Abstract
Primary hyperparathyroidism (pHPT) is characterized by an increase in the levels of PTH and Ca, or one of these (Ca, PTH) as a result of a dysregulation of calcium (Ca) metabolism due to inappropriate excess parathyroid hormone (PTH) autonomously produced from one or more than one parathyroid glands. Ninety to 95% of pHPT is a sporadic type, which is not associated with the familial history and other endocrine organ tumors, and 5-10% of it is hereditary. While 80-85% of pHPT arises from a single parathyroid adenoma, 4-5% is caused by a double adenoma, 10-15% by multigland hyperplasia and less than 1% by parathyroid cancer. The diagnosis of pHPT is reached biochemically. The only curative treatment of pHPT is surgery. The choice of surgery in pHPT may vary depending on whether the patient has hereditary HPT or thyroid disease requiring surgical treatment, preoperative localization studies and the findings in these studies, the possibilities of using intraoperative PTH and the preference of the surgeon.
The preoperatively determined surgical strategy can be revised according to intraoperative findings in case of need to achieve excellent results. The two main approaches in the surgical treatment of pHPT are BNE (bilateral neck exploration) and MIP (minimal invasive parathyroidectomy). Although BNE is a consistently valid option that has excellent results in the surgical treatment of pHPT and is considered the gold standard, MIP is the ideal approach in selected patients with clinically and radiologically considered a single-gland disease. Negative imaging is not a contraindication for parathyroid surgery and is not a criterion for the presence or absence of surgical indication. Although both methods are safe and effective in the surgical treatment of sporadic pHPT, there is still controversy regarding the effectiveness of both methods.
Surgical intervention should establish the risk-benefit balance well, minimize the risk of persistent and recurrent disease and provide the highest cure rate without increasing the risk of complications. Complication rates are higher in the secondary surgery, thus in secondary procedures, selective surgery should be performed under guidance of an imaging modality.
The surgical strategy should be determined to achieve maximum cure with minimum dissection and minimal morbidity. In this study, we aimed to determine the type of surgical treatment and pHPT patients suitable for the surgical treatment.
Keywords: Bilateral neck exploration, minimal invasive parathyroidectomy, unilateral neck exploration, primary hyperparathyroidism
Primary hyperparathyroidism (pHPT) is characterized by an increase in the levels of PTH and Ca, or one of these (Ca, PTH) as a result of dysregulation of calcium (Ca) metabolism due to inappropriate and autonomous production of excess parathyroid hormone (PTH) from one or more than one parathyroid glands.[1, 2] pHPT is the third most common endocrine disease after diabetes mellitus and thyroid diseases[3] and the most common cause of hypercalcemia in ambulatory patients.[4]
After pHPT was defined in the early 20th century, pHPT was usually diagnosed for 50 years based on severe hypercalcemia and severe skeletal and renal symptoms. With the development of the analyzer and diffuse serum Ca measurement in 1970, most of the patients were asymptomatic patients who were diagnosed incidentally with hypercalcemia. Due to this development, the prevalence of pHPT is increasing, and its prevalence in the USA has tripled between 1995 and 2010.[5] The actual prevalence is thought to be higher than reported in traditional studies.[6] The incidence of pHPT is estimated to be in a wide range of 0.4-82 cases per 100000 individuals.[1] It is seen three times more frequently in women than in men. Although of pHPT can be seen at any age, the incidence increases in both sexes with age, and dramatically after age 50.[5]
Ninety-95% of pHPT is a sporadic type, which is not associated with the familial history and other endocrine organ tumors, and 5-10% of them is hereditary. Types of hereditary hyperparathyroidism include multiple endocrine neoplasias (MEN) 1, 2A, 4, familial syndromes associated with pHPT component; multiple endocrine neoplasia MEN I, MEN IIA, MEN4, hyperparathyroidism-jaw tumor syndrome, familial isolated hyperparathyroidism, autosomal dominant moderate hyperparathyroidism and severe neonatal hyperparathyroidism. Hereditary pHPT is seen at an earlier age than the sporadic type. Gene penetration varies.[7] Although some risk factors for the development of sporadic PrHPT are known, the underlying cause is unknown in the majority of the patients.
Long-term lithium therapy, history of radioactive iodine therapy for thyroid disease, or external neck radiation is a risk factor for sporadic pHPT.[1, 3] Chronic low-dose calcium use, obesity, celiac disease, hypertension, furosemide use have been reported to be risk factors for the development of sporadic pHPT.[1–3] Eighty -85% of pHPT is caused by a single parathyroid adenoma, 4-5% by a double adenoma, 10-15% by multigland hyperplasia and less than 1% by parathyroid cancer.[8]
Diagnosis
The diagnosis of pHPT is made biochemically. The first standard biochemical panel that should be performed when pHPT is suspected; including serum total Ca, PTH, phosphorus, creatinine, albumin, 25-OH vitamin D (nonclassic presentation or suspected vitamin D deficiency) and 24-hour urinary calcium excretion (for presumptive familial hypocalciuric hypercalcemia).[2] When normocalcemic hyperparathyroidism is suspected or in hypoalbuminemic patients, serum ionized calcium should be evaluated.[9, 10] The negative feedback pathway in regulating serum calcium level is impaired due to the autonomy of the pHPT parathyroid gland(s). As a result, hypercalcemia and high PTH values are detected in classical pHPT. As a result of the increase in the incidence of diagnosed cases of pHPT, biochemically unpredictable types emerged.
These types may cause complex findings in biochemical evaluation. While serum Ca values of the type called nonclassic pHPT (normohormonal or non-suppressed) are at high levels or near the upper limit of the reference range, PTH is not suppressed due to inappropriate negative inhibition, so it remains within normal limits.[5] In normocalcemic HPT, despite normal vitamin 25 (OH) D3 values, increased PTH values were detected, and corrected serum Ca and ionized Ca values are within normal limits.[10] When making the diagnosis of pHPT, common causes of secondary HPT, such as renal insufficiency, vitamin D deficiency, abnormalities of intestinal absorption, and renal escape syndrome should be excluded.
In addition, the diagnosis of familial hypocalciuric hypercalcemia should be excluded with 24-hour urine Ca measurement.[2] It should also be considered that there may be hereditary pHPT at the time of diagnosis. Genetic testing is recommended in the presence of pHPT in the first-degree relatives of patients aged <45 years, male gender, presence of multigland disease, MEN 1, and one of the other clinical features of hyperparathyroidism-jaw tumor syndrome (MEN1, CASR, CDKN1A, CDKN1B, CDKN2C, RET genes).[11]
Surgical Indications
The only curative treatment of pHPT is surgery. Parathyroidectomy is indicated in all symptomatic patients with renal and bone findings.[12] However, medical treatment of the patients may be considered for patients who decline parathyroidectomy or the patients whose surgical treatment is not recommended by clinicians because of surgical comorbidies, and contraindications, symptomatic pHPT patients or individuals with a history of unsuccessful neck exploration.[2] Surgical indications proposed in the guideline published after the fourth international workshop on the treatment of asymptomatic pHPT were updated in 2014.
These criteria are as follows: age <50 years; serum Ca >1 mg/dL (>0.25 mmol/L); T-score below -2.5 in one of the following bone regions: lumbar spine, total hip, femoral neck or 1/3 lower end of radius bone or detection of asymptomatic vertebral fracture by any imaging method; creatinine clearance <60cc/min; 24-hour urine calcium >400 mg/d (<10 mmol/d) together with increased risk of kidney stones based on biochemical stone risk analysis, presence of asymptomatic nephrolithiasis or nephrocalcinosis detected by imaging methods. The presence of one of these criteria is sufficient for surgical indication. Patients with disease progression followed up because they do not meet these criteria are also candidates for surgical treatment.[13] However, surgery is always an option because it is the only definitive treatment of pHPT even in asymptomatic patients who do not meet the criteria for surgical indication.[13]
Even if normocalcemia persists in normocalcemic pHPT, patients these patients are advised to refer to surgery if they have signs of disease progression or worsening complications of pHPT, such as osteoporosis, fractures due to increased bone fragility or renal calculi.[13]
At the time of diagnosis, asymptomatic patients without complications can be monitored for disease progression. Parathyroidectomy reduces the risk of nephrolithiasis and fracture and increases bone mineral density.
Even limited data in normocalcemic pHPT suggest that parathyroidectomy may increase bone mineral density.[1] In addition, studies have shown that insomnia improved after parathyroidectomy, clinical dyspepsia complaints decreased, quality of life improved, neuropsychiatric symptoms improved, cardiovascular risks decreased, and survival increased.[4] Therefore, many authors believe that surgical treatment should be considered independent of chronological age in all patients with asymptomatic pHPT with minimal perioperative risk and adequate life expectancy (Fig. 1).[14]
Figure 1.
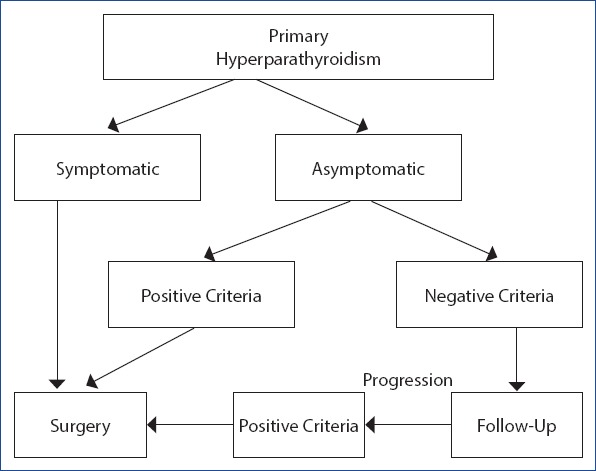
Treatment of hyperparathyroidism.
History of Parathyroidectomy
First successful parathyroidectomy: In 1925, Felix Mandel performed bilateral neck exploration (BNE) under local anesthesia in Austria in a patient with osteitis fibrosa cystica. Three normal-sized- and one enlarged gland were detected in the patient, and the enlarged gland was removed. Severe symptoms associated with the disease improved after the operation. This first successful surgery changed the practical dogma for the treatment of hyperparathyroidism.[15] Other pathological entities other than parathyroid adenoma were not identified until 1933.
In 1934, Albright et al.[16] described parathyroid hyperplasia involving all four glands. In many patients, subtotal parathyroidectomy or total parathyroidectomy with autotransplantation was recommended because of persistent disease after removal of the dominant adenoma.[17] In a series of 200 cases in 1958, Cope et al. indicated that although 80% of the cases were single adenomas, they described 4 -gland hyperplasia and double adenomas as different entities.[18] BNE in pHPT has become the preferred surgical approach since it allows the identification and removal of all pathologic glands. In 1966, Cope stated that the only way for the surgeon to know the diagnosis was to see at least two glands and that exposure of four glands would be more helpful.[19]
BNE performed under general anesthesia has become the gold standard treatment for pHPT. This approach can be applied in experienced centers with a success rate of more than 95%. Taking this into consideration, a unilateral exploration method was proposed as a surgical strategy in the late 1970s, in which solitary parathyroid adenoma is removed, and normal parathyroid on the same side is explored.[23]
Tibblin et al.[24] reported that the presence of multiglandular disease in the macroscopically normal-appearing gland can be reliably determined by frozen section examination of intraoperatively stained specimen using red oil staining (OIL) technique. Using this technique in 102 consecutive patients, the authors found out abnormal glands on the first explored side in 43 patients, and contralateral exploration was performed in 45 patients with normal glands on the first explored t side. The authors recommended unilateral exploration when adenoma was found in the first explored side.
In 1985, Dennison et al.[25] reported that unilateral parathyroidectomy using Tibblin unilateral parathyroidectomy method could be performed after preoperatively lateralizing the hyperfunctional gland using bilateral percutaneous subclavian venous sampling. In this method based on unilateral neck exploration, there is a possibility of secondary adenoma on the other side. In this method, the risk of postoperative hypocalcemia is low and the risk of contralateral recurrent laryngeal nerve (RLS) paralysis is avoided since the other parathyroid glands on the other side are not affected. The thought of lower complication risk has been an encouraging motivation for new interventions and has been one of the studies that underlie focused parathyroidectomy.[22]
Parathyroid scintigraphy was first reported by Young et al.[26] as thallium (Ta) 201-Tc99m subtraction scintigraphy. Between 1985 and 1988, 90 patients who had undergone preoperative Ta-201-Tc99m subtraction scintigraphy and candidates for neck exploration had undergone imaging- guided unilateral exploration for the first time. If pathological gland was found on the same side in patients with single focal involvement on scintigraphy, a biopsy was taken from the normal gland on the same side, and the other side was not explored. Unilateral exploration was performed in 48, and bilateral cervical exploration in 42 patients.
Operative time was shorter in unilateral exploration than in bilateral exploration (71 min vs 97 min, respectively, p<0.001). Any persistent or recurrent disease was not detected in solitary parathyroid adenoma patients who underwent unilateral exploration within an average of the follow-up period of 17 months.[27] In 1989, Tc99m sestamibi scintigraphy was reported using lipophilic sestamibi, which is accumulated in mitochondria-rich oxyphil cells in abnormal parathyroid tissue in parathyroid imaging.[28] This agent has greatly increased the sensitivity of scintigraphy and is currently the main agent of choice for parathyroid scintigraphy.
Preoperative imaging with parathyroid ultrasonography (US) was reported in 1975 by Arima et al.[29] US- guided unilateral exploration was evaluated between 1989 and 1996 in 77 patients who were thought to have single-gland disease and underwent preoperative parathyroid ultrasound performed by a radiologist experienced in parathyroid ultrasound. Forty-six patients had undergone unilateral and 31 patients had bilateral neck explorations. Unilateral exploration was performed if the surgical findings were compatible with the US and normal glands were seen on the same side, and bilateral exploration was performed if there was no normal gland on the same side or if hyperplasia was suspected.
In 69 of these patients, the US and surgical findings were concordant, and the concordance of US was detected in 90% of the cases. Unilateral exploration was shorter than bilateral exploration with lower complication rates (22% vs 45%, p=0.04, respectively). Most of the complications were asymptomatic and symptomatic hypocalcemia, and two patients who underwent bilateral exploration had transient hoarseness. The authors reported that unilateral exploration could be performed in patients who met the surgical criteria based on preoperative noninvasive US due to lower complication rates and shorter operative times.[30] However, in 1988, Nussbaum et al.[31] described intraoperative PTH measurement to demonstrate the suitability of resection after removal of the hyperfunctioning parathyroid gland. In 1991, Irvin et al.[32] reported rapid intraoperative PTH assay. The development of ultrasound technology and experience in this field has increased considerably. The introduction of sestamibi scintigraphy and SPECT (single photon emission tomography) into clinical practice, the combination of SPECT and computed tomography (CT) images have significantly increased the sensitivity of the SPECT/CT (fusion images) method.[33] These encouraging studies and advances formed the basis of focused (selective) parathyroidectomy in which only the pathological gland was removed, and the normal gland on the same side was not attempted to be seen in parathyroid surgery.
Since causative agent in most cases of pHPT is single gland disease, unilateral exploration in surgical treatment has been replaced by a graded focused (selective) parathyroidectomy also with the contribution of imaging methods and intraoperative PTH measurement.[33–37]
After the year 2000, focused parathyroidectomy has become a favorite approach among surgeons concentrated on the treatment of prHPT.[38]
BNE or MIP in Surgical Treatment of Primary Hyperparathyroidism
The success rate in the surgical treatment of pHPT is above 95% regardless of the preoperative localization study and surgical technique in the hands of experienced surgeons.[38] The two main approaches in the surgical treatment of pHPT are methods of BNE and MIP. Currently, MIP has become the standard of care in the treatment of pHPT. For selected patients with positive imaging findings, BNE is still the gold standard in the surgical treatment of pHPT.[39] Although both methods are safe and effective in the surgical treatment of sporadic pHPT, there is still controversy about the effectiveness of both methods.[38]
Basic surgical intervention should find the risk-benefit balance well, decrease the risk of persistent and recurrent disease and deliver the highest cure rate and should not increase the risk of complications. Advocates of BNE recommend this technique because it can be applied with a success rate of over 95% and a permanent complication rate of less than 1%.[38] Any method that reduces the cure rate raises the risk of secondary parathyroidectomy due to persistent and recurrent disease. Although the success rate of secondary parathyroidectomies carried out by experienced surgeons is over 90%, the patient’s lowest complication and the highest cure rate can be achieved in the first surgery. In addition, complication rates are higher in secondary surgery.[38] However, in selected patients, cure rates similar to BNE can be achieved with focused parathyroidectomy.[40]
One of the main arguments about the advantage of BNE is that preoperative localization studies and additional intraoperative methods are not mandatory. Without these, BNE can be applied with high cure and low complication rates. It has been stated that this method reduces the cost associated with preoperative studies.[38–40]
The procedural expenditures were 21% higher in patients who underwent preoperative US and scintigraphy compared to patients who did not undergo imaging studies before standard BNE.[41]
However, supporters of focused parathyroidectomy stated that this procedure could be performed with regional anesthesia and as day surgery, and that total hospital fees could be reduced by 50% compared to BNE.[34] Considering the higher likelihood of postoperative readmissions of patients who undergo BNE, which is indicated as one of the main factors that will increase hospital expenditures.[40]
Imaging has no place in patients with a biochemical diagnosis of pHPT without surgical indication or in whom surgery is contraindicated. Once the surgical indication has been established, the patient is referred to the surgery, preferably to an experienced surgeon. Parathyroid imaging should be performed in the surgical center according to the conditions of the center and the preference of the surgeon so as to plan the surgery. Today, pathological parathyroid glands can be localized and focused parathyroidectomy can be performed with the aid of preoperative imaging methods in 80-90% of the patients with pHPT.[33] However, 90% of the endocrinologists perform one or more imaging modalities before referring the patient to the surgeon. The applicability of MIP increases the number of patients referred for surgery by 79% of endocrinologists. Negative imaging is not a determinative criterion for surgical indication and is not a contraindication for parathyroid surgery.[43] The presence of negative scintigraphy reduces the rate of referrals to surgery and delays the referral for a median of 25 months.[44]
Although BNE can be performed without performing imaging modalities, parathyroid imaging has become a routine application in candidates for pHPT surgery. The pathologic gland cannot be localized by noninvasive methods in approximately 10-20% of the patients with pHPT. Although the rate of multigland disease (22-32%) is higher among patients with negative imaging, most frequently single gland disease is seen.[45]
Despite advances in imaging modalities and additional improvements in surgery, one of the main causes of persistent and recurrent disease is multiglandular disease (MGD).[46] Although it is difficult to predict true multigland disease in sporadic pHPT, multiple glands in 7-33% of the cases have been reported in the literature. One of the factors affecting this issue is the extent of surgery.[47] Multigland disease is better detected in BNE compared to -focused parathyroidectomy.[40] In studies with BNE, multigland disease was more frequently detected than those found in unilateral exploration or focused parathyroidectomy (19.3% vs 5.3%, p<0.001).[48] One of the drawbacks of limited exploration is that the enlarged gland is more likely to be left on the unexplored side or region. In their prospective studies, Siperstein et al. performed preoperative scintigraphy; US and intraoperative PTH guided limited exploration, followed by standard bilateral exploration, and evaluated the rate of enlarged glands that can be left behind. When focused parathyroidectomy was performed in cases where US and MIBI were compatible, then enlarged gland was detected in 20% of the cases who had undergone BNE.[21] When an appropriate drop in PTH levels was achieved inraoperatively, then, the detection rate of additional enlarged gland could be reduced to 16%. The authors suggested that these enlarged glands may be at risk for recurrence in at least 16% of the patients who cannot be identified by localization studies and intraoperative PTH assays.[21]
Although it is more likely to leave the enlarged parathyroid gland behind with limited exploration, its significance is unknown.[21, 48] There is no correlation between size and morphology of parathyroid gland and PTH secretion and not all enlarged parathyroid glands may secrete excess PTH and excision of these glands may not be necessary.[49] This suggests that leaving behind large parathyroid glands secreting normal levels of parathyroid hormone after limited surgical exploration will not increase the recurrence rate.[50] In a large case series, the recurrence rate was 11 times higher in unilateral exploration than BNE.[51] However, in another study, the type of surgery was not found to be an independent predictor for recurrent disease.[52]
In a recent meta-analysis of 12743 patients from 19 studies, focused parathyroidectomy was compared with BNE. Although multigland disease was higher in the BNE group than focused parathyroidectomy group (16.4% vs 5.7%), rates of persistent disease (2.4% vs 2.3%), recurrent disease (0.8% vs 1.25%), total failure (3.3% vs. 3.6%) and reoperation (1.3% vs 2.2%) were comparable.[53]
The high rate of multigland disease in the BNE group is related to patient selection for different types of surgery. Focused surgical imaging is performed in selected disease-positive patients. The high detection rate of multiple glands in the BNE group is related to the high rate of multigland disease in patients with negative and incompatible results in preoperative imaging methods, and in BNE performed in these patients led to insufficient decrease ie. less than 50% in intraoperative PTH.[53]
The overall complication rate was lower in the focused surgical group when compared with BNE (3.7% vs 17.1%, p=0.02). This difference could be attributed in particular to a lower rate of transient hypocalcemia (1.6% vs 13.2%). The rate of persistent hypoparathyroidism was lower in both types of surgery (focused parathyroidectomy: 0.05% vs BNE: 0.2%). Other complication rates were also similar. The operative time was shorter in the focused surgery group (mean: 64 minutes) than in the BNE (mean: 103 minutes). [53] In addition, lower postoperative pain, lesser analgesic requirement, shorter hospital stay, earlier cosmetic satisfaction, better cosmetic outcome and decreased fibrosis were reported in patients who underwent MİP compared to BNE.[54]
Finally, in the meta-analysis of 5282 patients from 14 trials with a follow-up of one year or more, the average cure and recurrence rates were found to be 96.9% (95.5-100%) and 1.6% (0-3.5%), respectively during 33.5 months of follow-up. In addition, compared with patients whose intraoperative PTH was measured, the rate of cure was higher (99.3% vs 98.1%, p<0.001) and the recurrence rate was lower (0.2% vs 1.5%, p<0.001) in patients who had not undergone intraoperative PTH assays. It is noteworthy that nearly 95% of the patients whose intraoperative PTH values were not determined consisted of patients with two compatible imaging methods, while patients whose intraoperative PTH values were determined comprised of patients with incompatible or two negative imaging methods and this fact appears to be the main factor affecting the outcome.[55]
MIP can only be performed in selected cases with single gland disease. In some special cases, it may be necessary to perform BNE. Especially in patients with negative imaging, it is necessary to perform BNE because the incidence of multigland disease is significantly higher in these patients (Table 1).[40]
Table 1.
| Advantages of BNE | Advantages of MIP |
|---|---|
| It can be performed through a small incision. | Relatively smaller incision. |
| Higher cure rates can be achieved. | Cure rate comparable to BNE. |
| No need for preoperative imaging or intraoperative PTH assay. | In many patients, pathologic gland can be localized. |
| Multigland disease can be more frequently detected. | Lower complication rate. |
| In some cases, BNE should be performed. | Shorter operative time. |
| Lower cost. | Lower cost. |
| Daycare procedure. | |
| Lower postoperative pain. |
BNE: Bilateral Neck Exploration; MIP: Minimal İnvasive Parathyroidectomy.
Preoperative Imaging Methods
Ultrasonography and Scintigraphy
Preoperative localization of the enlarged gland provides the possibility of performing MIP and does not increase the need for BNE. US and scintigraphic methods are the most commonly used methods for preoperative primary preoperative imaging in pHPT. These two imaging methods are routinely combined in many centers.[33] The combination of US and scintigraphic methods increases sensitivity.[56] The combination of SPECT or SPECT/CT with US before the first intervention appears to be the optimal combination option.[33]
4-Dimensional Computed Tomography (4d-ct)
4D-CT is a dynamic phase computed tomography (CT) imaging method. Although its sensitivity is limited in multigland disease, it is used in increasing frequency. The main disadvantages are the relatively high radiation dose and the lack of experience of some radiologists in this technique.[57] Although 4D-CT has been used among the first localization studies in primary cases in some centers, it is used in many centers as a secondary or confirmatory study in problematic primary cases or as imaging method in reoperative cases.[33–58]
Magnetic Resonance Imaging
Since the suitability of magnetic resonance imaging (MRI) is similar to US and scintigraphic methods, it is used less frequently. Similar to 4D-CT, it is used as a secondary imaging method to solve problems. Recently, dynamic multiphase 4D-MRI based on the evaluation of parathyroid perfusion properties similar to 4D-CT has been used to evaluate parathyroid lesions. This technique can be considered in place of 4D-CT, especially in difficult cases and in patients in whom exposure to radiation is contraindicated.[33]
PET/CT
In recent years, PET/CT studies with 18F-fluorocholine and 11C-methionine have been promising for pathological gland imaging especially in difficult cases. In patients with persistent or recurrent disease and negative imaging scheduled for reoperations, PET/CT with fluorocholine has started to take place among parathyroid imaging modalities that can be considered before invasive procedures.[33]
Invasive Localization Studies
Parathormone Measurement and Fine Needle Aspiration Biopsy
In some cases, it may be necessary to verify whether the suspected parathyroid nodule is really a parathyroid nodule. In scintigraphy-negative reoperative cases, US verifies suspicious lesions and may allow the application of focused parathyroidectomy.[33]
Parathormone Measurement with Bilateral Jugular Ven Sampling
This method is performed to evaluate PTH by drawing blood from both internal jugular veins at the lowest level of the neck under US guidance for lateralization of the pathological gand. The test is considered positive if PTH is 10% higher on one side. The contribution of this method in primary cases can be questioned. However, in persistent or recurrent cases, it may be considered before more complicated techniques, such as selective venous sampling or these techniques are not available.[33]
Selective Venous Sampling (SVS)
Venous angiography is used to determine increased PTH levels in blood samples taken from the point where the brachiocephalic vein, internal jugular vein and thyroid veins drain into the internal jugular vein. Selective venous sampling (CVS) is an invasive procedure and is almost always used in patients with persistent or recurrent hyperparathyroidism, and in patients where negative or incompatible results of noninvasive imaging methods are obtained
Intraoperative Parathyroid Hormone Measurement
Intraoperative PTH monitoring is based on demonstrating decrements in intraoperative measurements of PTH, which has a half-life of 3-5 minutes at certain intervals to confirm biochemical cure in parathyroidectomy. The most widely used criteria for predicting treatment success in intraoperative PTH monitoring are the Miami criteria.[37] A drop of more than 50% in PTH at the 10th minute of the excision of the suspected gland relative to the highest preincision or pre-excision PTH value is considered to be a sufficient decrease in PTH.[59]
Immunochemulsifying method that can be installed in the operating room for intraoperative rapid hormone measurement has been developed, and results can be obtained in approximately 10 minutes in the operating room. However, this method is more expensive than measuring PTH in the central laboratory and cannot be applied to many centers in our country and the world. However, delivery of blood samples to the central laboratory for the measurement of PTH may be an alternative to portable rapid PTH measurement in the operating room. The sensitivity, specificity, and suitability of this method are similar to the rapid PTH measurements performed in the operating room, and its most important disadvantage is the average waiting time of 25-30 minutes to obtain results.[33]
Other Additional Intraoperative Methods
In some centers, preoperatively intravenous Tc99m sestamibi scintigraphy is performed, and parathyroidectomy is realized with the help of intraoperative gamma probe. It has been reported that MIP can be applied successfully with this method.[4] There is no clear consensus on the use of gamma probes in parathyroidectomy. The use of the gamma probes is not widespread nowadays when preoperative imaging and intraoperative PTH monitoring have been developed and prevalently used. It has been stated that it can be considered in patients who had previously undergone ectopic parathyroidectomy or thyroidectomy, and in secondary interventions in centers that do not use this method routinely.[60] It has been reported that in reoperative cases, the pathological gland is localized intraoperatively with gamma probe, so as to allow its removal with minimal dissection in an area of fragile, fibrotic changes and dense scar tissue.[61]
Frozen cross-section examination and measurement of PTH in resected parathyroid tissue are used to determine whether the resected tissue is parathyroid; however, these are helpful in recognizing the tissue, but they are not indicators of intraoperative cure. Its routine use is not recommended.[39]
To increase the rate of unilateral exploration in patients with preoperative negative and incompatible results of imaging modalities, intraoperative lateralization can be performed by PTH measurement using bilateral jugular venous sampling.[62] It has been reported that the pathological gland could be successfully visualized in patients with pHPT who underwent secondary intervention with the aid of indocyanine green fluorescence imaging.[63] The ioPTH is the best method of demonstrating intraoperative biochemical cure.[58]
Surgical Selection in Sporadic Primary Hyperparathyroidism: Primarily Surgery
The choice of surgery in pHPT may vary depending on whether the patient has hereditary HPT or thyroid disease requiring surgical treatment, the results of preoperative localization studies, the possibilities of using intraoperative PTH and the preference of the surgeon.[23] The preoperative surgical strategy can be revised according to intraoperative findings in case of need to achieve excellent results. Although BNE is a consistently valid option that has excellent results in the surgical treatment of pHPT and is considered as the gold standard, MIP is the ideal approach in selected patients thought to have single-gland disease based on clinical and radiologic evidence. MIP is not routinely recommended in patients with known or highly suspected multigland disease based on clinical and radiological findings.[43]
In case of detection of multigland disease during MIP, presence of 2 normal glands or absence of enlarged gland on the explored side, and lack of adequate, and appropriate reduction in intraoperative PTH levels, BNE should be applied. Absolute indications for BNE have been reported as cases with negative preoperative imaging results, and presence of MEN-1 syndrome. There is not much debate on this issue. Relative indications for planned BNE include the presence of MEN-2 syndrome, isolated familial pHPT, hyperparathyroidism due to lithium treatment, history of radiation to the head and neck region, and incompatible imaging methods. In some studies, it is stated that MIP can be applied with the aid of preoperative imaging methods and intraoperative measurements of PTH.[47–65]
If there is one or more than one enlarged parathyroid adenomas in the patient thought to have sporadic pHPT, treatment is the removal of one or more enlarged adenomas.[4] Double adenomas are usually synchronous, and recurrence after surgery is rare in the presence of true double adenomas. However, when the surgeon detects a double adenoma during surgery, intraoperatively or postoperatively detected 4 - gland diseases (asymmetric hyperplasia or asynchronous hyperplasia) should be kept in mind. If there is 4 - gland hyperplasia, subtotal thyroidectomy should be performed by leaving 40-60 mg remnant with its vascular pedicle. If appropriate, it is more convenient to leave remnant from the inferior glands located in the anterior region, so that it can be reached more easily in the presence of persistent or recurrent disease.[4]
Nowadays, imaging methods are routinely applied. US and scintigraphy are combined in patients with surgical indications.[23] MIP can be performed if both imaging methods are consistent and show the presence of the same gland. Although some studies have reported that ioPTH measurement will contribute to the diagnosis, this contribution is only marginal.[66, 67] Therefore, it is generally accepted that MIP should be performed without using ioPTH in patients in whom two imaging modalities yielded positive and consistent results. If one of these two imaging modalities yielded positive, and the other negative results or both of them yielded positive and incompatible results, then, approximately 20% of the patients may have multigland disease.[39] When single imaging is positive, the additional contribution of ioPTH is approximately 20%. Therefore, use ioPTH is recommended if IMIP is to be planned in patients with positive single imaging. MIP or unilateral exploration with the aid of ioPTH can be performed in these patients. If ioPTH cannot be measured, and unilateral exploration reveals a large gland compatible with positive imaging with an additional normal gland on the same side, then, the surgery can be terminated after unilateral exploration. However, this is not a reliable method as use of ioPTH.
BNE should be preferred in the presence of 2 incompatible positive imaging results. However, if MRP or unilateral exploration is to be performed, they should be performed with the aid of ioPTH. If sufficient drop in ioPTH is achieved after removal of the large gland on the first side, surgery may be terminated.[69] If both imaging methods are negative, BNE should be preferred since multigland disease is likely.[39, 47, 65, 69] In some centers, additional 4D-CT is recommended when two imaging methods are negative. MRP with ioPTH is recommended if 4D-CT is positive and BNE is recommended if negative.[58]
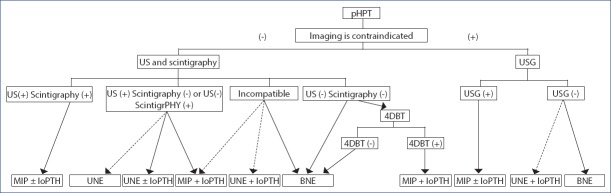
If the patient with negative imaging has one large and one normal parathyroid gland when the first side is explored, then, the opposite side may not be explored if sufficient drop in PTH is detected in ioPTH test performed after removal of the large gland. In this way, it has been reported that up to 30% cure can be achieved by unilateral exploration in patients with two negative imaging methods.[70] If US is positive in pregnant patients who should only undergo US examination, and refrain from exposure to radiation, preferably ioPTH- guided MRP is recommended. If USG is negative, BNE is recommended. If sufficient PTH drop is detected by ioPTH test after detecting and removing the pathological gland at the first side of exploration, then, surgery may be terminated (Fig. 2).[58]
Figure 2.
Surgical algorithm in patients who had not undergone thyroid or parathyroid surgery (pHPT: Primary hyperparathyroidism, US Ultrasonography, 4D-CT: 4- dimensional computed tomography, BNE: Bilateral neck exploration, UNE: Unilateral neck exploration, MIP: Minimal invasive parathyroidectomy, ioPTH: Intraoperative parathormone) (Straight arrows: first choice, dashed arrows: 2nd choice).
In hereditary pHPT, multigland involvement is common, but gene penetration varies, and in some types, metachronous involvement may be seen. In the hereditary pHPT, total parathyroidectomy is performed in which the entire parathyroid tissue is removed to provide a theoretical cure. However, permanent hypoparathyroidism as a result of this procedure is not the desired result. Therefore, the goal of surgery in hereditary pHPT is not to provide a simple surgical cure, but rather to minimize especially persistent hypoparathyroidism. Therefore, an approach that allows or facilitates surgical intervention in a potentially developed recurrent disease that will provide normocalcemia that can last as long as possible should be preferred. Since genetic characteristics are different in hereditary pHPT, the surgical approach should be determined by considering the patient’s preference and surgeon’s experience according to genetic involvement.[71]
In MEN1, subtotal parathyroidectomy or total parathyroidectomy and autotransplantation with cervical thymectomy and BNE have been recommended.[71] In MEN 2A, if all parathyroid glands are enlarged, subtotal parathyroidectomy or total parathyroidectomy and autotransplantation are recommended. If not all 4 glands are enlarged macroscopically, selective parathyroidectomy in which only enlarged glands are removed, can lead to long-term normocalcemia. There is no evidence in the literature regarding the role of thymectomy in patients with MEN 2A. In patients with MEN 2A, when prophylactic thyroidectomy is performed for medullary, cancer parathyroid glands should not be removed, but they should be spared when pHPT is not detected.[71] Optimal treatment of hyperparathyroidism-jaw tumor syndrome remains controversial. In the past, subtotal parathyroidectomy or total parathyroidectomy with or without autotransplantation with BNE has been recommended when imaging methods were negative. Since there is a risk of parathyroid cancer in these patients, it is reported that autotransplantation may be risky because of seeding or spread of parathyroid cancer cells.
Since patients often have a single gland disease, selective parathyroidectomy is recommended in recent studies if preoperative localization studies suggest the presence of a single gland disease. These patients should be followed up regularly at 6-month intervals for the development of parathyroid cancer and recurrence. If parathyroid cancer is suspected, en bloc resection with thyroid tissue and adjacent adipose tissue is recommended. Central dissection may be required in case of suspected lymph node involvement.[71] There is a limited number of case of isolated familial HPT, autosomal mild HPT, MEN 4 HPT in the literature, and case-specific approach is recommended in these cases.[71] Total parathyroidectomy without emergency autotransplantation is recommended if there is a hypercalcemic crisis in severe neonatal HPT.[71]
Surgery in Persistent or Recurrent Hyperparathyroidism
Persistent pHPT (P-pHPT) is defined as the persistence or recurrence of hypercalcemia within six months after surgery. Recurrent pHPT (R-pHPT) is the recurrence of hypercalcemia six months after normocalcemia is achieved in the first operation. The definition of reoperative parathyroid surgery includes persistent, the recurrent disease and parathyroid surgery in patients who have previously undergone anterior neck surgery (especially thyroidectomy).[58] Persistent disease is more common in Phpt, and in the literature, incidence rates of persistent and recurrent pHPT have been reported to range between 2-22%, and 1-10%, respectively.[46] The term of persistent or recurrent disease indicates inaccurate first diagnosis, inability to detect parathyroid adenoma, or to recognize multigland disease, the presence of the supernumerary parathyroid gland or ectopic parathyroid gland, recurrence from the remnant left behind in first subtotal resection or from autotransplanted remnant and inexperience of the surgeon.[72]
The main reason for failure in the first operation is unsuccessful exploration by the inexperienced surgeon and failure rates increase up to 30% in surgeons performing less than 10 parathyroid operations per year.[23] In the studies performed, advanced age of the patient (>70 years), obesity, ASA-3 disease, low-volume hospital (<50 cases/year), inexperience of the surgeon, suspicious sestamibi scintigraphy findings, first parathyroid pathology (single adenoma <double adenoma <hyperplasia), surgical strategy (absence of abnormal gland, failure to perform BNE despite inadequate drop in ioPTH levels) have been indicated as predictive factors for P-pHPT and R-pHPT.[73]
In the patient evaluated for persistent and recurrent disease, the accuracy of diagnosis, surgical indication, family history, first preoperative imaging tests, and first operative data should be evaluated before reoperation.
Confirmation of diagnosis: Confirmation of the diagnosis is extremely important. Some medications, such as lithium and thiazide diuretics, may stimulate PTH secretion by altering calcium (Ca) metabolism. In addition, renal Ca leakage, vitamin D insufficiency and mild secondary HPT due to gastrointestinal abnormalities may cause PTH increase in renal failure. Familial hypocalciuric hypercalcemia should be excluded. In addition, isolated PTH elevation may be seen in some patients, although the postoperative Ca value is normal or low. Familial hypocalciuric hypercalcemia should be excluded. The diagnosis of pHPT should be confirmed by taking these conditions into consideration, together with the new biochemical tests of the patient.[73–74]
Surgical Indication: Since the secondary procedures are more difficult to perform due to the disruption of the tissue plans, and developing scar tissue secondary to the first surgery. Thus, the risk of hypoparathyroidism and vocal cord paralysis is higher. These characteristics should be taken into consideration in the risk-benefit relationship. Re-exploration is required in patients with significant symptomatic symptoms. Patients with mild symptoms or asymptomatic cases can be followed up.[73, 74] In all parathyroid surgeries, the experienced surgeon is important for success, but especially secondary surgeries should be performed by experienced surgeons. In the surgical strategy evaluation of vocal cord function by preoperative vocal cord, examination carries utmost importance.[75]
Evaluation of the Findings of the First Surgery: It is very important for surgical strategy to examine the first images, surgical notes or drawings and pathology reports. Information about the extent of dissection and size of the lesion can be obtained from surgical notes. In addition, it can be understood whether the tissue indicated in the pathology report is parathyroid or normal parathyroid or a pathological gland. If there is no mention of parathyroid gland or normal parathyroid gland in the pathology report, the pathological lesion in the initial imaging may be the main indication for previous surgery.
In the first pathology report, indication of a multigland disease should remind us of the presence of a pathological gland. If subtotal parathyroidectomy was performed in the first operation, the recurrent or persistent disease might develop due to remnant or supernumerary gland.
Preoperative Imaging: Blind exploration should not be performed without the aid of imaging modality. Although an experienced surgeon is important for success in all parathyroid operations, imaging methods should be repeated before secondary interventions, even if imaging methods are negative, especially in the first operation. Blind exploration should not be performed without the aid of imaging.[4] Selective parathyroidectomy should be performed with the guidance of imaging whenever possible. The first imaging methods should be noninvasive tests.
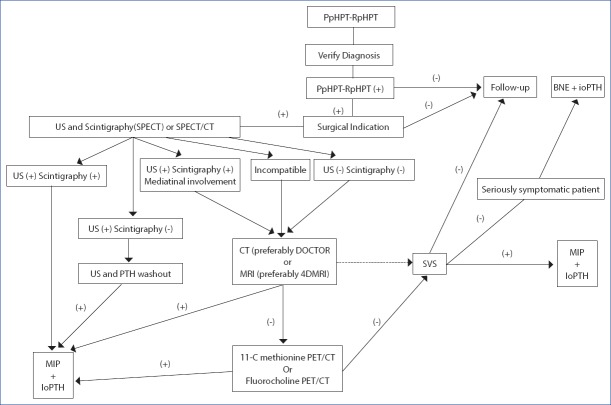
Before the second intervention, SPECT or SPECT/CT combined with US performed by an experienced ultrasonographer is seen as the optimal combination option.[33, 74] If these two imaging studies are positive and compatible, then, the patient may undergo imaging-guided selective parathyroidectomy.[73] To confirm the lesion with mediastinal ectopic involvement in scintigraphy, thoracic and neck CT, preferably dynamic 4D-CT, should be performed in patients with negative or incompatible US and scintigraphy results. In addition, MRI, preferably 4D-MRI, should be performed instead of CT in patients protected from adverse effects of radiation exposure.[73] PET/CT studies with 11C-methionine or fluorocholine can be performed before invasive procedures in patients whose pathological gland cannot be focused up to this stage.[33] Selective venous sampling should be performed in patients in whom noninvasive methods cannot be used (Fig. 3).
Figure 3.
Evaluation and treatment algorithm for persistent pHPT (PpHPT) and recurrent pHPT (RpHPT) (Straight arrows: first choice, dashed arrows: 2nd choice) (US: Ultrasonography, SPECT: Single- photon emission computed tomography, 4D-CT: 4-dimensional computed tomography, MRI: Magnetic resonance imaging, 4D-MRI: 4-dimensional magnetic resonance imaging, SVS: Selective venous sampling, PET: Positron Emission tomography, BNE: Bilateral neck exploration, UNE: Unilateral neck exploration, MRP: Minimally invasive parathyroidectomy, ioPTH: Intraoperative parathormone.
The reliability of all imaging modalities performed before secondary surgeries is lower than those obtained before primary cases, and false positive and false negative results are more frequently seen.[74] In patients in whom non-invasive imaging methods are negative or angiographic procedures such as selective venous sampling cannot be performed, the lesion can be lateralized by PTH measurement with the aid of the preoperative US or intraoperative bilateral jugular vein sampling.[33]
Additional Methods Which will Provide Intraoperative Contributions
Intraoperative nerve monitoring should be used in secondary surgeries.[75] The use of ioPTH is recommended, particularly in secondary interventions.[58] Intraoperative US may provide contribution during surgery. It has been reported that radio-guided surgery with the aid of the gamma probes may contribute even to the assessment of scintigraphy- negative patients.[61] t Radio guided preoperative occult lesion localization (ROLL) in patients who had preoperative US and lesions with typical parathyroid pathology or suspicious images confirmed to be parathyroid using PTH washout may make possible minimally invasive parathyroidectomy with the aid of the gamma probe and less dissection in fibrotic areas.[76]
Indocyanine-green fluorescence imaging has been shown to allow realization of focused parathyroidectomy with less dissection by providing images of the pathological gland in the early period of exploration.[63] The best method of demonstrating intraoperative biochemical cure is ioPTH.test.[58]
Which Surgical Approach
Before re-exploration, two compatible imaging studies should be preferred. In secondary interventions, selective surgery should be performed with imaging guidance as much as possible.[23] Surgical strategy which can provide maximum cure with minimum dissection and minimal morbidity should be determined. Blind reexploration should be performed in life-threatening diseases in patients with negative localization studies.[58]
Parathyromatosis
Parathyroimatosis is a rare condition associated with seeding of parathyroid cells in the neck due to iatrogenic rupture of the parathyroid gland. Parathyroimatosis usually develops after surgery performed for secondary hyperparathyroidism and does not occur in most of the patients after rupture of the capsule in pHPT surgery. Parathyromatosis is usually diagnosed as small nodular structures during reoperative neck exploration.[58] En bloc resection of all parathyroid tissue can provide curative resection. However, it is difficult to detect and remove all planted foci within the scar tissue.[58–73]
Parathyroid cancer
Parathyroid cancer accounts for less than 1% of cases with pHPT, and 20% of which are associated with hereditary HPT. Although it is difficult to diagnose parathyroid cancer preoperatively, 45% of the cases have a palpable mass. Intraoperatively, parathyroid cancer is typically gray-white in color, hard, and intensely adherent to the thyroid. En bloc resection with thyroid and soft tissue on the same side should be performed.[58]
Disclosures
Peer-review: Externally peer-reviewed.
Conflict of interest: None declared.
Authorship Contributions: Concept – N.A., M.U.; Design – N.A., M.U.; Supervision – M.U.; Materials – N.A.; Data collection &/or processing – N.A.; Analysis and/or interpretation – N.A., M.U.; Literature search – N.A., M.U.; Writing – N.A.; Critical review – M.U.
References
- 1.Walker MD, Bilezikian JP. Primary hyperparathyroidism:recent advances. Curr Opin Rheumatol. 2018;30:427–39. doi: 10.1097/BOR.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 2.Uludag M, Aygun N. Primary hyperparathyroidism:Current situation in the clinical and biochemical presentation. Med Bull Sisli Etfal Hosp. 2016;50:171–80. [Google Scholar]
- 3.Madkhali T, Alhefdhi A, Chen H, Elfenbein D. Primary hyperparathyroidism. Ulus Cerrahi Derg. 2016;32:58–66. doi: 10.5152/UCD.2015.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallick R, Chen H. Diagnosis and Management of Hyperparathyroidism. Adv Surg. 2018;52:137–53. doi: 10.1016/j.yasu.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Yeh MW, Ituarte PH, Zhou HC, Nishimoto S, Liu IL, Harari A, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98:1122–9. doi: 10.1210/jc.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Press DM, Siperstein AE, Berber E, Shin JJ, Metzger R, Monteiro R, et al. The prevalence of undiagnosed and unrecognized primary hyperparathyroidism:a population-based analysis from the electronic medical record. Surgery. 2013;154:1232–7. doi: 10.1016/j.surg.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 7.Iacobone M, Carnaille B, Palazzo FF, Vriens M. Hereditary hyperparathyroidism--a consensus report of the European Society of Endocrine Surgeons (ESES) Langenbecks Arch Surg. 2015;400:867–86. doi: 10.1007/s00423-015-1342-7. [DOI] [PubMed] [Google Scholar]
- 8.Felger EA, Kandil E. Primary hyperparathyroidism. Otolaryngol Clin North Am. 2010;43:417–32. doi: 10.1016/j.otc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Eastell R, Brandi ML, Costa AG, D'Amour P, Shoback DM, Thakker RV. Diagnosis of asymptomatic primary hyperparathyroidism:proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570–9. doi: 10.1210/jc.2014-1414. [DOI] [PubMed] [Google Scholar]
- 10.Uludağ M. Normocalcemic hyperparathyroidism:A new clinical type of primary hyperparathyroidism. Med Bull Sisli Etfal Hosp. 2014;48:264–73. [Google Scholar]
- 11.Machado NN, Wilhelm SM. Diagnosis and Evaluation of Primary Hyperparathyroidism. Surg Clin North Am. 2019;99:649–66. doi: 10.1016/j.suc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Callender GG, Udelsman R. Surgery for primary hyperparathyroidism. Cancer. 2014;120:3602–16. doi: 10.1002/cncr.28891. [DOI] [PubMed] [Google Scholar]
- 13.Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism:summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3561–9. doi: 10.1210/jc.2014-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JX, Yeh MW. Asymptomatic Primary Hyperparathyroidism:Diagnostic Pitfalls and Surgical Intervention. Surg Oncol Clin N Am. 2016;25:77–90. doi: 10.1016/j.soc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Mandl F. Versuch bei Ostitis fibrosa generalisata mittels Exstirpation eines Epithelkörperchen Tumors. Wien Klin Wehnschr. 1925;50:1343. [Google Scholar]
- 16.Albright F, Aub JC, Bauer W. A Common And Polymorphic Condition As Illustrated By Seventeen Proved Cases From One Clinic. JAMA. 1934;102:1276–87. [Google Scholar]
- 17.Churchill ED, Cope O. The Surgical Treatment Of Hyperparathyroidism:Based On 30 Casesconfirmed By Operation. Ann Surg. 1936;104:9–35. doi: 10.1097/00000658-193607000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cope O, Keynes WM, Roth SI, Castleman B. Primary chief-cell hyperplasia of the parathyroid glands:a new entity in the surgery of hyperparathyroidism. Ann Surg. 1958;148:375–88. doi: 10.1097/00000658-195809000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cope O. The study of hyperparathyroidism at the Massachusetts General Hospital. N Engl J Med. 1966;274:1174–82. doi: 10.1056/NEJM196605262742105. [DOI] [PubMed] [Google Scholar]
- 20.Allendorf J, DiGorgi M, Spanknebel K, Inabnet W, Chabot J, Logerfo P. 1112 consecutive bilateral neck explorations for primary hyperparathyroidism. World J Surg. 2007;31:2075–80. doi: 10.1007/s00268-007-9068-5. [DOI] [PubMed] [Google Scholar]
- 21.Siperstein A, Berber E, Barbosa GF, Tsinberg M, Greene AB, Mitchell J, et al. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestamibi, and intraoperative parathyroid hormone:analysis of 1158 cases. Ann Surg. 2008;248:420–8. doi: 10.1097/SLA.0b013e3181859f71. [DOI] [PubMed] [Google Scholar]
- 22.Kunstman JW, Udelsman R. Superiority of minimally invasive parathyroidectomy. Adv Surg. 2012;46:171–89. doi: 10.1016/j.yasu.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Mihai R, Barczynski M, Iacobone M, Sitges-Serra A. Surgical strategy for sporadic primary hyperparathyroidism an evidence-based approach to surgical strategy, patient selection, surgical access, and reoperations. Langenbecks Arch Surg. 2009;394:785–98. doi: 10.1007/s00423-009-0529-1. [DOI] [PubMed] [Google Scholar]
- 24.Tibblin S, Bondeson AG, Ljungberg O. Unilateral parathyroidectomy in hyperparathyroidism due to single adenoma. Ann Surg. 1982;195:245–52. doi: 10.1097/00000658-198203000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennison A, Ball M, Dudley N. Preoperative percutaneous localisation of parathyroid tumours:a preliminary report. Ann R Coll Surg Engl. 1985;67:276–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Young AE, Gaunt JI, Croft DN, Collins RE, Wells CP, Coakley AJ. Location of parathyroid adenomas by thallium-201 and technetium-99m subtraction scanning. Br Med J (Clin Res Ed) 1983;286:1384–6. doi: 10.1136/bmj.286.6375.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell CF, Laird JD, Ferguson WR. Scan-directed unilateral cervical exploration for parathyroid adenoma:a legitimateapproach? World J Surg. 1990;14:406–9. doi: 10.1007/BF01658540. [DOI] [PubMed] [Google Scholar]
- 28.Coakley AJ, Kettle AG, Wells CP, O'Doherty MJ, Collins RE. 99Tcm sestamibi-a new agent for parathyroid imaging. Nucl Med Commun. 1989;10:791–4. doi: 10.1097/00006231-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Arima M, Yokoi H, Sonoda T. Preoperative identification of tumor of the parathyroid by ultrasonotomography. Surg Gynecol Obstet. 1975;141:242–4. [PubMed] [Google Scholar]
- 30.Vogel LM, Lucas R, Czako P. Unilateral parathyroid exploration. Am Surg. 1998;64:693–6. [PubMed] [Google Scholar]
- 31.Nussbaum SR, Thompson AR, Hutcheson KA, Gaz RD, Wang CA. Intraoperative measurement of parathyroid hormone in the surgical management of hyperparathyroidism. Surgery. 1988;104:1121–7. [PubMed] [Google Scholar]
- 32.Irvin GL, 3rd, Dembrow VD, Prudhomme DL. Operative monitoring of parathyroid gland hyperfunction. Am J Surg. 1991;162:299–302. doi: 10.1016/0002-9610(91)90135-z. [DOI] [PubMed] [Google Scholar]
- 33.Uludağ M. Preoperative Localization Studies in Primary Hyperparathyroidism. Med Bull Sisli Etfal Hosp. 2019;53:7–15. doi: 10.14744/SEMB.2019.78476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002;235:665–70. doi: 10.1097/00000658-200205000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inabnet WB, 3rd, Dakin GF, Haber RS, Rubino F, Diamond EJ, Gagner M. Targeted parathyroidectomy in the era of intraoperative parathormone monitoring. World J Surg. 2002;26:921–5. doi: 10.1007/s00268-002-6619-7. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson SR, van Heerden JA, Farley DR, Grant CS, Thompson GB, Mullan BP, et al. Focused cervical exploration for primary hyperparathyroidism without intraoperative parathyroid hormone monitoring or use of the gamma probe. World J Surg. 2004;28:1127–31. doi: 10.1007/s00268-004-7469-2. [DOI] [PubMed] [Google Scholar]
- 37.Aygun N, Uludag M. Primer hiperparatiroidizmde intraoperatif yardımcıyöntemler. Med Bull Sisli Etfal Hosp. 2019 Epub doi:10.14744/SEMB.2019.93457. [Google Scholar]
- 38.Elaraj D, Sturgeon C. Operative treatment of primary hyperparathyroidism:balancing cost-effectiveness with successful outcomes. Surg Clin North Am. 2014;94:607–23. doi: 10.1016/j.suc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Egan RJ, Scott-Coombes DM. The surgical management of sporadic primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32:847–59. doi: 10.1016/j.beem.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Laird AM, Libutti SK. Minimally Invasive Parathyroidectomy Versus Bilateral Neck Exploration for Primary Hyperparathyroidism. Surg Oncol Clin N Am. 2016;25:103–18. doi: 10.1016/j.soc.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Aarum S, Nordenström J, Reihnér E, Zedenius J, Jacobsson H, Danielsson R, et al. Operation for primary hyperparathyroidism:the new versus the old order. A randomised controlled trial of preoperative localisation. Scand J Surg. 2007;96:26–30. doi: 10.1177/145749690709600105. [DOI] [PubMed] [Google Scholar]
- 42.Gallagher SF, Denham DW, Murr MM, Norman JG. The impact of minimally invasive parathyroidectomy on the way endocrinologists treat primary hyperparathyroidism. Surgery. 2003;134:910–7. doi: 10.1016/s0039-6060(03)00414-8. [DOI] [PubMed] [Google Scholar]
- 43.Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016;151:959–68. doi: 10.1001/jamasurg.2016.2310. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurthy VD, Sound S, Okoh AK, Yazici P, Yigitbas H, Neumann D, et al. The utility of repeat sestamibi scans in patients with primary hyperparathyroidism after an initial negative scan. Surgery. 2017;161:1651–8. doi: 10.1016/j.surg.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Bunch PM, Kelly HR. Preoperative Imaging Techniques in Primary Hyperparathyroidism:A Review. JAMA Otolaryngol Head Neck Surg. 2018;144:929–37. doi: 10.1001/jamaoto.2018.1671. [DOI] [PubMed] [Google Scholar]
- 46.Kartal AC, Çitgez B, Öden S, Yetkin SG, Mihmanlı M, Aygün N, et al. Risk factors in the occurance of persistent primary hyperparathyroidism. Med Bull Sisli Etfal Hosp. 2014;48:213–26. [Google Scholar]
- 47.Barczyński M, Bränström R, Dionigi G, Mihai R. Sporadic multiple parathyroid gland disease--a consensus report of the European Society of Endocrine Surgeons (ESES) Langenbecks Arch Surg. 2015;400:887–905. doi: 10.1007/s00423-015-1348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee NC, Norton JA. Multiple-gland disease in primary hyperparathyroidism:a function of operative approach? Arch Surg. 2002;137:896–9. doi: 10.1001/archsurg.137.8.896. [DOI] [PubMed] [Google Scholar]
- 49.Mun HC, Conigrave A, Wilkinson M, Delbridge L. Surgery for hyperparathyroidism:does morphology or function matter most? Surgery. 2005;138:1111–20. doi: 10.1016/j.surg.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Lew JI, Solorzano CC. Surgical management of primary hyperparathyroidism:state of the art. Surg Clin North Am. 2009;89:1205–25. doi: 10.1016/j.suc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Norman J, Lopez J, Politz D. Abandoning unilateral parathyroidectomy:why we reversed our position after 15,000 parathyroid operations. J Am Coll Surg. 2012;214:260–9. doi: 10.1016/j.jamcollsurg.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Schneider DF, Mazeh H, Chen H, Sippel RS. Predictors of recurrence in primary hyperparathyroidism:an analysis of 1386 cases. Ann Surg. 2014;259:563–8. doi: 10.1097/SLA.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jinih M, O'Connell E, O'Leary DP, Liew A, Redmond HP. Focused Versus Bilateral Parathyroid Exploration for Primary Hyperparathyroidism:A Systematic Review and Meta-analysis. Ann Surg Oncol. 2017;24:1924–34. doi: 10.1245/s10434-016-5694-1. [DOI] [PubMed] [Google Scholar]
- 54.Slepavicius A, Beisa V, Janusonis V, Strupas K. Focused versus conventional parathyroidectomy for primary hyperparathyroidism:a prospective, randomized, blinded trial. Langenbecks Arch Surg. 2008;393:659–66. doi: 10.1007/s00423-008-0408-1. [DOI] [PubMed] [Google Scholar]
- 55.Ishii H, Mihai R, Watkinson JC, Kim DS. Systematic review of cure and recurrence rates following minimally invasiveparathyroidectomy. BJS Open. 2018;2:364–70. doi: 10.1002/bjs5.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tunca F, Akici M, Işcan Y, Cem Sormaz I, Giles Senyurek Y, Terzioğlu T. The impact of combined interpretation of localization studies on image-guided surgical approaches for primary hyperparathyroidism. Minerva Endocrinol. 2017;42:213–22. doi: 10.23736/S0391-1977.16.02396-8. [DOI] [PubMed] [Google Scholar]
- 57.Bunch PM, Kelly HR. Preoperative Imaging Techniques in Primary Hyperparathyroidism:A Review. JAMA Otolaryngol Head Neck Surg. 2018;144:929–37. doi: 10.1001/jamaoto.2018.1671. [DOI] [PubMed] [Google Scholar]
- 58.Callender GG, Udelsman R. Surgery for primary hyperparathyroidism. Cancer. 2014;120:3602–16. doi: 10.1002/cncr.28891. [DOI] [PubMed] [Google Scholar]
- 59.Irvin GL, 3rd, Solorzano CC, Carneiro DM. Quick intraoperative parathyroid hormone assay:surgical adjunct to allow limited parathyroidectomy, improve success rate, and predict outcome. World J Surg. 2004;28:1287–92. doi: 10.1007/s00268-004-7708-6. [DOI] [PubMed] [Google Scholar]
- 60.Noureldine SI, Gooi Z, Tufano RP. Minimally invasive parathyroid surgery. Gland Surg. 2015;4:410–9. doi: 10.3978/j.issn.2227-684X.2015.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pitt SC, Panneerselvan R, Sippel RS, Chen H. Radioguided parathyroidectomy for hyperparathyroidism in the reoperative neck. Surgery. 2009;146:592–8. doi: 10.1016/j.surg.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barczynski M, Konturek A, Hubalewska-Dydejczyk A, Cichon S, Nowak W. Utility of intraoperative bilateral internal jugular venous sampling with rapid parathyroid hormone testing in guiding patients with a negative sestamibi scan for minimally invasive parathyroidectomy-a randomized controlled trial. Langenbecks Arch Surg. 2009;394:827–35. doi: 10.1007/s00423-009-0516-6. [DOI] [PubMed] [Google Scholar]
- 63.Sound S, Okoh A, Yigitbas H, Yazici P, Berber E. Utility of Indocyanine Green Fluorescence Imaging for Intraoperative Localization in Reoperative Parathyroid Surgery. Surg Innov. 2015 Oct 27; doi: 10.1177/1553350615613450. Epub ahead of print, pii:1553350615613450. [DOI] [PubMed] [Google Scholar]
- 64.Gasparri G. Updates in primary hyperparathyroidism. Updates Surg. 2017;69:217–23. doi: 10.1007/s13304-017-0477-1. [DOI] [PubMed] [Google Scholar]
- 65.Moalem J, Guerrero M, Kebebew E. Bilateral neck exploration in primary hyperparathyroidism-when is it selected and how is it performed? World J Surg. 2009;33:2282–91. doi: 10.1007/s00268-009-9941-5. [DOI] [PubMed] [Google Scholar]
- 66.Bobanga ID, McHenry CR. Is intraoperative parathyroid hormone monitoring necessary for primary hyperparathyroidism with concordant preoperative imaging? Am J Surg. 2017;213:484–8. doi: 10.1016/j.amjsurg.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 67.Morris LF, Zanocco K, Ituarte PH, Ro K, Duh QY, Sturgeon C, et al. The value of intraoperative parathyroid hormone monitoring in localized primary hyperparathyroidism:a cost analysis. Ann Surg Oncol. 2010;17:679–85. doi: 10.1245/s10434-009-0773-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barczynski M, Konturek A, Cichon S, Hubalewska-Dydejczyk A, Golkowski F, Huszno B. Intraoperative parathyroid hormone assay improves outcomes of minimally invasive parathyroidectomy mainly in patients with a presumed solitary parathyroid adenoma and missing concordance of preoperative imaging. Clin Endocrinol (Oxf) 2007;66:878–85. doi: 10.1111/j.1365-2265.2007.02827.x. [DOI] [PubMed] [Google Scholar]
- 69.Mihai R, Simon D, Hellman P. Imaging for primary hyperparathyroidism-an evidence-based analysis. Langenbecks Arch Surg. 2009;394:765–84. doi: 10.1007/s00423-009-0534-4. [DOI] [PubMed] [Google Scholar]
- 70.Scott-Coombes DM, Rees J, Jones G, Stechman MJ. Is Unilateral Neck Surgery Feasible in Patients with Sporadic Primary Hyperparathyroidism and Double Negative Localisation? World J Surg. 2017;41:1494–9. doi: 10.1007/s00268-017-3891-0. [DOI] [PubMed] [Google Scholar]
- 71.Iacobone M, Carnaille B, Palazzo FF, Vriens M. Hereditary hyperparathyroidism--a consensus report of the European Society of Endocrine Surgeons (ESES) Langenbecks Arch Surg. 2015;400:867–86. doi: 10.1007/s00423-015-1342-7. [DOI] [PubMed] [Google Scholar]
- 72.Wang TS, Udelsman R. Remedial surgery for primary hyperparathyroidism. Adv Surg. 2007;41:1–15. doi: 10.1016/j.yasu.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 73.Guerin C, Paladino NC, Lowery A, Castinetti F, Taieb D, Sebag F. Persistent and recurrent hyperparathyroidism. Updates Surg. 2017;69:161–9. doi: 10.1007/s13304-017-0447-7. [DOI] [PubMed] [Google Scholar]
- 74.Udelsman R. Approach to the patient with persistent or recurrent primary hyperparathyroidism. J Clin Endocrinol Metab. 2011;96:2950–8. doi: 10.1210/jc.2011-1010. [DOI] [PubMed] [Google Scholar]
- 75.Aygün A, Besler E, Yetkin G, Mihmanlı M, Işgör A, Uludağ M. Complication Risk in Secondary Thyroid Surgery. Med Bull Sisli Etfal Hosp. 2018;52:19–25. doi: 10.14744/SEMB.2017.87609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terzioğlu T, Senyurek YG, Tunca F, Türkmen C, Mudun A, Salmaslıoglu A, et al. Excision efficiency of radioguided occult lesion localization in reoperative thyroid and parathyroid surgery. Thyroid. 2010;20:1271–8. doi: 10.1089/thy.2009.0441. [DOI] [PubMed] [Google Scholar]