Abstract
Objectives:
Anesthetic applications may cause increased neuronal damage in infants and children. Commonly cognitive or learning disability tests were used to investigate the neurological progress in children. Visual Evoked Potential is a gross electrical signal generated by the occipital regions of the cerebral cortex in response to visual stimulation and an objective assessment of brain function. In this study, to acquire more objective results, Visual Evoked Potential responses of children who had multiple exposures to anesthesia during the treatment of corrosive esophagitis were compared to children who have never received anesthesia before.
Methods:
In this prospective, single-blinded, randomized, controlled study, 25 children, who were admitted to our pediatric surgery clinic because of corrosive esophagitis and who received general anesthesia more than 15 times composed Group-P; 25 children, who admitted to our well-child-clinic and who had never received anesthesia before consisted Group-C. The flash and pattern VEP responses of both groups were measured at the electrophysiology laboratory without any anesthetic drug application. The VEP responses of children in Group-P were recorded at least three days after the last exposure to anesthesia.
Results:
Latencies and amplitudes of the N2 and P2 components of the pattern and flash VEP responses were statistically significantly different between the two groups (p=0.000).
Conclusion:
This study shows that in children who had repeated anesthetic applications VEP parameters are significantly altered. We believe that VEP responses may be a reliable objective criterion for the evaluation of anesthesia neurotoxicity.
Keywords: Anesthesia, corrosive esophagitis, neurotoxicity, pediatric, visual evoked potential
Neurotoxicity, due to anesthesia, particularly in children, is one of the major concerns of anesthesiologists in recent years.[1–3] Present studies in the literature are mostly experimental and conducted on animals since it is difficult to practice such prospective studies on children because of ethical reasons and the excessive time they consume.[4–6]
The stage of brain development at the time of exposure to anesthesia and the frequency and cumulative anesthetic doses are some of the important factors causing neurotoxicity.[7]
Commonly cognitive or learning disability tests were used to investigate the neurological progress in children.[3] However, the learning disability tests used in the studies were not purely neuropsychological and the socioeconomic factors were also effective on the results. To acquire more objective results, we preferred to use the analysis of Visual Evoked Potential (VEP) responses to determine neurotoxicity of anesthesia in this study.
VEP is a gross electrical signal generated by the occipital regions of the cerebral cortex in response to visual stimulation.[8] The averaged VEP is a gross electrical signal generated by the occipital regions of the cerebral cortex in response to visual stimulation. VEP studies to pattern reversal stimulation have reported age-dependent waveform changes, decrease in latency and increase in amplitude during early development and decline thereafter. VEP is more specific than the EEG and more sensitive to the changes in the visual stimuli providing ophthalmologists and researchers information about the human visual system that is less available by other methods.[9] All of the studies have shown that VEP maturation is rapid in infants, gradual in preschool years and persists until adulthood.[10]
The clinical studies related to anesthetic neurotoxicity were generally conducted on children who were exposed to general anesthesia only once. In our study, a group of children who had multiple (more than 15 times) anesthesia during their treatment for corrosive esophagitis, created a rare and special group to conduct research on the anesthetic neurotoxicity. Accidental ingestion of household caustic cleaning material, such as bleaches or drain cleaner, causes esophagitis. These children have anesthesia from fifteen to forty times for treatment of esophagitis by balloon dilatation under general anesthesia.
In this study, we aimed to investigate whether anesthesia causes permanent neurotoxic harm or not, by comparing the VEP responses of 25 children who received repeated anesthesia with children who had never received anesthesia.
Methods
After obtaining local Ethics Committee approval (352/02.09.2014) and informed consent of the parents, this study was conducted. Because repeated anesthesia applications may be neurotoxic; data of children, who accidentally ingested liquid household cleaning material, such as bleach or drain cleaner, but who had no other systemic diseases, who underwent general anesthesia for at last 15 times for balloon dilatation and who were followed up in our pediatric surgery unit from 2011-2014 were included in this study in Group P (n=25). Patients with complications as perforation or undergoing major operations were taken as exclusion criteria. Randomly 25 children from this patient population were selected to participate in Group P by a computer program.
Since it is expected, that neurotoxicity is fewer in children, who never received anesthesia before, the control group consisted of children from our well-child clinic. That is why Group C was also randomly selected from 25 children, who were under observation from birth to the present day at our hospital’s well-child clinic.
VEP recordings were taken at least three days after receiving the last anesthesia. The dates and types of anesthesia received by the children of Group P were recorded retrospectively. All of the children had received 0.1mg/kg IV or 0.4mg/kg oral midazolam (Dormicum, Roche, Hamburg, Germany) as premedication. Induction was performed with 8% sevoflurane in50% O2-N2O to the children who were not allowed for the IV access. For induction of children who had IV access, propofol 2-3mg/kg (Propofol %1, Fresenius, Hamburg, Germany), fentanyl 1mcg/kg (Fentanyl Citrate, Abbott, İllinois, United States) and 0.5mg/kg atracurium (Tracrium, Glaxo SmithKline, Auckland, New Zealand) were used. Anesthesia maintenance was provided with 1-3% sevoflurane (Sevorane, Abbott, Mascot Australia) in 50% O2-N2O. For early pain management, paracetamol 10 mg/kg was used in all cases during the operations.
All children accompanied by their parents were randomly invited to the electrophysiology laboratory. Patients did not receive any anesthesia. VEP responses were recorded on the awake children.
The flash and pattern VEP (FVEP and PVEP) responses were taken at the electrophysiology laboratory of the Physiology Department of Istanbul University Istanbul Faculty of Medicine with a Nihon-Kohden RM 6000 polygraph system, and Ag/AgCl skin cup electrodes were used for both recordings. The mean luminance of the pattern VEP monitor was 105.35 cd/m2, and the contrast ratio between black and white squares was 110:1. Electrode impedances were kept below 5 kOhm using electrode paste. Refractive errors of all subjects were corrected while recording. Children were seated 1 m distant from the monitor, and signals were recorded as they looked at a fixation point in the middle of the screen with one eye while the other eye was occluded. The stimulus was an alternating, square black-and-white checkerboard pattern (100 stimuli) with 30 and 60 min check size, and stimulus reversal was 2 Hz. The stimulus was a light flash of one Joule with 1 µs duration at 1 Hz. Manual artifact rejection was applied to data for eye movement artifacts.
For FVEP and PVEP recordings, the active electrode was placed over the visual cortex at occipital zone according to the International 10/20 system, and the reference electrode was placed on the left earlobe, while the ground electrode was placed on the right earlobe. The data were band-pass filtered between 1 and 45 Hz and averaged. The peak-to-peak amplitude of N75–P100 (negative wave at ca. 75. ms and positive wave at ca. 100. ms after the stimulus) and peak implicit times were measured for each eye separately. The data of the left and right eyes were then merged in the same pool.
Statistical Analysis
Statistical calculations were performed with the IBM SPSS Statistics 21. One-way ANOVA test was used for the comparison of patient and control groups’ flash and pattern VEP responses components’ latencies and amplitudes.
Results
The children in Group P were under treatment for four years and were six to 14 years old. They were administered anesthesia for 15 to 40 times while their treatment was lasting four to 48 months. The periods between two anesthesia applications were minimum of seven days and maximum of 60 days (Table 1).
Table 1.
Demographic data
| Group P (n=25) | Group C (n=25) | |
|---|---|---|
| Age at the time of the study (years) | 9.7±3.1 (6-14) | 10.1±4.2 (6-14) |
| Female/Male | 2F/23M | 2F/23M |
| Age at the time of the first anesthesia (years) | 6.4±2.7 (2-10) | |
| Treatment period (months) | 34.6±13.3 (4-48) | |
| The number of anesthesia given | 31.7±9.0 (15-40) | |
| The average duration of anesthesia (minute) | 28.4±14.7 (20-45) |
Latencies and amplitudes of the N2 and P2 components of pattern VEPs taken with 30 and 60 min check sizes were evaluated statistically using one-way ANOVA, F-ratios and p-values. The latencies of the Group P were longer while their peak to peak amplitudes (N2-P2) were higher than those of the Group C (Table 2).
Table 2.
Pattern VEP data of the control and patient groups.One way ANOVA results (f ratio and p values)
| Group C | Group P | f | p | |
|---|---|---|---|---|
| 30N2Lat | 68.23±5.41 | 78.08±8.51 | 24.265 | 0.000 |
| 30P2Lat | 100.15±8.35 | 119.25±12.29 | 41.865 | 0.000 |
| 30Amp | 5.52±0.78 | 14.82±7.39 | 40.827 | 0.000 |
| 60N2Lat | 68.38±5.22 | 75.75±8.89 | 13.016 | 0.001 |
| 60P2Lat | 100.15±8.16 | 117.67±11.15 | 40.641 | 0.000 |
| 60Amp | 5.53±0.78 | 14.4±8.71 | 26.805 | 0.000 |
The latencies of the P2 components of the group P were longer while their peak to peak amplitudes (N2-P2) were higher than those of the group C. The N2 latencies were not statistically different between the two groups (Table 3).
Table 3.
Flash VEP data of the control and patient groups.
| Group C | Group P | f | p | |
|---|---|---|---|---|
| VEPN2Lat | 68.23±4.47 | 70.87±8.35 | 1.993 | 0.164 |
| VEPP2Lat | 100.23±7.25 | 121.83±16.94 | 35.306 | 0.000 |
| VEPAmp | 5.57±0.83 | 20.12±9.10 | 65.978 | 0.000 |
Discussion
Previous clinical studies related to anesthetic neurotoxicity were generally conducted on children who had only one exposure to general anesthesia. Our study was conducted on a group of children who had anesthesia for more than 15 times during their treatment for corrosive esophagitis. This group of children was a rare and special study group to conduct research on the anesthetic neurotoxicity. This study showed that in children who had repeated anesthetic applications VEP parameters are significantly altered, which indicates neurotoxicity.
Preclinical and also retrospective clinical studies suggested that anesthesia could be damaging brain functions in children.[7] The mechanism of anesthesia-induced neurotoxicity is complex: General anesthetics affect multiple ion channels, receptors and cell signaling systems in the central nervous system to produce anesthesia.[2] In the previous studies, it has been indicated that inhaled anesthetics by causing apoptosis and reducing neuro-genesis may have serious effects on neonatal animals during neuro-developmental periods.[2] The studies conducted on developing monkeys have shown that exposure to anesthesia causes neuro-apoptosis[4, 5] and permanent neuro-cognitive deficits in the developing monkey brain.[6]
On the other hand, the reflection of these laboratory findings to the clinical practice remains unclear because of very restricted knowledge existing in the literature. It is quite difficult to find or prove the neurological harm which might be caused by anesthesia in clinical studies conducted on children. These studies sometimes cannot be conducted due to ethical reasons, while studies which can be conducted consume too much time.
Pediatric Anesthesia NeuroDevelopment Assessment (PANDA Study) which compared children who were given general anesthesia for inguinal hernia surgery before the age of three with siblings who had no received anesthesia before age three, showed no significant difference in IQ scores between the siblings when assessed between the ages of 8 and 15. This study included healthy children with a single exposure to general anesthesia. A different study, including children with a more complicated medical history and with multiple general anesthesia exposures, may show different results.[11, 12]
One previous study, General Anesthesia compared to Spinal Anesthesia(GAS) found strong evidence that exposure of just under an hour to a sevoflurane GA in infancy does not increase the risk of adverse neurodevelopmental outcome at two years of age.[12, 13]
The Mayo Anesthesia Safety in Kids (MASK) study which analyses the condition of the children who had anesthesia for once or more is currently incomplete.[3] Our study group is a rare and specific group for investigating the neuro-detrimental effect of multiple anesthesias on children. In the literature, psychological tests and/or tests evaluating cognitive functions are frequently used to evaluate the neurologic harm.[9, 10, 14–16] These tests evaluate learning disability, behavior, autism and education difficulties. Children’s disabilities in paying attention, learning, memory and social activities are discussed. Children, who were hospitalized multiple times due to esophagitis might have psychological and scholar problems independent of repetitive anesthesia applications. That is why we preferred to use VEP responses in our study, contrary to the majority of researchers who investigated anesthesia neurotoxicity using subjective psychological tests. We believe that VEP responses give us more objective data to determine neurotoxicity of anesthesia. Moreover, VEP analysis is easy to use in child studies as a research indicator.
Latencies and amplitudes of VEP components are mostly related to myelination, brain development and organic defects rather than to psychological disorders. However, the excessive increase in amplitudes attributes to disinhibition and therefore cannot be evaluated as a positive sign. Increases in the amplitudes of the VEP components in our study, while their latencies were longer than the control group, can be evaluated as disinhibition pathology, rather than better brain development.
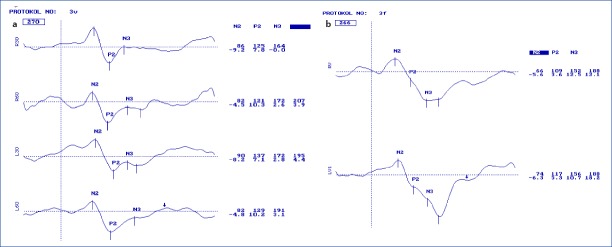
Todorovic-Jevkovic et al.[7] reported at BJA Salzburg seminar, that children, who received anesthesia before they were 12 months old, suffered weaknesses in memory at age 6-11 years. They mentioned that the brain cells may be affected in different degrees related to age during anesthesia application. In another animal study, they reported that brain cell death caused by anesthesia was observed in rats and that a long-lasting exposure to anesthesia caused worse effects rather than frequent application.[17] Di Maggio et al.[18] found that siblings who were younger than three years and who received anesthesia for operations had a risk elevation of 60% in developmental or behavioral disorders, when compared to a similar group of siblings, who did not undergo any operations.[18] Similarly, in our study, the most harmful effects were detected in four patients, who had their first anesthesia application before they were three years old (Figs. 1, 2). Besides, these children were exposed to anesthesia most frequently as totally 36-40 times.
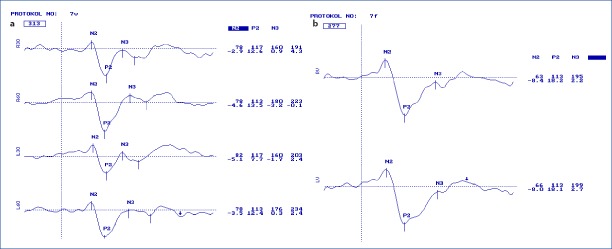
Figure 1.
Pattern (a) and Flash (b) VEP of a healthy child of seven years old. Latency and amplitudes are seen in the first and second lines consecutively at the right side of the curves. R for right and L for left eye responses. 30 and 60 attribute to check sizes of 30 and 60 mins.
Figure 2.
Pattern (a) and Flash (b) VEP of a child of six years old who had his first anesthesia before three years old. Latency and amplitudes are seen in the first and second lines consecutively at the right side of the curves. R for right and L for left eye responses. 30 and 60 attribute to check sizes of 30 and 60 mins. Note that the latency of the N2 and P2 components are longer than normal, and there are even later components.
Some studies underlined differences in cognitive dysfunction related to gender. They found that boys were affected significantly more than girls.[17, 19] In our study, such a comparison could not be make since the majority of the patients were males.
In previous studies, the neurotoxic effects of the anesthesia were investigated in children who had undergone only a single seance of anesthesia.[20, 21] In this study, our patients had at least 15 repetitive anesthesia applications. One of the limitations of this study was that we had no study group consisting of children, who received anesthesia once. Another limitation was that we could not measure VEP responses in our study group before their first anesthetic application because all patients underwent emergency interventions and we randomized the study group from a retrospective patient data pool. Further studies with a larger number of patients are required to support our findings.
Conclusion
In conclusion, our study shows that in children who had multiple anesthetic applications, VEP parameters are significantly altered. We believe that VEP responses may be a reliable objective criterion for the evaluation of anesthesia neurotoxicity.
Disclosures
Ethics Committee Approval: Sisli Hamidiye Etfal Education and Research Hospital; 352/02.09.2014.
Peer-review: Externally peer-reviewed.
Conflict of interest: None declared.
Authorship Contributions: Concept – S.O., S.K.; Design – S.O., S.K.; Supervision – A.İ.D.; Data collection &/or processing – C.T.I., H.Ş.T., S.A., M.K.; Analysis and/or interpretation – L.K., C.T.I., H.Ş.T.; Literature search – S.O., S.K., C.T.I., H.Ş.T.; Writing – S.O., L.K., H.Ş.T.; Critical review – A.İ.D.
References
- 1.Nemergut ME, Aganga D, Flick RP. Anesthetic neurotoxicity:what to tell the parents? Paediatr Anaesth. 2014;24:120–6. doi: 10.1111/pan.12325. [DOI] [PubMed] [Google Scholar]
- 2.Hudson AE, Hemmings HC., Jr Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30–7. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun L. Early childhood general anaesthesia exposure and neurocognitive development. Br J Anaesth. 2010;105(Suppl 1):i61–8. doi: 10.1093/bja/aeq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, Dissen GA, et al. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–35. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, et al. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–58. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–30. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jevtovic-Todorovic V, Absalom AR, Blomgren K, Brambrink A, Crosby G, Culley DJ, et al. Anaesthetic neurotoxicity and neuroplasticity:an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–51. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenassi E, Likar K, Stirn-Kranjc B, Brecelj J. VEP maturation and visual acuity in infants and preschool children. Doc Ophthalmol. 2008;117:111–20. doi: 10.1007/s10633-007-9111-8. [DOI] [PubMed] [Google Scholar]
- 9.Sprung J, Flick RP, Wilder RT, Katusic SK, Pike TL, Dingli M, et al. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;111:302–10. doi: 10.1097/ALN.0b013e3181adf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokol S. Visually evoked potentials:theory, techniques and clinical applications. Surv Ophthalmol. 1976;21:18–44. doi: 10.1016/0039-6257(76)90046-1. [DOI] [PubMed] [Google Scholar]
- 11.Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA. 2016;7(315):2312–20. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS):an international multicentre, randomised controlled trial. Lancet. 2016;16(387):239–50. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millar K, Bowman AW, Burns D, McLaughlin P, Moores T, Morton NS, et al. Children's cognitive recovery after day-case general anesthesia:a randomized trial of propofol or isoflurane for dental procedures. Paediatr Anaesth. 2014;24:201–7. doi: 10.1111/pan.12316. [DOI] [PubMed] [Google Scholar]
- 15.Kayaalp L, Bozkurt P, Odabasi G, Dogangun B, Cavusoglu P, Bolat N, et al. Psychological effects of repeated general anesthesia in children. Paediatr Anaesth. 2006;16:822–7. doi: 10.1111/j.1460-9592.2006.01867.x. [DOI] [PubMed] [Google Scholar]
- 16.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BH, Chan JT, Kraeva E, Peterson K, Sall JW. Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology. 2014;83:9–17. doi: 10.1016/j.neuropharm.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stratmann G. Review article:Neurotoxicity of anesthetic drugs in the developing brain. Anesth Analg. 2011;113:1170–9. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 20.Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res. 2010;17:179–88. doi: 10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]