Abstract
An interesting clinical feature of basal cell carcinoma (BCC) of the skin is a marked variation in tumor number, sites, and accrual. Some individuals develop only a single BCC lesion with no impact on health status, while a significant proportion is affected repeatedly with new primary tumors at various body sites. Approximately 29% of patients with a first BCC will develop at least 1 more lesion during their lifetime. The candidate predictors for multiple BCC development include younger age and a superficial BCC subtype at the time of the first diagnosis, red hair phenotype, initial or frequent tumor location on the trunk or on the upper limbs, and male gender. The pathogenesis of multiple BCC development does not seem to be related to greater UVR exposure. Individual genetic susceptibility may have a greater impact than extrinsic factors. In clinical practice, it is meaningful to estimate the probability of new BCC development in patients who have an initial lesion. A reliable prediction model for individualized risk stratification remains a subject of continued research; however, a focus on the risk factor profile is beneficial for clinical screening and may help clinicians to determine the individuals who should be followed up more closely.
Keywords: Basal cell carcinoma, multiple development, risk factors
Basal cell carcinoma (BCC) of the skin constitutes approximately 75% of all malignant skin tumors[1] and it is now the most common malignancy in the Caucasian population.[1, 2] The incidence rate varies significantly around the world, ranging from 2 in East Asia to 1600/100.000 per year in Queensland, Australia.[2] This oncological entity represents a heterogeneous group of tumors with a variable clinical manifestation, histomorphology, and biological behavior. Although it is usually a slow-growing neoplasia with only minimal metastatic potential, some subtypes grow aggressively, causing extensive tissue destruction.[1] The disease can be classified into sporadic (non-syndromic) and syndromic (hereditary) forms. In the first group, which comprises the vast majority of the cases, the affected individuals do not have any genodermatosis susceptible to developing cutaneous malignancies. BCC lesions usually occur in middle-aged and older adults. In the second group, the patients suffer from a genetic skin disorder (e.g., Gorlin-Goltz syndrome, Bazex syndrome, xeroderma pigmentosum), which predisposes them to BCC development at an early age. One of the interesting clinical features of this cancer is a marked variation in tumor number, sites, and accrual (number of tumors per year from the first presentation) between individuals.[3–5] Some patients have developed only 1 BCC lesion throughout their life with no impact on their health status. On the other hand, a significant proportion is affected several times by new primary tumors at various body sites. Furthermore, although most individuals demonstrate only a single lesion at each presentation, others may suffer many tumor clusters at different locations.[3–5] In this paper, the development of multiple cutaneous BCCs is discussed with an emphasis on the pathogenesis, risk factors, and prediction models for individualized risk stratification.
Classification of BCC Phenotypes
As previously mentioned, BCC patients display a considerable phenotypic diversity in tumor number and accrual. Two decades ago, Ramachandran et al.[3] proposed a classification of BCC cases on the basis of presentation with a single presentation phenotype (SPP) or multiple presentation phenotype (MPP). SPP was subdivided into categories of SPP-one and SPP-more. SPP-one comprises patients with only a single BCC lesion at the first presentation and no development of any additional lesions. The SPP-more group includes patients with only 1 primary BCC lesion at the first presentation but the development of additional single lesions during follow-up. MPP was subdivided into categories of MPP-cluster initial and MPP-cluster later. A cluster was defined as the presence of 2 or more new, primary BCC lesions at the same locality. The MPP-cluster initial definition includes patients who present with a cluster of BCC lesions at first presentation but may or may not develop additional BCCs during follow-up. The MPP-cluster later category comprises patients who had only 1 primary BCC lesion at the first presentation but develop a cluster at the next or a subsequent presentation. Although this classification is well-conceived, it has many practical limitations. Since the development of a cutaneous BCC is a dynamic process over a long period of time, the appropriate category may change. Furthermore, many patients with a single BCC lesion may die before they obtain adequate follow-up. Nevertheless, this scheme describes the phenotypic heterogeneity of the disease and may be useful for predicting the development of other new primary lesions.
Prevalence of Multiple BCC Cases
According to the literature data, the percentage of patients with more than 1 primary BCC lesion has varied from 7% to 46%[3, 6–13] and the mean number of tumors per individual has ranged from 1.5 to 1.9.[11, 14] This variability may be explained by several factors, such as the total number of participants, distinct ethnicity, different follow-up periods, and misclassification bias. A precise meta-analysis conducted by Dutch authors[13] demonstrated that approximately 29% of patients with a first BCC lesion will develop at least 1 more in their lifetime. In our recent study,[15] we analyzed a cohort of 899 patients with biopsy-proven cutaneous BCC who were registered in our pathology database. Among them, 171 (19%) had ≥2 primary lesions. The number of lesions per person ranged from 1 to 17, with a mean of 1.3. All but 1 patient were cases of the sporadic form of disease (Fig. 1). There was only 1 young man who suffered from Gorlin-Goltz syndrome. He had dozens of primary BCC lesions, located mainly on the trunk (Fig. 2).
Figure 1.
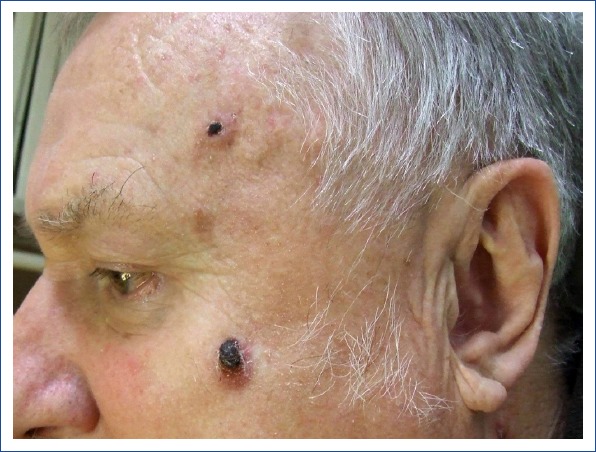
Two basal cell carcinoma lesions on the left cheek and the left frontotemporal region in a 66-year-old man. (Image courtesy of Dr. Milada Kullova).
Figure 2.
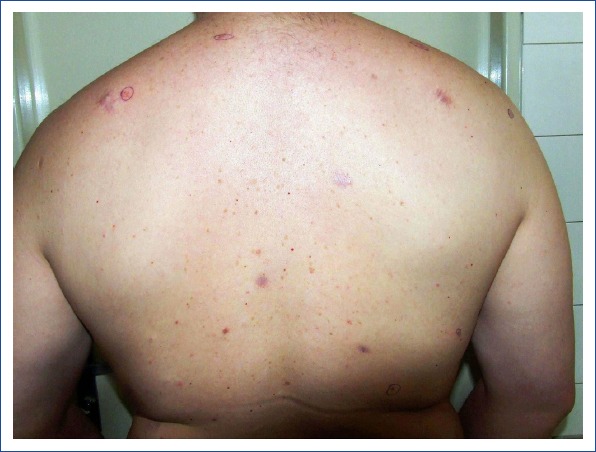
Multiple basal cell carcinoma lesions on the back in a 34-year-old man with Gorlin-Goltz syndrome (Image courtesy of Dr. Milada Kullova).
Pathogenesis and Risk Factors for BCC
BCC is a good example of an oncological disease caused by interactions between extrinsic (environmental) and several intrinsic (host-dependent) etiological factors.[1] While the first group mainly consists of extensive ultraviolet radiation (UVR) exposure, the second includes a light skin phototype, which is generally more prone to develop skin tumors.[16] Because the pathogenesis and molecular mechanisms of the disease are very complex, a detailed description would go far beyond the scope of this article. They are well described in other papers.[1, 16, 17] Herein, I only focus on some aspects of UVR exposure and genetic susceptibility related to individual response to UVR-induced DNA damage in the putative precursor cells.
A. Ultraviolet Radiation Exposure
Although UVR (sunlight or sunbed use) has been accepted as the key causal factor for cutaneous BCC, a lot of questions still remain regarding the relationship between sun exposure and tumor development. It is well known that areas commonly more exposed to sunlight are more frequently the sites of BCC lesions. BCC lesions arise most often on the head and neck, and amount to more than 80% of cases.[16] Since this body part represents the primary area of permanently sun-exposed skin in humans, this reinforces cumulative sun exposure as the main risk factor for BCC. However, the fact that about one-quarter of BCC lesions occur on anatomical sites that are not habitually exposed to the sun, such as the trunk,[18] indicates that discontinuous episodes of intense sunlight exposure may also play an important role in tumor pathogenesis. Epidemiological studies[19, 20] have confirmed such a hypothesis and found that intermittent solar exposure (including severe sunburns, especially in childhood), rather than chronic exposure, was a more prejudicial etiological determinant in BCC carcinogenesis. Currently there are suggestions that the pathophysiology of BCC arising on distinct (sun-exposed vs sun-protected) parts of the body and developing into different subtypes, differs from the patterns of UVR exposure that give rise to lesions arising on distinct body sites.[2, 18] Depending on timing (childhood, adolescence), pattern (intermittent, continuous), source (natural, artificial), and amount (cumulative sun exposure), the impact of UVR on BCC development seems to be far more complex.[16] In fact, only a fraction of the individuals who have been exposed to increasing levels of solar UV radiation during a lifetime will develop BCC and, vice versa, many who have reported only a modest lifetime exposure to sunlight will develop this malignancy. It obviously suggests a genetic susceptibility to UVR-induced carcinogenesis in the general population. Because the incidence of cutaneous BCC, in contrast to cutaneous squamous cell carcinoma, is clearly less dependent on solar exposure, genetic factors must play a role in the etiology. Therefore, BCC may serve as an excellent disease model for studying gene-environment interactions in skin cancer.
B. Genetic Susceptibility
The principal genetic alterations mediating a predisposition to BCC development, especially multiple tumor manifestation, are not fully understood. Genetic background significantly modulates skin response to UVR. The effectiveness of DNA reparative mechanisms undoubtedly modifies the process of BCC carcinogenesis. In a study conducted by Wang et al.,[21] reduced DNA repair capacity (DRC) for UVR-induced DNA damage was established as an independent risk factor for the development of BCC. They found that the DRC was significantly lower in BCC patients compared with controls, irrespective of tumor behavior, but interestingly, not among individuals who had more than 1 BCC lesion. Many other studies have indicated that there may be an influence of genetic polymorphisms in a variety of molecular markers (e.g., carcinogen-metabolizing enzymes, cell-signaling proteins) on susceptibility to this cancer. For example, a relationship has been demonstrated between glutathione S-transferase,[22, 23] reduced nicotinamide adenine dinucleotide NAD(+):quinone oxidoreductase,[24] cytochrome P450,[22, 23, 25] vitamin D receptor,[25] tumor necrosis factor alpha,[25] and the protein patched homolog (PTCH)[26] gene polymorphisms and the rate of development (number and accrual) of BCC lesions in individual patients. In addition, an association between the human leukocyte antigen (HLA) DR4 subtype[27] or HLA-DR1[28] and the pathogenesis of multiple BCCs was confirmed 2 decades ago. Because 1 of the effects of UVR is the creation of a sustained immunosuppressive environment, the crucial parameter in the promotion and progression of photo-induced skin cancers seems to be an activity of the immune system. The rate of tumor appearance may be mediated by the efficiency of the local immune surveillance of the skin. Ramachandran et al.[22] proposed a theory that the SPP-one, SPP-more, and MPP-cluster later phenotypes, which initially have a single BCC, reflect a modest reduction in the effectiveness of immune surveillance. However, in MPP-cluster initial patients, clustering at first presentation suggests a greater loss in immune surveillance. The authors proposed that infection and/or stress could cause temporary impairment of local, cutaneous immunity in the presence of UVR exposure via effects on expression of key cytokines.
Origin and Clonality of Neoplastic Cells
Identification of the cell origin of human neoplasias remains a challenging but important task in cancer research. Several cell types have been suggested as the precursor cells for BCC,[1] but it is currently thought to originate from pluripotential stem cells in the basal layer of the interfollicular epidermis or the hair follicle.[17] BCC does not have detectable precursor lesions and arises de novo,[16] which makes it different from most other skin malignancies. The question of local immune surveillance of neoplastic cells in patients with multiple BCCs has not yet been clarified. The results of Austrian authors[29, 30] indicated that the origin may be polyclonal. Heitzer et al.[29] sequenced the PTCH gene and analyzed the loss of heterozygosity (LOH) pattern of BCCs from a total of 6 patients with multiple lesions (3 lesions from anatomically distant locations per patient). They detected a total of 18 differential PTCH mutations in 14 of 18 BCCs. On the basis of the PTCH mutation and LOH pattern results, they concluded that BCCs in patients with multiple lesions were most likely polyclonal in origin and that multiple lesions at anatomically different body sites may not arise from the same progenitor cell. Similar results corroborating a theory of BCC polyclonality were also observed in their earlier research.[30] However, another molecular study[31] of 41 BCC lesions from 15 individuals contradicts such a hypothesis and suggests that the majority of anatomically distinct BCCs are monoclonal neoplasms.
Risk Factor Profile for Multiple BCC Development
The risk factor profile of individuals who develop many subsequent BCCs during their lifetime is relatively poorly understood. Some investigators[7, 8, 32] have tried to explore whether patients with a single BCC have a different scale for risk factors compared with those with multiple lesions. The results of several studies have suggested that candidate predictors for multiple BCC development include younger age and a superficial BCC subtype at the time of the first diagnosis,[7, 8, 33] red hair phenotype,[7] initial or frequent tumor location on the trunk[5, 7, 8, 32–34] or on the upper extremities[7] and clinical manifestation with tumor clusters.[3, 4] Further, some studies[9, 13, 14] have shown that men have a higher risk of developing multiple BCCs than women. In our recent study,[15] we found that there was a higher prevalence of male gender among the participants with multiple BCCs. While there was a nearly equal proportion of men and women in the subset of members with 2 BCCs, the male-to-female ratio continuously increased with the number of additional lesions. These findings confirm that men are more prone to develop a greater number of BCCs than women, corroborating observations from some previous research.[9, 13, 14] Furthermore, we found that in the subgroup of patients with multiple BCCs, the tumors developed more frequently on the back and upper limbs and much less frequently on the face. Histologically, these BCCs more commonly consisted of a superficial subtype and less commonly of a nodular or infiltrative type. The data were consistent with the results published by Kiiski et al.[7]
Several papers[8, 22, 32] have provided evidence that the risk determinants for multiple BCC development are not related to more UVR exposure during one’s life. Verkouteren et al.[8] have demonstrated that no significant influence was found for phenotype status or UVR-related characteristics on the development of a second BCC. Even in the study of Ramachandran et al.,[22] there was no proof that MPP patients had received more UVR exposure. In other research,[32] no significant differences were seen between multiple and single BCC in terms of outdoor occupation, skin type, or eye/hair color. The data indicate that an individual genetic susceptibility has a greater impact in the development of multiple BCCs of the skin than the effect of extrinsic factors.
Prediction Models for Individualized Risk Stratification
In clinical practice, it is worthwhile to estimate the probability of the development of new BCCs once an initial lesion has been detected. Information on the frequency and timing of subsequent BCCs may be crucial to guiding adequate follow-up care. It was calculated[35] that among individuals with at least 1 previous BCC, the 1-, 3-, and 5-year risk of a new BCC was 17%, 33%, and 41%, respectively. More detailed observations showed[34] that the 5-year risk of further BCC development in relation to the number of prior BCCs increased as follows: 27% in cases of 1 BCC, 49% with 2 BCCs, 68% with 3 BCCs, 73% with 4-5 BCCs, 78% with 6-9 BCCs, and 90% risk in those with ≥10 BCCs. However, these data cannot be universally accepted for the entire population and are insufficient for reliable individual prediction in dermatological practice. Therefore, Verkouteren et al.[8] recently developed a simple model to predict an individual´s risk of a second BCC at the time of a first BCC. They demonstrated that the absolute risk of a second BCC could be predicted with reasonable accuracy using simple phenotypic, lifestyle, and tumor-specific characteristics. The following 5 variables revealed the strongest associations with an increased risk of a new BCC: age at first BCC, male sex, coffee consumption, superficial subtype of the first BCC, and more than 1 BCC at first date of diagnosis. The patients are scored according to these predictors, and based on the total score, a corresponding percentage risk of a second BCC within 1, 3, and 5 years may be assessed. The physician can thus easily calculate the predicted risk of a second BCC for a patient with an initial BCC. The prediction model and simple scoring chart allow for identification of high-risk patients for more intensive follow-up while excluding low-risk patients. As this is a novel scoring system, further research is needed to confirm its validity and applicability in routine practice.
Conceptual and Practical Considerations
Although the occurrence of multiple cutaneous BCC harbors many conceptual and practical considerations, here I mention only 2 practical issues. From a clinical point of view, simultaneous or consecutive development of new BCCs has a negative impact on clinical outcome, substantially increases morbidity, and requires repeated treatment interventions. That imposes a greater healthcare cost burden. In addition, consistent evidence indicates[36] that patients with a history of BCCs also have an elevated risk of developing other primary cutaneous malignancies. Therefore, adequate clinical monitoring of these patients is necessary.
From an epidemiological point of view, due to the common occurrence of multiple primary lesions within a single individual, the statistically reported incidence of BCC underestimates the true incidence of this neoplasia in the population.[9] German authors[12] calculated an age-standardized incidence rate for the first BCC diagnosis (patient incidence) and for any diagnosis of BCC (case incidence). The case incidence was about 30% higher than the patient incidence, since 25% of the individuals had more than 1 primary lesion during the 5-year period of the study. Similarly, British investigators[37] found that between 1993 and 2002, there was a 62% increase in the overall number of BCC samples processed by local pathology laboratories but only a 13% increase in the number of affected patients.
Conclusion
Many patients with diagnosed BCC of the skin have a tendency to develop new primary lesions. A better understanding of why some individuals have many BCCs during their lifetime is fundamental to the identification and selection of such cases at the first presentation. A focus on the risk factor profile is beneficial for clinical screening and may help clinicians determine who should be followed up more closely. Early detection and removal of subsequent lesions may be enhanced by encouraging a regular personal skin inspections and professional skin assessments after an initial BCC diagnosis.
Disclosures
Peer-review: Externally peer-reviewed.
Conflict of interest: None declared.
References
- 1.Tilli CM, Van Steensel MA, Krekels GA, Neumann HA, Ramaekers FC. Molecular aetiology and pathogenesis of basal cell carcinoma. Br J Dermatol. 2005;152:1108–24. doi: 10.1111/j.1365-2133.2005.06587.x. [DOI] [PubMed] [Google Scholar]
- 2.Khalesi M, Whiteman DC, Doi SA, Clark J, Kimlin MG, Neale RE. Cutaneous markers of photo-damage and risk of Basal cell carcinoma of the skin:a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22:1483–9. doi: 10.1158/1055-9965.EPI-13-0424. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran S, Fryer AA, Smith AG, Lear JT, Bowers B, Griffiths CE, et al. Basal cell carcinoma. Cancer. 2000;89:1012–8. doi: 10.1002/1097-0142(20000901)89:5<1012::aid-cncr10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran S, Fryer AA, Strange RC. Genetic factors determining cutaneous basal cell carcinoma phenotype. Med Pediatr Oncol. 2001;36:559–63. doi: 10.1002/mpo.1130. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran S, Fryer AA, Smith A, Lear J, Bowers B, Jones PW, et al. Cutaneous basal cell carcinomas:distinct host factors are associated with the development of tumors on the trunk and on the head and neck. Cancer. 2001;92:354–8. doi: 10.1002/1097-0142(20010715)92:2<354::aid-cncr1330>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Adachi K, Yoshida Y, Noma H, Goto H, Yamamoto O. Characteristics of multiple basal cell carcinomas:The first study on Japanese patients. J Dermatol. 2018;45:1187–90. doi: 10.1111/1346-8138.14576. [DOI] [PubMed] [Google Scholar]
- 7.Kiiski V, de Vries E, Flohil SC, Bijl MJ, Hofman A, Stricker BH, et al. Risk factors for single and multiple basal cell carcinomas. Arch Dermatol. 2010;146:848–55. doi: 10.1001/archdermatol.2010.155. [DOI] [PubMed] [Google Scholar]
- 8.Verkouteren JAC, Smedinga H, Steyerberg EW, Hofman A, Nijsten T. Predicting the Risk of a Second Basal Cell Carcinoma. J Invest Dermatol. 2015;135:2649–56. doi: 10.1038/jid.2015.244. [DOI] [PubMed] [Google Scholar]
- 9.Richmond-Sinclair NM, Pandeya N, Ware RS, Neale RE, Williams GM, van der Pols JC, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution:longitudinal study of an Australian population. J Invest Dermatol. 2009;129:323–8. doi: 10.1038/jid.2008.234. [DOI] [PubMed] [Google Scholar]
- 10.Raasch BA, Buettner PG. Multiple nonmelanoma skin cancer in an exposed Australian population. Int J Dermatol. 2002;41:652–8. doi: 10.1046/j.1365-4362.2002.01573.x. [DOI] [PubMed] [Google Scholar]
- 11.Mantese OAS, Gomides ADM, Berbert VCLA, Rocha A. Basal cell carcinoma –analysis of 300 cases observed in Uberlândia - MG, Brazil. An Bras Dermatol. 2006;81:136–42. [Google Scholar]
- 12.Stang A, Ziegler S, Büchner U, Ziegler B, Jöckel KH, Ziegler V. Malignant melanoma and nonmelanoma skin cancers in Northrhine-Westphalia, Germany:a patient- vs. diagnosis-based incidence approach. Int J Dermatol. 2007;46:564–70. doi: 10.1111/j.1365-4632.2006.03056.x. [DOI] [PubMed] [Google Scholar]
- 13.Flohil SC, Koljenović S, de Haas ER, Overbeek LI, de Vries E, Nijsten T. Cumulative risks and rates of subsequent basal cell carcinomas in the Netherlands. Br J Dermatol. 2011;165:874–81. doi: 10.1111/j.1365-2133.2011.10488.x. [DOI] [PubMed] [Google Scholar]
- 14.Souza CF, Thomé EP, Menegotto PF, Schmitt JV, Shibue JR, Tarlé RG. Topography of basal cell carcinoma and their correlations with gender, age and histologic pattern:a retrospective study of 1042 lesions. [Article in English, Portuguese] An Bras Dermatol. 2011;86:272–7. doi: 10.1590/s0365-05962011000200010. [DOI] [PubMed] [Google Scholar]
- 15.Bartos V, Kullová M. Basal Cell Carcinoma Multiplicity - a Retrospective Analysis of 899 Biopsy-proven Patients from a Single Institute. Klin Onkol. 2017;30:197–201. doi: 10.14735/amko2017197. [DOI] [PubMed] [Google Scholar]
- 16.Göppner D, Leverkus M. Basal cell carcinoma:from the molecular understanding of the pathogenesis to targeted therapy of progressive disease. J Skin Cancer. 2011:650258. doi: 10.1155/2011/650258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehgal VN, Chatterjee K, Pandhi D, Khurana A. Basal cell carcinoma:pathophysiology. Skinmed. 2014;12:176–81. [PubMed] [Google Scholar]
- 18.Khalesi M, Whiteman DC, Rosendahl C, Johns R, Hackett T, Cameron A, et al. Basal cell carcinomas on sun-protected vs. sun-exposed body sites:a comparison of phenotypic and environmental risk factors. Photodermatol Photoimmunol Photomed. 2015;31:202–11. doi: 10.1111/phpp.12170. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy C, Bajdik CD, Willemze R, De Gruijl FR, Bouwes Bavinck JN Leiden Skin Cancer Study. The influence of painful sunburns and lifetime sun exposure on the risk of actinic keratoses, seborrheic warts, melanocytic nevi, atypical nevi, and skin cancer. J Invest Dermatol. 2003;120:1087–93. doi: 10.1046/j.1523-1747.2003.12246.x. [DOI] [PubMed] [Google Scholar]
- 20.Pelucchi C, Di Landro A, Naldi L, La Vecchia C Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology (GISED) Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an italian case-control study. J Invest Dermatol. 2007;127:935–44. doi: 10.1038/sj.jid.5700598. [DOI] [PubMed] [Google Scholar]
- 21.Wang LE, Li C, Strom SS, Goldberg LH, Brewster A, Guo Z, et al. Repair capacity for UV light induced DNA damage associated with risk of nonmelanoma skin cancer and tumor progression. Clin Cancer Res. 2007;13:6532–9. doi: 10.1158/1078-0432.CCR-07-0969. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran S, Lear JT, Ramsay H, Smith AG, Bowers B, Hutchinson PE, et al. Presentation with multiple cutaneous basal cell carcinomas:association of glutathione S-transferase and cytochrome P450 genotypes with clinical phenotype. Cancer Epidemiol Biomarkers Prev. 1999;8:61–7. [PubMed] [Google Scholar]
- 23.Lear JT, Heagerty AH, Smith A, Bowers B, Payne CR, Smith CA, et al. Multiple cutaneous basal cell carcinomas:glutathione S-transferase (GSTM1, GSTT1) and cytochrome P450 (CYP2D6, CYP1A1) polymorphisms influence tumour numbers and accrual. Carcinogenesis. 1996;17:1891–6. doi: 10.1093/carcin/17.9.1891. [DOI] [PubMed] [Google Scholar]
- 24.Clairmont A, Sies H, Ramachandran S, Lear JT, Smith AG, Bowers B, et al. Association of NAD(P)H:quinone oxidoreductase (NQO1) null with numbers of basal cell carcinomas:use of a multivariate model to rank the relative importance of this polymorphism and those at other relevant loci. Carcinogenesis. 1999;20:1235–40. doi: 10.1093/carcin/20.7.1235. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran S, Fryer AA, Lovatt TJ, Smith AG, Lear JT, Jones PW, et al. Combined effects of gender, skin type and polymorphic genes on clinical phenotype:use of rate of increase in numbers of basal cell carcinomas as a model system. Cancer Lett. 2003;189:175–81. doi: 10.1016/s0304-3835(02)00516-5. [DOI] [PubMed] [Google Scholar]
- 26.Strange RC, El-Genidy N, Ramachandran S, Lovatt TJ, Fryer AA, Smith AG, et al. PTCH polymorphism is associated with the rate of increase in basal cell carcinoma numbers during follow-up:preliminary data on the influence of an exon 12-exon 23 haplotype. Environ Mol Mutagen. 2004;44:469–76. doi: 10.1002/em.20068. [DOI] [PubMed] [Google Scholar]
- 27.Czarnecki D, Nicholson I, Tait B, Nash C. HLA DR4 is associated with the development of multiple basal cell carcinomas and malignant melanoma. Dermatology. 1993;187:16–8. doi: 10.1159/000247190. [DOI] [PubMed] [Google Scholar]
- 28.Czarnecki D, Lewis A, Nicholson I, Tait B. Multiple basal cell carcinomas and HLA frequencies in southern Australia. J Am Acad Dermatol. 1991;24:559–61. doi: 10.1016/0190-9622(91)70082-d. [DOI] [PubMed] [Google Scholar]
- 29.Heitzer E, Quehenberger F, Wolf P. Polyclonality of multiple sporadic basal cell carcinomas. J Invest Dermatol. 2009;129:1586–9. doi: 10.1038/jid.2008.411. [DOI] [PubMed] [Google Scholar]
- 30.Heitzer E, Wolf P. Nonmonoclonal PTCH gene mutations in psoralen plus UVA-associated basal cell carcinomas. J Invest Dermatol. 2008;128:746–9. doi: 10.1038/sj.jid.5701128. [DOI] [PubMed] [Google Scholar]
- 31.Shulman O, Laitman Y, Vilan A, Leviav A, Friedman E. Monoclonal origin of anatomically distinct basal cell carcinomas. J Invest Dermatol. 2006;126:676–9. doi: 10.1038/sj.jid.5700130. [DOI] [PubMed] [Google Scholar]
- 32.Lear JT, Tan BB, Smith AG, Bowers W, Jones PW, Heagerty AH, et al. Risk factors for basal cell carcinoma in the UK:case-control study in 806 patients. J R Soc Med. 1997;90:371–4. doi: 10.1177/014107689709000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovatt TJ, Lear JT, Bastrilles J, Wong C, Griffiths CE, Samarasinghe V, et al. Associations between ultraviolet radiation, basal cell carcinoma site and histology, host characteristics, and rate of development of further tumors. J Am Acad Dermatol. 2005;52:468–73. doi: 10.1016/j.jaad.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 34.Karagas MR, Greenberg ER. Unresolved issues in the epidemiology of basal cell and squamous cell skin cancer. In: Mukhtar H, editor. Skin Cancer:Mechanisms and Human Relevance. Boca Raton: CRC Press; 1995. pp. 79–86. [Google Scholar]
- 35.Karagas MR, Stukel TA, Greenberg ER, Baron JA, Mott LA, Stern RS. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin Cancer Prevention Study Group. JAMA. 1992;267:3305–10. [PubMed] [Google Scholar]
- 36.Flohil SC, van der Leest RJ, Arends LR, de Vries E, Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma:a systematic review and meta-analysis. Eur J Cancer. 2013;49:2365–75. doi: 10.1016/j.ejca.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Hoey SE, Devereux CE, Murray L, Catney D, Gavin A, Kumar S, et al. Skin cancer trends in Northern Ireland and consequences for provision of dermatologyservices. Br J Dermatol. 2007;156:1301–7. doi: 10.1111/j.1365-2133.2007.07936.x. [DOI] [PubMed] [Google Scholar]


