were adjustment techniques used that are likely to correct for the presence of selection biases? | N | |
| Risk of bias judgement | Moderate | |
| Optional: What is the predicted direction of bias due to selection of participants into the study? | Unpredictable | |
| Bias in classification of interventions | ||
| 3.1 Were intervention groups clearly defined? | No. Unclear what 'other' means. For handwashing at any time, the only options are soap/ash/water, however for handwashing after defecation and cleansing of body the options are soap/other/water. No details about the intervention are given. | N |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | No, so several problems could bias the definition of intervention, e.g. recall bias and social desirability bias. | N |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | PN | |
| Risk of bias judgement | Serious | |
| Optional: What is the predicted direction of bias due to classification of interventions? | Unpredictable | |
| Bias due to deviations from intended interventions | ||
| If your aim for this study is to assess the effect of assignment to intervention, answer questions 4.1 and 4.2 | ||
| 4.1. Were there deviations from the intended intervention beyond what would be expected in usual practice? | The actual handwashing was not observed, so there could be substantial deviations between individuals and groups. | PY |
| 4.2. If Y/PY to 4.1: Were these deviations from intended intervention unbalanced between groups and likely to have affected the outcome? | NI | |
| Risk of bias judgement | Serious | |
| Optional: What is the predicted direction of bias due to deviations from the intended interventions? | Unpredictable | |
| Bias due to missing data | ||
| 5.1 Were outcome data available for all, or nearly all, participants? | Data were missing for 19 out of 3952 women (< 0.5%) | Y |
| 5.2 Were participants excluded due to missing data on intervention status? | No information on missing participants | NI NI |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | No information on missing participants | |
| 5.4 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? | ||
| 5.5 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Is there evidence that results were robust to the presence of missing data? | ||
| Risk of bias judgement | Low | |
| Optional: What is the predicted direction of bias due to missing data? | Unpredictable | |
| Bias in measurement of outcomes | ||
| 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | The outcome is self‐reported symptoms of reproductive tract infection – it is possible that women engaging in hygienic practices considered unsafe would be more likely to notice such symptoms. On the other hand such women might be ashamed of their practice and unwilling to admit that it has caused them trouble. | PY |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | Yes – the outcome was assessed by the participants themselves (through interviews with community healthworkers) | Y |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | Probably, although it is not described in any detail | PY |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | NI | |
| Risk of bias judgement | Serious | |
| Optional: What is the predicted direction of bias due to measurement of outcomes? | Unpredictable | |
| Bias in selection of the reported result | ||
| Is the reported effect estimate likely to be selected, on the basis of the results, from... | ||
| 7.1. ... multiple outcome measurements within the outcome domain? | No protocol | NI |
| 7.2 ... multiple analyses of the intervention‐outcome relationship? | No protocol | NI |
| 7.3 ... different subgroups? | No protocol | NI |
| Risk of bias judgement | Moderate | |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Unpredictable | |
| Overall bias | ||
| Risk of bias judgement | Critical | |
| Optional: What is the overall predicted direction of bias for this outcome? | Unpredictable | |
| N/A: not applicable; NI: no information; PN: probably no; PY: probably yes; Y: yes | ||
| GEMS 2014 | |
| Design | |
| Participants | People of all ages |
| Experimental intervention | Hand cleaning with ash with clean water |
| Comparator | Hand cleaning with soap, mud, soil, water only, or any other material |
| Is your aim for this study…? | To assess the effect of assignment to intervention |
| Outcome | Number of cases of infections |
| Numerical result being assessed | RR for having diarrhoea when washing hands with ash versus not washing hands with ash (calculated based on data in Baker 2014, Table 3) RR 0.96, 95% CI 0.86 to 1.07) |
| RR: risk ratio | |
| Confounding | ||||
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary?* | Is the confounding domain measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is failure to adjust for this variable (alone) expected to favour the experimental intervention or the comparator? |
| Socioeconomic status | Wealth index quintile | No | Yes – A bit unclear how the wealth index was constructed, however it seems fairly comprehensive. | NI |
| Physical illness | Not measured | No | NA | NI |
| Toilet facilities | Not measured | No | NA | NI |
| Water source | Access to "improved drinking water source" | No | No – it is not certain that access to an improved drinking water source would necessarily mean that this source was used for hand cleaning. | NI |
| Occupation | Not measured | No | NA | NI |
| N/A: not applicable; NI: no information | ||||
| Risk of bias assessment | ||
| Signalling questions | Description | Response options |
| Bias due to confounding | ||
| 1.1 Is there potential for confounding of the effect of intervention in this study? | There is potential for confounding of the effect of intervention | Y |
| 1.2. Was the analysis based on splitting participants’ follow up time according to intervention received? | N | |
| 1.3. Were intervention discontinuations or switches likely to be related to factors that are prognostic for the outcome? | N/A | |
| Questions relating to baseline confounding only | ||
| 1.4. Did the authors use an appropriate analysis method that controlled for all the important confounding domains? | An adjusted model was presented; however, it was only adjusted for wealth index quintile but not for other relevant confounders. | N |
| 1.5. If Y/PY to 1.4: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | N/A | |
| 1.6. Did the authors control for any post‐intervention variables that could have been affected by the intervention? | N/A | |
| Questions relating to baseline and time‐varying confounding | ||
| 1.7. Did the authors use an appropriate analysis method that controlled for all the important confounding domains and for time‐varying confounding? | No adjustment done | N/A |
| 1.8. If Y/PY to 1.7: Were confounding domains that were controlled for measured validly and reliably by the variables available in this study? | N/A | |
| Risk of bias judgement | Critical | |
| Optional: What is the predicted direction of bias due to confounding? | Unpredictable | |
| Bias in selection of participants into the study | ||
| 2.1. Was selection of participants into the study (or into the analysis) based on participant characteristics observed after the start of intervention? If N/PN to 2.1: go to 2.4 |
Case‐control study – participants were selected based on their outcome | Y |
| 2.2. If Y/PY to 2.1: Were the post‐intervention variables that influenced selection likely to be associated with intervention? 2.3 If Y/PY to 2.2: Were the post‐intervention variables that influenced selection likely to be influenced by the outcome or a cause of the outcome? |
Y | |
| 2.4. Do start of follow‐up and start of intervention coincide for most participants? | Case‐control study – participants were selected based on their outcome | N |
| 2.5. If Y/PY to 2.2 and 2.3, or N/PN to 2.4: Were adjustment techniques used that are likely to correct for the presence of selection biases? | NI | |
| Risk of bias judgement | Critical | |
| Optional: What is the predicted direction of bias due to selection of participants into the study? | Unpredictable | |
| Bias in classification of interventions | ||
| 3.1 Were intervention groups clearly defined? | Only very broadly defined – used ash or not ash to wash hands | N |
| 3.2 Was the information used to define intervention groups recorded at the start of the intervention? | No – only at time of outcome (case‐control study) | N |
| 3.3 Could classification of intervention status have been affected by knowledge of the outcome or risk of the outcome? | Most likely not – study measured many variables of interest | PN |
| Risk of bias judgement | Critical | |
| Optional: What is the predicted direction of bias due to classification of interventions? | Unpredictable | |
| Bias due to deviations from intended interventions | ||
| If your aim for this study is to assess the effect of assignment to intervention, answer questions 4.1 and 4.2 | ||
| 4.1. Were there deviations from the intended intervention beyond what would be expected in usual practice? | There was no observation of actual handwashing practice – only whether ash was available at washing station | PY |
| 4.2. If Y/PY to 4.1: Were these deviations from intended intervention unbalanced between groups and likely to have affected the outcome? | NI | |
| 4.3. Were important co‐interventions balanced across intervention groups? | NI | |
| 4.4. Was the intervention implemented successfully for most participants? | NI | |
| 4.5. Did study participants adhere to the assigned intervention regimen? | NI | |
| 4.6. If N/PN to 4.3, 4.4 or 4.5: Was an appropriate analysis used to estimate the effect of starting and adhering to the intervention? | No analysis done | N/A |
| Risk of bias judgement | Critical | |
| Optional: What is the predicted direction of bias due to deviations from the intended interventions? | Unpredictable | |
| Bias due to missing data | ||
| 5.1 Were outcome data available for all, or nearly all, participants? | Case‐control study – outcome for all | Y |
| 5.2 Were participants excluded due to missing data on intervention status? | NI | |
| 5.3 Were participants excluded due to missing data on other variables needed for the analysis? | All participants seem to have been investigated for the intervention | PN |
| 5.4 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Are the proportion of participants and reasons for missing data similar across interventions? | NI | |
| 5.5 If PN/N to 5.1, or Y/PY to 5.2 or 5.3: Is there evidence that results were robust to the presence of missing data? | N/A | |
| Risk of bias judgement | Low | |
| Optional: What is the predicted direction of bias due to missing data? | Unpredictable | |
| Bias in measurement of outcomes | ||
| 6.1 Could the outcome measure have been influenced by knowledge of the intervention received? | Cases were defined based on hospital visit with diagnosis of diarrhoea | PN |
| 6.2 Were outcome assessors aware of the intervention received by study participants? | PN | |
| 6.3 Were the methods of outcome assessment comparable across intervention groups? | PY | |
| 6.4 Were any systematic errors in measurement of the outcome related to intervention received? | PN | |
| Risk of bias judgement | Low | |
| Optional: What is the predicted direction of bias due to measurement of outcomes? | Unpredictable | |
| Bias in selection of the reported result | ||
| Is the reported effect estimate likely to be selected, on the basis of the results, from... | ||
| 7.1. ... multiple outcome measurements within the outcome domain? | No protocol available | NI |
| 7.2 ... multiple analyses of the intervention‐outcome relationship? | No protocol available | NI |
| 7.3 ... different subgroups? | No protocol available | NI |
| Risk of bias judgement | Moderate | |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Unpredictable | |
| Overall bias | ||
| Risk of bias judgement | Critical | |
| Optional: What is the overall predicted direction of bias for this outcome? | Unpredictable | |
| N/A: not applicable; NI: no information; PN: probably no; PY: probably yes; Y: yes | ||
Appendix 4. Review authors' 'Risk of bias' assessments using RoB 2
| Hoque 1991 | |||
| Study design | Individually randomised parallel‐group trial | ||
| Intervention | Ash | ||
| Comparator | Soap, mud, water or no wash | ||
| Outcome | Risk ratio of contaminated hands | ||
| Numerical result being assessed | Ash vs soap: RR 0.75 (0.19 to 2.93) Ash vs mud: RR 0.75 (0.19 to 2.93) Ash vs water: RR 0.38 (0.12 to 1.21) Ash vs no wash: RR 0.25 (0.08 to 0.75) |
||
| Aim is to… | assess the effect of assignment to intervention (the ‘intention‐to‐treat’ effect) | ||
| Signalling questions | Comments | Response options | |
| Domain 1: Risk of bias arising from the randomisation process | |||
| 1.1 Was the allocation sequence random? | States random, but no information of sequence ‐ Quote: "To avoid any confounding effect, these women were randomly divided into five groups of four women, each of whom, at every session, washed their hands either with water, mud, ash or soap or did not wash their hands and were considered a control" – Based on the context adequate allocation concealment seems unlikely. | PY | |
| 1.2 Was the allocation sequence concealed until participants were enrolled and assigned to interventions? | PN | ||
| 1.3 Did baseline differences between intervention groups suggest a problem with the randomization process? | No baseline characteristics reported | NI | |
| ‘Risk of bias’ judgement | The trial is reported to be randomised; however, it is unclear how the sequence was generated, and proper allocation concealment is unlikely. As all women use all materials confounding may be unlikely. | Some concerns | |
| Optional: What is the predicted direction of bias arising from the randomization process? | Unpredictable | ||
| Domain 2: Risk of bias due to deviations from the intended interventions (effect of assignment to intervention) | |||
| 2.1. Were participants aware of their assigned intervention during the trial? | The women would know which material they were using | Y | |
| 2.2. Were carers and people delivering the interventions aware of participants' assigned intervention during the trial? | The researchers giving instructions to the women were aware of the assigned intervention and could potentially give different instructions based on assignment. | Y | |
| 2.3. If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the trial context? | NI | ||
| 2.4 If Y/PY to 2.3: Were these deviations likely to have affected the outcome? | NI | ||
| 2.5. If Y/PY/NI to 2.4: Were these deviations from intended intervention balanced between groups? | NI | ||
| 2.6 Was an appropriate analysis used to estimate the effect of assignment to intervention? | NI | ||
| 2.7 If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomized? | NI | ||
| 2.8 (Taken from archived cross‐over version) Was there sufficient time for any carry‐over effects to have disappeared before outcome assessment in the second period? | The measurements were done on subsequent days, so we consider the risk of carry‐over effects minimal | Y | |
| ‘Risk of bias’ judgement | The women would know which material they were using, and it is possible this knowledge could influence how they washed their hands. Additionally, instructions might have varied between groups. | Some concerns | |
| Optional: What is the predicted direction of bias due to deviations from intended interventions? | Unpredictable | ||
| Domain 3: Missing outcome data | |||
| 3.1 Were data for this outcome available for all, or nearly all, participants randomized? | Unless the trial is fraudulently reported, data are available from all women | Y | |
| 3.2 If N/PN/NI to 3.1: Is there evidence that the result was not biased by missing outcome data? | N/A | ||
| 3.3 If N/PN to 3.2: Could missingness in the outcome depend on its true value? | N/A | ||
| 3.4 If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value? | N/A | ||
| ‘Risk of bias’ judgement | Low | ||
| Optional: What is the predicted direction of bias due to missing outcome data? | Unpredictable | ||
| Domain 4: Risk of bias in measurement of the outcome | |||
| 4.1 Was the method of measuring the outcome inappropriate? | The method seems valid, however it is unclear how trained the personnel were, and the counting of colony‐forming units is somewhat subjective | PN | |
| 4.2 Could measurement or ascertainment of the outcome have differed between intervention groups? | Same method used for all groups | N | |
| 4.3 If N/PN/NI to 4.1 and 4.2: Were outcome assessors aware of the intervention received by study participants? | Not explicitly mentioned but based on the context and the description in the trial publication it seems clear that outcome assessors were not blinded. | Y | |
| 4.4 If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received? | Both the collection of samples and the counting of colony‐forming units could be biased, if the researchers had preferences. | Y | |
| 4.5 If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of intervention received? | NI | ||
| ‘Risk of bias’ judgement | High | ||
| Optional: What is the predicted direction of bias in measurement of the outcome? | |||
| Domain 5: Risk of bias in selection of the reported result | |||
| 5.1 Were the data that produced this result analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis? | NI | ||
| Is the numerical result being assessed likely to have been selected, on the basis of the results, from... | |||
| 5.2. ... multiple eligible outcome measurements (e.g. scales, definitions, time points) within the outcome domain? | The number of colonies forming units would also be available as an outcome, as we don’t have a protocol it is unclear whether 'contaminated hands' was a pre‐specified outcome. | PY | |
| 5.3 ... multiple eligible analyses of the data? | We collected the event counts, not the results of the analyses | Not relevant | |
| ‘Risk of bias’ judgement | As we do not know whether the outcome was prespecified I judge 'some concerns' | Some concerns | |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Unpredictable | ||
| Overall risk of bias | |||
| ‘Risk of bias’ judgement | Due to the High risk of bias for Domain 4 and some concerns for domains 1, 2, and 5 the overall risk of bias is judged to be High | High | |
| Optional: What is the overall predicted direction of bias for this outcome? | Unpredictable | ||
| N/A: not applicable; NI: no information; PN: probably no; PY: probably yes; RR: risk ratio; Y: yes | |||
| Hoque 1995 | |||
| Study design | Non‐randomised parallel‐group trial | ||
| Intervention | Ash | ||
| Comparator | Soap or soil | ||
| Outcome | Number of colony‐forming units | ||
| Numerical result being assessed | Not estimable, as there is no reported measure of variance ash vs no wash: RR 0.25 (0.08 to 0.75) | ||
| Aim is to… | assess the effect of assignment to intervention (the ‘intention‐to‐treat’ effect) | ||
| Signalling questions | Comments | Response options | |
| Domain 1: Risk of bias arising from the randomization process | |||
| 1.1 Was the allocation sequence random? | The allocation process is poorly described in all three papers; however, it seems highly unlikely that the study is randomised. Quote (1995a): "During this phase, visits were made by the same (Phase I) trained women workers to every household between 5:30 and 9:00a.m. Any women of the same area (including the 90 in phase I) who were seen coming out of the defecation sites and who had not yet washed their hands were requested to take part in the experiment by washing hands according to one of our instructions" |
PN | |
| 1.2 Was the allocation sequence concealed until participants were enrolled and assigned to interventions? | PN | ||
| 1.3 Did baseline differences between intervention groups suggest a problem with the randomization process? | No baseline characteristics reported | NI | |
| 'Risk of bias' judgement | High | ||
| Optional: What is the predicted direction of bias arising from the randomization process? | Unpredictable | ||
| Domain 2: Risk of bias due to deviations from the intended interventions (effect of assignment to intervention) | |||
| 2.1. Were participants aware of their assigned intervention during the trial? | The women would necessarily be aware of which material they were using. | Y | |
| 2.2. Were carers and people delivering the interventions aware of participants' assigned intervention during the trial? | Researchers giving instructions, could give different instructions to different groups. | Y | |
| 2.3. If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the trial context? | NI | ||
| 2.4 If Y/PY to 2.3: Were these deviations likely to have affected the outcome? | NI | ||
| 2.5. If Y/PY/NI to 2.4: Were these deviations from intended intervention balanced between groups? | NI | ||
| 2.6 Was an appropriate analysis used to estimate the effect of assignment to intervention? | NI | ||
| 2.7 If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomized? | NI | ||
| 'Risk of bias' judgement | Some concerns | ||
| Optional: What is the predicted direction of bias due to deviations from intended interventions? | Unpredictable | ||
| Domain 3: Missing outcome data | |||
| 3.1 Were data for this outcome available for all, or nearly all, participants randomized? | It is not described how many women were asked to use each material, so it is not possible to determine whether any outcome data is missing. | NI | |
| 3.2 If N/PN/NI to 3.1: Is there evidence that the result was not biased by missing outcome data? | N | ||
| 3.3 If N/PN to 3.2: Could missingness in the outcome depend on its true value? | Y | ||
| 3.4 If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value? | NI | ||
| 'Risk of bias' judgement | Some concerns | ||
| Optional: What is the predicted direction of bias due to missing outcome data? | Unpredictable | ||
| Domain 4: Risk of bias in measurement of the outcome | |||
| 4.1 Was the method of measuring the outcome inappropriate? | The method used seems valid (‘slightly modified finger‐tip count technique’), however, it is unclear whether it was done appropriately. | PN | |
| 4.2 Could measurement or ascertainment of the outcome have differed between intervention groups? | Seems method is the same | N | |
| 4.3 If N/PN/NI to 4.1 and 4.2: Were outcome assessors aware of the intervention received by study participants? | Not described in detail but seems highly likely that outcome assessors were aware of the intervention received. | PY | |
| 4.4 If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received? | If outcome assessors had any preference for one intervention over the others, there would be ample opportunity for influencing the results; whether this was likely is difficult to determine. | Y | |
| 4.5 If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of intervention received? | NI | ||
| 'Risk of bias' judgement | High | ||
| Optional: What is the predicted direction of bias in measurement of the outcome? | Unpredictable | ||
| Domain 5: Risk of bias in selection of the reported result | |||
| 5.1 Were the data that produced this result analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis? | NI | ||
| Is the numerical result being assessed likely to have been selected, on the basis of the results, from... | |||
| 5.2. ... multiple eligible outcome measurements (e.g. scales, definitions, time points) within the outcome domain? | Number of colony‐forming units is a relatively standard outcome for trials such at this one, and it would seem unlikely others have been used. | PN | |
| 5.3 ... multiple eligible analyses of the data? | We are not looking at the analyses, but at the geometric mean. | N | |
| 'Risk of bias' judgement | The measure of variance is not reported. | Some concerns | |
| Optional: What is the predicted direction of bias due to selection of the reported result? | Unpredictable | ||
| Overall risk of bias | |||
| ‘Risk of bias’ judgement | Based on the high risk of bias in domain 1, 2, and 4 and the concerns about domain 3 and 5 the overall risk of bias is judged to be High | High | |
| Optional: What is the overall predicted direction of bias for this outcome? | Unpredictable | ||
| N/A: not applicable; NI: no information; PN: probably no; PY: probably yes; RR: risk ratio; Y: yes | |||
| Khin 1997 | |||
| Study design | Individually randomised parallel‐group trial | ||
| Intervention | Ash | ||
| Comparator | Many others | ||
| Outcome | Count of colony‐forming units | ||
| Numerical result being assessed | Due to unclear reporting it was not possible to analyse data | ||
| Aim is to… | assess the effect of assignment to intervention (the ‘intention‐to‐treat’ effect) | ||
| Signalling questions | Comments | Response options | |
| Domain 1: Risk of bias arising from the randomization process | |||
| 1.1 Was the allocation sequence random? | It is not described how participants were allocated. | PN | |
| 1.2 Was the allocation sequence concealed until participants were enrolled and assigned to interventions? | PN | ||
| 1.3 Did baseline differences between intervention groups suggest a problem with the randomization process? | NI | ||
| 'Risk of bias' judgement | High | ||
| Optional: What is the predicted direction of bias arising from the randomization process? | Unpredictable | ||
| Domain 2: Risk of bias due to deviations from the intended interventions (effect of assignment to intervention) | |||
| 2.1. Were participants aware of their assigned intervention during the trial? | Participants would know what material they were using. | Y | |
| 2.2. Were carers and people delivering the interventions aware of participants' assigned intervention during the trial? | Instructions given by researchers could differ based on group. | Y | |
| 2.3. If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the trial context? | NI | ||
| 2.4 If Y/PY to 2.3: Were these deviations likely to have affected the outcome? | NI | ||
| 2.5. If Y/PY/NI to 2.4: Were these deviations from intended intervention balanced between groups? | NI | ||
| 2.6 Was an appropriate analysis used to estimate the effect of assignment to intervention? | NI | ||
| 2.7 If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomized? | NI | ||
| 'Risk of bias' judgement | The method of handwashing and instructions could potentially be influenced by knowledge of which material was used. | Some concerns | |
| Optional: What is the predicted direction of bias due to deviations from intended interventions? | Unpredictable | ||
| Domain 3: Missing outcome data | |||
| 3.1 Were data for this outcome available for all, or nearly all, participants randomized? | No sample size is given, so unclear | NI | |
| 3.2 If N/PN/NI to 3.1: Is there evidence that the result was not biased by missing outcome data? | N | ||
| 3.3 If N/PN to 3.2: Could missingness in the outcome depend on its true value? | Y | ||
| 3.4 If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value? | NI | ||
| 'Risk of bias' judgement | As no sample size is given, it is not possible to know whether any outcome data is missing. | Some concerns | |
| Optional: What is the predicted direction of bias due to missing outcome data? | Unpredictable | ||
| Domain 4: Risk of bias in measurement of the outcome | |||
| 4.1 Was the method of measuring the outcome inappropriate? | The method seems valid, however it is unclear whether the personnel was adequately trained. | PN | |
| 4.2 Could measurement or ascertainment of the outcome have differed between intervention groups? | PN | ||
| 4.3 If N/PN/NI to 4.1 and 4.2: Were outcome assessors aware of the intervention received by study participants? | Not described, but seems highly unlikely that outcome assessors were blinded | Y | |
| 4.4 If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received? | Y | ||
| 4.5 If Y/PY/NI to 4.4: Is it likely that assessment of the outcome was influenced by knowledge of intervention received? | NI | ||
| 'Risk of bias' judgement | High | ||
| Optional: What is the predicted direction of bias in measurement of the outcome? | Unpredictable | ||
| Domain 5: Risk of bias in selection of the reported result | |||
| 5.1 Were the data that produced this result analysed in accordance with a pre‐specified analysis plan that was finalized before unblinded outcome data were available for analysis? | NI | ||
| Is the numerical result being assessed likely to have been selected, on the basis of the results, from... | |||
| 5.2. ... multiple eligible outcome measurements (e.g. scales, definitions, time points) within the outcome domain? | Colony‐forming units is a standard outcome, so likely that this was the plan from the beginning, but unclear as we do not have access to a protocol. | PN | |
| 5.3 ... multiple eligible analyses of the data? | We are not looking at results of analyses | Not relevant | |
| 'Risk of bias' judgement | Some concerns | ||
| Optional: What is the predicted direction of bias due to selection of the reported result? | Unpredictable | ||
| Overall risk of bias | |||
| ‘Risk of bias’ judgement | Due to the high risk of bias in Domains 1 and 4 and some concerns for domains 2, 3, and 5 the overall risk of bias is judged to be High | High | |
| Optional: What is the overall predicted direction of bias for this outcome? | Unpredictable | ||
| N/A: not applicable; NI: no information; PN: probably no; PY: probably yes; Y: yes | |||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anuradha 1999.
| Study characteristics | ||
| Notes | ||
Aziz 1990.
| Study characteristics | ||
| Notes | ||
Baker 2017.
| Study characteristics | ||
| Notes | ||
Edward 2019.
| Study characteristics | ||
| Notes | ||
GEMS 2014.
| Study characteristics | ||
| Notes | ||
Hoque 1991.
| Study characteristics | ||
| Notes | ||
Hoque 1995.
| Study characteristics | ||
| Notes | ||
Huda 2010.
| Study characteristics | ||
| Notes | ||
Jha 2006.
| Study characteristics | ||
| Notes | ||
Khin 1997.
| Study characteristics | ||
| Notes | ||
Nguyen 2015.
| Study characteristics | ||
| Notes | ||
Nizame 2015.
| Study characteristics | ||
| Notes | ||
Ravindra 2019.
| Study characteristics | ||
| Notes | ||
Zeitlin 1986.
| Study characteristics | ||
| Notes | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afroz 2010 | |
| Bennett 1997 | |
| Blum 2017a | |
| Blum 2017b | |
| Hoffman 1997 | |
| Luby 2008 | |
| McMichael 2016 | |
| Min 1988 | |
| NCT01900912 | |
| Ngulube 2015 | |
| Russpatrick 2015 | |
| Yeboah‐Antwi 2017 |
Contributions of authors
Asger Sand Paludan‐Müller: Contributed to the design, co‐ordination, data collection, analysis and interpretation of data. Co‐wrote the first draft and made revisions.
Kim Boesen: Contributed to the design, co‐ordination, data collection, analysis and interpretation of data. Co‐wrote the first draft and made revisions.
Irma Klerings: Contributed to the design and undertaking of the search strategies. Read and commented on all drafts and the final version.
Karsten Juhl Jørgensen: Contributed to the design and co‐ordination of the review. Read and commented on all drafts and the final version.
Klaus Munkholm: Contributed to the design, co‐ordination, data collection, analysis and interpretation of data. Co‐wrote the first draft and made revisions.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
External sources
-
Department for International Development, UK
Project number 300342‐104
Declarations of interest
Asger Sand Paludan‐Müller: none known Kim Boesen: none known Irma Klerings: none known Karsten Juhl Jørgensen: none known Klaus Munkholm: none known
These authors contributed equally to this work
These authors contributed equally to this work
Edited (no change to conclusions)
References
References to studies included in this review
Anuradha 1999 {published data only}
- Anuradha P, Yasoda Devi P, Prakash MS. Effect of handwashing agents on bacterial contamination. Indian Journal of Pediatrics 1999;66(1):7-10. [CRSREF 13336096] [DOI] [PubMed] [Google Scholar]
Aziz 1990 {published data only}
- Aziz KM, Cairncross AM, Hasan Z, Hoque BA, Huttly SR, Minnatullah KM, et al. Water supply, sanitation and hygiene education: report of a health impact study in Mirzapur, Bangladesh. Available from Documents.worldbank.org/curated/en/797601468428690833/Water-supply-sanitation-and-hygiene-education-report-of-a-health-impact-study-in-Mirzapur-Bangladesh 1990. [CRSREF 13336098]
Baker 2017 {published data only}
- Baker KK, Padhi B, Torondel B, Das P, Dutta A, Sahoo KC, et al. From menarche to menopause: a population-based assessment of water, sanitation, and hygiene risk factors for reproductive tract infection symptoms over life stages in rural girls and women in India. PloS One 2017;12(12):e0188234. [CRSREF 13336100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edward 2019 {published data only}
- Edward A, Jung Y, Chhorvann C, Ghee AE, Chege J. Association of mother's handwashing practices and pediatric diarrhea: evidence from a multi-country study on community oriented interventions. Journal of Preventive Medicine and Hygiene 2019;60(2):E93-E102. [CRSREF 13336102] [DOI] [PMC free article] [PubMed] [Google Scholar]
GEMS 2014 {published data only}
- Baker KK, Dil Farzana F, Ferdous F, Ahmed S, Kumar Das S, Faruque AS, et al. Association between moderate-to-severe diarrhea in young children in the global enteric multicenter study (GEMS) and types of handwashing materials used by caretakers in Mirzapur, Bangladesh. American Journal of Tropical Medicine and Hygiene 2014;91(1):181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KK, O'Reilly CE, Levine MM, Kotloff KL, Nataro JP, Ayers TL, et al. Sanitation and hygiene-specific risk factors for moderate-to-severe diarrhea in young children in the Global Enteric Multicenter Study, 2007-2011: Case-Control Study. PLoS Medicine / Public Library of Science 2016;13(4):e1002010. [CRSREF 13336105] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoque 1991 {published data only}
- Hoque BA, Briend A. A comparison of local handwashing agents in Bangladesh. Journal of Tropical Medicine and Hygiene 1991;94(1):61-4. [PubMed] [Google Scholar]
Hoque 1995 {published data only}
- Hoque BA, Mahalanabis D, Alam MJ, Islam MS. Post-defecation handwashing in Bangladesh: practice and efficiency perspectives. Public Health 1995;109(1):15-24. [CRSREF 1336109] [DOI] [PubMed] [Google Scholar]
- Hoque BA, Mahalanabis D, Pelto B, Alam MJ. Research methodology for developing efficient handwashing options: an example from Bangladesh. Journal of Tropical Medicine and Hygiene 1995;98(6):469-75. [CRSREF 13336110] [PubMed] [Google Scholar]
- Hoque BA. Handwashing practices and challenges in Bangladesh. International Journal of Environmental Health Research 2003;13 Suppl 1(S):81-7. [CRSREF 13336111] [DOI] [PubMed] [Google Scholar]
Huda 2010 {published data only}
- Huda TM, Unicomb L, Halder AK, Johnston RB, Luby SP. Effect of a large-scale sanitation, hygiene education and water supply intervention in rural Bangladesh. American Journal of Tropical Medicine and Hygiene 2010;83(5):7-8. [CRSREF 13336113] [Google Scholar]
- Huda TM, Unicomb L, Johnston RB, Halder AK, Yushuf Sharker MA, Luby SP. Interim evaluation of a large scale sanitation, hygiene and water improvement programme on childhood diarrhea and respiratory disease in rural Bangladesh. Social Science & Medicine 2012;75(4):614-11. [CRSREF 13336114] [DOI] [PubMed] [Google Scholar]
- Huda TM, Unicomb L, Johnston RB, Tronchet C, Halder AK, Luby SP. Interim assessment of a sanitation, hygiene education and water supply intervention in rural Bangladesh, 2008. American Journal of Tropical Medicine and Hygiene 2009;81(4):306. [CRSREF 13336115] [Google Scholar]
Jha 2006 {published data only}
- Jha N, Kumar S, Yadav BK, Singh GC, Niraula SR. Impact of family health exercise program on health knowledge and practice of a rural population of eastern Nepal. Kathmandu University Medical Journal (KUMJ) 2006;4(1):44-7. [CRSREF 13336117] [PubMed] [Google Scholar]
Khin 1997 {published data only}
- Khin NO, Cho YW, Myat T. Effect of various cleansing materials on decontamination of hands. Myanmar Health Sciences Research Journal 1997;9(1):33-6. [CRSREF 13336119] [Google Scholar]
Nguyen 2015 {published data only}
- Nguyen T. Hand washing with ash and mud, an accepted practice in Malawi: findings from a knowledge, attitudes and practice study. Tropical Medicine & International Health 2015;20:360. [CRSREF 13336121] [Google Scholar]
Nizame 2015 {published data only}
- Nizame FA, Nasreen S, Halder AK, Arman S, Winch PJ, Unicomb L, et al. Observed practices and perceived advantages of different hand cleansing agents in rural Bangladesh: ash, soil, and soap. American Journal of Tropical Medicine and Hygiene 2015;92(6):1111-6. [CRSREF 13336123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravindra 2019 {published data only}
- Ravindra K, Mor S, Pinnaka VL. Water uses, treatment, and sanitation practices in rural areas of Chandigarh and its relation with waterborne diseases. Environmental Science and Pollution Research International 2019;26(19):19512-22. [CRSREF 13336125] [DOI] [PubMed] [Google Scholar]
Zeitlin 1986 {published data only}
- Zeitlin MF, Guldan G, Klein RE, Ahmad N. A vulnerable age: environment, behavior and the spread of diarrhoea. Dialogue on Diarrhoea 1986;Sep(26):4. [CRSREF 13336127] [PubMed] [Google Scholar]
References to studies excluded from this review
Afroz 2010 {published data only}
- Afroz A, Nasreen S, Unicomb L, Gurley ES, Arman S, Kadir MA, et al. Perceptions, practices and barriers of handwashing in rural Bangladesh. American Journal of Tropical Medicine and Hygiene 2010;83(5):104. [CRSREF 13336129] [Google Scholar]
Bennett 1997 {published data only}
- Bennett J, Macia J, Traverso H, Banoagha S, Malooly C, Boring J. Protective effects of topical antimicrobials against neonatal tetanus. International Journal of Epidemiology 1997;26(4):897-903. [CRSREF 13336131] [DOI] [PubMed] [Google Scholar]
Blum 2017a {published data only}
- Blum LS, Yemweni A, Trinies V, Kambere M, Tolani F, O'Reilly M, et al. A qualitative assessment of motivators and barriers to handwashing behaviors in an emergency setting in North Kivu, Democratic Republic of Congo. American Journal of Tropical Medicine and Hygiene 2017;97(5):401-2. [CRSREF 13336133] [Google Scholar]
Blum 2017b {published data only}
- Blum LS, Yemweni A, Trinies V, Kambere M, Tolani F, Allen JV, et al. Programmatic implications for promotion of handwashing behavior in an internally displaced persons camp in North Kivu, Democratic Republic of Congo. Conflict and Health 2019;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 1997 {published data only}
- Hoffman BH, Tuomanen B, Price IR, Beaulieu HJ. Biological monitoring of employees with potential exposures to inorganic lead and cadmium at municipal solid waste resource recovery, or trash-to-energy, facilities. Applied Occupational and Environmental Hygiene 1997;12(7):470-9. [CRSREF 13336135] [Google Scholar]
Luby 2008 {published data only}
- Luby SP. Commentary: Hand washing for preventing diarrhoea. International Journal of Epidemiology 2008;37(3):472. [DOI: 10.1093/ije/dyn069] [CRSREF 13336137] [DOI] [PubMed] [Google Scholar]
McMichael 2016 {published data only}
- McMichael C, Robinson P. Drivers of sustained hygiene behaviour change: a case study from mid-western Nepal. Social Science & Medicine 2016;163:28-36. [CRSREF 13336139] [DOI] [PubMed] [Google Scholar]
Min 1988 {published data only}
- Min CH, Jung-Han P. Causes of burn and emergency care on the spot for the patients admitted to three hospitals in Taegu. Korean Journal of Preventive Medicine 1988;21(2):238-44. [CRSREF 13336141] [Google Scholar]
NCT01900912 {published data only}
- NCT01900912. Evaluating the impact of community led total sanitation programs in Mali. Clinicaltrials.Gov/Show/NCT01900912 (first received 26 March 2020). [CRSREF 13336143]
Ngulube 2015 {published data only}
- Ngulube V, Zimba R, Chomba L, Crooks P, Katooka O, Larsen DA, et al. Chiengi district, Zambia open defecation free! American Journal of Tropical Medicine and Hygiene 2015;93(4):547. [CRSREF 13336145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Russpatrick 2015 {published data only}
- Russpatrick S, Berkowitz J, Katooka O, Lukama C, Manangi A, Markle L, et al. Mobility up and down the sanitation ladder in rural communities of Zambia following community-led total sanitation triggering. American Journal of Tropical Medicine and Hygiene 2015;93(4):175. [CRSREF 13336147] [Google Scholar]
Yeboah‐Antwi 2017 {published data only}
- Yeboah-Antwi K, Macleod W, Biemba G, Hamer D. Impact of improved sanitation and hygiene on stunting in rural Zambia improved sanitation and hygiene on stunting in rural Zambia. American Journal of Tropical Medicine and Hygiene 2017;97(5):26. [CRSREF 13336149] [Google Scholar]
References to studies awaiting assessment
Barlas 1998 {published data only}
- Barlas U. Cleaniness and hygiene from infancy to adolescence in Karabuk, Safranbolu, Eflani and Eskipazar. Yeni Tip Tarihi Arastirmalari [New History of Medicine Studies] 1998;4:165-80. [PubMed] [Google Scholar]
Kaur 1994 {published data only}
- Kaur P, Singh G. Food practices during diarrhoea. Indian Journal of Public Health 1994;38(2):58-61. [PubMed] [Google Scholar]
Mendiratta 2018 {published data only}
- Mendiratta SR, Choudhary M, Kumar S. Sustainability of hygiene behaviours in Nirmal Gram Puraskar awarded gram panchayats in Rajasthan: a big challenge. Indian Journal of Public Health Research and Development 2018;9(3):281-5. [Google Scholar]
Ray 2006 {published data only}
- Ray SK, Dobe M, Maji S, Chakrabarty D, Sinha Roy AK, Basu SS. A pilot survey on hand washing among some communities of West Bengal. Indian Journal of Public Health 2006;50(4):225-30. [PubMed] [Google Scholar]
Ray 2009 {published data only}
- Ray SK, Dobe M, Lahiri A, Basu SS. Hand washing practices in urban and rural communities in and around Kolkata, West Bengal. Indian Journal of Public Health 2009;53(3):192-5. [PubMed] [Google Scholar]
Additional references
Baker 2014
- Baker KK, Farzana FD, Ferdous F, Ahmed S, Das SK, Faruque AS, et al. Association between moderate-to-severe diarrhea in young children in the Global Enteric Multicenter Study (GEMS) and types of handwashing materials used by caretakers in Mirzapur, Bangladesh. American Journal of Tropical Medicine and Hygiene 2014;91(1):181-9. [DOI: 10.4269/ajtmh.13-0509] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloomfield 2009
- Bloomfield SF, Nath KJ. Use of ash and mud for handwashing in low income communities. International Scientific Forum on Home Hygiene: available from www.ifh-homehygiene.org/review-best-practice/use-ash-and-mud-handwashing-low-income-communities (accessed after 26 March 2020).
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 26 March 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Ejemot‐Nwadiaro 2015
- Ejemot‐Nwadiaro RI, Ehiri JE, Meremikwu MM, Critchley JA. Hand washing promotion for preventing diarrhoea. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD004265. [DOI: 10.1002/14651858.CD004265.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 26 March 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Li T, Deeks JJ (editors). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Howard 2002
- Howard G, Bogh C. Healthy villages: a guide for communities and community health workers. Geneva: World Health Organization, 2002. [Google Scholar]
Jefferson 2006
- Jefferson T, Demicheli V, Rivetti D, Jones M, Di Pietrantonj C, Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet 2006;367:303-13. [DOI] [PubMed] [Google Scholar]
Jefferson 2011
- Jefferson T, Del Mar CB, Dooley L, Ferroni E, Al‐Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD006207. [DOI: 10.1002/14651858.CD006207.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
R 2020 [Computer program]
- R Foundation for Statistical Computing R: A language and environment for statistical computing. R Core Team, Version 3.4.2. Vienna, Austria: R Foundation for Statistical Computing, 2020. Available at www.R-project.org.
RStudio 2019 [Computer program]
- RStudio, Inc RStudio: Integrated Development for R. RStudio Team. Boston, MA: RStudio, Inc, 2019.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
UNICEF 2020
- UNICEF. Everything you need to know about washing your hands to protect against coronavirus (COVID-19). Available from www.unicef.org/coronavirus/everything-you-need-know-about-washing-your-hands-protect-against-coronavirus-covid-19 2020.
WHO 2009
WHO 2019
- World Health Organization, UNICEF. Progress on household drinking water, sanitation and hygiene 2000-2017. Special focus on inequalities. Available from www.unicef.org/reports/progress-on-drinking-water-sanitation-and-hygiene-2019 2019.
WHO 2020a
- World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19, March 11 2020. Available from www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 2020.
WHO 2020b
- World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Available from www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it 2020.


