Abstract
Acid Ceramidase Deficiency (Farber Disease, FD) is an ultra-rare Lysosomal Storage Disorder that is poorly understood and often misdiagnosed as Juvenile Idiopathic Arthritis (JIA). Hallmarks of FD are accumulation of ceramides, widespread macrophage infiltration, splenomegaly, and lymphocytosis. The cytokines involved in this abnormal hematopoietic state are unknown. There are dozens of ceramide species and derivatives, but the specific ones that accumulate in FD have not been investigated. We used a multiplex assay to analyze cytokines and mass spectrometry to analyze ceramides in plasma from patients and mice with FD, controls, Farber patients treated by hematopoietic stem cell transplantation (HSCT), JIA patients, and patients with Gaucher disease. KC, MIP-1α, and MCP-1 were sequentially upregulated in plasma from FD mice. MCP-1, IL-10, IL-6, IL-12, and VEGF levels were elevated in plasma from Farber patients but not in control or JIA patients. C16-Ceramide (C16-Cer) and dhC16-Cer were upregulated in plasma from FD mice. a-OH-C18-Cer, dhC12-Cer, dhC24:1-Cer, and C22:1-Cer-1P accumulated in plasma from patients with FD. Most cytokines and only a-OH-C18-Cer returned to baseline levels in HSCT-treated Farber patients. Sphingosines were not altered. Chitotriosidase activity was also relatively low. A unique cytokine and ceramide profile was seen in the plasma of Farber patients that was not observed in plasma from HSCT-treated Farber patients, JIA patients, or Gaucher patients. The cytokine profile can potentially be used to prevent misdiagnosis of Farber as JIA and to monitor the response to treatment. Further understanding of why these signaling molecules and lipids are elevated can lead to better understanding of the etiology and pathophysiology of FD and inform development of future treatments.
Keywords: Chemokines, Cytokines, Juvenile Idiopathic Arthritis, Ceramides, MCP-1, IP-10
1. Introduction
Acid Ceramidase Deficiency (Farber Disease, FD; Farber Lipogranulomatosis, OMIM #228000) is an ultra-rare, autosomally recessive inherited Lysosomal Storage Disorder (LSD). It is caused by mutations in ASAH1, which encodes for the lipid-processing enzyme, acid ceramidase (ACDase, EC 3.5.1.23). This deficiency results in the accumulation of ceramides and many downstream effects, leading to a multisystem disorder that is often lethal in childhood. Hematopoietic stem cell transplantation (HSCT) is currently the only treatment available for FD, but it is not consistently successful and is associated with severe, even life-threatening, side effects. A better understanding of the biology of FD is needed to develop more effective treatments. As well, ceramides are fundamental sphingolipids involved in many cellular processes. A better understanding of their biology will have a far-reaching impact.1
One of the most pronounced results of FD is perturbation of the hematopoietic system. Patients and mice with FD have splenomegaly, lymphocytosis, and excess macrophage infiltration into many organs [1,2]. They also develop granuloma-like lesions, principally at cartilage sites, that are composed of immune cells including lipid-filled macrophages and neutrophils. We previously described the hematopoietic abnormalities in our mouse model of FD and found that by the end of life (9 weeks of age) the bone marrow, thymus, spleen, and lymph nodes of Farber mice were packed with foamy macrophages; B and T progenitor cells were almost completely absent; and hematopoietic stem and progenitor cells were significantly increased in the bone marrow [3]. In other LSDs that present with macrophage involvement, such as Gaucher disease and Niemann-Pick disease, chitotriosidase levels in plasma can be elevated [4, 5]. To our knowledge, however, chitotriosidase activity has not been evaluated in the plasma of patients with FD.
Hematopoiesis and immune cell control is complex, being regulated primarily by cis- and trans-acting cytokines. Sphingolipids also play a role in cell signaling. Normally, ACDase converts ceramide into sphingosine and a free fatty acid. In FD, this enzyme has minimal functionality, resulting in bulk ceramide accumulation in patients and mice [1,2]. There are dozens of ceramide molecular species that vary by their carbon chain length, degree of unsaturation and hydroxylation, and the presence or absence of phosphates. The specific ceramide species that accumulate in FD have not been investigated in detail. Identification of these ceramide species is critical to understand which lipids may be causing the detrimental effects of FD. Additionally, it may be expected that sphingosine levels are reduced in ACDase deficiency, but this has not been confirmed. Along these lines, we previously found that some cytokines were elevated in aged Farber mice [2]; here we supplement that study by adding younger mice to visualize the timeline of cytokine changes and extend such analyses to include samples obtained from FD patients as well. To examine whether this elevated cytokine pattern is unique to FD, we also compare the profile with that obtained from another macrophage-prominent LSD, Gaucher disease. In addition to analyzing cytokines and ceramides, we also measured the activity of chitotriosidase in plasma from patients with FD, which can be up to 1000-fold elevated in plasma from patients with Gaucher disease [6].
In addition to being poorly understood, FD is likely underdiagnosed or misdiagnosed. Definite diagnosis of FD patients can be established by measuring ACDase enzymatic activity and/or sequencing the ASAH1 gene. ACDase activity is determined in leukocytes collected from peripheral blood or from skin fibroblasts obtained by a biopsy. Analysis of variations in the ASAH1 sequence is done by comparisons with known Farber mutations, which are limited by the small number of case reports, and by software that predict the detrimental impact of mutations. Should a patient have a unique mutation, the geneticist may not be able to differentiate between a disease-causing or non-disease-causing ASAH1 polymorphism due to limited historical information.
Another obstacle is misdiagnosis. Joint abnormalities (e.g., contractures, pain) may also occur in Juvenile Idiopathic Arthritis (JIA), resulting in the misdiagnosis of Farber patients [7–9]. Several physicians submitting samples to this study (B.M., J.M., B.H., B.M.) have reported moderate responses of certain Farber symptoms (joint disease and inflammation) to treatment with biologic therapies used in treating JIA (TNF-α inhibitors, Interleukin-6 receptor blockers), which indicates an additional facet that may perpetuate a misdiagnosis of JIA in the clinic. Indeed, 36% of case reports of patients with moderate FD were initially misdiagnosed as JIA [10]. Here, we identify the cytokines and ceramides that are changed in the plasma of patients with FD. These results can help investigators understand the pathobiology of FD and allow for better differentiation between FD and inflammatory arthropathies such as JIA.
2. Materials and Methods
2.1 Sample collection
Blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-coated tubes from 5-, 7-, 9- and 11-week-old mice homozygous for the FD mutation Asah1P361R/P361R (Hom), heterozygous for the mutation (Het), or WT [2]. Three to four samples were collected for each genotype for 5-, 7-, and 9-week-old mice, and two samples per genotype for 11-week-old mice. Samples were centrifuged at 1377×g for 5 minutes at room temperature, and plasma samples were stored at −80°C until use.
Human samples were collected from 15 patients with FD, 5 patients with FD who underwent HSCT, 5 patients with JIA, and 11 patients with Gaucher disease. The unaffected parents and siblings of these patients were used as controls; for a total of 39 samples. Blood was collected in EDTA-coated tubes, plasma was separated from cells by centrifugation as above, and white blood cells were isolated. Treating physicians also filled in a questionnaire about the patient’s history with FD. Samples and patient information were collected in accordance with a protocol approved by the University Health Network Research Ethics Board. The data were kept anonymous.
2.2 Determination of ACDase activity
The in vitro activity of ACDase was measured from patient leukocytes using a modification of the method previously published [11]. Briefly, for each assay 3 µl of substrate buffer (0.2 M citrate-phosphate, pH 5 containing 0.2% Igepal CA-630, 0.3 M NaCl, and 200 µM C12-NBD ceramide (Cayman Chemical)) was mixed with 3 µl of leukocyte cell lysate, vortexed and then incubated at 37°C for 1 hour. The reaction was stopped by adding 100% ethanol followed by centrifugation at 13 000×g for 5 min. 35 µl of supernatant was removed and applied onto a UPLC system (Waters Acquity H-Class) with fluorescent detector for analysis.
2.3 Determinatino of chitotriosidase activity
Chitotriosidase activity was measured in the plasma of Farber patients and parental/sibling controls. Gaucher patient plasma was used as a reference. The chitotriosidase activity of the Gaucher patient plasma was independently determined by Dr. Levade, and the samples were classified as ‘Gaucher High’ and ‘Gaucher Low.’ Chitotriosidase activity was determined by incubation of 5 µl of plasma with 4-methylumbelliferyl β-D-N,N′,N″-triacetylchitotrioside (Sigma) for 1 hour. The reaction was stopped by adding 0.5 M glycine-NaOH at pH 10.5. Fluorescence intensity was measured at 358/448 nm.
2.4 Quantitation of lipids by mass spectrometry
Sphingolipid analysis was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) by the Lipidomics Shared Resource at the Medical University of South Carolina as described previously [12]. The specific sphingolipids measured were ceramides (Cer), dihydroceramides (dhCer), alpha-hydroxylated ceramides (a-OH-Cer), and phosphorylated ceramides (Cer-1P) of chain lengths C14 to C26. Sphingosine (Sph), phosphorylated sphingosine (Sph-1P), dihydrosphingosine (sphinganine, dhSph), and phosphorylated dihydrosphingosine (dhSph-1P) were also measured.
2.5 Cytokine analysis
Cytokine levels were analyzed from mouse plasma using the Bio-Plex Pro Mouse Cytokine 23-plex Assay (Bio-Rad) as per the manufacturer’s instructions. Human plasma was analyzed for cytokine levels using the Cytokine Human Magnetic 30-Plex Panel (Novex). Luminescence was quantified on the Luminex 100 instrument (Luminex). Data where low bead count was observed (<45 beads) was omitted. Where individual data points were below the detection limit of the kit, values were set to half of the detection limit. Where individual data points were above the detection limit, values were set to the top of the detection level. Heatmaps were generated in the statistical software program R using the gplots package [13].
2.6 Statistics
Comparisons of cytokine and ceramide levels between Farber mice of different ages and humans with different disease status was performed using Prism 5.0c. Each cytokine was analyzed by a one-way-ANOVA followed by Bonferroni’s Multiple Comparison Test. If Bartlett’s test for equal variances indicated that the variance was significantly different, then the Kruskal-Wallis test was performed followed by Dunn’s Multiple Comparison test. The significance between cytokines and gender of the patient was assessed using a two-tailed t-test in Microsoft Excel. The R2 value when correlating cytokines with the age at sample collection or ACDase activity, or when correlating one cytokine with another, was calculated using Microsoft Excel.
3. Results
3.1 Circulating cytokine levels
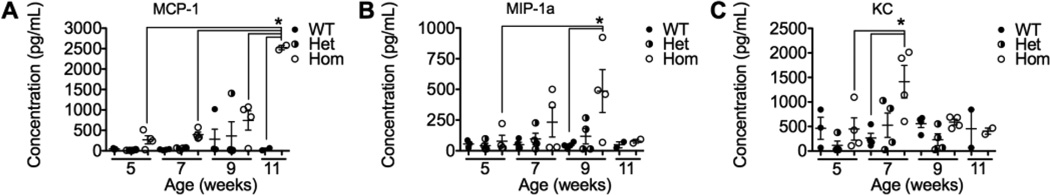
Plasma was isolated from wild-type (WT), heterozygous (Het), and homozygous (Hom) Asah1P361R mice aged 5, 7, and 9 weeks, and WT and Hom mice aged 11 weeks, and cytokines were analyzed by a multiplex assay. The selected data points span the start of the observable disease in this mouse model, as the immune system is compromised between 5 and 7 weeks [3], and the end of their life (9–11 weeks). Circulating levels of monocyte chemotactic protein 1 (MCP-1, CCL2), macrophage inflammatory protein1a (MIP-1a, CCL3), and keratinocyte chemoattractant (KC, CXCL1) were elevated in Hom mice compared to WT and Het mice during this time-course (Fig. 1). These cytokines were elevated early, but each peaked at a different age: KC at 7 weeks, MIP-1a at 9 weeks, and MCP-1 was highest at the end of life. Cytokine levels that were not significantly different (though some show trends towards elevation) were interleukin (IL)-1a, IL-1b, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-17, basic fibroblast growth factor (bFGF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFNg), interferon gamma-induced protein 10 (IP-10), monokine induced by gamma interferon (MIG), tumor necrosis factor alpha (TNFa), and vascular endothelial growth factor (VEGF) (Fig. S1).
Figure 1.
Cytokines elevated in the plasma of Farber mice. Cytokines were measured in plasma samples from WT, Het, and Hom Farber mice aged 5, 7, 9, and 11 weeks using a multiplex assay. MCP-1 (CCL2) (A), MIP-1a (CCL3) (B) and KC (CXCL1) (C) levels are illustrated. n=2–4 for each genotype at each time point. *p<0.05. MCP-1, monocyte chemotactic protein 1, MIP-1a, macrophage inflammatory 1a; KC, keratinocyte chemoattractant.
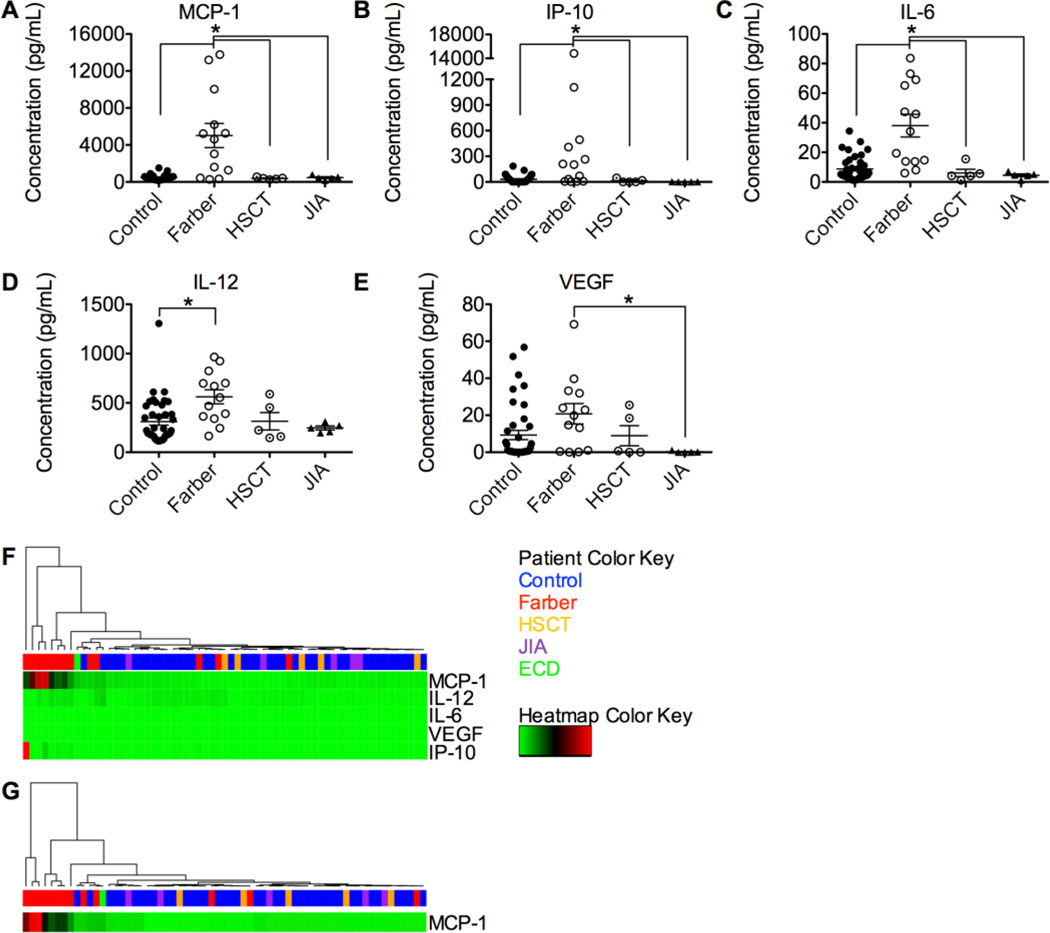
Plasma was also obtained from the following human patient populations: patients with confirmed FD, patients with FD treated by HSCT, and patients with JIA confirmed not to have FD. Cytokine levels in these human samples were analyzed by multiplex assay and compared to parental and sibling controls (when available). MCP-1, IP-10, and IL-6 were all dramatically elevated in samples from Farber patients compared to those obtained from control and JIA patients. Strikingly, levels of these cytokines were normalized in Farber patients that had received HSCT (Fig. 2A–C). IL-12 was also elevated significantly in Farber patients compared to controls, and VEGF was significantly elevated in Farber patients compared to controls and those patients with JIA (Fig. 2D–E). As observed in mice, the levels of several cytokines were not significantly different between FD and controls. These were IL-1b, IL-1RA, IL-2, IL-2R, IL-4, IL-5, IL-7, IL-8, IL-10, IL-13, IL-15, IL-17, bFGF, epidermal growth factor (EGF), eoxtaxin, granulocyte colony-stimulating factor (G-CSF), GM-CSF, hepatocyte growth factor (HGF), interferon alpha (IFNa), IFNg, MIG, MIP-1a, MIP-1b, regulated on activation, normal T cell expressed and secreted (RANTES), and TNFa (Fig. S2).
Figure 2.
Farber patients have a unique plasma cytokine profile that is normalized following HSCT and different from JIA. Cytokines were measured in plasma from controls (n=39), untreated Farber patients (n=13), HSCT-treated Farber patients (n=5), and JIA patients (n=5) using a multiplex assay. MCP-1 (A), IP-10 (B), IL-6 (C), IL-12 (D) and VEGF (E) levels are shown. Unbiased hierarchical clustering was performed on all patients using these five cytokines (F) or MCP-1 (G). A cluster dendrogram is seen at the top (branches), followed by the patient color key (single row of colored rectangles) and a heatmap of the relative cytokine levels (green, black, and red rectangles). The relative amount of each cytokine is seen horizontally corresponding to its row, where green represents relatively low expression, black medium expression, and red relatively high expression. *p<0.05. HSCT, hematopoietic stem cell transplanted Farber patients; JIA, Juvenile Idiopathic Arthritis; ECD, Erdheim-Chester disease; MCP-1, monocyte chemotactic protein 1; IP-10, interferon gamma-induced protein 10; IL-6, interleukin 6; IL-12, interleukin 12; VEGF, vascular endothelial growth factor; ACDase, acid ceramidase.
To examine whether the elevated cytokine profile we saw in plasma from patients with FD was specific to this disorder and not a general pattern seen in LSDs with inflammatory manifestations, we compared our results to those samples obtained from patients with Gaucher disease. Gaucher disease also has macrophage involvement, and has been reported to manifest with elevated inflammatory markers [14, 15]. The cytokine elevation was much more pronounced in FD than in Gaucher disease for MCP-1, IP-10, and IL-6. IL-12 was much more elevated in Gaucher disease than in FD (Figure S3).
The significantly altered cytokines in FD were informative in classifying the samples. When unbiased hierarchical clustering was performed utilizing the top five cytokines in terms of expression increases, 10 of 13 Farber patients clustered together, away from controls, HSCT, and JIA samples (Fig. 2F). Two of the three patients who did not cluster with the Farber patients had an attenuated Farber phenotype: relatively mild symptoms and little impairment of daily functioning at over 20 years old, a rare age for patients with FD to reach based on reports published in the literature so far. Importantly, MCP-1 levels generated the same clustering pattern alone (Fig. 2G). Using all of the cytokines together did not result in better clustering, nor did using any of the other top five cytokines individually (Fig. S4). Interestingly, plasma from the single Erdheim-Chester disease patient analyzed, a disease with a different clinical phenotype but similar histopathologic changes, clustered near the Farber patients [16].
To better understand how these cytokines could play a role in FD, we identified patterns through correlation analysis. Only IL-12 and VEGF positively correlated with each other (Fig. S5H). Other cytokine pairs did not correlate (Fig. S5). We also investigated the relationship between the ACDase activity measured in Farber patient leukocytes or the age of patients at sample collection with these cytokines. Only MCP-1 levels correlated in a strongly negative fashion with leukocyte ACDase activity (Fig. S6F). No cytokine levels correlated with the age at sample collection or the sex of the patient, nor did any additional cytokines correlate with ACDase activity (Fig. S6, Table S1).
3.2 Plasma ceramide levels
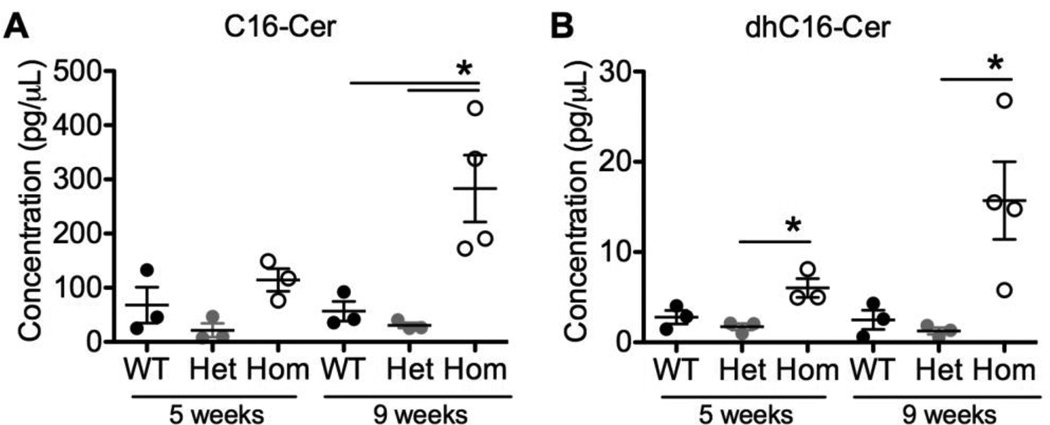
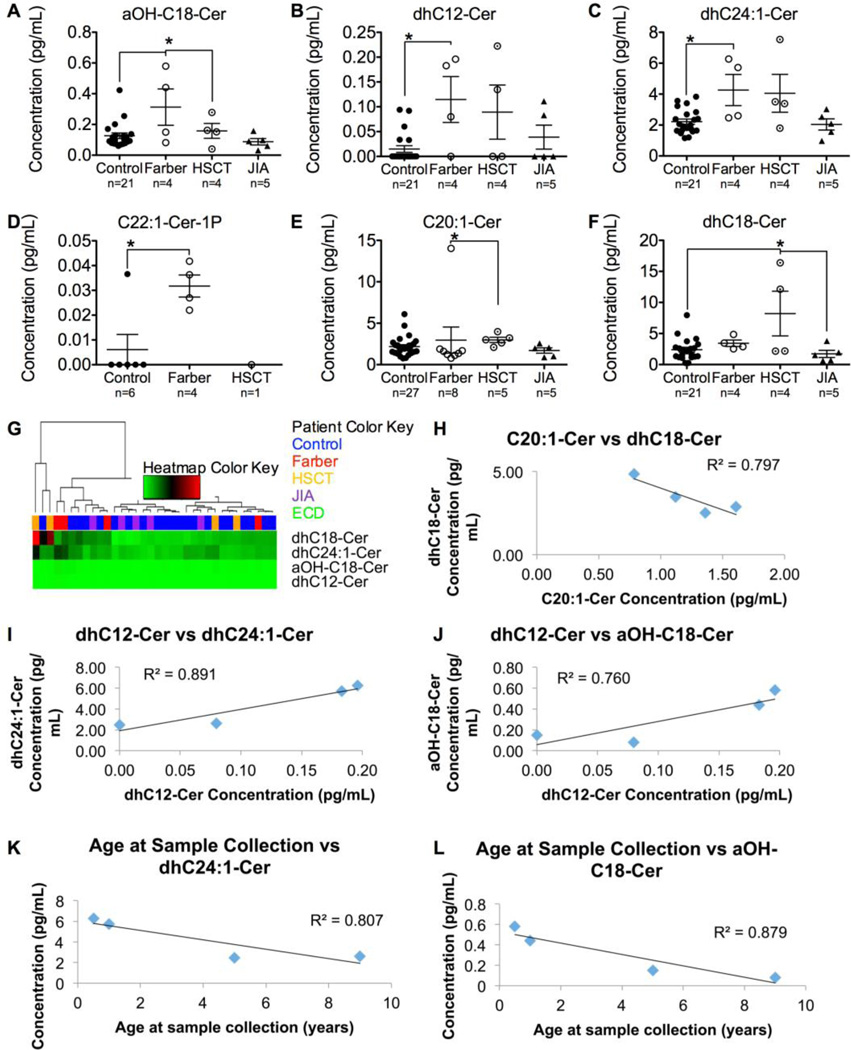
ACDase degrades ceramides into sphingosine and a free fatty acid. In FD, bulk ceramides accumulate due to deficient ACDase activity. To identify which of the dozens of ceramide species are altered in plasma from FD patients and mice, mass spectrometry was used. Ceramides were analyzed in the plasma of 5- and 9-week-old WT, Het, and Hom Farber mice, and human Farber and JIA patients, controls, and Farber patients treated with HSCT. Only C16-Ceramide (C16-Cer) and dhC16-Cer were significantly elevated in Hom Farber mouse plasma (Fig. 3), and in Farber patient plasmas only alpha-hydroxy-C18-Cer, dhC12-Cer, dhC24:1-Cer, and C22:1-Cer-1-phosphate (C22:1-Cer-1P) were significantly elevated compared to controls (Fig. 4A–D). Of those ceramides elevated in Farber patient plasma, only alpha-hydroxy-C18-Cer was normalized following HSCT. In samples from Farber patients that had received HSCT, C20:1-Cer was slightly elevated compared to samples from untreated Farber patients (Fig. 4E), and dhC18-Cer was higher than seen in control samples or those from JIA patients (Fig. 4F). None of these ceramides could be used to group Farber patients using unbiased hierarchical clustering (Fig. 4G, S12). Most were not significantly different (Fig. S7, S9, S10). Quite surprisingly, the levels of sphingosine and its derivatives were also unchanged (Fig. S8, S11).
Figure 3.
Ceramides with a chain length of 16 carbons are elevated in the plasma of Farber mice. Plasma was collected from 5- and 9-week-old WT, Het, and Hom Farber mice. Ceramides were analyzed by mass spectrometry. C16-Cer (A) and dhC16-Cer (B) levels are shown. *p<0.05. n=3–4 for each genotype at each time point. Cer, ceramide.
Figure 4.
Ceramide levels vary in plasma from patients with different diseases. Plasma was collected from controls (n=6–27), untreated Farber patients (n=4–8), and HSCT-treated Farber patients (n=1–5), and JIA patients (n=5). Ceramides were analyzed by mass spectrometry. a-OH-C18-Cer (A), dhC12-Cer (B), dhC24:1-Cer (C), C22:1-Cer-1P (D), C20:1-Cer (E) and dhC18-Cer (F) levels are illustrated. *p<0.05. Unbiased hierarchical clustering was performed using four significantly altered ceramides (G). A cluster dendrogram is seen at the top (branches), followed by the patient color key (single row of colored rectangles) and a heatmap of the relative ceramide levels (green, black, and red rectangles). The relative amount of each ceramide is seen horizontally corresponding to its row, where green represents relatively low expression, black medium expression, and red relatively high expression. There was a very strong negative correlation between the expression level of C20:1-Cer and dhC18-Cer (H). dhC12-Cer had a strong and very strong positive correlation, respectively, with dhC24:1-Cer (I) and aOH-C18-Cer (J). The age at sample collection correlated very strongly negatively with dhC24:1-Cer (K) and aOH-C18-Cer (L). Cer, ceramide; HSCT, hematopoietic stem cell transplanted Farber patients; JIA, Juvenile Idiopathic Arthritis.
There were strong correlations between some of the altered ceramide species: C20:1-Cer and dhC18-Cer had a very strong negative correlation, meaning that one may be metabolically related to the other. dhC12-Cer had a strong and very strong positive correlation, respectively, with dhC24:1-Cer and aOH-C18-Cer (Fig. 4H–J). Other correlations were not found (Fig. S13). There was also negative correlation of ceramide species with the age at sample collection: dhC24:1-Cer, and aOH-C18-Cer correlated very strongly with the age at sample collection (Fig. 4K–L). Other ceramides did not correlate with the age at sample collection, and none correlated with leukocyte ACDase activity (Fig. S14).
3.3 Cytokines and ceramides
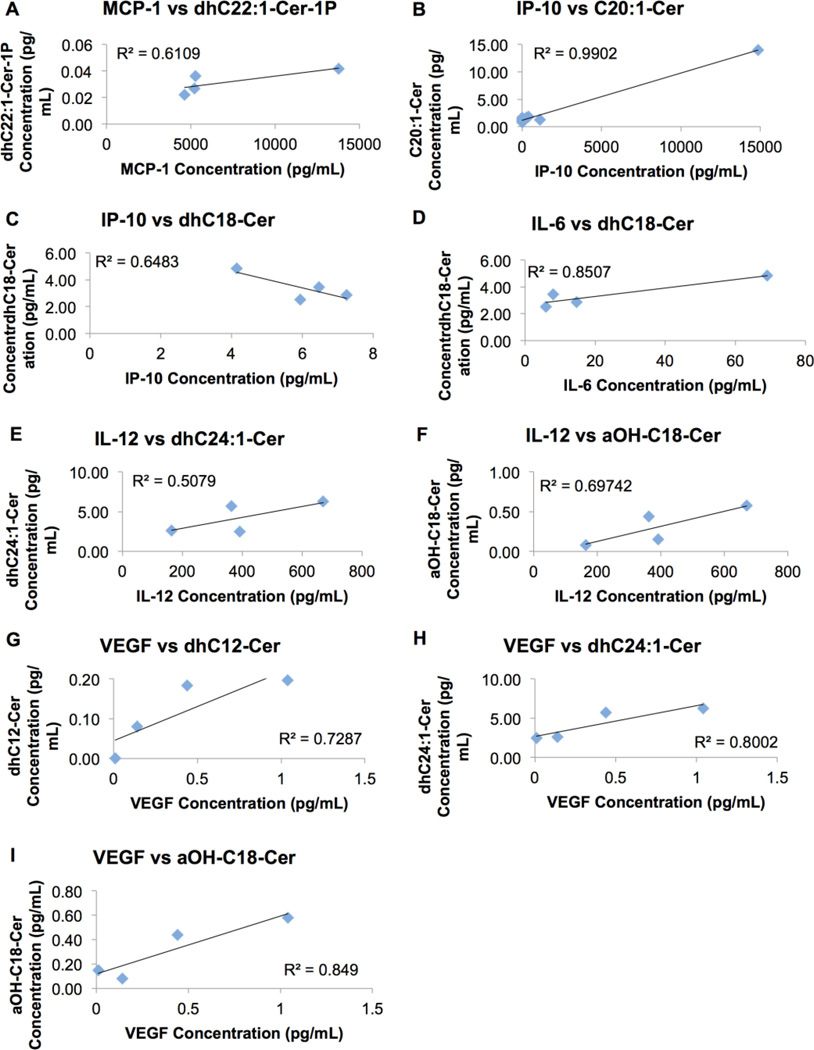
Inter-relationships between cytokine and ceramide levels were also probed. MCP-1, IP-10, and VEGF correlated positively with dhC22:1-Cer-1P, C20:1-Cer, and dhC12-Cer, respectively (Fig. 5). dhC24:1 and aOH-C18-Cer correlated positively with IL-12 and VEGF levels. dhC18-Cer correlated negatively with levels of IP-10 but positively with that of IL-6. There was no correlation between other cytokines and ceramides (Fig. S15, S16, S17).
Figure 5.
Cytokine changes correlate with ceramide changes in plasma. The change in significantly different cytokines was compared with the change in significantly different ceramides. There was a strong positive correlation between MCP-1 and dhC22:1-Cer-1P (A). IP-10 correlated very strongly positively with C20:1-Cer (B), and strongly negatively with dhC18-Cer (C). IL-6 correlated very strongly positively with dhC18-Cer (D). IL-12 correlated moderately with dhC24:1-Cer (E) and strongly with aOH-C18-Cer (F). VEGF correlated strongly positively with dhC12-Cer (G), and very strongly positively with dhC24:1-Cer (H), and aOH-C18-Cer (I). MCP-1, monocyte chemotactic protein 1; IP-10, interferon gamma-induced protein 10; IL, interleukin ; VEGF, vascular endothelial growth factor; Cer, ceramide.
3.4 Chitotriosidase activity
Given the dominant role of macrophages in FD, we investigated the activity of chitotriosidase in plasma. Chitotriosidase is a chitinase that is secreted by activated macrophages [4]. Plasma chitotriosidase activity is high in patients with macrophage-involved disorders, such as Gaucher disease and Niemann-Pick disease [5]. To determine if this biomarker was altered in FD, we evaluated its activity in plasma.
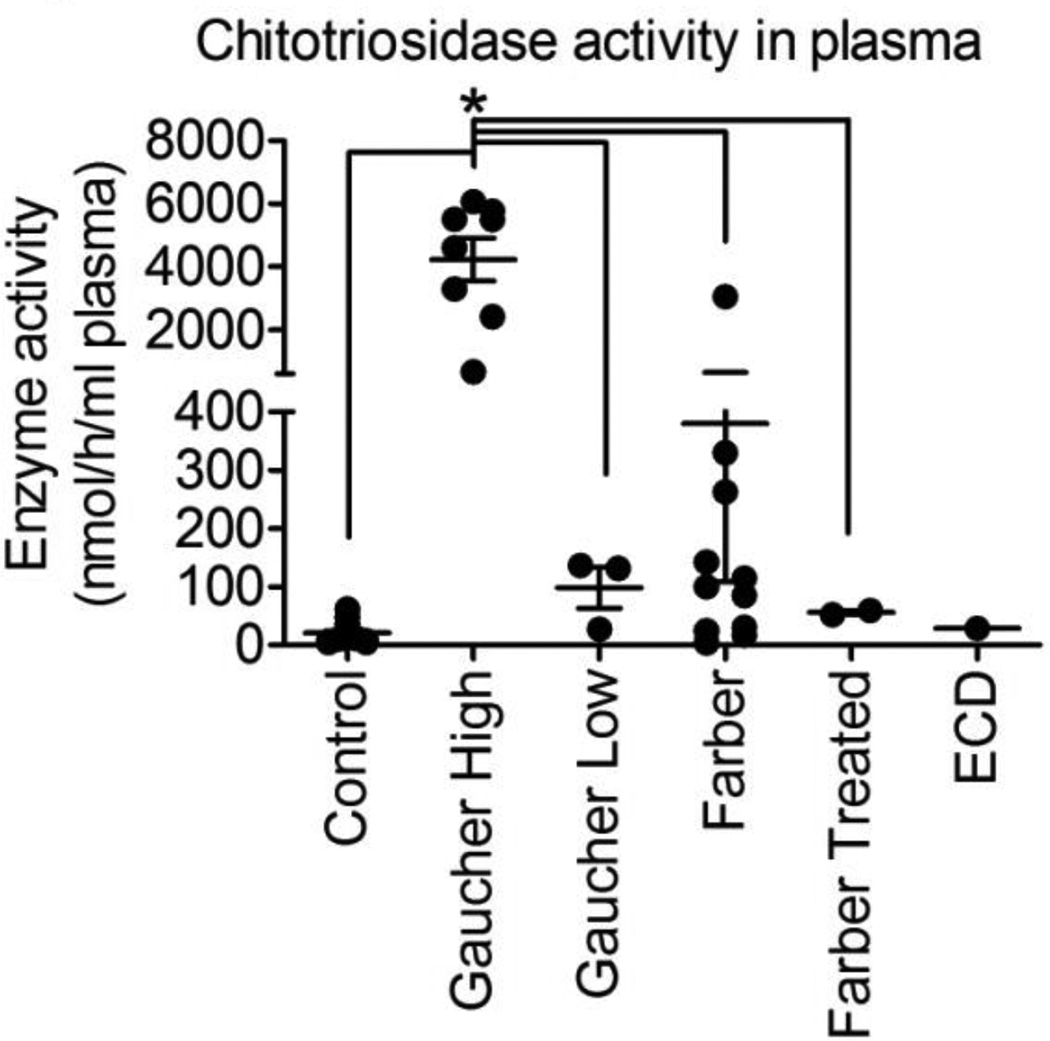
Chitotriosidase activity in plasma from patients with FD was higher than parental/sibling controls but lower than Gaucher patients with characteristically 'high' activity (Figure 6). The activity in FD was in the same range as Gaucher patients with characteristically 'low' activity.
Figure 6.
Chitotriosidase activity in Farber patient plasma. Plasma was collected from controls (n=18), Gaucher patients with independently identified 'high' chitotriosidase activity (n=8), Gaucher patients with independently identified 'low' chitotriosidase activity (n=3), untreated Farber patients (n=11), HSCT-treated Farber patients (n=2), and an ECD patient (n=1). Chitotriosidase activity was measured in vitro using a fluorescent substrate. *p<0.0001.
4. Discussion
We identified patterns in plasma cytokines and ceramides due to ACDase deficiency that are unique to mice and humans with FD and not present in controls of either species, Farber patients treated with HSCT, or JIA patients. These changes are critical to understanding of the pathophysiology of FD. The cytokines identified can also be useful for the differential diagnosis of FD and JIA.
4.1 Understanding the biology of Farber disease
4.1.1 The role of cytokines in the initiation and progression of Farber disease
In general, neutrophils are the first to arrive to a region of inflammation, followed by monocytes/macrophages. We observed the same sequence in the organs of Farber mice. The timing of the elevated cytokines in Farber mice (Fig. 1) matches this process. KC, an inflammatory cytokine that recruits neutrophils, peaks first at 7 weeks. Next, MIP-1a and MCP-1, inflammatory cytokines that recruit monocytes/macrophages, peak at 9 and 11 weeks, respectively. These cytokines are elevated in plasma and may be recruiting neutrophils and monocytes out of the bone marrow and into the circulation where they can travel to and infiltrate organs. MCP-1 levels were elevated in the plasma of 11 week old Farber mice compared to WT mice of the same age (Fig. 1). In addition, higher levels of MCP-1 were observed in organs compared to plasma, suggesting that circulating monocytes are the source cells that infiltrate organs in this disorder [2].
We previously demonstrated that the macrophage infiltration is so severe in the Farber mouse bone marrow, thymus, spleen, and lymph nodes that the endogenous architecture is disrupted [3]. Subcutaneous nodules, common to all reported FD patients, are composed of lipid-filled macrophages [1]. Activated macrophages are present, as seen by elevations in chitotriosidase plasma activity, but not to the levels of 'high' Gaucher disease.
Based on our results, future treatment studies in Farber disease may investigate the correlation between therapeutic efficacy and decrease in MCP-1 and MIP-1a as markers of lower levels of macrophage infiltration and tissue damage. The anecdotal evidence of the moderate effect of powerful anti-inflammatory medications targeting the innate immune system (Interleukin-6 receptor blockers) as symptomatic treatments in Farber disease, first related by BM and confirmed by other co-authors, also can be seen to support this proposition. As does the fact that even interruption of MCP-1 signaling has been suggested as a therapeutic intervention for rheumatoid arthritis and atherosclerosis [17, 18]. Modulation of these specific cytokines is critical, based on our findings (Fig. S1, S2). Highlighting the importance of MCP-1 in the etiology of FD, the absolute levels of the cytokine in plasma were 10-fold higher in patients with FD than in controls (Fig. S3). In another lysosomal storage disorder where macrophages are a key feature, Gaucher disease, this elevation was less than 2-fold [19].
As shown above, MCP-1 and MIP-1a are uniquely elevated in mice and humans with FD. Together they may be recruiting monocytes into organs. Furthermore, this may be a feed forward mechanism, as IP-10, IL-6, and IL-12 are themselves secreted by macrophages [20,21]. Increasing ceramide levels by stimulating its de novo formation resulted in MCP-1 and IL-6 expression in a macrophage cell line [22]. MIP-1a, IP-10, IL-6, and IL-12 are implicated in arthritis, where they may exacerbate inflammation [23–25]. C22:1-Cer-1P was also elevated in Farber patient plasma (Fig. 4D). Cer-1P has been shown in a macrophage cell line to stimulate MCP-1 release and cell migration through the phosphatidylinositol 3-kinase (PI3K)/Akt, mitogen-activated protein kinase kinase (MEK)/extracellularly regulated kinases (ERK), and p38 pathways [26]. Other specific cytokines were also elevated in human Farber patients. IP-10, IL-6, and IL-12 were all increased in Farber patients compared to controls (Fig. 2A–D).
It is important to note that three out of four of the elevated cytokines returned to baseline levels following HSCT. This may be a sign of the positive effects of HSCT on FD, or HSCT may reduce inflammatory cytokine secretion. Plasma cytokines can be added to the toolbox of read-outs when assessing the efficacy of experimental therapeutics for FD, such as enzyme replacement therapy and gene therapy [1,27,28].
4.1.2 Ceramides in Farber disease
The ceramide trends in non-Farber controls in this report corroborate previous mass spectrometry analyses of plasma ceramides in healthy humans [29–31]. C24-Cer was the main product, and C18-Cer was a relatively minor product in plasma samples from both controls and Farber patients. This study analyzed more ceramides than the others, and we found several additional ceramide species in non-Farber human controls at lower levels than C18-Cer, including C18:1-Cer, C20:1-Cer, C20:4-Cer, C22:1-Cer, C26-Cer, and most of the Cer-1P. In our analysis, C22:1-Cer-1P was a minor product in controls.
Interestingly, not all ceramide species were elevated in the plasma of Farber patients. This is not surprising as there are other ceramidases (one neutral ceramidase and three alkaline ceramidases) that also degrade ceramides. The only ceramides significantly elevated in the plasma from FD mice were C16-Cer and dhC16-cer (Fig. 3); 24 other ceramides analyzed were not changed (Fig. S7). This suggests that there is special significance of ceramides with a fatty acid chain length of 16 carbons. We did not find a unique pattern of ceramide accumulation in the Farber patients in this cohort, and were not able to demonstrate clustering by the unique ceramides identified in the Farber mouse model (Fig. 4G). However, a few ceramide species correlated with each other (Fig. 4H–J), suggesting a possible common defect. Specifically, dhC12-Cer, dhC24:1-Cer, and aOH-C18-Cer are positively correlated with each other, and negatively correlated with age at sample collection.
The product of ceramide degradation is sphingosine and a free fatty acid. The assumption is that reduced degradation of ceramide would lead to lower levels of sphingosine. This was not the case for plasma: sphingosine and its derivatives were unchanged (Fig. S8). The levels of sphingosine are critical to a cell: in the treatment of another Lysosomal Storage Disorder, Niemann-Pick Type C, too much sphingosine resulted in neurodegeneration [32]. These data suggest that either there is an alternate pathway through which sphingosine is formed (such as by other ceramidases), or that its degradation is reduced so that the small amount of sphingosine produced by ACDase is maintained at sufficient levels for its function.
4.2 Improving diagnosis of Farber disease: a new method of verification that distinguishes between Farber and JIA patients
We identified an alternate method of verification of FD: by analysis of plasma cytokines. We identified that MCP-1, IP-10, IL-6, and VEGF are elevated in Farber patients (Fig. 1A–E). These four markers may be useful for physicians to differentiate between FD and JIA. Expression of MCP-1, IP-10, IL-6, IL-12 and VEGF results in discrimination of 10 of 13 Farber patients from JIA patients, in cluster analysis (Fig. 2F). An identical success rate was achieved when clustering the patients based on MCP-1 expression (Fig. 2G). This result, coupled with the observation that MCP-1 was the only cytokine elevated in both mouse and human Farber plasma, suggests that MCP-1 is a key player or biomarker in the etiology of FD. It is not the only cytokine that attracts monocytes that was elevated, raising the possibility that the role of MCP-1 could be shared/replaced by other cytokines. A further benefit of MCP-1 as a differential marker between Farber and JIA patients is the possibility of large-scale screening for FD in JIA populations of unknown etiology to identify misdiagnosed patients.
Using MCP-1 and other cytokines may also be helpful in identifying misdiagnosed Farber patients in other populations. Spinal muscular atrophy with progressive myoclonic epilepsy (SMA-PME) is caused by ACDase deficiency but does not present the same way as FD [33–36]. Comparison of plasma from SMA patients with SMN1 or SMN2 mutations compared to SMA-PME patients with ASAH1 mutations may reveal cytokine differences that can be used to screen larger SMA populations. Samples from SMA-PME patients were not available at the time of analysis.
Collectively, our data support that ACDase deficiency results in changes in the cytokine and ceramide profiles found in the plasma of mice and patients. These changes are unique to untreated FD and are not seen in controls, HSCT treated FD patients, or JIA patients. These changes can be used to better understand the biology of FD and to develop novel treatments. MCP-1 plasma levels may also be useful as a tool to differentiate between a diagnosis of FD and JIA.
Supplementary Material
Highlights.
-
-
Specific cytokines are elevated in the plasma of Farber mice and Farber patients.
-
-
This set distinguishes Farber Disease from Juvenile Idiopathic Arthritis.
-
-
Treatment by bone marrow transplantation normalizes most of these cytokines.
-
-
While macrophages are involved in FD, plasma chitotriosidase levels are low.
Acknowledgments
The authors thank all of the families who participated in this study. The authors thank Dr. Dwayne Barber for critical reading of the manuscript.
Funding
This work was supported by Rare Disease Foundation & BC Children’s Hospital Foundation [Microgrant to J.A.M.]; the National Institutes of Health [1R21NS078191-01A1 to J.A.M., R01 DK54830 to E.H.S.]; Vaincre les Maladies Lysosomales [to T.L. and J.A.M.]; and Plexcera Therapeutics [to E.H.S.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: AC, acid ceramidase; bFGF, basic fibroblast growth factor; Cer, ceramide; EDTA, ethylenediaminetetraacetic acid; EGF, epidermal growth factor ERK, extracellularly regulated kinases; FD, Farber Disease; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; Het, heterozygous; HGF, hepatocyte growth factor; Hom, homozygous; HSCT, hematopoietic stem cell transplantation; IFNa, interferon alpha; IFNg, interferon gamma; IL, interleukin; IP-10, interferon gamma-induced protein 10; JIA, Juvenile Idiopathic Arthritis; KC, keratinocyte chemoattractant; LC-MS/MS, liquid chromatography-tandem mass spectrometry; LSD, Lysosomal Storage Disorder; MCP-1, monocyte chemotactic protein 1; MIG, monokine induced by gamma interferon; MIP-1a, macrophage inflammatory protein 1a; RANTES, regulated on activation, normal T cell expressed and secreted; PI3K, phosphatidylinositol 3-kinase; MEK, mitogenactivated protein kinase kinase; SMA-PME, spinal muscular atrophy with progressive myoclonic epilepsy; TNFa, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; WT, wild-type
Conflict of interest statement: Alexander Solyom is an employee of Plexcera Therapeutics. Edward H. Schuchman is a co-founder, equity holder, and consultant for Plexcera Therapeutics. Jeffrey A. Medin is on the Scientific Advisory Board of Plexcera Therapeutics. All other authors declare that they have no conflicts of interest.
References
- 1.Levade T, Sandhoff K, Schulze H, Medin J. Acid ceramidase deficiency: Farber lipogranulomatosis. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonorakis SE, Ballabio A, editors. Scriver’s OMMBID (The Online Metabolic & Molecular Bases of Inherited Disease) 2009. [Google Scholar]
- 2.Alayoubi AM, Wang JC, Au BC, Carpentier S, Garcia V, Dworski S, El-Ghamrasni S, Kirouac KN, Exertier MJ, Xiong ZJ, et al. Systemic ceramide accumulation leads to severe and varied pathological consequences. EMBO Mol. Med. 2013;5:827–842. doi: 10.1002/emmm.201202301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dworski S, Berger A, Furlonger C, Moreau JM, Yoshimitsu M, Trentadue J, Au BC, Paige CJ, Medin JA. Markedly perturbed hematopoiesis in acid ceramidase deficient mice. Haematologica. 2015;100:e162–e165. doi: 10.3324/haematol.2014.108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boot RG, Renkema GH, Strijland A, van Zonneveld AJ, Aerts JM. Cloning of a cDNA encoding chitotriosidase, a human chitinase produced by macrophages. J. Biol. Chem. 1995;270:26252–6. doi: 10.1074/jbc.270.44.26252. [DOI] [PubMed] [Google Scholar]
- 5.Ries M, Schaefer E, Lührs T, Mani L, Kuhn J, Vanier MT, Krummenauer F, Gal A, Beck M, Mengel E. Critical assessment of chitotriosidase analysis in the rational laboratory diagnosis of children with Gaucher disease and Niemann-Pick disease type A/B and C. J. Inherit. Metab. Dis. 2006;29:647–52. doi: 10.1007/s10545-006-0363-3. [DOI] [PubMed] [Google Scholar]
- 6.Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Invest. 1994;93:1288–92. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostik MM, Chikova IA, Avramenko VV, Vasyakina LI, Le Trionnaire E, Chasnyk VG, Levade T. Farber lipogranulomatosis with predominant joint involvement mimicking juvenile idiopathic arthritis. J. Inherit. Metab. Dis. 2013;36:1079–1080. doi: 10.1007/s10545-012-9573-z. [DOI] [PubMed] [Google Scholar]
- 8.Torcoletti M, Petaccia A, Pinto RM, Hladnik U, Locatelli F, Agostoni C, Corona F. Farber disease in infancy resembling juvenile idiopathic arthritis: identification of two new mutations and a good early response to allogeneic haematopoietic stem cell transplantation. Rheumatology (Oxford) 2014;53:1533–1534. doi: 10.1093/rheumatology/keu010. [DOI] [PubMed] [Google Scholar]
- 9.Erfan M, Haque AU, Ahmed SA. Farber’s disease: a case report. IntJPathol. 2015;13:115–119. [Google Scholar]
- 10.Schuchman E. A132: Farber disease explains subset of Juvenile Idiopathic Arthritis [abstract] Arthritis Rheum. 2014;66:S173. [Google Scholar]
- 11.He X, Li CM, Park JH, Dagan A, Gatt S, Schuchman EH. A fluorescence-based high-performance liquid chromatographic assay to determine acid ceramidase activity. Anal. Biochem. 1999;274:264–269. doi: 10.1006/abio.1999.4284. [DOI] [PubMed] [Google Scholar]
- 12.Pettus BJ, Baes M, Busman M, Hannun YA, Van Veldhoven PP. Mass spectrometric analysis of ceramide perturbations in brain and fibroblasts of mice and human patients with peroxisomal disorders. Rapid Commun. Mass Spectrom. 2004;18:1569–1574. doi: 10.1002/rcm.1520. [DOI] [PubMed] [Google Scholar]
- 13.Warnes GR, Bolker B, Bonebakker L, Gentleman R, Liaw WHA, Maechler TLM, Magnusson A, Moeller S, Schwartz M, Venables B. gplots: Various R programming tools for plotting data. R package version 2.10.1. 2011 Retrieved from http://cran.r-project.org/package=gplots.
- 14.Pandey MK, Grabowski GA. Immunological cells and functions in Gaucher disease. Crit. Rev. Oncog. 2013;18:197–220. doi: 10.1615/critrevoncog.2013004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tantawy AAG. Cytokines in Gaucher disease: Role in the pathogenesis of bone and pulmonary disease. Egyptian Journal of Medical Human Genetics. 2015;16:207–213. [Google Scholar]
- 16.Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, Ferrarini M, Abdel-Wahab O, Heaney ML, Scheel PJ, et al. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood. 2014;124:483–492. doi: 10.1182/blood-2014-03-561381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashida K, Nanki T, Girschick H, Yavuz S, Ochi T, Lipsky PE. Synovial stromal cells from rheumatoid arthritis patients attract monocytes by producing MCP-1 and IL-8. Arthritis Res. 2001;3:118–126. doi: 10.1186/ar149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusano KF, Nakamura K, Kusano H, Nishii N, Banba M, Ikeda T, Hashimoto K, Yamamoto M, Fujio H, Miura A, et al. Significance of the level of monocyte chemoattractant protein-1 in human atherosclerosis. Circ. J. 2004;68:671–676. doi: 10.1253/circj.68.671. [DOI] [PubMed] [Google Scholar]
- 19.Pavlova EV, Deegan PB, Tindall J, McFarlane I, Mehta A, Hughes D, Wraith JE, Cox TM. Potential biomarkers of osteonecrosis in Gaucher disease. Blood Cells Mol. Dis. 2011;46:27–33. doi: 10.1016/j.bcmd.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler. Thromb. Vasc. Biol. 2008;28:1928–1936. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 22.Hamada Y, Nagasaki H, Fujiya A, Seino Y, Shang QL, Suzuki T, Hashimoto H, Oiso Y. Involvement of de novo ceramide synthesis in pro-inflammatory adipokine secretion and adipocyte-macrophage interaction. J. Nutr. Biochem. 2014;25:1309–1316. doi: 10.1016/j.jnutbio.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Patel DD, Zachariah JP, Whichard LP. CXCR3 and CCR5 ligands in rheumatoid arthritis synovium. Clin. Immunol. 2001;98:39–45. doi: 10.1006/clim.2000.4957. [DOI] [PubMed] [Google Scholar]
- 24.Nishimoto N, Kishimoto T. Inhibition of IL-6 for the treatment of inflammatory diseases. Curr. Opin. Pharmacol. 2004;4:386–391. doi: 10.1016/j.coph.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Petrovic-Rackov L, Pejnovic N. Clinical significance of IL-18, IL-15, IL-12 and TNF-alpha measurement in rheumatoid arthritis. Clin. Rheumatol. 2006;25:448–452. doi: 10.1007/s10067-005-0106-0. [DOI] [PubMed] [Google Scholar]
- 26.Arana L, Ordoñez M, Ouro A, Rivera IG, Gangoiti P, Trueba M, Gomez-Muñoz A. Ceramide 1-phosphate induces macrophage chemoattractant protein-1 release: involvement in ceramide 1-phosphatestimulated cell migration. AmJPhysiol. Endocrinol. Metab. 2013;304:E1213–E1226. doi: 10.1152/ajpendo.00480.2012. [DOI] [PubMed] [Google Scholar]
- 27.Ramsubir S, Nonaka T, Girbes CB, Carpentier S, Levade T, Medin JA. In vivo delivery of human acid ceramidase via cord blood transplantation and direct injection of lentivirus as novel treatment approaches for Farber disease. Mol. Genet. Metab. 2008;95:133–141. doi: 10.1016/j.ymgme.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walia JS, Neschadim A, Lopez-Perez O, Alayoubi A, Fan X, Carpentier S, Madden M, Lee CJ, Cheung F, Jaffray DA, et al. Autologous transplantation of lentivector/acid ceramidase-transduced hematopoietic cells in nonhuman primates. Hum. Gene Ther. 2011;22:679–687. doi: 10.1089/hum.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drobnik W, Liebisch G, Audebert F, Fröhlich D, Glück T, Vogel P, Rothe G, Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003;44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, Rembiesa B, Klein RL, Hannun YA, Bielawski J, Bielawska A. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J. Lipid. Res. 2010;51:3074–3087. doi: 10.1194/jlr.D008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ichi I, Nakahara K, Miyashita Y, Hidaka A, Kutsukake S, Inoue K, Maruyama T, Miwa Y, Harada-Shiba M, Tsushima M, et al. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Evans E, Platt FM. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 2010;11:419–428. doi: 10.1111/j.1600-0854.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, Tawk M, Tiziano FD, Veillet J, Bayes M, Nolent F, Garcia V, Servidei S, Bertini E, Castro-Giner F, et al. Spinal muscular atrophy associated with progressive myoclonic epilepsy is caused by mutations in ASAH1. AmJHum. Genet. 2012;91:5–14. doi: 10.1016/j.ajhg.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dyment DA, Sell E, Vanstone MR, Smith AC, Garandeau D, Garcia V, Carpentier S, Le Trionnaire E, Sabourdy F, Beaulieu CL, et al. Evidence for clinical, genetic and biochemical variability in spinal muscular atrophy with progressive myoclonic epilepsy. Clin. Genet. 2014;86:558–563. doi: 10.1111/cge.12307. [DOI] [PubMed] [Google Scholar]
- 35.Rubboli G, Veggiotti P, Pini A, Berardinelli A, Cantalupo G, Bertini E, Tiziano FD, D'Amico A, Piazza E, Abiusi E, et al. Spinal muscular atrophy associated with progressive myoclonic epilepsy: A rare condition caused by mutations in ASAH1. Epilepsia. 2015;56:692–698. doi: 10.1111/epi.12977. [DOI] [PubMed] [Google Scholar]
- 36.Gan JJ, Garcia V, Tian J, Tagliati M, Parisi JE, Chung JM, Lewis R, Baloh R, Levade T, Pierson TM. Acid ceramidase deficiency associated with spinal muscular atrophy with progressive myoclonic epilepsy. Neuromuscul. Disord. 2015;25:959–963. doi: 10.1016/j.nmd.2015.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.