Abstract
About 11% of the adult global population is estimated to be living with type 2 diabetes mellitus (T2DM) by 2040. T2DM requires people to make decisions regarding complex therapeutic regimes, to maintain their well-being and quality of life, to manage symptoms and to reduce disease complications. All these behaviours, requiring knowledge, motivation, experience, and skills, have been referred to as the concept of self-care. The intricacy and multidimensionality of T2DM self-care requires a complex approach to its overall comprehension. This Embedded Mixed Method study aims to investigate the experience of self-care in Type 2 Diabetes Mellitus adult patients. It comprises a prospective observational design, and an interpretive description. Quantitative data will be collected with validated questionnaires from 300 patients at baseline and once a year for two years on: diabetes self-care, quality of life, diabetes related distress, and sleep quality. Socio-demographic and clinical data will be collected from medical records. Qualitative data will be collected using semi-structured interviews on circa 10-20 patients, at baseline and once a year for two years, and will be analysed according to interpretive description. Quantitative and qualitative data will be analysed separately and then merged and interpreted. This study will expand our understanding of self-care in people with T2DM. The expected outcome will be a better understanding of the effect of self-care on glycaemic control and therefore clinical outcomes and costs.
Keywords: Type 2 Diabetes Mellitus, Self-care, Mixed Method Research, Research Protocol, Self-management, Self-care of Diabetes Inventory
Abstract
Si stima che entro il 2040 circa l’11% della popolazione mondiale sarà affetta da diabete mellito di tipo 2 (T2DM). Il T2DM richiede che le persone che ne sono affette prendano decisioni rispetto a complessi regimi terapeutici, per mantenere il loro benessere e qualità di vita, per gestire sintomi e ridurre le complicanze legate alla malattia. Tutti questi comportamenti che richiedono conoscenze, motivazione, esperienza e abilità, vengono identificate col concetto di self-care. La complessità e la multidimensionalità del self-care richiedono un approccio adeguato alla loro comprensione. Questo studio mixed method ha lo scopo di esplorare l’esperienza del self-care nelle persone affette da diabete mellito di tipo 2. Comprende uno studio osservazionale longitudinale e una interpretive description. I dati quantitativi verranno raccolti attraverso la somministrazione a 300 persone, all’inizio dello studio e poi una volta all’anno per due anni, di questionari validati circa: il self-care nel diabete, la qualità di vita, il distress legato al diabete, la qualità del sonno. Dati socio-demografici e clinici verranno raccolti attraverso la consultazione delle cartelle cliniche. I dati qualitativi, raccolti attraverso interviste semi-strutturate con circa 10-20 pazienti, all’inizio dello studio e poi una volta all’anno per due anni, verranno analizzati secondo l’interpretive description. I dati quantitativi e qualitativi verranno analizzati separatamente, dopodiché uniti ed interpretati. Questo studio vuole aumentare la nostra comprensione del self-care nelle persone con T2DM. L’outcome atteso sarà una migliore comprensione del self-care e dell’effetto del self-care sul controllo glicemico e quindi sugli outcome clinici ed i costi.
Keywords: Diabete mellito di tipo 2, Self-care, metodi misti, protocollo di ricerca, Self-management, Self-care of Diabetes Inventory
INTRODUCTION
Diabetes affects 629 million people worldwide and, in 2017, the number of deaths directly due to diabetes was 4 million. About 90% of diabetes mellitus cases are type 2 diabetes mellitus (T2DM) (International Diabetes Federation, 2017). T2DM complications, such as stroke, heart attack, kidney failure, amputation, and blindness, lead to a poor quality of life and rising health care costs (Handelsman et al., 2015; International Diabetes Federation, 2017; Schunk et al., 2012; Scollan-Koliopoulos et al., 2013; World Health Organization, 2016). Collectively, T2DM complications account for up to 90% of diabetes health care expenditures (International Diabetes Federation, 2017) and costs are projected to rise (International Diabetes Federation, 2017). Cost effective strategies are critically needed to reduce T2DM complications. Without such strategies, people with T2DM will remain at high risk for a poor quality of life and T2DM will exact an increasing toll on the health care system.
Self-care is “a process of maintaining health through health promoting practices and managing illness” (Riegel, Jaarsma, & Strömberg, 2012). Self-care is able to reduce co-morbidities, to improve quality of life, and to reduce health care costs (Barlow, Wright, Sheasby, Turner, & Hainsworth, 2002; Powers et al., 2015; Riegel et al., 2012; Shrivastava, Shrivastava, & Ramasamy, 2013). Self-care requires knowledge, motivation, experience, and skills (Ausili, Masotto, Dall’Ora, Salvini, & Di Mauro, 2014; Baumann & Dang, 2012; Godfrey et al., 2011; Ryan & Sawin, 2009). Self-care is affected by habits, support from others, and access to care, as well as cultural beliefs and values (Riegel et al., 2012). Several studies have examined self-care in persons with T2DM (Holt et al., 2013; Kovacs Burns et al., 2013; Nicolucci et al., 2014; Peyrot et al., 2013) . Self-care effectively reduced glycated haemoglobin (HbA1c), hospitalisation and BMI in people with T2DM (Song, Ratcliffe, Tkacs, & Riegel, 2012; Ouyang et al., 2015; Ausili, Bulgheroni, et al., 2017). But these studies are limited by cross-sectional study designs, use of non-validated measurement tools , omission of potentially influential variables, lack of culturally diverse groups, and lack of a theoretical background guiding the study (Caro-Bautista, Martín-Santos, & Morales-Asencio, 2014; Lu, Xu, Zhao, & Han, 2016; Riegel & Weaver, 2009). Moreover, relationships between self-care, T2DM, and health care utilisation costs are understudied. Therefore, the overall purpose of this mixed method study is to describe and measure self-care and its experience in adults with T2DM.
Conceptual framework
This study is guided by the “Middle Range Theory of Self-Care of Chronic Illness”, developed by Riegel and colleagues (Riegel et al., 2012) (Table 1). According to this theory, self-care comprises self-care maintenance, self-care monitoring, and self-care management. Self-care maintenance includes “those behaviours used by patients with a chronic illness to maintain physical and emotional stability” (Riegel et al., 2012, p. 195). Self-care maintenance consists of the recommendations and advice both from providers and significant others, as well as a person’s adherence to treatment regimes. Self-care maintenance is improved by the ability of persons to reflect on and adapt to situations. Self-care monitoring is “the process of observing oneself for changes in signs and symptoms” (Riegel et al., 2012, p. 196). The objective of self-care monitoring is to recognise physiological changes. Self-care monitoring is influenced by one’s personal insight and interpersonal awareness. Self-care monitoring ignites the process of decision-making and, therefore, self-care management. Self-care management is a “response to signs and symptoms when they occur” (Riegel et al., 2012, p. 196). Once individuals recognise a change, they need to evaluate it and act accordingly. This requires simulating options in one’s mind, deciding the course of action, and evaluating its effectiveness. Consultation with health care providers may be required during self-care management (Riegel et al., 2012) . Self-care maintenance, self-care monitoring, and self-care management interact and function synchronously. “Mastery of self-care maintenance precedes mastery of self-care management” (Riegel et al., 2012, p. 200) due to the complexity of the latter. “Self-care monitoring is necessary for effective self-care management because one cannot make a decision about a change unless it has been noticed and evaluated” (Riegel et al., 2012, p. 200).
Table 1.
Antecedents and consequences of self-care in T2DM.
| Antecedents | Self-care | Consequences |
|---|---|---|
| Socio-demographic characteristics Experience and skill Functional and cognitive abilities Support from others Self-care Confidence Distress Sleep quality |
Self-care Maintenance Self-care Monitoring Self-care Management |
Hb1Ac Complications Quality of life Hospitalization Costs Mortality |
Several factors influence self-care (Table 1), including socio-demographic characteristics, experience and skills, functional and cognitive abilities, support from others, confidence, distress, and sleep quality. Self-care confidence underlies these factors and mediates cognitive abilities and self-care behaviours (Riegel & Dickson, 2008; Vellone et al., 2015; Vellone, Pancani, Greco, Steca, & Riegel, 2016; Jaarsma, Cameron, Riegel, & Strömberg, 2017; Ausili, Barbaranelli, et al., 2017). Self-care influences illness stability, quality of life, mortality, and health care utilization. Factors not included in the “Middle Range Theory of Self-Care” (Riegel et al., 2012), but relevant for this study include socio-demographic characteristics and sleep quality.
Antecedents
Socio-demographic characteristics.
Several socio-demographic characteristics influence T2DM such as: social support, gender, education and income (Clark & Utz, 2014; Glasgow & Toobert, 1988; Hill et al., 2013; Lidfeldt et al., 2003; Schub & Smith, 2016).
Experience and skill.
Experience is needed to develop skills that are essential for self-care. These skills include recognizing patterns, setting goals, making decisions, and predicting outcomes (Paterson, Thorne, & Dewis, 1998; Riegel et al., 2012).
Functional and Cognitive Abilities.
Functional abilities that impact self-care behaviours include difficulties with hearing, vision, manual dexterity, and energy (Riegel et al., 2012) . Impairments in these functional abilities may particularly impact elderly adults with diabetes (Hiltunen, Keinänen-Kiukaanniemi, Läärä, & Kivelä, 1996). Cognitive abilities that impair self-care (Riegel et al., 2012) include attention, awareness, memory, decision making, perception, planning, reasoning, judgement, language, general knowledge, visuospatial, and intuition (Cognitive Deficits, 2017). Cross-sectional studies have demonstrated that poorer cognitive abilities reduce self-care (Sinclair, Girling, & Bayer, 2000; Feil, Zhu, & Sultzer, 2012; Primožič, Tavčar, Avbelj, Dernovšek, & Oblak, 2012; Wykes, Lee, McKibbin, & Laurent, 2016). Prospective studies are needed to elucidate the direction of this relationship (Tomlin & Sinclair, 2016).
Support from Others.
Support from others can impede or facilitate self-care. More support is associated with improved self-care behaviours in some, but not all studies (Badedi et al., 2016; Bohanny et al., 2013; van Dam et al., 2005). Relationships between support and self-care may vary by gender or support type, such as formal versus informal. Consensus for social support measures and prospective study designs are needed to improve our understanding of these relationships (Stopford, Winkley, & Ismail, 2013).
Confidence.
It is the “confidence one has in the ability to perform a specific action and to persist in performing that action despite barriers” (Riegel et al., 2012, p. 201). Confidence is called self-care confidence when actions performed are related to self-care (Riegel & Dickson, 2008; Vellone et al., 2015, 2016; Jaarsma et al., 2017). It has been strongly correlated with self-care behaviours in T2DM (Aljasem, Peyrot, Wissow, & Rubin, 2001; Gherman et al., 2011; Bohanny et al., 2013; Adam & Folds, 2014; Badedi et al., 2016; Devarajooh & Chinna, 2017).
Distress.
Distress is defined as “an emotional response towards adverse or unpleasant stressors” (Snoek, Bremmer, & Hermanns, 2015, p. 452). Chronic illnesses impact distress and challenge coping strategies (Riegel et al., 2012; Snoek et al., 2015). T2DM distress is rooted in the demands of diabetes management and it is a product of emotional adjustment (Berry, Lockhart, Davies, Lindsay, & Dempster, 2015). T2DM distress is linked to self-care but it is uncertain how distress interacts with T2DM self-care (Berry et al., 2015; Devarajooh & Chinna, 2017; Snoek et al., 2015).
Sleep quality.
Sleep quality refers to an individual’s assessment of their sleep as ‘good’ or ‘poor’ (Daniel J. Buysse, 2014) . Sleep quality indicators include the ease of falling asleep, number of nocturnal awakenings, time spent awake after sleep onset, and the ratio of time in bed to time asleep. T2DM increases the risk for poor sleep quality (Foster et al., 2009; Lopes et al., 2005). Sleep quality is associated with quality of life (Chasens, Korytkowski, Sereika, & Burke, 2013; Lou et al., 2015; Marcum et al., 2013) and antecedents of self-care, such as self-care confidence, functional and cognitive abilities, and distress (Byun, Kim, & Riegel, 2017; Chasens et al., 2013; Seligowski et al., 2013). Prospective studies are needed to elucidate the direction of these associations.
Consequences
Hb1Ac.
HbA1c has a key role in clinical assessment of a patient’s metabolic control. HbA1c has been validated as a surrogate endpoint of microvascular complications associated with diabetes (FDA-NIH Biomarker Working Group, 2017) and is associated with T2DM control (Badedi et al., 2016).
Complications, hospitalisation, mortality, and costs.
Self-care behaviours have been linked to illness stability and reduced T2DM complications (Al-Khawaldeh, Al-Hassan, & Froelicher, 2012; Austin Boren, Gunlock, Schaefer, & Albright, 2007; Badedi et al., 2016; Powers et al., 2015; Riegel et al., 2012). Fewer T2DM complications leads to reduced hospitalisation and mortality, and therefore, costs (Haw, Tantry, Vellanki, & Pasquel, 2015; Powers et al., 2015).
Quality of Life.
Quality of Life is a complex multidimensional construct which includes the subjective perception of physical, emotional and social well-being (Rubin & Peyrot, 1999). A better quality of life is associated with improved self-care activities (D’Souza, Ruppert, Karkada, & Jacob, 2015; Timar et al., 2016), and adherence to self-care practices and medications (Saleh, Mumu, Ara, Hafez, & Ali, 2014) . Comorbidities (O’Shea, Teeling, & Bennett, 2014) and diabetes complications (Rubin & Peyrot, 1999; Laiteerapong et al., 2011; Timar et al., 2016) reduce the quality of life. However, the relationship between quality of life and Hb1Ac is undetermined (Timar et al., 2016; Trikkalinou, Papazafiropoulou, & Melidonis, 2017).
THE STUDY
Aims
The overall purpose of this study is to investigate the experience of self-care in adults with T2DM. To accomplish this, we will recruit 300 participants with T2DM and we will perform a mixed method longitudinal study over a 2 year follow-up.
The primary quantitative objective of this study is to estimate associations between self-care maintenance, self-care monitoring, and self-care management at baseline with HbA1c in adults with T2DM, after a follow up of 12 months. Secondary objectives will be: 1) to estimate associations between self-care maintenance, self-care monitoring, and self-care management at baseline with HbA1c level in adults with T2DM after a follow up of 24 months; 2) to identify longitudinal associations between self-care and HbA1c with self-care and clinical outcomes of T2DM patients (e.g. unplanned medical referral, access to emergency department, hospitalisation, indicators of metabolic control, incidence of diabetes complications, quality of life, and death over a 24 months follow-up); 3) to describe self-care maintenance, self-care monitoring, and self-care management of people with T2DM and their directions over a 24 months follow-up; 4) to identify clinical and socio-demographic determinants of self-care maintenance, self-care monitoring, and self-care management in T2DM patients; 5) to estimate the association between the trends of self-care and HbA1c levels over a 24 months follow-up. The qualitative objective of the study is to gain a better understanding of the self-care experience in people with T2DM and its evolution over time.
Study Design
The study is a mixed method embedded design (Figure 1) that uses a prospective observational design and an interpretive description. The mixed method design was chosen because it is an approach to knowledge that considers multiple perspectives (Holloway & Wheeler, 2010) while maintaining the focus on people and their perceptions and experiences (Johnson & Onwuegbuzie, 2004; Östlund, Kidd, Wengström, & Rowa-Dewar, 2011). Data will be integrated for corroboration (Halcomb & Hickman, 2015). Specifically, data will be collected concurrently and analysed separately. Integration will occur during the interpretation phase. The results of one method will be used to support and verify the findings of the other on the same topic (Greene, Caracelli, & Graham, 1989; Halcomb & Hickman, 2015). The prospective observational design was chosen to collect clinical and self-care behaviour data over time so that strong inferences about self-care and its relationship with health outcomes can be made. Annual follow up periods for 2 years will be able to detect significant changes in a slowly evolving disease. Interpretive description stems from nursing science and it was chosen because of the shared disciplinary lens and the fit to the research question (Thorne, 2016, p. 57).
Figure 1.
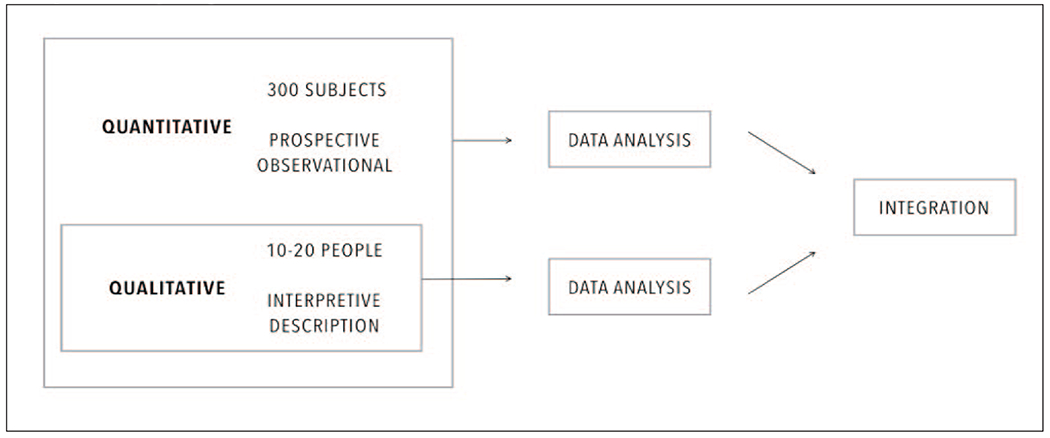
Study design.
Recruitment
Subjects will be contacted during their outpatient visit to the diabetes centre. Inclusion criteria will be: T2DM diagnosis, adequate comprehension of Italian language. Exclusion criteria will be: age < 18; Type 1 Diabetes or another type of diabetes (other than T2DM) as described in the available guidelines (Associazione Medici Diabetologi (AMD) & Società Italiana di Diabetologia (SID), 2018); documented psychiatric or neurodegenerative disease; pregnancy; inability to provide informed consent. Eligible participants will provide an informed consent prior to participating.
Sample size/Power evaluation
We will enroll an overall sample size of 300 subjects, of which 150 are expected to be at a low level of self-care maintenance. This sample size achieves 80% power at a 0.05 significance level to detect a difference of about 0.4% (with a standard deviation of 1.1%) in HbA1c levels between patient with lower and higher self-care maintenance after a follow-up of 12 months. This sample size accounts for a 20% loss to follow-up and has been based on self-care maintenance, but similar results could be expected also in the other scales of self-care.
From this sample of 300, a subsample will be recruited for the qualitative study using purposive sampling (Palinkas et al., 2015; Thorne, 2016, p. 99). We will recruit people who are either suggested by the center Principal Investigator for having a meaningful experience or whom have a “critical” score on the Self-care of Diabetes Inventory (very high or very low). An additional informed consent will be required prior to participation in this section of the study. We expect to enroll between 10 and 20 people in this subsample, according to the quality of data collected (Thorne, 2016).
Study Procedure
After signing the informed consent, socio-demographic and clinical data will be collected from medical records (Table 2). Clinical data will be updated every year (for a total of 2 years) at the beginning or at the end of the clinical standard outpatient visit. At the screening visit and at the follow-up visits, patients will be asked to complete five study questionnaires described in the paragraph “Measurements”. Quantitative data will be collected at baseline, 12 months, and 24 months. For the study procedures details, please refer to the Schedule of Events (Table 3).
Table 2.
Study Indicators
| STUDY INDICATORS | |
|---|---|
| Resource utilization/health outcomes indicators* | - Number of unplanned diabetes medical referral per year |
| - Number of all-cause accesses to emergency departments per year, | |
| - Number of all-cause hospitalizations per year; | |
| - Death. | |
| Clinical Indicators* | - Time from the diagnosis of T2DM |
| - Presence or absence and number of diabetes-related complications | |
| - Presence or absence and number of co-morbidities; | |
| - Ongoing medications and number of medications (or injections if patients are treated by insulin); | |
| - Smoking habits; | |
| - Glycated haemoglobin (HbA1c); | |
| - Lipid Profile (Total Cholesterol, HDL Cholesterol, LDL Cholesterol, Triglycerides); | |
| - Liver Profile (ALT, AST, GGT) | |
| - Renal Profile (Creatinine, estimated Glomerular Filtration Rate); | |
| - Height, weight, abdominal circumference and Body Mass Index (BMI); | |
| - Presence of insulin lipodystrophy; | |
| - Arterial Blood Pressure and Hearth Rate. | |
| - Self-care confidence (measured by the Self-Care of Diabetes Index, SCODI); | |
| Person-centered indicators | - Self-care maintenance of diabetes (measured by the Self-Care of Diabetes Index, SCODI); |
| - Self-care monitoring of diabetes (measured by the Self-Care of Diabetes Index, SCODI); | |
| - Self-care management of diabetes (measured by the Self-Care of Diabetes Index, SCODI); | |
| - Functional status (measured by the SF12 questionnaire); | |
| - Cognitive status (measured by the SF12 questionnaire); | |
| - Quality of life (measured by the EQ-5D-3L Quality of life Questionnaire); | |
| - Diabetes-related distress (measured by the Problem Areas in Diabetes Questionnaire Short Form, PAID-5); | |
| - Sleep quality (measured by the Pittsburgh Sleep Quality Index, PSQI). | |
| - Age; | |
| Socio-demographic indicators* | - Gender; |
| - Educational level; | |
| - Employment status; | |
| - Income level; | |
| - Presence of formal or informal caregiver. | |
Table 3.
Schedule of Events (quantitative)
| Protocol Activities | VISIT 1 Screening - Day 1 | VISIT 2 Month 12 ± 28 days | VISIT 3 Month 24± 28 days |
|---|---|---|---|
| BASELINE DOCUMENTATION | |||
| Informed consent No activity should be performed before obtaining signed and dated informed consent | × | ||
| Review of inclusion and exclusion criteria | × | ||
| Disease history Assess diabetes history, diabetes-related medications and complications, co-morbidities, ongoing drugs, smoking habits. | × | × | × |
| Physical assessment Height (at screening only), weight, abdominal circumference, presence of insulin lipodystrophy, BMI, blood pressure and pulse rate | × | × | × |
| LABORATORY STUDIES | |||
| HbA1c (mmol and %) | × | × | × |
| Lipid profileTotal Cholesterol, HDL Cholesterol, LDL Cholesterol, Triglycerides | × | × | × |
| Renal and Liver profile Creatinine, estimated GFR, ALT, AST, GGT | × | × | × |
| QUESTIONNAIRES | |||
| Short Form 12 (SF-12) | × | × | × |
| Self-Care of Diabetes Inventory (SCODI) | × | × | × |
| Quality of Life (EQ-5D-5L) | × | × | × |
| Problem Areas in Diabetes Questionnaire Short Form (PAID-5) | × | × | × |
| Pittsburgh Sleep Quality Index (PSQI) | × | × | × |
| OTHER ASSESSMENT | |||
| Events Any referral or unplanned diabetes visit, request for home care assistance, hospitalization, access to emergency care, death, received diabetes education in the last year. | × | × | × |
Qualitative data will be collected, within the framework of Interpretive Description, with in-depth semi-structured interviews. For each person enrolled, there will be one interview per time point. Eventually, participants will be contacted for additional interviews, in person or by phone. Interviews will be audiotaped and transcribed. Interviews will be directed by a topic guide (or interview schedule), that allows for the exploration of main themes, still leaving the possibility to probe emerging subjects.
Measurements
Quantitative data will be gathered from clinical charts and questionnaires. Short forms of the questionnaires have been chosen to reduce subject burden, improve response rates, and protocol adherence.
Outcome Variables
The primary outcome is HbAlc (measured in mmol and %). Secondary outcomes are unplanned medical referrals, hospitalisations, mortality, T2DM complications, and quality of life. Further information will be collected about the opportunity that patients had to participate in diabetes education programs during the previous year.
Unplanned medical referral is the number of unplanned medical visits.
ED visits is the number of visits to the Emergency Department.
Hospitalisations are defined as being admitted to a hospital overnight.
Mortality will be considered as all-causes mortality.
T2DM complications refer to the presence of one or more T2DM complications such as retinopathy, nephropathy, neuropathy, etc. These data will be collected from medical charts.
Quality of life will be measured by the EQ-5D-3L. The 5-item EQ-5D-3L queries participants about movement, hygiene, daily living activities tolerance, pain/discomfort, and anxiety/depression. General health status is also assessed. The EQ-5D-3L is a valid and reliable tool to measure quality of life in diabetes population (Konerding et al., 2014).
Independent Variables
Independent variables include self-care (maintenance, monitoring and management), functional and cognitive function, sleep quality, socio-demographic characteristics, and distress.
Self-care will be measured using the Self-care of Diabetes Inventory - SCODI (Italian). SCODI has been demonstrated to be a valid, reliable, and theoretically grounded tool for measuring self-care in patients with T2DM (Ausili, Barbaranelli, et al., 2017). SCODI comprises 40 items (5 points Likert type), developed according to the most recent evidence-based clinical recommendations. These items are grouped into 4 dimensions: self-care maintenance (12 items), self-care monitoring (8 items), self-care management (9 items), and self-care confidence (11 items). Factor analysis and model based reliability coefficients showed high internal consistency and construct validity was demonstrated through the association of SCODI scores with relevant clinical criteria such as glycated haemoglobin, body mass index and diabetes-related complications (Ausili, Barbaranelli, et al., 2017).
Functional and cognitive status will be measured using the Short Form 12 - SF-12. The 12-item, self-administered SF-12 queries participants about social discomfort, disabilities, and level of health perceived over the previous 4 weeks, physical functioning, role limitation due to physical health, pain, general health, and fatigue (Kodraliu et al., 2001; Ware, A. Kosinski, & D. Keller, 1998).
Sleep quality will be measured using the Pittsburgh Sleep Quality Index — PSQI (Italian). This 19-item self-rated questionnaire assesses sleep quality over the previous month across seven domains; sleep quality, sleep latency, sleep duration, sleep disturbances, sleep medication use, and daytime functioning (D. J. Buysse, Reynolds, Monk, Berman, & Kupfer, 1989; Curcio et al., 2013). Scores range from 0 to 21. PSQI scores higher than five indicate poor sleep quality. Reliability and validity of the PSQI (Italian version) have been established (Curcio et al., 2013).
Socio-demographic characteristics collected from medical records include age, gender, educational level, employment status, income level, and presence of formal or informal caregiver.
Distress. The 5-item PAID-5 (Problem Areas in Diabetes Questionnaire) measures diabetes related emotional distress concerning treatment-related, food-related, and social support-related problems. Scores range from 0 to 100 with > 40 points score indicating depression and other negative psychological outcomes. Good sensitivity (94%) and specificity (89%) for recognition of diabetes-related emotional distress have been established (McGuire et al., 2010).
Analysis
Study indicators are outlined in Table 2. HbA1c is the primary endpoint of this study. Clinical, person-centered indicators, and resources utilization indicators are the secondary endpoints. As visually explained in Figure 1, qualitative and quantitative data will be analyzed separately and integrated subsequently according to the embedded mixed-methods design (Creswell, 2014; Halcomb & Hickman, 2015; Yardley, 2017).
Continuous data will be summarized with number of observations, mean value, standard deviation, median, quartiles, minimum, maximum and 95% confidence intervals for the mean. Categorical data will be summarized by means of absolute and relative frequencies (counts and percentages). Scores of SCODI’s self-care scales will be standardized to 100 (Ausili et al., 2017).
Disposition of subjects enrolled will be presented and the number of subjects at each visit will be tabulated. The reason for inclusion/exclusion will be tabulated and listed. To estimate associations between self-care maintenance, monitoring, management and confidence at baseline and HbA1c level at 12 months, a t-test will be used. The incidences of health and clinical outcomes over the follow-up will be computed by the Kaplan-Meier estimator in patients with low and high self-care at baseline and they will be compared by the log-rank test. The Cox model will be used to account for confounders (age, gender, time from diagnosis, comorbidity, cognitive and functional status). A Cox model will be also used to evaluate the impact of the SCODI (as continuous score) on health and clinical outcomes. Self-care care behaviors of people with type 2 diabetes will be described by a box-plot and their relation with health/clinical outcomes over the whole follow-up will be modelled by longitudinal models. In order to identify clinical and socio-demographic determinants of self-care behaviours, multiple linear regression analyses will be performed. Explorative and confirmative factor analyses will be used to further evaluate the construct validity of the SCODI. Chronbach Alpha and other inter-item coefficients will be used to measure the internal consistency of the tool.
The focus of interpretive description is to generate new knowledge that can be relevant and meaningful for the applied practice context (Thorne, 2016), here nursing. Despite its ontological flexibility, the qualitative data analysis is composed by three main moments: sorting and organising data, making sense of pattern, and transforming pattern into findings (Thorne, 2016).
Ethical Considerations
Approval from Institutional Review Board (IRB)/Independent Ethics Committee (IEC) was obtained for the protocol and the informed consents. Participants will receive an informative sheet to read and discuss with the Principal Investigator (PI) or his delegate. The PI or his delegate will ensure that each study participant is fully informed about the nature and objectives of the study and possible risks associated with participation. Written informed consent will be obtained from each participant before any study-specific activity is performed. Personal data will be protected. Participants’ names will not be included on any forms, reports, publications, or in any other disclosures. A sequential identification number will be attributed to each patient enrolled in the study in order to maximise anonymity. Concerning the qualitative phase, names will be changed and details, which will make persons identifiable, will be removed. Participants will be made aware that in report and publication sentences with their quotes may be used. They will be informed that, despite every effort made to protect people’s privacy, their words might make them recognizable by their peers or acquaintances.
Validity and Reliability / Rigour
The first step undertaken to ensure the validity of this study is the design itself. Mixed methods allows the integration the quantitative and qualitative data to increase the integrity of the research (Greene et al., 1989) and its internal validity (Barbour, 2001). For the quantitative phase, we consulted a panel of experts to design the study, which is grounded in practice. Only validated and reliable instruments will be used to collect quantitative data. Sensitivity to context, commitment and rigour, transparency and coherence, impact and importance will be used to guide the qualitative data collection and analysis (Yardley, 2000). This study is grounded both in practice and in theory, and it is supported by a skilled multi-disciplinary team. Results from this study will have an impact on clinical practice, health expenditures, and the quality of life of people living with diabetes
Strengths and limitations
One limitation of this study is the single country nature of the study. To address this limitation, multiple centres will be represented. Another limitation of this study is that some variables influencing self-care, such as coping and resilience, will not be measured quantitatively. However, we expect some of these variables will be illuminated by the qualitative part of the study and therefore integrated by the merging process during interpretation (Yardley, 2017). A major strength of this study is the longitudinal study design. Few longitudinal studies have investigated self-care in T2DM. Self-care maintenance, self-care monitoring, and self-care management have never been studied prospectively and longitudinally in T2DM patients.
CONCLUSION
This study will expand our understanding of self-care in people with T2DM. The expected outcome will be a better understanding of the effect of self-care on glycaemic control and therefore clinical outcomes and costs. This will lead to more informed clinical interventions, educational programs, healthcare services, and future research developments in the field.
T2DM is widespread and increases both direct and indirect healthcare costs. Self-care in T2DM is a complex phenomenon that has not been thoroughly investigated and described. Therefore, we designed a mixed methods multi-centred study in order to shed insights on experience of self-care in people with T2DM.
FUNDING
The author(s) (SKM) were supported in part by the following grants T32HL7953 from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI), K99NR017416 from the NIH, National Institute of Nursing Research (NINR), and UL1TR001445 from the New York University Clinical and Translational Science Award.
REFERENCES
- Adam J, & Folds L (2014). Depression, Self-efficacy, and Adherence in Patients With Type 2 Diabetes. The Journal for Nurse Practitioners, 10(9), 646–652. https://doi.org/10.10l6/j.nurpra.2014.07.033 [Google Scholar]
- Aljasem LI, Peyrot M, Wissow L, & Rubin RR (2001). The Impact of Barriers and Self-Efficacy on Self-Care Behaviors in Type 2 Diabetes. The Diabetes Educator, 27(3), 393–404. 10.1177/014572170102700309 [DOI] [PubMed] [Google Scholar]
- Al-Khawaldeh OA, Al-Hassan MA, & Froelicher ES (2012). Self-efficacy, self-management, and glycemic control in adults with type 2 diabetes mellitus. Journal of Diabetes and Its Complications, 26(1), 10–16. 10.1016/j.jdiacomp.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Associazione Medici Diabetologi (AMD), & Società Italiana di Diabetologia (SID) (2018, April 27). Standard italiani per la cura del diabete mellito. [Google Scholar]
- Ausili D, Barbaranelli C, Rossi E, Rebora P, Fabrizi D, Coghi C, … Riegel B. (2017). Development and psychometric testing of a theory-based tool to measure self-care in diabetes patients: The Self-Care of Diabetes Inventory. BMC Endocrine Disorders, 17(1). 10.1186/s12902-017-0218-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausili D, Bulgheroni M, Ballatore P, Specchia C, Ajdini A, Bezze S, … Genovese S, (2017). Self-care, quality of life and clinical outcomes of type 2 diabetes patients: An observational cross-sectional study. Acta Diabetologica, 54(11), 1001–1008. 10.1007/s00592-017-1035-5 [DOI] [PubMed] [Google Scholar]
- Austin Boren S, Gunlock TL, Schaefer J, & Albright A (2007). Reducing Risks in Diabetes Self-management. The Diabetes Educator, 33(6), 1053–1077. 10.1177/0145721707309809 [DOI] [PubMed] [Google Scholar]
- Badedi M, Solan Y, Darraj H, Sabai A, Mahfouz M, Alamodi S, & Alsabaani A (2016). Factors Associated with Long-Term Control of Type 2 Diabetes Mellitus. Journal of Diabetes Research, 2016. 10.1155/2016/2109542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour RS (2001). Checklists for improving rigour in qualitative research: A case of the tail wagging the dog? BMJ (Clinical Research Ed.), 322(7294), 1115–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J, Wright C, Sheasby J, Turner A, & Hainsworth J (2002). Self-management approaches for people with chronic conditions: A review. Patient Education and Counseling, 48(2), 177–187. [DOI] [PubMed] [Google Scholar]
- Berry E, Lockhart S, Davies M, Lindsay JR, & Dempster M (2015). Diabetes distress: Understanding the hidden struggles of living with diabetes and exploring intervention strategies. Postgraduate Medical Journal, 91(1075), 278–283. 10.1136/postgradmedj-2014-133017 [DOI] [PubMed] [Google Scholar]
- Bohanny W, Wu S-FV, Liu C-Y, Yeh S-H, Tsay S-L, & Wang T-J (2013). Health literacy, self-efficacy, and self-care behaviors in patients with type 2 diabetes mellitus. Journal of the American Association of Nurse Practitioners, 25(9), 495–502. 10.1111/1745-7599.12017 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Buysse Daniel J. (2014). Sleep Health: Can We Define It? Does It Matter? Sleep, 37(1), 9–17. 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun E, Kim J, & Riegel B (2017). Associations of Subjective Sleep Quality and Daytime Sleepiness With Cognitive Impairment in Adults and Elders With Heart Failure. Behavioral Sleep Medicine, 15(4), 302–317. 10.1080/15402002.2015.1133418 [DOI] [PubMed] [Google Scholar]
- Caro-Bautista J, Martín-Santos FJ, & Morales-Asencio JM (2014). Systematic review of the psychometric properties and theoretical grounding of instruments evaluating self-care in people with type 2 diabetes mellitus. Journal of Advanced Nursing, 70(6), 1209–1227. 10.1111/jan.12298 [DOI] [PubMed] [Google Scholar]
- Chasens ER, Korytkowski M, Sereika SM, & Burke LE (2013). Effect of Poor Sleep Quality and Excessive Daytime Sleepiness on Factors Associated With Diabetes Self-Management. The Diabetes Educator, 39(1), 74–82. 10.1177/0145721712467683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ML, & Utz SW (2014). Social determinants of type 2 diabetes and health in the United States. World Journal of Diabetes, 5(3), 296–304. 10.4239/wjd.v5.i3.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognitive Deficits: Overview, Diagnosis, Risk Factors and Etiology. (2017). Retrieved from http://emedicine.medscape.com/article/917629-overview
- Creswell JW (2014). Research design: Qualitative, quantitative, and mixed methods approaches (4th ed). Thousand Oaks: SAGE Publications. [Google Scholar]
- Curcio G, Tempesta D, Scarlata S, Marzano C, Moroni F, Rossini PM, … De Gennaro L (2013). Validity of the Italian Version of the Pittsburgh Sleep Quality Index (PSQI). Neurological Sciences, 34 (4), 511–519. 10.1007/s10072-012-1085-y [DOI] [PubMed] [Google Scholar]
- Devarajooh C, & Chinna K (2017). Depression, distress and self-efficacy: The impact on diabetes self-care practices. PLOS ONE, 12(3), e0175096 10.1371/journal.pone.0175096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS, Ruppert SD, Karkada SN, & Jacob D (2015). Health Related Quality of Life among Omani Men and Women with Type 2 Diabetes. Journal of Diabetes Research, 2016. 10.1155/2016/8293579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA-NIH Biomarker Working Group. (2017). Validated Surrogate Endpoint (2016th ed.). Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK453484/
- Feil DG, Zhu CW, & Sultzer DL (2012). The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. Journal of Behavioral Medicine, 35(2), 190–199. 10.1007/s10865-011-9344-6 [DOI] [PubMed] [Google Scholar]
- Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, … for the Sleep AHEAD Research Group. (2009). Obstructive Sleep Apnea Among Obese Patients With Type 2 Diabetes. Diabetes Care, 32(6), 1017–1019. 10.2337/dc08-1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherman A, Schnur J, Montgomery G, Sassu R, Veresiu I, & David D (2011). How Are Adherent People More Likely to Think? A Meta-Analysis of Health Beliefs and Diabetes Self-Care. The Diabetes Educator, 37(3), 392–408. 10.1177/0145721711403012 [DOI] [PubMed] [Google Scholar]
- Glasgow RE, & Toobert DJ (1988). Social Environment and Regimen Adherence Among Type II Diabetic Patients. Diabetes Care, 11(5), 377–386. 10.2337/diacare.11.5.377 [DOI] [PubMed] [Google Scholar]
- Greene JC, Caracelli VJ, & Graham WF (1989). Toward a Conceptual Framework for Mixed-Method Evaluation Designs. Educational Evaluation and Policy Analysis, 11(3), 255–274. 10.3102/01623737011003255 [DOI] [Google Scholar]
- Halcomb E, & Hickman L (2015). Mixed methods research. Nursing Standard, 29(32), 41–47. 10.7748/ns.29.32.41.e8858 [DOI] [PubMed] [Google Scholar]
- Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, … Zangeneh F, (2015). American association of clinical endocrinologists and american college of endocrinology—Clinical practice guidelines for developing a diabetes mellitus comprehensive care plan— 2015. Endocrine Practice: Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, 21 Suppl 1, 1–87. 10.4158/EP15672.GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haw JS, Tantry S, Vellanki P, & Pasquel FJ (2015). National Strategies to Decrease the Burden of Diabetes and Its Complications. Current Diabetes Reports, 15(9), 65 10.1007/s11892-015-0637-y [DOI] [PubMed] [Google Scholar]
- Hill JO, Galloway JM, Goley A, Marrero DG, Minners R, Montgomery B, … Aroda VR (2013). Scientific statement: Socioecological determinants of prediabetes and type 2 diabetes. Diabetes Care, 36(8), 2430–2439. 10.2337/dc13-1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen L, Keinänen-Kiukaanniemi S, Läärä E, & Kivelä SL (1996). Functional ability of elderly persons with diabetes or impaired glucose tolerance. Scandinavian Journal of Primary Health Care, 14(4), 229–237. 10.3109/02813439608997090 [DOI] [PubMed] [Google Scholar]
- Holloway I, & Wheeler S (2010). Qualitative research in nursing and healthcare. Chichester, West Sussex, UK: Wiley-Blackwell. [Google Scholar]
- Holt RIG, Nicolucci A, Kovacs Burns K, Escalante M, Forbes A, Hermanns N, … DAWN2 Study Group. (2013). Diabetes Attitudes, Wishes and Needs second study (DAWN2TM): Cross-national comparisons on barriers and resources for optimal care--healthcare professional perspective. Diabetic Medicine: A Journal of the British Diabetic Association, 30(7), 789–798. 10.1111/dme.12242 [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. (2017). IDF Diabetes Atlas—8th Edition (8th ed.). Retrieved from www.diabetesatlas.org [PubMed]
- Jaarsma T, Cameron J, Riegel B, & Strömberg A (2017). Factors Related to Self-Care in Heart Failure Patients According to the Middle-Range Theory of Self-Care of Chronic Illness: A Literature Update. Current Heart Failure Reports, 14(2), 71–77. 10.1007/s11897-017-0324-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RB, & Onwuegbuzie AJ (2004). Mixed Methods Research: A Research Paradigm Whose Time Has Come. Educational Researcher, 33(7), 14–26. [Google Scholar]
- Kodraliu G, Mosconi P, Groth N, Carmosino G, Perilli A, Gianicolo EA, … Apolone G, (2001). Subjective health status assessment: Evaluation of the Italian version of the SF-12 Health Survey. Results from the MiOS Project. Journal of Epidemiology and Biostatistics, 6(3), 305–316. [DOI] [PubMed] [Google Scholar]
- Konerding U, Elkhuizen SG, Faubel R, Forte P, Malmström T, Pavi E, & Janssen MB (2014). The validity of the EQ-5D-3L items: An investigation with type 2 diabetes patients from six European countries. Health and Quality of Life Outcomes, 12(1), 181 10.1186/s12955-014-0181-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs Burns K, Nicolucci A, Holt RIG, Willaing I, Hermanns N, Kalra S, … DAWN2 Study Group. (2013). Diabetes Attitudes, Wishes and Needs second study (DAWN2TM): Cross-national benchmarking indicators for family members living with people with diabetes. Diabetic Medicine: A Journal of the British Diabetic Association, 30(7), 778–788. 10.1111/dme.12239 [DOI] [PubMed] [Google Scholar]
- Laiteerapong N, Karter AJ, Liu JY, Moffet HH, Sudore R, Schillinger D, … Huang ES (2011). Correlates of Quality of Life in Older Adults With Diabetes. Diabetes Care, 34(8), 1749–1753. 10.2337/dc10-2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidfeldt J, Nyberg P, Nerbrand C, Samsioe G, Schersten B, & Agardh CD (2003). Socio-demographic and psychosocial factors are associated with features of the metabolic syndrome. The Women’s Health in the Lund Area (WHILA) study. Diabetes, Obesity and Metabolism, 5(2), 106–112. 10.1046/j.1463-1326.2003.00250.x [DOI] [PubMed] [Google Scholar]
- Lopes LA, Lins C. de M. M., Adeodato VG, Quental DP, de Bruin PFC, Montenegro RM, & de Bruin VMS (2005). Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care, 28(11), 2633–2636. [DOI] [PubMed] [Google Scholar]
- Lou P, Qin Y, Zhang P, Chen P, Zhang L, Chang G, … Zhang N (2015). Association of sleep quality and quality of life in type 2 diabetes mellitus: A cross-sectional study in China. Diabetes Research and Clinical Practice, 107(1), 69–76. 10.1016/j.diabres.2014.09.060 [DOI] [PubMed] [Google Scholar]
- Lu Y, Xu J, Zhao W, & Han H-R (2016). Measuring Self-Care in Persons With Type 2 Diabetes: A Systematic Review. Evaluation & the Health Professions, 39(2), 131–184. 10.1177/0163278715588927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcum ZA, Zheng Y, Perera S, Strotmeyer E, Newman AB, Simonsick EM, … Hanlon JT (2013). Prevalence and correlates of self-reported medication non-adherence among older adults with coronary heart disease, diabetes mellitus, and/or hypertension. Research in Social and Administrative Pharmacy, 9(6), 817–827. 10.1016/j.sapharm.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BE, Morrison TG, Hermanns N, Skovlund S, Eldrup E, Gagliardino J, … Snoek FJ (2010). Short-form measures of diabetes-related emotional distress: The Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia, 53(1), 66–69. 10.1007/s00125-009-1559-5 [DOI] [PubMed] [Google Scholar]
- Nicolucci A, Rossi MC, Pellegrini F, Lucisano G, Pintaudi B, Gentile S, … Vespasiani G (2014). Benchmarking network for clinical and humanistic outcomes in diabetes (BENCH-D) study: Protocol, tools, and population. SpringerPlus, 3. 10.1186/2193-1801-3-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea MP, Teeling M, & Bennett K (2014). Comorbidity, health-related quality of life and self-care in type 2 diabetes: A cross-sectional study in an outpatient population. Irish Journal of Medical Science (1971 -), 184(3), 623–630. 10.1007/s11845-014-1190-4 [DOI] [PubMed] [Google Scholar]
- Östlund U, Kidd L, Wengström Y, & Rowa-Dewar N (2011). Combining qualitative and quantitative research within mixed method research designs: A methodological review. International Journal of Nursing Studies, 48(3), 369–383. 10.1016/j.ijnurstu.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang C-M, Dwyer JT, Jacques PF, Chuang L-M, Haas CF, & Weinger K (2015). Diabetes self-care behaviours and clinical outcomes among Taiwanese patients with type 2 diabetes. Asia Pacific Journal of Clinical Nutrition, 24(3), 438–443. [DOI] [PubMed] [Google Scholar]
- Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, & Hoagwood K (2015). Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Administration and Policy in Mental Health, 42(5), 533–544. 10.1007/s10488-013-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson BL, Thorne S, & Dewis M (1998). Adapting to and Managing Diabetes. Image: The Journal of Nursing cholarship, 30(1), 57–62. https://doi.org/10.1111Zj.1547-5069.1998.tb01237.x [DOI] [PubMed] [Google Scholar]
- Peyrot M, Burns KK, Davies M, Forbes A, Hermanns N, Holt R, … Skovlund SE (2013). Diabetes Attitudes Wishes and Needs 2 (DAWN2): A multinational, multi-stakeholder study of psychosocial issues in diabetes and person-centred diabetes care. Diabetes Research and Clinical Practice, 99(2), 174–184. 10.1016/j.diabres.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Powers MA, Bardsley J, Cypress M, Duker P, Funnell MM, Hess Fischl A, … Vivian E (2015). Diabetes Self-management Education and Support in Type 2 Diabetes: A Joint Position Statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care, 38(7), 1372–1382. 10.2337/dc15-0730 [DOI] [PubMed] [Google Scholar]
- Primožič S, Tavčar R, Avbelj M, Dernovšek MZ, & Oblak MR (2012). Specific cognitive abilities are associated with diabetes self-management behavior among patients with type 2 diabetes. Diabetes Research and Clinical Practice, 95(1), 48–54. 10.1016/j.diabres.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Riegel B, & Dickson VV (2008). A situation-specific theory of heart failure self-care. The Journal of Cardiovascular Nursing, 23(3), 190–196. 10.1097/01.JCN.0000305091.35259.85 [DOI] [PubMed] [Google Scholar]
- Riegel B, Jaarsma T, & Strömberg A (2012). A middle-range theory of self-care of chronic illness. ANS. Advances in Nursing Science, 35(3), 194–204. 10.1097/ANS.0b013e318261b1ba [DOI] [PubMed] [Google Scholar]
- Riegel B, & Weaver TE (2009). Poor sleep and impaired self-care: Towards a comprehensive model linking sleep, cognition, and heart failure outcomes. European Journal of Cardiovascular Nursing, 8(5), 337–344. 10.1016/j.ejcnurse.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RR, & Peyrot M (1999). Quality of life and diabetes. Diabetes/Metabolism Research and Reviews, 15(3), 205–218. [DOI] [PubMed] [Google Scholar]
- Saleh F, Mumu SJ, Ara F, Hafez MA, & Ali L (2014). Non-adherence to self-care practices & medication and health related quality of life among patients with type 2 diabetes: A cross-sectional study. BMC Public Health, 14, 431 10.1186/1471-2458-14-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schub T Bs, & Smith N Rn, Msn, Cnp. (2016). Diabetes Mellitus, Type 2: Ethnic and Other Demographic Risk Disparities CINAHL Nursing Guide. Retrieved from https://login.proxy.unimib.it/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=nup&AN=T701291&lang=it&site=eds-live&scope=site
- Schunk M, Reitmeir P, Schipf S, Völzke H, Meisinger C, Thorand B, … Holle R (2012). Health-related quality of life in subjects with and without Type 2 diabetes: Pooled analysis of five population-based surveys in Germany. Diabetic Medicine: A Journal of the British Diabetic Association, 29(5), 646–653. 10.1111/j.1464-5491.2011.03465.x [DOI] [PubMed] [Google Scholar]
- Scollan-Koliopoulos M, Bleich D, Rapp KJ, Wong P, Hofmann CJ, & Raghuwanshi M (2013). Health-Related Quality of Life, Disease Severity, and Anticipated Trajectory of Diabetes. The Diabetes Educator, 39(1), 83–91. 10.1177/0145721712467697 [DOI] [PubMed] [Google Scholar]
- Seligowski AV, Pless Kaiser AP, Niles BL, Mori DL, King LA, & King DW (2013). Sleep Quality as a Potential Mediator Between Psychological Distress and Diabetes Quality of Life in Veterans With Type 2 Diabetes: Sleep as a Potential Mediator. Journal of Clinical Psychology, 69(10), 1121–1131. 10.1002/jclp.21866 [DOI] [PubMed] [Google Scholar]
- Shrivastava SR, Shrivastava PS, & Ramasamy J (2013). Role of self-care in management of diabetes mellitus. Journal of Diabetes & Metabolic Disorders, 12, 14 10.1186/2251-6581-12-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AJ, Girling AJ, & Bayer AJ (2000). Cognitive dysfunction in older subjects with diabetes mellitus: Impact on diabetes self-management and use of care services. Diabetes Research and Clinical Practice, 50(3), 203–212. 10.1016/S0168-8227(00)00195-9 [DOI] [PubMed] [Google Scholar]
- Snoek FJ, Bremmer MA, & Hermanns N (2015). Constructs of depression and distress in diabetes: Time for an appraisal. The Lancet Diabetes & Endocrinology, 3(6), 450–460. 10.1016/S2213-8587(15)00135-7 [DOI] [PubMed] [Google Scholar]
- Song M, Ratcliffe SJ, Tkacs NC, & Riegel B (2012). Self-care and health outcomes of diabetes mellitus. Clinical Nursing Research, 21(3), 309–326. 10.1177/1054773811422604 [DOI] [PubMed] [Google Scholar]
- Stopford R, Winkley K, & Ismail K (2013). Social support and glycemic control in type 2 diabetes: A systematic review of observational studies. Patient Education and Counseling, 93(3), 549–558. 10.1016/j.pec.2013.08.016 [DOI] [PubMed] [Google Scholar]
- Thorne S (2016). Interpretive description: Qualitative research for applied practice (Second edition). New York, NY: Routledge. [Google Scholar]
- Timar R, Velea PI, Timar B, Lungeanu D, Oancea C, Roman D, & Mazilu O (2016). Factors influencing the quality of life perception in patients with type 2 diabetes mellitus. Patient Preference and Adherence, Volume 10, 2471–2477. 10.2147/PPA.S124858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin A, & Sinclair A (2016). The influence of cognition on self-management of type 2 diabetes in older people. Psychology Research and Behavior Management, 9, 7 10.2147/PRBM.S36238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikkalinou A, Papazafiropoulou AK, & Melidonis A (2017). Type 2 diabetes and quality of life. World Journal of Diabetes, 8(4), 120 10.4239/wjd.v8.i4.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam HA, van der Horst FG, Knoops L, Ryckman RM, Crebolder HFJM, & van den Borne BHW (2005). Social support in diabetes: A systematic review of controlled intervention studies. Patient Education and Counseling, 59(1), 1–12. 10.1016/j.pec.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Vellone E, Fida R, D’Agostino F, Mottola A, Juarez-Vela R, Alvaro R, & Riegel B (2015). Self-care confidence may be the key: A cross-sectional study on the association between cognition and self-care behaviors in adults with heart failure. International Journal of Nursing Studies, 52(11), 1705–1713. 10.1016/j.ijnurstu.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Vellone E, Pancani L, Greco A, Steca P, & Riegel B (2016). Self-care confidence may be more important than cognition to influence self-care behaviors in adults with heart failure: Testing a mediation model. International Journal of Nursing Studies, 60, 191–199. 10.1016/j.ijnurstu.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Ware J, A. Kosinski M, & D. Keller S (1998). SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales.
- World Health Organization. (2016). Global Report on Diabetes. Retrieved from http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1
- Wykes TL, Lee AA, McKibbin CL, & Laurent SM (2016). Self-Efficacy and Hemoglobin A1C Among Adults With Serious Mental Illness and Type 2 Diabetes: The Roles of Cognitive Functioning and Psychiatric Symptom Severity. Psychosomatic Medicine, 78(3), 263–270. 10.1097/PSY.0000000000000295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley L (2000). Dilemmas in qualitative health research. Psychology & Health, 15(2), 215–228. 10.1080/08870440008400302 [DOI] [Google Scholar]
- Yardley L (2017). The SAGE Handbook of Qualitative Research in Psychology (By pages 352–369; F. Bishop, Ed.). 10.4135/9781848607927 [DOI]


