Abstract
Objective
We aimed to assess the association between migraine headache and incident dementia.
Background
Migraine is a risk factor for white matter hyperintensities and ischemic stroke, which are both associated with increased risk of dementia. However, it is unknown whether migraine is independently associated with dementia.
Methods
History of migraine was ascertained via questionnaire. Adjudicated cases of dementia were identified using cognitive tests, neuropsychological exams, and clinician review of suspected cases. Incident dementia was identified using adjudicated cases, follow-up calls, and surveillance of hospital and death codes. We assessed hazards of incident dementia by migraine status. Sex differences were also examined and stratified results were presented.
Results
Analysis included 12,495 White and African American participants ages 51–70 with a median follow-up time of 21 years. Prevalence of dementia was 18.5% (1821/9955) among those with no migraine history, 15.8% (196/1243) among those with severe non-migraine heading, and 16.7% (233/1397) among migraineurs. There was no association between migraine and incident dementia [hazard ratio: 1.04 (0.91, 1.20)]. There was also no statistically significant interaction between sex and migraine status on risk of dementia.
Conclusion
Despite evidence of brain abnormalities in migraineurs, there was no association between migraine and incident dementia in this prospective cohort.
Keywords: epidemiology, dementia, headache, migraine
BACKGROUND
Migraine headache is a complex neurological disorder characterized by throbbing, severe, and typically unilateral pain in the head.1 Among those ages 12 and older, prevalence is 6.5% in men and 18.2% in women and noted to cluster in families.2–4 Prevalence peaks between the ages of 30 and 39 before falling to reach the lowest prevalence in those 60 and older.5
The significant vascular component in migraine6 has led to interest in the connection between migraine, stroke, and cognitive decline. Several studies have found migraineurs to be at increased risk of ischemic stroke with a pooled relative risk (95% CI) of 1.7 (1.3, 2.3); this association was significant in women [RR (95% CI): 2.1 (1.1, 3.8)], but not in men [RR (95% CI): 1.4 (0.9, 2.1)].7 In the Atherosclerosis Risk in Communities (ARIC) study, as with other cohorts,8 a history of migraine symptoms was associated cross-sectionally with cerebral white matter hyperintensities.9 In addition, a recent review concluded that a history of migraine was associated with increased odds of white matter abnormalities, subclinical infarct-like lesions, and volumetric changes in the brain.10 Stroke, white matter hyperintensities, silent infarcts, and volumetric brain changes are all associated with increased risk of cognitive impairment, suggesting migraine may be a risk factor for cognitive decline and dementia.11
Despite these prior published reports suggesting a potential link, few studies have evaluated the association between migraine and risk of developing dementia.11–19 The majority of studies assessing this relationship were small, had short or retrospective follow-up periods, and measured cognitive decline instead of dementia as an outcome. Further, results from these studies have been mixed, and to our knowledge, no studies in U.S. cohorts have assessed the prospective association between migraine and dementia.
Our aim was to examine the association between migraine status (based on self-reported symptom history) and incident dementia hypothesizing that those who experienced migraine would be at increased risk of dementia compared to those who did not. Our second aim was to examine potential interaction by sex on the association of migraine with incident dementia, hypothesizing women with history of migraine would be at increased risk of dementia compared to men with a history of migraine.
METHODS
ARIC is a prospective cohort study that enrolled 15,792 primarily White and African American participants ages 45–64 at baseline (1987–1989) from Forsyth County, North Carolina, Jackson, Mississippi, the northwest suburbs of Minneapolis, Minnesota, and Washington County, Maryland.20 After IRB approval and written informed consent, ARIC followed participants continuously for hospitalization and mortality. ARIC also completed 6 clinic visits from 1987 to 2017, as well as cognitive assessments, which were integrated into regular clinic visits as part of the ARIC Neurocognitive Study (ARIC-NCS). For this analysis, baseline started at ARIC visit 3 (1993–1995) and cognitive status was ascertained through visit 6 (2016–2017). Participants were excluded if they did not attend visit 3 (n = 2886), were missing migraine status based on self-reported headache symptoms (n = 57), had prevalent stroke (n = 245), prevalent dementia (identified via ICD codes) (n = 6), or were non-White or African American or were African American from Maryland or Minnesota (n = 103) (due to small numbers), for a final sample of 12,495 participants.
Migraine status, our exposure of interest, was assessed via a questionnaire adapted from the International Headache Society (IHS) diagnostic criteria21 and administered at ARIC visit 3 (Supporting Table S1). The questionnaire assessed participants’ lifetime history of migraine symptoms, and similar IHS-based questionnaires have been validated in population-based studies.22–24 Two population-based studies using questionnaires adapted from the IHS diagnostic criteria, from which the ARIC questionnaire was derived, confirmed the validity and reliability of using abbreviated diagnostic criteria to accurately identify migraineurs estimating Cohen’s kappa coefficients ranging from 0.43 to 0.68. Migraine was defined as: (1) headache lasting 4 or more hours; (2) headache with throbbing, pounding, or pulsating pain, or that was unilateral; (3) symptoms of nausea, vomiting, or sensitivity to light or sound; and (4) one or more years with history of headaches.9 Those who reported headache lasting 4 or more hours, but did not meet all the other criteria for migraine were defined as suffering from severe non-migraine headache, and participants who denied having a headache lasting 4 or more hours were classified as having no headache.12 Participants meeting the definition of migraine were defined as having aura if they reported the occurrence of visual aura (eg, spots, jagged lines, etc.).12
Covariates included age, sex, race-center (MS-African Americans, NC-Whites, NC-African Americans, MN-Whites, and MD-Whites), APOE ε4, income, and education from visit 1, and body mass index (BMI), smoking status, hypertension, diabetes, prevalent coronary heart disease (CHD), drinking status, high-density lipoprotein (HDL) cholesterol, and total cholesterol from visit 3. BMI was calculated from measured weight and height. Hypertension was defined as having a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self-report of antihypertensive medication use. Diabetes was defined as non-fasting glucose ≥200 mg/dL, fasting blood glucose ≥126 mg/dL, self-report of diabetes diagnosis from a physician, or report of taking medication for diabetes or high blood sugar. Prevalent CHD was ascertained via hospital surveillance and self-report with clinical events adjudicated by a panel of experts.25 These covariates were identified a priori as potential confounders on the association between migraine and dementia. Model 1 was minimally adjusted, including demographics and APOE ε4. All of these factors are (relatively) stable across time. Model 2 additionally adjusted for behaviors (ie, smoking status, drinking status) and physiologic characteristics that may be associated with both migraine prevalence and dementia development (ie, BMI, smoking status, HDL cholesterol, total cholesterol, and prevalent hypertension, diabetes, and CHD).
The outcome of interest, incident dementia, was ascertained several ways. Adjudicated cases were primarily identified using data from ARIC-NCS clinic examinations conducted at visits 5 (2011–2013) and 6 (2016–2017).26 This included a neuropsychological battery using standardized protocols with scores converted to z-scores in order to assess change over time. Cognitively impaired participants were identified as those with significant decline or poor test performance. These participants, as well as a random sample of cognitively normal participants, were given additional physical and neurological exams including brain magnetic resonance imaging, and their informants were interviewed using the Clinical Dementia Rating scale and the Functional Activities Questionnaire.26 Using all the cognitive data available, suspected cases were then adjudicated by a committee of clinicians.26 For participants who could not attend visits 5 and 6, additional dementia cases were identified via surveillance of hospital discharge and death certificate codes related to dementia as well as screening during annual and semi-annual follow-up calls. Informant interviews for deceased participants suspected to have had dementia and telephone-based cognitive assessments were used to help identify these cases. Our analysis included all incident dementia cases available in ARIC between visits 3 and 6 identified through adjudication of cases in ARIC-NCS, surveillance of hospital and death records, and telephone interviews for cognitive status.
Statistical Analysis
Statistical analyses, including sensitivity analyses, were planned prior to accessing the data and reviewed by the ARIC Steering Committee (submission approval: July 2018). Poisson regression was used to estimate incidence of dementia between visits 3 (1993–1995) and 6 (2016–2017) stratified by headache subtype (migraine, severe non-migraine headache, no headache) and sex. We then evaluated the association between self-reported lifetime history of migraine symptoms with risk of dementia using Cox regression. We calculated hazard of dementia stratified by headache subtype with no headache as the reference. The association between migraine with aura (MWA) and dementia was assessed by repeating the Cox regression using an added “migraine with aura” headache subtype. To determine whether there was effect modification on the association between migraine and dementia by sex, a multiplicative sex by migraine interaction term was tested in the Cox models and sex-stratified results were presented.
We tested 2 models with adjustment for baseline (visit 3) covariates. Model 1 was adjusted for potential confounders of age, race-center, APOE ε4, income, and education. Model 2 was adjusted for model 1 covariates in addition to potential confounders of BMI, smoking status, hypertension, diabetes, prevalent CHD, alcohol drinking status, HDL cholesterol, and total cholesterol. All hypothesis testing was done using a 2-tailed alpha to test a significance level of 0.05. To test the proportional hazards assumption, models were run with a migraine status*log follow-up time interaction term, and the assumption was met. Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
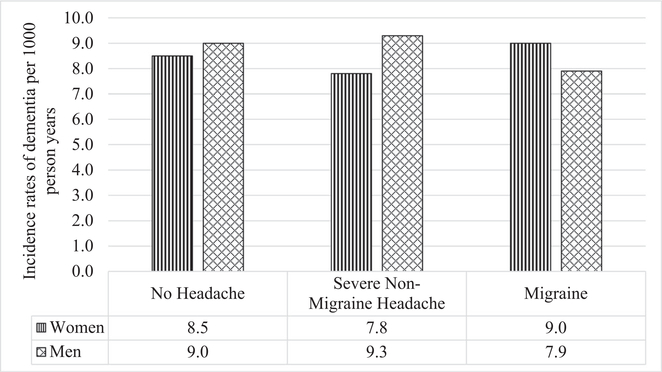
After exclusions, analysis included 12,495 participants with a mean age of 60 and median follow-up time of 21 years. Through follow-up, cumulative incidence of dementia was 18.5% (1821/9955) among those with no migraine history, 15.8% (196/1243) among those with severe non-migraine heading, and 16.7% (233/1397) among migraineurs. Participants who reported experiencing migraine symptoms were more likely to be younger, White, female, and had higher HDL and total cholesterol than those who reported no headache symptoms or severe non-migraine headache symptoms (Table 1). In addition, migraineurs were less likely to have hypertension, diabetes, or be current alcohol drinkers. There was no statistically significant difference in the overall incidence of dementia between those who experienced migraine symptoms compared to those who experienced severe non-migraine headaches or no headaches between visits 3 and 6 (Fig. 1). There was also no statistically significant interaction by sex on the association between migraine status and dementia.
Table 1.—
Baseline Participant Characteristics Stratified by Migraine Status, ARIC, 1993–1995
| No Headache | Severe Non-Migraine Headache | Migraine | |
|---|---|---|---|
| Characteristic | n Total = 9855 | n Total = 1243 | n Total = 1397 |
| Age, years | 60.4 ± 5.7 | 58.7 ± 5.5 | 58.3 ± 5.5 |
| African American, % | 24.7 | 15.3 | 14.5 |
| Male, % | 48.2 | 36.0 | 22.1 |
| ApoE4 carriers, % | 29.3 | 27.0 | 29.3 |
| Basic education*, % | 20.8 | 15.5 | 18.5 |
| Family income <$16,000†, % | 23.9 | 19.2 | 23.4 |
| Current alcohol drinker, % | 52.5 | 53.9 | 49.5 |
| Current tobacco smoker, % | 18.0 | 16.4 | 16.7 |
| BMI, kg/m2 | 28.6 ± 5.6 | 27.9 ± 5.3 | 28.4 ± 5.9 |
| Hypertension‡, % | 41.7 | 36.1 | 35.9 |
| Diabetes§, % | 15.9 | 12.4 | 11.6 |
| HDL cholesterol, mg/dL | 51.7 ± 18.2 | 53.9 ± 18.4 | 55.1 ± 18.3 |
| Total cholesterol, mg/dL | 207.0 ± 37.4 | 207.0 ± 37.9 | 211.6 ± 39.3 |
Mean ± standard deviation.
Based on self-report of some high school education or less at visit 1.
Based on self-report of income at visit 1 (1987–1989).
Defined as diastolic blood pressure ≥90 mm Hg, systolic blood pressure ≥140 mm Hg, or use of hypertensive medication.
Defined as non-fasting blood glucose ≥200 mg/dL, fasting blood glucose ≥ 126 mg/dL, self-report of diabetes, or reporting taking medication for diabetes or high blood sugar.
Fig. 1.—
Incidence rates of dementia adjusted for age and stratified by baseline headache subtype and sex, ARIC, 1993–2017.
Those who experienced migraine symptoms at baseline did not have a significantly increased hazard of incident dementia [HR (95% CI): 1.04 (0.91, 1.20)] after full adjustment for model 2 covariates compared to those with no history of headache symptoms (Table 2). There was also no significant association between lifetime history of MWA symptoms and hazard of dementia after full adjustment [HR (95% CI): 1.12 (0.88, 1.43)]. Severe non-migraine headache was also not associated with increased risk of dementia compared to no headache. Associations did not change between model 1 and model 2 covariate adjustments.
Table 2.—
Hazard Ratios (95% CI) of Dementia in Relation to Baseline Headache Status, ARIC 1993–2017
| No Headache | Severe Non-Migraine Headache | Migraine | ||
| n Total = 9855 | n Total = 1243 | n Total = 1397 | ||
| n Events = 1821 | n Events = 196 | n Events = 233 | ||
| Model 1 HR | 1 (Ref) | 1.00 (0.86, 1.16) | 1.02 (0.89, 1.17) | |
| Model 2 HR | 1 (Ref) | 1.00 (0.87, 1.17) | 1.04 (0.91, 1.20) | |
| No Headache | Severe Non-Migraine Headache | Migraine Without Aura | Migraine With Aura | |
| n Total = 9855 | n Total = 1243 | n Total = 992 | n Total = 405 | |
| n Events = 1821 | n Events = 196 | n Events = 165 | n Events = 68 | |
| Model 1 HR | 1 (Ref) | 1.00 (0.86, 1.16) | 0.99 (0.84, 1.16) | 1.11 (0.87, 1.42) |
| Model 2 HR | 1 (Ref) | 1.00 (0.87, 1.17) | 1.01 (0.86, 1.19) | 1.12 (0.88, 1.43) |
Model 1: age, sex, race-center, APOE ε4, income, and education.
Model 2: Model 1 + BMI, smoking status, hypertension, diabetes, prevalent CHD, drinking status, HDL cholesterol, and total cholesterol.
CI = confidence interval; HR = hazard ratio.
We did not find a statistically significant interaction between sex and migraine status on risk of dementia (Table 3). The associations between migraine status and risk of dementia were similarly null for men and women.
Table 3.—
Hazard Ratios (95% CI) of Dementia in Relation to Baseline Headache Status and Sex, ARIC 1993–2017
| Women (n = 6988) | Men (n = 5507) | |||||
|---|---|---|---|---|---|---|
| No Headache | Severe Non-Migraine Headache | Migraine | No Headache | Severe Non-Migraine Headache | Migraine | |
| n Total = 5105 | n Total = 795 | n Total = 1088 | n Total = 4750 | n Total = 375 | n Total = 309 | |
| n Events = 1012 | n Events = 123 | n Events = 190 | n Events = 809 | n Events = 73 | n Events = 43 | |
| Model 1 HR | 1 (Ref) | 0.96 (0.80, 1.16) | 1.09 (0.93, 1.28) | 1 (Ref) | 1.09 (0.85, 1.38) | 0.82 (0.60, 1.11) |
| Model 2 HR | 1 (Ref) | 0.97 (0.80, 1.17) | 1.13 (0.96, 1.32) | 1 (Ref) | 1.09 (0.85, 1.39) | 0.79 (0.58, 1.08) |
Model 1: age, race-center, APOE ε4, income, and education.
Model 2: Model 1 + BMI, smoking status, hypertension, diabetes, prevalent CHD, drinking status, HDL cholesterol, and total cholesterol.
CI = confidence interval; HR = hazard ratio.
DISCUSSION
This large population-based prospective study found no association between history of migraine symptoms and risk of dementia. This lack of association was found in both men and women. While migraine is associated with cerebrovascular lesions, migraine-related lesions are reported to be stable over time and may not contribute to dementia pathophysiology later in life.9
There were several limitations to this analysis. Despite the size of our cohort, power was somewhat limited. A hazard ratio of approximately 1.4 or greater would have been needed to detect an effect with 80% power and a type I error rate of 0.5. Thus, this study was not powered to detect weak associations between lifetime history of migraine symptoms and dementia. In addition, there was potential selection bias due to attrition and misclassification of the dementia cases that were not examined directly. However, ARIC used a variety of strategies to prevent attrition and identify possible dementia cases among participants who did not attend all clinic visits including surveillance of hospital and death records as well as follow-up telephone interviews. Additionally, a sensitivity analysis was done incorporating inverse probability of attrition weights and associations (or lack thereof) did not differ (results not shown).
There was potential for misclassification of migraine status due to reliance on a single self-report of lifetime history of migraine symptoms measured when participants ranged in age from 51 to 70 years.5 Participants were past the peak ages of migraine prevalence (30–39 years old), and cases may have been missed due to recall bias leading to misclassification of the exposure, which, in turn, may have biased effect estimates. However, 2 population-based studies using shortened questionnaires adapted from the IHS diagnostic criteria, have reported that the validity and reliability of using abbreviated diagnostic criteria to assess migraine is reasonable.22,23 Additionally, the symptoms associated with migraine, including unilateral, severe pain, prodromes, and aura, would not likely be forgotten and should be easily distinguishable from a severe non-migraine headache in most cases. By asking participants about their lifetime migraine history after prevalence peaked in young adulthood and middle age, it is less likely migraine cases that developed later in life were missed while also ensuring temporality (ie, that migraine was assessed before dementia). Further, because migraine in ARIC is associated with increased stroke risk,27 as in other studies,7,28,29 the validity of the ARIC migraine classification is likely adequate to ascertain meaningful associations. Finally, we were unable to account for migraine burden. We did not have information on age of onset, duration, frequency, or severity of migraines. Migraine headache often begins around puberty. This study cannot address whether age of onset, number, and duration of migraines over one’s lifetime might have a differential effect dementia risk.
In summary, we found no association between history of migraine headache and incident dementia in ARIC. While there is evidence that migraine is associated with brain alterations that have been linked to cognitive changes, these alterations may not be clinically meaningful or they may resemble white matter hyperintensities associated with vascular disease, but have a different underlying pathophysiologic process.9–11 Additional research with a larger sample size, greater power, and more extensive migraine history is warranted to investigate the possible association between migraine and dementia.
Supplementary Material
Acknowledgments
We would like to thank Sheila Burgard at the Collaborative Studies Coordinating Center at the University of North Carolina Gillings School of Global Public Health, Chapel Hill, NC for technical data assistance. Dr. George is supported by National Heart, Lung, and Blood Institute (NHLBI) Training Grant T32HL007779. Dr. Hamedani is supported by NIH NINDS T32 NS061779-10. Dr. Gottesman is supported by NIH grant K24 AG052573. The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA, and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. The authors thank the staff and participants of the ARIC study for their important contributions.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CHD
coronary heart disease
- HDL
high-density lipoprotein
- IHS
International Headache Society
- NCS
neurocognitive study
Footnotes
Conflict of Interest: None
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web site.
Contributor Information
Kristen M. George, Division of Epidemiology, Department of Public Health Sciences, University of California Davis Medical Center, Davis, CA, USA; Division of Epidemiology and Community Health, University of Minnesota School of Public Health, Minneapolis, MN, USA.
Aaron R. Folsom, Division of Epidemiology and Community Health, University of Minnesota School of Public Health, Minneapolis, MN, USA.
A. Richey Sharrett, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Thomas H. Mosley, Memory Impairment and Neurodegenerative Dementia (MIND) Center, University of Mississippi Medical Center, Jackson, MS, USA.
Rebecca F. Gottesman, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA; Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ali G. Hamedani, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Pamela L. Lutsey, Division of Epidemiology and Community Health, University of Minnesota School of Public Health, Minneapolis, MN, USA.
REFERENCES
- 1.Goadsby PJ, Lipton RB, Ferrari MD. Migraine – Current understanding and treatment. N Engl J Med. 2002;346:257–270. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–69. [PubMed] [Google Scholar]
- 3.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache. 2001;41:646–657. [DOI] [PubMed] [Google Scholar]
- 4.Cutrer FM, Smith JH. Human studies in the pathophysiology of migraine: Genetics and functional neuroimaging. Headache. 2013;53:401–412. [DOI] [PubMed] [Google Scholar]
- 5.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- 6.Silberstein SD. Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24(Suppl. 2):2–7. [DOI] [PubMed] [Google Scholar]
- 7.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: Systematic review and meta-analysis. BMJ. 2009; 339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palm-Meinders IH, Koppen H, Terwindt GM, et al. Structural brain changes in migraine. JAMA. 2012;308:1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamedani AG, Rose KM, Peterlin BL, et al. Migraine and white matter hyperintensities: The ARIC MRI study. Neurology. 2013;81:1308–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bashir A, Lipton RB, Ashina S, Ashina M. Migraine and structural changes in the brain: A systematic review and meta-analysis. Neurology. 2013;81:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paemeleire K Brain lesions and cerebral functional impairment in migraine patients. J Neurol Sci. 2009; 283:134–136. [DOI] [PubMed] [Google Scholar]
- 12.Rist PM, Kurth T. Migraine and cognitive decline: A topical review. Headache. 2013;53:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalaydjian A, Zandi PP, Swartz KL, Eaton WW, Lyketsos C. How migraines impact cognitive function: Findings from the Baltimore ECA. Neurology. 2007;68:1417–1424. [DOI] [PubMed] [Google Scholar]
- 14.Rist PM, Kang JH, Buring JE, Glymour MM, Grodstein F, Kurth T. Migraine and cognitive decline among women: Prospective cohort study. BMJ. 2012; 345:e5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rist PM, Dufouil C, Glymour MM, Tzourio C, Kurth T. Migraine and cognitive decline in the population-based EVA study. Cephalalgia. 2011;31: 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagen K, Stordal E, Linde M, Steiner TJ, Zwart JA, Stovner LJ. Headache as a risk factor for dementia: A prospective population-based study. Cephalalgia. 2014;34:327–335. [DOI] [PubMed] [Google Scholar]
- 17.Kostev K, Bohlken J, Jacob L. Association between migraine headaches and dementia in more than 7400 patients followed in general practices in the United Kingdom. J Alzheimers Dis. 2019;71:353–360. [DOI] [PubMed] [Google Scholar]
- 18.Tzeng NS, Chung CH, Lin FH, et al. Headaches and risk of dementia. Am J Med Sci. 2017;353:197–206. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Lim JS, Oh DJ, Kong IG, Choi HG. Increased risk of neurodegenerative dementia in women with migraines: A nested case-control study using a national sample cohort. Medicine (Baltimore). 2019;98:e14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 21.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 22.Hagen K, Zwart JA, Vatten L, Stovner LJ, Bovim G. Head-HUNT: Validity and reliability of a headache questionnaire in a large population-based study in Norway. Cephalalgia. 2000;20:244–251. [DOI] [PubMed] [Google Scholar]
- 23.Lipton RB, Dodick D, Sadovsky R, et al. A self-administered screener for migraine in primary care: The ID migraine validation study. Neurology. 2003;61:375–382. [DOI] [PubMed] [Google Scholar]
- 24.Martin VT, Penzien DB, Houle TT, Andrew ME, Lofland KR. The predictive value of abbreviated migraine diagnostic criteria. Headache. 2005;45: 1102–1112. [DOI] [PubMed] [Google Scholar]
- 25.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: Methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 26.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive study (ARIC-NCS). Alzheimers Dement (Amst). 2016; 2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stang PE, Carson AP, Rose KM, et al. Headache, cerebrovascular symptoms, and stroke: The Atherosclerosis Risk in Communities study. Neurology. 2005;64:1573–1577. [DOI] [PubMed] [Google Scholar]
- 28.Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischaemic stroke in people with migraine: Systematic review and meta-analysis of observational studies. BMJ. 2005;330:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol. 2005;4:533–542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.