Abstract
Mood disorders are highly prevalent and are the leading cause of disability worldwide. The neurobiological mechanisms underlying depression remain poorly understood, although theories regarding dysfunction within various neurotransmitter systems have been postulated. Over 50 years ago, clinical studies suggested that increases in central acetylcholine could lead to depressed mood. Evidence has continued to accumulate suggesting that the cholinergic system plays a important role in mood regulation. In particular, the finding that the antimuscarinic agent, scopolamine, exerts fast-onset and sustained antidepressant effects in depressed humans has led to a renewal of interest in the cholinergic system as an important player in the neurochemistry of major depression and bipolar disorder. Here, we synthesize current knowledge regarding the modulation of mood by the central cholinergic system, drawing upon studies from human postmortem brain, neuroimaging, and drug challenge investigations, as well as animal model studies. First, we describe an illustrative series of early discoveries which suggest a role for acetylcholine in the pathophysiology of mood disorders. Then, we discuss more recent studies conducted in humans and/or animals which have identified roles for both acetylcholinergic muscarinic and nicotinic receptors in different mood states, and as targets for novel therapies.
INTRODUCTION
Mood disorders are the leading cause of disability worldwide. Two major categories of mood disorder, major depression and bipolar disorder, are estimated to occur in the general population at rates of 18% and 2–3%, respectively1–3. Dysfunction within various neurotransmitter systems, including the serotonergic, noradrenergic, dopaminergic, GABAergic, glutamatergic, and endorphinergic systems have been hypothesized to underlie mood disorders due to the mechanism of action of pharmacological treatments that target these systems, and biological findings4–6. The recent finding that the antimuscarinic agent, scopolamine, induces fast-onset and sustained antidepressant effects in depressed patients has renewed interest in understanding the role of the cholinergic nervous system in mood regulation7–11.
As early as 1950, clinical research observations suggested that increases in central acetylcholine (ACh) could lead to depressed mood12–14. In the 1970s, this possibility was further elaborated as the adrenergic-cholinergic balance hypothesis of mania and depression15. As proposed by Janowsky et al. (1972), this hypothesis posited that depression involved a central predominance of acetylcholinergic to noradrenergic tone, while mania resulted from the converse15. This theory was then reformulated as the catecholaminergic-cholinergic balance hypothesis of mania and depression, which integrated more recent findings incorporating the role of dopamine, a neurotransmitter integral to the regulation of mood16.
Since the role of catecholamines, and dopamine in particular, have been reviewed previously17–19, this review will primarily summarize current knowledge regarding the modulation of mood by the central cholinergic system. Human postmortem brain, neuroimaging, drug challenge, and animal model studies have examined the role of the cholinergic system in mood regulation. Here, we first describe an illustrative series of early discoveries which have suggested a role for acetylcholine in the pathophysiology of mood disorders. We will then discuss more recent studies conducted in humans and/or animals which have investigated the role of acetylcholinergic muscarinic and nicotinic receptors in different mood states, and as targets for therapeutics.
CHOLINERGIC REGULATION OF MOOD
Acetylcholinesterase Inhibitors, ACh receptor agonists, and ACh Precursor Effects on Mood in Humans
The earliest observations of a potential link between acetylcholine and depression were based on clinical observations of the effects of ACh receptor (AChR) agonists, which activate AChRs, and acetylcholinesterase inhibitors (AChEIs), which prevent the breakdown of ACh by acetylcholinesterases (AChEs). AChEIs compose a class of insecticides used in agriculture, and nerve agents used in wartime. Exposure to AChEIs increases acetylcholine levels in both the central nervous system and the periphery, causing toxic and potentially lethal respiratory, central nervous system, and cardiovascular effects.
Several early reports indicated that individuals exposed to AChEI insecticides or potential nerve agent weapons developed psychiatric symptoms including psychotic phenomena, anxiety, and most commonly, depression. In 1950, Rowntree et al. found that administration of the irreversible AChEI diisopropylflurophosphonate (DFP) to bipolar patients reduced manic symptoms and induced depression12. Furthermore, when administered to normal or depressed patients, DFP increased depressive symptoms and in some, activated psychotic symptoms. Gershon and Shaw (1961) reported that agricultural workers chronically exposed to AChEI insecticides became depressed or psychotic13. Bowers et al. (1964) tested the AChEI, EA 1701, in army volunteers and reported onset of depression14. Moreover, early animal behavioral pharmacological studies reported similar findings. For example, both the cholinergic muscarinic agonist arecoline and the AChEI physostigmine reduced rodent intracranial self–stimulation (ICSS), a measure of reward threshold20, 21.
Subsequently, a number of investigators formally studied the psychiatric and behavioral effects of AChEI challenges (Table 1). Janowsky et al. (1973) reported that in patients with bipolar or major depressive disorder, an acute challenge with the centrally active and reversible AChEI, physostigmine, antagonized manic symptoms and induced depressive symptoms, including apathy, slowness of thought, and psychomotor retardation22. Similar studies replicated the antimanic effects of physostigmine in bipolar patients23, 24. Furthermore, an acute physostigmine challenge also precipitated depressive symptoms in psychiatric inpatients, especially those with a history of major depression22. Other reports indicated that physostigmine treatment also induces depressive symptoms in a minority of control subjects25–27. Recently, the AChEI donepezil was reported to induce more frequent relapse of depression in older patients with mild cognitive impairment28, 29. Furthermore, Altinyazer et al. (2016) showed that among individuals with major depression living in agricultural districts, red blood cell acetylcholinesterase activity levels negatively correlated with the number of past suicide attempts and hopelessness levels30. These reports are consistent with more recent SPECT imaging studies suggesting that levels of acetylcholine are increased throughout the brain in depressed unipolar and bipolar patients31. Thus, considerable evidence suggests that AChEIs reduce elevated mood during mania, increase depressive symptoms in depressed patients, and induce depression in euthymic individuals with a personal or family history of depression.
Table 1.
Summary of pharmacological evidence for cholinergic regulation of mood
| Illness | Drug | Mechanism of action | Effect |
|---|---|---|---|
| Unipolar depression | Physostigmine | AChEI | Worsened depression22 |
| Arecoline | mAChR agonist | Worsened depression182 | |
| Scopolamine | Antimuscarinic | Antidepressant7, 9–11 | |
| Nicotine | nAChR agonist | Antidepressant in depressed non-smokers134 | |
| Bipolar depression | Physostigmine | AChEI | Worsened depression22 |
| Arecoline | mAChR agonist | Worsened depression182 | |
| Scopolamine | Antimuscarinic | Antidepressant7, 9–11 | |
| Bipolar mania | Physostigmine | AChEI | Reduced mania22–24 |
| RS 86 | M1-AChR agonist | Reduced mania95 | |
| Controls | Physostigmine | AChEI | Induced depression in those with positive history25–27 |
| Induced depression in marihuana-intoxicated controls90 | |||
| Donepezil | AChEI | Induced depression in cognitively impaired with positive history28, 29 | |
| Deanol | ACh precursor | Induced depression or hypomania in those with positive history183 | |
| Choline | ACh precursor | Induced depression in tardive dyskinesia patients33 | |
| Arecoline | mAChR agonist | Induced depression in controls and euthymic bipolars patients92, and Alzheimers disease93 | |
| Oxotremorine | ACh agonist | Induced depression in Alzheimers disease94 | |
| Nicotine | nAChR agonist | Antidepressant in non-smokers133 |
Abbreviations: ACh, acetylcholine; AChEI, acetylcholinesterase inhibitor; nAChR, nicotinic acetylcholine receptor; mAChR, muscarinic acetylcholine receptor; M1-AChR, muscarinic 1 acetylcholine receptor.
Acetylcholine precursors, such as deanol, choline, and lecithin, have also been reported to cause depression, and paradoxically hypomania in some cases. Tamminga et al. (1976) reported that choline precipitated depression in 2 out of 4 patients with tardive dyskinesia32, and Growdon et al. (1977) reported that 2 out of 20 patients with tardive dyskinesia became withdrawn and apathetic33. Casey (1979) reported that high doses of deanol caused severe depression in 5 out of 33 movement disorder patients, and 3 of the 33 became hypomanic34. Overall, patients who developed mood symptoms had a history of affective disorder. Subsequent studies with the AChergic precursor citicoline showed mixed effects, with either no mood effects or reduced depressive symptoms when given along with agents including rivastigmine, citalopram, or lithium35,36,37,38.
Procholinergic Agents Induce Biomarkers of Depression in Humans
Cholinergic agents including AChEIs and muscarinic agonists have also been reported to influence the expression of biomarkers linked to mood disorders, including effects on neuroendocrine measures, sleep, and pupillary size. Serum levels of adrenocorticotropic hormone (ACTH)39, 40, cortisol41–43, and beta endorphin44–46, have been reported to be increased in depressed patients, as has cortisol non-suppression by dexamethasone. Similarly, increasing acetylcholine levels with physostigmine treatment has been reported to increase serum ACTH, cortisol, and beta endorphin27, 47, 48. Furthermore, physostigmine-induced increases in ACTH and beta endorphin were exaggerated in depressed patients compared to normal volunteers49. Physostigmine also induced non-suppression of cortisol by dexamethasone in normal adults50. Subsequently, a sex difference was reported in which low doses of physostigmine increased serum levels of ACTH and cortisol in females with a history of depression51, but not in males.
Specific changes in sleep architecture are also endophenotypic markers of clinical depression observed in depressed patients and their asymptomatic relatives52. These changes include increases in rapid eye movement (REM) sleep duration, increases in REM density, reductions in REM latency, decreases in slow wave sleep, and disturbances in sleep continuity52, 53, 54, 55. These sleep alterations result in part from perturbations in the cholinergic system, with procholinergic influences inducing depression-relevant sleep effects56. Thus, the AChEI, galantamine, increases REM tonic activity and decreases REM latency57. Administration of procholinergic agents including physostigimine58, donepezil59, arecoline60–63, or pilocarpine64, 65 to controls reduces REM latency, and increases REM density. Furthermore, Laurer et al. (2004) found that the cholinergic muscarinic agonist RS 86 induced REM latency super-shortening in high risk individuals without a history of depression, and this was a predictor of a subsequent first major depressive episode66. Similarly, Perlis et al. (2002) discriminated control versus depressed patients by their response to donepezil. Controls showed no REM latency changes following low dose treatment, whereas depressed patients showed decreased REM latency67. Similarly, asymptomatic relatives of depressed patients showed a parallel vulnerability to cholinergic stimulation67. Furthermore, vulnerability to physostigmine-induced REM hypersensitivity is highly correlated in monozygotic twins, suggesting a genetic component to this phenomenon68.
In contrast to the effects of cholinomimetic agents on sleep, several reports have indicated that the centrally active antimuscarinic, scopolamine, increases REM latency and suppresses REM density and duration in depressed patients69, 70. Furthermore, these scopolamine effects are evident following remission71. Thus, antagonizing muscarinic receptors produces effects on REM sleep that are opposite of those observed in depressed patients.
Another biomarker observed in depressed patients is pupillary sensitivity to cholinergic agonists. Depressed patients exhibited significantly greater reductions in pupillary diameter following administration of the muscarinic agonist pilocarpine than did controls, suggesting increased muscarinic sensitivity72. Conversely, a report by Sokolski and DeMet (2000) showed decreased pupillary sensitivity to pilocarpine in manic patients, a phenomenon which was reduced with lithium treatment73.
AChEIs and ACh Agonist Effects on Mood in Rodents
Similar to findings in humans, a number of rodent studies have also demonstrated that treatment with AChEIs or cholinergic agonists induce depression-like behaviors (Table 2). Picciotto and colleages, as well as others, have shown that systemic administration of physostigmine to mice increases depression-like behaviors including social avoidance following social defeat74, immobility in the forced swim test (FST)75–77, and immobility in the tail suspension test (TST)76, 78. Furthermore, some of these pro-depressive effects were more pronounced in male than female mice76, 77. The pro-depressive effects of systemic physostigmine treatment in the TST were blocked by acute treatment with either scopolamine, a broad muscarinic antagonist, or mecamylamine, a broad nicotinic antagonist74. Additionally, physostigmine treatment increased anxiety-like behavior in mice, including time spent in the dark side of the light/dark box, and time spent in the center of the open field76, 77. Treatment with either physostigmine or pilocarpine also decreased rates of intracranial self-stimulation (ICSS) of the ventral tegmental area (VTA), reflecting increased anhedonia79. These depressogenic effects of physostigmine are observed despite tight regulation of AChE through negative feedback pathways at the mRNA, protein, and activity levels. For example, significant adaptation has been observed following knockout of AChE isoforms80, and exposure to AChEIs leads rapidly to upregulation of AChE mRNAs81. Furthermore, stress induces alternative splicing of AChE mRNAs and modulation of AChE activity82, 83.
Table 2.
Effects of manipulations of the cholinergic system on depression-like behavior in rodents
| Species | Drug | Mechanism | Route | Test | Effect |
|---|---|---|---|---|---|
| Mice | Physostigmine | AChEI | Systemic | FST | Depression-like76–78 |
| TST | Depression-like74, 78 | ||||
| Intra-VTA | FST | Depression-like75 | |||
| Intra-Hipp | TST | Depression-like74 | |||
| Social defeat | Depression-like74 | ||||
| Scopolamine | Antimuscarinic | Systemic | FST | Antidepressant-like118–121 | |
| CMS | Antidepressant-like119 | ||||
| LH | Antidepressant-like,122 | ||||
| NSF | Antidepressant-like118, 120 | ||||
| SP | Antidepressant-like119 | ||||
| Nicotine | nAChR agonist | Systemic | FST | Antidepressant-like154, 156 | |
| TST | Antidepressant-like154, 156 | ||||
| Mecamylamine | nAChR antagonist | Systemic | FST | Antidepressant-like148, 156 | |
| TST | Antidepressant-like156 | ||||
| Methyllycaconite | a7 nAChR antagonist | Systemic | FST | Antidepressant-like76 | |
| Varenicline | a4b2 nAChR partial agonist | Systemic | FST | Antidepressant-like160 | |
| TST | Antidepressant-like76 | ||||
| Rats | Physostigmine | AChEI | Systemic | FST | Depression-like75 |
| Intra-VTA | SP | Depression-like84 | |||
| Pilocarpine | mAChR agonist | Intra-VTA | FST | Depression-like84 | |
| Scopolamine | Antimuscarinic | Intra-VTA | FST | Antidepressant-like75 | |
| Mecamylamine | nAChR antagonist | Intra-VTA | FST | Antidepressant-like75 | |
| Systemc | FST | Antidepressant-like158 | |||
| CMS | Antidepressant-like158 | ||||
| SP | Antidepressant-like158 | ||||
| Arecoline | mAChR agonist | Intra-NAc | FST | Depression-like86 | |
| Pirenzepine | M1-AChR antagonist | Intra-NAc | FST | Antidepressant-like86, 88 | |
| Gallamine | M2-AChR antagonist | Intra-NAc | FST | Depression-like86 | |
| Scopolamine | Antimuscarinic | Intra-NAc | FST | Antidepressant-like86 | |
| Nicotine | nAChR agonist | Systemic | FST | Antidepressant-like152, 153 | |
| OBX | Antidepressant-like155 |
Abbreviations: AChEI, acetylcholinesterase inhibitor; nAChR, nicotinic acetylcholine receptor; mAChR, muscarinic acetylcholine receptor; M1-AChR, muscarinic 1 acetylcholine receptor; Intra-VTA, intra-ventral tegmental area; Intra-Hipp, intra-hippocampal; Intra-NAc, intra-nucleus accumbens; FST, forced swim test; TST, tail suspension test; SP, sucrose preference; CMS, chronic mild stress; LH, learned helplessness; NSF, novelty suppressed feeding.
Local intracerebral drug infusion studies in rodents have implicated several brain regions in the depressogenic effects of procholinergic drug treatments. For example, intra-VTA infusion of physostigmine increased immobility time in the FST75, decreased time spent on open arms in the elevated plus maze, and decreased sucrose preference84, indicating increased anxiety and anhedonia, respectively. Furthermore, intra-VTA infusion of pilocarpine produced similar effects84. Conversely, intra-VTA infusion of scopolamine or mecamylamine decreased baseline immobility time in the FST, indicating antidepressant-like effects75.
Mineur et al. (2013) assessed the effects of chronic fluoxetine treatment on AChE activity in multiple brain regions, and found that chronic fluoxetine increased AChE activity, specifically in the hippocampus74. To test the hypothesis that signaling at Ach receptors could contribute to depression-like behavior, physostigmine was infused locally into the hippocampus and behavior in the TST was assessed. Consistent with this hypothesis, intra-hippocampal physostigmine increased immobility, reflecting depression-like behavior74. Additionally, knockdown of AChE in the hippocampus using an adeno-associated virus (AAV) approach increased anxiety in the open field test and the light/dark test, and increased depression-like behavior in the FST, TST, and social defeat paradigm74. Additionally, 15 days of treatment with fluoxetine prevented the effects of viral hippocampal AChE knockdown in the social defeat paradigm74.
Work by Chau et al. (2011) suggests that fluoxetine treatment produces antidepressant-like effects in the FST by reducing cholinergic activity in the nucleus accumbens (NAc)85. Specifically, intra-NAc infusions of fluoxetine reduced extracellular acetylcholine and increased active behavior in the FST85. Furthermore, exposure to the FST, a potent stressor, increased basal extracellular acetylcholine in the NAc shell for up to 14 days, while chronic fluoxetine treatment prevented this effect85. Similarly, intra-NAc infusions of the muscarinic acetylcholine receptor (AChR) agonist arecoline reduced active behavior86, and intra-NAc infusions of neostigmine produced a conditioned taste aversion 87. This depressant-like effect may be mediated in part through activation of muscarinic 1 acetylcholine receptors (M1-AChRs)88, since blocking these postsynaptic receptors with the specific M1-AChR antagonist pirenzepine increased swimming in the FST86. On the other hand, activation of M2 acetylcholine autoreceptors, which are Gi-coupled, reduces ACh release and has antidepressant-like effects. Accordingly, the acetylcholine M2-AChR antagonist gallamine induces pro-depressant effects86. Finally, intra-NAc infusion of scopolamine increases active behavior in the FST, indicating an antidepressant-like effect86. However, Warner-Schmidt et al. (2012) reported that silencing cholinergic interneurons in the NAc induced a depression-like phenotype in mice89. Since the cholinergic interneurons of the NAc synapse locally onto other neurons within the NAc, and are the only source of ACh in the striatum, these results appear to contrast with findings that intra-NAc scopolamine infusions produced antidepressant effects86. This inconsistency might be explained by the positioning of cholinergic interneurons within microcircuits of the NAc, which theoretically could preferentially influence M2-over M1-AChRs. In sum, AChRs in the VTA, hippocampus, and NAc appear to regulate depression-like behaviors in rodent models.
Muscarinic Effects of Physostigmine and Arecoline in Humans
Early studies suggested that muscarinic AChRs (M-AChRs) are important for the mood altering effects of acetylcholine. The depressogenic effects of low dose physostigmine were reversed by administration of atropine, a centrally active antimuscarinic agent90, or scopolamine91. Direct muscarinic agonists have also been reported to reduce mood. Specifically, the direct muscarinic agonists arecoline92, 93, and oxotremorine94 worsened mood state in both euthymic and bipolar patients. Similarly, the M1-AChR agonist, RS 86, was reported to have antimanic effects95.
Similarly, a series of studies specifically investigated whether the depression-like effects of physostigmine are mediated by muscarinic receptors, and whether these effects are mediated centrally or peripherally. Studies dissecting the mechanisms of action of physostigmine on behavioral, neuroendocrine, cardiovascular, and sleep measures have largely implicated central muscarinic receptors in these actions. Whereas centrally acting physostigmine causes significant behavioral, neuroendocrine, cardiovascular, and sleep effects in humans, equipotent doses of the non-centrally acting AChEI, neostigmine, does not91, 96, 97. Furthermore, these effects of physostigmine can be blocked by the centrally acting antimuscarinic agent, scopolamine91, which is a high affinity antagonist at the five known muscarinic receptors and does not act at nicotinic receptors98. The effects of physostigmine are not prevented by treatment with the peripherally acting antimuscarinic agent, methscopolamine91, suggesting a central effect.
Muscarinic Receptor Alterations in Mood Disorders
A body of research has explored central muscarinic receptor expression in controls, depressives and/or bipolar patients using in vivo imaging, positron emission tomography (PET), or radioligand binding in postmortem brain samples. Several reports have identified a reduction in M2-AChR and M4-AChR density in both bipolar and major depressive disorder patients. Activation of M2-AChRs or M4-AChRs, which are autoreceptors, reduces acetylcholine release from cholinergic terminals99. Thus, reduced expression of these receptors might increase Ach release. Cannon et al. (2006) used PET imaging to investigate central M2-AChR receptor density using [(18)F]FP-TZTP in unmedicated major depressive and bipolar disorder patients. They found that bipolar patients showed a lower distribution volume of M2-AChR in the anterior cingulate cortex (ACC) compared to both major depressive disorder and control groups100. This decrease in distribution volume could have resulted from reduced M2-AChR density or affinity, or increased endogeneous acetylcholine levels which could reduce radioligand binding to M2-AChRs. Indeed, increased central choline levels have been found in depressed patients101, 102. Studies applying the M2/4 receptor antagonist, [3H] AFDX-384, to post-mortem brain tissue from patients with major depressive or bipolar disorder also reported reduced M2-AChR and M4-AChR binding in Broadman’s area 46 of the dorsolateral prefrontal cortex103. Furthermore, a decrease in binding of the M3-AChR antagonist, [3H]4-DAMP, in Broadman’s area 10 indicated a decrease in M3-AChR expression in the rostral prefrontal cortex in bipolar patients103. More recently, Gibbons et al. (2016) measured M2-AChR and M4-AChR binding in Broadman’s Area 24 and 46 of the dorsolateral prefrontal cortex using post-mortem tissue from controls, bipolar disorder patients, or major depressive patients103. Results showed that both M2-AChR and M4-AChR binding was lower in Broadman’s area 24 and 46 of the dorsolateral prefrontal cortex in mood disorder patients relative to controls.
However, other reports have not replicated muscarinic receptor density changes in depression or bipolar disorder. For example, Zavitsanou et al (2005) assessed [(3)H]AF-DX 384 binding in the ACC to determine M2-AChR and M4-AChR binding in control, major depression, and bipolar patients104. However, no differences in receptor binding were found. Furthermore, an experiment using quantitative autoradiography to measure [(3)H]pirenzepine binding to M1-AChR and M4-AChR receptors in post-mortem tissue also found no difference in binding in bipolar and major depression groups compared to controls105. Additionally, a study using a [3H]4-DAMP radioligand binding assay which was modified to increase selectivity for the M3-AChR showed that cortical M3-AChR levels were not altered in major depression or bipolar disorder106. Heterogeneity in the etiology underlying mood disorders may be a factor leading to discrepant findings between studies107, 108.
ANTIMUSCARINIC TREATMENT STUDIES
Fast-onset Antidepressant Effects of Scopolamine in Humans
In the early 1980’s, Janowsky et al. (1983) proposed that centrally active anticholinergic drugs might be effective antidepressants109, 110. Although anticholinergics were reported to cause a “high” in recreational users111–113, and scopolamine was found to be effective in antagonizing the behavioral, cardiovascular, sleep, and neuroendocrine effects of AChEIs91, definitive proof that anticholinergics like scopolamine alleviated depression in humans remained elusive. In the next two decades, several randomized controlled trials (RCTs) assessed the effects of scopolamine or the antimuscarinic biperiden in depressed patients, but did not conclusively identify antidepressant effects of these drug treatments114, 115. However, Gillin et al. (1991) reported a small but significant antidepressant effect of scopolamine administered intramuscularly for three consecutive nights compared to placebo116.
Studies published beginning in the mid-2000s by the National Institute of Mental Health (NIMH) Intramural Mood and Anxiety Disorders Program demonstrated that when administered at a higher dosage by the intravenous (i.v.) route, 4 μg/kg scopolamine exerted fast-onset antidepressant effects. After an open placebo infusion, major depressive disorder and bipolar patients received a series of three 15 min infusions of placebo followed by a series of three infusions of scopolamine, or the reverse sequence, each infusion pulsed 3–5 days apart. The placebo adjusted remission rate with scopolamine was 56%, with an onset of 3 days, persisting for at least 15 days7. Anti-cholinergic side effects were well tolerated. The same group replicated these findings in a major depressive group. Results showed that scopolamine also decreased anxiety, and produced larger reductions in anxiety symptoms in women than men9. Subsequent trials by the NIMH group have also shown the rapid-onset antidepressant effects of i.v. scopolamine, and identified characteristics of responders. Scopolamine induced antidepressant effects in treatment resistant major depressive and bipolar patients, and also produced larger antidepressant effects in treatment naïve patients11. Furthermore, scopolamine was more effective in patients with greater self-rated depressive symptoms at baseline10. In summary, i.v. infusion of scopolamine has been identified as a rapid-onset antidepressant with sustained effects for major depression and bipolar disorders. Also, one study by Khajavi et al. (2012) reported antidepressant effects of orally administered scopolamine. This study evaluated whether scopolamine could augment the effects of a classical antidepressant in major depressive disorder patients. In this RCT, a combination of the SSRI antidepressant citalopram (40 mg/day) plus oral scopolamine hydrobromide (1 mg/d) for six weeks was more effective (65% remission) than citalopram plus placebo (20% remission)117.
Although a number of RTCs have demonstrated fast-onset antidepressant effects of scopolamine infusion in patients with unipolar or bipolar depression, none have reported any effects on mania symptoms. Based on the catecholaminergic-cholinergic balance hypothesis of mania and depression, scopolamine treatment might be expected to not only reduce depression, but possibly increase mania symptoms. The catecholaminergic-cholinergic balance hypothesis is also supported by work in rodents showing antidepressant-like effects of scopolamine treatment in normal or stressed animals. Future RTCs should be designed to assess the effects of scopolamine infusion on manic or hypomanic symptoms in bipolar patients, including those with rapid cycling.
Fast-onset Antidepressant-like Effects of Scopolamine in Rodents
A number of recent studies in rats and mice have shed light on the mechanisms by which scopolamine induces fast-onset antidepressant-like effects118–121. Similar to ketamine, a single, low dose injection of scopolamine in rodents rapidly induces an antidepressant-like behavioral response in several paradigms, including the FST118–121, the chronic mild stress paradigm119, the novelty-induced feeding paradigm118, 120, the sucrose preference test119, and learned helplessness122, 123. Acute scopolamine treatment induces molecular changes in the medial prefrontal cortex (mPFC). These effects include induction of brain-derived neurotrophic factor (BDNF) release118, activation of mammalian target of rapamycin complex 1 (mTORC1), and increases in the number and function of spine synapses in layer V pyramidal neurons in the mPFC. These molecular changes induced by scopolamine have also been implicated in the fast-onset antidepressant effects of ketamine124–126. Pretreatment with a mTORC1 inhibitor or by a glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor antagonist blocks the antidepressant effects of scopolamine121. Scopolamine has been reported to initiate this molecular cascade by antagonizing the M1-AChR in the mPFC. Although both GABAergic (GAD67+) interneurons and glutamatergic (CaMKII+) interneurons in the mPFC express M1-AChR, viral-mediated knockdown of M1-AChR in GABAergic, but not glutamatergic, neurons diminishes the antidepressant-like effects of scopolamine120. Further immunohistological and electrophysiological studies have shown that somatostatin interneurons in the mPFC highly express M1-AChR, and receptor knockdown studies demonstrated that M1-AChR expression in these neurons is required for the rapid antidepressant-like effects of scopolamine120. In addition, the antagonism of M1-AChR on somatostatin interneurons in the mPFC results in disinhibition of pyramidal glutamatergic neurons, leading to a burst of glutamate and downstream molecular pathways thought to mediate the antidepressant response through synaptogenesis120. More details regarding the molecular mechanisms mediating the rapid-onset antidepressant-like effects of scopolamine in rodents has been reviewed elsewhere118.
The role of M2-AChRs in the mechanism of antidepressant action of scopolamine has also been investigated. Studies using M1-AChR or M2-AChR knockout mice treated with agonists that are preferentially selective for each of these receptors have shown that antagonists of M2 receptors, as well as M1 receptors, induce antidepressant-like effects127. Conversely, mice lacking M3-AChRs, M4-AChRs, or M5-AChRs do not show a reduced antidepressant-like response to scopolamine127. Thus, work to date suggests that M1-AChRs, and likely M2-AChRs, mediate the antidepressant-like effects of acute scopolamine treatment.
NICOTINIC REGULATION OF MOOD:
Nicotinic Treatment Studies in Humans:
Evidence suggests that nicotinic receptors also contribute significantly to the regulation of mood. Nicotine withdrawal due to cigarette smoking cessation has been well established to induce depression, anxiety, and dysphoria, which may continue for as long as 10 weeks128, 129. This especially occurs in subjects with a history of major depression130, 131. Furthermore, administration of nicotine using transdermal patches has been shown to alleviate depression during smoking cessation132. However, controlled studies assessing the effects of nicotine on depression in non-smokers are very rare. Of those, McClernon et al. (2006) found that chronic nicotine administration by patch produced antidepressant effects in non-smokers, compared to placebo133. Furthermore, Salin-Pascual et al. (1996) noted improvement in mood in major depression patients after only 2 days of treatment with nicotine patches134. Observations of improved mood following nicotine treatment134, 135, or smoking, have led to the hypothesis that smoking nicotine tobacco is sometimes used to self-medicate symptoms of depression136.
A small number of controlled studies have examined the effects of nicotinic agents besides nicotine in depressed patients. One study assessed the ability of CP-601,927, an α4β2 nicotinic acetylcholine receptor (nAChR) partial agonist, to augment a sub-optimal antidepressant response to SSRIs; however, no therapeutic effect was found137. Furthermore, a meta-analysis of six randomized-controlled trials including 2067 participants failed to confirm preliminary positive evidence for the efficacy of nAChR antagonists in treatment-resistant depression138. For example, the non-selective, non-competitive antagonist of nAChRs, desmecamylamine (TC-5214) failed Phase III trials as a treatment for major depression139. Although no agents acting primarily at nicotinic receptors are currently in use for the treatment of depression, it should be noted that many current antidepressants including tricyclics, SSRIs, and buproprion also act as α4β2 nAChR antagonists in cell-based assays140, 141.
Nicotinic Treatment Studies in Rodents
Nicotine withdrawal-induced anhedonia in rodents provides a well validated model for aspects of major depression142. For example, the affective aspects of nicotine withdrawal can be assessed in rats and mice as elevations in brain-stimulation reward thresholds and conditioned place aversion. nAChR subtypes including the α5, α2, α3, and β4 subunits have been implicated in the aversive properties of nicotine withdrawal143–146. Furthermore, nicotine self-administration in rats results in an increase in the sensitivity of natural brain reward systems, detected by post-nicotine lowering of intracranial self-stimulation (ICSS) thresholds147. Surprisingly, nicotine-induced excitation of reward systems has been reported to persist for at least 36 days after cessation of nicotine self-administration had ceased147. Since nicotine increases serotonergic and noradrenergic neuronal activity and facilitates serotonin and noradrenaline release, the effects of coadministration of nicotine with SSRIs or noradrenaline reuptake inhibitors (NRIs) have been explored in preclinical studies. For example, nicotine enhances the antidepressant-like effects of low-dose citalopram or reboxetine in the FST in mice148. In addition, chronic mild stress-induced anhedonia can be alleviated by either nicotine or sertraline, but the two treatments did not have a synergistic effect149.
Human Studies of Nicotinic Receptors
Like muscarinic receptors, nicotinic receptor function has also been reported to be altered in depressed patients. Single-photon emission computed tomography (SPECT) studies have found a reduction of β2 subunit-containing nAChR (β2* nAChR) availability across all brain regions in major depression patients compared to healthy controls. Furthermore, acutely depressed patients in this study were also found to have lower β2* nAChR availability than remitted patients31. This reduction in β2* nAChR availability in depressed patients has been suggested to result from increased central acetylcholine levels, which reduce the number of receptors available for binding to a SPECT ligand. For example, a SPECT study by Esterlis et al. (2013) showed that increases in ACh levels induced by physostigmine challenge reduces SPECT ligand binding150. Furthermore, a negative correlation between lifetime number of depressive episodes and β2* nAChR availability in temporal cortex, occipital cortex, thalamus, and striatum was reported in patients with major depressive disorder31. Consistent with findings in patients with major depression, a SPECT imaging study using [(123)I]5IA-85380 to quantify β2* nAChR total volume of distribution found significantly lower β2* nAChR availability (20–38% less) in subjects with bipolar depression compared to euthymic patients and controls in frontal, parietal, temporal, anterior cingulate cortex, hippocampus, amygdala, thalamus, and striatum151. In contrast, post-mortem studies using acetylcholine washout showed identical β2* nAChR density in controls, major depression patients, and bipolar disorder patients. Therefore, depressed and bipolar patients likely have increased endogeneous acetylcholine levels but similar β2* nAChR expression levels relative to controls.
Rodent Studies of Nicotinic Receptors
Rodent studies have also implicated nicotinic receptors in the regulation of depression-like behavior. However, differences between rats and mice have been found, with the antidepressant effects of nicotine being observed more frequently in rats. Nicotine treatment has antidepressant-like effects in several animal models of depression-like behavior, including the FST152, 153, the TST154 and the olfactory bulbectomy model155. Yet, the non-selective, non-competitive nicotinic antagonist mecamylamine also produces antidepressant-like effects in the FST in rodents148, 156, a result which was not due to a generalized stimulant effect157. Furthermore, in rats exposed to chronic restraint stress, mecamylamine blocked depression-like behaviors including reductions in sucrose preference, increased anxiety, and hypothalamic pituitary adrenal hyperactivity158. However, a meta-analysis including six randomized-controlled trials examining effects of nAChR antagonists in treatment-resistant depression found no antidepressant effects138.
Rodent studies suggest that targeting specific nAChR subtypes might provide a therapeutic strategy for treating depression159. Nicotinic antagonists at β2* or α7 nAChRs have been reported to have antidepressant-like effects in mice. The α7 nAChR antagonist methyllycaconite reduced immobility in the FST and TST, and decreased physostigmine-induced c-fos immunoreactivity in the hippocampus. These effects were observed in male, but not female, mice76. The α4β2 nAChR partial agonist varenicline showed antidepressant-like activity in the FST in two mouse strains, and also enhanced the effects of the SSRI sertraline160; α4β2 nAChR partial agonists may induce antidepressant-like effects by inducing dopamine release from VTA-NAc projections161. Furthermore, one small open-label study of varenicline augmentation was associated with significant improvement in mood in outpatient smokers with persistent depression162. However, the α4β2 nAChR partial agonist, CP-601,927, which was derived from varenicline, was not found to be effective in mouse models of antidepressant efficacy163.
In a series of studies, Mineur et al. (2016) showed that viral-mediated knockdown of either the β2* or α7 nAChR subunit within specific brain regions induced robust antidepressant-like effects in several behavioral tests. Specifically, α7 subunit knockdown in the amygdala produced antidepressant-like effects in the TST159, while β2* subunit knockdown produced antidepressant-like effects in the FST, TST, and the social defeat paradigm159. β2* subunit knockdown in the amygdala was also found to be critical for the antidepressant-like effects of the alpha2-noradrenergic receptor agonist guanfacine, while the antidepressant-like effects of the nicotinic partial agonist cytisine required noradrenergic signaling in the amygdala, highlighting an interaction between ACh and noradrenergic signaling in the regulation of depression-like behaviors in the mouse164. Furthermore, ACh signaling through α7 nAChRs in the hippocampus was reported to regulate depression-like behaviors when ACh levels are increased, which can occur under stressful conditions76. More work in humans and animals will be required to determine which nAChR subtypes provide the most promising targets for the treatment of mood disorders.
DISCUSSION:
We have highlighted a number of experiments which suggest that increasing central acetylcholine causes depression in humans and depression-like behaviors in animals. These results are remarkably consistent across species, and suggest that blocking or lowering acetylcholinesterase activity, increasing central acetylcholine levels, or stimulating specific cholinergic receptors, all lead to depression15, 23, 24. Furthermore, depression can be rapidly alleviated in humans by the pan-muscarinic receptor blocking agent, scopolamine7, 9–11. Depression-like behavior is also rapidly reduced by acute scopolamine treatment in rodent models118–122. Preclinical studies in rodents have made progress in understanding the specific muscarinic receptors, neural circuits, and molecular mechanisms by which altering cholinergic neurotransmission regulates affect74, 76, 118–121, 159, and may provide novel therapeutics for mood disorders.
Work to date has also revealed an important role for nicotinic receptors in mood regulation. Extensive evidence has shown that nicotine withdrawal in both humans and animals produces a syndrome which includes symptoms of depression142. Furthermore, nicotine administration to rodents produces antidepressant-like effects149, and several small studies suggest that nicotine may be antidepressant in nonsmokers133, 135 in addition to those undergoing smoking cessation. Furthermore, nicotinic partial agonists and antagonists at β2* and α7 nAChRs, respectively, have antidepressant-like effects in mice76, 159, 160, and one small study reported that varenicline augmentation improved mood in outpatient smokers with persistent depression162. Yet, it remains unclear whether activation, desensitization, or interference with temporally precise ACh signaling is more important for the antidepressant effects of nicotinic agents165. More work in humans and animals will be required to dissect the specific nicotinic receptor subtypes and that mediate the effects of Ach on mood, and could lead to the development of novel therapeutics.
The finding that scopolamine exerts a fast-onset and sustained antidepressant effects in humans has led to a renaissance of interest in the cholinergic system as an important factor in the neurochemistry of major depression and bipolar disorder. Controlled studies have indicated that acute i.v. treatment with scopolamine induces fast-onset and sustained antidepressant effects, even in treatment resistant patients with depression7, 9–11. Yet over the last decade, a far greater number of basic and clinical studies have investigated the therapeutic effects and mechanism of action of the NMDA antagonist, ketamine, compared to scopolamine124–126. Since no relative disadvantage of scopolamine regarding efficacy or safety has been reported compared to ketamine, and some common mechanisms of action have been identified, further investigation of the fast-onset antidepressant effects of scopolamine are highly warranted. Possibly, a subpopulation of depressed patients might respond to treatment with scopolamine who do not respond to classical antidepressants, ketamine, or nonpharmacological treatments. Furthermore, a better understanding of the mechanism of action of scopolamine could lead to the development of novel fast-onset antidepressants with improved efficacy and fewer side effects.
The reviewed literature indicates that scopolamine treatment produces rapid-onset antidepressant-like effects in rodents by antagonizing M1-AChRs on somatostatin interneurons in the mPFC, resulting in disinhibition of pyramidal glutamatergic neurons, a glutamate surge, and ultimately an increase in synapses120, 121. On the other hand, activating M1-AChRs increases depression-like behaviors in humans and animals86, 95, 166. Stimulation of presynaptic M2-AChRs and M4-AChRs decreases acetylcholine release when activated, and hence agonists at these receptors might decrease depressive symptoms by reducing acetylcholine availability99. More work is needed to determine a potential role for M3–5-AChRs in the regulation of mood.
Animal studies have suggested that co-treatment with scopolamine and other antidepressants such as venlafaxine or noradrenaline reuptake inhibitors may potentiate antidepressant-like effects167, 168. As discussed above, more selective muscarinic or nicotinic agents such as M1-AChR antagonists, and nicotinic α7 and β2* agents, also require further investigation as potential therapeutics for the treatment of depression. Both nicotinic and muscarinic receptors also regulate other neurotransmitter systems implicated in mood regulation, including noradrenergic169, 170, serotonergic171–173, dopaminergic84, 174, GABAergic175–177, glutamaniergic178–180 and cannabinoid181 neurotransmitter systems. Investigation into how the acetylcholine neurotransmitter system interacts with other neurotransmitters, neuromodulators, and epigenetic factors to modulate mood state will be an important direction for future research.
CONCLUSIONS
The catecholaminergic-cholinergic balance hypothesis of depression and mania proposes that a central predominance of acetylcholinergic to catecholaminergic tone underlies depression, while mania results from the converse15. Evidence to date implicates higher central acetylcholine levels in depression, and antagonism of M1-AChRs by scopolamine in fast-onset antidepressant effects. Blockade of α7 nAChRs receptors and partial agonism of β2* nAChRs are also implicated in antidepressant-like effects. Future studies are needed to identify optimal pharmacological strategies for treating depression by harnessing the acetylcholinergic system.
Figure 1.
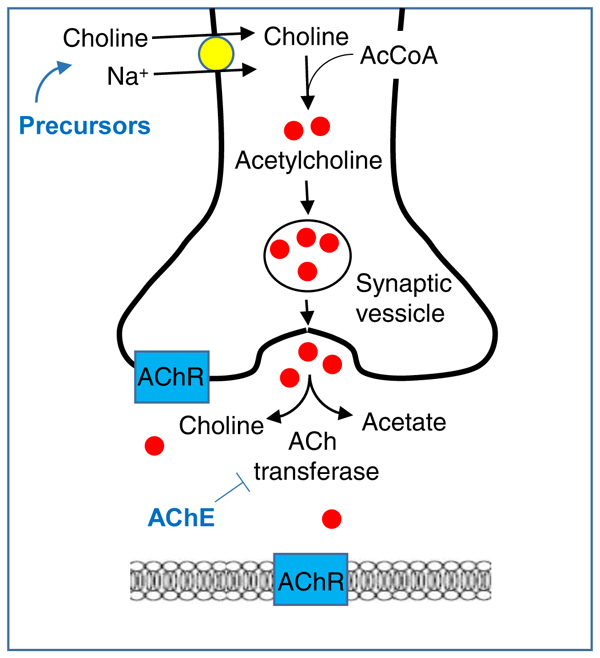
Acetylcholine synthesis and degradation, and the actions of pharmacological interventions. Acetylcholine (ACh) is synthesized in neurons from choline and acetyl-coenzyme A by the enzyme acetyltransferase. ACh is protected from degradation by packaging within synaptic vesicles. ACh is released into the synaptic cleft where it acts upon pre- and postsynaptic muscarinic and nicotinic receptors, and degraded into choline and acetate by the enzyme acetylcholinesterase (AChE). Choline is recycled back into neurons. AChE inhibitors (AChEIs) such as physostigmine and donepezil prevent the breakdown of ACh. Precursors such as deanol and choline contribute to ACh synthesis. Abbreviations: AcCoA, acetyl coenzyme A; AChR, acetylcholine receptor.
Figure 2.
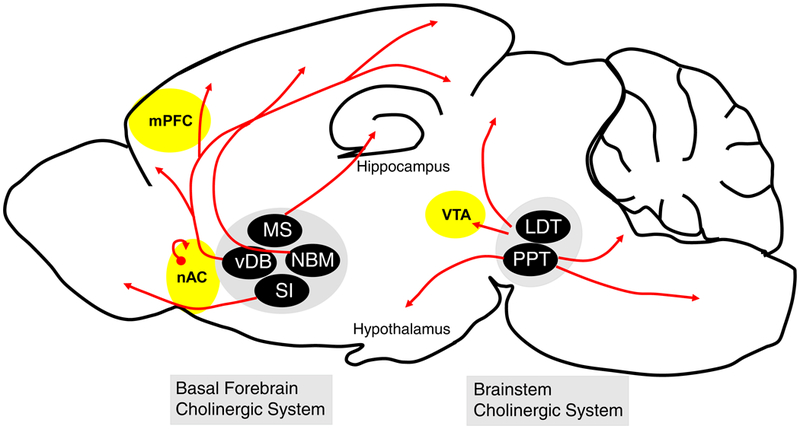
Sites of action of scopolamine’s rapid antidepressant-like effects within cholinergic circuitry. Rodent studies have shown that acute scopolamine treatment can induce fast-onset antidepressant effects when administered within the mPFC, nAC, and VTA (shown in yellow). Cholinergic innervation of the mPFC and VTA is supplied by the basal forebrain and brainstem cholinergic systems, respectively. The only source of acetylcholine within the nAC comes from local cholinergic interneurons (shown in red). Abbreviations: nAC, nucleus accumbens; mPFC, medial prefrontal cortex; VTA, ventral tegmental area; MS, medial septal nucleus; vDB, vertical diagonal band; NBM, nucleus basalis of Meynert; SI, substantia innominata; LDT, laterodorsal tegmental nucleus; PPT, pedunculopontine tegmental nucleus.
Box 1.
Future research directions
Further explore potential antidepressant effects of nAChR agents in humans and animals.
Further elucidate the similarities and differences in the mechanisms of antidepressant action of scopolamine versus ketamine using animal models.
Longitudinal imaging studies assessing M2-AChR receptor expression during illness and remission.
Further elucidate the mechanism of action of scopolamine to identify novel targets for antidepressant development.
Identify biomarkers that identify responders to scopolamine versus ketamine treatment.
Replicate oral and i.v. trials of scopolamine in affective disorder patients.
Use human imaging studies and animal models to identify the neural circuits involved in scopolamine’s fast-onset antidepressant effects.
Abbreviations: M2-AChR, muscarinic 2 acetylcholine receptor.
Acknowledgements:
S.C.D. was funded by a Rising Star Depression Research Award in Memory of George Largay, R21-MH115395-01, and a NARSAD Independent Investigator Award.
Footnotes
Conflict of interest:
Drs. Dulawa and Janowsky declare no conflict of interest.
Bibliography
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289(23): 3095–3105. [DOI] [PubMed] [Google Scholar]
- 2.Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry 2011; 68(3): 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet 2016; 387(10027): 1561–1572. [DOI] [PubMed] [Google Scholar]
- 4.Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin GM, Young AH et al. The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry 2017; 22(5): 666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenblat JD, McIntyre RS, Alves GS, Fountoulakis KN, Carvalho AF. Beyond Monoamines-Novel Targets for Treatment-Resistant Depression: A Comprehensive Review. Curr Neuropharmacol 2015; 13(5): 636–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 1996; 29(1): 2–11. [DOI] [PubMed] [Google Scholar]
- 7.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry 2006; 63(10): 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furey ML, Drevets WC, Szczepanik J, Khanna A, Nugent A, Zarate CA Jr. Pretreatment Differences in BOLD Response to Emotional Faces Correlate with Antidepressant Response to Scopolamine. Int J Neuropsychopharmacol 2015; 18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology 2010; 35(12): 2479–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furey ML, Nugent AC, Speer AM, Luckenbaugh DA, Hoffman EM, Frankel E et al. Baseline mood-state measures as predictors of antidepressant response to scopolamine. Psychiatry Res 2012; 196(1): 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellis JS, Zarate CA Jr., Luckenbaugh DA, Furey ML. Antidepressant treatment history as a predictor of response to scopolamine: clinical implications. J Affect Disord 2014; 162: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowntree DW, Nevin S, Wilson A. The effects of diisopropylfluorophosphonate in schizophrenia and manic depressive psychosis. J Neurol Neurosurg Psychiatry 1950; 13(1): 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet 1961; 1(7191): 1371–1374. [DOI] [PubMed] [Google Scholar]
- 14.Bowers MB Jr., Goodman E, Sim VM. Some Behavioral Changes in Man Following Anticholinesterase Administration. J Nerv Ment Dis 1964; 138: 383–389. [DOI] [PubMed] [Google Scholar]
- 15.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet 1972; 2(7778): 632–635. [DOI] [PubMed] [Google Scholar]
- 16.van Enkhuizen J, Janowsky DS, Olivier B, Minassian A, Perry W, Young JW et al. The catecholaminergic-cholinergic balance hypothesis of bipolar disorder revisited. Eur J Pharmacol 2015; 753: 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter WZ, Manji HK. Catecholamines in depression: an update. Clin Chem 1994; 40(2): 279–287. [PubMed] [Google Scholar]
- 18.Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol 2007; 21(5): 461–471. [DOI] [PubMed] [Google Scholar]
- 19.Grossman F, Potter WZ. Catecholamines in depression: a cumulative study of urinary norepinephrine and its major metabolites in unipolar and bipolar depressed patients versus healthy volunteers at the NIMH. Psychiatry Res 1999; 87(1): 21–27. [DOI] [PubMed] [Google Scholar]
- 20.Domino EF, Olds ME. Cholinergic inhibition of self-stimulation behavior. J Pharmacol Exp Ther 1968; 164(1): 202–211. [PubMed] [Google Scholar]
- 21.Olds ME, Domino EF. Comparison of muscarinic and nicotinic cholinergic agonists on self-stimulation behavior. J Pharmacol Exp Ther 1969; 166(2): 189–204. [PubMed] [Google Scholar]
- 22.Janowsky DS, el-Yousef MK, Davis JM, Hubbard B, Sekerke HJ. Cholinergic reversal of manic symptoms. Lancet 1972; 1(7762): 1236–1237. [DOI] [PubMed] [Google Scholar]
- 23.Carroll BJ, Frazer A, Schless A, Mendels J. Cholinergic reversal of manic symptoms. Lancet 1973; 1(7800): 427–428. [DOI] [PubMed] [Google Scholar]
- 24.Davis KL, Berger PA, Hollister LE, Defraites E. Physostigmine in mania. Arch Gen Psychiatry 1978; 35(1): 119–122. [DOI] [PubMed] [Google Scholar]
- 25.Janowsky DS, Risch C, Parker D, Huey L, Judd L. Increased vulnerability to cholinergic stimulation in affective-disorder patients [proceedings]. Psychopharmacol Bull 1980; 16(4): 29–31. [PubMed] [Google Scholar]
- 26.Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. J Clin Psychopharmacol 1981; 1(4): 186–192. [DOI] [PubMed] [Google Scholar]
- 27.Risch SC, Cohen RM, Janowsky DS, Kalin NH, Murphy DL. Mood and behavioral effects of physostigmine on humans are accompanied by elevations in plasma beta-endorphin and cortisol. Science 1980; 209(4464): 1545–1546. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds CF 3rd, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry 2011; 68(1): 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katona C. Dementia: Does depression predict donepezil response in MCI? Nat Rev Neurol 2009; 5(11): 585–586. [DOI] [PubMed] [Google Scholar]
- 30.Altinyazar V, Sirin FB, Sutcu R, Eren I, Omurlu IK. The Red Blood Cell Acetylcholinesterase Levels of Depressive Patients with Suicidal Behavior in an Agricultural Area. Indian J Clin Biochem 2016; 31(4): 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A et al. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry 2012; 169(8): 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamminga C, Smith RC, Chang S, Haraszti JS, Davis JM. Depression Associated with Oral Choline. Lancet 1976; 2(7991): 905–905. [DOI] [PubMed] [Google Scholar]
- 33.Growdon JH, Hirsch MJ, Wurtman RJ, Wiener W. Oral Choline Administration to Patients with Tardive-Dyskinesia. New Engl J Med 1977; 297(10): 524–527. [DOI] [PubMed] [Google Scholar]
- 34.DE C. Affective changes with deanol In: Davis KL B P (ed). Brain acetylcholine and neuropsychiatric disease. Plenum Press: New York, 1979. [Google Scholar]
- 35.Brown ES, Gabrielson B. A randomized, double-blind, placebo-controlled trial of citicoline for bipolar and unipolar depression and methamphetamine dependence. J Affect Disord 2012; 143(1–3): 257–260. [DOI] [PubMed] [Google Scholar]
- 36.Castagna A, Cotroneo AM, Ruotolo G, Gareri P. The CITIRIVAD Study: CITIcoline plus RIVAstigmine in Elderly Patients Affected with Dementia Study. Clin Drug Investig 2016; 36(12): 1059–1065. [DOI] [PubMed] [Google Scholar]
- 37.Gareri P, Castagna A, Cotroneo AM, Putignano D, Conforti R, Santamaria F et al. The Citicholinage Study: Citicoline Plus Cholinesterase Inhibitors in Aged Patients Affected with Alzheimer’s Disease Study. J Alzheimers Dis 2017; 56(2): 557–565. [DOI] [PubMed] [Google Scholar]
- 38.Roohi-Azizi M, Arabzadeh S, Amidfar M, Salimi S, Zarindast MR, Talaei A et al. Citicoline Combination Therapy for Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin Neuropharmacol 2017; 40(1): 1–5. [DOI] [PubMed] [Google Scholar]
- 39.Yerevanian BI, Woolf PD, Iker HP. Plasma ACTH levels in depression before and after recovery: relationship to the dexamethasone suppression test. Psychiatry Res 1983; 10(3): 175–181. [DOI] [PubMed] [Google Scholar]
- 40.Young EA, Ribeiro SC. Sex differences in the ACTH response to 24H metyrapone in depression. Brain Res 2006; 1126(1): 148–155. [DOI] [PubMed] [Google Scholar]
- 41.Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A et al. Pathophysiology of hypercortisolism in depression. Acta Psychiatr Scand Suppl 2007; (433): 90–103. [DOI] [PubMed] [Google Scholar]
- 42.Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ et al. Hypercortisolemia and hippocampal changes in depression. Psychiatry Res 1993; 47(2): 163–173. [DOI] [PubMed] [Google Scholar]
- 43.Bremmer MA, Deeg DJ, Beekman AT, Penninx BW, Lips P, Hoogendijk WJ. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry 2007; 62(5): 479–486. [DOI] [PubMed] [Google Scholar]
- 44.Risch SC. beta-Endorphin hypersecretion in depression: possible cholinergic mechanisms. Biol Psychiatry 1982; 17(10): 1071–1079. [PubMed] [Google Scholar]
- 45.Hegadoren KM, O’Donnell T, Lanius R, Coupland NJ, Lacaze-Masmonteil N. The role of beta-endorphin in the pathophysiology of major depression. Neuropeptides 2009; 43(5): 341–353. [DOI] [PubMed] [Google Scholar]
- 46.Goodwin GM, Austin MP, Curran SM, Ross M, Murray C, Prentice N et al. The elevation of plasma beta-endorphin levels in major depression. J Affect Disord 1993; 29(4): 281–289. [DOI] [PubMed] [Google Scholar]
- 47.Rubin RT, Rhodes ME, Miller TH, Jakab RL, Czambel RK. Sequence of pituitary-adrenal cortical hormone responses to low-dose physostigmine administration in young adult women and men. Life Sci 2006; 79(24): 2260–2268. [DOI] [PubMed] [Google Scholar]
- 48.Peskind ER, Raskind MA, Wingerson D, Pascualy M, Thal LJ, Dobie DJ et al. Enhanced hypothalamic-pituitary-adrenocortical axis responses to physostigmine in normal aging. J Gerontol A Biol Sci Med Sci 1995; 50(2): M114–120. [DOI] [PubMed] [Google Scholar]
- 49.Risch SC, Janowsky DS, Gillin JC. Muscarinic supersensitivity of anterior pituitary ACTH and B-endorphin release in major depressive illness. Peptides 1983; 4(5): 789–792. [DOI] [PubMed] [Google Scholar]
- 50.Doerr P, Berger M. Physostigmine-induced escape from dexamethasone suppression in normal adults. Biol Psychiatry 1983; 18(2): 261–268. [PubMed] [Google Scholar]
- 51.Rubin RT, O’Toole SM, Rhodes ME, Sekula LK, Czambel RK. Hypothalamo-pituitary-adrenal cortical responses to low-dose physostigmine and arginine vasopressin administration: sex differences between major depressives and matched control subjects. Psychiatry Res 1999; 89(1): 1–20. [DOI] [PubMed] [Google Scholar]
- 52.Modell S, Lauer CJ. Rapid eye movement (REM) sleep: an endophenotype for depression. Curr Psychiatry Rep 2007; 9(6): 480–485. [DOI] [PubMed] [Google Scholar]
- 53.Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev 2013; 17(5): 377–390. [DOI] [PubMed] [Google Scholar]
- 54.Modell S, Ising M, Holsboer F, Lauer CJ. The Munich vulnerability study on affective disorders: premorbid polysomnographic profile of affected high-risk probands. Biol Psychiatry 2005; 58(9): 694–699. [DOI] [PubMed] [Google Scholar]
- 55.Giles DE, Kupfer DJ, Roffwarg HP, Rush AJ, Biggs MM, Etzel BA. Polysomnographic parameters in first-degree relatives of unipolar probands. Psychiatry Res 1989; 27(2): 127–136. [DOI] [PubMed] [Google Scholar]
- 56.Sitaram N, Nurnberger JI Jr., Gershon ES, Gillin JC. Faster cholinergic REM sleep induction in euthymic patients with primary affective illness. Science 1980; 208(4440): 200–202. [DOI] [PubMed] [Google Scholar]
- 57.Biard K, Douglass AB, De Koninck J. The effects of galantamine and buspirone on sleep structure: Implications for understanding sleep abnormalities in major depression. J Psychopharmacol 2015; 29(10): 1106–1111. [DOI] [PubMed] [Google Scholar]
- 58.Gillin JC, Sitaram N, Mendelson WB, Wyatt RJ. Physostigmine alters onset but not duration of REM sleep in man. Psychopharmacology (Berl) 1978; 58(1): 111–114. [DOI] [PubMed] [Google Scholar]
- 59.Schredl M, Weber B, Leins ML, Heuser I. Donepezil-induced REM sleep augmentation enhances memory performance in elderly, healthy persons. Exp Gerontol 2001; 36(2): 353–361. [DOI] [PubMed] [Google Scholar]
- 60.Gillin JC, Sutton L, Ruiz C, Kelsoe J, Dupont RM, Darko D et al. The cholinergic rapid eye movement induction test with arecoline in depression. Arch Gen Psychiatry 1991; 48(3): 264–270. [DOI] [PubMed] [Google Scholar]
- 61.Sitaram N, Gillin JC. Development and use of pharmacological probes of the CNS in man: evidence of cholinergic abnormality in primary affective illness. Biol Psychiatry 1980; 15(6): 925–955. [PubMed] [Google Scholar]
- 62.Sitaram N, Nurnberger JI Jr., Gershon ES, Gillin JC. Cholinergic regulation of mood and REM sleep: potential model and marker of vulnerability to affective disorder. Am J Psychiatry 1982; 139(5): 571–576. [DOI] [PubMed] [Google Scholar]
- 63.Sitaram N, Dube S, Keshavan M, Davies A, Reynal P. The association of supersensitive cholinergic REM-induction and affective illness within pedigrees. J Psychiatr Res 1987; 21(4): 487–497. [DOI] [PubMed] [Google Scholar]
- 64.Lauriello J, Kenny WM, Sutton L, Golshan S, Ruiz C, Kelsoe J et al. The cholinergic REM sleep induction test with pilocarpine in mildly depressed patients and normal controls. Biol Psychiatry 1993; 33(1): 33–39. [DOI] [PubMed] [Google Scholar]
- 65.Berkowitz A, Sutton L, Janowsky DS, Gillin JC. Pilocarpine, an orally active muscarinic cholinergic agonist, induces REM sleep and reduces delta sleep in normal volunteers. Psychiatry Res 1990; 33(2): 113–119. [DOI] [PubMed] [Google Scholar]
- 66.Lauer CJ, Modell S, Schreiber W, Krieg JC, Holsboer F. Prediction of the development of a first major depressive episode with a rapid eye movement sleep induction test using the cholinergic agonist RS 86. J Clin Psychopharmacol 2004; 24(3): 356–357. [DOI] [PubMed] [Google Scholar]
- 67.Perlis ML, Smith MT, Orff HJ, Andrews PJ, Gillin JC, Giles DE. The effects of an orally administered cholinergic agonist on REM sleep in major depression. Biol Psychiatry 2002; 51(6): 457–462. [DOI] [PubMed] [Google Scholar]
- 68.Nurnberger J Jr., Sitaram N, Gershon ES, Gillin JC. A twin study of cholinergic REM induction. Biol Psychiatry 1983; 18(10): 1161–1165. [PubMed] [Google Scholar]
- 69.Kim EJ, Jeong DU. Transdermal scopolamine alters phasic REM activity in normal young adults. Sleep 1999; 22(4): 515–520. [DOI] [PubMed] [Google Scholar]
- 70.Rao U, Lutchmansingh P, Poland RE. Age-related effects of scopolamine on REM sleep regulation in normal control subjects: relationship to sleep abnormalities in depression. Neuropsychopharmacology 1999; 21(6): 723–730. [DOI] [PubMed] [Google Scholar]
- 71.Poland RE, McCracken JT, Lutchmansingh P, Lesser IM, Tondo L, Edwards C et al. Differential response of rapid eye movement sleep to cholinergic blockade by scopolamine in currently depressed, remitted, and normal control subjects. Biol Psychiatry 1997; 41(9): 929–938. [DOI] [PubMed] [Google Scholar]
- 72.Sokolski KN, Demet EM. Increased pupillary sensitivity to pilocarpine in depression. Prog Neuropsychopharmacol Biol Psychiatry 1996; 20(2): 253–262. [DOI] [PubMed] [Google Scholar]
- 73.Sokolski KN, DeMet EM. Cholinergic sensitivity predicts severity of mania. Psychiatry Res 2000; 95(3): 195–200. [DOI] [PubMed] [Google Scholar]
- 74.Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM et al. Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A 2013; 110(9): 3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Addy NA, Nunes EJ, Wickham RJ. Ventral tegmental area cholinergic mechanisms mediate behavioral responses in the forced swim test. Behav Brain Res 2015; 288: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mineur YS, Mose TN, Blakeman S, Picciotto MR. Hippocampal alpha7 nicotinic ACh receptors contribute to modulation of depression-like behaviour in C57BL/6J mice. Br J Pharmacol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mineur YS, Bentham MP, Zhou WL, Plantenga ME, McKee SA, Picciotto MR. Antidepressant-like effects of guanfacine and sex-specific differences in effects on c-fos immunoreactivity and paired-pulse ratio in male and female mice. Psychopharmacology (Berl) 2015; 232(19): 3539–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Enkhuizen J, Milienne-Petiot M, Geyer MA, Young JW. Modeling bipolar disorder in mice by increasing acetylcholine or dopamine: chronic lithium treats most, but not all features. Psychopharmacology (Berl) 2015; 232(18): 3455–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Druhan JP, Fibiger HC, Phillips AG. Differential effects of cholinergic drugs on discriminative cues and self-stimulation produced by electrical stimulation of the ventral tegmental area. Psychopharmacology (Berl) 1989; 97(3): 331–338. [DOI] [PubMed] [Google Scholar]
- 80.Dori A, Soreq H. ARP, the cleavable C-terminal peptide of “readthrough” acetylcholinesterase, promotes neuronal development and plasticity. J Mol Neurosci 2006; 28(3): 247–255. [DOI] [PubMed] [Google Scholar]
- 81.Kaufer D, Friedman A, Seidman S, Soreq H. Anticholinesterases induce multigenic transcriptional feedback response suppressing cholinergic neurotransmission. Chem Biol Interact 1999; 119–120: 349–360. [DOI] [PubMed] [Google Scholar]
- 82.Shaked I, Zimmerman G, Soreq H. Stress-induced alternative splicing modulations in brain and periphery: acetylcholinesterase as a case study. Ann N Y Acad Sci 2008; 1148: 269–281. [DOI] [PubMed] [Google Scholar]
- 83.Nijholt I, Farchi N, Kye M, Sklan EH, Shoham S, Verbeure B et al. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol Psychiatry 2004; 9(2): 174–183. [DOI] [PubMed] [Google Scholar]
- 84.Small KM, Nunes E, Hughley S, Addy NA. Ventral tegmental area muscarinic receptors modulate depression and anxiety-related behaviors in rats. Neurosci Lett 2016; 616: 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chau DT, Rada PV, Kim K, Kosloff RA, Hoebel BG. Fluoxetine alleviates behavioral depression while decreasing acetylcholine release in the nucleus accumbens shell. Neuropsychopharmacology 2011; 36(8): 1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chau DT, Rada P, Kosloff RA, Taylor JL, Hoebel BG. Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience 2001; 104(3): 791–798. [DOI] [PubMed] [Google Scholar]
- 87.Taylor KM, Mark GP, Hoebel BG. Conditioned taste aversion from neostigmine or methyl-naloxonium in the nucleus accumbens. Physiol Behav 2011; 104(1): 82–86. [DOI] [PubMed] [Google Scholar]
- 88.Chau D, Rada PV, Kosloff RA, Hoebel BG. Cholinergic, M1 receptors in the nucleus accumbens mediate behavioral depression. A possible downstream target for fluoxetine. Ann N Y Acad Sci 1999; 877: 769–774. [DOI] [PubMed] [Google Scholar]
- 89.Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A 2012; 109(28): 11360–11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El-Yousef MK, Janowsky DS, Davis JM, Rosenblatt JE. Induction of severe depression by physostigmine in marihuana intoxicated individuals. Br J Addict Alcohol Other Drugs 1973; 68(4): 321–325. [DOI] [PubMed] [Google Scholar]
- 91.Janowsky DS, Risch SC, Kennedy B, Ziegler M, Huey L. Central muscarinic effects of physostigmine on mood, cardiovascular function, pituitary and adrenal neuroendocrine release. Psychopharmacology (Berl) 1986; 89(2): 150–154. [DOI] [PubMed] [Google Scholar]
- 92.Nurnberger JI Jr., Jimerson DC, Simmons-Alling S, Tamminga C, Nadi NS, Lawrence D et al. Behavioral, physiological, and neuroendocrine responses to arecoline in normal twins and “well state” bipolar patients. Psychiatry Res 1983; 9(3): 191–200. [DOI] [PubMed] [Google Scholar]
- 93.Sunderland T, Tariot PN, Newhouse PA. Differential responsivity of mood, behavior, and cognition to cholinergic agents in elderly neuropsychiatric populations. Brain Res 1988; 472(4): 371–389. [DOI] [PubMed] [Google Scholar]
- 94.Davis KL, Hollander E, Davidson M, Davis BM, Mohs RC, Horvath TB. Induction of depression with oxotremorine in patients with Alzheimer’s disease. Am J Psychiatry 1987; 144(4): 468–471. [DOI] [PubMed] [Google Scholar]
- 95.Krieg JC, Berger M. Treatment of mania with the cholinomimetic agent RS 86. Br J Psychiatry 1986; 148: 613. [DOI] [PubMed] [Google Scholar]
- 96.Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med 1974; 36(3): 248–257. [DOI] [PubMed] [Google Scholar]
- 97.Janowsky DS, Risch SC, Kennedy B, Ziegler MG, Huey LY. Acute effects of physostigmine and neostigmine in man. Mil Med 1986; 151(1): 48–51. [PubMed] [Google Scholar]
- 98.Dohanich GP, Johnson AE, Nock B, McEwen BS, Feder HH. Distribution of cholinergic muscarinic binding sites in guinea-pig brain as determined by in vitro autoradiography of [3H]N-methyl scopolamine binding. Eur J Pharmacol 1985; 119(1–2): 9–16. [DOI] [PubMed] [Google Scholar]
- 99.Iannazzo L, Majewski H. M(2)/M(4)-muscarinic receptors mediate automodulation of acetylcholine outflow from mouse cortex. Neurosci Lett 2000; 287(2): 129–132. [DOI] [PubMed] [Google Scholar]
- 100.Cannon DM, Carson RE, Nugent AC, Eckelman WC, Kiesewetter DO, Williams J et al. Reduced muscarinic type 2 receptor binding in subjects with bipolar disorder. Arch Gen Psychiatry 2006; 63(7): 741–747. [DOI] [PubMed] [Google Scholar]
- 101.Charles HC, Lazeyras F, Krishnan KR, Boyko OB, Payne M, Moore D. Brain choline in depression: in vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry 1994; 18(7): 1121–1127. [DOI] [PubMed] [Google Scholar]
- 102.MacMaster FP, Kusumakar V. Choline in pediatric depression. Mcgill J Med 2006; 9(1): 24–27. [PMC free article] [PubMed] [Google Scholar]
- 103.Gibbons AS, Scarr E, McLean C, Sundram S, Dean B. Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord 2009; 116(3): 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zavitsanou K, Katsifis A, Yu Y, Huang XF. M2/M4 muscarinic receptor binding in the anterior cingulate cortex in schizophrenia and mood disorders. Brain Res Bull 2005; 65(5): 397–403. [DOI] [PubMed] [Google Scholar]
- 105.Zavitsanou K, Katsifis A, Mattner F, Huang XF. Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology 2004; 29(3): 619–625. [DOI] [PubMed] [Google Scholar]
- 106.Jeon WJ, Gibbons AS, Dean B. The use of a modified [3H]4-DAMP radioligand binding assay with increased selectivity for muscarinic M3 receptor shows that cortical CHRM3 levels are not altered in mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 2013; 47: 7–12. [DOI] [PubMed] [Google Scholar]
- 107.Milaneschi Y, Lamers F, Peyrot WJ, Abdellaoui A, Willemsen G, Hottenga JJ et al. Polygenic dissection of major depression clinical heterogeneity. Mol Psychiatry 2016; 21(4): 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peterson RE, Cai N, Dahl AW, Bigdeli TB, Edwards AC, Webb BT et al. Molecular Genetic Analysis Subdivided by Adversity Exposure Suggests Etiologic Heterogeneity in Major Depression. Am J Psychiatry 2018: appiajp201717060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry 1983; 7(2–3): 297–307. [DOI] [PubMed] [Google Scholar]
- 110.Janowsky DS, Overstreet DH, Nurnberger JI Jr. Is cholinergic sensitivity a genetic marker for the affective disorders? Am J Med Genet 1994; 54(4): 335–344. [DOI] [PubMed] [Google Scholar]
- 111.Coid J, Strang J. Mania secondary to procyclidine (“Kemadrin”) abuse. Br J Psychiatry 1982; 141: 81–84. [DOI] [PubMed] [Google Scholar]
- 112.Smith JM. Abuse of the antiparkinson drugs: a review of the literature. J Clin Psychiatry 1980; 41(10): 351–354. [PubMed] [Google Scholar]
- 113.Jellinek T, Gardos G, Cole JO. Adverse effects of antiparkinson drug withdrawal. Am J Psychiatry 1981; 138(12): 1567–1571. [DOI] [PubMed] [Google Scholar]
- 114.Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM et al. The effects of acute scopolamine in geriatric depression. Arch Gen Psychiatry 1988; 45(10): 906–912. [DOI] [PubMed] [Google Scholar]
- 115.Gillin JC, Lauriello J, Kelsoe JR, Rapaport M, Golshan S, Kenny WM et al. No antidepressant effect of biperiden compared with placebo in depression: a double-blind 6-week clinical trial. Psychiatry Res 1995; 58(2): 99–105. [DOI] [PubMed] [Google Scholar]
- 116.Gillin JC, Sutton L, Ruiz C, Darko D, Golshan S, Risch SC et al. The effects of scopolamine on sleep and mood in depressed patients with a history of alcoholism and a normal comparison group. Biol Psychiatry 1991; 30(2): 157–169. [DOI] [PubMed] [Google Scholar]
- 117.Khajavi D, Farokhnia M, Modabbernia A, Ashrafi M, Abbasi SH, Tabrizi M et al. Oral scopolamine augmentation in moderate to severe major depressive disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2012; 73(11): 1428–1433. [DOI] [PubMed] [Google Scholar]
- 118.Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ et al. Activity-Dependent Brain-Derived Neurotrophic Factor Release Is Required for the Rapid Antidepressant Actions of Scopolamine. Biol Psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Navarria A, Wohleb ES, Voleti B, Ota KT, Dutheil S, Lepack AE et al. Rapid antidepressant actions of scopolamine: Role of medial prefrontal cortex and M1-subtype muscarinic acetylcholine receptors. Neurobiol Dis 2015; 82: 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest 2016; 126(7): 2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 2013; 74(10): 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geoffroy M, Scheel-Kruger J, Christensen AV. Effect of imipramine in the “learned helplessness” model of depression in rats is not mimicked by combinations of specific reuptake inhibitors and scopolamine. Psychopharmacology (Berl) 1990; 101(3): 371–375. [DOI] [PubMed] [Google Scholar]
- 123.Ramaker MJ, Dulawa SC. Identifying fast-onset antidepressants using rodent models. Mol Psychiatry 2017. [DOI] [PubMed] [Google Scholar]
- 124.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475(7354): 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533(7604): 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329(5994): 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. J Pharmacol Exp Ther 2014; 351(2): 448–456. [DOI] [PubMed] [Google Scholar]
- 128.Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol 1992; 60(5): 689–697. [DOI] [PubMed] [Google Scholar]
- 129.Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry 1991; 48(1): 52–59. [DOI] [PubMed] [Google Scholar]
- 130.Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Compr Psychiatry 1990; 31(4): 350–354. [DOI] [PubMed] [Google Scholar]
- 131.Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry 1997; 154(2): 263–265. [DOI] [PubMed] [Google Scholar]
- 132.Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology (Berl) 2006; 184(3–4): 637–644. [DOI] [PubMed] [Google Scholar]
- 133.McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2006; 189(1): 125–133. [DOI] [PubMed] [Google Scholar]
- 134.Salin-Pascual RJ, Rosas M, Jimenez-Genchi A, Rivera-Meza BL, Delgado-Parra V. Antidepressant effect of transdermal nicotine patches in nonsmoking patients with major depression. J Clin Psychiatry 1996; 57(9): 387–389. [PubMed] [Google Scholar]
- 135.Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R. Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology (Berl) 1995; 121(4): 476–479. [DOI] [PubMed] [Google Scholar]
- 136.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 1998; 18(3): 135–174. [DOI] [PubMed] [Google Scholar]
- 137.Fava M, Ramey T, Pickering E, Kinrys G, Boyer S, Altstiel L. A randomized, double-blind, placebo-controlled phase 2 study of the augmentation of a nicotinic acetylcholine receptor partial agonist in depression: is there a relationship to leptin levels? J Clin Psychopharmacol 2015; 35(1): 51–56. [DOI] [PubMed] [Google Scholar]
- 138.Wang HR, Woo YS, Bahk WM. Ineffectiveness of nicotinic acetylcholine receptor antagonists for treatment-resistant depression: a meta-analysis. Int Clin Psychopharmacol 2016; 31(5): 241–248. [DOI] [PubMed] [Google Scholar]
- 139.Moller HJ, Demyttenaere K, Olausson B, Szamosi J, Wilson E, Hosford D et al. Two Phase III randomised double-blind studies of fixed-dose TC-5214 (dexmecamylamine) adjunct to ongoing antidepressant therapy in patients with major depressive disorder and an inadequate response to prior antidepressant therapy. World J Biol Psychiatry 2015; 16(7): 483–501. [DOI] [PubMed] [Google Scholar]
- 140.Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry 2002; 7(6): 525–535. [DOI] [PubMed] [Google Scholar]
- 141.Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther 2000; 295(1): 321–327. [PubMed] [Google Scholar]
- 142.Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav 2001; 70(4): 531–549. [DOI] [PubMed] [Google Scholar]
- 143.Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S et al. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron 2011; 70(3): 522–535. [DOI] [PubMed] [Google Scholar]
- 144.Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci 2004; 24(45): 10035–10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol 2003; 63(5): 1059–1066. [DOI] [PubMed] [Google Scholar]
- 146.Upton M, Lotfipour S. alpha2-Null mutant mice have altered levels of neuronal activity in restricted midbrain and limbic brain regions during nicotine withdrawal as demonstrated by cfos expression. Biochem Pharmacol 2015; 97(4): 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 2006; 31(6): 1203–1211. [DOI] [PubMed] [Google Scholar]
- 148.Andreasen JT, Redrobe JP. Nicotine, but not mecamylamine, enhances antidepressant-like effects of citalopram and reboxetine in the mouse forced swim and tail suspension tests. Behav Brain Res 2009; 197(1): 150–156. [DOI] [PubMed] [Google Scholar]
- 149.Andreasen JT, Henningsen K, Bate S, Christiansen S, Wiborg O. Nicotine reverses anhedonic-like response and cognitive impairment in the rat chronic mild stress model of depression: comparison with sertraline. J Psychopharmacol 2011; 25(8): 1134–1141. [DOI] [PubMed] [Google Scholar]
- 150.Esterlis I, Hannestad JO, Bois F, Sewell RA, Tyndale RF, Seibyl JP et al. Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med 2013; 54(1): 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hannestad JO, Cosgrove KP, DellaGioia NF, Perkins E, Bois F, Bhagwagar Z et al. Changes in the cholinergic system between bipolar depression and euthymia as measured with [123I]5IA single photon emission computed tomography. Biol Psychiatry 2013; 74(10): 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Villegier AS, Gallager B, Heston J, Belluzzi JD, Leslie FM. Age influences the effects of nicotine and monoamine oxidase inhibition on mood-related behaviors in rats. Psychopharmacology (Berl) 2010; 208(4): 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E Jr., Janowsky DS et al. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 1999; 142(2): 193–199. [DOI] [PubMed] [Google Scholar]
- 154.Haj-Mirzaian A, Kordjazy N, Haj-Mirzaian A, Ostadhadi S, Ghasemi M, Amiri S et al. Evidence for the involvement of NMDA receptors in the antidepressant-like effect of nicotine in mouse forced swimming and tail suspension tests. Psychopharmacology (Berl) 2015; 232(19): 3551–3561. [DOI] [PubMed] [Google Scholar]
- 155.Vieyra-Reyes P, Mineur YS, Picciotto MR, Tunez I, Vidaltamayo R, Drucker-Colin R. Antidepressant-like effects of nicotine and transcranial magnetic stimulation in the olfactory bulbectomy rat model of depression. Brain Res Bull 2008; 77(1): 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacol 2009; 20(3): 286–295. [DOI] [PubMed] [Google Scholar]
- 157.Andreasen JT, Olsen GM, Wiborg O, Redrobe JP. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol 2009; 23(7): 797–804. [DOI] [PubMed] [Google Scholar]
- 158.Aboul-Fotouh S. Behavioral effects of nicotinic antagonist mecamylamine in a rat model of depression: prefrontal cortex level of BDNF protein and monoaminergic neurotransmitters. Psychopharmacology (Berl) 2015; 232(6): 1095–1105. [DOI] [PubMed] [Google Scholar]
- 159.Mineur YS, Fote GM, Blakeman S, Cahuzac EL, Newbold SA, Picciotto MR. Multiple Nicotinic Acetylcholine Receptor Subtypes in the Mouse Amygdala Regulate Affective Behaviors and Response to Social Stress. Neuropsychopharmacology 2016; 41(6): 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA et al. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline’s effect. Eur J Pharmacol 2009; 605(1–3): 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reperant C, Pons S, Dufour E, Rollema H, Gardier AM, Maskos U. Effect of the alpha4beta2* nicotinic acetylcholine receptor partial agonist varenicline on dopamine release in beta2 knock-out mice with selective re-expression of the beta2 subunit in the ventral tegmental area. Neuropharmacology 2010; 58(2): 346–350. [DOI] [PubMed] [Google Scholar]
- 162.Philip NS, Carpenter LL, Tyrka AR, Whiteley LB, Price LH. Varenicline augmentation in depressed smokers: an 8-week, open-label study. J Clin Psychiatry 2009; 70(7): 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mineur YS, Einstein EB, Seymour PA, Coe JW, O’Neill BT, Rollema H et al. alpha 4 beta 2 nicotinic acetylcholine receptor partial agonists with low intrinsic efficacy have antidepressant-like properties. Behavioural Pharmacology 2011; 22(4): 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mineur YS, Cahuzac EL, Mose TN, Bentham MP, Plantenga ME, Thompson DC et al. Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016; 91(6): 1199–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rada P, Colasante C, Skirzewski M, Hernandez L, Hoebel B. Behavioral depression in the swim test causes a biphasic, long-lasting change in accumbens acetylcholine release, with partial compensation by acetylcholinesterase and muscarinic-1 receptors. Neuroscience 2006; 141(1): 67–76. [DOI] [PubMed] [Google Scholar]
- 167.Singh P, Singh TG. Modulation of muscarinic system with serotonin-norepinephrine reuptake inhibitor antidepressant attenuates depression in mice. Indian J Pharmacol 2015; 47(4): 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Palucha-Poniewiera A, Podkowa K, Lenda T, Pilc A. The involvement of monoaminergic neurotransmission in the antidepressant-like action of scopolamine in the tail suspension test. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79(Pt B): 155–161. [DOI] [PubMed] [Google Scholar]
- 169.Beiranvand F, Zlabinger C, Orr-Urtreger A, Ristl R, Huck S, Scholze P. Nicotinic acetylcholine receptors control acetylcholine and noradrenaline release in the rodent habenulo-interpeduncular complex. Br J Pharmacol 2014; 171(23): 5209–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Barik J, Wonnacott S. Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol 2006; 69(2): 618–628. [DOI] [PubMed] [Google Scholar]
- 171.Kenny PJ, File SE, Neal MJ. Evidence for a complex influence of nicotinic acetylcholine receptors on hippocampal serotonin release. J Neurochem 2000; 75(6): 2409–2414. [DOI] [PubMed] [Google Scholar]
- 172.Dougherty JJ, Nichols RA. Cross-regulation between colocalized nicotinic acetylcholine and 5-HT3 serotonin receptors on presynaptic nerve terminals. Acta Pharmacol Sin 2009; 30(6): 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schwartz RD, Lehmann J, Kellar KJ. Presynaptic nicotinic cholinergic receptors labeled by [3H]acetylcholine on catecholamine and serotonin axons in brain. J Neurochem 1984; 42(5): 1495–1498. [DOI] [PubMed] [Google Scholar]
- 174.Sharples CG, Kaiser S, Soliakov L, Marks MJ, Collins AC, Washburn M et al. UB-165: a novel nicotinic agonist with subtype selectivity implicates the alpha4beta2* subtype in the modulation of dopamine release from rat striatal synaptosomes. J Neurosci 2000; 20(8): 2783–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol 2001; 536(Pt 1): 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aracri P, Consonni S, Morini R, Perrella M, Rodighiero S, Amadeo A et al. Tonic modulation of GABA release by nicotinic acetylcholine receptors in layer V of the murine prefrontal cortex. Cereb Cortex 2010; 20(7): 1539–1555. [DOI] [PubMed] [Google Scholar]
- 177.Li DP, Pan YZ, Pan HL. Acetylcholine attenuates synaptic GABA release to supraoptic neurons through presynaptic nicotinic receptors. Brain Res 2001; 920(1–2): 151–158. [DOI] [PubMed] [Google Scholar]
- 178.Garduno J, Galindo-Charles L, Jimenez-Rodriguez J, Galarraga E, Tapia D, Mihailescu S et al. Presynaptic alpha4beta2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus. J Neurosci 2012; 32(43): 15148–15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bortz DM, Mikkelsen JD, Bruno JP. Localized infusions of the partial alpha 7 nicotinic receptor agonist SSR180711 evoke rapid and transient increases in prefrontal glutamate release. Neuroscience 2013; 255: 55–67. [DOI] [PubMed] [Google Scholar]
- 180.Reno LA, Zago W, Markus RP. Release of [(3)H]-L-glutamate by stimulation of nicotinic acetylcholine receptors in rat cerebellar slices. Neuroscience 2004; 124(3): 647–653. [DOI] [PubMed] [Google Scholar]
- 181.Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci 2002; 22(23): 10182–10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Risch SC, Siever LJ, Gillin JC, Janowsky DS, Sitaram N, Weker J et al. Differential Mood Effects of Arecoline in Depressed-Patients and Normal Volunteers. Psychopharmacology Bulletin 1983; 19(4): 696–698. [Google Scholar]
- 183.Casey DE. Mood alterations during deanol therapy. Psychopharmacology (Berl) 1979; 62(2): 187–191. [DOI] [PubMed] [Google Scholar]


