Abstract
The only curative treatment for primary hyperparathyroidism (pHPT) is surgery. The most important factors that increase the success rate of a parathyroidectomy are the establishment of the correct diagnosis and the surgeon’s good knowledge of anatomy and embryology. The lower parathyroid glands develop from the dorsal portion of the third pharyngeal pouch, and the upper parathyroid glands from the fourth pharyngeal pouch. Humans typically have 4 parathyroid glands; however, more than 4 and fewer than 4 have been observed. Typically, the upper parathyroid glands are located in the cricothyroid junction area on the posterolateral portion of the middle and upper third of the thyroid, while the lower parathyroids are located in an area 1 cm in diameter located posterior, lateral, or anterolateral to the lower thyroid pole. Ectopic locations of parathyroid glands outside the normal anatomical regions due to the abnormal migration during embryological development or acquired ectopy due to migration of enlarged parathyroids are not uncommon. There are various surgical techniques to treat HPT; however, 2 main surgical options are used: bilateral neck exploration (BNE) and minimally invasive parathyroidectomy (MIP). While there are open, endoscopic, and video-assisted MIP (MIVAP) approaches, most often an open lateral MIP technique is used. In addition, endoscopic or robotic parathyroidectomy methods performed from remote regions outside the neck have been reported. Although currently MIP is the standard treatment option in selected patients with positive imaging, BNE remains the gold standard procedure in parathyroid surgery. In 80% to 90% of patients with pHPT, a pathological parathyroid gland can be detected with preoperative imaging methods and MIP can be applied. However, the pathological gland may not be found during a MIP procedure as a result of false positive results. The parathyroid surgeon must also know the BNE technique and be able to switch to BNE and change the surgical strategy if necessary. If the intended gland is not found in its normal anatomical site, possible embryological and acquired ectopic locations should be investigated. It should be kept in mind that MIP and BNE are not alternatives to each other, but rather complementary techniques for successful treatment in parathyroid surgery.
Keywords: Bilateral neck exploration, minimal invasive parathyroidectomy, primary hyperparathyroidism, surgical techniques
The most important variables that contribute to greater success rates in parathyroidectomy operations planned for primary hyperparathyroidism (pHPT) and other parathyroid diseases, are the establishment of an accurate diagnosis based on a multidisciplinary approach and the application of the appropriate surgical intervention. Therefore, it is important that the surgeon be very knowledgeable of the embryology and anatomy of the parathyroid glands for a successful parathyroidectomy.[1]
Embryology, Anatomy, and Morphology of the Parathyroid Gland
Embryology
In the sixth gestational week, the lower parathyroid develops from the dorsal portion of the third pharyngeal pouch, and the primitive thymus from the ventral part. The upper parathyroid arises from the dorsal portion of the fourth pharyngeal pouch, and the ultimobranchial body (lateral thyroid) cells coming from the neural crista develop from the ventral part.
The lower parathyroid and the thymus separate from the pharyngeal wall and migrate in a medial and caudal direction in front of the fourth pouch. The inferior parathyroid glands become localized near the lower poles of the thyroid, and the thymus descends to the lower neck region and the mediastinum (Fig. 1).
Figure 1.

Embryological development of the parathyroid glands.
FC: Foramen cecum; P1: Pharyngeal pouch; P2: Pharyngeal pouch; P3: Pharyngeal pouch; P4: Pharyngeal pouch; PT3: Lower parathyroid; PT4: Upper parathyroid; T: Thyroid.
The upper parathyroid and lateral thyroid leave the pharynx and migrate inferiorly. The lateral thyroid merges with the lateral lobes of the median thyroid descending from above and differentiates into parafollicular thyroid cells (C cells), which secrete calcitonin. The upper parathyroid glands settle posteromedial to the thyroid capsule around the cricothyroid joint (Fig. 1).[2]
Anatomy
Size, Weight, and Color
The average dimensions of a normal parathyroid gland are 5x3x1 mm with a weight of 35 to 40 mg (10-78 mg).[3] In a cadaver series, the average weight of the parathyroid gland was determined to be greater in the Black race, in individuals with a greater stromal fat ratio, and in males.[4–6] The weight of the parathyroid may increase until the age of 30 years in men and 50 years in women. The parathyroid glands are generally spherical, oval, or bean-shaped.[6] Although it is accepted that there is no relationship between the shape and function of the parathyroid, hyperactive parathyroid glands are often reported to be spherical in shape.[7] The color of the parathyroid is often light yellow or mustard yellow. The color varies with the age of the individual, fat content, and degree of vascularization. It is grayish in color in the newborn, and light pink in a child. In cases of an excess of fatty tissue, it may be light brown, while a light red or brown color may be seen with an excessive cellular development or vascularization.[7, 8] Parathyroid glands may also be embedded in fat lobules.
Quantity
Humans typically have 4 parathyroid glands; however, varied counts have been reported. In a large autopsy series of individuals without HPT, 4 parathyroid glands were found in 80% to 93%, while more than 4 were observed in 3.7% to 13% (supernumerary glands), and fewer than 4 in 4% to 13%.[9–12] The total weight of in cases of fewer than 4 parathyroid glands was found to be less than the general average. However, it should be kept in mind that an existing parathyroid may be overlooked.
A parathyroid gland in addition to the normal 4 that weighs at least 5 mg is considered a supernumerary gland. Extra parathyroid glands weighing less than 5 mg are considered rudimentary or accessory parathyroid glands.
Another feature of accessory parathyroids is that they are usually located next to the main parathyroid. Although Akerstörm et al.[9] reported findings of more than 4 glands in 13% of cases, true supernumerary parathyroid glands weighing more than 5 mg are found in a different location in only some 5% of cases. Accessory parathyroid glands may grow and become pathological, particularly in cases of continuous stimulation, such as multiple endocrine neoplasia, type 1 (MEN 1) syndrome, or secondary HPT.[13]
In a meta-analysis, supernumerary parathyroidism was found in 4.9% in a cadaveric series and in 6.3% of patients with HPT.[14] Supernumerary parathyroid glands are often found in the thymus.
Other ectopic locations mainly detected intraoperatively during HPT surgery include the piriform sinus, vagus nerve, carotid sheath, posterior cervical triangle, aortopulmonary window (anterior pulmonary artery, posterior aortic arch and tracheal carina), pericardium, and the diaphragmatic dome.[15–24]
Location
The anatomical location of the parathyroid glands in adults varies depending on embryological migration and the migration of enlarged parathyroid glands from their normal site (Fig. 2a, b).
Figure 2.

Anatomical locations of (a) upper parathyroid and (b) lower parathyroid glands according to embryological migration. (The percentages indicated were reported in large anatomical series; the rates of any single study may vary.)
CA: Common carotid artery; E: Esophagus; ITA: Inferior thyroid artery; RLN: Recurrent laryngeal nerve; Tr: Trachea.
The term congenital ectopy is used for parathyroid glands in an abnormal anatomical location due to abnormal migration during embryological development, whereas acquired ectopy refers to the migration of pathologically enlarged parathyroid glands. Acquired ectopy is most often seen in the upper parathyroids and congenital ectopy in the lower parathyroids.
Subcapsular parathyroid describes a parathyroid gland located under the surgical capsule of the thyroid, which can be seen in some 15% of cases.[25, 26] In a meta-analysis that examined the anatomy of the parathyroid in cadaveric series and in patients with HPT and provides general information about the location of the parathyroids, a total of 26 studies were evaluated and it was found that parathyroid glands were orthotopic in 94.3% of cadavers and 82.5% of HPT patients studied. Ectopic glands were determined in a total of 15.9%: 11.6% in the cervical region and 4.3% in the mediastinum.[14] The results indicated that 31.4% of ectopic parathyroid glands in the neck were localized in the retroesophageal/paraesophageal space, 20.3% in the thyroid gland, 17.7% in the carotid sheath, 17% in the thyrothymic ligament, 5.1% in the tracheolesophageal groove, and 8.4% in other locations (thyroid cartilage and in the vicinity of the hyoid bone, retropharyngeal space). The majority of mediastinal parathyroid glands were found in the thymus.[14]
Intrathyroidal parathyroids are ectopic parathyroid glands that are completely surrounded by thyroid tissue. Subcapsular parathyroid glands remaining between the enlarging nodules in a nodular goiter may be mistaken for intrathyroid parathyroid. In autopsy studies, the incidence of intrathyroid parathyroidism in thyroidectomy and parathyroidectomy series has been reported to range between 0.2% and 3.2%.[9, 27–30] Although the upper parathyroid glands descend with the ultimobranchial body and can be predicted to settle near the thyroid, no similar mechanism has been proposed as yet for the lower parathyroids.[3] However, the lower parathyroid glands are more often observed in an intrathyroidal location than the upper parathyroid glands.
In general, the upper parathyroid glands are located symmetrically in 80% of cases and the lower parathyroids in 70%.[9]
Normal location of upper parathyroids The typical location of the superior parathyroid glands is the junction of the cricothyroid and the posterolateral aspect of the middle and upper third of the thyroid.[3, 9] (Fig. 2a). They are most commonly found 1 cm above the intersection of the inferior thyroid artery (ITA) and the recurrent laryngeal nerve (RLN), and 80% of the upper parathyroids are located in an area within 2 cm of this central position.[3, 9, 31] This area can also be defined according to the Zuckerkandl tubercle; the upper parathyroids are located in a 1 cm diameter area around the point where the Zuckerkandl tubercle and the RLN meet. The Zuckerkandl tubercle can often be used as a guide in the search for upper parathyroids since they are mostly located in the cranial portion of this tubercle and beneath the superficial vascular layer covering the RLN. In surgical procedures, most of these parathyroid glands are observed as freely moving structures on the thyroid capsule.[1] (Fig. 2a).
Congenital ectopic location of upper parathyroids Some 13% of upper parathyroid glands are located behind the upper thyroid pole, lateral to the cricoid cartilage and the pharynx, or in the intercricothyroid area between the thyroid and the cricoid cartilage, and in 1% to 4% they may be found in the esophagus, pharynx, or behind the larynx.[13, 32] (Fig. 2a). The upper parathyroids may occasionally (1-2%) be located in the cranial part of the upper pole or a higher location (0.8%). The upper parathyroids are located caudal to the ITA in around 4% of cases, and can be mistaken for a lower parathyroid gland[9] (Fig. 2a). It is also very rarely found in the lateral neck region (lateral to the carotid sheath).[19]
Acquired ectopic location of upper parathyroids Enlarged parathyroid glands may be displaced due to regional dynamics such as recurrent muscle contractions during swallowing, negative intrathoracic pressure, cervicomediastinal fascial planes, and gravity. Enlarged upper parathyroid glands, especially adenomas, may be found in the epithelial layer over the prevertebral fascia and may descend through the paraesophageal and retroesophageal pathway to the inferior part of the neck and even the posterior mediastinum in as many as 40% of cases. These glands are located posterior to the ITA and posterolateral to the RLN (Fig. 3b).[13]
Figure 3.

Acquired ectopic locations of (a) lower parathyroid and (b) upper parathyroid glands as a result of migration of enlarged glands.
CA: Common carotid artery; C&M: Clavicle and sternum; ITA: Inferior thyroid artery; L: Lower parathyroid; MA: Anterior mediastinum; MP: Posterior mediastinum; SA: Subclavian artery; U: Upper parathyroid.
Normal location of the lower parathyroids: Approximately 60% to 70% of the lower parathyroids are located in the 1-cm diameter area posterior, lateral, or anterolateral to the lower pole of the thyroid. In about 26% of the cases it is located in the thyrothymic ligament and in nearly 6% of cases in the more posterior part of the middle third of the thyroid (Fig. 2b).[9]
Congenital ectopic location of lower parathyroids As a result of incomplete migration, the lower parathyroids may have a more cranial or a more caudal location. Outside the normal settlement areas, they can be found anywhere between the pericardium and the angle of the mandible. Insufficient migration leads to a location in the cranial aspect of the ITA in approximately 3% of cases and they can be mistaken for upper parathyroid glands (Fig. 2b). High ectopia of the lower parathyroids occurs in approximately 1% of cases. These parathyroid glands are frequently located 2 to 3 cm lateral to the upper thyroid pole and at the level of the carotid bifurcation.
They may also be located more cranially in the carotid bifurcation near the angle of the mandible or near the hyoid bone.[13] In some 2% of cases they may be located in the upper and middle parts of the cervical thymus due to separation failure from the thymus during the process of migration.[9] They have also been located in the anterior mediastinum inferior to the thymus in 3% of cases. Rarely, they can also be found more inferiorly on the pericardium.[13, 23] If remnants of the thymus are found around a parathyroid, this gland should be considered a lower parathyroid gland.[9]
Acquired ectopic location of lower parathyroids Enlarged lower parathyroid glands may migrate through the thyrothymic ligament and thymus to the anterior mediastinum, and in some cases, to the posterior mediastinum (Fig. 3a).
Relationship between Parathyroids and the Recurrent Laryngeal Nerve This relationship may provide important clues about the location of the parathyroids when the RLN is observed before the glands. The expected relationship of both the upper and lower parathyroids with the RLN can be defined within a rectangular prism area. This prism can be imagined when the thyroid lobe is turned medially and pulled in a cranial direction.
The posterior surface of the prism is formed by the coronal plane tangential to the anterior surface of the esophagus, and the anterior surface is formed by the coronal plane tangential to the anterior face of the trachea. The upper surface of the prism is formed by the transverse plane passing through the apex of the thyroid lobe, and the lower surface by the transverse plane passing 4 cm below the lowest point of the thyroid lobe. The outer surface of the prism is the vertical plane passing through the edge of the carotid artery and the medial surface is the vertical plane passing through the anterolateral section of the trachea.
This rectangular prism is generally divided into 2 triangular prisms from anterior to posterior with an oblique plane that extends from lateral to medial corresponding to the RLN. Accordingly, the upper parathyroids are located in the superoposterior and the lower parathyroids are located in the anteroposterior prism (Fig. 4). Another description uses the assumption of the RLN coursing through a coronal plane: the upper parathyroids remain deep in the dorsal plane and the lower parathyroids remain in a superficial ventral position. The course of the RLN is a reliable guide in the location and identification of parathyroid glands during surgery.
Figure 4.

Locations of parathyroid glands according to the recurrent laryngeal nerve.
CA: Common carotid artery; CPM: Cricopharyngeal muscle; CTM: Cricothyroid muscle; E: Esophagus; ICFM: Inferior constrictor pharyngeal muscle; ICPM: Inferior pharyngeal constrictor muscle; ITA Inferior thyroid artery; L: Lower parathyroid gland; L-RLN: Left recurrent laryngeal nerve; R-RLN: Recurrent laryngeal nerve; T: Thyroid; TC: Thyroid cartilage; Tr: Trachea; U: Upper parathyroid gland. *Posterolateral part of the thyroid has been removed to demonstrate the course of the nerve.
Parathyroid Vessels
One or more branches of the main thyroid arteries may supply the parathyroid glands. In a few instances, the arteria thyroidea ima and arterial branches from the esophagus, trachea, and mediastinum may also supply parathyroids. Some studies have found that most parathyroid glands are fed by the ITA and at a lesser extent by the superior thyroid artery (STA); however, other studies contain different data. Delattre et al.[34] reported that 77.1% of the upper parathyroid glands and 90.3% of the lower parathyroid glands were perfused by the ITA, while 15.3% of the upper parathyroids were fed from anastomoses between branches of the STA and the ITA. In some 10% of cases, the lower parathyroids were supplied by a branch coming from the STA or anastomoses between the STA and the ITA.
In a study using a contrast agent injected into the ipsilateral STA, the upper parathyroids were identified in 98% of the cases, and lower and upper parathyroid glands could be visualized on the injected side in 50%. In this study, 9 (45%) of the 20 cases examined in detail demonstrated that the artery coming from the anastomosis between the STA and the ITA supplied the upper parathyroid and it was concluded that the upper parathyroid gland is usually perfused by the STA.[35] In a study using laser Doppler flowmetry, it was determined that the blood flow to the parathyroid glands decreased by one-third when the inferior or superior thyroid arteries were occluded separately.[36]
Hypoparathyroidism does not develop in a significant proportion of thyroidectomies in which the ITA is ligated proximally. Therefore, it can be said that the upper parathyroids are fed mainly by the STA, and mostly from the posterior branch of this artery, and most of the lower parathyroids are fed by the ITA. Especially in cases where there is no ITA on the right side, the lower parathyroids may be supplied by either the STA or the arteria thyroidea ima. Intrathymically localized lower parathyroid glands almost always have branches to the ITA. Some parathyroids located anterolaterally to the thyroid, on or under the thyroid capsule of the thyroid are supplied by capsular vessels of the thyroid.[1]
It has been reported that parathyroids received their blood supply from a single terminal branch in 80%, 2 branches in 15%, and 3 or more different branches in 5% of cases, and that a single terminal branch was separated into 3 or more smaller branches before entering the parathyroid in 35% of cases.[34] The length of parathyroid arteries ranges from 1 to 40 mm, but they are usually 8 to 12 mm in length. It is usually curved when long in length. When the artery is short, it pulls the parathyroid toward the originating vessel. In general, the pedicle of the upper parathyroid is shorter.[9]
Venous drainage of parathyroid glands is provided by the venous network of the thyroid capsule and/or the main veins of the thyroid. The parathyroid veins are important since they can easily be thrombosed during dissection, which may lead to disruption of the blood circulation of the parathyroid glands and the development of hypoparathyroidism.
Considering all of the data, it is important to note that the number, shape, location, and especially the arterial nutrition of both pathological and normal parathyroid glands vary from case to case and it is necessary to plan and perform thyroid or parathyroid surgery with great care in order to properly identify the parathyroid glands and protect them from possible trauma.
Basic Principles in Parathyroid Surgery
Currently, the only curative treatment for pHPT is surgery.[37] The most important factors in surgical success are the establishment of the correct diagnosis and the surgeon’s knowledge of anatomy and embryology. In addition, it is important for the parathyroid surgeon to distinguish a normal gland from a pathological gland (the hypercellular gland is usually darker, stiffer, and more vascular), the well-perfused gland from the gland with impaired perfusion, and benign characteristics from malignant pathology.[38]
Once a decision to pursue surgical treatment has been made, the surgical strategy should be determined with consideration for several factors. A preoperative localization study should be performed, any risk factors for multiple-gland disease should be investigated, and the facilities of the surgical center should be evaluated.[38] The 2 primary surgical options for the treatment of HPT are bilateral neck exploration (BNE) and minimally invasive parathyroidectomy (MIP).[39] The terminologies and definitions used for these options are discussed in a later section.
Although a significant proportion of patients with pHPT (80-85%) have a single adenoma, the possibility of multiple-gland disease should be considered both during the planning process for surgery and during the procedure. If a pathological gland cannot be identified preoperatively, if coexistent thyroid disease requiring surgery is present, or if there is a suspicion of multiple-gland disease or familial HPT, the preferred method of intervention is BNE. In addition, BNE should be the standard option in secondary and tertiary HPT.[38]
While MIP is now an accepted treatment option for imaging-positive patients, BNE remains the gold standard intervention technique in parathyroid surgery. MIP is only applicable to selected patients, while BNE is an option that can be applied to all patients. In addition, false positivity may lead to the inability to find the pathological gland during a MIP procedure. The surgeon must be familiar with the BNE technique as well, in order to switch to a BNE procedure based on intraoperative evaluation.
With the development of minimally invasive surgery, laparoscopic cholecystectomy became the standard surgical treatment for cholelithiasis; however, as a consequence, surgical residents had fewer opportunities to observe open cholecystectomy operations and gained limited experience in this procedure.[40] A pathological parathyroid gland can be detected with preoperative imaging methods in 80% to 90% of patients with pHPT and MIP is often applied.[41] The results of a survey noted that endocrinologists referred patients with imaging results to MIP before surgery and referred imaging-negative patients to surgery much later or elected to follow up these patients.[42] MIP is generally used in the treatment of pHPT, while BNE is performed less frequently.[43] This may contribute to young surgeons learning the MIP technique but not developing sufficient training in a BNE procedure. It should be kept in mind is that in parathyroid surgery, MIP and BNE are not alternatives to each other, but rather they are complementary techniques for successful treatment. Therefore, a surgeon performing parathyroid surgery should be proficient in both techniques.
The use of magnifying glasses (surgical loop) and good illumination during parathyroid surgery provide a more detailed observation of the surgical field. Performing dissection in a bloodless environment prevents staining of tissue with blood and reduces the likelihood that pathological parathyroids or darker brown-colored normal parathyroids will be overlooked. The role of palpation is limited, but in some cases it may provide an additional contribution. Since the symmetry rate is high in parathyroids, this mirror image should be used when exploring the contralateral side. In addition, as described in the anatomy section, identification of the RLN and the ITA and determination of the relationship between them may contribute to the preservation of the nerve and facilitate the distinction between the upper and lower parathyroid glands. However, the cricothyroid joint is a more reliable landmark in the identification of upper parathyroid.[13] In parathyroid surgery, dissection is performed in the opposite direction to that used in a thyroidectomy: Dissection starts from the medial aspect of the carotid artery, and extends in a lateral to a medial direction.
Another method that may help to reach the relevant parathyroid is to find the ITA, and if needed the STA, and pursue the course of the artery medially.[13] When a visible parathyroid is freed from the surrounding tissue, the direction of dissection should be from the distal end of the gland to the hilus. In other words, dissection should be performed in the inferomedial direction for the lower parathyroids and superomedial direction for the upper parathyroids. In this way, the vascular pedicle and nutrition of the gland can be better protected. Furthermore, since the parathyroid vessels are extremely thin and fragile, they can be easily damaged during dissection. Therefore, a fine and gentle dissection should be performed and every parathyroid gland should be carefully protected as if it were the last.[44] Nonetheless, despite these efforts, parathyroid nutrition may still deteriorate.
Although color change in a parathyroid glad indicates malnutrition, it is not a safe indicator of permanent dysfunction. However, it indicates that at least transient parathyroid dysfunction may occur in these patients.[45] A normal parathyroid capsule suspected of having arterial devascularization with venous congestion or a hematoma should be drained with a syringe needle or scalpel. Detection of oozing blood indicates that the arterial supply remains intact, and improvement in gland color indicates that congestion has been resolved.[44]
Recently, new optical technologies such as autofluorescence and laser contrast imaging have been used to evaluate the viability of parathyroid glands and promising results have been reported.[46] Normal parathyroids with intact nutrition should not be removed; autotransplantation (parathyroid implantation) should be performed if a gland that is determined to be malnourished is removed.[47] The function of a parathyroid gland that is permanently impaired is best restored with intraoperative autotransplantation; graft viability is more than 90% in these cases. Due to the possibility of recurrent HPT, it is generally accepted that the first choice for autotransplantation is the brachioradialis muscle of the non-dominant forearm and 1 to 2 cm inferior to the antecubital fossa. The production of excess parathyroid hormone from this gland can be easily detected with antecubital venous sampling. However, parathyroid implantation can also be performed using the sternocleidomastoid muscle (SCM) or in the fat tissue of the presternal region because the gland can maintain its function in adipose tissue.[44]
During dissection, examination should be conducted for an adenoma adhered to the normal parathyroid. Otherwise a preexisting adenoma may be overlooked. Generally, an experienced surgeon can visually recognize a parathyroid gland and easily distinguish pathological glands from normal glands. Therefore, a biopsy is often not required to determine if the tissue is a parathyroid gland. However, as with asymmetric hyperplasia, a small piece may be retrieved from the avascular distal end for histological determination of dissected tissue. If a biopsy is to be performed for a normal parathyroid gland and a discolored or dark brown parathyroid gland is found, then biopsy material should be obtained from this parathyroid instead of the normal parathyroid gland. Cautery should not be used on the parathyroid.[13] In patients scheduled for subtotal parathyroidectomy, all 4 parathyroid glands should be identified before resection.
When remnants are to be left behind, the selection depends on the pathological appearance and location of the glands. It is generally accepted that the smallest or least abnormal in appearance should be selected. However, since the lower parathyroids are usually anterior to the RLN, it may be more appropriate to select 1 of the lower parathyroids to the extent permitted by the pathology. Re-exploration for recurrent or persistent HPT has less risk of RLN injury and the gland is more easily accessible.[48] The part to be left behind should remain on the side of the pedicle. In other words, the distal half or third portion of the parathyroid (so as to leave approximately 50 mg of parathyroid behind) should be clipped transversely and the distal avascular side of the gland should be dissected with a scalpel just below the edge of the marker clip. Another method of is to leave a remnant 2 times the size of the normal parathyroid gland.[48] One of the important points to keep in mind is that the viability of the remnant should be ensured before removing other parathyroid glands.[44]
In patients with MEN 1, autotransplantation should be performed together with a subtotal parathyroidectomy or total parathyroidectomy, and the addition of a thymectomy to the procedure should be considered. An intrathymic gland is common in these patients. There is also a risk of the development of a thymic carcinoid in the long term.[48]
Terminology and Types of Parathyroid Surgery
BNE, which is the gold standard in parathyroid surgery, is well defined. Other more limited procedures can be grouped under the heading of MIP. Many of these approaches are similar or identical with various names, including unilateral neck exploration, and focused, selective, targeted, and scan-directed parathyroidectomy.[49] In general, minimally invasive surgery describes entering the surgical field with minimal trauma to perform the surgical procedure. There is also less inflammatory response to surgical stress.
In this context, in order to use the term “minimally invasive” for a parathyroidectomy, the intervention should include some specific features. Localization of the pathological parathyroid should be determined with preoperative imaging methods, and the operation should be completed with a unilateral procedure (open, endoscopic, or video-assisted) using a smaller incision and limited dissection. In recent years, endoscopic and robotic methods have been developed in order to prevent visible scarring on the neck featuring incisions made in the axilla, breast, chest wall, retroauricular region, or in the mouth. Therefore, these methods should not be defined as MIP, as they usually require dissection of a wider area to reach the surgical site. The use of the term “remote access approaches” has been recommended for such procedures. The 2 classifications of surgical methods used for a parathyroidectomy are described in Tables 1 and 2 (Figs. 5, 6, 7).
Table 1.
Types of surgery
| Types of surgery | Surgical approach | Method | |
|---|---|---|---|
| Open surgery | Midline cervical approach Lateral cervical approach |
Bilateral or unilateral exploration Unilateral exploration* |
|
| Endoscopic or Robotic Surgery | Cervical approach | Bilateral or unilateral | Total endoscopic Video-assisted |
| Remote access approaches | Bilateral or unilateral | Axillary approach Thoracic approach Breast approach Retroauricular approach Transoral vestibular approach |
|
Exploration of the contralateral side can be similarly performed when necessary.
Table 2.
Types of surgery
| Method of exploration | Types of surgery | Approach |
|---|---|---|
| Bilateral neck exploration | Open | Midline or *lateral cervical approach |
| Endoscopic or robotic surgery | Cervical or **remote access approach | |
| Minimally invasive *** | Open | Midline or *lateral cervical approach |
| Endoscopic | Cervical remote access approach Cervical total endoscopic |
|
| Remote access | Endoscopic or robotic | Axillary approach Thoracic approach Breast approach Retroauricular approach Transoral vestibular approach |
Separate lateral incisions or extending the incision to the contralateral side;
Axillary, breast, retroauricular and transoral;
Unilateral neck exploration, focused, selective, targeted scan-directed parathyroidectomy (Fig. 5, 6, and 7).
Figure 5.

Incision sites for a parathyroidectomy with a neck approach.
1 (green): Standard bilateral neck exploration; 2 (red): Open minimal invasive parathyroidectomy via lateral approach; 3 (turquoise): Minimal invasive video- assisted parathyroidectomy; 4 (brown): Total endoscopic parathyroidectomy port site incisions.
Figure 6.

Incision sites for remote access parathyroidectomy methods.
Suprasternal notch 1 (light green): Anterior chest approach with carbon dioxide insufflation; 2 (turquoise): Video-assisted cervicothoracic approach; 3 (blue): Axillary approach with carbon dioxide insufflation; 4 (orange): Gasless axillary approach using an anterior chest port; 5 (pink): A single-incision transaxillary approach with carbon dioxide insufflation; 6 (red): Gasless unilateral axillary approach; 7 (purple): Gasless unilateral axillary-breast approach; 8 (medium green): Breast approach with carbon dioxide insufflation; 9 (gray): Bilateral axillary-breast approach with carbon dioxide insufflation; 10 (brown): Unilateral axillary-breast approach with carbon dioxide insufflation; 11 (dark green): Bilateral axillary-breast approach with carbon dioxide insufflation. SCM: Sternocleidomastoid muscle.
Figure 7.
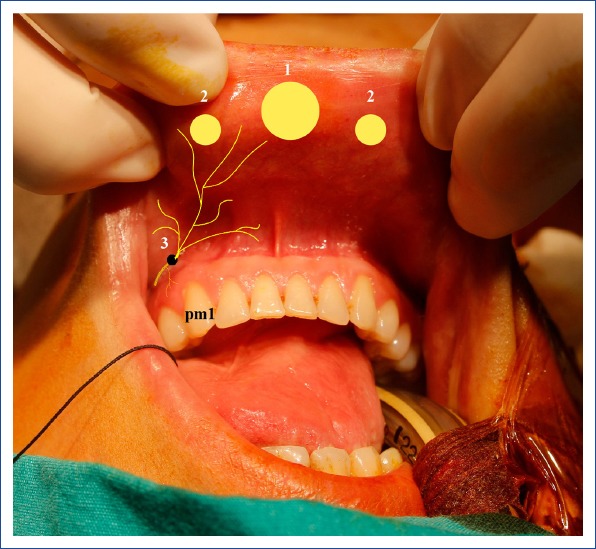
Port site incisions for a transoral endoscopic parathyroidectomy vestibular approach.
1: A 10-mm midvestibular camera port incision site; 2: A 5-mm working port incision site on the lateral side; 3: Mental foramen, source of the mental nerve; pm1: First premolar tooth.
Bilateral Neck Exploration
Generally, this term describes dissection on both sides of the neck with an open, endoscopic or video-assisted intervention, but it is used in practice only for open bilateral exploration (Fig. 5). All of the parathyroid glands are exposed and the parathyroids or macroscopically enlarged parathyroids are removed (Fig. 8). [49, 52]
Figure 8.

Bilateral neck exploration.
CA: Common carotid artery; E: Esophagus; IJV: Internal jugular vein; SCM: Sternocleidomastoid muscle; Tr: Trachea; V: Vertebra. 1, 2: Superficial layers of the deep fascia (prethyroid fascia and pretracheal fascia); 3: Superficial leaf of the deep layer of the deep fascia; 4: Deep leaf of the deep layer of the deep fascia (alar and prevertebral fascia); 5: Carotid sheath (1+2+3).
Unilateral Neck Exploration
Generally, open, endoscopic, or video-assisted dissection is performed on only 1 side of the neck. This open method is performed through a standard cervical midline 2 to 3-cm incision (Figs. 5, 8), or through a lateral incision made between the anterior edge of the SCM muscle and the strap muscles and only 1 side of the neck is explored (Figs. 5, 9).[52, 53] This method allows visualization of both ipsilateral parathyroid glands. When the pathological gland is removed, the normal gland is left in place or biopsied, if necessary.[49] In unilateral exploration, the cervical incision used is the same as that used in BNE, but since more limited dissection is performed, this procedure may be also considered MIP.
Figure 9.

Lateral minimal invasive parathyroidectomy using open surgery.
E: Esophagus; SCM: Sternocleidomastoid muscle; Tr: Trachea; V: Vertebra. 1, 2: Superficial layers of the deep fascia (prethyroid fascia and pretracheal fascia); 3: Superficial leaf of the deep layer of the deep fascia; 4: Deep leaf of the deep layer of the deep fascia (alar and prevertebral fascia); 5: Carotid sheath (1+2+3).
Minimally Invasive Parathyroidectomy
MIP is usually performed using open, endoscopic, or video-assisted (MIVAP) approaches.[49] In a review conducted in 2015, it was found that MIP was most commonly performed as open MIP (approximately 70%), and MIVAP and total endoscopic parathyroidectomy rates were 20% and 10%, respectively.[54] As mentioned earlier, if parathyroidectomy with unilateral neck exploration is left aside, the term MIP is commonly used for focused, selective, or targeted parathyroidectomies.[49] In fact, these methods differ from unilateral exploration only in that they are directed towards diseased parathyroid glands and no effort is made to detect unilateral normal parathyroid glands.
Open MIP: This technique is used in unilateral exploration. It can be applied using a lateral (Figs. 5, 9) or midline 2- to 3-cm incision. In cases where a lateral incision is used, if necessary, a similar incision is made on the contralateral side, and in cases where a midline incision is used, it may be extended to both sides and then a standard BNE can be performed. This procedure can be performed under general anesthesia or under sedation and a cervical block.[51] Successful results of up to 98% have been reported using open MIP.[55]
Gamma probe-guided (radio-guided or scan-directed) parathyroidectomy: This method is based on the identification and removal of an adenoma or hyperplastic glands with maximum radioactivity uptake determined by counting radioactive particles in both thyroid lobes with an intraoperative gamma probe. Technetium Tc99m sestamibi is administered intravenously 1 to 2 hours before surgery. A selective parathyroidectomy is more commonly performed with this technique, especially in preoperative scintigraphy-positive patients.[56] However, gamma probe-guided BNE can be performed in patients with negative preoperative scintigraphy results.[56, 57] Although it is a very effective method, it is currently used as a standard procedure only in some centers.[56, 57]
Video-assisted parathyroidectomy: This method was first reported by Miccoli et al.[38] It is an endoscopic procedure performed over the suprasternal notch with a 1.5 to 2-cm skin incision in the midline without gas insufflation (Fig. 5). The working area is exposed with small conventional retractors. The pathological gland is removed through the same incision without placing a port using a 5-mm, 30o camera and small surgical instruments. Bilateral exploration can also be performed with this technique without an additional incision. General anesthesia or a cervical block may be used.[58]
A success rate of more than 98% has been reported in experienced centers.[59, 60] Since appropriate patient selection and surgical experience are important factors affecting the switch to open surgery, rates of conversion to open surgery vary widely (0.9-53%).[61] This method, which requires special endoscopic instruments, is easier to learn than the total endoscopic method.
Total endoscopic parathyroidectomy: Total endoscopic parathyroidectomy through cervical approach was first described by Gagner[62] in 1996. Subtotal parathyroidectomy was performed by inserting 4 trocars each 5 mm in diameter into the neck under 15 mmHg CO2 of insufflation pressure. However, this first patient developed extensive subcutaneous emphysema and hypercarbia. Later, Henry et al.[63] published the first series describing total endoscopic selective parathyroidectomy using a lateral method in which the parathyroidectomy is performed using 8 mmHg CO2 pressure and inserting one 10- to 12-mm trocar 3 to 4 cm cranial to the sternal notch through the anterior edge of the SCM and inserting two 2- to 3-mm trocars superoinferior to the sternal notch (Fig. 5).[63]
In later large-scale studies, the same group used a totally endoscopic lateral method (Henry technique) with ports placed at the anterior edge of the SCM for posteriorly located parathyroid glands. For anteriorly located adenomas, they used a gas-free technique with a 5-mm camera and surgical instruments placed through a 15-mm incision in the midline, and suprasternal region (Fig. 5).[64] The endoscopic parathyroidectomy technique has also been performed with a camera port placed in the suprasternal notch and a working port through the anterior edges of the SCM.[65, 66] It has been reported that the lateral approach can be applied robotically.[54] Importantly, conversion to an open technique has been reported in 13% to 28% of cases.[64, 67] Total endoscopic parathyroidectomy techniques are performed in only a limited number of centers.
Remote access parathyroidectomy (Fig. 6): After remote endoscopic or robotic thyroidectomy outside the neck, this method has been applied successfully to perform a parathyroidectomy. As mentioned before, the endoscopic methods of this type are called remote access approaches since the entry points are outside the neck. These include axillary, thoracic, breast, retroauricular, and transoral approaches.[68–70] The reason to use a remote access method for parathyroid pathologies located in the neck is to avoid leaving any visible scar on the neck. However, due to extensive dissection in subcutaneous areas, fibrotic bands and long-term paresthesia may develop in the dissected areas. Therefore, it is generally accepted that remote access parathyroidectomies other than a thoracoscopic parathyroidectomy should not be included in the class of minimally invasive surgery. Pathological parathyroid glands have a mediastinal localization in approximately 20% of patients with pHPT. A significant portion of these glands can be removed from inside the neck, but a thoracic approach is required in 2% to 5% of all patients. It is generally more reasonable to perform a less invasive thoracoscopic (classical or robotic) parathyroidectomy instead of a highly invasive classical thoracotomy.[70, 71]
Potential complications and the need for adequate experience with endoscopic surgery in this region are the main challenges with these methods.[54, 61] The retroauricular method developed in the United States, and other remote access methods mostly from the Far East have not been widely used yet. The transoral endoscopic technique with a vestibular approach may find a widespread use in the future. Therefore, this method will be discussed in more detail.
Transoral parathyroidectomy: A transoral parathyroidectomy has been included in the classification of Natural Orifice Transluminal Endoscopic Surgery (NOTES), and was first performed through the base of the mouth.[72] However, this pathway was not often used, and it was eventually abandoned due to severe tissue damage, a high complication rate, a high rate of conversion to open surgery, and limited technical mobility during surgery. In recent years. Anuwong[73] described a transoral endoscopic thyroidectomy (TOETVA) with a vestibular approach and reported that it is a safe method that can be performed with minimal complications.
A 10-mm camera port was used with a 2-cm incision made from the superior of the inferior frenulum from the midline, and two 5-mm working ports at the lateral junction between the canine and first premolar teeth were inserted in the oral vestibular area with 6 mmHg CO2 insufflation pressure. Subsequently, parathyroidectomy (TOEPVA) was performed with this technique, and a transoral vestibular robotic parathyroidectomy was introduced.[74–76] (Fig. 7). Although use of TOETVA is becoming more widespread in the world, the number of TOEPVA cases is still limited. While this method does not leave visible scars on the neck, it requires greater exposure of the subplatysmal area and more tissue dissection than a lateral open MIP. This appears to be a feature preventing widespread use.
No matter which approach is used in parathyroid surgery, the surgical principles to be applied after reaching the surgical field are the same. Therefore, in this section, open BNE and MIP techniques applying the most commonly used open lateral method will be explained in detail.
Bilateral Neck Exploration Technique
This technique is generally performed without preoperative imaging studies or additional auxiliary methods. However, at least 1 or 2 imaging methods are typically used today.[41, 46] Although general anesthesia is frequently used, a deep cervical block and sedation can also be applied.[77] If intraoperative nerve monitoring (IONM) is to be performed, additional muscle relaxant should not be used after the dose administered during anesthesia induction.[78]
After the patient is placed in the appropriate position, a transverse incision (Kocher incision) is made beginning 2 cm cranial to the sternal notch, and skin-subcutaneous flaps are prepared from the avascular subplatysmal plane. The length of incision varies according to the anatomical structure of the neck and the choice of the surgeon, but an incision longer than 4 to 6 cm is not usually required.[77] The thyroid isthmus is accessed by opening the midline fascia. If a suspect parathyroid gland has been observed with imaging methods, the surgeon begins from that side; otherwise, the procedure may be initiated from either side. The strap muscles are dissected from the thyroid lobe to reach the area between the thyroid and the carotid sheath (Fig. 8). It is important to note that superficial lower parathyroid glands may be localized within fat lobules, and in particular, may adhere to the posterior surface of the sternothyroid muscle. The sternothyroid muscle should be retracted laterally. Pulling the strap muscles laterally and medial to the thyroid facilitates visualization of the area between the thyroid and the carotid sheath and allows for dissection in the area between the cricoid cartilage cranially, and the thymus caudally (Fig. 8). In cases where IONM is used, at this stage, the vena thyroidea media is either cut or gently pulled out of the operative field without further dissection. Then the vagus nerve is stimulated through the carotid sheath or by opening the carotid sheath (V1 response). A vagus probe is used if continuous vagal stimulation is to be applied.[78] At this stage, exploration should be carried out according to certain principles. The first basic principle is taking some anatomical landmarks into consideration, namely, exploration of the areas where normal parathyroids are frequently found including subcapsular locations of normal parathyroid glands. The second basic principle is that dissection performed to search for parathyroid glands should extend from the medial edge of the carotid artery to the trachea, in contrast to the standard thyroidectomy. It should be noted that parathyroids may be lodged in fat lobules, especially in the central region. After retracting the strap muscles laterally and then anteriorly, intermittently stretching the carotid artery in the lateral direction and pulling the thyroid medially creates a fluctuation in the central region. The abnormal parathyroid may move within the fat lobule, resembling a buoy floating on the water. Intermittent pressure can also be applied to fatty tissue using a peanut sponge to create a similar effect.[13, 77] Although there have been reports that identification of the RLN is not necessary in parathyroid surgery,[77] exposure of the RLN makes a significant contribution to the preservation of the nerve and detection of parathyroid glands in relation to the ITA.
As discussed in detail in the anatomy section, many parathyroid glands are located close to the intersection of the RLN with RTA, and the course of the RLN also helps to differentiate between upper and lower parathyroid glands.[79]
The lower parathyroid glands are anteriorly located in the region of lower pole of the thyroid and easier to reach and to visualize. Therefore, determination of the lower parathyroids should be attempted first, because the thyroid gland may need to be rotated more medially to explore for the upper parathyroids. Approximately 50% of normally located lower parathyroid glands are situated caudal to the ITA, anteromedial to the RLN, and within an area of about 1cm in diameter, including the inferior, lateral, and posterior parts of the lower thyroid pole.
The upper parathyroid glands can be seen more easily by retracting the thyroid medially. Nearly 80% of upper parathyroids are located in an area of 1 to 2 cm in diameter, approximately 1 cm superior to the intersection point between the ITA and the RLN in the cricothyroid joint area near the entry point of the RLN to the larynx, and posterolateral to the RLN.[13] In addition, fatty tissue close to the points of entry of the inferior and superior branches of the thyroid artery into the thyroid may be helpful to localizing the parathyroids.[77] If exploration is performed without seeing the RLN, damage to the RLN can easily occur in this area where it enters the larynx.[13] IONM may make a significant contribution to the detection and tracing of the RLN.[79]
If the parathyroid glands cannot be found in the normal anatomical sites, possible embryological and acquired ectopic locations of the glands are investigated. If all 4 parathyroid glands are found to be normal, a fifth supernumerary gland should be considered as the cause of disease and potential locations should be explored.[13]
Exploration for Ectopic Parathyroid Glands
It is important to determine whether a parathyroid gland that cannot be detected in its normal anatomical site is an upper or lower parathyroid gland because the ectopic areas to be explored will differ. If the missing gland is anteromedial to the RLN, then it is an upper parathyroid, while if the gland is localized posterolateral to the RLN it is a lower parathyroid gland.
Exploration for ectopic localization of lower parathyroids: In order to identify ectopic parathyroids, the thyroid must be freed from all surrounding soft tissue connections by retracting it completely in the medial direction. This maneuver allows for visualization of the lower parathyroid glands, often hidden behind the thyroid. It also makes the lateral and medial aspects of the lower pole easily explorable. However, it should be remembered that excessive retraction may cause strain trauma to the RLN.[44] The thyrothymic area should then be examined and, if necessary, the upper part of the thymus should be removed using an incision in the neck. If the gland cannot be identified after these dissections, the presence of a parathyroid located more inferiorly should be suspected. In particular, it should be considered that a pathologically enlarged lower parathyroid gland may have migrated to the anterior mediastinum or may be located in the middle and lower thymus. A thymectomy can be performed by retracting the thymus slightly from the cervical region and bluntly dissecting the distal portion.[80] When dissecting the thymus, the veins leading into the innominate vein should be occluded with ligation or electrocautery. Enlarged parathyroids in the middle and upper thymus can be removed with a cervical thymectomy (Fig. 10).
Figure 10.

CC: Cricoid cartilage; E: Esophagus; EP: Epiglottis; ET: Endotracheal tube with electrodes; HB: Hyoid bone; IA: Innominate artery; IV: Innominate vein; M: Mandible; OP: Oropharynx; St: Sternum; T: Thyroid; TC: Thyroid cartilage; THY: Thymus; Tr: Trachea; V: Vertebra; VC: Vocal cord.
A lower parathyroid that cannot be found in the usual ectopic locations in the lower neck raises the possibility of a lower thyroid gland that did not complete its migration or a lower parathyroid gland located at the level of the carotid bifurcation or the hyoid bone. An undescended parathyroid gland, or parathymus, often has remnants of thymic tissue. It is usually located medial to the carotid sheath and if the surgeon does not know of this possibility, it will be overlooked.[81] Another area to be investigated is the carotid sheath. To explore the area, the carotid sheath should be opened from the hyoid bone to the level of the innominate artery on the right and to its projection on the shoulder on the left side.[13] Intravagal parathyroid growth may also occur within the carotid sheath. Although one case of an adenoma that developed from the fourth parathyroid has been reported,[82] intravagal adenomas usually develop from a supernumerary parathyroid.[23]
Though the incidence is low, an ectopic gland may also be located intrathyroidally. After excluding all other possible ectopic locations, intraoperative ultrasound can be performed for intrathyroid parathyroidism, or it can be diagnosed by either preoperatively or intraoperatively with a parathormone washout test. There are also reports suggesting use of a blind hemithyroidectomy if there is suspicion of intrathyroidal parathyroidism.[83, 84]
Other than cases of an ectopic parathyroid gland in the neck, a blind procedure is not generally recommended because it can lead to accidental removal of a normal subcapsular parathyroid or devascularization of normal parathyroids. In addition, intense adhesions that may occur during the postoperative period may create more challenging difficulties for secondary surgery.[13] However, success has been reported in the removal of an adenoma with a thyroidotomy rather than a lobectomy or thyroidectomy in patients with intrathyroidal pathological parathyroid (adenoma) previously detected using imaging modalities.[85]
In spite of all these approaches, simultaneous blind sternotomy is generally not recommended in patients who do not have an ectopic parathyroid gland in the neck. Instead, more detailed noninvasive imaging methods should be used in the postoperative period and selective venous catheterization should be considered in patients with negative, uncertain, or conflicting results. However, if the calcium level is more than 13 mg/dL and there is an experienced team, a sternotomy may be appropriate.[80] A total or partial sternotomy that can be extended to the right or left may be performed as necessary.
Exploration for ectopic localization of the upper parathyroids: A search for an ectopic upper parathyroid can be initiated with palpation of the tracheoesophageal groove and paraesophageal area and gentle dissection of this region. Since the upper parathyroids are more stable embryologically, migration of an enlarged gland to the paraesophageal area or the tracheoesophageal groove in accordance with the fascial planes of the neck is not an uncommon finding. In this area, migration may lead to localization behind the ITA .
Migration may also continue to the upper posterior mediastinum. If the gland cannot be detected in these areas, retroesophageal and retrotracheal areas should be examined by opening the corresponding layers of the deep neck fascia, as mentioned in the open MIP technique. It is more appropriate to start the investigation of the upper parts of the neck after exploration of the mentioned areas. The upper pole vessels of the thyroid will need to be cut and the upper pole liberated to examine the posterior aspect of the upper pole, the region of the upper pole vessels, and the parapharyngeal and retropharyngeal areas.[13, 44]
The upper parathyroid glands may be subcapsular, intrathyroidal, or intravagal within the carotid sheath, as is the case with the lower parathyroids. Since the details of these structures are similar to the lower parathyroids they will not be repeated here.
Open Minimal Invasive Parathyroidectomy Technique
Open MIP can be performed with a midline approach, although it is usually applied using a lateral approach, also known as a backdoor technique. A midline, open MIP is performed in a similar manner to unilateral neck exploration, but as mentioned earlier, the intervention is performed only for parathyroid glands with known pathology and terminologically it does not include ipsilateral normal parathyroid exploration. Open MIP with a midline approach may be more advantageous than a lateral MIP in cases of an ectopic localization of lower parathyroids anterior to the trachea or medial to the upper lobe.[38]
Lateral or midline open MIP can be performed under general anesthesia, cervical block, or local anesthesia.[86]
Open Minimally Invasive Parathyroidectomy Using Lateral Approach
If the intervention is performed for deep and posteriorly localized parathyroid pathology, this is a better option than the midline approach.[53] It is also a very suitable method for patients who have had a previous thyroidectomy or have recurrent or persistent pHPT.
The patient is placed in the normal thyroid surgery position, and the neck can be turned slightly to the opposite side.
Since the surgical field is narrow, a forehead lamp makes an additional contribution to illumination of this area. From the anterior edge of the SCM to the medial side, a 2 to 3-cm incision parallel to the skin folds is made to reach the subplatysmal area (Fig. 9).[87] It is useful to make the incision near or directly over an adenoma detected with imaging methods or where the most activity is measured in cases using gamma probes.[53] The risk of capsule rupture is greater in enlarged parathyroid adenoma, and care should be taken to remove the complete capsule.
Postoperative parathyromatosis may develop, especially in pathologies demonstrating cystic growth. Therefore, the extent and location of the incision in the MIP procedure should be adjusted according to the size of the parathyroid and should be of sufficient length to allow the parathyroid to be easily removed.
After the subplatysmal area is exposed using cautery or blunt dissection, the superficial layer of the deep neck fascia between the anterior edge of the SCM and the strap muscle is opened. The SCM is retracted laterally, the strap muscle medially, and the branches of the ansa cervicalis, the internal jugular vein, and the carotid artery are visible immediately medial to the vein.
If there is no difficulty in dissection, the branches of the ansa cervicalis should be preserved. If the vena jugularis media is seen in the dissection area, it is cut. If IONM is used, V1 response is obtained by opening the carotid sheath or stimulating the vagus nerve through the sheath. Retracting the carotid sheath laterally, and strap muscle and the thyroid anteromedially exposes the area between the carotid artery and the thyroid.[88, 89] After this stage, dissection should be directed toward the area determined with cross-sectional imaging methods.
If cross-sectional imaging is indicates the upper parathyroid, the first fascial structure to be opened is the prethyroid fascia, which is the middle leaf of the superficial layer of the deep neck fascia extending from the anterior surface of the carotid sheath. The next layer of the deep fascia (pretracheal fascia) should be opened so that a pathological gland located in the paraesophageal and retroesophageal regions can be easily seen and removed.[89] Identification of the targeted pathological upper parathyroid contributes to easier visualization and protection of the RLN adjacent to its medial aspect.[90] The upper thyroid pole may need to be stabilized in order to reach the upper parathyroid through a more cranial incision. In this case, cutting the omohyoid muscle may provide additional benefit.
If imaging indicates the presence of a possible lower parathyroid, the thyroid lobe is released and retracted in the mediosuperior direction for exploration. If the parathyroid gland cannot be seen at this stage, the RLN should be found first and a search conducted of the medial aspect of the RLN around the lower pole of the lobe.[89] If parathyroid glands are not found in this region, the thyrothymic ligament and its surroundings should be examined as described for bilateral exploration and cervical thymectomy should be performed if necessary.
Disclosures
Peer-review: Externally peer-reviewed.
Conflict of interest: None declared.
Authorship Contributions: Concept – M.U.; Design – M.U.; Supervision – M.U., A.İ.; Materials – M.U., N.A.; Data collection &/or processing – M.U., N.A.; Analysis and/or interpretation – M.U., A.İ.; Literature search – M.U., N.A.; Writing – M.U.; Critical review – M.U., A.İ.
References
- 1.İşgör A, Ulıudağ M. Tiroidin fonksiyonel ve cerrahi anatomisi. In: İşgör A, Uludağ M, editors. Tiroit. 1. baskı. İstanbul: Nobel Tıp Kitabevleri; 2013. pp. 775–800. [Google Scholar]
- 2.Tiroidin fonksiyonel ve cerrahi anatomisi. In: İşgör A, Ulıudağ M, editors; İşgör A, Uludağ M, editors. Tiroit. 1. baskı. İstanbul: Nobel Tıp Kitabevleri; 2013. pp. 13–22. [Google Scholar]
- 3.Wang C. The anatomic basis of parathyroid surgery. Ann Surg. 1976;183:271–5. doi: 10.1097/00000658-197603000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufour DR, Wilkerson SY. Factors related to parathyroid weight in normal persons. Arch Pathol Lab Med. 1983;107:167–72. [PubMed] [Google Scholar]
- 5.Ghandur-Mnaymneh L, Cassady J, Hajianpour MA, Paz J, Reiss E. The parathyroid gland in health and disease. Am J Pathol. 1986;125:292–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Obara T, Fujimoto Y, Aiba M. Stromal fat content of the parathyroid gland. Endocrinol Jpn. 1990;37:901–5. doi: 10.1507/endocrj1954.37.901. [DOI] [PubMed] [Google Scholar]
- 7.Al-Sobhi S, Clark OH. Parathroid hyperplasia;Parathroidectomy. In: Clark OH, Duh QY, editors. Textbook of Endocrine Surgery. 1st ed. Philadelphia: WB Saunders Company; 1997. pp. 372–79. [Google Scholar]
- 8.Bonjer HJ, Bruining HA. Technique of Parathyroidectomy. In: Clark OH, Duh QY, editors. Textbook of Endocrine Surgery. 1st ed. Philadelphia: WB Saunders; 1997. pp. 372–79. [Google Scholar]
- 9.Akerström G, Malmaeus J, Bergström R. Surgical anatomy of human parathyroid glands. Surgery. 1984;95:14–21. [PubMed] [Google Scholar]
- 10.Gilmour JR. Gross anatomy of the parathyroid glands. J Pathol Bacteriol. 1938;46:133–49. [Google Scholar]
- 11.Alveryd A. Parathyroid glands in thyroid surgery. I. Anatomy of parathyroid glands. II. Postoperative hypoparathyroidism-identification and autotransplantation of parathyroid glands. Acta Chir Scand. 1968;389:1–120. [PubMed] [Google Scholar]
- 12.Lappas D, Noussios G, Anagnostis P, Adamidou F, Chatzigeorgiou A, Skandalakis P. Location, number and morphology of parathyroid glands:results from a largeanatomical series. Anat Sci Int. 2012;87:160–4. doi: 10.1007/s12565-012-0142-1. [DOI] [PubMed] [Google Scholar]
- 13.Randolph GW, Grant CS, Kamani D. Principles in surgical management of primary hyperparathyroidism. In: Randolph GW, editor. Surgery of the Thyroid and Parathyroid Glands. 2nd ed. Philadelpia: Elsevier Saunders; 2013. pp. 546–66. [Google Scholar]
- 14.Taterra D, Wong LM, Vikse J, Sanna B, Pękala P, Walocha J, et al. The prevalence and anatomy of parathyroid glands:a meta-analysis with implications for parathyroid surgery. Langenbecks Arch Surg. 2019;404:63–70. doi: 10.1007/s00423-019-01751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beahrs OH, Edis AJ, Purnell DC. Unusual problems in parathyroid surgery. Am J Surg. 1977;134:502–4. doi: 10.1016/0002-9610(77)90387-7. [DOI] [PubMed] [Google Scholar]
- 16.Jaskowiak N, Norton JA, Alexander HR, Doppman JL, Shawker T, Skarulis M, et al. A prospective trial evaluating a standard approach to reoperation for missedparathyroid adenoma. Ann Surg. 1996;224:308–20. doi: 10.1097/00000658-199609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHenry C, Walsh M, Jarosz H, Henkin R, Tope J, Lawrence AM, et al. Resection of parathyroid tumor in the aorticopulmonary window without prior neckexploration. Surgery. 1988;104:1090–4. [PubMed] [Google Scholar]
- 18.Stocks AE, Hartley LC. Hypercalcaemia. The case of the missing adenoma. Med J Aust. 1986;145:92–4. [PubMed] [Google Scholar]
- 19.Udekwu AO, Kaplan EL, Wu TC, Arganini M. Ectopic parathyroid adenoma of the lateral triangle of the neck:report of twocases. Surgery. 1987;101:114–8. [PubMed] [Google Scholar]
- 20.Saky MT, Hasinski S, Rose LI. Ectopic primary hyperparathyroidism. Endocr Pract. 2001;7:272–4. [PubMed] [Google Scholar]
- 21.Pawlik TM, Richards M, Giordano TJ, Burney R, Thompson N. Identification and management of intravagal parathyroid adenoma. World J Surg. 2001;25:419–23. doi: 10.1007/s002680020067. [DOI] [PubMed] [Google Scholar]
- 22.Fukumoto A, Nonaka M, Kamio T, Kamura E, Ozu C, Baba S, et al. A case of ectopic parathyroid gland hyperplasia in the pyriform sinus. Arch Otolaryngol Head Neck Surg. 2002;128:71–4. doi: 10.1001/archotol.128.1.71. [DOI] [PubMed] [Google Scholar]
- 23.Uludag M, Isgor A, Yetkin G, Atay M, Kebudi A, Akgun I. Supernumerary ectopic parathyroid glands. Persistent hyperparathyroidism due to mediastinal parathyroid adenoma localized by preoperative single photon emission computed tomography and intraoperative gamma probe application. Hormones (Athens) 2009;8:144–9. doi: 10.14310/horm.2002.1231. [DOI] [PubMed] [Google Scholar]
- 24.Noussios G, Anagnostis P, Natsis K. Ectopic parathyroid glands and their anatomical, clinical and surgical implications. Exp Clin Endocrinol Diabetes. 2012;120:604–10. doi: 10.1055/s-0032-1327628. [DOI] [PubMed] [Google Scholar]
- 25.Harach HR, Vujanić GM. Intrathyroidal parathyroid. Pediatr Pathol. 1993;13:71–4. doi: 10.3109/15513819309048194. [DOI] [PubMed] [Google Scholar]
- 26.Kraas J, Clark PB, Perrier ND, Morton KA. The scintigraphic appearance of subcapsular parathyroid adenomas. Clin Nucl Med. 2005;30:213–7. doi: 10.1097/01.rlu.0000155982.79457.2b. [DOI] [PubMed] [Google Scholar]
- 27.Thompson NW, Eckhauser FE, Harness JK. The anatomy of primary hyperparathyroidism. Surgery. 1982;92:814–21. [PubMed] [Google Scholar]
- 28.Lee NJ, Blakey JD, Bhuta S, Calcaterra TC. Unintentional parathyroidectomy during thyroidectomy. Laryngoscope. 1999;109:1238–40. doi: 10.1097/00005537-199908000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Sorgato N, Pennelli G, Boschin IM, Ide EC, Pagetta C, Piotto A, et al. Can we avoid inadvertent parathyroidectomy during thyroid surgery? In Vivo. 2009;23:433–9. [PubMed] [Google Scholar]
- 30.Ros S, Sitges-Serra A, Pereira JA, Jimeno J, Prieto R, Sancho JJ, et al. Intrathyroid parathyroid adenomas:right and lower. [Article in Spanish] Cir Esp. 2008;84:196–200. doi: 10.1016/s0009-739x(08)72619-8. [DOI] [PubMed] [Google Scholar]
- 31.Sadler GP, Clark OH, Van Heerden JA. Thyroid and parathyroid. In: Schwartz SI, Shires GM, Spencer FC, editors. Principles of Surgery. 7th ed. New York: Mc Graw Hill; 1999. pp. 1661–714. [Google Scholar]
- 32.Henry JF. Surgical anatomy and embriyology of the thyroid and parathyroid glands and recurrent and external laryngeal nerves. In: Clark OH, Duh QY, editors. Textbook of Endocrine Surgery. Philadelpia: WB Saunders; 1997. pp. 8–14. [Google Scholar]
- 33.Herrera MF, Gambao-Dominguez A. Parathyroid embryology, anatomy, and pathology. In: Clark OH, Duh QY, editors. Textbook of Endocrine Surgery. 1st ed. Philadelphia: WB Saunders; 1997. pp. 277–83. [Google Scholar]
- 34.Delattre JF, Flament JB, Palot JP, Pluot M. Variations in the parathyroid glands. Number, situation and arterial vascularization. Anatomical study and surgical application. [Article in French] J Chir (Paris) 1982;119:633–41. [PubMed] [Google Scholar]
- 35.Nobori M, Saiki S, Tanaka N, Harihara Y, Shindo S, Fujimoto Y. Blood supply of the parathyroid gland from the superior thyroid artery. Surgery. 1994;115:417–23. [PubMed] [Google Scholar]
- 36.Johansson K, Ander S, Lennquist S, Smeds S. Human parathyroid blood supply determined by laser-Doppler flowmetry. World J Surg. 1994;18:417–20. doi: 10.1007/BF00316825. [DOI] [PubMed] [Google Scholar]
- 37.Uludağ M. Normocalcemic hyperparathyroidism:A new clinical type of primary hyperparathyroidism. Med Bull Sisli Etfal Hosp. 2014;48:264–73. [Google Scholar]
- 38.Cheatem DM, Surgeon C. Tecnique of parathyroidectomy. In: Clark OH, Duh QY, Kebebew E, Gosnell JE, Shen WT, editors. Textbook of Endocrine Surgery. 3rd ed. Jeypee Brothers Medical Publishers; 2016. pp. 747–56. [Google Scholar]
- 39.Callender GG, Udelsman R. Surgery for primary hyperparathyroidism. Cancer. 2014;120:3602–16. doi: 10.1002/cncr.28891. [DOI] [PubMed] [Google Scholar]
- 40.Nebiker CA, Mechera R, Rosenthal R, Thommen S, Marti WR, von Holzen U, et al. Residents'performance in open versus laparoscopic bench-model cholecystectomy in a hands-on surgical course. Int J Surg. 2015;19:15–21. doi: 10.1016/j.ijsu.2015.04.072. [DOI] [PubMed] [Google Scholar]
- 41.Uludağ M. Preoperative Localization Studies in Primary Hyperparathyroidism. Med Bull Sisli Etfal Hosp. 2019;53:7–15. doi: 10.14744/SEMB.2019.78476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallagher SF, Denham DW, Murr MM, Norman JG. The impact of minimally invasive parathyroidectomy on the way endocrinologists treat primary hyperparathyroidism. Surgery. 2003;134:910–7. doi: 10.1016/s0039-6060(03)00414-8. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Oh HB, Parameswaran R, Gorelik A, Miller JA. Practice Patterns in Parathyroid Surgery:A Survey of Asia-Pacific Parathyroid Surgeons. World J Surg. 2019;43:1964–71. doi: 10.1007/s00268-019-04990-4. [DOI] [PubMed] [Google Scholar]
- 44.Machado NN, Wilhelm SM. Diagnosis and Evaluation of Primary Hyperparathyroidism. Surg Clin North Am. 2019;99:649–66. doi: 10.1016/j.suc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Promberger R, Ott J, Kober F, Mikola B, Karik M, Freissmuth M, et al. Intra- and postoperative parathyroid hormone-kinetics do not advocate for autotransplantation of discolored parathyroid glands during thyroidectomy. Thyroid. 2010;20:1371–5. doi: 10.1089/thy.2010.0157. [DOI] [PubMed] [Google Scholar]
- 46.Aygun N, Uludag M. Intraoperative Adjunct Methods for Localization in Primary Hyperparathyroidism. Med Bull Sisli Etfal Hosp. 2019;53:84–95. doi: 10.14744/SEMB.2019.37542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UludağM Yetkin G, Çitgez B, İşgör A, Kebudi A. Parathyroid autotransplantation during thyroidectomy. Med Bull Sisli Etfal Hosp. 2009;43:33–7. [Google Scholar]
- 48.Moalem J, Guerrero M, Kebebew E. Bilateral neck exploration in primary hyperparathyroidism-when is it selected and how is it performed? World J Surg. 2009;33:2282–91. doi: 10.1007/s00268-009-9941-5. [DOI] [PubMed] [Google Scholar]
- 49.Mihai R, Barczynski M, Iacobone M, Sitges-Serra A. Surgical strategy for sporadic primary hyperparathyroidism an evidence-based approach to surgical strategy, patient selection, surgical access, and reoperations. Langenbecks Arch Surg. 2009;394:785–98. doi: 10.1007/s00423-009-0529-1. [DOI] [PubMed] [Google Scholar]
- 50.Uludag M, İsgor A. Scarless thyroidectomy:transoral endoscopic thyroidectomy by vestibular approach. Med Bull Sisli Etfal Hosp. 2017;51:169–83. [Google Scholar]
- 51.Kunstman JW, Udelsman R. Superiority of minimally invasive parathyroidectomy. Adv Surg. 2012;46:171–89. doi: 10.1016/j.yasu.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Egan RJ, Scott-Coombes DM. The surgical management of sporadic primary hyperparathyroidism. Best Pract Res Clin Endocrinol Metab. 2018;32:847–59. doi: 10.1016/j.beem.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Mallick R, Chen H. Diagnosis and Management of Hyperparathyroidism. Adv Surg. 2018;52:137–53. doi: 10.1016/j.yasu.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Brunaud L, Li Z, Van Den Heede K, Cuny T, Van Slycke S. Endoscopic and robotic parathyroidectomy in patients with primary hyperparathyroidism. Gland Surg. 2016;5:352–60. doi: 10.21037/gs.2016.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suliburk JW, Sywak MS, Sidhu SB, Delbridge LW. 1000 minimally invasive parathyroidectomies without intra-operative parathyroid hormone measurement:lessons learned. ANZ J Surg. 2011;81:362–5. doi: 10.1111/j.1445-2197.2010.05488.x. [DOI] [PubMed] [Google Scholar]
- 56.Murphy C, Norman J. The 20% rule:a simple, instantaneous radioactivity measurement defines cure and allows elimination of frozen sections and hormone assays during parathyroidectomy. Surgery. 1999;126:1023–8. doi: 10.1067/msy.2099.101578. [DOI] [PubMed] [Google Scholar]
- 57.Chen H, Sippel RS, Schaefer S. The effectiveness of radioguided parathyroidectomy in patients with negativetechnetium tc 99m-sestamibi scans. Arch Surg. 2009;144:643–8. doi: 10.1001/archsurg.2009.104. [DOI] [PubMed] [Google Scholar]
- 58.Miccoli P, Pinchera A, Cecchini G, Conte M, Bendinelli C, Vignali E, et al. Minimally invasive, video-assisted parathyroid surgery for primary hyperparathyroidism. J Endocrinol Invest. 1997;20:429–30. doi: 10.1007/BF03347996. [DOI] [PubMed] [Google Scholar]
- 59.Miccoli P, Berti P, Materazzi G, Massi M, Picone A, Minuto MN. Results of video-assisted parathyroidectomy:single institution's six-year experience. World J Surg. 2004;28:1216–8. doi: 10.1007/s00268-004-7638-3. [DOI] [PubMed] [Google Scholar]
- 60.Lombardi CP, Raffaelli M, Traini E, De Crea C, Corsello SM, Sollazzi L, et al. Advantages of a video-assisted approach to parathyroidectomy. ORL J Otorhinolaryngol Relat Spec. 2008;70:313–8. doi: 10.1159/000149833. [DOI] [PubMed] [Google Scholar]
- 61.Bellantone R, Raffaelli M DE, Crea C, Traini E, Lombardi CP. Minimally-invasive parathyroid surgery. Acta Otorhinolaryngol Ital. 2011;31:207–15. [PMC free article] [PubMed] [Google Scholar]
- 62.Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg. 1996;83:875. doi: 10.1002/bjs.1800830656. [DOI] [PubMed] [Google Scholar]
- 63.Henry JF, Defechereux T, Gramatica L, de Boissezon C. Minimally invasive videoscopic parathyroidectomy by lateral approach. Langenbecks Arch Surg. 1999;384:298–301. doi: 10.1007/s004230050207. [DOI] [PubMed] [Google Scholar]
- 64.Henry JF, Sebag F, Tamagnini P, Forman C, Silaghi H. Endoscopic parathyroid surgery:results of 365 consecutive procedures. World J Surg. 2004;28:1219–23. doi: 10.1007/s00268-004-7601-3. [DOI] [PubMed] [Google Scholar]
- 65.Yeung GH, Ng JW. The technique of endoscopic exploration for parathyroid adenoma of the neck. Aust N Z J Surg. 1998;68:147–50. doi: 10.1111/j.1445-2197.1998.tb04727.x. [DOI] [PubMed] [Google Scholar]
- 66.Cougard P, Goudet P, Bilosi M, Peschaud F. Videoendoscopic approach for parathyroid adenomas:results of a prospective study of 100 patients. [Article in French] Ann Chir. 2001;126:314–9. doi: 10.1016/s0003-3944(01)00520-x. [DOI] [PubMed] [Google Scholar]
- 67.Fouquet T, Germain A, Zarnegar R, Klein M, De Talance N, Claude Mayer J, et al. Totally endoscopic lateral parathyroidectomy:prospective evaluation of 200patients. ESES 2010 Vienna presentation. Langenbecks Arch Surg. 2010;395:935–40. doi: 10.1007/s00423-010-0687-1. [DOI] [PubMed] [Google Scholar]
- 68.Mohamed HE, Bhatia P, Aslam R, Moulthrop T, Kandil E. Robotic transaxillary and retroauricular parathyroid surgery. Gland Surg. 2015;4:420–8. doi: 10.3978/j.issn.2227-684X.2015.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikeda Y, Takami H. Endoscopic parathyroidectomy. Biomed Pharmacother. 2000;54(Suppl 1):52s–6s. doi: 10.1016/s0753-3322(00)80011-6. [DOI] [PubMed] [Google Scholar]
- 70.Arora A, Garas G, Tolley N. Robotic Parathyroid Surgery:Current Perspectives and Future Considerations. ORL J Otorhinolaryngol Relat Spec. 2018;80:195–203. doi: 10.1159/000488355. [DOI] [PubMed] [Google Scholar]
- 71.Randone B, Costi R, Scatton O, Fulla Y, Bertagna X, Soubrane O, et al. Thoracoscopic removal of mediastinal parathyroid glands:a critical appraisal of an emerging technique. Ann Surg. 2010;251:717–21. doi: 10.1097/SLA.0b013e3181c1cfb0. [DOI] [PubMed] [Google Scholar]
- 72.Karakas E, Steinfeldt T, Gockel A, Westermann R, Kiefer A, Bartsch DK. Transoral thyroid and parathyroid surgery. sSurg Endosc. 2010;24:1261–7. doi: 10.1007/s00464-009-0757-z. [DOI] [PubMed] [Google Scholar]
- 73.Anuwong A. Transoral Endoscopic Thyroidectomy Vestibular Approach:A Series of the First 60 Human Cases. World J Surg. 2016;40:491–7. doi: 10.1007/s00268-015-3320-1. [DOI] [PubMed] [Google Scholar]
- 74.Sasanakietkul T, Jitpratoom P, Anuwong A. Transoral endoscopic parathyroidectomy vestibular approach:a novel scarless parathyroid surgery. Surg Endosc. 2017;31:3755–63. doi: 10.1007/s00464-016-5397-5. [DOI] [PubMed] [Google Scholar]
- 75.Hurtado-López LM, Gutiérrez-Román SH, Basurto-Kuba E, Luna-Ortiz K. Endoscopic transoral parathyroidectomy:Initial experience. Head Neck. 2019;41:3334–7. doi: 10.1002/hed.25828. [DOI] [PubMed] [Google Scholar]
- 76.Ozdenkaya Y, Ersavas C, Arslan NC. Robotic transoral vestibular parathyroidectomy:Two case reports and review of literature. World J Clin Cases. 2018;6:542–7. doi: 10.12998/wjcc.v6.i12.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siperstein AE, Stephen AE, Milas M. Standard bilateral parathyroid exploration. In: Randolph GW, editor. Surgery of the Thyroid and Parathyroid Glands. 2nd ed. Philadelpia: Elsevier Saunders; 2013. pp. 567–79. [Google Scholar]
- 78.Uludag M, Aygun N, Kaya C, Tanal M, Oba S, Isgor A. Basic principles and standardization of intraoperative nerve monitoring in thyroid surgery. Med Bull Sisli Etfal Hosp. 2017;51:13–25. [Google Scholar]
- 79.Uludag M, Tanal M, İşgör A. A Review of Methods for the Preservation of Laryngeal Nerves During Thyroidectomy. Med Bull Sisli Etfal Hosp. 2018;52:79–91. doi: 10.14744/SEMB.2018.37928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lal G, Clark OH. Thyroid, parathyroid, and adrenal. In: Brunicardi FC, Andersen DK, Billiar TR, Dunn DL, Hunter JG, Kao LS, et al., editors. Schwartz's Principles of Surgery. 11th ed. New York: McGraw Hill; 2019. pp. 1625–704. [Google Scholar]
- 81.Edis AJ, Purnell DC, van Heerden JA. The undescended “parathymus”. An occasional cause of failed neck explorationfor hyperparathyroidism. Ann Surg. 1979;190:64–8. doi: 10.1097/00000658-197907000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daruwalla J, Sachithanandan N, Andrews D, Miller JA. Ectopic intravagal parathyroid adenoma. Head Neck. 2015;37:E200–4. doi: 10.1002/hed.24068. [DOI] [PubMed] [Google Scholar]
- 83.de la Cruz Vigo F, Ortega G, González S, Martinez JI, Cruz Leiva J, Gálvez R, et al. Pathologic intrathyroidal parathyroid glands. Int Surg. 1997;82:87–90. [PubMed] [Google Scholar]
- 84.Proye C, Bizard JP, Carnaille B, Quiévreux JL. Hyperparathyroidism and intrathyroid parathyroid gland. 43 cases. [Article in French] Ann Chir. 1994;48:501–6. [PubMed] [Google Scholar]
- 85.Simeone DM, Sandelin K, Thompson NW. Undescended superior parathyroid gland:a potential cause of failed cervical exploration for hyperparathyroidism. Surgery. 1995;118:949–56. doi: 10.1016/s0039-6060(05)80099-6. [DOI] [PubMed] [Google Scholar]
- 86.Carling T, Donovan P, Rinder C, Udelsman R. Minimally invasive parathyroidectomy using cervical block:reasons for conversion to general anesthesia. Arch Surg. 2006;141:401–4. doi: 10.1001/archsurg.141.4.401. [DOI] [PubMed] [Google Scholar]
- 87.Shindo ML, Rosenthal JM. Minimal access parathyroidectomy using the focused lateral approach:technique, indication, and results. Arch Otolaryngol Head Neck Surg. 2007;133:1227–34. doi: 10.1001/archotol.133.12.1227. [DOI] [PubMed] [Google Scholar]
- 88.Uludag M, Yetkin G, Citgez B. Minimal invasive thyroid lobectomy with lateral mini-incision tecnique:Our first experience. Med Bull Sisli Etfal Hosp. 2007;41:36–41. [Google Scholar]
- 89.Delbridge L, McMullen T. Minimally invasive parathyroidectomy. In: Clark OH, Duh QY, Kebebew E, editors. Atlas of Endocrine Surgical Tecniques. 1st ed. Saunders Elsevier; 2011. pp. 145–64. [Google Scholar]
- 90.Moses WO, Suh I. Surgical approach to primary hyperparathyroidism (unilateral approach) In: Clark OH, Duh QY, Kebebew E, Gosnell JE, Shen WT, editors. Textbook of Endocrine Surgery. 3rd ed. Jaypee Brothers Medical Publishers; 2016. pp. 769–76. [Google Scholar]


