Dear Editor,
As with all new virus diseases with no drug therapy, identifying key pharmacological targets for SARS-coronavirus 2 (SARS-CoV-2 or 2019-nCoV) based on our knowledge of the viral entry and replication mechanisms in host cells is critical. The present insight highlights the possibility of targeting the Transmembrane Serine Protease 2 (TMPRSS2) to tackle COVID-19, based on similarities with other known coronaviruses such as severe acute respiratory syndrome-related coronavirus (SARS-CoV) and Middle East respiratory syndrome (MERS).
The entry of coronavirus into host cells requires proteolytic activation by protease enzymes and several such potential targets have already been identified. New evidence suggests that TMPRSS2 is involved in MERS and SARS-CoV S protein processing for infection in a number of susceptible host cells in cell lines obtained from various organs [1]. In the murine models of infection by SARS-CoV and MERS-CoV, for example, TMPRSS2-knockout mice exhibit lower level of viral spread in the lungs coupled with reduced severity in immunopathology [2].
Extending the above-mentioned research to SARS-CoV-2, Matsuyama et al. [3] have shown that VeroE6 cells expressing TMPRSS2 are highly susceptible to infection. By using TMPRSS2 overexpression as a tool, the isolation of SARS-CoV-2 is more readily possible [3]. While both ACE2 and TMPRSS2 have been shown to be involved in the entry of SARS-CoV-2 via S protein activation, sera from convalescent SARS patients have been shown to cross-neutralize SARS-CoV2-S-mediated entry into a large number of sensitive cell lines [4]. This is in addition to the demonstrated inhibitory role of cellular serine protease TMPRSS2 inhibitor in experimental SARS-CoV-2 entry [4]. A recent study on the expression level of TMPRSS2 showed that it is widely expressed in lung tissues while ACE2 is predominantly expressed in a transient secretory cell types or differentiating cells [5]. Activation of TMPRSS2 further plays a key role in other viral respiratory diseases such as influenza A (as with MERS) and inhibition of viral activation by a serine protease inhibitor, the hepatocyte growth factor activator inhibitor type-1 (HAI-1), has been demonstrated in vitro [6]. The putative role of TMPRSS2 as the major activating protease for influenza B virus has also been established [7].
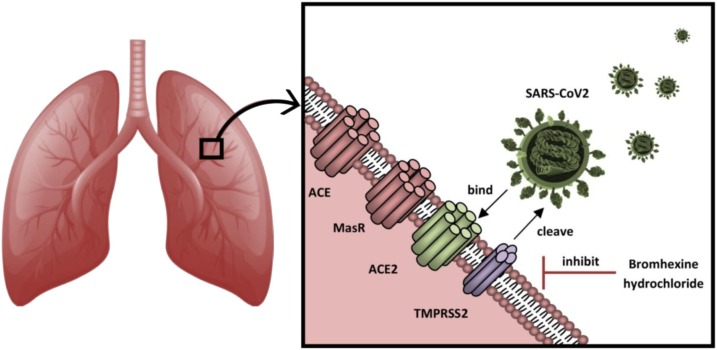
Based on our knowledge on SARS-CoV and MERS-CoV entry into host cells, the therapeutic potential of TMPRSS2 inhibitors for SARS-CoV-2 can be postulated (Fig. 1 ). While S1/S2 precleavage was shown to promote S protein activation, processing by TMPRSS2 appears to be critical for infection by these viruses [6]. The detailed mechanism of proteolytic activation of these viruses by TMPRSS2 both at entry and release phases of the infection cycle has been reviewed [1]. Further research is however required to better appreciate the extent by which the proteases and endosomal pathway are involved in viral entry for SARS-CoV-2. The preference of wild-type human coronavirus for cell surface TMPRSS2 when compared to the endosomal route has already been shown [7]. The FDA-approved expectorant/mucolytic agent and cough suppressant, bromhexine hydrochloride, is a well-known potent inhibitor of TMPRSS2 with IC50 equal to 0.75 μM [1]. Since epithelisin is expressed primarily in the apical surface of airway epithelial cells, inhibiting pulmonary TMPRSS2 with bromhexine hydrochloride could represent a prophylactic strategy against the airborne transmission of SARS-CoV2. Because there is no substantial adverse effect, it is reasonable to suggest that this well-known TMPRSS2 inhibitor cold be regarded as a novel prophylactic agent against SARS-CoV-2 infection and research on other potential inhibitors should be encouraged. Clinical trials are needed to evaluate its prophylactic potential against SARS-CoV-2.
Fig. 1.
Strategy of inhibiting SARS-CoV-2 entry into host lung cells via targeting TMPRSS2 by bromohexine hydrochloride.
Declaration of Competing Interest
There is no conflict of interest.
References
- 1.Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwata-Yoshikawa N., Okamura T., Shimizu Y., Hasegawa H., Takeda M., Nagata N. TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection. J. Virol. 2019;93:e01815–e01818. doi: 10.1128/JVI.01815-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U. S. A. 2020 doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020 doi: 10.15252/embj.2020105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zmora P., Hoffmann M., Kollmus H., Moldenhauer A.S., Danov O., Braun A. TMPRSS11A activates the influenza A virus hemagglutinin and the MERS coronavirus spike protein and is insensitive against blockade by HAI-1. J. Biol. Chem. 2018;293:13863–13873. doi: 10.1074/jbc.RA118.001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limburg H., Harbig A., Bestle D., Stein D.A., Moulton H.M., Jaeger J. TMPRSS2 is the major activating protease of influenza A virus in primary human airway cells and influenza B virus in human type II pneumocytes. J. Virol. 2019;93:e00649–19. doi: 10.1128/JVI.00649-19. [DOI] [PMC free article] [PubMed] [Google Scholar]