Graphical abstract
Keywords: SARS CoV-2, COVID-19, Nitazoxanide, Azithromycin, Interferons
Abstract
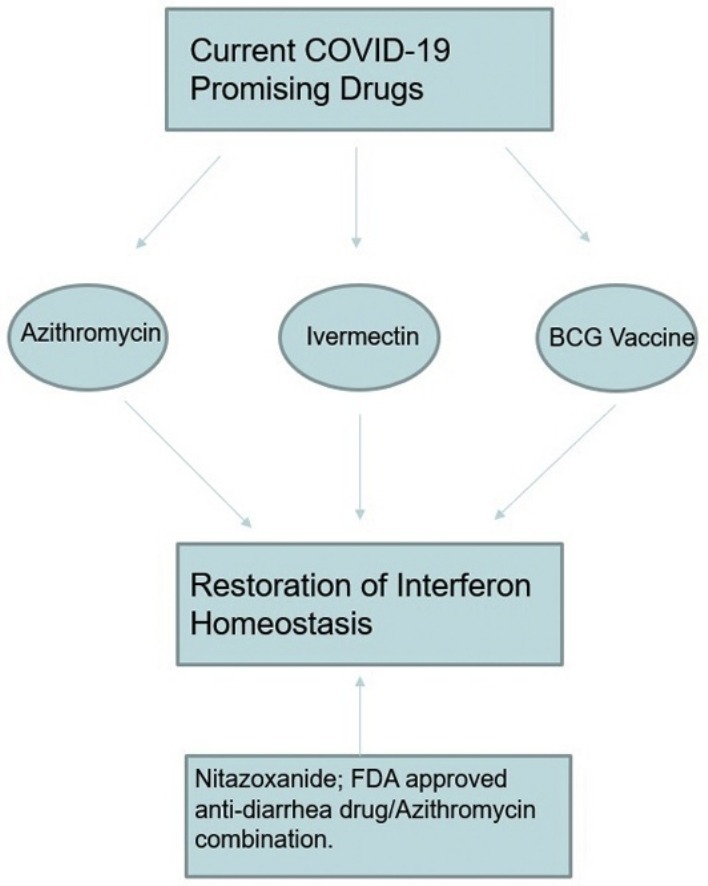
Azithromycin has been shown to have a clinical efficacy against severe acute respiratory syndrome coronavirus 2; ivermectin has also demonstrated a remarkable experimental efficacy with a potential to be used for Coronavirus disease 2019. Further, BCG vaccination is being considered for clinical trials aiming to test its potential for lowering COVID-19 morbidity and mortality. This article illustrates some structural and functional relationships that may gather these drugs and the author, basing on a combined pathophysiological and pharmacological approach, recommends the FDA-approved antidiarrhea drug; nitazoxanide, which has been previously suggested but unfortunately widely ignored, to be tested in combination with azithromycin for their potential activity against SARS CoV-2, soonest. The author also recommends testing their combined administration as early during the clinical course of COVID-19 as possible. Further, basing on the same represented concept, the author suggests more trials for interferons to be tested against SARS CoV-2, especially in severe and critical COVID-19 cases.
Ivermectin (Fig. 1 ) is a broad spectrum macrolide endectocide macrocyclic lactone produced by Streptomyces avermitilis and it’s widely used for treatment of pediculosis, scabies, onchocerciasis and lymphatic filariasis [[1], [2], [3]]. It’s also been shown to possess an invitro antiviral activity against influenza A, dengue and Venezuelan equine encephalitis viruses. Importantly, Ivermectin has been recently proved, in an invitro experiment, to produce approximately 5000-fold reduction in the RNA of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) causing Coronavirus disease 2019 (COVID-19) at 48 h of its single addition [4]. Interestingly, ivermectin therapy has been shown to reverse onchocerciasis-associated immunosuppression through sustained production of IFN-γ in amicrofilaridermic individuals [5], to be also noted that it was previously shown that recombinant human IFN-γ had a particular effect on the incidence of herpes simplex, and cytomegalovirus infections opportunistic infections in advanced HIV disease [6]. Similarly, another macrolide macrocyclic lactone antibiotic; azithromycin (Fig. 2 ) has been reported to have an invitro antiviral activity against Zika and Ebola viruses, it has also significantly decreased rhinovirus-16 viral load in bronchial epithelial cells inducing the expression of type I and III interferons in cells of donors suffering from chronic obstructive pulmonary disease, 24 h post infection [7,8]. Noteworthy, only azithromycin which is a synthetic derivative of erythromycin; the prototype of macrolides antibiotics first isolated from the soil bacterium Streptomyces erythraeus, but not erythromycin or telithromycin, has been shown to significantly increase rhinovirus 1B- and rhinovirus 16-induced interferons and interferon-stimulated gene mRNA expression as well as protein production leading to a significant reduction of rhinovirus replication and release in bronchial epithelial cells [9]. This differential peculiarity associated with azithromycin should be properly investigated as regards to its potential structure activity relationship. Further, it’s also been recently reported that when azithromycin was added to hydroxychloroquine, the efficiency of SARS CoV-2 elimination was significantly improved leading to a significant more rapid virologic cure as evident by a negative nasopharyngeal PCR, and healing in COVID-19 patients [7]. Surprisingly, a recent study has suggested an association of increased overall mortality that was identified in patients treated with hydroxychloroquine without azithromycin as compared to the standard care alone and it’s also pointed no evidence that the use of hydroxychloroquine, either with or without azithromycin, reduced the risk of mechanical ventilation in patients hospitalized with Covid-19 [10]. The author wonders if the previous reported beneficial effects might be more properly attributed to azithromycin alone; further studies might explore this potential and hopefully this manuscript might also help other colleagues in their search for answers. Taken together, ivermectin and azithromycin share a potential structural similarity and more importantly both drugs are potentiating the interferons’ immune response which is essential to combat viral infections. Noteworthy, BCG vaccination which is currently being considered for clinical trials to test its potential against COVID-19, was previously shown when given once a month for 3 consecutive months to produce a significant reduction on the prevalence of adult upper respiratory tract infections in the elderly, at least partially through its induced significant increase of IFN-γ level as compared to the placebo group [11]. Taken together, the author agrees that ivermectin deserves its enrollment for clinical trials to explore its potential for COVID-19 treatment and suggests that BCG vaccination might not be the proper fast response we need. On the other hand, more research for other macrolides to assess their potential antiviral effect might be fertile, not only for this COVID-19 pandemic but also afterwards. Similarly, the author recommends more COVID-19 clinical trials for recombinant human IFN-γ, interferon alfa-1, recombinant interferon beta and while a preliminary version of this manuscript was being submitted on the 8th of April, 2020 elsewhere; other colleagues have also been pointing out, both clinically and theoretically, the significance of interferons as potential drug candidates for COVID-19 [[12], [13], [14]]. Further, it might also be beneficial to test the related possibility that SARS CoV-2 may produce interferon antagonist activities similar to the effect Middle East respiratory syndrome coronavirus (MERS-CoV) has previously exhibited [15,16].
Fig. 1.
Ivermectin.
Fig. 2.
Azithromycin.
Most importantly, the author basing on the same concept, would like to recommend the start of clinical trials for the widely available FDA-approved, for both adults and immunocompetent children, antidiarrhea drug; nitazoxanide, [17] in combination with azithromycin [18], soonest. Nitazoxanide (Fig. 3 ), is an orally active nitrothiazoly-salicylamide broad-spectrum antiparasitic and antiviral prodrug that is converted rapidly to the active metabolites tizoxanide and tizoxanide conjugates and unlike metronidazole, these metabolites appear to be safer and free of mutagenic effects [19]. Similarly, nitazoxanide is also known to potentiate interferon alfa and interferon beta production and it has been previously shown to exhibit an in vitro activity against MERS-CoV and other coronaviruses [20]. Moreover, when nitazoxanide was given as 600 mg twice daily for 5 days, it’s proved to reduce of the duration of symptoms in patients with acute uncomplicated influenza with minor adverse effects [21] and this dose regimen might be reasonably considered to be used combined with azithromycin in a suggested new COVID-19 protocol aiming to test their integrated potential to decrease SARS CoV-2 morbidity and mortality. Noteworthy, the author would like to stress that on the 9th of March, 2020, nitazoxanide was previously suggested, among other drugs, by French colleagues to be evaluated for its potential against SARS CoV-2; unfortunately, their recommendation wasn’t popular and has been practically ignored [22]. Similarly, the author has recently found another research, though also unpopular and ignored, that has been published on the 15th of March, 2020 to recommend nitazoxanide to be used with hydroxychloroquine for COVID-19 patients pointing to some links with the interferon immune system [23]. To date, the 30 th of April, 2020, only eight nitazoxanide clinical trials are undergoing worldwide [24] with remarkable difficulties in recruitment of patients, who might have also doubted the potential of nitazoxanide for COVID-19, and only two of them are currently recruiting; one wishing to collect 50 participants with an estimated start date on the 12th of April, 2020 to compare nitazoxanide 600 mg, without any added drugs, against placebo for hospitalized patients with moderate COVID-19 condition and the author suggests that it’s for the best interests of patients and researchers not to compare any drug against placebo in COVID-19 as this might hinder the enrollment as well as the compliance of patients in their clinical trials. Similarly, the second currently recruiting nitazoxanide trial is wishing to enroll 86 participants with an actual start date on the 6th of April, 2020 to compare nitazoxanide alone to nitazoxanide/hydroxychloroquine combination in patients with COVID-19 risk factors for poor outcome. The author also suggests that their combination might be better considered for early cases not for late ones and wishes that they might also consider the new regimen suggested in this manuscipt. Interestingly 4 out of the 8 nitazoxanide clinical trials for COVID-19 are undergoing in Egypt and 2 of them were posted on the same day the first version of this manuscript has been published at Preprints platform. However, none of the eight trials used an arm for nitazoxanide/azithromycin and the author wishes this might be changed, soonest. The author also recommends comparing the suggested nitazoxanide/azithromycin combination, against any other currently used protocol like hydroxychloroquine/azithromycin combination [25] which is still being used in most countries all over the world. Unfortunately, if we may just compare the very small number with their difficulties in recruitment, of nitazoxanide clinical trials which have been only adopted countries like Egypt, Mexico and Brazil as well as a single pharmaceutical company to the progressively increasing 150 COVID-19 registered and undergoing studies for hydroxychloroquine all over the world [26], we might eventually consider that this was not, after all and after this manuscript becomes available, scientifically justified especially after the recent report that showed no evidence for hydroxychloroquine to decrease the risk of mechanical ventilation in patients hospitalized with Covid-19 [10]. The author wishes also to suggest that regardless of the clinical outcomes of this newly suggested protocol, the reasons for this potentially misfortunate underestimation of nitazoxanide should be properly investigated, and to stress that all FDA approved drugs recommended by experts for COVID-19, should be properly dealed with to explore their potential against SARS CoV-2, to be also noted that for the safe drugs among them, including nitazoxanide, they deserve to be added to any already used protocol in an arm for any undergoing clinical trial, as soon as possible. The author also suggests that we should make this concept clear for any upcoming pandemics. Thus, and as discussed above, nitazoxanide/azithromycin combination for early management of COVID-19 is pharmacologically and sceintfically justified to be considered and popularized for clinical trials, soonest. Finally, The author humbly suggests the approach represented in this manuscript might be considered a first of its kind as regards to its combined pathophysiological as well as pharmacological COVID-19 displayed concept just to raise an Afro-Egyptian voice on behalf of COVID-19 patients that nitazoxanide/azithromycin should be considered as a safe and available regimen to be tested immediately for early stages of COVID-19. Further, based on the same concept and supported by the recent release of clinical data disputing hydroxychloroquine efficacy, the author suggests that nitazoxanide/azithromycin combination might eventually appear to be a safer, more effective regimen that might replace hydroxychloroquine/azithromycin combination as a standard care for early COVID-19, especially if the preliminary data regarding the inefficacy of hydroxychloroquine has been confirmed or as soon as nitazoxanide/azithromycin combination proved clinically effective, as wished. There’re also multiple questions and suggestions presented in this manuscript which are waiting to be researched and answered by our colleagues who might examine this paper. If the concept presented in this manuscript proved right, many lives might be saved as wished but if not, then the author wishes to finally declare: we, humans, will never lose hope, abandon courage, or stop researching and we can’t afford to do so.
Fig. 3.
Nitazoxandie.
Funding
None.
Declaration of Competing Interest
None.
Acknowledgments
The author wishes to thank Prof. Emilio Clementi, Editor in Chief of Pharmacological as well as Prof. Elaine Leung, guest editor of the special issue: “Pharmacoepidemiology and pathogenetics of 2019-nCoV” for their precious remarks and their integrative extra-ordinary and highly professional handling of this manuscript in such a timely manner.
References
- 1.Coscione S., Esau T., Kekeubata E. Impact of ivermectin administered for scabies treatment on the prevalence of head lice in Atoifi, Solomon Islands. PLoS Negl. Trop. Dis. 2018;12(9) doi: 10.1371/journal.pntd.0006825. [published Online First: 2018/09/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandeel M., Elgazar W., Kitade Y. The binding interactions of the macrolide endectocide ivermectin with the antibiotics ampicillin, chloramphenicol and tetracycline HCL. Indian J. Pharm. Sci. 2012;74(6):592–596. doi: 10.4103/0250-474X.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smit M.R., Ochomo E.O., Aljayyoussi G. Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2018;18(6):615–626. doi: 10.1016/s1473-3099(18)30163-4. [published Online First: 2018/04/01] [DOI] [PubMed] [Google Scholar]
- 4.Caly L., Druce J.D., Catton M.G. The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020 doi: 10.1016/j.antiviral.2020.104787. [published Online First: 2020/04/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soboslay P.T., Luder C.G., Hoffmann W.H. Ivermectin-facilitated immunity in onchocerciasis; activation of parasite-specific Th1-type responses with subclinical Onchocerca volvulus infection. Clin. Exp. Immunol. 1994;96(2):238–244. doi: 10.1111/j.1365-2249.1994.tb06548.x. [published Online First: 1994/05/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riddell L.A., Pinching A.J., Hill S. A phase III study of recombinant human interferon gamma to prevent opportunistic infections in advanced HIV disease. AIDS Res. Hum. Retroviruses. 2001;17(9):789–797. doi: 10.1089/088922201750251981. [published Online First: 2001/06/29] [DOI] [PubMed] [Google Scholar]
- 7.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [published Online First: 2020/03/25] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Menzel M., Akbarshahi H., Bjermer L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci. Rep. 2016;6 doi: 10.1038/srep28698. 28698-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36(3):646–654. doi: 10.1183/09031936.00095809. [published Online First: 2010/02/13] [DOI] [PubMed] [Google Scholar]
- 10.Magagnoli J., Narendran S., Pereira F. medRxiv; 2020. Outcomes of Hydroxychloroquine Usage in United States Veterans Hospitalized With Covid-19. 2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardhana Datau E.A., Sultana A. The efficacy of Bacillus calmette-guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 2011;43(3):185–190. [published Online First: 2011/10/08] [PubMed] [Google Scholar]
- 12.Sallard E., Lescure F.-X.-X., Yazdanpanah Y. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020 doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams Eni. 2020. Interferon: Potential COVID-19 Treatment.https://www.medicinenet.com/interferon/article.htm#what_are_interferons_and_how_do_they_work April 8 [Available from: [Google Scholar]
- 14.Zhou Q., Wei X.-S.-S., Xiang X. medRxiv; 2020. Interferon-a2b Treatment for COVID-19. 2020.04.06.20042580. [DOI] [Google Scholar]
- 15.Rossignol J.-F. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 2014;110:94–103. doi: 10.1016/j.antiviral.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zumla A., Chan J.F.W., Azhar E.I. Coronaviruses - drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [published Online First: 2016/02/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetzel D.M., Phillips M.A. Chemotherapy of protozoal infections: amebiasis, giardiasis, trichomoniasis, trypanosomiasis, leishmaniasis, and other protozoal infections. In: Brunton L.L., Hilal-Dandan R., Knollmann B.C., editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e. McGraw-Hill Education; New York, NY: 2017. [Google Scholar]
- 18.Ramos-Soriano A.G., Black J. Nitazoxanide use as part of an empiric multi-drug regimen in treating children with suspected helicobacter pylori infection. Case Rep. Gastroenterol. 2015;9(1):36–42. doi: 10.1159/000375116. [published Online First: 2015/03/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenthal P.J. Antiprotozoal drugs. In: Katzung B.G., editor. Basic & Clinical Pharmacology, 14e. McGraw-Hill Education; New York, NY: 2017. [Google Scholar]
- 20.Rossignol J.-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public Health. 2016;9(3):227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haffizulla J., Hartman A., Hoppers M. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 2014;14(7):609–618. doi: 10.1016/s1473-3099(14)70717-0. [published Online First: 2014/05/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SRLF S, SFMU, GFRUP, SPILF . 2020. Recommendations from Experts on the Resuscitation Care of Patients during an SARS-CoV2 Epidemic Version 1.https://www.srlfS.org/wp-content/uploads/2020/03/Recommandations-dexperts-COVID-19-10-Mars-2020.pdf March 9 [Available from: [Google Scholar]
- 23.Padmanabhan S. 2020. Potential Dual Therapeutic Approach against SARS-CoV-2/COVID-19 with Nitazoxanide and Hydroxychloroquine. [Google Scholar]
- 24.https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=Nitazoxanide&cntry=&state=&city=&dist=
- 25.Gautret Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=Hydroxychloroquine&cntry=&state=&city=&dist=&Search=Search