Abstract
Objective:
With the advent of new treatment options for Chronic Rhinosinusitis (CRS) comes the ability for physicians to provide more individualized patient care. Physicians are now tasked with identifying who may be the best candidate for a particular therapy. In this review, existing biomarkers and potentially new methods that could guide treatment choices in CRS patients will be discussed.
Data Sources:
Published literature obtained through PubMed searches.
Study Selections:
Studies relevant to inflammatory endotypes, phenotypes, and biomarkers in CRS were included.
Results:
Currently, there are no clinically validated tools that determine the best therapeutic modality for CRS patients with or without nasal polyps (CRSwNP or CRSsNP). Patients with CRS can be classified into three endotypes based on the presence of type 1, type 2, or type 3 inflammation. CRS endotypes can be influenced by age and geographic location. Clinical application however may be limited since endotyping current requires basic research laboratory support. Clinical symptoms may also predict inflammatory endotypes with smell loss being indicative of type 2 inflammation. Numbers of tissue and/or peripheral eosinophils as well as levels of IgE may predict disease severity in CRSwNP but not necessarily treatment responses. Unique clinical phenotypes or biomarkers are especially lacking that predict type 1 or type 3 inflammation in CRSwNP or type 1, type 2, or type 3 inflammation in CRSsNP.
Conclusion:
While significant progress has been made in characterizing endotypes, phenotypes, and biomarkers in CRS, additional studies are needed to determine if and how these factors could assist physicians in providing more individualized clinical care.
Keywords: Chronic Rhinosinusitis, nasal polyps, CRSsNP, CRSwNP, endotype, phenotype, biomarker, inflammation
Introduction
Chronic Rhinosinusitis (CRS) is estimated to affect 3-6% of the general population1 and is characterized by chronic inflammation of the sinonasal mucosa. To fulfill the clinical diagnosis, patients must have cardinal symptoms (nasal congestion, anterior and/or posterior rhinorrhea, sinus pressure/pain, and/or smell loss) for at least 12 weeks duration and have objective evidence of sinonasal inflammation on either sinus CT scan or nasal endoscopy2. CRS is an important disease to study given it is associated with significant reductions in patient quality of life 3, substantial losses in productivity4, 5, and estimated direct and indirect healthcare costs of $22 billion in 2014 alone6, 7.
CRS can be divided into two major clinical phenotypes based upon the presence or absence of nasal polyps. Smaller sub-groups of CRS exist including cystic fibrosis8, allergic fungal rhinosinusitis9, and aspirin exacerbated respiratory disease10, 11 but these are outside the scope of the current review. While the majority of patients with CRS do not have nasal polyps (CRSsNP), the 20% with nasal polyps (CRSwNP) tend to have more severe clinical disease12. However, the nature, severity, and frequency of clinical symptoms can significantly vary between individual patients.
Despite the clinical heterogeneity, treatment options for patients with CRS were mainly limited to corticosteroids (topical or systemic) or sinus surgery until recently. In 2019, Dupilumab, a monoclonal antibody targeting the alpha-chain of the IL-4 receptor that blocks both IL-4- and IL-13-mediated signals, was the first biologic approved in the US for the treatment of CRSwNP13. Other biologics targeting distinct inflammatory mediators including soluble IL-5 (Mepolizumab, NCT03085797)14, the IL-5 receptor (Benralizumab, NCT03401229), and IgE (Omalizumab, NCT03280550 and NCT03280537)15, 16 are currently in clinical trials for CRSwNP. In contrast, there are no biologics currently approved for the treatment of CRSsNP but it is possible a subset of CRSsNP patients could respond to these agents.
With the advent of new treatment options for CRS comes the ability for physicians to provide more individualized patient care. As such, physicians will have to identify which patients may be the best candidates for a particular therapy. However, few criteria are currently available to help guide these decisions. In this review, existing biomarkers and potentially new methods that could guide treatment choices in patients with CRS will be addressed using a bench to bedside approach.
Inflammatory Endotypes in Chronic Rhinosinusitis
To fully appreciate the clinical presentation of a disease, it is important to understand the basic cellular and molecular mechanisms contributing to its pathology. In CRS, chronic inflammation in the sinonasal mucosa is thought in part to be secondary to disruptions in the epithelial barrier and dysregulation of the ongoing immune response17, 18. Interestingly, different types of inflammatory processes have been observed in patients with CRS. Characterizing and understanding these variations may help to explain the clinical heterogeneity of the disease.
Overview of Inflammatory Endotypes
In general, an immune response can be classified into 3 inflammatory endotypes based upon a unique signature profile comprised of specific inflammatory mediators, immune cells, and physiological functions 19, 20 (FIGURE 1). Type 1 inflammation is characterized by the preferential expression of the cytokines IFNγ and IL-12. In contrast, type 2 inflammation is associated with enhanced production of the cytokines IL-4, IL-5, and IL-13. More recently, a third inflammatory pattern, referred to as type 3 (or type 17) inflammation, has been described with elevated levels of IL-17 and IL-22.
Figure 1. Associations between Inflammatory Endotypes and Clinical Phenotypes in Chronic Rhinosinusitis.
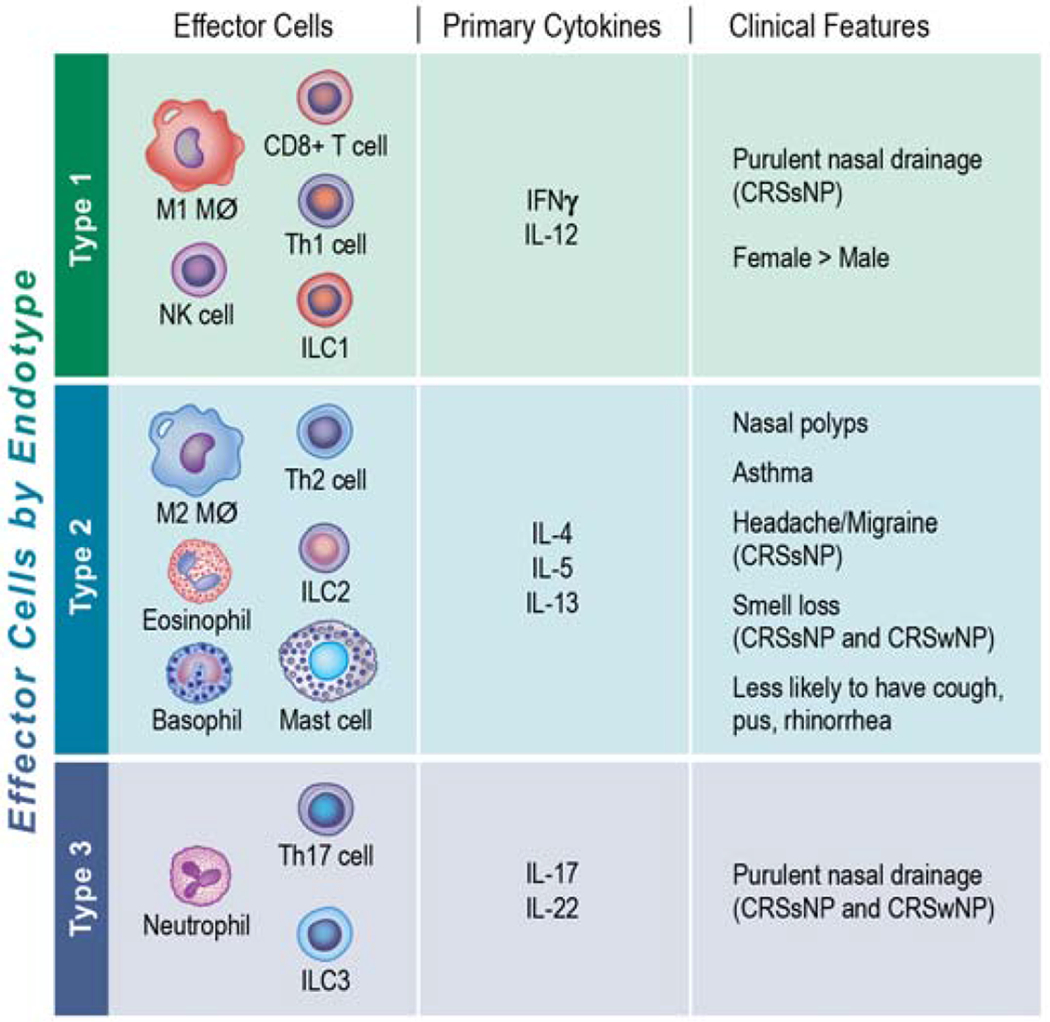
Immune responses can be broadly classified into 3 inflammatory endotypes (type 1, type 2, or type 3) based upon a unique signature profile comprised of specific immune cells, inflammatory mediators, and physiological functions. Several associations between inflammatory endotypes and clinical features in patients with CRS have been identified 33. For example, smell loss strongly associated with type 2 inflammation in all patients with CRS. In contrast, the presence of purulent nasal discharge was associated with a type 1 endotype in CRSsNP but type 1 and type 3 endotypes in CRSwNP.
Initial studies identified distinct populations of CD4+ T cells, named Th1, Th2, and Th17, that contributed to the cytokine patterns observed in type 1, type 2, and type 3 inflammatory endotypes respectively. However, other cell types including macrophages and innate lymphoid cells can also be polarized towards a type 1, type 2, and/or type 3 inflammatory profile. Other cells specifically associated with type 2 inflammation include eosinophils, mast cells, and basophils while NK cells and CD8+ T cells are associated with type 1 inflammation and neutrophils with type 3 inflammation.
Type 1 inflammation is predominantly involved in mediating host protection from intracellular microbes, including viruses. Type 2 immune responses help the host defend against parasitic infections and are also classically associated with driving allergic disease. Finally, type 3 inflammation primarily is thought to protect against extracellular bacteria and fungi.
Inflammatory Endotypes in CRS
It was initially hypothesized that CRSwNP was characterized by type 2 inflammation and CRSsNP by type 1 inflammation. Several reports showed elevated levels of IL-4, IL-5, and IL-13, numbers of eosinophils, and levels of eosinophil granule proteins such as eosinophil cationic protein (ECP) in nasal polyps compared to healthy sinonasal tissue21–23. However, studies are conflicted as to whether IFNγ levels (a marker of type 1 inflammation) were22,24–26 or were not 21, 24, 27 elevated in sinonasal tissue from patients with CRSsNP compared to those with CRSwNP or healthy controls.
With recent advancements in bioinformatics, it is has become easier to simultaneously evaluate expression patterns of hundreds if not thousands of inflammatory mediators within a single sinonasal specimen in an unbiased manner. This in turn has allowed for the inflammatory milieu, and by extension endotypes, to be comprehensively profiled in CRS. A European study using a cluster-based analysis identified 10 unique endotypes of CRS that could be distinguished in part by expression of IL-5, a major cytokine responsible for eosinophil survival and migration28. When a post-hoc analysis of this data was linked to clinical phenotypes, the authors found the majority of patients with undetectable levels of IL-5 had CRSsNP. Of patients with moderate IL-5 levels, there was a mixture of both CRSsNP and CRSwNP. However, those with the highest levels of IL-5 exclusively had CRSwNP.
The observation that CRSsNP and CRSwNP are not dichotomous but instead have overlapping inflammatory signatures was supported by more recent work in the US and Europe. Again, type 2 inflammation was the predominant endotype in both CRSsNP and CRSwNP29–31. Type 1 and type 3 inflammatory endotypes were also detected in smaller subsets of patients with CRSsNP or CRSwNP29. Interestingly, some patients with CRS expressed a mixture of two or more inflammatory endotypes. In contrast, other CRS patients did not significantly express any cytokines measured. It is possible this latter untypeable subgroup represents another distinct endotype of CRS whose inflammatory signature has yet to be identified but does not include significant levels of IFNγ, IL-5, ECP, or IL-17A.
Geographical Variations in Inflammatory Endotypes in CRS
Studies have suggested that environmental and genetic factors can also influence inflammatory endotypes in CRS (FIGURE 2). Second-generation Asian patients living in the US, for example, had lower levels of eosinophils in nasal polyps when compared to non-Asian controls32. Historically, patients with CRSwNP from Asian countries were considered to have a lower prevalence of type 2 inflammation than patients living in Western countries. As previously discussed, a study from the US found 87% of patients with CRSwNP had a type 2 inflammatory endotype33. This was similar to 85% and 73% of CRSwNP patients having a type 2 inflammatory endotype according to studies in Europe and Australia31. While the type 2 endotype was observed in Chinese patients with CRSwNP (61% in Beijing and 20% in Chengdu), there were similar percentages of patients with type 1 inflammatory endotypes (58% and 17%)31. Longitudinal studies of CRS patients in Asia have suggested the prevalence of type 2 inflammatory endotypes has risen over the past two decades possibly in part due to the adoption of a more Westernized lifestyle in these countries34.
Figure 2. Geographical Diversity of Inflammatory Endotypes in Chronic Rhinosinusitis.
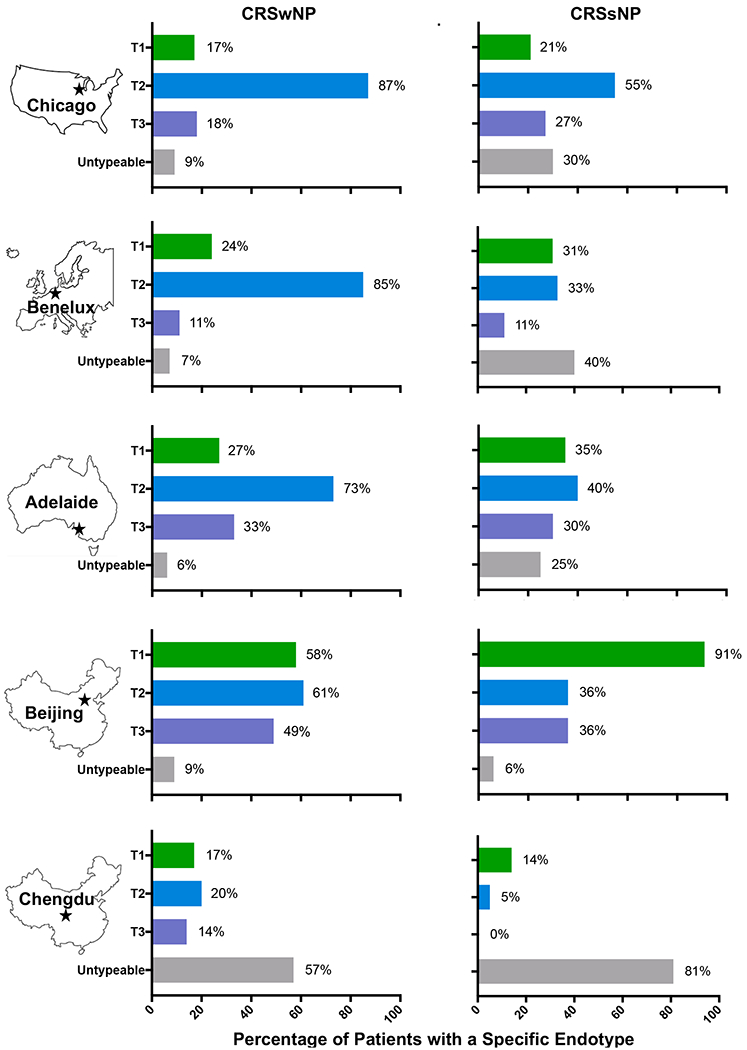
There is a marked global heterogeneity in inflammatory endotypes among patients with CRSsNP or CRSwNP. In a study from the United States, type 1, type 2, and type 3 endotypes were determined by expression levels of IFNγ, Eosinophil Cationic Protein/Charcot Leiden Crystal, and IL-17A being above the 90% of expression in control ethmoid tissues respectively 33. In a separate study from Europe, Australia, and China, type 1, type 2, and type 3 endotypes were determined if levels of IFNγ, IL-5, and IL-17A respectively were detected in nasal polyps of patients with CRSwNP and in ethmoid tissue of patients with CRSsNP 31. Among patients living in the US, Europe, and Australia, the type 2 endotype was the predominant endotype observed in patients with CRSwNP. Type 2 inflammation was also the predominant endotype in patients with CRSsNP living in the US. Meanwhile in China, a larger percentage of patients were characterized by a type 1 inflammatory endotype, especially those patients with CRSsNP living in Beijing.
There is also heterogeneity among CRSsNP patients throughout the world (FIGURE 2). Type 2 inflammation was seen in a significant proportion of patients in the US (55%)33 and to a lesser extent in Europe (33%) and Australia (40%)31. Strikingly, type 1 inflammation was the dominant endotype in Chinese patients with CRSsNP living in Beijing while the majority of patients living in Chendgu had an untypeable phenotype not characterized by elevated IFNγ, IL-5, or IL-1731. Taken together, these finding suggest that CRS endotypes can vary based on geographical location. How environmental triggers, genetic factors, or combinations of both may impact the development of type 1, type 2, or type 3 inflammatory endotypes warrants further study.
Current Limitations for Utilizing Endotypes to Guide Treatment Choices in CRS
Overall, significant progress has been made in characterizing the inflammatory endotypes in CRS.
One limitation is the current lack of a standard definition for each endotype since different study groups have used different markers and nomenclature to define endotypes to date. Additionally, how endotypes impact clinical disease and if endotypes could be predicted by clinical biomarkers are still not fully understood. Additional studies are thus needed to determine if endotypes could indeed be predictive of treatment responses and possibly identify which patients would be better candidates for biologic therapy versus surgical intervention.
An important consideration when determining CRS endotypes is that the levels of inflammatory mediators can not only vary by patient but also by anatomical location within the sinonasal cavity35. For example, Tan and colleagues reported marked variation in expression of type 2 inflammatory mediators between inferior turbinate (IT), uncinate tissue (UT), and ethmoid tissue (ET) of patients with CRSsNP29. There is currently no general consensus as to which sinonasal tissue is the most appropriate to study but most recent work has utilized ethmoid tissue in CRSsNP and nasal polyps in CRSwNP30, 31, 33.
To assess whether endotypes could be determined using less invasive techniques, Turner and colleagues identified inflammatory endotypes in mucus collected in absorbent polyurethane sponges temporarily placed in the nasal cavity36. Similar to tissue studies, the authors reported significant variation in inflammatory markers with CRS subtypes. While CRSwNP was associated with the highest levels of type 2 inflammation, levels of type 2 cytokines were measured, albeit in lower levels, in mucus from patients with CRSsNP as well36. This suggests that CRS endotypes could be determined without the need for sinus surgery but the approach would still require processing and analysis of samples by a basic research laboratory. While this arguably provides the most detailed characterization of inflammatory endotypes, it remains difficult to translate this method to routine, rapid, and convenient clinical use.
Clinical Phenotypes, age and comorbid asthma in Chronic Rhinosinusitis
All patients with CRS, by definition, have sinonasal symptoms. However, the type of symptoms reported (i.e., post-nasal drainage, sinus pressure, smell loss) and the severity of these symptoms can vary between patients and even disease subtypes. It may be that specific constellations of clinical symptoms, or phenotypes, could serve as another means to predict treatment responses in patients with CRS. However, it is more likely that clinical symptoms could provide a non-invasive way to determine inflammatory endotypes which then would be used to guide choice of treatment. Associations between inflammatory endotypes and clinical phenotypes as well as how specific clinical features might impact inflammatory endotypes has been the focus of ongoing investigations.
Associations between Clinical Symptoms and Endotypes in CRS
A recent study examining phenotypes and endotypes in matched patients with CRS found significant associations between certain clinical presentations and specific inflammatory signature profiles33. The presence of smell loss was most strongly associated with type 2 inflammation (OR 2.80) and the presence of purulent nasal discharge with type 3 inflammation (OR 5.42) even after controlling for age, sex, nasal polyps, asthma, and atopy33. Among patients with CRSsNP, purulent nasal discharge was associated with type 1 and type 3 inflammation while smell loss and headache/migraine was associated with type 2 inflammation. Among patients with CRSwNP, purulent nasal discharge again correlated with the presence of type 3 inflammation. However, in contrast to patients with CRSsNP, those with CRSwNP and a type 2 inflammatory endotype were less likely to report headache/migraine.
Influence of Age on Inflammatory Endotypes in CRS
Turner and colleagues found that older patients with CRS were more likely to have a neutrophilic response with increased mucus levels of IL-1β, IL-6, IL-8, and TNF-α compared to younger CRS patients37. In a separate tertiary care surgical cohort of patients with CRSwNP, nasal polyp levels of ECP were significantly lower in patients aged 60-77 years compared to those between 16 and 59 years of age38. This limited data suggests that age can affect CRS inflammatory endotypes as older patients with CRSwNP were less likely to have a type 2 inflammatory endotype. These findings raise the possibility that biologics targeting type 2 inflammation could potentially be less efficacious in older individuals. However, larger studies are needed for confirmation and to better characterize the influence age has on CRS phenotypes and endotypes39.
Influence of Asthma on Inflammatory Endotypes in CRS
As many as 36% of patients with CRSsNP and 48% of patients with CRSwNP have comorbid asthma12,40,41. Asthma has been linked to more severe sinonasal disease in patients with CRSwNP42 and more recently was associated with a type 2 inflammatory endotype in all patients with CRS33. Similar to CRS, asthma can be divided into 3 distinct endotypes-type 1, type 2, and type 3. Few studies have simultaneously profiled inflammatory patterns of the upper and lower respiratory tract in a patient with both diseases. In a small study, similar inflammatory endotypes (type 1 or type 2) were observed in nasal polyps and bronchial tissue43. Separately, severe asthmatics with CRS had elevated levels of type 2 inflammatory cytokines in sinonasal tissues when compared to CRS patients with less severe asthma44. How endotypes in the lung impact that of the sinuses and vice versa is not well established and should be the focus of additional investigations.
Current Limitations for Utilizing Clinical Symptoms and Phenotypes to Guide Treatment Choices in CRS
Medical histories are routinely obtained from patients as part of a standard clinic visit and would arguably be the easiest means by which clinicians could gather information to determine the best therapeutic modality for a given patient. However, the ability, sensitivity, and specificity of a clinical symptom to predict endotype or treatment response have not been rigorously confirmed or validated to date. Symptoms indicative of type 1 and type 3 endotypes are especially lacking and it may be that a currently unrecognized symptom could be a better indicator of these inflammatory responses. It may also be that a constellation of several clinical symptoms will needed that one specific symptom alone.
Another limitation to utilizing clinical phenotypes to guide treatment choices in CRS is that symptoms can be highly subjective45 and may evolve over a period of time. In a primary care population, Sundaresan and colleagues found that the majority of patients with CRS reported a waxing and waning of their sinonasal symptoms over a 6-month period46. It is unclear if inflammatory endotypes change along with clinical symptoms or instead is constant. Additionally, it is unknown if the extent by which clinical symptoms fluctuate over a period of time could also be a means to help select treatment modalities.
Clinical Biomarkers in Chronic Rhinosinusitis
Given the current limitations in utilizing endotypes and clinical phenotypes to guide treatment choices in CRS, a third approach involving clinical biomarkers has been more extensively examined. There remain no known clinical biomarkers indicative of type 2 inflammation in CRSsNP or of type 1 or 3 endotypes in CRSwNP or CRSsNP. In contrast, eosinophils and IgE levels have been studied as potential biomarkers in type 2 CRSwNP more as a means to predict clinical disease severity than treatment response.
Eosinophils in Sinonasal Tissue
Eosinophils have long been a hallmark of type 2 inflammation. Consistent with recent endotype studies, there is variability in the number of eosinophils detected in nasal polyps. Further complicating matters is that studies have used different protocols and numerical cutoffs to define eosinophilia within sinonasal tissue47. Patients with greater than 5 eosinophils per high-power field (hpf) in nasal polyps had more severe sinonasal inflammation on sinus CT scan compared to those patients with fewer than 5 eosinophils/hpf48. In a separate study, the presence of more than 70 mucosal eosinophils/hpf in nasal polyps was associated with a higher rate of polyp recurrence following surgery compared to patients with lower numbers of tissue eosinophils49. Higher levels of eosinophil granule proteins in UT of CRS patients was also associated with a greater risk of polyp recurrence following surgery50. Despite the link between numbers of eosinophils in nasal polyps and objective evidence of sinus disease severity, the severity of patient-reported sinonasal symptoms did not correlate with eosinophil numbers in nasal polyps of patients with CRSwNP51.
Eosinophils in Peripheral Blood
The number of tissue eosinophils has been shown by several groups to correlate with the number of eosinophils detected in the peripheral blood51,52. In 2015, Japanese researchers determined that having greater than 10% eosinophils in the peripheral blood was associated with a higher rate of CRS recurrence53. Furthermore, using a novel scoring system, the presence of eosinophilic CRS could be predicted by factoring the presence of nasal polyps, percentage of peripheral blood eosinophils, and extent of sinonasal opacification on sinus CT scan53. In this scoring system, the greater the percentage of peripheral blood eosinophils, the higher the likelihood of having eosinophilic CRS.
In a US study, a significant but weak correlation was found between peripheral eosinophil numbers and degree of polyp burden on sinus CT scan pre-operatively (r=0.35, p<0.01)54. However, following sinus surgery, the change in peripheral eosinophil numbers strongly and significantly correlated with changes in endoscopic measurements of sinonasal inflammation (r=0.82, p<0.001)54. In a separate analysis, peripheral eosinophil numbers measured prior to surgery were significantly higher in those patients who had recurrence of nasal polyps following sinus surgery55,56. Taken together, eosinophil levels in the peripheral blood (or tissue) may help predict disease severity and recurrence in patients with CRSwNP but the use of eosinophils as a biomarker in type 2 CRSsNP remains unknown.
Levels of Total and Specific IgE
The presence of IgE antibodies is another hallmark of type 2 inflammation. In nasal polyps, total IgE levels were elevated compared to that measured in healthy sinonasal tissue but no concomitant elevation was observed in the systemic circulation of patients with CRSwNP57. Clinical trials of omalizumab in CRSwNP show promising clinical benefit supporting the idea that IgE plays an important, albeit unclear, role in CRS pathogenesis15, 16.
The specificities of IgE antibodies detected in nasal polyps is not well defined. In a Chinese population, local IgE in nasal polyps was predominantly against common aeroallergens58 while other studies suggest a percentage of specific IgE is against common nasal bacteria59. There has been a larger number of studies reporting elevated levels of specific IgE against Staphylococcus aureus enterotoxins (SAE) in a proportion of European patients with CRSwNP60,61 and specific IgE to SAE has been associated with more severe clinical disease28. Levels of total IgE and specific IgE against SAE significantly correlated with levels of IL-5 and total number of eosinophils in nasal polyps62. Levels of total IgE or specific IgE did not significantly correlate between serum and nasal polyp but a combination of serum total IgE, serum specific IgE to SAE, and serum periostin levels has been suggested to predict patients having more severe CRSwNP63.
Current Limitations for Utilizing Biomarkers to Guide Treatment Choices in CRS
Numbers of peripheral eosinophils and levels of IgE are routinely and easily measured by physicians without the requirement of basic research laboratory support. Compared to clinical symptoms, the utilization of these biomarkers is also less subjective. What remains unclear is how best these biomarkers could be used to guide clinical decisions about treatment options in CRS.
In the large phase 3 study of Dupilumab in CRSwNP, the majority of patients reported benefit with the drug compared to placebo but this was irrespective of their number of peripheral eosinophils13. Patients with higher peripheral eosinophil numbers prior to treatment tended to report greater symptom improvement with the drug than those with lower eosinophils numbers. However, no specific numerical cut-off could be established that predicted a responder versus a non-responder to therapy. As a result, there is no minimum peripheral eosinophil number required to start Dupilumab in patients with CRSwNP.
Peripheral eosinophils were also not a biomarker of response in studies evaluating Mepolizumab in CRSwNP. In a post hoc analysis, baseline peripheral eosinophil numbers were not predictive of patients who had a 1-point reduction in nasal polyp size with treatment14. Another drug, dexpramipexole significantly depleted eosinophils in the peripheral blood and in polyp tissue from patients with CRSwNP albeit by unknown mechanisms. Despite this reduction in eosinophils, treatment with the drug did not reduce nasal polyp size or improve patient-reported symptoms64. It may be that eosinophils are instead a better indicator of a high-risk group of patients with CRS, those with significant morbidity and increased healthcare costs. These such patients may be the ones in which a biologic should be considered. Additional studies are needed to identify biomarkers (phenotypes and endotypes) that could help guide other CRS treatment choices including systemic steroids and surgery.
Summary
In the past decade, significant progress has been made in defining the cellular and molecular mechanisms responsible for CRS pathogenesis. Likewise, epidemiological and cohort studies of patients with CRS have advanced the clinical understanding of this disease. Novel therapeutics have been approved or are in clinical trials to expand treatment options for affected patients. However, the physician is now faced with translating these findings into providing more personalized clinical care. Ideally, a factor easily and routinely assessed at the bedside would guide physicians in determining the most appropriate CRS treatment modality for a patient. While such a clinically validated marker does not currently exist, novel approaches are being explored.
Patients with CRS can be classified into 3 distinct endotypes based on the type of underlying inflammatory response. CRSsNP and CRSwNP in the US are predominantly characterized by a type 2 inflammatory endotype but smaller subsets with type 1, type 3, or a mixture of inflammatory patterns have been identified. It may be that CRSsNP and CRSwNP patients with type 2 inflammatory endotypes would respond similarly to a biologic therapy. In contrast, patients with type 1 or type 3 inflammation may report less clinical benefit from these same agents. Clinical characteristics could also be used as a surrogate to predict inflammatory endotypes. Smell loss, for example, has been shown to be indicative of type 2 inflammation. However, large validated studies confirming associations between CRS phenotypes and endotypes have yet to be performed. Finally, numbers of tissue and/or peripheral eosinophils as well as levels of IgE may predict disease severity in CRSwNP but not necessarily treatment responses.
Conclusion
In conclusion, significant advancements have been made in characterizing endotypes, phenotypes, and biomarkers in CRS. While more work is needed, these tools may assist physicians in providing more individualized care of patients with CRS.
Learning Objectives:
To become familiar with how inflammatory endotypes, clinical phenotypes, and biomarkers could be used to guide treatment choices in patients with CRS.
To define the different inflammatory endotypes observed in CRS and to identify associations with specific clinical features.
Acknowledgements:
The authors gratefully acknowledge Ms. Jacqi Schaffer for her illustrations of Figure 1.
Funding Source: National Institutes of Health (K23AI141694, PO1AI145818, and R01AI137174) and the Ernest S. Bazley Foundation
Conflicts of Interest: Anna Staudacher reports no conflicts of interest. Anju Peters has research support from Astra Zeneca and Optinose and has received consultancy fees from Sanofi Regeneron and Astra Zeneca. Atsushi Kato has received consultancy fees from Astellas Pharma and has received a gift for his research from Lyra Therapeutics. Whitney Stevens has served on an advisory board for GlaxoSmithKline.
Question 1.
Each of the following clinical symptoms meets criteria for diagnosing Chronic Rhinosinusitis except:
Smell loss
Anterior rhinorrhea
Sinus pressure/pain
Sneezing
Nasal congestion
Answer: D, sneezing
Rationale: According to the EPOS 2012 guidelines, patients with CRS must report the cardinal symptoms of nasal congestion, anterior and/or posterior rhinorrhea, sinus pressure/pain, and/or smell loss for at least 12 weeks duration. Additionally, these patients must also have objective evidence of sinonasal inflammation on either sinus CT scan or nasal endoscopy.
Reference:
1. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012; 23:3 p preceding table of contents, 1-298.
Question 2.
The type 1 inflammatory endotype is characterized by expression of which of the following cytokines?
IL-4
IL-5
IFNγ
IL-17A
IL-13
Answer: C, IFNγ
Rationale: Type 1 inflammation is characterized by the preferential expression of the cytokines IFNg and IL-12. In contrast, type 2 inflammation is associated with enhanced production of the cytokines IL-4, IL-5, and IL-13. Type 3 (or type 17) inflammation is characterized by elevated levels of IL-17 and IL-22.
Reference:
1. Steinke JW, Rosenwasser LJ, Borish L. Cytokines in Allergic Inflammation. In: Adkinson NF, Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF, et al., editors. Middleton’s Allergy Priniciples and Practice. Philadelphia: Elsevier; 2014. p. 65-82.
Question 3.
In the United States, patients with CRSsNP were most likely to have which of the following inflammatory endotypes?
Type 1
Type 2
Type 3
Type 4
Untypeable
Answer: B, type 2 inflammatory endotype
Rationale: In a US study of patients living in Chicago, IL, 55% of patients with CRSsNP were characterized as having a type 2 inflammatory endotype. This is compared to 21% with a type 1 endotype, 27% with a type 3 endotype, and 30% with an untypeable endotype. A type 4 inflammatory pattern has not been extensively described to date.
Reference:
1. Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations Between Inflammatory Endotypes and Clinical Presentations in Chronic Rhinosinusitis. J Allergy Clin Immunol Pract 2019.
Question 4.
Of the following clinical symptoms, which is most associated with a type 2 inflammatory endotype in patients with CRS?
Smell loss
Fatigue
Sinus pressure/pain
Nasal congestion
Purulent nasal drainage
Answer: A, smell loss
Rationale: Even after controlling for age, sex, nasal polyps, asthma, and atopy, the presence of smell loss was most strongly associated with type 2 inflammation (OR 2.80) and the presence of purulent nasal drainage with type 3 inflammation (OR 5.42) among patients with CRS. There was no association identified between any inflammatory endotype and patient-reported fatigue, nasal congestion, or sinus pressure/pain.
Reference:
1. Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations Between Inflammatory Endotypes and Clinical Presentations in Chronic Rhinosinusitis. J Allergy Clin Immunol Pract 2019.
Question 5.
Which decade of life is most associated with having a reduced type 2 inflammatory endotype?
10-19 years
20-29 years
30-39 years
40-49 years
> 60 years
Answer: E, > 60 years
Rationale: In a tertiary care surgical cohort of patients with CRSwNP, nasal polyp levels of ECP, a type 2 inflammatory marker, were significantly lower in patients aged 60-77 years compared to those between 16 and 59 years of age. Furthermore, another study found that older patients with CRS were more likely to have a neutrophilic response with increased mucus levels of IL-1P, IL-6, IL-8, and TNF-α than younger CRS patients.
Reference:
1. Cho SH, Hong SJ, Han B, Lee SH, Suh L, Norton J, et al. Age-related differences in the pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol 2012; 129:858-60 e2.
2. Morse JC, Li P, Ely KA, Shilts MH, Wannemuehler TJ, Huang LC, et al. Chronic rhinosinusitis in elderly patients is associated with an exaggerated neutrophilic proinflammatory response to pathogenic bacteria. J Allergy Clin Immunol 2019; 143:990–1002 e6.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: not applicable
References:
- 1.Dietz de Loos D, Lourijsen ES, Wildeman MAM, Freling NJM, Wolvers MDJ, Reitsma S, et al. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J Allergy Clin Immunol 2019; 143:1207–14. [DOI] [PubMed] [Google Scholar]
- 2.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012; 23:3 p preceding table of contents, 1-298. [PubMed] [Google Scholar]
- 3.Mattos JL, Rudmik L, Schlosser RJ, Smith TL, Mace JC, Alt J, et al. Symptom importance, patient expectations, and satisfaction in chronic rhinosinusitis. Int Forum Allergy Rhinol 2019; 9:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudmik L, Smith TL, Schlosser RJ, Hwang PH, Mace JC, Soler ZM. Productivity costs in patients with refractory chronic rhinosinusitis. Laryngoscope 2014; 124:2007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudmik L Economics of Chronic Rhinosinusitis. Curr Allergy Asthma Rep 2017; 17:20. [DOI] [PubMed] [Google Scholar]
- 6.Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: A systematic review. Laryngoscope 2015; 125:1547–56. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya N, Villeneuve S, Joish VN, Amand C, Mannent L, Amin N, et al. Cost burden and resource utilization in patients with chronic rhinosinusitis and nasal polyps. Laryngoscope 2019; 129:1969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilos DL. Chronic Rhinosinusitis in Patients with Cystic Fibrosis. J Allergy Clin Immunol Pract 2016; 4:605–12. [DOI] [PubMed] [Google Scholar]
- 9.Tyler MA, Luong AU. Current understanding of allergic fungal rhinosinusitis. World J Otorhinolaryngol Head Neck Surg 2018; 4:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laidlaw TM. Clinical updates in aspirin-exacerbated respiratory disease. Allergy Asthma Proc 2019; 40:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White AA, Stevenson DD. Aspirin-Exacerbated Respiratory Disease. N Engl J Med 2018; 379:2281–2. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin MR, Stevens WW, Li N, Bose S, Grammer LC, Kern RC, et al. Clinical Characteristics of Patients with Chronic Rhinosinusitis without Nasal Polyps in an Academic Setting. J Allergy Clin Immunol Pract 2019; 7:1010–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394:1638–50. [DOI] [PubMed] [Google Scholar]
- 14.Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol 2017; 140:1024–31 e14. [DOI] [PubMed] [Google Scholar]
- 15.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 2013; 131:110–6 e1. [DOI] [PubMed] [Google Scholar]
- 16.Gevaert P, Bachert C, Corren J, Mullol J, Han JK, Ow R, et al. Omalizumab efficacy and safety in nasal polyposis: results from two parallel double-blind placebo-controlled trials. Ann Allergy Asthma Immunol 2019; 123:Supplement, S1–S168. [Google Scholar]
- 17.Schleimer RP. Immunopathogenesis of Chronic Rhinosinusitis and Nasal Polyposis. Annu Rev Pathol 2017; 12:331–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao PP, Wang ZC, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol 2019; 122:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 2015; 348:aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbas AK, Lichtman AH, Pillai S. Differentiation and Functions of CD4+ Effector T cells In: Cellular and Molecular Immunology. 9th ed. Philadelphia, PA: Saunders; 2018. p. 225–42. [Google Scholar]
- 21.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med 2015; 192:682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006; 61:1280–9. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Novo CA, Watelet JB, Claeys C, Van Cauwenberge P, Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol 2005; 115:1189–96. [DOI] [PubMed] [Google Scholar]
- 24.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol 2008; 121:1435–41, 41, e1–3. [DOI] [PubMed] [Google Scholar]
- 25.Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One 2014; 9:e97581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SJ, Kim TH, Jun YJ, Lee SH, Ryu HY, Jung KJ, et al. Chronic rhinosinusitis with polyps and without polyps is associated with increased expression of suppressors of cytokine signaling 1 and 3. J Allergy Clin Immunol 2013; 131:772–80. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol 2008; 122:961–8. [DOI] [PubMed] [Google Scholar]
- 28.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol 2016; 137:1449–56 e4. [DOI] [PubMed] [Google Scholar]
- 29.Tan BK, Klingler AI, Poposki JA, Stevens WW, Peters AT, Suh LA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol 2017; 139:699–703 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyler MA, Russell CB, Smith DE, Rottman JB, Padro Dietz CJ, Hu X, et al. Large-scale gene expression profiling reveals distinct type 2 inflammatory patterns in chronic rhinosinusitis subtypes. J Allergy Clin Immunol 2017; 139:1061–4 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol 2016; 138:1344–53. [DOI] [PubMed] [Google Scholar]
- 32.Mahdavinia M, Suh LA, Carter RG, Stevens WW, Norton JE, Kato A, et al. Increased noneosinophilic nasal polyps in chronic rhinosinusitis in US second-generation Asians suggest genetic regulation of eosinophilia. J Allergy Clin Immunol 2015; 135:576–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations Between Inflammatory Endotypes and Clinical Presentations in Chronic Rhinosinusitis. J Allergy Clin Immunol Pract 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Gevaert E, Lou H, Wang X, Zhang L, Bachert C, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol 2017; 140:1230–9. [DOI] [PubMed] [Google Scholar]
- 35.Seshadri S, Rosati M, Lin DC, Carter RG, Norton JE, Choi AW, et al. Regional differences in the expression of innate host defense molecules in sinonasal mucosa. J Allergy Clin Immunol 2013; 132:1227–30 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner JH, Chandra RK, Li P, Bonnet K, Schlundt DG. Identification of clinically relevant chronic rhinosinusitis endotypes using cluster analysis of mucus cytokines. J Allergy Clin Immunol 2018; 141:1895–7 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morse JC, Li P, Ely KA, Shilts MH, Wannemuehler TJ, Huang LC, et al. Chronic rhinosinusitis in elderly patients is associated with an exaggerated neutrophilic proinflammatory response to pathogenic bacteria. J Allergy Clin Immunol 2019; 143:990–1002 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho SH, Hong SJ, Han B, Lee SH, Suh L, Norton J, et al. Age-related differences in the pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol 2012; 129:858–60 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song WJ, Lee JH, Won HK, Bachert C. Chronic Rhinosinusitis with Nasal Polyps in Older Adults: Clinical Presentation, Pathophysiology, and Comorbidity. Curr Allergy Asthma Rep 2019; 19:46. [DOI] [PubMed] [Google Scholar]
- 40.Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy 2009; 23:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Promsopa C, Kansara S, Citardi MJ, Fakhri S, Porter P, Luong A. Prevalence of confirmed asthma varies in chronic rhinosinusitis subtypes. Int Forum Allergy Rhinol 2016; 6:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin DC, Chandra RK, Tan BK, Zirkle W, Conley DB, Grammer LC, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy 2011; 25:205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakansson K, Bachert C, Konge L, Thomsen SF, Pedersen AE, Poulsen SS, et al. Airway Inflammation in Chronic Rhinosinusitis with Nasal Polyps and Asthma: The United Airways Concept Further Supported. PLoS One 2015; 10:e0127228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee TJ, Fu CH, Wang CH, Huang CC, Huang CC, Chang PH, et al. Impact of chronic rhinosinusitis on severe asthma patients. PLoS One 2017; 12:e0171047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips KM, Bergmark RW, Hoehle LP, Shu ET, Caradonna DS, Gray ST, et al. Differential perception and tolerance of chronic rhinosinusitis symptoms as a confounder of gender-disparate disease burden. Int Forum Allergy Rhinol 2019; 9:1119–24. [DOI] [PubMed] [Google Scholar]
- 46.Sundaresan AS, Hirsch AG, Young AJ, Pollak J, Tan BK, Schleimer RP, et al. Longitudinal Evaluation of Chronic Rhinosinusitis Symptoms in a Population-Based Sample. J Allergy Clin Immunol Pract 2018; 6:1327–35 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope 2011; 121:2262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kountakis SE, Arango P, Bradley D, Wade ZK, Borish L. Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope 2004; 114:1895–905. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama T, Yoshikawa M, Asaka D, Okushi T, Matsuwaki Y, Otori N, et al. Mucosal eosinophilia and recurrence of nasal polyps - new classification of chronic rhinosinusitis. Rhinology 2011; 49:392–6. [DOI] [PubMed] [Google Scholar]
- 50.Weibman AR, Huang JH, Stevens WW, Suh LA, Price CPE, Lidder AK, et al. A prospective analysis evaluating tissue biopsy location and its clinical relevance in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 2017; 7:1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinke JW, Smith AR, Carpenter DJ, Patrie JT, Payne SC, Borish L. Lack of Efficacy of Symptoms and Medical History in Distinguishing the Degree of Eosinophilia in Nasal Polyps. J Allergy Clin Immunol Pract 2017; 5:1582–8 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho J, Hamizan AW, Alvarado R, Rimmer J, Sewell WA, Harvey RJ. Systemic Predictors of Eosinophilic Chronic Rhinosinusitis. Am J Rhinol Allergy 2018; 32:252–7. [DOI] [PubMed] [Google Scholar]
- 53.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015; 70:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drake VE, Rafaels N, Kim J. Peripheral blood eosinophilia correlates with hyperplastic nasal polyp growth. Int Forum Allergy Rhinol 2016; 6:926–34. [DOI] [PubMed] [Google Scholar]
- 55.Guo M, Alasousi F, Okpaleke C, Habib AR, Javer A. Prognosis of Chronic Rhinosinusitis With Nasal Polyps Using Preoperative Eosinophil/Basophil Levels and Treatment Compliance. Am J Rhinol Allergy 2018; 32:440–6. [DOI] [PubMed] [Google Scholar]
- 56.Brescia G, Barion U, Zanotti C, Giacomelli L, Martini A, Marioni G. The prognostic role of serum eosinophil and basophil levels in sinonasal polyposis. Int Forum Allergy Rhinol 2017; 7:261–7. [DOI] [PubMed] [Google Scholar]
- 57.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol 2013; 131:1075–83, 83, e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao PP, Zhang YN, Liao B, Ma J, Wang BF, Wang H, et al. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy 2014; 44:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takeda K, Sakakibara S, Yamashita K, Motooka D, Nakamura S, El Hussien MA, et al. Allergic conversion of protective mucosal immunity against nasal bacteria in patients with chronic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol 2019; 143:1163–75 e15. [DOI] [PubMed] [Google Scholar]
- 60.Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol 2004; 114:981–3. [DOI] [PubMed] [Google Scholar]
- 61.Huvenne W, Hellings PW, Bachert C. Role of staphylococcal superantigens in airway disease. Int Arch Allergy Immunol 2013; 161:304–14. [DOI] [PubMed] [Google Scholar]
- 62.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol 2001; 107:607–14. [DOI] [PubMed] [Google Scholar]
- 63.Jonstam K, Westman M, Holtappels G, Holweg CTJ, Bachert C. Serum periostin, IgE, and SE-IgE can be used as biomarkers to identify moderate to severe chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2017; 140:1705–8 e3. [DOI] [PubMed] [Google Scholar]
- 64.Laidlaw TM, Prussin C, Panettieri RA, Lee S, Ferguson BJ, Adappa ND, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope 2019; 129:E61–E6. [DOI] [PubMed] [Google Scholar]


