ABSTRACT
Lung transplantation has become an accepted therapeutic procedure for the treatment of end‐stage pulmonary parenchymal and vascular disease. Despite improved survival rates over the decades, lung transplant recipients have lower survival rates than other solid organ transplant recipients. The morbidity and mortality following lung transplantation is largely due to infection‐ and rejection‐related complications. This article will review the common infections that develop in the lung transplant recipient, including the general risk factors for infection in this population, and the most frequent bacterial, viral, fungal and other less frequent opportunistic infections. The epidemiology, diagnosis, prophylaxis, treatment and outcomes for the different microbial pathogens will be reviewed. The effects of infection on lung transplant rejection will also be discussed.
Keywords: Aspergillus, bacterial pneumonia, cytomegalovirus, immunosuppression, lung transplantation
INTRODUCTION
Significant progress has been made since the first human lung transplant (LT) in 1963, and although survival after transplantation was initially plagued by issues of rejection, the advent of immunosuppression ushered in a new era in transplantation science and made long‐term survival a possibility. With this success came the dilemma of post‐transplant infectious complications, which, to this day, remain a significant contributor to overall morbidity and mortality in the lung transplant recipient (LTR). Of all solid organ transplants, lungs are the most prone to infection, and this is likely due to several factors unique to the lung allograft. Apart from constant exposure to the outside environment, the lungs are exposed to the colonized native airway and have been stripped of their usual mechanisms of defence including the cough reflex, bronchial circulation and lymphatic drainage. These factors, coupled with the induction of an immunosuppressed state collaborate to produce an environment that is ripe for the development of infection.
Apart from direct injury, infection leads to several complications that may then have an effect on overall survival including the development of both acute and chronic rejection with eventual graft failure. The immune modulating effects of some pathogens, such as cytomegalovirus (CMV), can also augment the risk of developing other infections further leading to increased morbidity. 1
A thorough and comprehensive screening and management approach must be undertaken to optimize the survival of these patients and minimize the risk of infectious complications. We present a review of the major infectious complications following LT as well as recent recommendations for the evaluation and management of these entities.
EPIDEMIOLOGY
The respiratory tract is the most common area of infection after LT, and bacterial pneumonia is the most common infectious complication. CMV is the second most common complication, and its occurrence is much higher than in other solid organ recipients. 2 It appears that the critical period for infections after LT is within the first 90 days. In a recent epidemiological study in which 51 LTR were followed for a mean of 38.2 months, 75% of infectious episodes occurred within the first year after transplantation, and nearly half (42%) occurred within the first 3 months. 3 Bacterial disease accounted for the largest proportion of infections (48%) followed by viral, fungal and mycobacterial disease (35%, 13% and 4%, respectively). In the early post‐LT period (days to 1 month), nosocomial organisms account for the majority of infections. Following this period and for the next several months, at a time when immunosuppression is at the highest level, opportunistic organisms such as CMV and fungi account for the majority of infections. In the late post‐transplant period, community‐acquired bacterial and viral infections develop, although infection with health care‐associated organisms remains common (Fig. 1).
Figure 1.
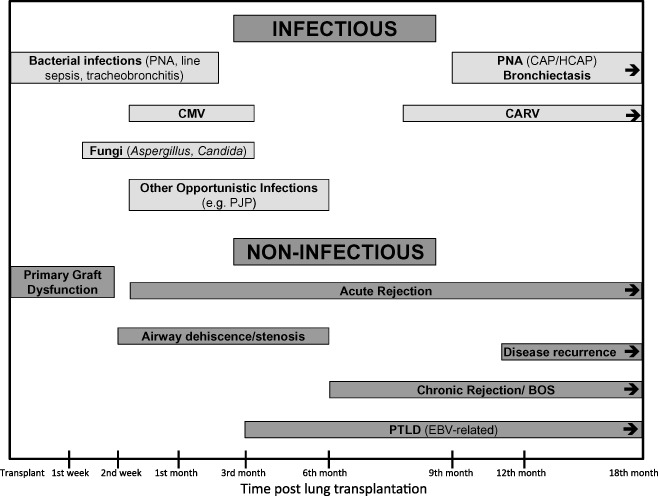
Infectious and non‐infectious complications after lung transplantation and the typical time frame in which they occur. Modified with permission from Levine. 4 BOS, bronchiolitis obliterans syndrome; CAP, community‐acquired pneumonia; CARV, community‐acquired respiratory virus; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HCAP, health care‐associated pneumonia; PJP, Pneumocystis jiroveci pneumonia; PNA, pneumonia; PTLD, post‐transplant lymphoproliferative disorder.
It is within the first year that infection makes the biggest impact on mortality. According to the Registry of the International Society for Heart and Lung Transplantation, infection is listed as the leading cause of mortality, accounting for 31% of deaths within the first year after transplant. 5 Thereafter, infection is a close second to bronchiolitis obliterans syndrome (BOS) as a cause of death. Recently, it has been increasingly recognized that infection may both predispose the airways to the development of BOS and increase the mortality of those with BOS, thus still contributing significantly to this mortality. 6
PREDISPOSING FACTORS FOR INFECTION
The lungs are unique organs in that they are constantly exposed to antigens from both the environment (inhaled antigens) and the bloodstream (blood‐borne antigens). The upper airways and pulmonary tissue have defence mechanisms composed of physical barriers and cellular components. Physical barriers include hairs in the nasal cavity, mucus secretions, cilia and turbulent airflow generated by the nasal cavity that prevent pathogens from reaching the lower airways. Despite these barriers, pathogens may still reach and infect the pulmonary tissue.
Anatomical factors
There are several risk factors that make LTR more vulnerable to infection (Table 1). Immediately post‐surgery, LTR may have disruption of normal physical barriers and are at risk of aspiration and infection (e.g. use of nasogastric and endotracheal tubes). 7 , 8 There are also other important changes that happen post‐surgery. First, during the surgical procedure of LT, there is a complete disruption of the bronchial circulation, and this may cause a loss of epithelium integrity, ciliary function and mucus production. 9 These effects are transient because of the development of collateral circulation but remain at risk of infection during the initial stages. 9 , 10 , 11 Second, denervation of the allograft may suppress the cough reflex and promote bronchial hyperresponsiveness. 2 Third, the lymphatic drainage of the allograft is also severed promoting stasis and oedema in the bronchial tissues impairing normal healing. 2 Fourth, stenosis or necrosis may occur at the site of the bronchial anastomosis, which may in turn facilitate colonization and invasion by opportunistic pathogens and decrease the clearance of secretions beyond the anastomosis. 12
Table 1.
Predisposing factors for infection in the transplant host
| Interruption of bronchial circulation |
| Disruption of the integrity of the epithelium |
| Abnormal ciliary function |
| Decreased sputum production |
| Denervation of the allograft |
| Diminished cough reflex |
| Bronchial hyperresponsiveness |
| Interruption of lymphatic drainage |
| Anastomosis site complications |
| Ischaemia, necrosis or dehiscence promoting colonization |
| Stenosis with impairment of secretion clearance |
| Previous colonizing pathogens |
| Contralateral lung (i.e. single lung transplant recipient) |
| Donor‐harboured pathogens |
| Recipient‐harboured pathogens |
| Immunosuppression |
| T‐lymphocyte dysfunction (e.g. calcineurin inhibitors) |
| B‐lymphocyte dysfunction (e.g. mycophenolate mofetil) |
| Macrophage and cytokine dysregulation (e.g. corticosteroids) |
Immunosuppression
At the cellular level, the LTR is vulnerable to infection due to the immunosuppression regimen used to prevent rejection affecting multiple inflammatory cellular lines and cytokines. The regimen consists of induction agents (medications used immediately post‐transplant) and maintenance agents for prolonged use. Because immunosuppression is needed indefinitely, LTR has a life‐long increased risk for opportunistic pathogens to proliferate and cause significant complications.
Maintenance agents
The maintenance immunosuppression regimen consists typically of a calcineurin inhibitor, an antimetabolite and corticosteroids. 13 , 14 The calcineurin inhibitors used in LT are cyclosporine A and tacrolimus. Cyclosporine A binds to cyclophylin preventing the activation of the nuclear factor of activated T‐lymphocytes (T cells) by calcineurin. Tacrolimus binds to FK‐binding protein 12 inhibiting calcineurin and preventing the activation of the nuclear factor of activated T cells. 13 , 15 By reducing the activation of nuclear factor of activated T cells, both drugs reduce the production of interleukin‐2 limiting the clonal expansion of activated T cells (Fig. 2). 16
Figure 2.
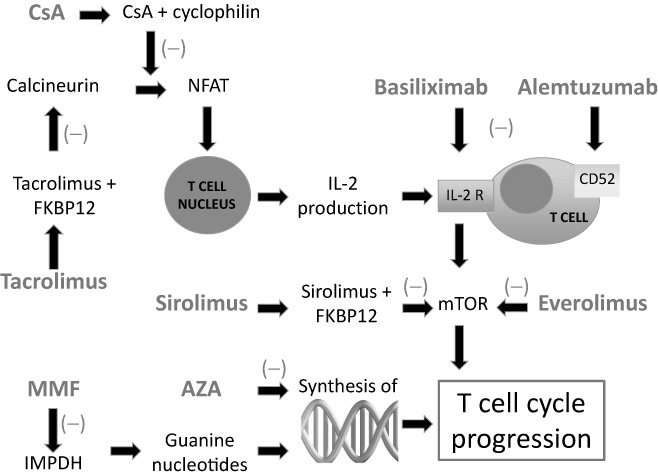
A schematic overview of the mechanisms of action of medications used for immunosuppression. IL‐2 is required for the activation of the mTOR pathway and progression of the T cell cycle. Both CsA and tacrolimus reduce the activation of NFAT, which in turn results in a decreased production of IL‐2. Basiliximab is a monoclonal antibody that inhibits the IL‐2 receptor. Sirolimus and everolimus inhibit the mTOR pathway through inhibition of specific enzymes. Alemtuzumab targets protein CD52 causing T cell dysfunction. Both MMF and AZA disrupt key elements of the deoxyribonucleic acid synthesis affecting the progression of the T cell cycle. AZA, azathioprine; Csa, cyclosporine A; FKBP12, FK‐binding protein 12; IMPDH,inosine‐50‐monophosphate dehydrogenase; IL‐2, interleukin‐2; IL‐2R, IL‐2 receptor; MMF, mycophenolate mofetil; mTOR, mammalian target of rapamycin; NFAT, nuclear factor of activated T‐lymphocytes.
Azathioprine and mycophenolate mofetil (MMF) are the commonly used antimetobolites after LT. Azathioprine, a derivative of 6‐mercaptopurine, inhibits both ribonucleic acid and deoxyribonucleic acid production, reducing the proliferation of both T cells and B‐lymphocytes. MMF is a prodrug of mycophenolic acid, an inhibitor of the inosine monophosphate dehydrogenase (Fig. 2). This enzyme is responsible for the synthesis of guanine nucleotides, which both T cells and B‐lymphocytes are critically dependent of. 17
Other maintenance agents that have been used less frequently to maintain immunosuppression include sirolimus and everolimus. Sirolimus binds to the FK‐binding protein 12 and through the mammalian target of rapamycin pathway prevents the synthesis of deoxyribonucleic acid and proteins by T cells (Fig. 2). 18 Through an independent mechanism, sirolimus also affects B‐lymphocytes and decreases cytokine and antigen production. 19 Everolimus reduces the mammalian target of rapamycin kinase activity, inhibiting the downstream pathways of proliferation and activation of T cells. 20
Finally, through the alteration of gene transcription factors, corticosteroids can exert a wide variety of immunosuppressive effects: interruption of antigen presentation, changes in the production of cytokines and alteration in the proliferative responses of various cell lines. 21
Induction agents
The use of induction agents after LT varies among centres. These agents include OKT3, antithymocyte globulin (ATG), alemtuzumab and basiliximab. OKT3 is a murine monoclonal antibody that inactivates the T cell receptor–CD3 complex preventing the activation of circulating T cells with a partial sparing of T regulatory cells. ATG is a polyclonal antibody directed against lymphocytes. It depletes circulating lymphocytes through complement‐mediated lysis and destruction by the reticuloendothelial system after opsonization. 13 Basiliximab is a chimeric monoclonal antibody that targets the α subunit of the interleukin‐2 receptor inhibiting the differentiation and proliferation of T cells. 22 , 23 Alemtuzumab is a murine monoclonal antibody that targets CD52. This receptor is present in macrophages, monocytes, B‐lymphocytes and T cells among other inflammatory cells. The binding of CD52 causes complement‐mediated cytolysis and activation of pathways leading to apoptosis. 13
The use of OKT3 is now significantly limited due to an increase risk of infection. 24 , 25 , 26 , 27 For this reason, most centres have elected to use ATG, basiliximab or alemtuzumab, in combination with corticosteroids for induction of immunosuppression after LT. 28 Evaluation of large series of solid organ recipients has shown that this combination prevents graft rejection and improves survival. 29 ATG does not increase the rate of infections in transplant recipients and has been associated with a survival benefit. 30 , 31 Basiliximab compared with ATG does not increase the risk of infection and was safer than OKT3 in heart and LTR. 22 , 23 , 26 , 32 Alemtuzumab was recently shown to improve survival compared with ATG. 33 Despite these positive outcomes, the immunosuppression is more profound during induction, and patients should be monitored closely for infection during this period.
Recipient‐harboured infection in patients with suppurative lung disease
Despite the removal of both lungs during bilateral procedures, residual colonization and/or infection can remain in the thoracic cavity, the bloodstream, the upper airways or the sinuses. Those patients with cystic fibrosis (CF) present the highest risk for recipient‐harboured infection due to the frequent colonization and infection with multiresistant microorganisms including bacteria (Gram‐negative rods and Gram‐positive cocci) and fungi. Resistant Gram‐negative organisms pose perhaps the greatest risk, and some studies suggest an association between pre‐transplant colonizing organisms from patients with suppurative lung disease and pneumonias following LT. 34 The majority of recent data suggests that patients colonized with multi‐drug‐resistant pseudomonas appear to have acceptable outcomes, including survival following LT, and should not be excluded on that criterion alone. 35 , 36
In contrast, a former subspecies of pseudomonas, now subspeciated as Burkholderia cenocepacia due to its unique resistance patterns, can pose significant problems in transplant recipients. There have now been at least nine distinct genotypic variants (genomovars) identified in the Burkholderia cenocepacia complex. 37 Colonization with Burkholderia cenocepacia complex (genomovar 3) can result in significant morbidity and mortality post‐transplant and should be considered a strong relative contraindication to LT, 38 , 39 although isolated reports of successful outcomes have been reported. 40 In one study of 75 patients, 38 there was a significant difference in 1‐year‐survival between those patients not infected (92%) and those colonized with a non‐Burkholderia cenocepacia strain (89%) compared with those colonized with Burkholderia cenocepacia (29%). Similar results of variable survival rates based on Burkholderia cenocepacia species have been found in other studies. 37 , 39 Because of these overwhelming data, the majority of transplant centres will not transplant colonized or infected patients with this organism.
Donor‐harboured infection
When evaluating the potential LT donor, routine screening is done to prevent transmission of donor‐harboured infection to the recipient. 41 Donor screening includes routine serology for viral infection including CMV, Epstein–Barr virus, varicella‐zoster, hepatitis B and C, and human immunodeficiency virus, among others. In addition, the potential donor lungs are evaluated radiographically and bronchoscopically.
Despite these measures, infection may still occur. To potentially pre‐empt the development of donor‐transmitted infection at the time of the transplant procedure, a culture swab or wash, or a portion of the donor bronchus is sent for culture. In contrast with some older studies, 42 , 43 more recent data suggest that recovery of an organism from the donor lung, including a positive Gram stain, or subsequent growth in culture does not always translate into infection and/or poor outcomes in the recipient. 34 , 44 , 45 In one study of 80 LTR, the investigators noted that organisms were grown from 57% or 89% of donors for a total number of isolates of 149. 44 Of these, most isolates were staphylococci or streptococci. Post‐transplant pneumonias were found in 41% of recipients in this study; however, pseudomonas, and not Gram‐positive organisms, was the most prevalent causative organism. The results of this study and others 45 suggest that the presence of organisms in the donor does not necessarily predict post‐transplant pneumonia, and perhaps this donor criterion should be re‐evaluated. Despite these suggestions and because empirical bacterial prophylaxis was used in the majority of these studies, the general practice is to routinely initiate prophylactic, broad‐spectrum antibiotics (regimens are discussed later) and then narrow the antibiotic therapy based on donor isolates. 41
Native lung infection
Any patient with suppurative lung disease, such as CF or bronchiectasis, being considered for LT will receive a bilateral procedure with attempts at avoiding infection from a remaining native lung. However, in those diagnoses where a single LT may be performed, such as chronic obstructive pulmonary disease or interstitial lung disease, the native lung may harbour infectious organisms that can infect the new graft, particularly when the patient is subjected to immunosuppression. Alternatively, the native lung can develop severe infection leading to sepsis and further compromise. Although attempts at avoiding this risk are undertaken by routine pretransplant screening, examples of infection that can be harboured in the native lung include bacteria, fungi (perhaps contained in a mycetoma) or non‐tuberculous mycobacteria (NTM). 46
General recipient screening
As part of the initial pretransplant evaluation, all potential transplant recipients should undergo careful screening for infection. Although there may be some variation between transplant centres, routine screening includes serological measurement for CMV, Epstein–Barr virus, varicella‐zoster, hepatitis B and C, and human immunodeficiency virus, and screening for latent tuberculous infection. The results obtained from this screening are used to assess the patient's overall candidacy for LT (e.g. human immunodeficiency virus is generally an exclusion) and also to stratify the patient for screening and prophylaxis in the post‐LT period (e.g. CMV and Epstein–Barr virus). Recommendations for recipient and donor presolid organ transplant screening are published from the American Society of Transplantation. 41
BACTERIAL INFECTION
Pneumonia
Early pneumonia
Pneumonias comprise the most common cause of infection following LT, and bacterial pathogens remain the most common cause of all pneumonias. 34 , 47 In a multicentre, prospective study from Spain, with a median follow‐up of 180 days, 85 episodes of pneumonia were documented in 236 LTR for an incidence of 72 episodes/100 LT years. 47 Of these, bacteria were the most common pathogen accounting for 82.7% of the pneumonias.
Bacterial pneumonia is most common in the early post‐transplant period (1–30 days) usually due to infection with health care‐associated and nosocomial organisms (Fig. 1). In the Spanish study, 40 of 85 of pneumonias (44%) occurred in the first 30 days following transplant. Nearly 3/4 of all bacterial pneumonias (72%) were due to Gram‐negative organisms—most commonly pseudomonas (incidence 118.6 episodes per 1000 LTR/year). Staphylococcus aureus and Acinetobacter infections were the second most common bacterial isolates (each with an incidence of 67.8 episodes/1000 LTR/year). The median time to development of Gram‐negative pneumonia was 31 days with a range of 3–394 days. Gram‐positive cocci‐related pneumonias also occurred in the early post‐transplant period at a median of 35.5 days (range 2–486 days) post‐transplant. Other bacterial isolates from this and other studies span the spectrum of health care‐acquired infectious organisms. Similarly, P. aeruginosa was found to be the most common isolate accounting for 33.3%, Staphylococcus aureus comprised 26.8%, and Aspergillus 16%. 34
Late pneumonia
Pneumonia is also seen in the late post‐transplant period. Throughout the lifespan of the LTR, ongoing contact with hospital settings, both outpatient and inpatient, and frequent antibiotic exposure commonly result in infections with health care‐associated, often resistant, pathogens. Community‐acquired pneumonias can also develop in the late post‐transplant period. 48 In a single‐centre study, 14 out of 220 LTR (6.4%) developed invasive pneumococcal infection (pneumonia and/or sepsis) at a median of 1.3 years after transplantation (incidence rate: 22.7 cases per 1000 person‐years). Routine vaccination for pneumococcus with the pneumococcal polysaccharide vaccine is recommended both before and every 5 years following LT. 41
Diagnosis
In general, the approach to suspected pneumonia at any time period post‐transplant includes sputum, blood cultures and often bronchoscopy with bronchoalveolar lavage (BAL), sterile brush and sometimes biopsy. The role of new biomarkers such as procalcitonin for diagnosis or follow‐up has not been well established in the LTR.
Prophylaxis
Due to the high incidence of early post‐transplant pneumonia, whether derived from the recipient, donor or nosocomially acquired, broad‐spectrum postoperative prophylaxis is routinely used. Prophylaxis in the post‐transplant period varies by centre but typically includes a third generation cephalosporin and vancomycin and is then tailored to the results of donor and recipient cultures, or as clinically indicated for 7–10 days. Prophylactic antibiotic treatment should be extended to 14 days for known pretransplant recipient colonization. For specific prophylactic regimens for viral and fungal pathogens, see later.
Treatment
Treatment of bacterial pneumonia includes standard regimens as outlined by the American Thoracic Society and Infectious Disease Society of America treatment for health care‐acquired pneumonia. 49 In the setting of known prior colonization or infection, initial antibiotic selection may be based on prior culture and sensitivity results. Typical antibiotics used should include coverage for Gram‐negative (including pseudomonas) and Gram‐positive (including Staphylococcus aureus) pathogens. In general, 8–14 days of therapy is recommended. In the case of resistant organisms, inhaled aminoglycosides may also be added to the treatment regimen.
Outcomes
Pneumonia has significant impact on overall post‐transplant survival and the eventual complication of chronic rejection. In the Spanish study, attributable 1‐year survival was reduced in those patients developing pneumonia of any aetiology (29.5% mortality) versus those without pneumonia (14% mortality), although bacterial pneumonia alone was not separated out in this analysis. These authors also found that the probability of survival during the first year of follow‐up was significantly higher in the multivariate analysis in LT recipients who did not have a pneumonia episode compared with those that had at least one episode of pneumonia. 47 In the Bonde et al. study, pneumonia was found to be an independent predictor of overall mortality. 44
VIRAL INFECTION
Viral infection after LT is common and classified into disease caused by CMV or caused by other community‐acquired respiratory viruses (CARV). A recent study showed that a viral pathogen was responsible for 25 of 71 infectious episodes in a cohort of LTR, with CMV accounting for 68% of those cases. Additionally, the majority of CMV episodes occurred within the first 3 months following LT, while the majority of the later infections were due to influenza and occurred after 1 year (Fig. 1). 3
CMV
Among the opportunistic infections following LT, CMV is the most prevalent and most important despite significant advances in both diagnosis and management. As well as contributing directly to both morbidity and mortality, mounting evidence suggests a relationship between CMV pneumonitis and chronic rejection in the form of BOS and decreased survival despite treatment. 50 CMV seropositivity can range from 30% to 97% in the general population, and after infection, the patient will harbour the virus for life. Of all solid organ transplants, LTR has the highest risk of developing CMV disease. 51 The incidence of CMV infection has been reported to range from 30% to 86% in post‐LTR, with a mortality of 2–12%. 52 This increased incidence is thought to be due partly to the high viral load of CMV transmitted in the lymphatics of the lung compared with other solid organs, as well as the high level of immunosuppression required for lung allograft.
The most important risk factor for the development of CMV infection is the donor‐positive/recipient‐negative serostatus of a transplant patient, as these patients will lack immunity to CMV. The lowest risk occurs in donor‐negative/recipient‐negative patients. 51 Other important risk factors include type and intensity of both induction and maintenance immunosuppression, concurrent infections, rejection and host factors such as age or comorbities. 51 , 52
There is almost a symbiotic relationship between rejection and CMV infection. Both of these individual processes induce a cytokine cascade that in essence promotes the development of the other. Tumour necrosis factor‐alpha, a key signal in the reactivation of CMV from latency, is released during allograft rejection, thereby facilitating the onset of viral replication and subsequent infection. Conversely, infection of the vascular endothelium and smooth muscle by CMV leads to an upregulation of adhesion molecules promoting an increase in the quantity of inflammatory cells in the graft and subsequent development of rejection. Additionally, molecular mimicry and the production of anti‐endothelial antibodies with CMV may also play a role in the development of rejection. 52 CMV serology of both donor and recipient must be checked prior to transplant. 53
Diagnosis
There is an important distinction between CMV infection and disease. Infection is defined as ‘CMV replication regardless of symptoms’, while disease is defined as ‘evidence of CMV infection with attributable symptoms’, such as ‘a viral syndrome with fever and/or malaise, leukopenia, thrombocytopenia or as tissue invasive disease’. 51 , 54
Recent technologies have effected a shift in the diagnosis of CMV infection and disease. The previous method of diagnosis, pp65 antigen detection, has been replaced by quantitative nucleic acid‐based amplification testing via polymerase chain reaction (PCR) for the recognition of viraemia by most centres, with 85% of institutions using this method for monitoring and diagnosis. 55 There are no universally accepted viral load cut‐offs for positive and negative results, and that reported values may be dissimilar between different laboratories. Despite this, current guidelines on the management of CMV in solid organ transplant patients do not clearly favour one test over the other and cite both as acceptable options for diagnosis. Additionally, viral culture of blood or urine has a limited role for diagnosis and is not routinely recommended. 53
Most recently, tests for cell‐mediated immunity against CMV have shown promise for predicting risk of developing disease. Lisboa and colleagues demonstrated that cell‐mediated immunity to CMV, as shown by a CD8+ T cell response assay, was associated with decreased risk of developing disease in patients with detectable low‐level viraemia. Twenty four of 26 patients (92.3%) with a positive interferon‐gamma release assay were able to clear their viraemia without disease compared with 5 of 11 (45.5%) in patients with a negative cell‐mediated immunity at onset (P = 0.004). 56 In a similar study, the same group was able to show that a negative assay was associated with a higher chance of developing late‐onset CMV after prophylaxis. In their study, CMV disease occurred in 2/38 (5.3%) patients with a detectable interferon‐gamma response versus 16/70 (22.9%) patients with a negative response (P = 0.038). 57
Prophylaxis
There are two accepted approaches to the prevention of disease from CMV, universal prophylaxis and pre‐emptive therapy, and although there are no randomized trials comparing one strategy versus the other in LTR, most centres favour the former or may sometimes employ both. 55 The first, universal prophylaxis, involves administration of antivirals to all transplant patients deemed to be at high risk by serostatus. The second, pre‐emptive therapy, is comprised of monitoring at‐risk patients for viral replication and administering antivirals at a predetermined level of replication in the hopes of treating patients prior to the onset of disease. A Cochrane Review comparing prophylaxis in different groups of solid organ transplant patients with antivirals versus placebo or no treatment showed a significant reduction in disease (relative risk 0.42), infection (relative risk 0.61), mortality from CMV disease (relative risk 0.26) and all‐cause mortality (relative risk 0.63). Interestingly, the review also found a decrease in the risk of developing herpes‐simplex virus, varicella‐zoster virus and bacterial infections. 58
Prophylaxis may not only be beneficial in decreasing direct morbidity and mortality from CMV disease but may also have secondary effects by decreasing the morbidity and mortality of both acute and chronic rejection. The Cochrane Review mentioned earlier failed to show a difference in acute rejection episodes, but other small studies have shown statistically significant differences in LTR specifically and it is generally believed that prevention of CMV decreases the risk for acute rejection. 58 , 59 , 60 The data for BOS are more encouraging. A recent study by Chmiel and colleagues was able to show a 23% absolute risk reduction of developing BOS in a group of LTR on CMV prophylaxis as compared with a historical cohort that was not prophylaxed and a 35% absolute risk reduction compared with data in the literature (P = 0.002). 1
Most centres provide prophylaxis for a period of 3–6 months after transplantation; however, the optimal duration of prophylaxis has not been well established and is currently under debate. 55 The guidelines recommend a minimum of 6 months for LTR. 53 Recent data suggest that this window of prophylaxis should possibly be extended, especially for donor‐positive/recipient‐negative patients. Palmer and colleagues report the first randomized, placebo‐controlled trial showing a decrease in the risk of CMV disease with extended prophylaxis. In this study, 136 LTR who completed 3 months of valganciclovir prophylaxis were randomized to an additional 9 months of valganciclovir versus placebo. The risk of CMV disease was reduced (32% vs 4%; P < 0.001) in the extended‐course group versus the short‐course group. There were also statistically significant reductions in CMV infection (64% vs 10%; P < 0.001) and disease severity as measured by viral load with extended treatment. Acute rejection episodes, opportunistic infections, adverse events and CMV UL97 ganciclovir‐resistance mutations were similar between both groups. 61 The international consensus guidelines list valganciclovir and ganciclovir (oral or intravenous (IV)) as the antivirals of choice for the prevention of CMV disease and state that CMV immunoglobulin may also be used in combination with these two, but there are limited data to support its use. 53
Treatment
Although foscarnet was commonly used in the past for CMV disease, the significant risk of nephrotoxicity with concomitant calcineurin‐inhibitor use has made it fall out of favour for the relatively safer agents ganciclovir and valganciclovir. 55 And, although the recommendation for treatment of severe disease is still IV ganciclovir, the results of the Valcyte in CMV disease Treatment of Solid Organ Recipients trial have made valganciclovir a viable choice in the treatment of less severe CMV. 53 The in CMV disease Treatment of Solid Organ Recipients trial randomized 321 solid organ transplant recipients with non‐life‐threatening CMV disease to either oral valganciclovir or IV ganciclovir. Valganciclovir demonstrated non‐inferiority in regard to clinical resolution of disease as well as eradication of viraemia in both the intent‐to‐treat and the per‐protocol arms of the study. 62 The current guidelines recommend oral valganciclovir at twice‐daily dosing or IV ganciclovir for the treatment of non‐severe CMV disease. As there are no efficacy data for valganciclovir in severe or life‐threatening disease, IV ganciclovir is still the ‘gold standard’ for those patients. In both groups, serial monitoring of viraemia should occur optimally at 1‐week intervals, and treatment should be continued for a minimum of 2 weeks and until viral eradication has been documented with two consecutive tests. The use of secondary prophylaxis is generally recommended for 1–3 months after treatment of disease. 53
CARV
Infection with a CARV is common after LT, and with the development of new diagnostic techniques, the incidence quoted in older literature is likely underestimated. A study of LTR undergoing serial surveillance and diagnostic BAL over a 3‐year period showed that a respiratory virus was isolated in 51.6% of patients on at least one BAL sample. Rhinovirus was the most common pathogen isolated, followed by parainfluenza, coronavirus, influenza, metapneumovirus and respiratory syncytial virus (RSV). 63 , 64 CARV is being increasingly recognized as contributors to significant morbidity in immunocompromised hosts and can cause severe and life‐threatening pneumonitis. Additionally, there appears to be evidence that infection with these organisms can also lead to a decrease in graft survival. A retrospective cohort study of 259 LTR followed over 5 years showed a significantly increased risk of developing BOS or death from BOS in the group that was diagnosed with a CARV infection. 65
Given the paucity of effective antiviral treatment for most of these viruses, early diagnosis is essential for both treatment and to minimize spread among other immunocompromised patients. With the exception of influenza and RSV, for which treatments exist, supportive care and a reduction in immunosuppression remain the cornerstones of care for the treatment of CARV. A complete listing of all the viruses that commonly affect LTR would be beyond the scope of this article so we will focus on those that have the most clinical bearing, namely influenza, RSV, human metapneumovirus and parainfluenza. As it typically does not cause respiratory tract disease, we will not discuss Epstein–Barr virus, except to mention its known association with post‐transplant lymphoproliferative disorder after LT.
Influenza
Infection of normal hosts with influenza most commonly causes a self‐limited disease with upper respiratory symptoms, myalgias and fever; however, infection in LTR appears to be associated with increased risk of lower respiratory tract involvement by either a primary viral or a concomitant bacterial superinfection. This was illustrated in a small series of LTR admitted for influenza where all appeared to have pulmonary parenchymal involvement on imaging and by BAL as well as in another series by Vilchez and colleagues, where 7 of 15 patients with influenza were found to have pulmonary infiltrates, 5 of which were attributed to a primary viral pneumonia after BAL. 66 , 67 Novel H1N1 influenza appears to have similar clinical features, although there appears to be an increased rate of gastrointestinal symptoms such as nausea and diarrhoea; which may be prominent. 68 Due to the increased severity of disease, all LTR and their household contacts should receive annual influenza vaccination for prevention of disease. 69
Diagnosis is essential, and efforts should be made to establish the type, as specific therapy will depend on resistance patterns. 69 Diagnosis of seasonal influenza is made by rapid antigen detection of nasopharyngeal swabs, but this method appears to be unsatisfactory for detection of novel H1N1 and molecular real‐time PCR methods are currently approved for use when swine flu is suspected. 70 In addition to supportive care and isolation, treatment involves the use of the antiviral agents amantadine and rimantidine for susceptible influenza A strains, and zanamavir and oseltamivir for both influenza A and B strains. Due to the variation in circulating strains from year to year, it is important to stay abreast of the current recommendations from the Centers for Disease Control and Prevention 71 for appropriate treatment. 72 In addition, given the prolonged viral shedding, the typical treatment course of 5 days may be insufficient in LTR, and prolonged therapy may be required. Some experts advocate treating influenza even if symptom onset is greater than 48 h and treating until viral replication ceases. 73 Treatment of novel H1N1 is limited by the resistance of the strain to the M2 inhibitors: amandatine and rimantidine. As such, current guidelines recommend treatment with oseltamivir or perhaps even zanamavir if resistance is suspected to this agent. IV or higher dose therapy is recommended for critically ill patients, and immunosuppression should be decreased. 63 , 64
RSV
By the age of 2, virtually, all children have been infected with RSV, although reinfection can occur throughout life, and early acquisition after transplant or with augmented immunosuppression is a risk factor for severe disease. 72 As with influenza, infection can vary from a self‐limited upper respiratory illness to severe pneumonia and occurs through inhalation of infectious droplets and contact with fomites, making isolation precautions paramount for prevention.
There are currently no available vaccines for RSV and no recommended therapies for prevention. Due to a lack of data for effective antiviral treatment, the only universally accepted recommendations for therapy are supportive care and a reduction of immunosuppression. 72 Ribavirin, which has shown in vitro activity against RSV, is approved for treatment of lower tract disease by showing benefit in stem cell recipients. 74 , 75 There are otherwise no controlled studies showing efficacy with the use of inhaled ribavirin in transplant patients. Despite this, inhaled ribavirin remains the most commonly used treatment for RSV with one report showing a multidrug regimen of ribavirin, steroids, RSV‐IV immunoglobulin and palivizumab to be safe, effective and associated with stability of lung function. 76 Two small case series have shown promise for parenteral and oral ribavirin in LTR. 77 , 78 An optimal treatment strategy for disease due to RSV is yet to be determined, and further studies are needed to better delineate effective agents that can safely be used in the LT setting.
Other paramyxoviruses
Like RSV, human metapneumovirus and parainfluenza are members of the paramyxovirus family and present similarly to RSV. Although typically they are milder than RSV, they have been shown to cause severe disease and have also been associated with both acute rejection and BOS. 67 , 79 , 80 real‐time PCR is the diagnostic modality of choice, and a diagnosis should be pursued, as clinical features alone are not specific enough to distinguish between the CARV. Supportive care remains the mainstay of treatment although inhaled ribavirin appears to be increasingly used for the treatment of these pathogens in patients with lower respiratory tract involvement despite a lack of controlled trials. Furthermore, some experts also consider the use of IV immunoglobulin with significant disease for both parainfluenza and human metapneumovirus. 72 , 80
FUNGAL INFECTIONS
Fungal infections are a common complication after LT with an estimated incidence of 15–35% and an overall mortality of 80%. 81 Complications at the site of the anastomosis (i.e. stenosis or necrosis) create the ideal environment for these infections to thrive. Other risk factors include the immunomodulatory effect of coexistent infections (i.e. viral) and neutropenia. 82 , 83 , 84 As previously mentioned, transmission of infection from donor to host after LT can occur, or the native lung may serve as a reservoir of fungal organisms during single LT. 85 This is particularly important in chronic obstructive pulmonary disease patients in whom the lung surfaces are irregular and may have colonized bullae. 84 Pretransplant fungal colonization is common, especially in patients with CF and chronic obstructive pulmonary disease, and it has been associated with post‐transplant fungal infection and BOS, 86 although not all colonized patients develop active/invasive infection. 83
The most common fungal pathogens in LTR are Candida and Aspergillus species, while Zygomycetes, Scedosporium, Fusarium, Cryptococcus species, histoplasmosis and coccidiomycosis occur less commonly. In general, these infections are more prevalent during the first few months after transplantation and, in some cases such as with Cryptococcus species, histoplasmosis or coccidiomyocosis, can present as a reactivation of a latent infection. Fungal infections can manifest as invasive disease with a reported 1‐year cumulative incidence of 8.6% in LTR. 87 Similarly, disseminated disease, post‐transplant empyema, and airway and anastomotic infection have been reported.
Aspergillus
Aspergillus species are the most common cause of invasive fungal infection after LT with an incidence of 32%. 84 More than half the cases occur within the first six months following LT, 84 (Fig. 1) and more often involve LTR than other solid organ recipients. 88 Several species have been described as pathogenic: Aspergillus terreus, Aspergillus flavus, Aspergillus fumigatus and Aspergillus niger. Among these species, Aspergillus fumigatus remains the most common cause of invasive disease. 89
The majority of Aspergillus isolates in sputum or BAL represent colonization (23%), and only a fraction of these will develop invasive disease (<10%), which carries a high mortality. 69 , 90 , 91 In LTR, the risk of invasive pulmonary aspergillosis rises with airway colonization by Aspergillus species. 84 , 89 , 92 Colonization is found in up to 50% of patients with CF. Despite higher colonization compared with other populations, these patients have lower risk of invasive aspergillosis, but a higher risk for aspergillus tracheobronchitis. 93 In addition to colonization, airway ischaemia and BOS have also been implicated as risk factors for invasive aspergillosis. 84 , 89 , 92 Disseminated disease has been reported with an incidence of 22%, occurring as reactivation from an occult focus and/or as a new post‐transplant infection. 84 Other less common manifestations, such as mediastinal masses, skin, soft‐tissue, sinus, orbit, central nervous system, sternal wound and chest wall infections, have also been described. 89 , 91
Diagnosis
There are limited data on the role of minimally invasive tests such galactomannan, PCR and 1,3‐β‐D‐glucan assay for the diagnosis of invasive aspergillosis in LTR. 94 , 95 1,3‐β‐D‐glucan, a cell component of all fungi, has been used in the diagnosis of multiple invasive fungal infections, but unfortunately, the role in LTR has limitations. 96 Diagnosis of invasive aspergillosis may require aggressive procedures (i.e. biopsy) to verify tissue involvement; however, this is not always possible, and often, the diagnosis is reached on evaluation of computed tomography chest findings and fungal staining/culture from bronchoscopy (i.e. BAL). The radiological findings of invasive aspergillosis include consolidations, nodules, cavitary lesions and mass‐like opacities, often with a ‘halo sign’. 84 In cases where the diagnosis is not possible with a less invasive approach, a biopsy with fungal stain/culture and histopathology may be required. Once the diagnosis of invasive pulmonary aspergillosis is made, computed tomography or magnetic resonance of the central nervous system is suggested to rule out disseminated disease.
Treatment
Over the years, the use of antifungal prophylaxis has decreased the overall risk of aspergillosis. Despite this, the risk of late infection after discontinuation of prophylaxis or even while using it is still present. 97 The treatment of pretransplant colonization has not been shown to reduce the incidence of post‐transplant aspergillosis, but invasive disease in the pretransplant setting should be treated. 90
Recent data has shown the superiority of voriconazole compared with amphotericin B deoxycholate in patients with invasive pulmonary aspergillosis, but solid organ transplant patients were poorly represented in the study. 98 A major concern with the use of voriconazole in LTR is the interaction with most of the immunosuppressants used in this population. Tacrolimus, sirolimus and cyclosporine can potentially increase the serum concentrations of voriconazole. For this reason, close monitoring of drug levels is needed. Other options for the treatment of invasive aspergillosis are posaconazole and itraconazole, but their roles as first‐line agents are not well established. The echinocandins (caspofungin, micafungin and anidulafungin) have shown some in vitro activity against Aspergillus species, but their utility as first‐line antifungals for this infection has not been studied either. The evidence for combined therapy with two or more agents as initial therapy is limited and not recommended.
Despite several alternatives, voriconazole remains the standard therapy for invasive aspergillosis along with reduction of immunosuppression. 99 Voriconazole levels should be monitored carefully, especially in CF patients where serum concentrations can be variable. 99 , 100 In general, target trough levels should range between 1 and 5 µg/µL. Duration is typically recommended for a minimum of 12 months and depends on clinical and radiographical improvement. Finally, surgical resection might be indicated when there is progression of disease despite optimal antifungal therapy, life‐threatening haemoptysis, sinus infection or lesions in the proximity of great vessels, pericardium or in the brain. 82
Candida
Severe candidal infections can appear within weeks to months after transplant, especially in the presence of heavier donor or recipient colonization. 91 Typically candida infections occur within the first 30 days after LT and appear to be the second most common cause of invasive fungal infection in LTR. 69 Candidaemia usually occurs during the first 4 weeks and is often related to the intensive care unit stay and the surgical procedure; however, parenchymal lung infection is rare. 101 Mortality for invasive candidal infections, excluding anastomotic infections, has been estimated at more than 50%. 102
Cultures are essential for the diagnosis of candidal infection in LTR. Identification of species and susceptibilities need to be obtained as intrinsic resistance and dose‐dependent susceptibility has been reported in different Candida species. 103 Other methods such as β‐D‐glucan have not reached significant accuracy for clinical use, 104 while others such as PCR are still experimental. Candida species are commonly found in the oropharynx and can potentially colonize the airway. Their presence in respiratory secretions may make it difficult to differentiate between invasive infection and colonization. Invasive lung infection with Candida is very infrequent even in the LT recipient colonized with Candida. 97 Clinical suspicion, culture results and direct bronchoscopic findings should guide any decision for treatment of candidal infections.
Echinocandins and liposomal amphotericin B are the first‐line agents for empirical therapy of suspected candidal infection. 69 This is especially true in LTR who are at risk of developing severe candidal disease. Fluconazole has been put forward as an empirical agent as well but is frequently reserved for patients with mild‐to‐moderate disease, non‐neutropenic and at low risk for Candida glabrata and Candida krusei, for which it has less activity. Empirical therapy should then be adjusted based on susceptibilities. For Candida albicans infections, fluconazole and echinocandins have been effective, but in widespread disease, amphotericin B might be considered. Finally, the duration of therapy varies among patients and with the degree and severity of infection. In candidaemia, treatment can extend up to 2 weeks but may be even longer in cases of more invasive disease. 69
Endemic mycoses
Histoplasmosis, coccidioidomycosis and rarely, blastomycosis are endemic mycoses that can potentially cause infection in transplant recipients. When present in this population, pulmonary and disseminated disease can occur with a high mortality. 105 These are especially important in endemic areas of the United States such as the Midwest for histoplasmosis and the Southwest for coccidiomycosis. 106
Histoplasmosis can present in the early or late post‐transplant period as a consequence of reactivation of a latent infection, new exposure or donor‐derived infection. 106 The diagnosis can be delayed, but in LTR, urinary antigen appears to be a better diagnostic tool than the fungal antibody serologies. 106 The presence of fever without a clear source should raise clinical suspicion for disseminated histoplasmosis in any transplant patient, especially when pancytopenia and absence of pulmonary manifestations are present. In patients whose explanted lung is found to have histoplasmosis, antifungal prophylaxis after transplant seems effective at preventing reactivation of this infection. 106 There is no clear consensus about the duration of prophylaxis, and 18 months has been reported to be effective. 106
Coccidioidomycosis is typically acquired when patients are exposed to the desert soil of the Southwestern United States and Northern Mexico. The most common mechanism of infection in LT recipients is reactivation, but donor‐derived transmission has also been reported. 107 Patients in whom there is evidence of prior coccidioidomycosis, either radiographically or serologically, may require lifelong antifungal prophylaxis after transplant. 91
Miscellaneous fungi
Cryptococcus infections can present in solid organ transplant recipients as a pulmonary or extrapulmonary process. 108 The incidence of Cryptococcus infection in LTR has been estimated around 2% and has been commonly associated with exposure to pigeons and other birds. 90 Interestingly, LTR may be less likely to have a positive cryptococcal antigen test in the setting of isolated pulmonary cryptococcosis. 38 , 108 An immunosuppressive regimen containing a calcineurin inhibitor has been associated with decreased mortality possibly due to synergistic effects between calcineurin inhibitors and antifungal agents use to treat Cryptococcus. 109 However, a recent study has reported the occurrence of an immune reconstitution syndrome‐like illness in some transplant patients after the initiation of antifungal therapy for cryptococcal infection. 110
Zygomycotic infections appear to be escalating in frequency in immunosuppressed patients, and this trend has been partially attributed to the increasing use of voriconazole for therapy and prophylaxis. 111 This infection is characterized by vascular invasion of affected tissues with subsequent infarction and necrosis. In LTR, it can manifest as bronchial anastomotic or parenchymal infection with a mortality of 87% in the latter. 112 , 113 Its management includes the combination of surgical debridement and antifungal agents.
Fungal prophylaxis
In the United States, 80% of transplant centres use antifungal prophylaxis, 114 and approximately 81% perform pretransplant surveillance for fungal colonization. 115 Despite this, there is still no general consensus regarding the most appropriate prophylactic strategy in the peritransplant window.
Although there are no randomized trials evaluating their efficacy, several antifungal agents have been used for prophylaxis in LTR. For universal prophylaxis, voriconazole, itraconazole and amphotericin B are commonly used, while targeted prophylaxis with fluconazole (Candida), voriconazole and itraconazole (Aspergillus) are used based on the results of surveillance bronchoscopy. 114 In general, the choice for antifungal prophylaxis depends, in part, on the presence of specific risk factors such as colonization with Aspergillus, presence of airway stents or ischaemia, single lung transplantation, CMV infection, hypogammaglobulinaemia or treatment of acute rejection. 69
Despite a lack of controlled trials, several studies suggest potential prevention of invasive aspergillosis with the use of either compound of amphotericin B. 116 , 117 Inhaled amphotericin B has lower systemic toxicity, better delivery to the site of fungal exposure and a lower likelihood of resistance when compared with systemic antifungal therapy. 116 , 118 , 119 The data regarding voriconazole for prophylaxis in LTR is promising, especially given the excellent bioavailability, broad antifungal coverage and good drug levels achieved in lung tissue. 120 , 121 Unfortunately, the numerous drug interactions with some of the immunosuppressants, and its potential adverse effects may preclude its use as a first‐line prophylactic agent. Itraconazole has clinical effectiveness similar to the combination of voriconazole and inhaled amphotericin B and may have lower hepatotoxicity when compared with voriconazole. 114
Duration of antifungal prophylaxis varies from centre to centre. The use of voriconazole or itraconazole for 3–6 months with or without amphotericin B has been shown to decrease the incidence of Aspergillus infection after transplantation. 88 The use of inhaled amphotericin B is typically for 2 weeks or is discontinued at the moment of discharge. In cases where pretransplant fungal colonization is present, patients may be treated for several weeks before LT and continued for up to 3 months after transplantation. Because LTR is at high risk for fungal infections, antifungal prophylaxis should be started in most patients after LT with careful consideration of side‐effects and interactions to improve outcomes and be guided by cultures from donor, graft and recipient.
MYCOBATERIAL INFECTIONS
Mycobacterial infection after LT is rare. Previously, most of these infections were secondary to Mycobacterium tuberculosis. 122 More recently, data have shown an increase in the incidence of NTM, particularly Mycobacterium abscessus, ranging between 3% and 9%. 123 , 124 Chalermskulrat et al., reported higher isolation of NTM in end‐stage CF patients undergoing pre‐LT evaluation (19.7%) than in post‐LT CF patients (13.7%). 124 Colonization, especially when M. abscessus was isolated, was associated with an increased risk for invasive mycobacterial infection in CF patients. 124
Over the last 10 years, multiple cases of M. abcessus in LT recipients have been reported with pleuropulmonary and disseminated disease. 125 , 126 , 127 In addition, there is an increase in both mortality and disseminated disease associated with M. abcessus in solid organ transplant recipients. 128 On the other hand, M. avium complex and other NTM infections are less common, and their impact on morbidity and mortality is less severe compared with M. abcessus. 129 If during the pretransplant evaluation, the clinical presentation and radiographical findings are suggestive of NTM infection, diagnostic testing and therapy should be considered before transplantation. In the CF population, the presence of NTM should not preclude LT, but careful monitoring for recurrence after transplant should be performed. 124
The diagnostic criteria of the American Thoracic Society and Infectious Disease Society of America apply to pre‐ and post‐LTR (symptoms, radiological findings and microbiology). 130 Similarly, the antimicrobial therapy recommended in the NTM guidelines is applicable to LTR. 130 Therapy for mycobacterial infection in the immunosuppressed patient can be problematic particularly due to drug interactions and increased toxicity. Nevertheless, these infections can be controlled, and some patients achieve an appropriate response and cure.
TRACHEOBRONCHITIS AND OTHER INFECTIONS
Anastomotic tracheobronchitis is a unique form of pulmonary infection 131 that usually develops in the first 6 weeks to 3 months following LT. During the transplant procedure, the bronchial circulation is not reanastomosed, and thus, the bronchial anastomosis must receive collateral blood flow from the pulmonary circulation, is subject to ischaemia and may be susceptible to infection. This diagnosis is easily confirmed with bronchoscopic examination revealing purulence, ulcerations, pseudomembranes, necrotic material, dehiscence and sometimes narrowing at the site of the anastomoses, and histological and culture results. The organisms most commonly causing tracheobronchitis in this setting are bacteria‐ (Pseudomonas, Staphylococcus) and fungi Aspergillus (an incidence of 32% and 20%, respectively) and Candida. 84 , 132 , 133
Treatment includes appropriate antibacterial and/or antifungal antimicrobials. The treatment of airway anastomotic infections with fungi is with a combination of both systemic and sometimes inhaled antifungal agents. 134 , 135 For aspergillosis, the combination of voriconazole and nebulized amphotericin B along with reduction of immunosuppression has been advocated. 99 , 134 Duration of therapy for tracheobronchitis is usually determined by resolution under bronchoscopic surveillance. Late sequelae may include stenosis and or stricture requiring intervention with balloon dilation or occasionally endobronchial stent placement. A study demonstrated a decrease in 5‐year survival in single LTR who developed bronchial anastomosis fungal infections. 132
Other types of bacterial infection described in LTR include those of the pleural space, blood stream and wounds, with organisms often isolated in the nosocomial setting, and Clostridium difficile.
Pneumocystis jiroveci
Pneumocystis jiroveci pneumonia (PJP) occurs exclusively in immunosuppressed states. The risk of infection is higher during the first 6 months after LT due to the degree of immunosuppression during this period. 136 CMV infection is also an independent risk factor for PJP. 137 Despite this, PJP remains a rare complication after LT. 138 The low rate of infection is due to the use of prophylaxis with trimethoprim‐sulfamethoxazole as a first‐line agent, and dapsone, pentamidine and atovaquone as alternatives. 139 , 140 Trimethoprim‐sulfamethoxazole has been shown to have better tolerance, potentially treat a wider range of infections, and has fewer side‐effects. 139 There is controversy regarding the duration of prophylaxis after transplant. A study revealed that the rate of PJP did not decline after 1 year of transplantation, suggesting that prophylaxis should be continued beyond this period. 141 LTR should receive at least 6 months of prophylaxis post–transplant, and if tolerated, adequately, it should be continued indefinitely. In those patients in whom prophylaxis has been discontinued, it should be resumed if the patient develops acute or chronic rejection requiring augmented immunosuppression. The standard therapy for PJP is trimethoprim‐sulfamethoxazole in combination with corticosteroids.
As previously noted, MMF is used frequently as part of the immunosuppression regimen after LT. Interestingly, this medication has shown antimicrobial properties against several pathogens including Pneumocysitis spp. 142 , 143 In three comparative studies, none of a total of 1152 transplant patients who received MMF developed PJP compared with an infection rate of 1.8% in a similar group that did not receive MMF. 144 , 145 , 146 The mechanism for these effects remains unknown, but it is likely that MMF may benefit LTR by two different mechanisms.
Nocardia species
In LT, Nocardia remains an important pathogen with a frequency of 0.6–2.1% and a directly attributable mortality of up to 30%. 147 It is important to note that some of these patients (60–100%) were on treatment with prophylactic trimethoprim‐sulfamethoxazole, a medication to which Nocardia is classically susceptible to, underscoring the resistance of some strains to prophylaxis therapy. 147 The treatment for Nocardia is trimethoprim‐sulfamethoxazole, but resistance has been documented and other alternatives have been used successfully: imipenem, amikacin, third generation cephalosporins, minocycline, moxifloxacin, linezolid and dapsone. 148 Despite the relatively low frequency of Nocardia in LT, because of the high risk of mortality and the ability to mimic other infections, clinicians must have awareness of this pathogen to improve an early diagnosis to initiate appropriate therapy.
BRONCHIOLITS OBLITERANS SYNDROME
Chronic rejection following LT is manifested pathologically by bronchiolitis obliterans and clinically by worsening obstructive dysfunction on pulmonary function, the BOS. BOS is the rate‐limiting factor in long‐term survival following LT, and up to 50% of LTR will develop BOS. 5 , 149 The aetiology remains unclear, although acute rejection is one of the identified risk factors. Emerging evidence continues to point towards infectious aetiologies as important factors in the pathogenesis of BOS. Several different viral, bacterial and fungal pathogens have been implicated in this process. 150 , 151 These findings are critical regarding the understanding the mechanisms of rejection and possible therapies to prevent it.
CMV was the first pathogen linked to the development of BOS. CMV pneumonitis is associated not only with BOS but also with decreased survival despite treatment. 50 Furthermore, there has been an absolute risk reduction in the development of BOS with the use of CMV prophylaxis, supporting the evidence that this virus may play an important role in the pathogenesis of rejection. 1 CARV infections, including RSV, human metapneumovirus and parainfluenza virus, were also identified as a significant risk factor for developing BOS. 65 , 67 , 79 , 80
Bacterial colonization and infection may be a contributing risk factor to the development of BOS. 152 , 153 , 154 , 155 Because macrolides are felt to slow the progression of BOS, it has been postulated that this response is due to the potential treatment of a chronic infection with Mycoplasma pneumoniae or Chlamydia pneumoniae, 154 , 156 although macrolide immunomodulation also plays an important role. It has been shown that a positive serology and PCR testing for Chlamydia pneumoniae on BAL samples increases the rate of BOS and early mortality. 157 , 158 Supporting this theory further, a study recently demonstrated that macrolides can prevent the development of BOS. 153
Fungal pathogens have been also associated with the development of BOS. 159 Fungal pneumonitis and aspergillus colonization have been identified as independent risk factors for BOS and mortality related to rejection. 151 , 159 , 160 Moreover, the combination of late‐onset aspergillosis and chronic allograft dysfunction was a risk factor for poorer survival. 132
CONCLUSION
Despite several advances in surgical technique, immunosuppression and prophylaxis, infection continues to remain an important cause of death and disease in the LTR. Although there are non‐modifiable factors that are innate to the patient or to the nature of the procedure, there are several modifiable factors that can be recognized and changed so as to optimize the patient's chances for survival and further extend life. Prompt recognition and treatment of these factors is paramount for appropriate management. Prophylaxis strategies continue to evolve and show promise for several of the infectious agents. Avoidance of these infectious complications may not only lead to a decrease in the direct consequences of infection but also to a reduction in the subsequent causes of ultimate graft failure including both acute and chronic rejection. Antimicrobial resistance is a growing problem, and although newer antimicrobials will likely be of benefit, especially against viral and fungal pathogens, prevention of these diseases remains the best approach. Careful consideration and further research are needed regarding the mechanisms by which infection and subsequent inflammation alters the immunoregulatory machinery of the host and subsequently leads to the development failure of the allograft. Factors that are important in evaluating an infectious episode include time after transplant, immunosuppression, CMV serostatus, prophylaxis regimen and treatment for acute rejection. 3 Given that outcomes appear to be improved with early recognition and treatment of disease, all practitioners must always maintain a high index of suspicion caring for these patients.
The Authors: S. Rodrigo Burguete, MD, is a Pulmonary and Critical Care Fellow with research interests in lung transplantation and critical care. Diego J. Maselli, MD, is a Pulmonary and Critical Care Fellow with research interests in obstructive lung disease, lung transplantation and pneumonia. Juan F. Fernandez, MD, is a Pulmonary and Critical Care Fellow with research interests in pneumonia, lung transplantation and critical care. Stephanie M. Levine, MD, is a Professor and Fellowship Program Director with research interests in lung transplantation, pulmonary issues in women and medical education.
SERIES EDITORS: JOHN E HEFFNER AND DAVID CL LAM
REFERENCES
- 1. Chmiel C, Speich R, Hofer M et al Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus‐related events and bronchiolitis obliterans syndrome after lung transplantation. Clin. Infect. Dis. 2008; 46: 831–9. [DOI] [PubMed] [Google Scholar]
- 2. Speich R, van der Bij W. Epidemiology and management of infections after lung transplantation. Clin. Infect. Dis. 2001; 33(Suppl. 1): S58–65. [DOI] [PubMed] [Google Scholar]
- 3. Parada MT, Alba A, Sepulveda C. Early and late infections in lung transplantation patients. Transplant. Proc. 2010; 42: 333–5. [DOI] [PubMed] [Google Scholar]
- 4. Levine SM. ACCP Pulm Crit Care Update. 1999; 13:lesson 16.
- 5. Christie JD, Edwards LB, Kucheryavaya AY et al The registry of the international society for heart and lung transplantation: twenty‐eighth adult lung and heart‐lung transplant report–2011. J. Heart Lung Transplant. 2011; 30: 1104–22. [DOI] [PubMed] [Google Scholar]
- 6. Parada MT, Alba A, Sepulveda C. Bronchiolitis obliterans syndrome development in lung transplantation patients. Transplant. Proc. 2010; 42: 331–2. [DOI] [PubMed] [Google Scholar]
- 7. Cook DJ, Kollef MH. Risk factors for ICU‐acquired pneumonia. JAMA 1998; 279: 1605–6. [DOI] [PubMed] [Google Scholar]
- 8. Desmond P, Raman R, Idikula J. Effect of nasogastric tubes on the nose and maxillary sinus. Crit. Care Med. 1991; 19: 509–11. [DOI] [PubMed] [Google Scholar]
- 9. Verleden GM, Vos R, van Raemdonck D et al Pulmonary infection defense after lung transplantation: does airway ischemia play a role? Curr. Opin. Organ. Transplant. 2010; 15: 568–71. [DOI] [PubMed] [Google Scholar]
- 10. Norgaard MA, Andersen CB, Pettersson G. Airway epithelium of transplanted lungs with and without direct bronchial artery revascularization. Eur. J. Cardiothorac Surg. 1999; 15: 37–44. [DOI] [PubMed] [Google Scholar]
- 11. Gade J, Qvortrup K, Andersen CB et al Bronchial transsection and reanastomosis in pigs with and without bronchial arterial circulation. Ann. Thorac. Surg. 2001; 71: 332–6. [DOI] [PubMed] [Google Scholar]
- 12. Murthy SC, Gildea TR, Machuzak MS. Anastomotic airway complications after lung transplantation. Curr. Opin. Organ. Transplant. 2010; 15: 582–7. [DOI] [PubMed] [Google Scholar]
- 13. Bhorade SM, Stern E. Immunosuppression for lung transplantation. Proc. Am. Thorac. Soc. 2009; 6: 47–53. [DOI] [PubMed] [Google Scholar]
- 14. Floreth T, Bhorade SM. Current trends in immunosuppression for lung transplantation. Semin. Respir. Crit. Care Med. 2010; 31: 172–8. [DOI] [PubMed] [Google Scholar]
- 15. Floreth T, Bhorade SM, Ahya VN. Conventional and novel approaches to immunosuppression. Clin. Chest Med. 2011; 32: 265–77. [DOI] [PubMed] [Google Scholar]
- 16. Parekh K, Trulock E, Patterson GA. Use of cyclosporine in lung transplantation. Transplant. Proc. 2004; 36(Suppl. 2): 318S–22S. [DOI] [PubMed] [Google Scholar]
- 17. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of mycophenolate in solid organ transplant recipients. Clin. Pharmacokinet. 2007; 46: 13–58. [DOI] [PubMed] [Google Scholar]
- 18. Terada N, Lucas JJ, Szepesi A et al Rapamycin blocks cell cycle progression of activated T cells prior to events characteristic of the middle to late G1 phase of the cycle. J. Cell. Physiol. 1993; 154: 7–15. [DOI] [PubMed] [Google Scholar]
- 19. Saunders RN, Metcalfe MS, Nicholson ML. Rapamycin in transplantation: a review of the evidence. Kidney Int. 2001; 59: 3–16. [DOI] [PubMed] [Google Scholar]
- 20. Kirchner GI, Meier‐Wiedenbach I, Manns MP. Clinical pharmacokinetics of everolimus. Clin. Pharmacokinet. 2004; 43: 83–95. [DOI] [PubMed] [Google Scholar]
- 21. Barnes PJ. Corticosteroid effects on cell signalling. Eur. Respir. J. 2006; 27: 413–26. [DOI] [PubMed] [Google Scholar]
- 22. de la Torre M, Pena E, Calvin M et al Basiliximab in lung transplantation: preliminary experience. Transplant. Proc. 2005; 37: 1534–6. [DOI] [PubMed] [Google Scholar]
- 23. Flaman F, Zieroth S, Rao V et al Basiliximab versus rabbit anti‐thymocyte globulin for induction therapy in patients after heart transplantation. J. Heart Lung Transplant. 2006; 25: 1358–62. [DOI] [PubMed] [Google Scholar]
- 24. Johnson MR, Mullen GM, O'Sullivan EJ et al Risk/benefit ratio of perioperative OKT3 in cardiac transplantation. Am. J. Cardiol. 1994; 74: 261–6. [DOI] [PubMed] [Google Scholar]
- 25. Smart FW, Naftel DC, Costanzo MR et al Risk factors for early, cumulative, and fatal infections after heart transplantation: a multiinstitutional study. J. Heart Lung Transplant. 1996; 15: 329–41. [PubMed] [Google Scholar]
- 26. Segovia J, Rodriguez‐Lambert JL, Crespo‐Leiro MG et al A randomized multicenter comparison of basiliximab and muromonab (OKT3) in heart transplantation: SIMCOR study. Transplantation 2006; 81: 1542–8. [DOI] [PubMed] [Google Scholar]
- 27. Brock MV, Borja MC, Ferber L et al Induction therapy in lung transplantation: a prospective, controlled clinical trial comparing OKT3, anti‐thymocyte globulin, and daclizumab. J. Heart Lung Transplant. 2001; 20: 1282–90. [DOI] [PubMed] [Google Scholar]
- 28. Anonymous Organ Procurement and Transplantation Network (OPTN) , Scientific Registry of Transplant Recipients (SRTR) . OPTN/SRTR 2010 Annual Data Report. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD, 2011, 117. [Google Scholar]
- 29. Cai J, Terasaki PI. Induction immunosuppression improves long‐term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation 2010; 90: 1511–15. [DOI] [PubMed] [Google Scholar]
- 30. Di Filippo S, Boissonnat P, Sassolas F et al Rabbit antithymocyte globulin as induction immunotherapy in pediatric heart transplantation. Transplantation 2003; 75: 354–8. [DOI] [PubMed] [Google Scholar]
- 31. Hachem RR, Edwards LB, Yusen RD et al The impact of induction on survival after lung transplantation: an analysis of the International Society for Heart and Lung Transplantation Registry. Clin. Transplant. 2008; 22: 603–8. [DOI] [PubMed] [Google Scholar]
- 32. Clinckart F, Bulpa P, Jamart J et al Basiliximab as an alternative to antithymocyte globulin for early immunosuppression in lung transplantation. Transplant. Proc. 2009; 41: 607–9. [DOI] [PubMed] [Google Scholar]
- 33. Shyu S, Dew MA, Pilewski JM et al Five‐year outcomes with alemtuzumab induction after lung transplantation. J. Heart Lung Transplant. 2011; 30: 743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campos S, Caramori M, Teixeira R et al Bacterial and fungal pneumonias after lung transplantation. Transplant. Proc. 2008; 40: 822–4. [DOI] [PubMed] [Google Scholar]
- 35. Dobbin C, Maley M, Harkness J et al The impact of pan‐resistant bacterial pathogens on survival after lung transplantation in cystic fibrosis: results from a single large referral centre. J. Hosp. Infect. 2004; 56: 277–82. [DOI] [PubMed] [Google Scholar]
- 36. Hadjiliadis D, Steele MP, Chaparro C et al Survival of lung transplant patients with cystic fibrosis harboring panresistant bacteria other than Burkholderia cepacia, compared with patients harboring sensitive bacteria. J. Heart Lung Transplant. 2007; 26: 834–8. [DOI] [PubMed] [Google Scholar]
- 37. Murray S, Charbeneau J, Marshall BC et al Impact of Burkholderia infection on lung transplantation in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2008; 178: 363–71. [DOI] [PubMed] [Google Scholar]
- 38. Alexander BD, Petzold EW, Reller LB et al Survival after lung transplantation of cystic fibrosis patients infected with Burkholderia cepacia complex. Am. J. Transplant. 2008; 8: 1025–30. [DOI] [PubMed] [Google Scholar]
- 39. Boussaud V, Guillemain R, Grenet D et al Clinical outcome following lung transplantation in patients with cystic fibrosis colonised with Burkholderia cepacia complex: results from two French centres. Thorax 2008; 63: 732–7. [DOI] [PubMed] [Google Scholar]
- 40. Nash EF, Coonar A, Kremer R et al Survival of Burkholderia cepacia sepsis following lung transplantation in recipients with cystic fibrosis. Transpl. Infect. Dis. 2010; 12: 551–4. [DOI] [PubMed] [Google Scholar]
- 41. Fischer SA, Avery RK, Infectious AST. Disease community of practice. screening of donor and recipient prior to solid organ transplantation. Am. J. Transplant. 2009; 9(Suppl. 4): S7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Avlonitis VS, Krause A, Luzzi L et al Bacterial colonization of the donor lower airways is a predictor of poor outcome in lung transplantation. Eur. J. Cardiothorac Surg. 2003; 24: 601–7. [DOI] [PubMed] [Google Scholar]
- 43. Orens JB, Boehler A, de Perrot M et al A review of lung transplant donor acceptability criteria. J. Heart Lung Transplant. 2003; 22: 1183–200. [DOI] [PubMed] [Google Scholar]
- 44. Bonde PN, Patel ND, Borja MC et al Impact of donor lung organisms on post‐lung transplant pneumonia. J. Heart Lung Transplant. 2006; 25: 99–105. [DOI] [PubMed] [Google Scholar]
- 45. Weill D, Dey GC, Hicks RA et al A positive donor gram stain does not predict outcome following lung transplantation. J. Heart Lung Transplant. 2002; 21: 555–8. [DOI] [PubMed] [Google Scholar]
- 46. King CS, Khandhar S, Burton N et al Native lung complications in single‐lung transplant recipients and the role of pneumonectomy. J. Heart Lung Transplant. 2009; 28: 851–6. [DOI] [PubMed] [Google Scholar]
- 47. Aguilar‐Guisado M, Givalda J, Ussetti P et al Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am. J. Transplant. 2007; 7: 1989–96. [DOI] [PubMed] [Google Scholar]
- 48. de Bruyn G, Whelan TP, Mulligan MS et al Invasive pneumococcal infections in adult lung transplant recipients. Am. J. Transplant. 2004; 4: 1366–71. [DOI] [PubMed] [Google Scholar]
- 49. American Thoracic Society , Infectious Diseases Society of America . Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare‐associated pneumonia. Am. J. Respir. Crit. Care Med. 2005; 171: 388–416. [DOI] [PubMed] [Google Scholar]
- 50. Snyder LD, Finlen‐Copeland CA, Turbyfill WJ et al Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am. J. Respir. Crit. Care Med. 2010; 181: 1391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Humar A, Snydman D, Infectious AST. Diseases community of practice. Cytomegalovirus in solid organ transplant recipients. Am. J. Transplant. 2009; 9(Suppl. 4): S78–86. [DOI] [PubMed] [Google Scholar]
- 52. Zamora MR. Cytomegalovirus and lung transplantation. Am. J. Transplant. 2004; 4: 1219–26. [DOI] [PubMed] [Google Scholar]
- 53. Kotton CN, Kumar D, Caliendo AM et al International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 2010; 89: 779–95. [DOI] [PubMed] [Google Scholar]
- 54. Humar A, Michaels M, AST ID Working Group on Infectious Disease Monitoring . American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am. J. Transplant. 2006; 6: 262–74. [DOI] [PubMed] [Google Scholar]
- 55. Zuk DM, Humar A, Weinkauf JG et al An international survey of cytomegalovirus management practices in lung transplantation. Transplantation 2010; 90: 672–6. [DOI] [PubMed] [Google Scholar]
- 56. Lisboa LF, Kumar D, Wilson LE et al Clinical Utility of cytomegalovirus cell‐mediated immunity in transplant recipients with cytomegalovirus viremia. Transplantation 2012; 93: 195–200. [DOI] [PubMed] [Google Scholar]
- 57. Kumar D, Chernenko S, Moussa G et al Cell‐mediated immunity to predict cytomegalovirus disease in high‐risk solid organ transplant recipients. Am. J. Transplant. 2009; 9: 1214–22. [DOI] [PubMed] [Google Scholar]
- 58. Hodson EM, Craig JC, Strippoli GF et al Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst. Rev. 2008; (2): CD003774. [DOI] [PubMed] [Google Scholar]
- 59. Jaksch P, Zweytick B, Kerschner H et al Cytomegalovirus prevention in high‐risk lung transplant recipients: comparison of 3‐ vs 12‐month valganciclovir therapy. J. Heart Lung Transplant. 2009; 28: 670–5. [DOI] [PubMed] [Google Scholar]
- 60. Snydman DR, Limaye AP, Potena L et al Update and review: state‐of‐the‐art management of cytomegalovirus infection and disease following thoracic organ transplantation. Transplant. Proc. 2011; 43(Suppl. 3): S1–17. [DOI] [PubMed] [Google Scholar]
- 61. Palmer SM, Limaye AP, Banks M et al Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann. Intern. Med. 2010; 152: 761–9. [DOI] [PubMed] [Google Scholar]
- 62. Asberg A, Humar A, Rollag H et al Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am. J. Transplant. 2007; 7: 2106–13. [DOI] [PubMed] [Google Scholar]
- 63. Kumar D, Husain S, Chen MH et al A prospective molecular surveillance study evaluating the clinical impact of community‐acquired respiratory viruses in lung transplant recipients. Transplantation 2010; 89: 1028–33. [DOI] [PubMed] [Google Scholar]
- 64. Kumar D, Morris MI, Kotton CN et al Guidance on novel influenza A/H1N1 in solid organ transplant recipients. Am. J. Transplant. 2010; 10: 18–25. [DOI] [PubMed] [Google Scholar]
- 65. Khalifah AP, Hachem RR, Chakinala MM et al Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am. J. Respir. Crit. Care Med. 2004; 170: 181–7. [DOI] [PubMed] [Google Scholar]
- 66. Garantziotis S, Howell DN, McAdams HP et al Influenza pneumonia in lung transplant recipients: clinical features and association with bronchiolitis obliterans syndrome. Chest 2001; 119: 1277–80. [DOI] [PubMed] [Google Scholar]
- 67. Vilchez R, McCurry K, Dauber J et al Influenza and parainfluenza respiratory viral infection requiring admission in adult lung transplant recipients. Transplantation 2002; 73: 1075–8. [DOI] [PubMed] [Google Scholar]
- 68. Danziger‐Isakov LA, Husain S, Mooney ML et al The novel 2009 H1N1 influenza virus pandemic: unique considerations for programs in cardiothoracic transplantation. J. Heart Lung Transplant. 2009; 28: 1341–7. [DOI] [PubMed] [Google Scholar]
- 69. Sims KD, Blumberg EA. Common infections in the lung transplant recipient. Clin. Chest Med. 2011; 32: 327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Faix DJ, Sherman SS, Waterman SH. Rapid‐test sensitivity for novel swine‐origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009; 361: 728–9. [DOI] [PubMed] [Google Scholar]
- 71. Centers for Disease Control and Prevention (CDC) . Pneumonia and influenza death rates—United States, 1979–1994. MMWR Morb. Mortal. Wkly Rep. 1995; 44: 535–7. [PubMed] [Google Scholar]
- 72. Ison MG, Michaels MG, Infectious AST. Diseases community of practice. RNA respiratory viral infections in solid organ transplant recipients. Am. J. Transplant. 2009; 9(Suppl. 4): S166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ison MG. Respiratory viral infections in transplant recipients. Antivir. Ther. 2007; 12(4 Pt B): 627–38. [PubMed] [Google Scholar]
- 74. Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin. Respir. Crit. Care Med. 2007; 28: 222–42. [DOI] [PubMed] [Google Scholar]
- 75. Sparrelid E, Ljungman P, Ekelof‐Andstrom E et al Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplant. 1997; 19: 905–8. [DOI] [PubMed] [Google Scholar]
- 76. Liu V, Dhillon GS, Weill D. A multi‐drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart‐lung transplant recipients. Transpl. Infect. Dis. 2010; 12: 38–44. [DOI] [PubMed] [Google Scholar]
- 77. Glanville AR, Scott AI, Morton JM et al Intravenous ribavirin is a safe and cost‐effective treatment for respiratory syncytial virus infection after lung transplantation. J. Heart Lung Transplant. 2005; 24: 2114–19. [DOI] [PubMed] [Google Scholar]
- 78. Pelaez A, Lyon GM, Force SD et al Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J. Heart Lung Transplant. 2009; 28: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shah PD, McDyer JF. Viral infections in lung transplant recipients. Semin. Respir. Crit. Care Med. 2010; 31: 243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weinberg A, Lyu DM, Li S et al Incidence and morbidity of human metapneumovirus and other community‐acquired respiratory viruses in lung transplant recipients. Transpl. Infect. Dis. 2010; 12: 330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sole A, Salavert M. Fungal infections after lung transplantation. Curr. Opin. Pulm. Med. 2009; 15: 243–53. [DOI] [PubMed] [Google Scholar]
- 82. Singh N, Husain S, Infectious AST. Diseases community of practice. Invasive aspergillosis in solid organ transplant recipients. Am. J. Transplant. 2009; 9(Suppl. 4): S180–91. [DOI] [PubMed] [Google Scholar]
- 83. Danziger‐Isakov LA, Worley S, Arrigain S et al Increased mortality after pulmonary fungal infection within the first year after pediatric lung transplantation. J. Heart Lung Transplant. 2008; 27: 655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Singh N, Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J. Heart Lung Transplant. 2003; 22: 258–66. [DOI] [PubMed] [Google Scholar]
- 85. Ruiz I, Gavalda J, Monforte V et al Donor‐to‐host transmission of bacterial and fungal infections in lung transplantation. Am. J. Transplant. 2006; 6: 178–82. [DOI] [PubMed] [Google Scholar]
- 86. Luong ML, Morrissey O, Husain S. Assessment of infection risks prior to lung transplantation. Curr. Opin. Infect. Dis. 2010; 23: 578–83. [DOI] [PubMed] [Google Scholar]
- 87. Pappas PG, Alexander BD, Andes DR et al Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010; 50: 1101–11. [DOI] [PubMed] [Google Scholar]
- 88. Minari A, Husni R, Avery RK et al The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl. Infect. Dis. 2002; 4: 195–200. [DOI] [PubMed] [Google Scholar]
- 89. Gordon SM, Avery RK. Aspergillosis in lung transplantation: incidence, risk factors, and prophylactic strategies. Transpl. Infect. Dis. 2001; 3: 161–7. [DOI] [PubMed] [Google Scholar]
- 90. Avery RK. Infections after lung transplantation. Semin. Respir. Crit. Care Med. 2006; 27: 544–51. [DOI] [PubMed] [Google Scholar]
- 91. Avery RK. Antifungal prophylaxis in lung transplantation. Semin. Respir. Crit. Care Med. 2011; 32: 717–26. [DOI] [PubMed] [Google Scholar]
- 92. Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin. Microbiol. Rev. 2005; 18: 44–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Helmi M, Love RB, Welter D et al Aspergillus infection in lung transplant recipients with cystic fibrosis: risk factors and outcomes comparison to other types of transplant recipients. Chest 2003; 123: 800–8. [DOI] [PubMed] [Google Scholar]
- 94. Husain S, Paterson DL, Studer SM et al Aspergillus galactomannan antigen in the bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in lung transplant recipients. Transplantation 2007; 83: 1330–6. [DOI] [PubMed] [Google Scholar]
- 95. Walsh TJ, Wissel MC, Grantham KJ et al Molecular detection and species‐specific identification of medically important Aspergillus species by real‐time PCR in experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 2011; 49: 4150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Alexander BD, Smith PB, Davis RD et al The (1,3){beta}‐D‐glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J. Clin. Microbiol. 2010; 48: 4083–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hosseini‐Moghaddam SM, Husain S. Fungi and molds following lung transplantation. Semin. Respir. Crit. Care Med. 2010; 31: 222–33. [DOI] [PubMed] [Google Scholar]
- 98. Herbrecht R, Denning DW, Patterson TF et al Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002; 347: 408–15. [DOI] [PubMed] [Google Scholar]
- 99. Walsh TJ, Anaissie EJ, Denning DW et al Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008; 46: 327–60. [DOI] [PubMed] [Google Scholar]
- 100. Berge M, Guillemain R, Boussaud V et al Voriconazole pharmacokinetic variability in cystic fibrosis lung transplant patients. Transpl. Infect. Dis. 2009; 11: 211–19. [DOI] [PubMed] [Google Scholar]
- 101. Palmer SM, Alexander BD, Sanders LL et al Significance of blood stream infection after lung transplantation: analysis in 176 consecutive patients. Transplantation 2000; 69: 2360–6. [DOI] [PubMed] [Google Scholar]
- 102. Sole A, Salavert M. Fungal infections after lung transplantation. Transplant. Rev. (Orlando) 2008; 22: 89–104. [DOI] [PubMed] [Google Scholar]
- 103. Pappas PG, Silveira FP, Infectious AST. Diseases Community of Practice. Candida in solid organ transplant recipients. Am. J. Transplant. 2009; 9(Suppl. 4): S173–9. [DOI] [PubMed] [Google Scholar]
- 104. Wheat LJ. Approach to the diagnosis of invasive aspergillosis and candidiasis. Clin. Chest Med. 2009; 30: 367–77. viii. [DOI] [PubMed] [Google Scholar]
- 105. Wheat LJ, Kauffman CA. Histoplasmosis. Infect. Dis. Clin. North Am. 2003; 17: 1–19. vii. [DOI] [PubMed] [Google Scholar]
- 106. Cuellar‐Rodriguez J, Avery RK, Lard M et al Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin. Infect. Dis. 2009; 49: 710–16. [DOI] [PubMed] [Google Scholar]
- 107. Miller MB, Hendren R, Gilligan PH. Posttransplantation disseminated coccidioidomycosis acquired from donor lungs. J. Clin. Microbiol. 2004; 42: 2347–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Singh N, Forrest G, Infectious AST. Diseases Community of Practice. Cryptococcosis in solid organ transplant recipients. Am. J. Transplant. 2009; 9(Suppl. 4): S192–8. [DOI] [PubMed] [Google Scholar]
- 109. Kontoyiannis DP, Lewis RE, Alexander BD et al Calcineurin inhibitor agents interact synergistically with antifungal agents in vitro against Cryptococcus neoformans isolates: correlation with outcome in solid organ transplant recipients with cryptococcosis. Antimicrob. Agents Chemother. 2008; 52: 735–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Singh N, Lortholary O, Alexander BD et al An immune reconstitution syndrome‐like illness associated with Cryptococcus neoformans infection in organ transplant recipients. Clin. Infect. Dis. 2005; 40: 1756–61. [DOI] [PubMed] [Google Scholar]
- 111. Ambrosioni J, Bouchuiguir‐Wafa K, Garbino J. Emerging invasive zygomycosis in a tertiary care center: epidemiology and associated risk factors. Int. J. Infect. Dis. 2010; 14(Suppl. 3): e100–3. [DOI] [PubMed] [Google Scholar]
- 112. Silveira FP, Husain S. Fungal infections in lung transplant recipients. Curr. Opin. Pulm. Med. 2008; 14: 211–18. [DOI] [PubMed] [Google Scholar]
- 113. McGuire FR, Grinnan DC, Robbins M. Mucormycosis of the bronchial anastomosis: a case of successful medical treatment and historic review. J. Heart Lung Transplant. 2007; 26: 857–61. [DOI] [PubMed] [Google Scholar]
- 114. Cadena J, Levine DJ, Angel LF et al Antifungal prophylaxis with voriconazole or itraconazole in lung transplant recipients: hepatotoxicity and effectiveness. Am. J. Transplant. 2009; 9: 2085–91. [DOI] [PubMed] [Google Scholar]
- 115. Dummer JS, Lazariashvilli N, Barnes J et al A survey of anti‐fungal management in lung transplantation. J. Heart Lung Transplant. 2004; 23: 1376–81. [DOI] [PubMed] [Google Scholar]
- 116. Monforte V, Ussetti P, Lopez R et al Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: pharmacokinetics and safety. J. Heart Lung Transplant. 2009; 28: 170–5. [DOI] [PubMed] [Google Scholar]
- 117. Monforte V, Ussetti P, Gavalda J et al Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. J. Heart Lung Transplant. 2010; 29: 523–30. [DOI] [PubMed] [Google Scholar]
- 118. Palmer SM, Drew RH, Whitehouse JD et al Safety of aerosolized amphotericin B lipid complex in lung transplant recipients. Transplantation 2001; 72: 545–8. [DOI] [PubMed] [Google Scholar]
- 119. Sole A. Invasive fungal infections in lung transplantation: role of aerosolised amphotericin B. Int. J. Antimicrob. Agents 2008; 32(Suppl. 2): S161–5. [DOI] [PubMed] [Google Scholar]
- 120. Husain S, Paterson DL, Studer S et al Voriconazole prophylaxis in lung transplant recipients. Am. J. Transplant. 2006; 6: 3008–16. [DOI] [PubMed] [Google Scholar]
- 121. Capitano B, Potoski BA, Husain S et al Intrapulmonary penetration of voriconazole in patients receiving an oral prophylactic regimen. Antimicrob. Agents Chemother. 2006; 50: 1878–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dromer C, Nashef SA, Velly JF et al Tuberculosis in transplanted lungs. J. Heart Lung Transplant. 1993; 12(6 Pt 1): 924–7. [PubMed] [Google Scholar]
- 123. Malouf MA, Glanville AR. The spectrum of mycobacterial infection after lung transplantation. Am. J. Respir. Crit. Care Med. 1999; 160(5 Pt 1): 1611–16. [DOI] [PubMed] [Google Scholar]
- 124. Chalermskulrat W, Sood N, Neuringer IP et al Non‐tuberculous mycobacteria in end stage cystic fibrosis: implications for lung transplantation. Thorax 2006; 61: 507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Taylor JL, Palmer SM. Mycobacterium abscessus chest wall and pulmonary infection in a cystic fibrosis lung transplant recipient. J. Heart Lung Transplant. 2006; 25: 985–8. [DOI] [PubMed] [Google Scholar]
- 126. Gilljam M, Schersten H, Silverborn M et al Lung transplantation in patients with cystic fibrosis and Mycobacterium abscessus infection. J. Cyst. Fibros. 2010; 9: 272–6. [DOI] [PubMed] [Google Scholar]
- 127. Chernenko SM, Humar A, Hutcheon M et al Mycobacterium abscessus infections in lung transplant recipients: the international experience. J. Heart Lung Transplant. 2006; 25: 1447–55. [DOI] [PubMed] [Google Scholar]
- 128. Garrison AP, Morris MI, Doblecki Lewis S et al Mycobacterium abscessus infection in solid organ transplant recipients: report of three cases and review of the literature. Transpl. Infect. Dis. 2009; 11: 541–8. [DOI] [PubMed] [Google Scholar]
- 129. Doucette K, Fishman JA. Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin. Infect. Dis. 2004; 38: 1428–39. [DOI] [PubMed] [Google Scholar]
- 130. Griffith DE, Aksamit T, Brown‐Elliott BA et al An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007; 175: 367–416. [DOI] [PubMed] [Google Scholar]
- 131. Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc. Am. Thorac. Soc. 2009; 6: 79–93. [DOI] [PubMed] [Google Scholar]
- 132. Sole A, Morant P, Salavert M et al Aspergillus infections in lung transplant recipients: risk factors and outcome. Clin. Microbiol. Infect. 2005; 11: 359–65. [DOI] [PubMed] [Google Scholar]
- 133. Hamacher J, Spiliopoulos A, Kurt AM et al Pre‐emptive therapy with azoles in lung transplant patients. Geneva Lung Transplantation Group. Eur. Respir. J. 1999; 13: 180–6. [DOI] [PubMed] [Google Scholar]
- 134. Palmer SM, Perfect JR, Howell DN et al Candidal anastomotic infection in lung transplant recipients: successful treatment with a combination of systemic and inhaled antifungal agents. J. Heart Lung Transplant. 1998; 17: 1029–33. [PubMed] [Google Scholar]
- 135. Hadjiliadis D, Howell DN, Davis RD et al Anastomotic infections in lung transplant recipients. Ann. Transplant. 2000; 5(3): 13–19. [PubMed] [Google Scholar]
- 136. Rodriguez M, Fishman JA. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus‐negative immunocompromised patients. Clin. Microbiol. Rev. 2004; 17: 770–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Arend SM, Westendorp RG, Kroon FP et al Rejection treatment and cytomegalovirus infection as risk factors for Pneumocystis carinii pneumonia in renal transplant recipients. Clin. Infect. Dis. 1996; 22: 920–5. [DOI] [PubMed] [Google Scholar]
- 138. Arthurs SK, Eid AJ, Deziel PJ et al The impact of invasive fungal diseases on survival after lung transplantation. Clin. Transplant. 2010; 24: 341–8. [DOI] [PubMed] [Google Scholar]
- 139. Subramanian AK. Antimicrobial prophylaxis regimens following transplantation. Curr. Opin. Infect. Dis. 2011; 24: 344–9. [DOI] [PubMed] [Google Scholar]
- 140. Thomas CF, Jr , Limper AH. Pneumocystis pneumonia. N. Engl. J. Med. 2004; 350: 2487–98. [DOI] [PubMed] [Google Scholar]
- 141. Gordon SM, LaRosa SP, Kalmadi S et al Should prophylaxis for Pneumocystis carinii pneumonia in solid organ transplant recipients ever be discontinued? Clin. Infect. Dis. 1999; 28: 240–6. [DOI] [PubMed] [Google Scholar]
- 142. Ritter ML, Pirofski L. Mycophenolate mofetil: effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl. Infect. Dis. 2009; 11: 290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Oz HS, Hughes WT. Novel anti‐Pneumocystis carinii effects of the immunosuppressant mycophenolate mofetil in contrast to provocative effects of tacrolimus, sirolimus, and dexamethasone. J. Infect. Dis. 1997; 175: 901–4. [DOI] [PubMed] [Google Scholar]
- 144. Sollinger HW, Belzer FO, Deierhoi MH et al RS‐61443 (mycophenolate mofetil). A multicenter study for refractory kidney transplant rejection. Ann. Surg. 1992; 216: 513–18. Discussion 518–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. The Mycophenolate Mofetil Renal Refractory Rejection Study Group . Anonymous mycophenolate mofetil for the treatment of refractory, acute, cellular renal transplant rejection. Transplantation 1996; 61: 722–9. [PubMed] [Google Scholar]
- 146. The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group . Anonymous A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 1996; 61: 1029–37. [PubMed] [Google Scholar]
- 147. Khan BA, Duncan M, Reynolds J et al Nocardia infection in lung transplant recipients. Clin. Transplant. 2008; 22: 562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Martinez R, Reyes S, Menendez R. Pulmonary nocardiosis: risk factors, clinical features, diagnosis and prognosis. Curr. Opin. Pulm. Med. 2008; 14: 219–27. [DOI] [PubMed] [Google Scholar]
- 149. Christie JD, Carby M, Bag R et al Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2005; 24: 1454–9. [DOI] [PubMed] [Google Scholar]
- 150. Estenne M, Maurer JR, Boehler A et al Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J. Heart Lung Transplant. 2002; 21: 297–310. [DOI] [PubMed] [Google Scholar]
- 151. Valentine VG, Gupta MR, Walker JE, Jr et al Effect of etiology and timing of respiratory tract infections on development of bronchiolitis obliterans syndrome. J. Heart Lung Transplant. 2009; 28: 163–9. [DOI] [PubMed] [Google Scholar]
- 152. Botha P, Archer L, Anderson RL et al Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation 2008; 85: 771–4. [DOI] [PubMed] [Google Scholar]
- 153. Vos R, Vanaudenaerde BM, Verleden SE et al A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur. Respir. J. 2011; 37: 164–72. [DOI] [PubMed] [Google Scholar]
- 154. Vos R, Vanaudenaerde BM, Ottevaere A et al Long‐term azithromycin therapy for bronchiolitis obliterans syndrome: divide and conquer? J. Heart Lung Transplant. 2010; 29: 1358–68. [DOI] [PubMed] [Google Scholar]
- 155. Gottlieb J, Mattner F, Weissbrodt H et al Impact of graft colonization with gram‐negative bacteria after lung transplantation on the development of bronchiolitis obliterans syndrome in recipients with cystic fibrosis. Respir. Med. 2009; 103: 743–9. [DOI] [PubMed] [Google Scholar]
- 156. Verleden GM, Dupont LJ. Azithromycin therapy for patients with bronchiolitis obliterans syndrome after lung transplantation. Transplantation 2004; 77: 1465–7. [DOI] [PubMed] [Google Scholar]
- 157. Glanville AR, Gencay M, Tamm M et al Chlamydia pneumoniae infection after lung transplantation. J. Heart Lung Transplant. 2005; 24: 131–6. [DOI] [PubMed] [Google Scholar]
- 158. Kotsimbos TC, Snell GI, Levvey B et al Chlamydia pneumoniae serology in donors and recipients and the risk of bronchiolitis obliterans syndrome after lung transplantation. Transplantation 2005; 79: 269–75. [DOI] [PubMed] [Google Scholar]
- 159. Weigt SS, Elashoff RM, Huang C et al Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. Am. J. Transplant. 2009; 9: 1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Valentine VG, Bonvillain RW, Gupta MR et al Infections in lung allograft recipients: ganciclovir era. J. Heart Lung Transplant. 2008; 27: 528–35. [DOI] [PubMed] [Google Scholar]


