ABSTRACT
Background and objective: Viral nucleic acid may be detected for up to 6 months after an acute asthma deterioration, but the pattern and consequences of viral persistence after acute asthma are incompletely understood. This study investigates the frequency of viral persistence after acute asthma, assesses viral infectivity and determines the host inflammatory responses to viral persistence.
Methods: Adults and children presenting to hospital with acute asthma and a confirmed respiratory virus infection were studied acutely and at recovery 4–6 weeks later by clinical evaluation and induced sputum for viral and inflammatory mediator detection.
Results: Viral RNA was detected during both acute asthma and recovery visits in 17 subjects (viral persistence), whereas in 22 subjects viral RNA had cleared by recovery (viral clearance). The following viruses were detected at recovery: human rhinovirus: 16; respiratory syncytial virus: 2; influenza: 2. In subjects with viral persistence, eight isolates were different to the virus detected at Visit 1. Forty‐four per cent of the human rhinovirus isolates were infective at recovery. Asthma and infection severity were similar in the viral clearance and viral persistence groups. Viral persistence was associated with elevated IL‐10 mRNA and inducible protein‐10 gene expression.
Conclusions: Respiratory viral detection after acute asthma is common, and most often persistence is with non‐infective human rhinovirus. There is a host inflammatory response with an altered cytokine environment, and the viral RNA can be source of persistent infection. These effects may have longer‐term consequences in asthma.
Keywords: asthma, IP‐10, rhinovirus, sputum, viral persistence
Short abstract
Viral persistence is a recently recognized phenomenon. It is poorly characterized in asthma. This work defines the problem of viral persistence in asthma and examines the inflammatory consequences of this phenomenon.
INTRODUCTION
Asthma exacerbation is a significant problem for adults and children. Respiratory viral infections, in particular human rhinovirus (HRV) infection, represent the main causal factor. 1 An innate immune defect in asthmatic epithelium 2 can result in enhanced virus replication in virally induced asthma. 3 , 4 HRV, an RNA virus, is readily degraded by RNase activity and, consequently, shedding of HRV is typically expected to occur only briefly during a viral infection. The impaired interferon response in asthmatic epithelium that has been observed in vitro could result in delayed viral clearance, 2 and several reports have documented persistence of HRV nucleic acid in 44–50% of children, at 6 weeks after presentation to the emergency department with acute asthma 5 or wheezing. 6 Several studies have also reported detection of HRV nucleic acid in up to 35% of asymptomatic subjects. 7 , 8 , 9 The unexpected persistence of viral RNA in the upper respiratory tract of asthmatic children indicates a possible connection between viral infection and recurrent asthma. The clinical implications of these findings are unknown; however, airway hyperresponsiveness persists after virus infection in asthmatic children.
There are several possible explanations for the persistence of HRV after acute asthma with viral infection. These include:
-
•
Residual infection with the same viral serotype.
-
•
The virus is non‐infectious, and represents nucleic acid remnants, or viral quasispecies 10 , 11 representing viral mutations.
-
•
Re‐infection with a different virus or viral serotype.
-
•
The virus is colonizing, and not eliciting a host response.
The consequences of viral persistence could include enhancing chronic inflammation, or changing the pattern of local cytokine or chemokine responses. In RSV infection, latent or persistent viral infection has been linked to adverse asthma outcomes in the long term, and similar results have been reported following HRV infection. 12 Thus viral persistence could have long‐term adverse effects.
This study sought to address the pattern and consequences of viral persistence after acute asthma exacerbation by investigating the frequency of viral persistence following acute asthma, assessing the infectivity of the persistent virus, and determining the host inflammatory and cytokine response in asthmatic subjects with viral persistence.
METHODS
Subjects
The 39 subjects with acute asthma were a subset of a previously described cohort 13 who were selected based on: age >7 years; presentation to John Hunter Hospital Emergency Department with acute asthma; virus detected by PCR and/or culture; and attendance for a follow‐up visit. The study was approved by the Hunter New England Research Ethics Committee and written informed consent obtained.
Study design
Subjects were assessed on two occasions, within 48 h of hospital admission and 4–6 weeks later. During hospital admission, subjects were managed according to hospital guidelines and discharged when stable. At both visits subjects completed asthma history and asthma control questionnaires, 14 provided nasal and throat swabs and underwent spirometry combined with sputum induction using ultrasonic nebulization of isotonic saline as previously described. 15 Selected portions of induced sputum were allocated to: (i) a lytic solution (buffer RLT; Qiagen, Hilden, Germany) for RNA extraction and subsequent mRNA expression analysis and virus PCR; (ii) in vitro culture in virus‐permissive human epithelial cell lines (HeLa and HEp‐2) for outgrowth of respiratory viruses; 16 and (iii) sequential dithiothreitol and PBS for cellular dispersion and profiling and storage of cell free supernatant as previously described. 15 Briefly, cytospins were prepared, stained (May–Grunwald Geimsa) and a differential cell count obtained from 400 non‐squamous cells. The remaining solution was centrifuged (400 g, 10 min, 4°C) and the cell‐free supernatant was aspirated and stored at −70°C.
Molecular processing, PCR and HRV sequencing
RNA was extracted (RNeasy RNA Mini Kit, Qiagen) and reverse transcription performed using random hexamers and Superscript II RNase H+ reverse transcriptase enzyme as previously described. 13 Samples were assayed for the presence of rhinovirus (HRV), 13 enterovirus (HEV), 13 influenza virus types A and B (IFA, IFB), respiratory syncytial virus types A and B (RSVA, RSVB), 13 metapneumovirus (MPV) 13 and non‐SARS coronavirus (CoV) 13 virus RNA transcripts using real‐time ‘TaqMan’ methodology PCR assays (Table 1).
Table 1.
Virus real‐time PCR Taqman assays
| Primer or probe | Oligonucleotide sequences (5′‐3′) and fluorescent labels | Target |
|---|---|---|
| Rhinovirus (HRV) | ||
| Primer RHFWD | GCACTTCTGTTTCCCC | 5′ UTR |
| Primer RHRVS | GGCAGCCACGCAGGCT | |
| Probe RHP1 | FAM‐AGCCTCATCTGCCAGGTCTA‐TAMRA | |
| Probe RHP2 | FAM‐AGCCTCATCGACCAAACTA‐TAMRA | |
| Enterovirus (EV) | ||
| Primer EVFWD | TCCTCCGGCCCCTGA | 5′ UTR |
| Primer EVRVS | RATTGTCACCATAAGCAGCCA | |
| Probe EVP | FAM‐CGGAACCGACTACTTTGGGTGWCCGT‐TAMRA | |
| Respiratory syncytial virus type A | ||
| Primer RSVAFWD | AGATCAACTTCTGTCATCCAGCAA | N gene |
| Primer RSVARVS | GCACATCATAATTAGGAGTATCAAT | |
| Probe RSVAP | FAM‐CACCATCCAACGGAGCACAGGAGAT‐TAMRA | |
| Respiratory syncytial virus type B | ||
| Primer RSVBFWD | AAGATGCAAATCATAAATTCACAGGA | N gene |
| Primer RSVBRVS | TGATATCCAGCATATTTAAGTATCTTTATAGTG | |
| Probe RSVBP | FAM‐AGGTATGTTATATGCTATGTCCAGGTTAGGAAGGGAA‐TAMRA | |
| Metapneumovirus | ||
| Primer ALTNFWD | CAACAACATAATGCTAGGACATGTATC | nucleoprotein gene |
| Primer ALTNRVS | CCGAGAACAACACTAGCAAAGTTG | |
| Probe ALTNP | FAM‐TGGTGCGAGAAATGGGTCCTGAATCTGG‐TAMRA | |
| Primer NLNFWD | CATATAAGCATGCTATATTAAAAGAGTCTC | |
| Primer NLNRVS | CCTATTTCTGCAGCATATTTGTAATCAG | |
| Probe NLNP | FAM‐TGYAATGATGAGGGTGTCACTGCGGTTG‐TAMRA | |
| Non‐SARS coronavirus | ||
| Primer N229EFWD | CAGTCAAATGGGCTGATGCA | N gene |
| Primer N229ERVS | AAAGGGCTATAAAGAGAATAAGGTATTCT | |
| Probe N229EP | FAM‐CCCTGACGACCACGTTGTGGTTCA‐TAMRA | |
| Primer NOC43FWD | CGATGAGGCTATTCCGACTAGGT | |
| Primer NOC43RVS | CCTTCCTGAGCCTTCAATATAGTAACC | |
| Probe NOC43P | FAM‐TCCGCCTGGCACGGTACTCCCT‐TAMRA |
FAM, 6‐carboxyfluorescein; TAMRA, 6‐carboxy‐tetramethyl‐rhodamine; degenerate primers: W = A/T, R = A/G, Y = C/T.
A fragment of approximately 549 nucleotides encompassing the VP4/VP2 region and the hypervariable region in the 5′‐non‐coding region was amplified with one‐step RT‐PCR using HRV specific primers. 17 RT‐PCR products were visualized, purified (QIAquick Gel Extraction kit, Qiagen), and nucleotide sequences determined in cycle sequencing reactions using DYEnamic ET Dye terminator cycle sequencing kit (MegaBACE) (GE Healthcare, Fairfield, CT, USA). Sequence data were analysed (Sequencher, version 3.1.1) and nucleotide–nucleotide alignments performed using NCBI BLAST for assessment of percentage identity with prototype strains (GenBank).
Real‐time semi‐quantitative mRNA PCR for cytokines
TaqMan primers and probes specific for IL‐8, IL‐10, IP‐10, MIP‐1α, and RANTES mRNA were combined with those for the housekeeping gene human 18S ribosomal RNA (rRNA) in duplex real‐time PCR reactions as described. 18 Relative quantification of mRNA expression was determined 18 as the expression ratio of cytokine mRNA to 18S rRNA and compared with the same ratio measured in a calibrator sample (phytohaemagglutinin‐stimulated peripheral blood mononuclear cells obtained from a healthy volunteer). 13
Sputum supernatant measurement
Induced sputum concentrations of IL‐8 and soluble ICAM‐1 were measured by ELISA (R&D Systems, Minneapolis, MN, USA), validated for the use in sputum supernatant.
Statistical analyses
Analysis was performed using Stata 7 (Stata Corporation, College Station, TX, USA), with results presented as median (interquartile range) or n (%) as appropriate. Significance was determined (P < 0.05) from chi‐square test or non‐parametric Wilcoxon rank sum test, Kruskal–Wallis test or Fisher's exact test.
RESULTS
Thirty‐nine subjects presented with acute asthma and were assessed at recovery, a mean of 5.4 weeks later. The majority of subjects recruited for the study (30/39, 77%), were <18 years of age. Viral RNA was detected by PCR at both visits in 17 subjects (43.6%, viral persistence) (Table 2). In 22 subjects (56.4%), viral RNA was detected at the acute asthma episode, but had cleared by recovery (viral clearance). Subjects with viral persistence were more often male and reported less rhinitis (Table 3). Subjects in the viral persistence and viral clearance groups had a similar prevalence of atopy, background asthma therapy, severity of acute episode, frequency and types of viruses detected in the acute episode (3, 4).
Table 2.
Viral isolates at visits 1 and 2 in subjects with viral persistence after acute asthma
| Virus identification—Visit 1 | Virus identification—Visit 2 |
|---|---|
| Picornavirus † | Rhinovirus ‡ |
| Enterovirus ‡ | Picornavirus § |
| Influenza type A | Picornavirus § |
| Rhinovirus + enterovirus ‡ | RSV |
| Rhinovirus § | Rhinovirus § |
| Influenza type A + rhinovirus 53 | Rhinovirus ‡ |
| Picornavirus † | Picornavirus † |
| Rhinovirus ‡ | Rhinovirus ‡ |
| Rhinovirus (EV76—but sequencing match very poor) | Picornavirus † |
| Enterovirus ‡ | Influenza type B + rhinovirus ‡ |
| Rhinovirus (RV53) + enterovirus | Picornavirus † |
| Rhinovirus (RV29) | Picornavirus † |
| Enterovirus‐68 | Rhinovirus‐85 |
| Rhinovirus § | Rhinovirus‐46 |
| Picornavirus † | Picornavirus † + influenza type A |
| RSV | Rhinovirus ‡ |
| Rhinovirus‐43 | RSV type B + rhinovirus‐43 |
Sub‐classification to rhinovirus or enterovirus was negative.
No RNA left for serotype identification by direct RNA sequencing.
Unable to amplify the available RNA to perform sequencing.
Table 3.
Characteristics of subjects at Visit 1 with acute asthma and viral persistence (acute asthma, virus positive at visits 1 and 2), and acute asthma with viral clearance (virus positive at Visit 1 and negative at Visit 2)
| Viral persistence | Viral clearance | P‐value | |
|---|---|---|---|
| Acute asthma, virus positive Visit 1, positive Visit 2, | Acute asthma, virus positive Visit 1, negative Visit 2, | ||
| n | 17 | 22 | |
| Age (years) | 11.4 (10.5, 14.1) | 13.1 (8.6, 24.7) | 0.865 |
| Males, n (%)* | 10 (58.8) | 6 (27.3) | 0.047 |
| BMI (kg/m2) | 22.1 (20.7, 29.0) | 21.6 (18.6, 27.0) | 0.871 |
| Never smoked, n (%) | 13 (86.7) | 19 (86.4) | 0.482 |
| Age asthma diagnosed (years) | 3.0 (2, 8) | 2.5 (2, 5) | 0.719 |
| Atopy, n (%) | 15 (93.8) | 17 (85.0) | 0.613 |
| Rhinitis/hayfever, n (%) | 6 (42.9) | 13 (86.7) | 0.021 |
| ICS, n (%) | 10 (58.8) | 15 (68.2) | 0.738 |
| ICS, BDP equivalent (µg/day) | 1500 (500, 2000) | 650 (400, 1000) | 0.174 |
| LABA, n (%) | 6 (35.3) | 5 (22.7) | 0.387 |
| OCS, n (%) | 0 | 2 (9.1) | 0.495 |
| ER visits past year | 0 (0, 1) | 0 (0, 1) | 0.688 |
Results are median (IQR), Wilcoxon rank sum test, or n (%), Fisher's exact test.
BDP, beclomethasone dipropionate; ICS, inhaled corticosteroid; ER, emergency room; IQR, interquartile range; LABA, long‐acting beta agonist; OCS, oral corticosteroid.
Table 4.
Characteristics of the acute asthma exacerbation and viral isolates for acute asthma with viral persistence and acute asthma with viral clearance
| Viral persistence | Viral clearance | P‐value | |
|---|---|---|---|
| Acute asthma, virus positive Visit 1, positive Visit 2 | Acute asthma, virus positive Visit 1, negative Visit 2 | ||
| FEV1 at Visit 1 (L) | 1.5 (1.21, 1.87) | 1.39 (1.08, 1.61) | 0.150 |
| FEV1% predicted at Visit 1 | 64 (59, 71) | 60 (47, 67) | 0.158 |
| %Change in FEV1 at Visit 2 | 35.5 (22.8, 53.2) | 41.1 (17.6, 53.8) | 0.536 |
| ACQ (max = 7) at Visit 1 | 2.1 (0.9, 4.0) | 2.8 (2.0, 3.4) | 0.503 |
| %Change in ACQ at Visit 2 | −57.7 (−69.4, −3.6) | −70.8 (−82.6, −21.7) | 0.389 |
| CCS (max = 30) at Visit 1 | 7.0 (6.0, 13.0) | 9 (4, 17) | 0.757 |
| %Change in CCS at Visit 2 | −81.6 (−96.7, −38.1) | −62.3 (−90.0, −33.8) | 0.689 |
| Length of hospital stay (days) | 2.0 (1.8, 3.5) | 2.5 (1.0, 3.8) | 0.769 |
| Virus type, n (%) | |||
| Rhinovirus | 12 (70.5) | 17 (77.3) | |
| Enterovirus | 6 (35.3) | 10 (45.5) | |
| Influenza A | 2 (11.8) | 2 (9.1) | |
| Influenza B | 0 | 1 (4.5) | |
| RSV | 1 (5.9) | 0 |
Results are median (IQR), Wilcoxon rank sum test or n (%).
ACQ, Asthma Control Questionnaire; CCS, Common Cold Score.
Viruses detected at Visit 2
At recovery, viral RNA was detected in 17 subjects (43.6%, Table 2). The infectivity of the viruses was examined by their ability to infect a host cell line. At Visit 2, 43.8% (7/16) of the HRV isolates were able to infect a host cell line, whereas during the acute asthma exacerbation (Visit 1), 44.8% (13/29) isolates were infectious (P = 0.946). Of all the samples that were PCR positive at either visit, 22/32 (68.8%) were both culture and PCR positive at Visit 1 compared with 4/14 (28.9%) at Visit 2 (P = 0.011). These results indicate that viral nucleic acids are frequently detected at recovery for HRV infections, but not for other respiratory viral isolates, and that HRV detected at Visit 2 was less able to infect host cells than Visit 1 isolates.
Similarity of the viruses at visits 1 and 2
Of the 20 viral species detected at recovery, seven were from the same genus to the virus detected at Visit 1 (Table 2). Differences in viruses isolated at the two different visits occurred due to detection of RSV (n = 3) or influenza virus (n = 4) at either visit, and detection of a picornavirus at the alternate visit, or detection of an enterovirus at Visit 1 and a rhinovirus at Visit 2 (n = 2). Where the virus detected at recovery was able to infect a host cell line, we sequenced the viral VP 2/4 regions to determine the viral serotype. In one instance the same serotype was present at visits 1 and 2 (HRV‐43); however at Visit 2, HRV‐43 was detected in association with RSV type B. In another case the virus detected at recovery was a different serotype to the virus detected at the first visit: enterovirus 68 at Visit 1 and HRV 85 at Visit 2. Overall, eight of the 17 subjects with viral persistence at Visit 2 had isolates that were different to the virus detected during the acute asthma exacerbation. These data indicate that when a virus is detected at recovery and is capable of infection, it is often a different virus to that associated with the acute asthma exacerbation.
Clinical features of viral persistence and viral clearance groups
The clinical severity of the asthma exacerbation and the severity of the respiratory infection were similar in the viral clearance and viral persistence groups (3, 4). Both groups showed clinical recovery from the asthma exacerbation and from the respiratory infection (Fig. 1).
Figure 1.
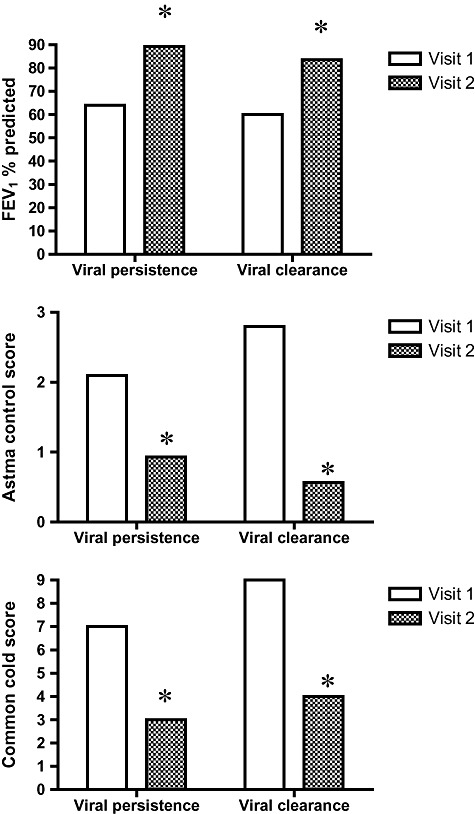
Clinical features during acute asthma (Visit 1) and after clinical recovery (Visit 2) in groups with viral persistence and viral clearance at Visit 2. *P < 0.05, Visit 1 versus Visit 2. Graphs indicate median values, differences between visits 1 and 2 are analysed using the Wilcoxon rank sum test.
Airway inflammation in viral persistence
5, 6 describe markers of airway inflammation in the subset of subjects for which adequate samples were available. The cytokine environment was altered in the viral persistence group, with elevated IL‐10 mRNA and IP‐10 gene expression (Table 5, Fig. 2). Viral persistence group was not altered, with similar gene expression for IL‐8, RANTES and MIP‐1α. Similarly, protein levels of IL‐8 and ICAM‐1 were similar in both groups (Table 5). Induced sputum cell counts at recovery were similar in the viral persistence and viral clearance groups (Table 6).
Table 5.
Cytokine gene expression and mediator results at Visit 2 for acute asthma with viral persistence and acute asthma with viral clearance
| n | Viral persistence | n | Viral clearance | P‐value | |
|---|---|---|---|---|---|
| Acute asthma, virus positive Visit 1, positive Visit 2 | Acute asthma, virus positive Visit 1, negative Visit 2 | ||||
| IP‐10 (RER%) | 13 | 118.9 (9.1, 1679.6) | 16 | 6.7 (1.1, 57.4) | 0.028 |
| RANTES RER% | 17 | 62.4 (18.6, 167.0) | 19 | 42.0 (11.7, 80.7) | 0.537 |
| MIP‐1α RER% | 17 | 18.8 (10.2, 22.0) | 19 | 13.5 (3.0, 33.7) | 0.350 |
| IL‐10 RER% | 17 | 12.59 (5.0, 30.6) | 19 | 3.15 (1.95, 5.0) | 0.011 |
| IL‐8 RER% | 17 | 78.4 (58.2, 202.8) | 19 | 57.0 (16.2, 298.9) | 0.537 |
| IL‐8 (ng/mL) | 11 | 4.48 (2.1, 20.7) | 8 | 6.1 (5.1, 10.1) | 0.804 |
| Soluble ICAM‐1 (pg/mL) | 8 | 2306 (792, 9989) | 8 | 2734 (761, 5698) | 0.916 |
Results are median (IQR), Wilcoxon rank sum test.
RER, relative expression ratio; see Methods section for details.
Table 6.
Sputum cell differentials at Visit 2 for acute asthma with viral persistence and viral clearance
| Viral persistence | Viral clearance | P‐value | |
|---|---|---|---|
| Acute asthma, virus positive Visit 1, positive Visit 2 | Acute asthma, virus positive Visit 1, negative Visit 2 | ||
| n | 15 | 14 | |
| Total cell count (×106/mL) | 4.77 (1.89, 5.76) | 2.88 (1.17, 4.68) | 0.327 |
| Neutrophils (%) | 34.8 (10.3, 53.5) | 14.3 (9.0, 42.3) | 0.316 |
| Neutrophils (×104/mL) | 130.4 (82.0, 200.2) | 111.7 (7.6, 165.9) | 0.462 |
| Eosinophils (%) | 1.9 (1, 4.4) | 5.3 (0.3, 15.6) | 0.303 |
| Eosinophils (×104/mL) | 5.9 (0, 7.3) | 3.3 (0, 40.8) | 0.967 |
| Macrophages (%) | 57.0 (43.3, 68.3) | 64.6 (42.3, 83.9) | 0.601 |
| Macrophages (×104/mL) | 255.5 (81.7, 431.9) | 100.3 (78.2, 269.9) | 0.414 |
| Lymphocytes (%) | 0.8 (0, 1.9) | 0.5 (0, 3.5) | 0.930 |
| Lymphocytes (×104/mL) | 3.0 (0, 7.2) | 1.2 (0, 3.5) | 0.481 |
| Columnar epithelial cells (%) | 0.5 (0.3, 2.5) | 0.6 (0, 1.2) | 0.343 |
| Columnar epithelial cells (×104/mL) | 4.0 (1.7, 10.9) | 3.1 (0, 3.4) | 0.151 |
| Squamous contamination (%) | 8.3 (3.2, 25.0) | 3.3 (0.7, 12.0) | 0.106 |
Results are median (IQR), Wilcoxon rank sum test.
Figure 2.

IP‐10 and IL‐10 mRNA expression at Visit 2. *P < 0.05, viral persistence versus viral clearance. RER, relative expression ratio (see Methods section for details). Graphs indicate median values; difference between groups is analysed using Kruskal–Wallis test.
Effect of persistent infection
The impact of persistent infective virus was examined by comparing the subjects with persistent infective (culture positive) virus at recovery (Visit 2) to culture positive subjects at Visit 1 and culture negative subjects at Visit 2 (Fig. 3). There were no differences in clinical features, sputum differentials or gene expression of IP‐10 or IL‐10 at follow up for those culture positive compared with those negative culture (P > 0.05). There were also no differences in change from Visit 1 in clinical features, sputum differentials, IP‐10 or IL‐10 gene expression for those culture positive at Visit 2 compared with those culture negative at Visit 2 (Fig. 3).
Figure 3.
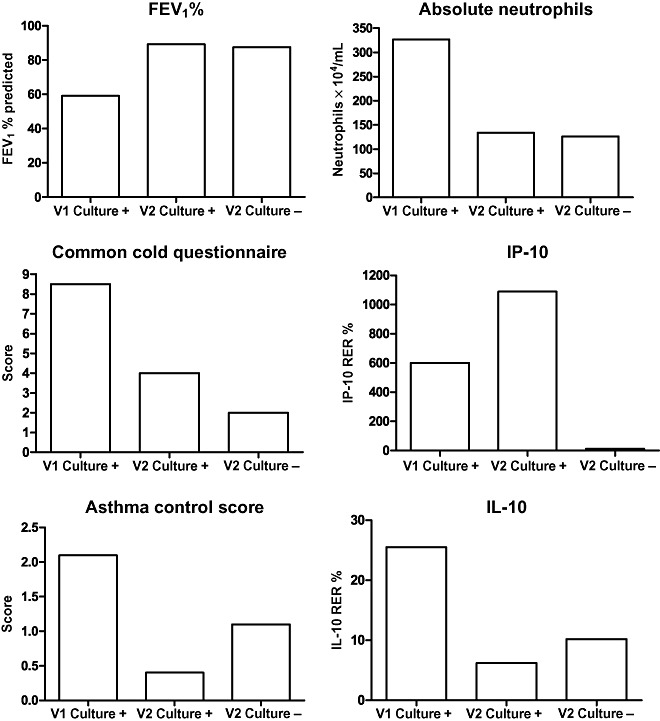
Effect of persistent infective virus (Visit 2 culture positive) on clinical and inflammatory measures compared with non‐infective virus (Visit 2 culture negative). Graphs indicate median values, difference between Visit 2 culture‐positive and Visit 2 culture‐negative groups analysed using Kruskal–Wallis test. V1, Visit 1; V2, Visit 2.
DISCUSSION
Respiratory viral infection plays a role in >80% of asthma exacerbations requiring hospitalization. The main virus detected during acute exacerbation is HRV, which has been shown to be present in 89% of cases of virus‐induced asthma. 3 In this study, we have shown that persistence of viral nucleic acid is common in asthmatics after an acute exacerbation and is associated with an altered cytokine environment, but no persistent clinical or inflammatory abnormality. The viral RNA observed most frequently to persist was HRV; however, persistence of the same infectious HRV was uncommon. When infectious virus was detected at recovery, it was often a different virus. The consequences of persistence of viral RNA were examined by assessing several disease domains. The clinical severity of the exacerbation and its recovery were similar whether there was clearance or persistence of viral RNA. In addition, the extent of cellular airway inflammation was similar between groups. However, viral persistence was associated with altered cytokine production, with elevated IL‐10 and IP‐10 gene expression. These results suggest that the cellular inflammation that results from viral replication is responsible for the clinical manifestations of a viral infection in asthma, rather than viral replication itself.
The period between infection by HRV and elimination of the virus with a negative PCR assay is not well described; however, HRV persistence has been seen in other circumstances. In vitro RV culture has been shown to decline rapidly (over less than 2 days). In most experimental infections, RV shedding detected by virus culture reaches a peak around 48 h after viral challenge, then rapidly declines, remaining positive for up to 3 weeks. 19 Gern et al. 20 found that experimental RV infection cleared by 14 days in 50% of adults. In clinical samples, HRV RNA positivity has remained detectable longer than culture positivity. Among young adults with a common cold, PCR was positive after 7 days in 45% of cases. 21 Enteroviruses may be shed for up to 2 weeks from the nasopharynx and several months in faeces. 22 Asymptomatic carriage of HRV has been identified in 5–35% of subjects. 9 , 23 This could represent the early subclinical phase of a new infection, or viral persistence. In asymptomatic children with no past (4 weeks) or future (2 weeks) respiratory symptoms indicating a clinical infection, the viral detection rate was as low as 5%. 9
Persistence of RNA viral infection has been previously reported. Measles virus persistence has been associated with subacute sclerosing panencephalitis (SSPE). 24 , 25 More recently persistent hepatitis C virus infection has been identified. 25 It has been suggested that several conditions must be met for viral persistence to occur. First, the virus should infect the cell without being cytopathic. The most common persistent virus in this study was HRV. HRV typically infects a host cell without causing cell death, and in asthma, apoptosis is suppressed after HRV infection. 2 , 4 Second, the viral genome must be maintained. For those RNA viruses that do not integrate into the host genome, such as HRV, continued replication at a low level is required to maintain infection. This has been shown to occur with RSV where gene transcription continues after the acute infection. 26 Third, the virus must avoid immune detection and elimination. Although specific antibodies to HRV develop after infection, their ability to protect against infection can be limited. After RSV infection, viral persistence can occur despite the presence of virus‐specific IgG. 26 Effective T‐cell responses are critical to eradicate acute viral infections and prevent viral persistence. It has recently been shown that virus‐specific CD4 T cells can be functionally inactivated early during the transition into viral persistence and fail to produce effector cytokines, thereby compromising an efficient and effective antiviral immune response. 27 If these mechanisms were to occur after HRV infection, this could lead to viral persistence.
When persistent viral infection does occur, there is an accumulation of viral mutations, as shown for measles, 24 SARS‐coV, 27 hepatitis C 25 , 28 , 29 and enteroviral persistence. 30 These mutations, or quasispecies, are particularly important for RNA viruses as they generate high levels of genetic variation that may facilitate viral adaptation to changing environments, including the development of drug resistance or escape from the immune system. 11 , 31 The development of picornavirus quasispecies can favour viral persistence, as frequently these species contain deletions in the 5′NTR that reduce replication to a level where cytopathic effect is not observed. 30 , 32 This may allow escape from the adaptive immune response that clears cytolytic wild‐type virus. 30 Increasing quasispecies diversity through chemical mutagenesis was recently found to restore pathogenicity of a poliovirus isolate. 11 It will be important in future work to characterize the RNA species of HRV that persist after an asthma exacerbation and to detect viral mutations that may be associated with viral persistence.
In this study the host response to viral persistence was limited. Subjects with viral persistence experienced a recovery of clinical features and improvement in inflammatory cell counts, even in subjects with persistent infective virus. This observation is interesting, as it implies that there are host defence mechanisms controlling the response to virus that are adequate to inhibit viral‐induced airway inflammation and clinical disease. Future identification of these responses could be very important. Viral persistence was associated with altered cytokine responses, with an ongoing elevation of IL‐10 and IP‐10 gene expression. A similar observation has been made with RSV persistence, where RSV‐RNA could be detected in lungs of infected mice for up to 150 days after the initial infection, 26 associated with persistent chemokine gene transcription. 13 The longer‐term consequences of viral persistence and this modified host response warrant further study. In addition, validation of these gene expression findings with protein measurements is an important area for further research.
When infectious virus was detected after recovery from acute asthma, it was often a different virus to that found during the acute exacerbation. In only one case did we confirm persistence of the same HRV serotype that was still able to infect host cells. The fact that a different virus was detected suggests that re‐infection is a significant mechanism for persistent viral detection after acute asthma exacerbation. This is also supported by the observation that IP‐10 gene expression was elevated in subjects with infective virus at Visit 2.
The role of HRV infection in leading to persistent asthma is an important and emerging research area. Our data deal with adults and children aged over 7 years studied during and post acute exacerbation. As such, we are studying populations where asthma is established and any effects of early HRV infection during the development of immunocompetence have already occurred. Further research, conducted in very young children without established disease, is necessary to determine the effects of viral persistence on asthma development.
In our study, 71% and 94% of subjects had HRV detected at visits 1 and 2, respectively. Thus the viruses identified in this study are representative of those typically associated with acute asthma. The PCR primers and probes used in this study detect all species of HRV. Hence, it is possible that the three HRV species, HRV‐A, HRV‐B and HRV‐C are represented among the samples with a generic HRV result. Indeed, a recent report of virus infection in children with acute asthma found that HRV was almost universally present and identified 52 HRV strains, including 16 HRV‐C strains. 33 Differences in the persistence of different strains and serotypes of HRV are not well understood. This highlights the need for further typing of HRV species in future work.
In conclusion, we have described that RNA viruses are commonly detected in airway samples after recovery from acute asthma. The viral species detected are frequently different to the infection that caused the original exacerbation; however, detection of non‐infectious HRV is also common. This virus is persistent, does not elicit a host clinical response, but may alter the host cytokine environment by leading to a persistent elevation of IL‐10 and IP‐10 gene expression. There may be longer‐term effects of viral persistence in asthma.
ACKNOWLEDGEMENT
This study was funded by the National Health and Medical Research Council of Australia.
REFERENCES
- 1. Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993; 307: 982–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wark P, Johnston S, Buccheri F et al Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 2005; 201: 934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wark PA, Johnston SL, Moric I et al Neutrophil degranulation and cell lysis is associated with clinical severity in virus‐induced asthma. Eur. Respir. J. 2002; 19: 68–75. [DOI] [PubMed] [Google Scholar]
- 4. Wark P, Gibson P. Pathogenesis of asthma exacerbation. Thorax 2006; 61: 909–15. doi:10.1136/thx.2005.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kling S, Donninger H, Williams Z et al Persistence of rhinovirus RNA after asthma exacerbation in children. Clin. Exp. Allergy 2005; 35: 672–8. [DOI] [PubMed] [Google Scholar]
- 6. Jartti T, Lehtinen P, Vuorinen T et al Persistence of rhinovirus and enterovirus RNA after acute respiratory illnes in children. J. Med. Virol. 2004; 72: 695–9. [DOI] [PubMed] [Google Scholar]
- 7. Johnston S, Sanderson G, Pattemore P et al Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J. Clin. Microbiol. 1993; 31: 111–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rakes G, Arruda E, Ingram J et al Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am. J. Respir. Crit. Care Med. 1999; 159: 785–90. [DOI] [PubMed] [Google Scholar]
- 9. Nokso‐Koivisto J, Kinnari T, Lindahl P et al Human picornavirus and coronavirus RNA in nasopharynx of children without concurrent respiratory symptoms. J. Med. Virol. 2002; 66: 417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Domingo E, Escarmis L, Menedez‐Arias L, Holland JJ. Viral quasispecies and fitness variations In: Domingo E, Holland JJ. (ed.) Origin and evolution of viruses. Academic Press, London, 1999; 141–61. [Google Scholar]
- 11. Vignuzzi M, Stone J, Arnold J et al Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 2006; 439: 344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lemanske RJ, Jackson D, Gangnon R et al Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J. Allergy Clin. Immunol. 2005; 116: 571–7. [DOI] [PubMed] [Google Scholar]
- 13. Grissell T, Powell H, Shafren D et al Interleukin‐10 gene expression in acute virus‐induced asthma. Am. J. Respir. Crit. Care Med. 2005; 172: 433–9. [DOI] [PubMed] [Google Scholar]
- 14. Juniper E, O'Byrne P, Guyatt G et al Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 1999; 14: 902–7. [DOI] [PubMed] [Google Scholar]
- 15. Wark PAB, Simpson JL, Hensley MJ et al Safety of sputum induction with isotonic saline in adults with acute severe asthma. Clin. Exp. Allergy 2001; 31: 1745–53. [DOI] [PubMed] [Google Scholar]
- 16. Payment P, Trudel M. (eds). Isolation and Identification of Viruses in ‘Methods and Techniques in Virology’. Marcel Dekker Inc, New York, 1993. [Google Scholar]
- 17. Savolainen C, Blomqvist S, Mulders M et al Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 2002; 83: 333–40. [DOI] [PubMed] [Google Scholar]
- 18. Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res. 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gwaltney J. Clinical significance and pathogenesis of viral respiratory infections. Am. J. Med. 2002; 112 (Suppl. 6A): 13S–18S. [DOI] [PubMed] [Google Scholar]
- 20. Gern J, Vrtis R, Grindle K et al Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am. J. Respir. Crit. Care Med. 2000; 162: 2226–31. [DOI] [PubMed] [Google Scholar]
- 21. Puhakka T, Makela M, Malmstrom K et al The common cold: effects of intranasal fluticasone propionate treatment. J. Allergy Clin. Immunol. 1998; 101: 726–31. [DOI] [PubMed] [Google Scholar]
- 22. Rotbart H. Enteroviruses In: Richman D, Whitley R, Hayden F. (eds) Clinical Microbiology. ASM Press, Washington, DC, 2002; 995–1018. [Google Scholar]
- 23. Marin J, Jeler‐Kacar D, Levstek V et al Persistence of viruses in upper respiratory tract of children with asthma. J. Infect. 2000; 41: 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rima B, Duprex W. Molecular mechanisms of measles virus persistence. Virus Res. 2005; 111: 132–47. [DOI] [PubMed] [Google Scholar]
- 25. Yagi S, Mori K, Tanaka E et al Identification of novel HCV subgenome replicating persistently in chronic active hepatitis C patients. J. Med. Virol. 2005; 77: 399–413. [DOI] [PubMed] [Google Scholar]
- 26. Schwarze J, O'Donnell D, Rohwelder A et al Latency and persistence of respiratory syncytial virus despite T cell immunity. Am. J. Respir. Crit. Care Med. 2004; 169: 801–5. [DOI] [PubMed] [Google Scholar]
- 27. Brooks D, Teyton L, Oldstone M et al Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 2005; 79: 10514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bowen D, Walker C. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J. Exp. Med. 2005; 201: 1709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen S, Wang Y. Multigene tracking of quasispecies in viral persistence and clearance of hepatitis C virus. World J. Gastroenterol. 2005; 11: 2874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim K, Tracy S, Tapprich W et al 5′‐Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative‐strand viral RNA. J. Virol. 2005; 79: 7024–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sullivan S, Mandava U, Evans‐Strickfaden T et al Diversity, divergence, and evolution of cell‐free human immunodeficiency virus type 1 in vaginal secretions and blood of chronically infected women: associations with immune status. J. Virol. 2005; 79: 9799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andino R, Rieckhof G, Baltimore D. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell 1990; 63: 369–80. [DOI] [PubMed] [Google Scholar]
- 33. Olenec JP, Kim WK, Lee WM et al Weekly monitoring of children with asthma for infections and illness during common cold seasons. J. Allergy Clin. Immunol. 2010; 125: 1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


