Summary of Paper:
Applying novel technology developed in immuno-oncology to cardiovascular biology and disease may be transformative.
Keywords: cardio-oncology, immuno-oncology, immunoprofiling, myocarditis, mass cytometry
The burgeoning field of cardio-oncology is continuing to grow in step with major scientific developments in oncology that have improved cancer prognosis and survivorship. Once focused primarily on addressing effects of conventional chemotherapy, cardio-oncology is now increasingly defining itself in relation to acute and chronic cardiovascular and metabolic risks of targeted oncologic therapies.1 In a bidirectional interaction, cardio-oncology holds the potential for bridging the application of fundamental molecular advances from hematology and oncology in the cardiovascular field. These concepts are now amalgamating in “cardio-immuno-oncology” or the cardiovascular sequelae of cancer therapies modulating the immune system.
Drugs that modulate the immune system have emerged as important treatments for many human diseases ranging from autoimmune diseases to cancer. What started with “biologics” in rheumatology for modulation of cytokines has extended to unprecedented proportions in cancer therapy. Immunotherapies in oncology include antibodies directed against cancer antigens and cell-based therapies. Perhaps most impactful in the current anti-cancer therapeutic armamentarium, immune checkpoint inhibitors (ICI), antibodies targeting brakes or “checkpoints” in immune cells, including T lymphocytes, have been effective in several previously deadly cancer types, such as melanoma and lung cancer, and are now being tested, often in combination, in virtually every cancer type. Seven ICI have been approved. Between 2011 and 2018 the percentage of cancer patients eligible for ICI therapy increased from 1.54% to 46.6%. The overall landscape of immuno-oncology (IO) is staggering. As of September 2017, there were 940 IO agents in clinical development owned by 462 different companies or academic institutes, with another 1064 compounds in pre-clinical phase.2 At the same time there were 3042 active clinical trials evaluating efficacy in nearly 600,000 patients.2 It is estimated that more than 50% of research and development in pharmaceutical companies is now focused on immuno-oncology.
While immunotherapies can lead to cure in a subset of cancer patients, they can also result in immune-related side-effects (irAE). Cell-based therapies (e.g., chimeric antigen receptor T cell therapy or CAR-T) can lead to a cytokine release syndrome. ICI-associated cardiac complications include myocarditis, pericarditis, vasculitis and arrhythmias and can occur in 1% of treated patients.3 Among irAEs induced by ICI, cardiovascular inflammatory complications have been most challenging to recognize and treat. For example, ICI-associated myocarditis can be difficult to diagnose due to its unusual presentation (electrocardiographic instability rather than heart failure) and has proven hard to treat (about 50% mortality).3 It is conceivable that severe, acute ICI-associated myocarditis represents only the tip of the iceberg, with lower intensity, chronic cardiovascular effects also occurring, but less well characterized.
The best strategies to identify patients at risk and to prevent and manage immunotherapy-associated cardiovascular effects are unclear. This calls for further investigation of the immune mechanisms involved and the mastery of combination therapies with ICI and immune activators to achieve effective anti-cancer immune activation and dial down immune-related reactions. The complexity of fine-tuning immune responses also makes the prospect of re-challenging with immuno-oncologic therapy after cardiovascular toxicity daunting. Moreover, conventional cardiotoxic therapies such as anthracyclines followed by immunotherapy have been shown to improve antitumor immunity – but raise the concern of exacerbating both immune-related and non-immune related cardiovascular toxicities.
Viewed from a broader perspective, scientific advances in understanding the biology of the immune system and ensuing therapeutic strategies to manipulate immune responses for treating human disease have generated a systemic paradigm shift. Immune effects on the cardiovascular system are also being unveiled, with new directions of investigation such as clonal hematopoiesis and the immune system’s role in hypertension and atherosclerosis, suggesting wide-ranging interactions between the cardiovascular and immune systems.4 These observations point to inflammation playing a critical role in cardiovascular disease pathogenesis but better understanding of the specific mechanisms can lead to more effective patient interventions.5 For example, the results of Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) resulted in statistically significant improvements in cardiovascular disease outcomes but did not lead to approval of the canakinumab by the regulatory bodies for patients with atherosclerosis. In 2019, there is a need for more fundamental and mechanistic understandings of the immune-cardiovascular interactions to realize the full potential of translating the modulation of the immune system in treating cardiovascular diseases.
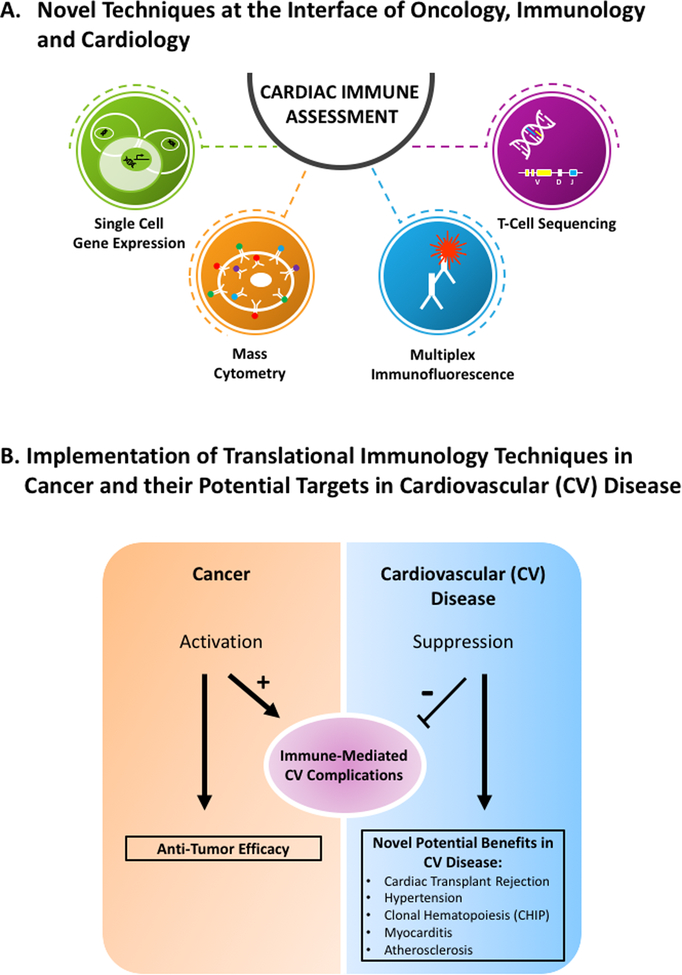
Fortunately, rapid translation from basic science experimentation to clinical science investigation and personalized clinical application are models established in cancer therapy and immuno-oncology. In particular, development of new immunological technological platforms has been critical in bench to bedside translation. In immuno-oncology, these novel technologies include single cell gene expression analysis, high-frequency and deeper T-cell receptor sequencing, multiplex immunofluorescence, mass cytometry, among others (FIGURE 1A). Application of these novel techniques can improve prevention, prognostication and therapies for cardiovascular complications of cancer immunotherapies. For example, next-generation sequencing of T-cell receptors has been applied to clinical cases of ICI-associated myocarditis to analyze the distribution, clonality and diversity of T-cell infiltrates in the myocardium.3 Combined with transcriptome analysis (via RNA sequencing) of the tumor, this has resulted in the hypothesis that tumor-specific genetic risk factors may better define patients with cancer at risk for myocarditis. Not the least, these new approaches will shed new light onto mechanisms involved in broader cardiovascular areas, such as atherosclerosis, myocarditis, heart transplant, hypertension and clonal hematopoiesis (FIGURE 1B). Application of these technologies may lead to better classification of other forms of myocarditis, better understanding and identifying patients at risk of cardiac transplant-associated rejection, and improved modulation of the immune system in the treatment of other cardiovascular diseases such as atherosclerosis.
FIGURE 1.
The world of cardio-immuno-oncology: Cardio-oncology facilitates the transfer of novel, breakthrough translational techniques from immuno-oncology to cardiovascular science.
A. Novel techniques at the interface of oncology, immunology and cardiology.
B. Implementation of translational immunology techniques in cancer and their potential in cardiovascular (CV) disease. Abbreviations: CV-Cardiovascular
Besides incorporation of these novel technologies, a closer collaboration for investigation between disciplines is needed. This includes clinicians and basic scientists, cardiovascular researchers and immunologists, and academicians with those in the pharmaceutical industry. More broadly, there needs to be better collaboration to systematically collect samples from patients with inflammatory cardiovascular disease. Funding agencies, including the National Institutional of Health, need to recognize this new reality. In conclusion, cardio-immuno-oncology is expanding the interconnectedness of cardiovascular medicine with immunology and oncology, highlighting the need for interdisciplinary research, specific emphasis in training the next generation of cardiologists and awareness of currently practicing cardiologists. Cardio-immuno-oncology also affords the ability to translate new scientific advances in immunology to the cardiovascular space. The burden is upon the cardiovascular research and clinical community to embrace this opportunity.
Acknowledgements
VGZ is supported by the Cancer Prevention and Research Institute of Texas (CPRIT) award RP180404. WCM was supported by funding from the Niels Stensen Fellowship and the Netherlands Heart Institute. JM is supported by NIH R56 HL141466 and R01 HL141466.
Footnotes
Conflicts of Interest
JM has served on an advisory board for Pfizer, Novartis, Bristol Myers Squibb, Deciphera, Audentes Pharmaceuticals, Nektar, Takeda, Ipsen, Myokardia, AstraZeneca and Regeneron. Other authors report no potential conflicts.
References
- 1.Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N Engl J Med. 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 2.Tang J, Shalabi A and Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol. 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 3.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB and Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutgens E, Atzler D, Doring Y, Duchene J, Steffens S and Weber C. Immunotherapy for cardiovascular disease. Eur Heart J. 2019. [DOI] [PubMed] [Google Scholar]
- 5.Libby P and Kobold S. Inflammation: a common contributor to cancer, aging, and cardiovascular diseases-expanding the concept of cardio-oncology. Cardiovasc Res. 2019;115:824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]