To comply with the National Institutes of Health (NIH) guidelines for rigor and reproducibility on reporting sex as a biological variable, the editors of ATVB previously reviewed 295 preclinical articles published in ATVB 2017 and 2016 and provided detailed analysis on “Reporting sex and sex differences in preclinical studies” (Published in the October issue of 20181). The editors have decided to continuously monitor and document the analytic results of reporting sex and sex differences in ATVB on an annual basis. Here we provide the annual report of 2018 in this Recent Highlights series.
A total of 186 preclinical science research articles2–187 published in 2018 were reviewed by the editors. Twenty-five articles were excluded for further analysis because they only studied human samples or cell lines. There were 6 species reported in the rest 161 articles (Table 1), including zebrafish, mouse, rat, rabbit, pig, and nonhuman primates. Among these 161 articles, 6 articles only studied embryos or neonates. Therefore, 155 publications with in vivo animal studies on reporting sex were analyzed.
Table 1.
Reporting Sex of Animals in Preclinical Studies of ATVB Publications in 2018
| Species | Total Article Number | Reporting Sex Number (%) | Reporting Both Sexes Number (%) |
|---|---|---|---|
| Zebrafish | 4 | - | - |
| Mouse | 142 | 132 (94%) | 42 (30%) |
| Rat | 8 | 7 (88%) | 0 |
| Rabbit | 4 | 3 (75%) | 0 |
| Pig | 1 | 1 (100%) | 1 (100%) |
| Primate | 2 | 2 (100%) | 1 (50%) |
In 2018, one hundred and forty five articles (94%) reported sex for in vivo models, compared to 92% and 79% of those published in 2017 and 2016, respectively. Among all species, 28% reported studies from both male and female animals, compared to 21% and 11% of publications in 2017 and 2016, respectively. Eighty two of the 155 articles (53%) articles reported primary cells isolated from either mouse or rat. Forty nine of the 82 articles (60%) reported sex, whereas 27% articles published in 2017 and 28% in 2016 provided sex information for primary cells. Eight articles in 2018 and 2 in both 2017 and 2016, respectively, reported cells from both male and female animals.
For in vivo studies among the 161 publications in 2018 (Table 1), 4 articles reported data from zebrafish during embryonic development, 4 articles studied rabbits with 3 reporting only male data and one did not define the sex that was studied, 1 article studied both male and female pigs, 2 articles studied non human primates with one reporting only male data and one reporting both male and female data.
Rodents are the predominantly used species, with most publications using mice and a lesser number using rats. Of the 161 articles published in 2018, 142 (88%) reported mouse models, and 8 (5%) reported rat models. For in vivo studies, 132 (94%) of 140 articles reported sex in mouse models after excluding 2 articles that only studied embryos or neonates, and 7 of 8 (88%) articles reported sex in rat models. For primary cells, 44 of 74 (59%) articles reported sex in mice, and 5 of 8 (63%) articles reported sex in rats.
For mouse studies (Table 2), 78 of 140 (56%) articles published in 2018 reported data only from male mice, 12 (9%) articles reported data only from female mice, and 42 (30%) reported data from both male and female animals. Among the 90 articles that only studied one sex, 39 (43%) provided a brief justification why only one sex was studied in mice. For the 8 rat studies, 7 (88%) articles reported data only from male rats, and 1 article did not provide the sex information.
Table 2.
Percent of Articles Reporting Sex in Mouse Studies of ATVB Publications in 2018
| Reporting Sex | Articles (Number) | Articles (%) |
|---|---|---|
| Male | 78 | 56 |
| Female | 12 | 9 |
| Both Male and Female | 42 | 30 |
On the basis of this data analysis, three significant improvements were noted in the 2018 publications, compared to the publications in 2017 (Figure 1). First, more animal studies provided a justification why a specific sex (either male or female) were used. Second, more studies reported data derived from both male and female animals. Third, increased number of articles reported the sex of primary cells isolated from animals. The latter improvement is largely attributed to the implantation of Major Resources Tables started in the late 2018.
Figure 1: Percent of articles that reported both sexes or provided a justification why only one sex was studied.
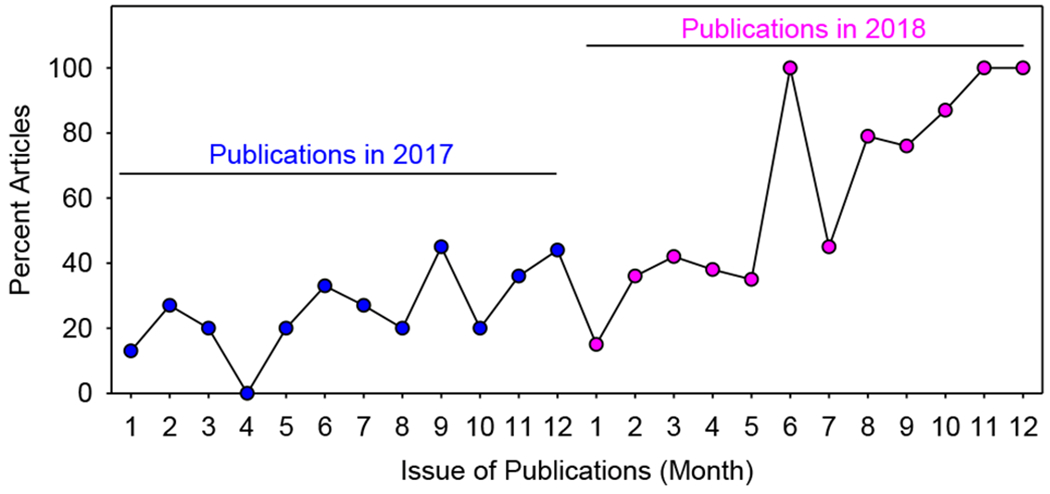
ATVB Publications of preclinical research in 2017 and 2018 were analyzed. X axis represents the month of publications. Y axis represents percent of monthly publications that either reported data from both male and female animals or provided a justification why the authors only studied a specific sex.
Table 2 shows clearly that most animal studies published in 2018 reported data only from male animals (89 articles for male versus 12 articles for female), similar to the publications in 2017 (75 articles for male versus 18 articles for female). The major reason the authors stated for studying only male animals is that the short estrus cycle in murine females may have confounding effects on data interpretation. Many provided literature evidence that estrogen had effects on the physiological or pathophysiological phenotype, while some did not provide solid evidence to support their use of male animals. Other reasons include (1) previous studies in the literature or the authors’ laboratory showed that female animals had milder effects on cardiovascular diseases; (2) the literature has exclusively reported data only from male mice; (3) previous studies did not note sexual dimorphism; or (4) the authors have studied the same sex in their previous studies and they hope to be consistent with their previous study designs. For the articles that only studied female animals, the authors state that (1) male animals tend to show aggressive behavior; (2) no sex difference was found, but female animals were easier to handle; (3) female animals had more severe disease; or (4) female had more modest phenotype. Among the 44 articles that reported both male and female animals, two articles found sex difference, which opened a door for the authors to understand sex-related mechanisms.
The editors acknowledge the significant improvement on reporting sex in most preclinical studies published in 2018. The editors also acknowledge that experimental designs and reporting sex in preclinical studies still need to be improved. For example, for articles that reported both male and female data, many studies pooled data from male and female animals. Some stated this approach was appropriate because male and female animals had no sex difference, whereas some did not provide a reason. The editorial process has now modified to require analysis of data in a sex specific manner. Pooling of data from both sexes may be permitted following the demonstration of no statistically significant difference between the two sexes.188 ATVB will continuously take efforts in encouraging the authors to report both sexes and analyze data from each sex separately to enhance opportunity to explore sex difference. In addition to the ATVB Technical Review mechanism and the editors’ annual review, the editors hope that more authors spontaneously study both sexes to explore new mechanisms of sex-mediated cardiovascular functions and diseases.1,189
Acknowledgment
The ATVB editors in this writing group would like to thank the ATVB editorial office, the editors, the reviewers, and the authors for their tremendous efforts in supporting publishing high quality research articles that report sex and sex differences.
Footnotes
Disclosures
None.
References
- 1.Lu HS, Schmidt AM, Hegele RA, Mackman N, Rader DJ, Weber C, Daugherty A. Reporting sex and sex differences in preclinical studies. Arterioscler Thromb Vasc Biol. 2018;38 :e171–e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu P, Xiong T, Tenedero CB, Lebeau P, Ni R, MacDonald ME, Gross PL, Austin RC, Trigatti BL. Rosuvastatin reduces aortic sinus and coronary artery atherosclerosis in SR-B1 (Scavenger Receptor Class B Type 1)/ApoE (Apolipoprotein E) double knockout mice independently of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2018;38:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curaj A, Wu Z, Rix A, Gresch O, Sternkopf M, Alampour-Rajabi S, Lammers T, van Zandvoort M, Weber C, Koenen RR, Liehn EA, Kiessling F. Molecular ultrasound imaging of junctional adhesion molecule A depicts acute alterations in blood flow and early endothelial dysregulation. Arterioscler Thromb Vasc Biol. 2018;38:40–48. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L, Hedin U, Maegdefessel L, Fish JE, Rayner KJ. Extracellular vesicles secreted by atherogenic macrophages transfer microRNA to inhibit cell migration. Arterioscler Thromb Vasc Biol. 2018;38:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Master E, Huang RT, Zhang C et al. Proatherogenic flow increases endothelial stiffness via enhanced CD36-mediated uptake of oxidized low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2018;38:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Raghavan A, Peters DT, Pashos EE, Rader DJ, Musunuru K. Interrogation of the atherosclerosis-associated SORT1 (sortilin 1) locus with primary human hepatocytes, induced pluripotent stem cell-hepatocytes, and locus-humanized mice. Arterioscler Thromb Vasc Biol. 2018;38:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hai Q, Ritchey B, Robinet P, Alzayed AM, Brubaker G, Zhang J, Smith JD. Quantitative trait locus mapping of macrophage cholesterol metabolism and CRISPR/Cas9 editing implicate an ACAT1 truncation as a causal modifier variant. Arterioscler Thromb Vasc Biol. 2018;38:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park K, Li Q, Evcimen ND et al. Exogenous insulin infusion can decrease atherosclerosis in diabetic rodents by improving lipids, inflammation, and endothelial function. Arterioscler Thromb Vasc Biol. 2018;38:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K, Wu Q, Hu K, Yang C, Wu X, Cheung P, Williams KJ. Suppression of hepatic FLOT1 (Flotillin-1) by type 2 diabetes mellitus impairs the disposal of remnant lipoproteins via syndecan-1. Arterioscler Thromb Vasc Biol. 2018;38:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeboudj L, Giraud A, Guyonnet L, Zhang Y, Laurans L, Esposito B, Vilar J, Chipont A, Papac-Milicevic N, Binder CJ, Tedgui A, Mallat Z, Tharaux PL, Ait-Oufella H. Selective EGFR (Epidermal Growth Factor Receptor) deletion in myeloid cells limits atherosclerosis-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:114–119. [DOI] [PubMed] [Google Scholar]
- 11.Biwer LA, Good ME, Hong K, Patel RK, Agrawal N, Looft-Wilson R, Sonkusare SK, Isakson BE. Non-endoplasmic reticulum-based calr (Calreticulin) can coordinate heterocellular calcium signaling and vascular function. Arterioscler Thromb Vasc Biol. 2018. ;38:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberzettl P , Conklin DJ, Abplanalp WT, Bhatnagar A, O’Toole TE. Inhalation of fine particulate matter impairs endothelial progenitor cell function via pulmonary oxidative stress. Arterioscler Thromb Vasc Biol. 2018;38:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsiraj Y, Thatcher SE, Blalock E, Fleenor B, Daugherty A, Cassis LA. Sex chromosome complement defines diffuse versus focal angiotensin II-induced aortic pathology. Arterioscler Thromb Vasc Biol. 2018;38:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karoor V, Fini MA, Loomis Z, Sullivan T, Hersh LB, Gerasimovskaya E, Irwin D, Dempsey EC. Sustained activation of Rho GTPases promotes a synthetic pulmonary artery smooth muscle cell phenotype in neprilysin null mice. Arterioscler Thromb Vasc Biol. 2018;38:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu CK, Xu T, Assoian RK, Rader DJ. Mining the stiffness-sensitive transcriptome in Human vascular smooth muscle cells identifies long noncoding RNA stiffness regulators. Arterioscler Thromb Vasc Biol. 2018;38:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue Y, Shimazawa M, Nakamura S, Takata S, Hashimoto Y, Izawa H, Masuda T, Tsuruma K, Sakaue T , Nakayama H, Higashiyama S, Hara H. Both autocrine signaling and paracrine signaling of HB-EGF enhance ocular neovascularization. Arterioscler Thromb Vasc Biol. 2018;38:174–185. [DOI] [PubMed] [Google Scholar]
- 17.Leenders GJ, Smeets MB, van den Boomen M, Berben M, Nabben M, van Strijp D, Strijkers GJ, Prompers JJ, Arslan F, Nicolay K, Vandoorne K. Statins promote cardiac infarct healing by modulating endothelial barrier function revealed by contrast-enhanced magnetic resonance imaging. Arterioscler Thromb Vasc Biol. 2018;38:186–194. [DOI] [PubMed] [Google Scholar]
- 18.Bai H, Lee JS, Hu H et al. Transforming growth factor-beta1 inhibits pseudoaneurysm formation after a ortic patch angioplasty. Arterioscler Thromb Vasc Biol. 2018;38:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wacker BK, Dronadula N, Bi L, Stamatikos A, Dichek DA. Apo A-I (Apolipoprotein A-I) vascular gene therapy provides durable protection against atherosclerosis in hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2018;38:206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gromovsky AD , Schugar RC, Brown AL, Helsley RN, Burrows AC, Ferguson D, Zhang R, Sansbury BE, Lee RG, Morton RE, Allende DS, Parks JS, Spite M, Brown JM. Delta-5 fatty a cid desaturase FADS1 impacts metabolic disease by balancing proinflammatory and proresolving lipid mediators. Arterioscler Thromb Vasc Biol. 2018;38:218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Bras A, Yu B, Issa Bhaloo S, Hong X, Zhang Z, Hu Y, Xu Q. Adventitial Sca1+ cells transduced with ETV2 are committed to the endothelial fate and improve vascular remodeling after injury. Arterioscler Thromb Vasc Biol. 2018;38:232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinne P, Kadiri JJ, Velasco-Delgado M, Nuutinen S, Viitala M, Hollmen M, Rami M, Savontaus E, Steffens S. Melanocortin 1 receptor deficiency promotes atherosclerosis in apolipoprotein E(−/−) mice. Arterioscler Thromb Vasc Biol. 2018;38:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vozenilek AE , Navratil AR, Green JM, Coleman DT, Blackburn CMR, Finney AC, Pearson BH, Chrast R, Finck BN, Klein RL, Orr AW, Woolard MD. Macrophage-associated lipin-1 enzymatic activity contributes to modified low-density lipoprotein-induced proinflammatory signaling and atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu S, Liu Y, You T et al. Vascular semaphorin 7A upregulation by disturbed flow promotes atherosclerosis through endothelial beta1 integrin. Arterioscler Thromb Vasc Biol. 2018;38:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotru SK, Chen W, Kraft P et al. TRPM7 kinase controls calcium responses in arterial thrombosis and stroke in mice. Arterioscler Thromb Vasc Biol. 2018;38:344–352. [DOI] [PubMed] [Google Scholar]
- 26.Krispin S, Stratman AN, Melick CH, Stan RV, Malinverno M, Gleklen J, Castranova D, Dejana E, Weinstein BM. Growth differentiation factor 6 promotes vascular stability by restraining vascular endothelial growth factor signaling. Arterioscler Thromb Vasc Biol. 2018. ;38:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gourgas O, Marulanda J, Zhang P, Murshed M, Cerruti M. Multidisciplinary approach to understand medial arterial calcification. Arterioscler Thromb Vasc Biol. 2018;38:363–372. [DOI] [PubMed] [Google Scholar]
- 28.Lu Q, Xie Z, Yan C, Ding Y, Ma Z, Wu S, Qiu Y, Cossette SM, Bordas M, Ramchandran R, Zou MH. SNRK (Sucrose Nonfermenting 1-related kinase) promotes angiogenesis in vivo. Arterioscler Thromb Vasc Biol. 2018;38:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez JJ, Albarran L, Jardin I, Sanchez-Collado J, Redondo PC, Bermejo N, Bobe R, Smani T, Rosado JA. Filamin A modulates store-operated Ca(2+) entry by regulating STIM1 (Stromal Interaction Molecule 1)-Orai1 association in human platelets. Arterioscler Thromb Vasc Biol. 2018;38:386–397. [DOI] [PubMed] [Google Scholar]
- 30.Wang LJ, Xiao F, Kong LM, Wang DN, Li HY, Wei YG, Tan C, Zhao H, Zhang T, Cao GQ, Zhang K, Wei YQ, Yang HS, Zhang W. Intermedin enlarges the vascular lumen by inducing the quiescent endothelial cell proliferation. Arterioscler Thromb Vasc Biol. 2018;38:398–413. [DOI] [PubMed] [Google Scholar]
- 31.Holmberg J, Bhattachariya A, Alajbegovic A, Rippe C, Ekman M, Dahan D, Hien TT, Boettger T, Braun T, Sward K, Hellstrand P, Albinsson S. Loss of vascular myogenic tone in miR-143/145 knockout mice is associated with hypertension-induced vascular lesions in small mesenteric arteries. Arterioscler Thromb Vasc Biol. 2018;38:414–424. [DOI] [PubMed] [Google Scholar]
- 32.Karamariti E , Zhai C, Yu B et al. DKK3 (Dickkopf 3) alters atherosclerotic plaque phenotype involving vascular progenitor and fibroblast differentiation into smooth muscle cells. Arterioscler Thromb Vasc Biol. 2018;38:425–437. [DOI] [PubMed] [Google Scholar]
- 33.Batra R, Suh MK, Carson JS, Dale MA, Meisinger TM, Fitzgerald M, Opperman PJ, Luo J, Pipinos II, Xiong W, Baxter BT. IL-1beta (Interleukin-1beta) and TNF-alpha (Tumor Necrosis Factor-alpha) impact abdominal aortic aneurysm formation by differential effects on macrophage polarization. Arterioscler Thromb Vasc Biol. 2018;38:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doddapattar P, Jain M, Dhanesha N, Lentz SR, Chauhan AK. Fibronectin containing extra domain A induces plaque destabilization in the innominate artery of aged apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2018;38:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang CL, Garcia-Arcos I, Nyren R, Olivecrona G, Kim JY, Hu Y, Agrawal RR, Murphy AJ, Goldberg IJ, Deckelbaum RJ. Lipoprotein lipase deficiency impairs bone marrow myelopoiesis and reduces circulating monocyte levels. Arterioscler Thromb Vasc Biol. 2018;38:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doddapattar P, Dhanesha N, Chorawala MR, Tinsman C, Jain M, Nayak MK, Staber JM, Chauhan AK. Endothelial cell-derived Von Willebrand Factor, but not platelet-derived, promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2018;38:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudhahar V, Okur MN, Bagi Z, O’Bryan JP, Hay N, Makino A, Patel VS, Phillips SA, Stepp D, Ushio-Fukai M, Fukai T. Akt2 (Protein Kinase B Beta) stabilizes ATP7A, a copper transporter for extracellular superoxide dismutase, in vascular smooth muscle: Novel mechanism to limit endothelial dysfunction in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2018. ;38:529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong K, Cope EL, DeLalio LJ, Marziano C, Isakson BE, Sonkusare SK. TRPV4 (Transient Receptor Potential Vanilloid 4) channel-dependent negative feedback mechanism regulates Gq protein-coupled receptor-induced vasoconstriction. Arterioscler Thromb Vasc Biol. 2018;38:542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, Fellows A, Foote K, Yang Z, Figg N, Littlewood T, Bennett M. FOXO3a (Forkhead Transcription Factor O Subfamily Member 3a) links vascular smooth muscle cell apoptosis, matrix breakdown, atherosclerosis, and vascular remodeling through a novel pathway involving MMP13 (Matrix Metalloproteinase 13). Arterioscler Thromb Vasc Biol. 2018;38:555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lother A, Bergemann S, Deng L, Moser M, Bode C, Hein L. Cardiac endothelial cell transcriptome. Arterioscler Thromb Vasc Biol. 2018;38:566–574. [DOI] [PubMed] [Google Scholar]
- 41.Viegas CSB, Santos L, Macedo AL, Matos AA, Silva AP, Neves PL, Staes A, Gevaert K, Morais R, Vermeer C, Schurgers L, Simes DC. Chronic kidney disease circulating calciprotein particles and extracellular vesicles promote vascular calcification: a role for GRP (Gla-Rich Protein). Arterioscler Thromb Vasc Biol. 2018;38:575–587. [DOI] [PubMed] [Google Scholar]
- 42.Galatioto J, Caescu CI, Hansen J, Cook JR, Miramontes I, Iyengar R, Ramirez F. Cell type-specific contributions of the angiotensin II type 1a receptor to aorta homeostasis and aneurysmal disease-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Shao Y, Sha X, Fang P, Kuo YM, Andrews AJ, Li Y, Yang WY, Maddaloni M, Pascual DW, Luo JJ, Jiang X, Wang H, Yang X. IL-35 (Interleukin-35) suppresses endothelial cell activation by i nhibiting mitochondrial reactive oxygen species-mediated site-specific acetylation of H3K14 (Histone 3 Lysine 14). Arterioscler Thromb Vasc Biol. 2018;38:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starke RM, Thompson JW, Ali MS, Pascale CL, Martinez Lege A, Ding D, Chalouhi N, Hasan DM, Jabbour P, Owens GK, Toborek M, Hare JM, Dumont AS. Cigarette smoke initiates oxidative stress-induced cellular phenotypic modulation leading to cerebral aneurysm pathogenesis. Arterioscler Thromb Vasc Biol. 2018;38:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma C, Beyer AM, Durand M, Clough AV, Zhu D, Norwood Toro L, Terashvili M, Ebben JD, Hill RB, Audi SH, Medhora M, Jacobs ER. Hyperoxia causes mitochondrial fragmentation in pulmonary endothelial cells by increasing expression of pro-fission proteins. Arterioscler Thromb Vasc Biol. 2018;38:622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulin A, Anstine LJ, Kim AJ, Potter SJ, DeFalco T, Lincoln J, Yutzey KE. Macrophage transitions in heart valve development and myxomatous valve disease. Arterioscler Thromb Vasc Biol. 2018. ;38:636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuffe H, Liu M, Key CC, Boudyguina E, Sawyer JK, Weckerle A, Bashore A, Fried SK, Chung S, Parks JS. Targeted deletion of adipocyte Abca1 (ATP-Binding Cassette Transporter A1) impairs diet-induced obesity. Arterioscler Thromb Vasc Biol. 2018;38:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karasawa T, Kawashima A, Usui-Kawanishi F et al. Saturated fatty acids undergo intracellular crystallization and activate the NLRP3 inflammasome in macrophages. Arterioscler Thromb Vasc Biol. 2018;38:744–756. [DOI] [PubMed] [Google Scholar]
- 49.Honda K, Matoba T, Antoku Y, Koga JI, Ichi I, Nakano K, Tsutsui H, Egashira K. Lipid-lowering therapy with ezetimibe decreases spontaneous atherothrombotic occlusions in a rabbit model of plaque erosion: A role of serum oxysterols. Arterioscler Thromb Vasc Biol. 2018;38:757–771. [DOI] [PubMed] [Google Scholar]
- 50.Stark K, Schubert I, Joshi U et al. Distinct pathogenesis of pancreatic cancer microvesicle-associated venous thrombosis identifies new antithrombotic targets in vivo. Arterioscler Thromb Vasc Biol. 2018;38:772–786. [DOI] [PubMed] [Google Scholar]
- 51.Wersall A, Williams CM, Brown E, Iannitti T, Williams N, Poole AW. Small GTPases in platelet membrane trafficking. Arterioscler Thromb Vasc Biol. 2018;38:787–800. [DOI] [PubMed] [Google Scholar]
- 52.Schwertz H, Rowley JW, Schumann GG, Thorack U, Campbell RA, Manne BK, Zimmerman GA, Weyrich AS, Rondina MT. Endogenous LINE-1 (Long Interspersed Nuclear Element-1) reverse transcriptase activity in platelets controls translational events through RNA-DNA hybrids. Arterioscler Thromb Vasc Biol. 2018;38:801–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plautz WE, Sekhar Pilli VS, Cooley BC, Chattopadhyay R, Westmark PR, Getz T, Paul D, Bergmeier W, Sheehan JP, Majumder R. Anticoagulant protein S targets the factor IXa heparin-binding exosite to prevent thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:816–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Praetner M, Zuchtriegel G, Holzer M et al. Plasminogen activator inhibitor-1 promotes neutrophil infiltration and tissue injury on ischemia-reperfusion. Arterioscler Thromb Vasc Biol. 2018;38:829–842. [DOI] [PubMed] [Google Scholar]
- 55.Meher AK, Spinosa M, Davis JP, Pope N, Laubach VE, Su G, Serbulea V, Leitinger N, Ailawadi G, Upchurch GR Jr. Novel role of IL (Interleukin)-1beta in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poulsen LC, Edelmann RJ, Kruger S et al. Inhibition of endothelial NOTCH1 s ignaling attenuates inflammation by reducing cytokine-mediated histone acetylation at inflammatory enhancers. Arterioscler Thromb Vasc Biol. 2018;38:854–869. [DOI] [PubMed] [Google Scholar]
- 57.Lee MY, Gamez-Mendez A, Zhang J, Zhuang Z, Vinyard DJ, Kraehling J, Velazquez H, Brudvig GW, Kyriakides TR, Simons M, Sessa WC. Endothelial cell autonomous role of Akt1: Regulation of vascular tone and ischemia-induced arteriogenesis. Arterioscler Thromb Vasc Biol. 2018;38:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J, Heagerty AM. Role of sympathetic nerves and adipocyte catecholamine uptake in the vasorelaxant function of perivascular adipose tissue. Arterioscler Thromb Vasc Biol. 2018. ;38:880–891. [DOI] [PubMed] [Google Scholar]
- 59.Cui XB, Luan JN, Dong K, Chen S, Wang Y, Watford WT, Chen SY. RGC-32 (Response Gene to Complement 32) deficiency protects endothelial cells from inflammation and attenuates atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:e36–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arroyo AB, de Los Reyes-Garcia AM, Rivera-Caravaca JM, Valledor P, Garcia-Barbera N, Roldan V, Vicente V, Martinez C, Gonzalez-Conejero R. MiR-146a regulates neutrophil extracellular trap formation that predicts adverse cardiovascular events in patients with atrial fibrillation. Arterioscler Thromb Vasc Biol. 2018;38:892–902. [DOI] [PubMed] [Google Scholar]
- 61.Umebayashi R , Uchida HA, Kakio Y, Subramanian V, Daugherty A, Wada J. Cilostazol attenuates angiotensin II-induced abdominal aortic aneurysms but not atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2018;38:903–912. [DOI] [PubMed] [Google Scholar]
- 62.Pandey D, Nomura Y, Rossberg MC, Hori D, Bhatta A, Keceli G, Leucker T, Santhanam L, Shimoda LA, Berkowitz D, Romer L. Hypoxia triggers SENP1 (Sentrin-Specific Protease 1) modulation of KLF15 (Kruppel-Like Factor 15) and transcriptional regulation of Arg2 (Arginase 2) in pulmonary endothelium. Arterioscler Thromb Vasc Biol. 2018;38:913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamada S, Senokuchi T, Matsumura T et al. Inhibition of local macrophage growth ameliorates focal inflammation and suppresses atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:994–1006. [DOI] [PubMed] [Google Scholar]
- 64.Rami M, Guillamat-Prats R, Rinne P, Salvermoser M, Ring L, Bianchini M, Blanchet X, Megens RTA, Doring Y, Walzog B, Soehnlein O, Weber C, Faussner A, Steffens S. Chronic intake of the selective serotonin reuptake inhibitor fluoxetine enhances atherosclerosis. Arterioscler Thromb Vasc Biol. 2018. ;38:1007–1019. [DOI] [PubMed] [Google Scholar]
- 65.Tay C, Liu YH, Kanellakis P, Kallies A, Li Y, Cao A , Hosseini H, Tipping P, Toh BH, Bobik A, Kyaw T. Follicular B cells promote atherosclerosis via T cell-mediated differentiation into plasma cells and secreting pathogenic immunoglobulin G. Arterioscler Thromb Vasc Biol. 2018;38:e71–e84. [DOI] [PubMed] [Google Scholar]
- 66.Adamson SE, Polanowska-Grabowska R, Marqueen K, Griffiths R, Angdisen J, Breevoort SR, Schulman IG, Leitinger N. Deficiency of Dab2 (Disabled Homolog 2) in myeloid cells exacerbates inflammation in liver and atherosclerotic plaques in LDLR (Low-Density Lipoprotein Receptor)-null mice-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li W, Luehmann HP, Hsiao HM, Tanaka S, Higashikubo R, Gauthier JM, Sultan D, Lavine KJ, Brody SL , Gelman AE, Gropler RJ, Liu Y, Kreisel D. Visualization of monocytic cells in regressing atherosclerotic plaques by intravital 2-Photon and positron emission tomography-based imaging-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adam F, Kauskot A, Kurowska M, Goudin N, Munoz I, Bordet JC, Huang JD, Bryckaert M, Fischer A, Borgel D, de Saint Basile G, Christophe OD, Menasche G. Kinesin-1 is a new actor involved in platelet secretion and thrombus stability. Arterioscler Thromb Vasc Biol. 2018;38:1037–1051. [DOI] [PubMed] [Google Scholar]
- 69.Lehmann M, Schoeman RM, Krohl PJ, Wallbank AM, Samaniuk JR, Jandrot-Perrus M, Neeves KB. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI-dependent manner in an in vitro venous thrombosis model. Arterioscler Thromb Vasc Biol. 2018;38:1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lutshumba J, Liu S, Zhong Y, Hou T, Daugherty A, Lu H, Guo Z, Gong MC. Deletion of BMAL1 in smooth muscle cells protects mice from abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38:1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusters PJH, Seijkens TTP, Beckers L, Lievens D, Winkels H, de Waard V, Duijvestijn A, Lindquist Liljeqvist M, Roy J, Daugherty A, Newby A, Gerdes N, Lutgens E. CD40L deficiency protects against aneurysm formation. Arterioscler Thromb Vasc Biol. 2018;38:1076–1085. [DOI] [PubMed] [Google Scholar]
- 72.Huang X, Yue Z, Wu J, Chen J, Wang S, Wu J, Ren L, Zhang A, Deng P, Wang K, Wu C, Ding X, Ye P, Xia J. MicroRNA-21 knockout exacerbates angiotensin II-induced thoracic aortic aneurysm and dissection in mice with abnormal transforming growth factor-beta-SMAD3 signaling. Arterioscler Thromb Vasc Biol. 2018;38:1086–1101. [DOI] [PubMed] [Google Scholar]
- 73.Kimball A, Schaller M, Joshi A, Davis FM, denDekker A, Boniakowski A, Bermick J, Obi A, Moore B, Henke PK, Kunkel SL, Gallagher KA. Ly6C(Hi) blood monocyte/macrophage drive chronic inflammation and impair wound healing in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2018;38:1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hao H, Hu S, Wan Q, Xu C, Chen H, Zhu L, Xu Z, Meng J, Breyer RM, Li N, Liu DP, FitzGerald GA, Wang M. Protective role of mPGES-1 (Microsomal Prostaglandin E Synthase-1)-derived PGE2 (Prostaglandin E2) and the endothelial EP4 (Prostaglandin E Receptor) in vascular responses to injury. Arterioscler Thromb Vasc Biol. 2018;38:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okwan-Duodu D, Hansen L, Joseph G, Lyle AN, Weiss D, Archer DR, Taylor WR. Impaired collateral vessel formation in sickle cell disease. Arterioscler Thromb Vasc Biol. 2018;38:1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Briot A, Decaunes P, Volat F, Belles C, Coupaye M, Ledoux S , Bouloumie A. Senescence alters PPARgamma (Peroxisome Proliferator-Activated Receptor Gamma)-dependent fatty acid handling in human adipose tissue microvascular endothelial cells and favors inflammation. Arterioscler Thromb Vasc Biol. 2018;38:1134–1146 . [DOI] [PubMed] [Google Scholar]
- 77.Pitter B, Werner AC, Montanez E. Parvins are required for endothelial cell-cell junctions and cell polarity during embryonic blood vessel formation. Arterioscler Thromb Vasc Biol. 2018. ;38:1147–1158. [DOI] [PubMed] [Google Scholar]
- 78.Tagashira T, Fukuda T, Miyata M, Nakamura K, Fujita H, Takai Y, Hirata KI, Rikitake Y. Afadin facilitates vascular endothelial growth factor-induced network formation and migration of vascular endothelial cells by inactivating Rho-associated kinase through ArhGAP29. Arterioscler Thromb Vasc Biol. 2018. ;38:1159–1169. [DOI] [PubMed] [Google Scholar]
- 79.Wagner JUG, Chavakis E, Rogg EM, Muhly-Reinholz M, Glaser SF, Gunther S, John D, Bonini F, Zeiher AM, Schaefer L, Hannocks MJ, Boon RA, Dimmeler S. Switch in laminin beta2 to laminin beta1 isoforms during aging controls endothelial cell functions-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:1170–1177. [DOI] [PubMed] [Google Scholar]
- 80.Burke AC, Telford DE, Sutherland BG, Edwards JY, Sawyez CG, Barrett PHR, Newton RS, Pickering JG, Huff MW. Bempedoic acid lowers low-density lipoprotein cholesterol and attenuates atherosclerosis in low-density lipoprotein receptor-deficient (LDLR(+/−) and LDLR(−/−)) Yucatan miniature pigs. Arterioscler Thromb Vasc Biol. 2018;38:1178–1190. [DOI] [PubMed] [Google Scholar]
- 81.Bowden KL, Dubland JA, Chan T, Xu YH, Grabowski GA, Du H, Francis GA. LAL (Lysosomal Acid Lipase) promotes reverse cholesterol transport in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2018. ;38:1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin YC, Liu CY, Kannagi R, Yang RB. Inhibition of endothelial SCUBE2 (Signal Peptide-CUB-EGF Domain-Containing Protein 2), a novel VEGFR2 (Vascular Endothelial Growth Factor Receptor 2) coreceptor, suppresses tumor angiogenesis. Arterioscler Thromb Vasc Biol. 2018;38:1202–1215. [DOI] [PubMed] [Google Scholar]
- 83.Alsina-Sanchis E, Garcia-Ibanez Y, Figueiredo AM, Riera-Domingo C, Figueras A, Matias-Guiu X, Casanovas O, Botella LM, Pujana MA, Riera-Mestre A, Graupera M, Vinals F. ALK1 loss results in vascular hyperplasia in mice and humans through PI3K activation. Arterioscler Thromb Vasc Biol. 2018;38:1216–1229. [DOI] [PubMed] [Google Scholar]
- 84.Jones SM, Mann A, Conrad K, Saum K, Hall DE, McKinney LM, Robbins N, Thompson J, Peairs AD, Camerer E, Rayner KJ, Tranter M, Mackman N, Owens AP 3rd. PAR2 (Protease-Activated Receptor 2) deficiency attenuates atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2018;38:1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Richardson GD, Sage A, Bennaceur K, Al Zhrany N, Coelho-Lima J, Dookun E, Draganova L, Saretzki G, Breault DT, Mallat Z, Spyridopoulos I. Telomerase mediates lymphocyte proliferation but not the atherosclerosis-suppressive potential of regulatory T-cells. Arterioscler Thromb Vasc Biol. 2018;38:1283–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ray M, Gabunia K, Vrakas CN, Herman AB, Kako F, Kelemen SE, Grisanti LA, Autieri MV. Genetic deletion of IL-19 (Interleukin-19) exacerbates atherogenesis in Il19(−/−)xLdlr(−/−) double knockout mice by dysregulation of mRNA stability protein HuR (Human Antigen R). Arterioscler Thromb Vasc Biol. 2018;38:1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yao D, Xu L, Xu O, Li R, Chen M, Shen H, Zhu H, Zhang F, Yao D, Chen YF, Oparil S, Zhang Z, Gong K. O-Linked beta-N-acetylglucosamine modification of A20 enhances the inhibition of NF-kappaB (Nuclear Factor-kappaB) activation and elicits vascular protection after acute endoluminal arterial injury. Arterioscler Thromb Vasc Biol. 2018;38:1309–1320. [DOI] [PubMed] [Google Scholar]
- 88.Mong EF, Akat KM, Canfield J, Lockhart J, VanWye J, Matar A, Tsibris JCM, Wu JK, Tuschl T, Totary-Jain H. Modulation of LIN28B/Let-7 signaling by propranolol contributes to infantile hemangioma involution. Arterioscler Thromb Vasc Biol. 2018;38:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen EK, Koval OM, Noble P, Broadhurst K, Allamargot C, Wu M, Strack S, Thiel WH, Grumbach IM. CaMKII (Ca(2+)/calmodulin-dependent kinase II) in mitochondria of smooth muscle cells controls mitochondrial mobility, migration, and neointima formation. Arterioscler Thromb Vasc Biol. 2018;38:1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morfoisse F, Tatin F, Chaput B et al. Lymphatic vasculature requires estrogen receptor-alpha signaling to protect from lymphedema. Arterioscler Thromb Vasc Biol. 2018. ;38:1346–1357. [DOI] [PubMed] [Google Scholar]
- 91.Cho YK, Kang YM, Lee SE, Lee Y, Seol SM, Lee WJ, Park JY, Jung CH. Effect of SFRP5 (Secreted Frizzled-Related Protein 5) on the WNT5A (Wingless-Type Family Member 5A)-induced endothelial dysfunction and its relevance with arterial stiffness in human subjects. Arterioscler Thromb Vasc Biol. 2018;38:1358–1367. [DOI] [PubMed] [Google Scholar]
- 92.Rana R, Huang T, Koukos G, Fletcher EK, Turner SE, Shearer A, Gurbel PA, Rade JJ, Kimmelstiel CD, Bliden KP, Covic L, Kuliopulos A. Noncanonical matrix metalloprotease 1-protease-activated receptor 1 signaling drives progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:1368–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu Y, Yan R, Zhang C, Zhou Z, Liu M, Wang C, Zhang H, Dong L, Zhou T, Wu Y, Dong N, Wu Q. High-mobility group box 1 from hypoxic trophoblasts promotes endothelial microparticle production and thrombophilia in preeclampsia. Arterioscler Thromb Vasc Biol. 2018;38:1381–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toyama K, Spin JM, Deng AC et al. MicroRNA-mediated therapy modulating blood-brain barrier disruption improves vascular cognitive impairment. Arterioscler Thromb Vasc Biol. 2018;38:1392–1406. [DOI] [PubMed] [Google Scholar]
- 95.Oldoni F, van Capelleveen JC, Dalila N, Wolters JC, Heeren J, Sinke RJ, Hui DY, Dallinga-Thie GM, Frikke-Schmidt R, Hovingh KG, van de Sluis B, Tybjaerg-Hansen A, Kuivenhoven JA. Naturally occurring variants in LRP1 (Low-Density Lipoprotein Receptor-Related Protein 1) affect HDL (High-Density Lipoprotein) metabolism through ABCA1 (ATP-Binding Cassette A1) and SR-B1 (Scavenger Receptor Class B Type 1) in humans. Arterioscler Thromb Vasc Biol. 2018;38:1440–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takiguchi S, Ayaori M, Yakushiji E, Nishida T, Nakaya K, Sasaki M, Iizuka M, Uto-Kondo H, Terao Y, Yogo M, Komatsu T, Ogura M, Ikewaki K. Hepatic overexpression of endothelial lipase lowers high-density lipoprotein but maintains reverse cholesterol transport in mice: Role of scavenger receptor class B type I/ATP-binding cassette transporter A1-dependent pathways. Arterioscler Thromb Vasc Biol. 2018;38:1454–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang F, Liu Z, Park SH, Gwag T, Lu W, Ma M, Sui Y, Zhou C. Myeloid beta-catenin deficiency exacerbates atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2018;38:1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lallemand T, Rouahi M, Swiader A, Grazide MH, Geoffre N, Alayrac P, Recazens E, Coste A, Salvayre R , Negre-Salvayre A, Auge N. nSMase2 (type 2-neutral sphingomyelinase) deficiency or inhibition by GW4869 reduces inflammation and atherosclerosis in Apoe(−/−) mice. Arterioscler Thromb Vasc Biol. 2018;38:1479–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Molusky MM, Hsieh J, Lee SX, Ramakrishnan R, Tascau L, Haeusler RA, Accili D, Tall AR. Metformin and AMP kinase activation increase expression of the sterol transporters ABCG5/8 (ATP-Binding Cassette Transporter G5/G8) with potential antiatherogenic consequences. Arterioscler Thromb Vasc Biol. 2018. ;38:1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin X, Dimitriadis EK, Liu Y, Combs CA, Chang J, Varsano N, Stempinski E, Flores R, Jackson SN, Muller L, Woods AS, Addadi L, Kruth HS. Macrophages shed excess cholesterol in unique extracellular structures containing cholesterol microdomains. Arterioscler Thromb Vasc Biol. 2018;38:1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilhelmson AS, Lantero Rodriguez M, Svedlund Eriksson E, Johansson I, Fogelstrand P, Stubelius A, Lindgren S, Fagman JB, Hansson GK, Carlsten H, Karlsson MCI, Ekwall O, Tivesten A. Testosterone protects against atherosclerosis in male mice by targeting thymic epithelial cells-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu S, Chen J, Diamond SL. Establishing the transient mass balance of thrombosis: From tissue factor to rhrombin to fibrin under venous flow. Arterioscler Thromb Vasc Biol. 2018. ;38:1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fava M, Barallobre-Barreiro J, Mayr U, Lu R, Didangelos A, Baig F, Lynch M, Catibog N, Joshi A, Barwari T, Yin X, Jahangiri M, Mayr M. Role of ADAMTS-5 in aortic dilatation and extracellular matrix remodeling. Arterioscler Thromb Vasc Biol. 2018;38:1537–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schillemans M, Karampini E, van den Eshof BL et al. Weibel-palade body localized syntaxin-3 modulates Von willebrand factor secretion from endothelial cells. Arterioscler Thromb Vasc Biol. 2018;38:1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chowdhury TA, Koceja C, Eisa-Beygi S, Kleinstiver BP, Kumar SN, Lin CW, Li K, Prabhudesai S, Joung JK, Ramchandran R. Temporal and spatial post-transcriptional regulation of zebrafish tie1 mRNA by long noncoding RNA during brain vascular assembly. Arterioscler Thromb Vasc Biol. 2018;38:1562–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peterson SM , Turner JE, Harrington A, Davis-Knowlton J, Lindner V, Gridley T, Vary CPH, Liaw L. Notch2 and proteomic signatures in mouse neointimal lesion formation. Arterioscler Thromb Vasc Biol. 2018;38:1576–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cameron SJ, Mix DS, Ture SK, Schmidt RA, Mohan A, Pariser D, Stoner MC, Shah P, Chen L, Zhang H, Field DJ, Modjeski KL, Toth S, Morrell CN. Hypoxia and ischemia promote a maladaptive platelet phenotype. Arterioscler Thromb Vasc Biol. 2018;38:1594–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shafiq M, Zhang Q, Zhi D, Wang K, Kong D, Kim DH, Kim SH. In situ blood vessel regeneration using SP (substance P) and SDF (stromal cell-derived factor)-1alpha peptide eluting vascular grafts. Arterioscler Thromb Vasc Biol. 2018;38:e117–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ , Bhatnagar A, Hamburg NM. Flavorings in tobacco products induce endothelial cell dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He L, Fu Y, Deng J et al. Deficiency of FAM3D (Family With Sequence Similarity 3, Member D), a novel chemokine, attenuates neutrophil recruitment and ameliorates abdominal aortic aneurysm development. Arterioscler Thromb Vasc Biol. 2018. ;38:1616–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tourdot BE, Stoveken H, Trumbo D, Yeung J, Kanthi Y, Edelstein LC, Bray PF, Tall GG, Holinstat M. Genetic variant in human PAR (Protease-Activated Receptor) 4 enhances thrombus formation resulting in resistance to antiplatelet therapeutics. Arterioscler Thromb Vasc Biol. 2018;38:1632–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Williams JW , Martel C, Potteaux S, Esaulova E, Ingersoll MA, Elvington A, Saunders BT, Huang LH, Habenicht AJ, Zinselmeyer BH, Randolph GJ. Limited macrophage positional dynamics in progressing or regressing murine atherosclerotic plaques-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:1702–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Centa M, Prokopec KE, Garimella MG et al. Acute loss of apolipoprotein E triggers an autoimmune response that accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:e145–e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jones PD, Kaiser MA, Ghaderi Najafabadi M et al. JCAD, a gene at the 10p11 coronary artery disease locus, regulates hippo signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2018;38:1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cansby E, Magnusson E, Nunez-Duran E, Amrutkar M, Pedrelli M, Parini P, Hoffmann J, Stahlman M, Howell BW, Marschall HU, Boren J, Mahlapuu M. STK25 regulates cardiovascular disease progression in a mouse model of hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2018. ;38:1723–1737. [DOI] [PubMed] [Google Scholar]
- 116.Xiong W, Zhao X, Villacorta L, Rom O, Garcia-Barrio MT, Guo Y, Fan Y, Zhu T, Zhang J, Zeng R, Chen YE, Jiang Z, Chang L. Brown adipocyte-specific PPARgamma (Peroxisome Proliferator-Activated Receptor gamma) deletion impairs perivascular adipose tissue development and enhances atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2018;38:1738–1747 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zilberman-Rudenko J, Reitsma SE, Puy C et al. Factor XII activation promotes platelet consumption in the presence of bacterial-type long-chain polyphosphate in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2018;38:1748–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salas-Perdomo A, Miro-Mur F, Urra X, Justicia C, Gallizioli M, Zhao Y, Brait VH, Laredo C, Tudela R, Hidalgo A, Chamorro A, Planas AM. T cells prevent hemorrhagic transformation in ischemic stroke by P-selectin binding. Arterioscler Thromb Vasc Biol. 2018;38:1761–1771. [DOI] [PubMed] [Google Scholar]
- 119.Claushuis TAM, de Stoppelaar SF, de Vos AF, Grootemaat AE, van der Wel NN, Roelofs JJTH, Ware J, Van’t Veer C, van der Poll T. Nbeal2 deficiency increases organ damage but does not affect host defense during gram-negative pneumonia-derived sepsis. Arterioscler Thromb Vasc Biol. 2018;38:1772–1784. [DOI] [PubMed] [Google Scholar]
- 120.van Loon NM , Ottenhoff R, Kooijman S, Moeton M, Scheij S, Roscam Abbing RLP, Gijbels MJJ, Levels JHM, Sorrentino V, Berbee JFP, Rensen PCN, Zelcer N. Inactivation of the E3 ubiquitin ligase IDOL attenuates diet-induced obesity and metabolic dysfunction in mice. Arterioscler Thromb Vasc Biol. 2018. ;38:1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adam M, Kooreman N, Jagger A et al. Systemic upregulation of IL-10 (Interleukin-10) using a nonimmunogenic vector reduces growth and rate of dissecting abdominal a ortic aneurysm. Arterioscler Thromb Vasc Biol. 2018;38:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Das S, Reddy MA, Senapati P, Stapleton K, Lanting L, Wang M, Amaram V, Ganguly R, Zhang L, Devaraj S, Schones DE, Natarajan R. Diabetes mellitus-induced long noncoding RNA Dnm3os regulates macrophage functions and inflammation via nuclear mechanisms. Arterioscler Thromb Vasc Biol. 2018;38:1806–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Akla N, Viallard C, Popovic N, Lora Gil C, Sapieha P, Larrivee B. BMP9 (Bone Morphogenetic Protein-9)/Alk1 (Activin-Like Kinase Receptor Type I) signaling prevents hyperglycemia-induced vascular permeability. Arterioscler Thromb Vasc Biol. 2018;38:1821–1836. [DOI] [PubMed] [Google Scholar]
- 124.Zhao G, Yang W, Wu J, Chen B, Yang X, Chen J, McVey DG, Andreadi C, Gong P, Webb TR, Samani NJ, Ye S. Influence of a coronary artery disease-associated genetic variant on FURIN expression and effect of furin on macrophage behavior. Arterioscler Thromb Vasc Biol. 2018;38:1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mehta V, Fields L, Evans IM, Yamaji M, Pellet-Many C, Jones T, Mahmoud M, Zachary I. VEGF (Vascular Endothelial Growth Factor) induces NRP1 (Neuropilin-1) cleavage via ADAMs (a Disintegrin and Metalloproteinase) 9 and 10 to generate novel carboxy-terminal NRP1 fragments that regulate angiogenic signaling. Arterioscler Thromb Vasc Biol. 2018;38:1845–1858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manchanda K, Kolarova H, Kerkenpass C, Mollenhauer M, Vitecek J, Rudolph V, Kubala L, Baldus S, Adam M, Klinke A. MPO (Myeloperoxidase) reduces endothelial glycocalyx thickness dependent on its cationic charge. Arterioscler Thromb Vasc Biol. 2018;38:1859–1867. [DOI] [PubMed] [Google Scholar]
- 127.Biswas I, Panicker SR, Cai X, Mehta-D’souza P, Rezaie AR. Inorganic polyphosphate amplifies high mobility group box 1-mediated von Willebrand factor release and platelet string formation on endothelial cells. Arterioscler Thromb Vasc Biol. 2018;38:1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lino M, Wan MH, Rocca AS, Ngai D, Shobeiri N, Hou G, Ge C, Franceschi RT, Bendeck MP. Diabetic vascular calcification mediated by the collagen receptor discoidin domain receptor 1 via the phosphoinositide 3-kinase/Akt/Runt-related transcription factor 2 signaling axis. Arterioscler Thromb Vasc Biol. 2018;38:1878–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilson PG, Thompson JC, Shridas P, McNamara PJ, de Beer MC, de Beer FC, Webb NR, Tannock LR. Serum amyloid A is an exchangeable apolipoprotein. Arterioscler Thromb Vasc Biol. 2018;38:1890–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1alpha and cathepsin G. Arterioscler Thromb Vasc Biol. 2018. ;38:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jarrett KE, Lee C, De Giorgi M, Hurley A, Gillard BK, Doerfler AM, Li A, Pownall HJ, Bao G, Lagor WR. Somatic editing of Ldlr with adeno-associated viral-CRISPR is an efficient tool for atherosclerosis research. Arterioscler Thromb Vasc Biol. 2018;38:1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asztalos BF , Horvath KV, Schaefer EJ. High-density lipoprotein particles, cell-cholesterol efflux, and coronary heart disease risk. Arterioscler Thromb Vasc Biol. 2018;38:2007–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Erbilgin A, Seldin MM, Wu X, Mehrabian M, Zhou Z, Qi H, Dabirian KS, Sevag Packard RR, Hsieh W, Bensinger SJ, Sinha S, Lusis AJ. Transcription factor Zhx2 deficiency reduces atherosclerosis and promotes macrophage apoptosis in mice. Arterioscler Thromb Vasc Biol. 2018;38:2016–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Muthuramu I , Amin R, Aboumsallem JP, Mishra M, Robinson EL, De Geest B. Hepatocyte-specific SR-BI gene transfer corrects cardiac dysfunction in Scarb1-deficient mice and improves pressure overload-induced cardiomyopathy. Arterioscler Thromb Vasc Biol. 2018;38:2028–2040. [DOI] [PubMed] [Google Scholar]
- 135.Laurent PA, Hechler B, Solinhac R, Ragab A, Cabou C, Anquetil T, Severin S, Denis CV, Mangin PH , Vanhaesebroeck B, Payrastre B, Gratacap MP . Impact of PI3Kalpha (Phosphoinositide 3-Kinase Alpha) inhibition on hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:2041–2053. [DOI] [PubMed] [Google Scholar]
- 136.Karlsen TV, Nikpey E, Han J, Reikvam T, Rakova N, Castorena-Gonzalez JA, Davis MJ, Titze JM, Tenstad O, Wiig H. High-salt diet causes expansion of the lymphatic network and increased lymph flow in skin and muscle of rats. Arterioscler Thromb Vasc Biol. 2018;38:2054–2064. [DOI] [PubMed] [Google Scholar]
- 137.DeLalio LJ, Keller AS, Chen J et al. Interaction between pannexin 1 and caveolin-1 in smooth muscle can regulate blood pressure. Arterioscler Thromb Vasc Biol. 2018. ;38:2065–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Badi I, Mancinelli L, Polizzotto A, Ferri D, Zeni F, Burba I, Milano G, Brambilla F, Saccu C, Bianchi ME, Pompilio G, Capogrossi MC, Raucci A. miR-34a promotes vascular smooth muscle cell calcification by downregulating SIRT1 (Sirtuin 1) and Axl (AXL Receptor Tyrosine Kinase). Arterioscler Thromb Vasc Biol. 2018;38:2079–2090. [DOI] [PubMed] [Google Scholar]
- 139.Stott JB, Barrese V, Suresh M, Masoodi S, Greenwood IA. Investigating the role of G protein betagamma in Kv7-dependent relaxations of the rat vasculature. Arterioscler Thromb Vasc Biol. 2018;38:2091–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang L, Wang Y, Zhang C, Li J, Meng Y, Dou M, Noguchi CT, Di L. Inhibiting glycogen synthase kinase 3 reverses obesity-induced white adipose tissue inflammation by regulating apoptosis inhibitor of macrophage/CD5L-mediated macrophage migration. Arterioscler Thromb Vasc Biol. 2018;38:2103–2116. [DOI] [PubMed] [Google Scholar]
- 141.Descamps B, Saif J, Benest AV, Biglino G, Bates DO, Chamorro-Jorganes A, Emanueli C. BDNF (Brain-Derived Neurotrophic Factor) promotes embryonic stem cells differentiation to endothelial cells via a molecular pathway, including MicroRNA-214, EZH2 (Enhancer of Zeste Homolog 2), and eNOS (Endothelial Nitric Oxide Synthase). Arterioscler Thromb Vasc Biol. 2018;38:2117–2125. [DOI] [PubMed] [Google Scholar]
- 142.Yuan S, Yurdagul A Jr, Peretik JM, Alfaidi M, Al Yafeai Z, Pardue S, Kevil CG, Orr AW. Cystathionine gamma-lyase modulates flow-dependent vascular remodeling. Arterioscler Thromb Vasc Biol. 2018;38:2126–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Villa-Bellosta R. Synthesis of extracellular pyrophosphate increases in vascular smooth muscle cells during phosphate-induced calcification. Arterioscler Thromb Vasc Biol. 2018;38:2137–2147. [DOI] [PubMed] [Google Scholar]
- 144.Parra-Izquierdo I, Castanos-Mollor I, Lopez J, Gomez C, San Roman JA, Sanchez Crespo M, Garcia-Rodriguez C. Calcification induced by type I interferon in human aortic valve interstitial cells Is larger in males and blunted by a janus kinase inhibitor. Arterioscler Thromb Vasc Biol. 2018;38:2148–2159. [DOI] [PubMed] [Google Scholar]
- 145.Herrero D, Canon S, Pelacho B, Salvador-Bernaldez M, Aguilar S, Pogontke C, Carmona RM, Salvador JM, Perez-Pomares JM, Klein OD, Prosper F, Jimenez-Borreguero LJ, Bernad A. Bmi1-progenitor cell ablation impairs the angiogenic response to myocardial infarction. Arterioscler Thromb Vasc Biol. 2018;38:2160–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Caolo V, Peacock HM, Kasaai B, Swennen G, Gordon E, Claesson-Welsh L, Post MJ, Verhamme P, Jones EAV. Shear stress and VE-cadherin. Arterioscler Thromb Vasc Biol. 2018;38:2174–2183. [DOI] [PubMed] [Google Scholar]
- 147.Lyu Q, Dhagia V, Han Y, Guo B, Wines-Samuelson ME, Christie CK, Yin Q, Slivano OJ, Herring P, Long X, Gupte SA, Miano JM. CRISPR-Cas9-mediated epitope tagging provides accurate and versatile assessment of myocardin-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:2184–2190 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hirai H, Yang B, Garcia-Barrio MT, Rom O, Ma PX, Zhang J, Chen YE. Direct reprogramming of fibroblasts into smooth muscle-like cells with defined transcription factors-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:2191–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Verges B, Duvillard L, Pais de Barros JP, Bouillet B, Baillot-Rudoni S, Rouland A, Sberna AL, Petit JM, Degrace P, Demizieux L. Liraglutide reduces postprandial hyperlipidemia by increasing ApoB48 (Apolipoprotein B48) catabolism and by reducing ApoB48 production in patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2018;38:2198–2206. [DOI] [PubMed] [Google Scholar]
- 150.Basu D, Huggins LA, Scerbo D, Obunike J, Mullick AE, Rothenberg PL, Di Prospero NA, Eckel RH, Goldberg IJ. Mechanism of increased LDL (Low-Density Lipoprotein) and decreased triglycerides with SGLT2 (Sodium-Glucose Cotransporter 2) inhibition. Arterioscler Thromb Vasc Biol. 2018;38:2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harari E, Guo L, Smith SL et al. Direct targeting of the mTOR (Mammalian Target of Rapamycin) kinase improves endothelial permeability in drug-eluting stents-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38:2217–2224. [DOI] [PubMed] [Google Scholar]
- 152.Ghaffari S, Naderi Nabi F, Sugiyama MG, Lee WL. Estrogen inhibits LDL (Low-Density Lipoprotein) transcytosis by human coronary artery endothelial cells via GPER (G-Protein-Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1). Arterioscler Thromb Vasc Biol. 2018;38:2283–2294. [DOI] [PubMed] [Google Scholar]
- 153.Jean-Charles PY, Wu JH, Zhang L, Kaur S, Nepliouev I, Stiber JA, Brian L, Qi R, Wertman V, Shenoy SK, Freedman NJ. USP20 (Ubiquitin-Specific Protease 20) inhibits TNF (Tumor Necrosis Factor)-triggered smooth muscle cell inflammation and attenuates atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:2295–2305 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sukhanov S, Higashi Y, Shai SY, Snarski P, Danchuk S, D’Ambra V, Tabony M, Woods TC, Hou X, Li Z, Ozoe A, Chandrasekar B, Takahashi SI, Delafontaine P. SM22alpha (Smooth Muscle Protein 22-alpha) promoter-driven IGF1R (Insulin-Like Growth Factor 1 Receptor) deficiency promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2018;38:2306–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lindskog Jonsson A, Caesar R, Akrami R, Reinhardt C, Fak Hallenius F, Boren J, Backhed F. Impact of gut microbiota and diet on the development of atherosclerosis in Apoe(−/−) mice. Arterioscler Thromb Vasc Biol. 2018;38:2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tirronen A, Vuorio T, Kettunen S, Hokkanen K, Ramms B, Niskanen H, Laakso H, Kaikkonen MU, Jauhiainen M, Gordts PLSM, Yla-Herttuala S. Deletion of lymphangiogenic and angiogenic growth factor VEGF-D leads to severe hyperlipidemia and delayed clearance of chylomicron remnants. Arterioscler Thromb Vasc Biol. 2018;38:2327–2337. [DOI] [PubMed] [Google Scholar]
- 157.Ni R, Vaezzadeh N, Zhou J, Weitz JI, Cattaneo M, Gross PL. Effect of different doses of acetylsalicylic acid on the antithrombotic activity of clopidogrel in a mouse arterial thrombosis model. Arterioscler Thromb Vasc Biol. 2018;38:2338–2344. [DOI] [PubMed] [Google Scholar]
- 158.Joris V, Gomez EL, Menchi L, Lobysheva I, Di Mauro V, Esfahani H, Condorelli G, Balligand JL, Catalucci D, Dessy C. MicroRNA-199a-3p and microRNA-199a-5p take part to a redundant network of regulation of the NOS (NO Synthase)/NO pathway in the endothelium. Arterioscler Thromb Vasc Biol. 2018;38:2345–2357. [DOI] [PubMed] [Google Scholar]
- 159.Minoshima A, Kabara M, Matsuki M et al. Pericyte-specific ninjurin1 deletion attenuates vessel maturation and blood flow recovery in hind limb ischemia. Arterioscler Thromb Vasc Biol. 2018. ;38:2358–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hashad AM, Harraz OF, Brett SE, Romero M, Kassmann M, Puglisi JL, Wilson SM, Gollasch M, Welsh DG. Caveolae link CaV3.2 channels to BKCa-mediated feedback in vascular smooth muscle. Arterioscler Thromb Vasc Biol. 2018;38:2371–2381. [DOI] [PubMed] [Google Scholar]
- 161.Liu L, Zeng P, Yang X et al. Inhibition of vascular calcification. Arterioscler Thromb Vasc Biol. 2018;38:2382–2395. [DOI] [PubMed] [Google Scholar]
- 162.Serra A, Gallart-Palau X, Park JE, Lim GGY, Lim KL, Ho HH, Tam JP, Sze SK. Vascular bed molecular profiling by differential systemic decellularization in vivo. Arterioscler Thromb Vasc Biol. 2018;38:2396–2409. [DOI] [PubMed] [Google Scholar]
- 163.Xu W, Wittchen ES, Hoopes SL, Stefanini L, Burridge K, Caron KM. Small GTPase Rap1A/B is required for lymphatic development and adrenomedullin-induced stabilization of lymphatic endothelial junctions. Arterioscler Thromb Vasc Biol. 2018;38:2410–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vukelic S, Xu Q, Seidel-Rogol B, Faidley EA, Dikalova AE, Hilenski LL, Jorde U, Poole LB, Lassegue B, Zhang G, Griendling KK. NOX4 (NADPH Oxidase 4) and poldip2 (Polymerase delta-Interacting Protein 2) induce filamentous actin oxidation and promote its interaction with vinculin during integrin-mediated cell adhesion. Arterioscler Thromb Vasc Biol. 2018;38:2423–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lo RW, Li L, Leung R, Pluthero FG, Kahr WHA. NBEAL2 (Neurobeachin-Like 2) is required for retention of cargo proteins by alpha-granules during their production by megakaryocytes. Arterioscler Thromb Vasc Biol. 2018;38:2435–2447. [DOI] [PubMed] [Google Scholar]
- 166.Singh AB, Dong B, Kraemer FB, Xu Y, Zhang Y, Liu J. Farnesoid X receptor activation by obeticholic acid elevates liver low-density lipoprotein receptor expression by mRNA stabilization and reduces plasma low-density lipoprotein cholesterol in mice. Arterioscler Thromb Vasc Biol. 2018;38:2448–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nishino T, Horie T, Baba O et al. SREBF1/MicroRNA-33b axis exhibits potent effect on unstable atherosclerotic plaque ormation in vivo. Arterioscler Thromb Vasc Biol. 2018;38:2460–2473. [DOI] [PubMed] [Google Scholar]
- 168.Masi S, Colucci R, Duranti E et al. Aging modulates the influence of arginase on endothelial dysfunction in obesity. Arterioscler Thromb Vasc Biol. 2018;38:2474–2483. [DOI] [PubMed] [Google Scholar]
- 169.Carnevale D , Facchinello N, Iodice D et al. Loss of EMILIN-1 enhances arteriolar myogenic tone through TGF-beta (Transforming Growth Factor-beta)-dependent transactivation of EGFR (Epidermal Growth Factor Receptor) and is relevant for hypertension in mice and humans. Arterioscler Thromb Vasc Biol. 2018;38:2484–2497. [DOI] [PubMed] [Google Scholar]
- 170.Rinne P, Guillamat-Prats R, Rami M, Bindila L, Ring L, Lyytikainen LP, Raitoharju E, Oksala N, Lehtimaki T, Weber C, van der Vorst EPC, Steffens S. Palmitoylethanolamide promotes a proresolving macrophage phenotype and attenuates atherosclerotic plaque formation. Arterioscler Thromb Vasc Biol. 2018;38:2562–2575. [DOI] [PubMed] [Google Scholar]
- 171.Wakabayashi T, Takahashi M, Yamamuro D, Karasawa T, Takei A, Takei S, Yamazaki H, Nagashima S, Ebihara K, Takahashi M, Ishibashi S. Inflammasome activation aggravates cutaneous xanthomatosis and atherosclerosis in ACAT1 (Acyl-CoA Cholesterol Acyltransferase 1) deficiency in bone marrow. Arterioscler Thromb Vasc Biol. 2018;38:2576–2589. [DOI] [PubMed] [Google Scholar]
- 172.Sakai K, Nagashima S, Wakabayashi T et al. Myeloid HMG-CoA (3-Hydroxy-3-Methylglutaryl-Coenzyme A) reductase determines atherosclerosis by modulating migration of macrophages. Arterioscler Thromb Vasc Biol. 2018;38:2590–2600. [DOI] [PubMed] [Google Scholar]
- 173.Al-Yafeai Z , Yurdagul A Jr, Peretik JM, Alfaidi M, Murphy PA, Orr AW. Endothelial FN (Fibronectin) deposition by alpha5beta1 integrins drives atherogenic inflammation. Arterioscler Thromb Vasc Biol. 2018;38:2601–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lorkowski SW, Brubaker G, Gulshan K, Smith JD. V-ATPase (Vacuolar ATPase) activity required for ABCA1 (ATP-Binding Cassette Protein A1)-mediated cholesterol efflux. Arterioscler Thromb Vasc Biol. 2018;38:2615–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Loyau S, Ho-Tin-Noe B, Bourrienne MC, Boulaftali Y, Jandrot-Perrus M. Microfluidic modeling of thrombolysis. Arterioscler Thromb Vasc Biol. 2018;38:2626–2637. [DOI] [PubMed] [Google Scholar]
- 176.Nosaka M, Ishida Y, Kimura A, Kuninaka Y, Taruya A, Furuta M, Mukaida N, Kondo T. Contribution of the TNF-alpha (Tumor Necrosis Factor-alpha)-TNF-Rp55 (Tumor Necrosis Factor Receptor p55) axis in the resolution of venous thrombus. Arterioscler Thromb Vasc Biol. 2018;38:2638–2650. [DOI] [PubMed] [Google Scholar]
- 177.Au DT, Ying Z, Hernandez-Ochoa EO, Fondrie WE, Hampton B, Migliorini M, Galisteo R, Schneider MF, Daugherty A, Rateri DL, Strickland DK, Muratoglu SC. LRP1 (Low-Density Lipoprotein Receptor-Related Protein 1) regulates smooth muscle contractility by modulating Ca(2+) signaling and expression of cytoskeleton-related proteins. Arterioscler Thromb Vasc Biol. 2018;38:2651–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shi D, Qi M, Zhou L et al. Endothelial mitochondrial preprotein translocase Tomm7-Rac1 signaling axis dominates cerebrovascular network homeostasis. Arterioscler Thromb Vasc Biol. 2018;38:2665–2677. [DOI] [PubMed] [Google Scholar]
- 179.Orsini F, Fumagalli S, Csaszar E, Toth K, De Blasio D, Zangari R, Lenart N, Denes A, De Simoni MG. Mannose-binding lectin drives platelet inflammatory phenotype and vascular damage after cerebral ischemia in mice via IL (Interleukin)-1alpha. Arterioscler Thromb Vasc Biol. 2018. ;38:2678–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang C, Lai MB, Pedler MG, Johnson V, Adams RH, Petrash JM, Chen Z, Junge HJ. Endothelial cell-specific inactivation of TSPAN12 (Tetraspanin 12) reveals pathological consequences of barrier defects in an otherwise intact vasculature. Arterioscler Thromb Vasc Biol. 2018;38:2691–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zheng Z, Ai J, Guo L, Ye X, Bondada S, Howatt D, Daugherty A, Li XA. SR-BI (Scavenger Receptor Class B Type 1) is critical in maintaining normal T-cell development and enhancing thymic regeneration. Arterioscler Thromb Vasc Biol. 2018;38:2706–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chai JT, Ruparelia N, Goel A, Kyriakou T, Biasiolli L, Edgar L, Handa A, Farrall M, Watkins H, Choudhury RP. Differential gene expression in macrophages from human atherosclerotic plaques shows convergence on pathways implicated by Genome-Wide Association Study risk variants. Arterioscler Thromb Vasc Biol. 2018. ;38:2718–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Abdelgawwad MS, Cao W, Zheng L, Kocher NK, Williams LA, Zheng XL. Transfusion of platelets loaded with recombinant ADAMTS13 (A Disintegrin and Metalloprotease With Thrombospondin Type 1 Repeats-13) is efficacious for inhibiting arterial thrombosis associated with thrombotic thrombocytopenic purpura. Arterioscler Thromb Vasc Biol. 2018;38:2731–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang M, Zeng X, Yang Q et al. Ablation of myeloid ADK (adenosine kinase) epigenetically suppresses atherosclerosis in ApoE−/− (Apolipoprotein E deficient) mice. Arterioscler Thromb Vasc Biol. 2018;38:2780–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu Y, Ouyang X, Yan L, Zhang M, Hu Z, Gu J, Fan X, Zhang L, Zhang J, Xue S, Chen G, Su B, Liu J. Sin1 (Stress-Activated Protein Kinase-Interacting Protein) regulates ischemia-induced microthrombosis through integrin alphaIIbbeta3-mediated outside-in signaling and hypoxia responses in platelets. Arterioscler Thromb Vasc Biol. 2018;38:2793–2805. [DOI] [PubMed] [Google Scholar]
- 186.Eisa-Beygi S, Benslimane FM, El-Rass S, Prabhudesai S , Abdelrasoul MKA, Simpson PM, Yalcin HC, Burrows PE, Ramchandran R. Characterization of endothelial cilia distribution during cerebral-vascular development in zebrafish ( Danio rerio). Arterioscler Thromb Vasc Biol. 2018. ;38:2806–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen L, Yang G, Zhang J, Ren B, Tang S, Li X, FitzGerald GA. Time-dependent hypotensive effect of aspirin in mice. Arterioscler Thromb Vasc Biol. 2018;38:2819–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-Statement From ATVB Council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Daugherty A, Lu HS, Hegele RA, Mackman N, Rader DJ, Schmidt AM, Weber C. Response by Daugherty et al to Letter Regarding Article, “Consideration of sex differences in design and reporting of experimental arterial pathology studies: A statement from the Arteriosclerosis, Thrombosis, and Vascular Biology Council”. Arterioscler Thromb Vasc Biol. 2018;38:e101–e102. [DOI] [PubMed] [Google Scholar]


