Abstract
The population structure of Sclerotium rolfsii from autumn‐sown sugar beet crops in Mediterranean‐type climate regions of Chile, Italy, Portugal and Spain was determined by analyses of mycelial compatibility groups (MCGs) and pathogenicity to 11 economically important plant species. Twelve MCGs (i–xii) were identified among 459 S. rolfsii isolates. MCG iii was the most prevalent group in all countries except Italy. MCG i, the most abundant group (64·7% of isolates) was identified in Portugal and Spain. The remaining MCGs were restricted to various regions within one country (ii, vi, ix) or different countries (v), or to specific localities (iv, vii, viii, x, xi, xii). MCGs iv, vii and x each comprised one isolate. Fields extensively sampled in southern Spain were infected with one to three MCGs. Plant species differed in susceptibility to MCG tester isolates with a MCG by species interaction. Cluster analyses allowed selection into five MCG groupings and grouped plant species into species‐groups 1 (broccoli, chickpea, sunflower, tomato) and 2 (cotton, pepper, sugar beet, watermelon). MCG groupings 1 (i, ix), 2 (ii, iii, vi, viii) and 5 (x, xii) were moderately virulent to species‐group 1 and mildly virulent to species‐group 2. MCG groupings 3 (iv, v, xi) and 4 (vii) were mildly virulent to both species‐groups. Across MCG groups, species were rated highly susceptible (chickpea, sunflower), susceptible (cotton, pepper, tomato, watermelon), moderately resistant (broccoli, melon, sugar beet) and resistant (corn, wheat). Establishing the MCG population structure and virulence variability among S. rolfsii isolates should help in the management of sclerotium root rot diseases.
Keywords: Beta vulgaris, cluster analysis, host range, MCG
Introduction
Sclerotium rolfsii is the mycelial stage of the basidiomycete Athelia rolfsii, a worldwide soilborne plant pathogenic fungus that lacks a conidial stage. The host range of S. rolfsii comprises over 500 plant species, mostly dicotyledons, but also some monocotyledons (Harlton et al., 1995; Okabe et al., 1998; Cilliers et al., 2000). The main disease symptoms caused by this pathogen include crown and root rot, stem canker or damping‐off. The resulting disease is referred to as southern blight, southern stem rot, or sclerotium root rot (Aycock, 1966; Punja, 1985). S. rolfsii produces abundant coarse, white mycelia on infected host tissues and forms sclerotia (Punja, 1985). The fungus overwinters in soil by means of sclerotia, as well as by mycelia in infected plants or infested plant debris (Punja, 1985). It can form a sexual stage, but this rarely occurs in nature and its role in the life cycle of the fungus is unknown (Nalim et al., 1995).
Sclerotium rolfsii has been reported from nearly every country between northern and southern latitudes of 38°. However, disease epidemics are most severe in warm temperate and subtropical regions that favour sclerotial germination, mycelial growth, infection and subsequent disease development (Aycock, 1966; Punja, 1985). These climatic conditions occur in the Mediterranean basin, southern California and Chile, where outbreaks of S. rolfsii often occur on autumn‐sown sugar beet (Beta vulgaris) crops.
Disease surveys conducted in Andalusia, southern Spain, in 2004 showed that sclerotium root rot of sugar beet is widespread in autumn‐sown crops in the southernmost part of the region, causing 5–80% yield loss (AIMCRA, 2005). Similarly, the disease severely affects sugar beet crops in central and southern Portugal (M. Paim, Associação para Desenvolvimento da Beterraba, Portugal, personal communication) and the central area of Chile (regions VI to VII) (R. Paillalef‐Monnrad, IANSAGRO, Chile, personal communication).
As a cosmopolitan soilborne plant pathogen, knowledge of its genetic diversity and distribution in infested areas would be useful for the management of sclerotium root rot diseases. Characterization of fungal populations into mycelial compatibility groups (MCGs) has been widely used for indirectly assessing the genetic variability among isolates of fungal plant pathogens (Leslie, 1993). MCGs play an important role both in defining field populations of fungi and facilitating genetic exchange in fungal species. This is particularly relevant for fungi for which sexual reproduction has a minimal impact on the disease cycle (Kohn et al., 1991; Leslie, 1993) because MCGs help to preserve the identity of genetically dissimilar mycelia and restrict the exchange of cytoplasm, genetic material and extrachromosomal elements (Caten, 1972). Mycelial incompatibility results in separate and distinct gene pools, which may differ in ecological, physiological and pathological traits. Mycelial compatibility studies have been used to estimate genetic variability or relatedness within many fungal species of the Basidiomycota such as Sclerotium cepivorum (Earnshaw et al., 2000) and Rhizoctonia solani (Carling et al., 2002; Ciampi et al., 2008), as well as ascomycetes such as Fusarium spp. (Puhalla, 1985; Elias et al., 1993; Chulze et al., 2000; Bayraktar et al., 2010), Cryphonectria spp. (van Heerden & Wingfield, 2001), Verticillium dahliae (Korolev et al., 2000; Collado‐Romero et al., 2006) and Sclerotinia sclerotiorum (Kohn et al., 1991).
Mycelial compatibility was demonstrated to occur between field isolates of S. rolfsii, and has been used to designate MCGs (Punja & Grogan, 1983) as well as to assess the genetic variability among S. rolfsii populations (Punja & Grogan, 1983; Harlton et al., 1995; Nalim et al., 1995; 2000, 2002; Okabe & Matsumoto, 2000; Almeida et al., 2001; Punja & Sun, 2001; Sarma et al., 2002; Adandonon et al., 2005). These studies suggested that MCGs in S. rolfsii can be associated with either the host source or geographical area of isolates, but also a single MCG can comprise isolates from diverse geographical origins and host sources. The variability of mycelial compatibility among S. rolfsii populations occurring in particular geographic regions or specific crops has also been demonstrated. Studies in Brazil (Almeida et al., 2001), India (Sarma et al., 2002) and South Africa (Cilliers et al., 2002) indicated that a considerable genetic variability existed among S. rolfsii isolates from various hosts and localities without clear correlation between the MCG to which they belonged and their host source or geographic origin (Almeida et al., 2001; Sarma et al., 2002), although a certain relationship between MCGs and host source of isolates was suggested for S. rolfsii populations in South Africa (Cilliers et al., 2002). On the other hand, in peanut, wide variation was reported among isolates within a field in Texas, USA (Nalim et al., 1995) and Japan (Okabe & Matsumoto, 2000), with a single peanut field harbouring up to three (Okabe & Matsumoto, 2000) or five (Nalim et al., 1995) MCGs.
Control of sclerotium root rot diseases is difficult because of the extensive mycelial growth, persistence in soil, genetic variability of populations and wide host range of S. rolfsii. In sugar beet, no resistant cultivars are available and attempts to control the disease with fungicides or biological control agents have been unsuccessful (Lal et al., 1997). However, a degree of resistance to S. rolfsii was found in some cultivars of cowpea (Fery & Dukes, 2002), alfalfa (Pratt & Rowe, 2002), peanut (Branch & Brenneman, 1999), pepper, sweet potato (Dukes et al., 1983) and chickpea (Akram et al., 2008). Reports from California and India indicate that the pathogen inoculum density can be effectively reduced by rotations of sugar beet with crops that are slightly susceptible to S. rolfsii, such as alfalfa, asparagus, barley, corn and wheat (Schneider & Whitney, 1996). This same strategy was also effective with rotations of susceptible carrot with sweet potato or buckwheat (Jenkins & Averre, 1986) and peanut with bahiagrass, corn, cotton (Johnson et al., 1999) or wheat (Minton et al., 1991). Knowledge about the genetic and virulence diversity in local populations of S. rolfsii associated with different crops is a key component for the management of sclerotium root rot diseases, particularly through the use of host resistance and crop rotation in a given region. In addition, knowledge of the host range of S. rolfsii populations present in a given area is essential for recommending suitable crops as an alternative to sugar beet production. Unfortunately, information of that nature is lacking in areas of Mediterranean‐type climate. Furthermore, the sugar beet production area in the Mediterranean Basin has steadily decreased in Italy, Portugal and southern Spain during the last few years, being replaced by vegetable crops that can be potentially affected by sclerotium root rot.
In the present study, a large collection of S. rolfsii isolates from sclerotium root rot‐affected sugar beet crops in four countries with a Mediterranean‐type climate and eight intensively sampled fields in southern Spain were used to: (i) assess S. rolfsii MCG diversity and geographical distribution; (ii) determine within‐field diversity and prevalence of S. rolfsii MCGs in sugar beet fields of southern Spain; (iii) determine any correlation that might exist between MCGs of S. rolfsii isolates from sugar beet and their pathogenicity and virulence to 11 agricultural crops; and (iv) determine the pathogenic variability within MCGs on three selected susceptible hosts.
Materials and methods
Sampling and isolation of the pathogen
Two sampling strategies were used to account for variability of S. rolfsii on different spatial scales. Sugar beet field plots located in southern Spain were intensively sampled to determine variability at field level. For that purpose, an area of c. 0·06 ha in each field plot was divided into a regular grid of 80 (4‐ × 2‐m) quadrants, and one affected sugar beet root was sampled from each quadrant. For the second spatial scale, whole fields in Chile, Italy and Portugal were arbitrarily selected to determine pathogen variability among fields with at least four arbitrarily chosen affected roots sampled from each field. In all cases, the sampled area was representative of the sugar beet fields affected by sclerotium root rot in each country.
For isolation of S. rolfsii, root tissues with symptoms were washed under running tap water, surface‐sterilized in 0·5% NaOCl for 1 min and blotted dry between sterile filter papers. Pieces of surface‐sterilized tissues (2–4 mm2) were plated onto potato dextrose agar (PDA, Difco Laboratories) amended with 0·25 mL 85% lactic acid and incubated at 25 ± 1°C in the dark for 2–5 days. Pure cultures forming sclerotia were obtained from each root sample. Single‐sclerotia cultures obtained from pure cultures were incubated to form abundant sclerotia and were allowed to dry at room temperature. Sclerotia from dry cultures were collected, dried and stored in paper bags at room temperature until use.
Isolate collection
A total of 459 S. rolfsii isolates were obtained from sclerotium‐root‐rot‐affected sugar beet crops sampled at a total of 18 localities from Chile (four localities, 22 isolates), Italy (one locality, one isolate), Portugal (five localities, 63 isolates) and Spain (eight localities, 373 isolates) from 2004 to 2007 (Table 1).
Table 1.
Geographic origin and mycelial compatibility group (MCG) of 459 Sclerotium rolfsii isolates infecting sugar beet used in this study
| Country | Geographic origin | No. of isolates | MCG (no. of isolates)a | Isolate code | |
|---|---|---|---|---|---|
| Province | Locality | ||||
| Chile | VI | Colchagua | 1 | vii (1) | Sr1 |
| VII | Curicó | 2 | iii (2) | Sr2, Sr3 | |
| Linares | 8 | viii (3), ix (1), xii (4) | viii: Sr4–Sr6; ix: Sr7; xii: Sr8–Sr11 | ||
| VIII | Chillán | 11 | iii (1), ix (9), x (1) | iii: Sr12; ix: Sr13–21; x: Sr22 | |
| Spain | Cádiz | Arcos Ftra. | 48 | i (48) | Sr23–Sr70 |
| Jerez Ftra. | 50 | i (43), iii (7) | i: Sr71–Sr113; iii: Sr114–Sr120 | ||
| Vejer Ftra. | 35 | i (19), v (16) | i: Sr121–Sr139; v: Sr140–Sr155 | ||
| Córdoba | Fuente Palmera | 37 | i (37) | Sr156–Sr192 | |
| Posadas | 31 | i (4), ii (17), iii (10) | i: Sr193–Sr196; ii: Sr197–Sr213; iii: Sr214–Sr223 | ||
| Huelva | Paterna Campo | 64 | i (62), xi (2) | i: Sr224–Sr285; xi: Sr286, Sr287 | |
| Seville | Lebrija | 28 | i (3), iii (25) | i: Sr288–Sr290; iii: Sr291–Sr315 | |
| Los Palacios | 80 | i (79), ii (1) | i: Sr316–Sr394; ii: Sr395 | ||
| Italy | Bari | Bari | 1 | iv (1) | iv: Sr396 |
| Portugal | Lisbon | Vila Franca de Xira | 26 | i (2), iii (5), v (10), vi (8) | i: Sr406,Sr407; iii: Sr408–Sr413; v: Sr414–Sr423; vi: Sr424–Sr431 |
| Portalegre | Avis | 12 | iii (12) | Sr432–Sr443 | |
| Benavila | 8 | iii (8) | Sr444–Sr451 | ||
| Elvas | 10 | iii (10) | Sr452–Sr461 | ||
| Santarém | Coruche | 7 | iii (6), vi (1) | iii: Sr462–Sr467; vi: Sr468 | |
aMCG typing was done in this study.
Determination of mycelial compatibility groups
A single sclerotium from each of the S. rolfsii isolates was placed on a PDA plate and incubated in the dark at 25 ± 1°C for 5 days. Then, small plugs from the inner colony were transferred to modified Patterson’s medium (MPM) in new plates (90‐mm diameter) and incubated for 5 days in the same conditions. This MPM allows improved assessment of mycelial interactions among S. rolfsii isolates (E. Remesal & J. A. Navas‐Cortés, unpublished results). Mycelial discs from the edges of actively growing MPM colonies of isolates were paired on MPM plates. Three isolates were paired on a 90‐mm‐diameter plate by placing discs 50 mm apart and incubating at 25 ± 1°C in the dark. There were four replicated plates per pairing. All isolates were paired with themselves to ensure self‐compatibility. All pairings were repeated twice. Pairings were examined macroscopically after 5 and 10 days of incubation for the presence of an antagonism zone in the region of mycelial contact (Punja & Grogan, 1983) and the presence of a coloured red line in the reaction zone between the colonies (Fig. 1a,b). Mycelial compatibility reactions showed no antagonism and were distinguished by merging colonies with no detectable coloured red line between the interacting colonies (Fig. 1c). MCGs were identified based on data from compatible reactions among tested isolates. Because of the high number of S. rolfsii isolates and difficulty of pairing them in all possible combinations, MCGs were defined first at field plot level, pairing isolates from a plot in all possible combinations. Then, one tester isolate was arbitrarily selected from each of the identified MCGs for further analyses. Representative tester isolates from all the identified MCGs in each field plot were paired in all possible combinations (Nalim et al., 1995).
Figure 1.
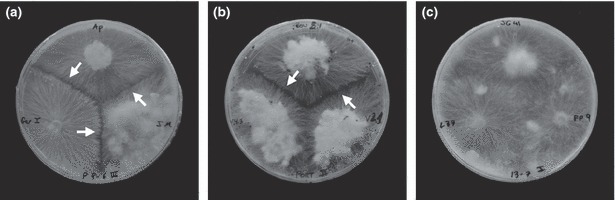
Mycelial interactions between isolates of Sclerotium rolfsii tested on modified Patterson’s medium: (a) and (b) incompatible reactions [different mycelial compatibility groups (MCGs)]; (c) compatible reactions (same MCG). Arrow indicates incompatible reaction zone.
The diversity of MCGs in each country and/or field plot in the study was estimated by the Shannon–Weaver diversity index: H′ = −Σp i × lnp i, where p i is the frequency of the ith MCG (Shannon & Weaver, 1963).
Pathogenic diversity
Plant species
Pathogenicity and virulence of S. rolfsii isolates were determined on 11 cultivated plant species of eight botanical families, including Amaranthaceae: sugar beet (Beta vulgaris cv. Markus); Asteraceae: sunflower (Helianthus annuus cv. SH‐25); Brassicaceae: broccoli (Brassica oleracea var. italica cv. Romanesco); Cucurbitaceae: melon (Cucumis melo L. var. cantalupensis cv. Piel de Sapo) and watermelon (Citrullus lanatus cv. Sugar Baby); Fabaceae: chickpea (Cicer arietinum cv. P‐2245); Malvaceae: cotton (Gossypium hirsutum cv. Theka); Poaceae: corn (Zea mays cv. Girona) and bread wheat (Triticum aestivum cv. Pinzón); and Solanaceae: pepper (Capsicum annuum cv. Cristal) and tomato (Solanum lycopersicum cv. Early‐Pack). Seeds were obtained from commercial seed companies. These plant species were selected as representative of the host range of the pathogen as well as of mono‐ and dicotyledonous species that could be an alternative to sugar beet crops in Mediterranean‐type climate areas.
Experimental design
Three experiments (I–III) were carried out in a growth chamber to determine the reaction of plant species to S. rolfsii isolates. Experiments I and II were first performed to determine reaction of the 11 plant species to inoculation with 12 tester isolates representative of each of the 12 identified MCGs. Because of space limitations in the growth chamber, experiments I and II consisted of six and five plant species, respectively. To test for reproducibility of reactions to the 12 MCG tester isolates, sunflower and tomato were common plant species in the two experiments.
In experiment III, a total of 23 S. rolfsii isolates representative of five of the more abundant MCGs identified, which comprised 5% of the total number of isolates in the study, were used to assess within‐MCG variation in pathogenicity and virulence. Isolates tested included 12 isolates for MCG i, four for MCG iii, three for MCG v and two for each of MCG vi and MCG xii. For this experiment, sunflower, tomato and watermelon were selected as susceptible hosts based on the consistency of their reaction to tester MCG isolates in experiments I and II.
In all three experiments, there were eight replications (eight pots, one plant per pot) of each treatment combination of plant species and S. rolfsii isolate. Each experiment was repeated partially and preliminary analyses of common treatments indicated no significant differences (P > 0·05) among experiments (see below); consequently, data were pooled for further analyses.
For each plant species, seeds were surface‐sterilized in 2% (v/v) NaOCl for 30 s, and germinated on autoclaved layers of filter paper in moist chambers at 25 ± 1°C in the dark for 2 days. Germinated seeds, selected for uniformity (length of radicle = 0·5–1 cm), were sown in sterile plastic pots filled with an autoclaved (121°C, 1 h, twice, on two consecutive days) soil mixture (silt:peat, 2:1).
Sclerotium rolfsii inoculum, plant inoculation and growth conditions
Inoculum for experiments consisted of infested oat (Avena sativa) seeds. One hundred grams of seeds were moistened in 50 mL water and autoclaved in 1‐L flasks for 60 min at 121°C. The sterilized seeds were then infested using 10 mycelial discs, 5 mm in diameter, from the growing edge of a S. rolfsii colony on PDA and incubated at 25 ± 1°C in the dark for 2 weeks.
Plants were inoculated at the two‐leaf stage. For inoculation, two selected oat seeds heavily and homogenously colonized with mycelia of an isolate were buried at a depth of 0·5 cm and a distance of 0·5–1 cm from the plant stem. Plants in pots with non‐infested seeds served as controls. Plants were incubated in a walk‐in growth chamber adjusted to 28 ± 1°C with a 14‐h photoperiod of fluorescent light of 360 μE m−2 s−1 and 60–90% relative humidity for 18 days. Plants were watered as needed to maintain field capacity and fertilized weekly with 100 mL 0·1% hydro‐sol fertilizer solution (Haifa Chemicals, Ltd, 20‐5‐32 N‐P‐K + micronutrients).
Disease assessment and data analyses
Disease reaction was characterized by the incidence of dead plants, established as the number of dead plants at the end of the experiment, 18 days after inoculation. S. rolfsii isolates were classified in terms of virulence based on the disease incidence (DI) that they induced: highly virulent (DI ≥ 66%), moderately virulent (66 < DI ≥ 33%), mildly virulent (33 < DI > 0%) and non‐pathogenic (DI = 0%). Similarly, plant species were classified according to DI values as: highly susceptible (DI ≥ 66%), susceptible (66 < DI ≥ 33%), moderately resistant (33 < DI > 0%) or resistant (DI = 0%).
The number of dead plants per treatment was used for statistical analyses. Data were analysed with the genmod procedure using the binomial distribution and the logit as link function in sas (version 9·2, SAS Institute Inc.) (Agresti, 2002). Least squares means were computed for all response variables to allow multiple comparisons between/among treatments by using the sas macro mult (https://www.uni-hohenheim.de/bioinformatik/beratung/toolsmacros/sasmacros/mult.sas) (Piepho, 2004). Linear single‐degree‐of‐freedom contrasts were computed to test the effect of selected experimental treatment combinations. The stability of disease reactions in the plant‐species by MCG combination was explored by cluster analyses of the number of dead plants to identify associated groupings of MCGs and plant species in two separate analyses. To find functional groupings of correlated MCGs or plant species, respectively, an agglomerative clustering based on the Spearman correlation matrix was performed among MCGs and among plant species using the Ward clustering method. For MCGs, the optimum number of clusters was estimated on the basis of the average silhouette width according to the Mantel statistic. Thus, the number of clusters in which the within‐group mean intensity of the link of the objects (MCG) to their groups was highest (i.e. with the largest average silhouette width) indicated the optimum cluster number. A new dendrogram was then produced representing the identified groupings of MCGs (Borcard et al., 2011). All calculations for cluster analyses were made using R version 2·13·0 (R Foundation for Statistical Computing, http://www.R-project.org/) with the cluster (Maechler et al., 2005) and vegan (Oksanen et al., 2011) packages.
The degree of virulence variation within each of five MCGs tested in experiment III was estimated by a separate likelihood analysis using the genmod procedure of sas, as described above, followed by multiple comparison of mean number of dead plants induced by the S. rolfsii isolates tested. In addition, the disease reaction induced by the tester isolate of each of the MCGs was compared with that of each of the rest of isolates within the same MCG using linear single‐degree‐of‐freedom contrast at P < 0·05.
Homogeneity of disease reactions across experiments
The consistency of results between experiments I and II was tested by a preliminary analysis in which the number of dead plants for treatments common to both experiments (i.e. the plant species sunflower and tomato inoculated with each of 12 MCG‐representative isolates) were analysed with the genmod procedure of sas as described above. The likelihood ratio test indicated no significant effects of the two experimental runs (χ2 = 0·35, P = 0·5536), or their interaction with MCG isolate (χ2 = 9·22, P = 0·6017), plant species (χ2 = 2·21, P = 0·1367), or both factors (χ2 = 13·14, P = 0·2844).
Similarly, homogeneity of disease reactions in experiments I, II and III was tested with the same methodology as indicated above using the number of dead plants of common treatments in all three experiments (i.e. the plant species sunflower and tomato inoculated with isolates representative of each of MCGs i, iii, v, vi and xii included in experiment III). The likelihood ratio test indicated no significant effects of the three experimental runs (χ2 = 0·27, P = 0·8740), or their interactions with MCG isolate (χ2 = 4·25, P = 0·8336), plant species (χ2 = 2·05, P = 0·3595), or both factors (χ2 = 11·53, P = 0·1732).
Results
Mycelial compatibility groups
Twelve MCGs were identified among the 459 isolates of S. rolfsii tested in the study. MCGs were designated in roman numerals from i to xii. The MCGs of isolates and their geographical locations are shown in Table 1. Mycelia compatibility among isolates of a MCG was characterized by their mycelia intermingling at the zone of interaction without aversion (Fig. 1c). Conversely, isolates assigned to different MCGs showed aversion at the interaction zone, together with thinned mycelium and formation of a red reaction line (Fig. 1a,b). All 459 isolates were self‐compatible, showing a reaction similar to that described for compatible isolates. Replicated experiments and replicated pairings within each experiment produced identical results.
Distribution and frequency of the 12 identified MCGs differed among sampling regions and countries. MCGs i and iii were the most prevalent among sampled isolates, representing 64·71 and 18·95% of the isolates, respectively. MCG i was identified only in the Iberian Peninsula, being present in all eight locations sampled in southern Spain and one location sampled in central Portugal (Table 1; Fig. 2). MCG iii was less abundant than MCG i but was the most widely distributed MCG, being identified in 10 of the 18 sampled locations from all sampled countries [Chile (two), Portugal (five) and southern Spain (three)] except Italy (Table 1; Fig. 2). MCG v comprised 5·66% of the isolates and was restricted to two distant field plots located in Vejer de la Frontera (Cádiz province) and Vila Franca de Xira (Lisbon province) in southern Spain and central Portugal, respectively. MCG ii comprised 3·92% of the isolates from two locations in Córdoba and Seville provinces in southern Spain. The remaining eight MCGs accounted for 6·75% of the isolates and were locally distributed. MCGs ix, vi, xii, viii and xi comprised 10, nine, four, three and two isolates, respectively; among these five multimember MCGs, MCGs viii, ix and xii were identified only in three locations in Chile, MCG vi in two locations in Portugal, and MCG xi in one location in Spain. MCGs iv, vii and x each consisted of a single isolate, identified in Bari (southern Italy), Colchagua (Chile) and Chillán (Chile), respectively.
Figure 2.
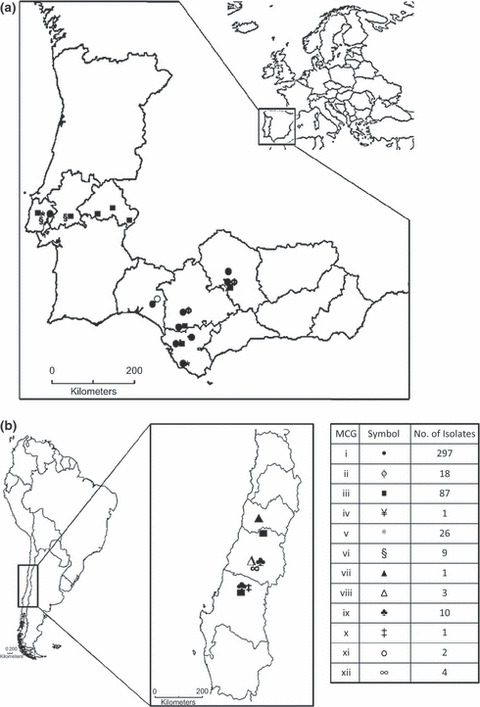
Geographic distribution of mycelial compatibility groups (MCGs) identified among isolates of Sclerotium rolfsii infecting sugar beet in (a) Iberian Peninsula (Spain and Portugal); and (b) Chile.
Regarding sampled countries, six, five and four MCGs were identified in Chile (MCGs iii, vii, viii, ix, x and xii), Spain (MCGs i, ii, iii, v and xi) and Portugal (MCGs i, iii, v and vi), respectively. The isolate sampled in southern Italy was assigned to MCG iv and occurred in this location only. Accordingly, the highest Shannon–Weaver H′ index of diversity, 1·49, was estimated in Chile, decreasing to 0·95 and 0·74 in Portugal and Spain, respectively. In southern Spain, MCG i was present in all locations sampled, whereas MCGs iii and v were identified in Cádiz, in the southwest of the region, MCG xi was found only at Huelva in the northwest of the region, and MCGs ii and iii occurred at Córdoba and Seville, in the centre of the region in the Guadalquivir valley (Fig. 2).
At the field level, the eight intensively sampled fields in four provinces in southern Spain showed a high degree of MCG homogeneity (Table 1; Fig. 3). The number of MCGs per field ranged from one to three and MCG i was present in all fields. A single field located at Posadas (Córdoba province) consisted of MCGs i, ii and iii, 12·9, 54·8 and 32·3% of a total of 31 isolates, respectively, and yielding a Shannon–Weaver index of H′ = 0·96. Two MCGs were identified in five fields; they included MCG i together with either MCGs ii (1·3% of isolates, H′ = 0·07), iii (two fields, 14·0 and 89·3% of isolates, H′ = 0·40 and H′ = 0·34, respectively), v (45·7% of isolates, H′ = 0·69) or xi (3·1% of isolates, H′ = 0·14) (Table 1; Fig. 3). In those fields, S. rolfsii isolates of a MCG tended to form discrete clusters of different sizes distributed along the plot, but there was no discernible spatial pattern for individual MCGs (Fig. 3).
Figure 3.
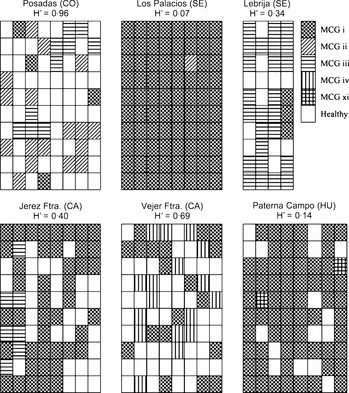
Schematic distribution of mycelial compatibility groups (MCGs) of Sclerotium rolfsii within six intensively sampled sugar beet fields in southern Spain, and Shannon–Weaver diversity index: H′. The sampled area (640 m2) was divided into 80 subplots, except for the field at Lebrija, in which the sampled area was 320 m2 divided into 40 subplots. Provinces sampled in southern Spain: CA, Cádiz; CO, Córdoba; SE, Seville; and HU, Huelva.
Pathogenic diversity
Susceptible reactions consisted of initial pale green and wilted leaves, followed by complete collapse and death of plants. Most plant mortality occurred within the first 8 days after inoculation. Symptoms developed neither in non‐inoculated controls nor in resistant plants. Overall, MCGs iii and MCGs vi had the widest host range, with their isolates being pathogenic to all plant species tested, except for wheat and corn, which were resistant to all 12 MCGs.
Isolates of MCGs i, ii, vii, ix and x were pathogenic to eight plant species, whereas isolates of MCGs viii, xi and xii were pathogenic to seven plant species, and those of MCGs iv and v were pathogenic to six (Fig. 4a). Three of the 12 tested plant species, chickpea, pepper and watermelon, were infected by all 12 MCGs, whereas cotton and sunflower were infected by 11 MCGs, tomato by 10, sugar beet by nine, broccoli by eight and melon by only six MCGs (Fig. 4b). Melon plants were not infected by six of the 12 MCGs tested. DI values were low for the remaining MCGs, reaching 12·5% in inoculations with the representative isolate of each of MCGs iii to vi, and 25 and 37·5% with those of MCG ii and MCG i, respectively, and were not included in the statistical analyses (data not shown).
Figure 4.
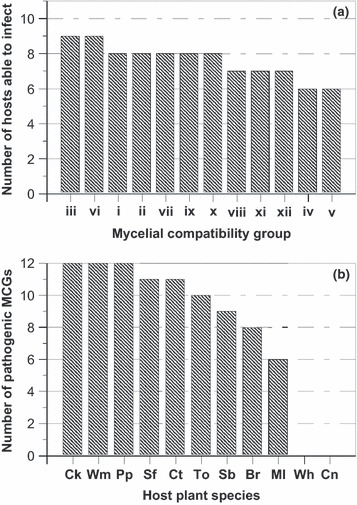
Disease reactions of 11 plant species to infection by 12 Sclerotium rolfsii isolates representative of mycelial compatibility groups (MCGs). (a) Number of plant species to which each MCG were pathogenic. (b) Number of MCGs pathogenic to each plant species. Plant species: Br, broccoli; Ck, chickpea; Cn, corn; Ct, cotton; Ml, melon; Pp, pepper; Sb, sugar beet; Sf, sunflower; To, tomato; Wh, wheat; and Wm, watermelon.
Global likelihood ratio analysis of the number of dead plants for the combinations of isolates representative of each of MCGs i to xii and the remaining eight plant species indicated a significant effect of MCG (χ2 = 33·37, P = 0·0005), plant species (χ2 = 68·04, P < 0·0001) and the MCG by plant‐species interaction (χ2 = 104·17, P < 0·0213) (data not shown). To further analyse the MCG by plant‐species interaction, functional groupings of MCGs were first identified using clustering silhouettes. In this analysis, four to seven cluster groupings could be appropriate according to the silhouettes’ mean width. This allowed selection of five functional MCG groupings among the 12 MCGs of the study which included: MCG grouping 1 (MCGs i and ix), MCG grouping 2 (MCGs ii, iii, vi and viii), MCG grouping 3 (MCGs iv, v and xi), MCG grouping 4 (MCG vii) and MCG grouping 5 (MCGs x and xii) (Fig. 5a). Similarly, plant species could be grouped into two plant species (SP) groups, namely SP group 1 (chickpea, broccoli, sunflower and tomato) and SP group 2 (cotton, pepper, sugar beet and watermelon) according to the two‐first‐order cluster groups identified in the cluster analysis (Fig. 5b). Thereafter, a new analysis was performed using MCG groupings and SP groups as independent variables, which indicated significant effects of both factors (P ≤ 0·0495), as well as of their interaction (P = 0·0012) (Table 2). This interaction was the result of disease incidence induced by isolates of MCG groupings 1, 2 and 5 being higher (P ≤ 0·0319) on SP group 1 than on SP group 2, whereas isolates of MCG groupings 3 and 4 induced similar disease incidence (P ≥ 0·0902) on SP groups 1 and 2 (Table 2). Additionally, a separate analysis was performed for each SP group to further assess the effects of MCG groupings on disease incidence induced by S. rolfsii isolates on the plant species included in each SP group. Results indicated no significant (P ≥ 0·05) MCG‐grouping by plant‐species interaction for the two SP groups (Table 3). Overall, for SP group 1, disease reaction to inoculation was significantly influenced both by the MCG grouping and plant species (P < 0·0001) but not by their interaction (P = 0·2026) (Table 3). DI was highest (P < 0·05) for isolates of MCG groupings 1, 2 and 5, which showed moderate virulence, and decreased significantly (P < 0·05) for MCG groupings 3 and 4, which were mildly virulent (Fig. 6a). Among plant species, DI was highest (P < 0·05) on chickpea, which was categorized as highly susceptible, decreased significantly (P < 0·05) on sunflower, which was categorized as susceptible, and was least (P < 0·05) on tomato and broccoli, which were considered moderately resistant with no significant differences (P ≥ 0·05) between these two species (Fig. 6a). No significant differences (P = 0·0681) occurred among MCG groupings on SP group 2 (Table 3; Fig. 6b). Across plant species in SP group 2, disease incidence was highest (P < 0·05) on cotton and pepper, which showed susceptible reactions, and decreased significantly (P < 0·05) to a moderately susceptible reaction on sugar beet. DI on watermelon was intermediate between those occurring on the three species of SP group 2 and did not differ significantly (P ≥ 0·05) from any of them (Fig. 6b).
Figure 5.
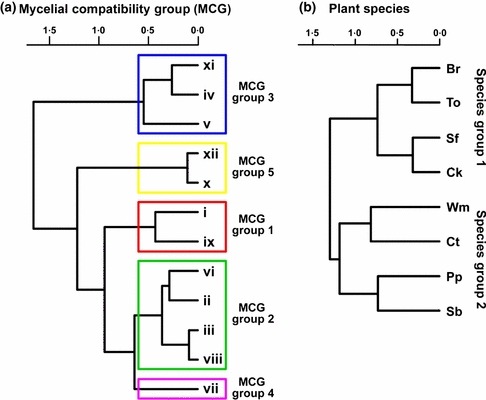
Dendrogram showing results of cluster analyses of disease reaction to infection by 12 Sclerotium rolfsii isolates representative of (a) mycelial compatibility groups (MCGs) and (b) eight plant species. Agglomerative cluster analyses were performed based on the Spearman correlation matrix calculated using number of dead plants among MCGs or among plant species using the Ward method. MCG groupings were estimated on the basis of the average silhouette width according to the Mantel statistic. Plant species: Br, broccoli; Ck, chickpea; Ct, cotton; Pp, pepper; Sb, sugar beet; Sf, sunflower; To, tomato; and Wm, watermelon.
Table 2.
Global likelihood ratio statistics to test the effects of groupings of Sclerotium rolfsii mycelial compatibility groups (MCG groupings) on sclerotium root rot incidence in groups of plant species (SP groups)
| Source of variationa | d.f. | χ2 | P > χ2 |
|---|---|---|---|
| MCG grouping | 4 | 22·36 | 0·0002 |
| SP group | 1 | 3·86 | 0·0495 |
| MCG grouping*SP group | 4 | 18·07 | 0·0012 |
| Contrasts | |||
| MCG grouping 1: SP group 1 vs. 2 | 1 | 13·53 | 0·0002 |
| MCG grouping 2: SP group 1 vs. 2 | 1 | 4·60 | 0·0319 |
| MCG grouping 3: SP group 1 vs. 2 | 1 | 2·87 | 0·0902 |
| MCG grouping 4: SP group 1 vs. 2 | 1 | 0·24 | 0·6249 |
| MCG grouping 5: SP group 1 vs. 2 | 1 | 4·70 | 0·0301 |
aMCG groups included: MCG grouping 1: MCGs i and ix; MCG grouping 2: MCGs ii, iii, vi and viii; MCG grouping 3: MCGs iv, v and xi; MCG grouping 4: MCG vii; and MCG grouping 5: MCGs x and xii. SP group 1: chickpea, broccoli, sunflower and tomato; SP group 2: cotton, pepper, sugar beet and watermelon.
Table 3.
Likelihood ratio statistics to test the effects of groupings of Sclerotium rolfsii mycelial compatibility groups (MCG groupings) on sclerotium root rot incidence in plant species of two groups of species (SP groups)
| MCG grouping | Source of variationa | d.f. | Species group 1 | Species group 2 | ||
|---|---|---|---|---|---|---|
| χ2 | P > χ2 | χ2 | P > χ2 | |||
| Global | MCG‐group (A) | 4 | 33·30 | <0·0001 | 8·73 | 0·0681 |
| Species (B) | 3 | 27·03 | <0·0001 | 8·41 | 0·0382 | |
| A*B | 12 | 15·76 | 0·2026 | 17·91 | 0·1184 | |
| 1 | MCG (A) | 1 | 0·05 | 0·8280 | 0·49 | 0·4818 |
| Species (B) | 3 | 22·28 | <0·0001 | 0·93 | 0·8186 | |
| A*B | 3 | 1·77 | 0·6207 | 5·48 | 0·1398 | |
| 2 | MCG (A) | 3 | 6·56 | 0·0874 | 6·88 | 0·0759 |
| Species (B) | 3 | 46·34 | <0·0001 | 12·05 | 0·0072 | |
| A*B | 9 | 9·41 | 0·3999 | 9·93 | 0·3565 | |
| 3 | MCG (A) | 2 | 0·29 | 0·8650 | 0·09 | 0·9571 |
| Species (B) | 3 | 21·41 | <0·0001 | 2·69 | 0·4412 | |
| A*B | 6 | 2·36 | 0·8840 | 11·38 | 0·0773 | |
| 4 | Species | 3 | 0·66 | 0·8837 | 5·41 | 0·1439 |
| 5 | MCG (A) | 1 | 3·63 | 0·0566 | 0·15 | 0·7025 |
| Species (B) | 3 | 12·43 | 0·0060 | 11·56 | 0·0090 | |
| A*B | 3 | 0·54 | 0·9107 | 0·83 | 0·8417 | |
aMCG groupings included: MCG grouping 1: MCGs i and ix; MCG grouping 2: MCGs ii, iii, vi and viii; MCG grouping 3: MCGs iv, v and xi; MCG grouping 4: MCG vii; and MCG grouping 5: MCGs x and xii. SP group 1: chickpea, broccoli, sunflower and tomato; SP group 2: cotton, pepper, sugar beet and watermelon.
Figure 6.
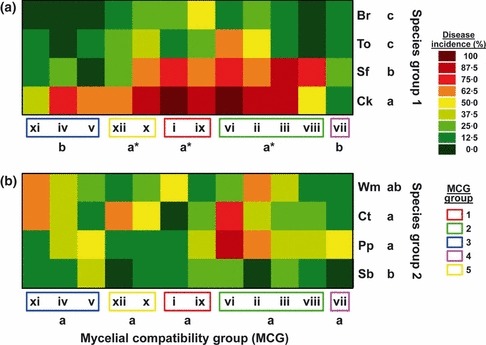
Two‐way plots showing results of likelihood analyses of disease reaction estimated by the number of dead plants to infection by 12 Sclerotium rolfsii isolates representative of mycelial compatibility groups (MCGs) grouped into five MCG groupings (x‐axis) and eight plant species (y‐axis) grouped into species‐group (SP) 1 (a) and SP group 2 (b). Plant species: Br, broccoli; Ck, chickpea; Ct, cotton; Pp, pepper; Sb, sugar beet; Sf, sunflower; To, tomato; and Wm, watermelon. For each SP group, MCG groupings or plant species followed by a different letter showed significant differences on the least squares means at P < 0·05. MCG groupings with an asterisk indicate a significantly higher least squares mean for disease incidence induced on SP group 1, compared to that on SP group 2 at P < 0·05.
Finally, the disease reaction induced by isolates of MCGs included in each of the four multimember MCG groupings (i.e. MCG groupings 1, 2, 3 and 5) on SP groups 1 and 2 was fairly homogeneous (P ≥ 0·0566). This supports the consistency of the five MCG groupings identified in the cluster analysis.
Within‐MCG pathogenic variability
There was little variation in the disease reaction induced on sunflower, tomato and watermelon by S. rolfsii isolates belonging to the same MCG (Fig. 7). Thus, likelihood analyses showed no significant differences (P ≥ 0·5350) among S. rolfsii isolates belonging to either MCG iii, v, vi or xii (Table 4). Moreover, disease reactions induced by isolates of each of these four MCGs were not significantly different (P ≥ 0·05) from those in experiments I and II. An exception to this occurred for isolates of MCG i, which showed significant differences between each other (P < 0·0001) (Table 4, Fig. 7). In any case, of the 12 MCG i isolates tested, only the disease reaction induced by isolate Sr033 differed significantly (P = 0·0068) from that induced by the MCG i type isolate Sr078 (Fig. 7). Comparison of the disease reaction induced by this isolate with those induced by the rest of the MCG i isolates indicated that Sr033 was more virulent to all three plant species (Fig. 7). For all five MCGs tested, there were no significant (P ≥ 0·4638) isolate by plant‐species interactions (Table 4).
Figure 7.
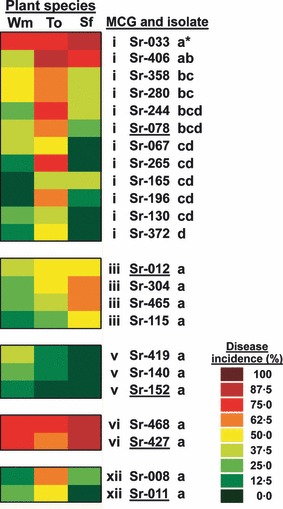
Two‐way plot showing results of likelihood analyses of disease reaction estimated by the number of dead plants of sunflower (Sf), tomato (To) and watermelon (Wm) (x‐axis) to infection by 23 Sclerotium rolfsii isolates representative of five of the more prevalent mycelial compatibility groups (MCG) (y‐axis) identified in this study. Isolates tested included 12 isolates for MCG i, four isolates for MCG iii, three for MCG v, and two for each of MCG vi and MCG xii. The type isolate for each MCG is underlined. For each MCG, isolates followed by a different letter showed significant differences in the least squares means at P < 0·05. Isolates with an asterisk indicate significantly different least squares means from that of the type isolate at P < 0·05.
Table 4.
Likelihood ratio statistics to test the pathogenic variability among Sclerotium rolfsii isolates belonging to different mycelial compatibility groups (MCG)
| MCG | Source of variationa | d.f. | χ2 | P > χ2 |
|---|---|---|---|---|
| MCG i | Isolate (A) | 11 | 41·83 | <0·0001 |
| Species (B) | 2 | 28·42 | <0·0001 | |
| A*B | 22 | 20·64 | 0·5431 | |
| MCG iii | Isolate (A) | 3 | 2·18 | 0·5350 |
| Species (B) | 2 | 6·93 | 0·0312 | |
| A*B | 6 | 1·32 | 0·9708 | |
| MCG v | Isolate (A) | 2 | 0·57 | 0·7522 |
| Species (B) | 2 | 2·99 | 0·2246 | |
| A*B | 4 | 0·41 | 0·9819 | |
| MCG vi | Isolate (A) | 1 | 0·07 | 0·7889 |
| Species (B) | 2 | 1·68 | 0·4307 | |
| A*B | 2 | 0·17 | 0·9185 | |
| MCG xii | Isolate (A) | 1 | 0·30 | 0·5864 |
| Species (B) | 2 | 8·10 | 0·0174 | |
| A*B | 2 | 1·54 | 0·4638 |
aIsolates tested included 12 isolates for MCG i, four isolates for MCG iii, three for MCG v, and two for each of MCG vi and MCG xii. Plant species tested included sunflower, tomato and watermelon.
Discussion
Genetic and pathogenic characterization of S. rolfsii isolates from different agricultural regions can provide important insights into the epidemiology and management of the diseases they cause. In this study, the structure of S. rolfsii populations obtained from a single host crop (autumn‐sown sugar beet) and regions of the Iberian Peninsula (Portugal and Spain), Italy and Chile sharing Mediterranean‐type climate were characterized by means of MCG analysis and pathogenicity to 11 economically important plant species cultivated in those regions. It was demonstrated that differences in virulence occur among isolates from certain locations and that these differences can be associated with genetic diversity based on MCGs of isolates. In addition, variation at field level was assessed by intensive sampling of plots at several locations in southern Spain.
A system of mycelial incompatibility was previously demonstrated to occur among field isolates of S. rolfsii and was used to designate MCGs (Punja & Grogan, 1983). This study was able to identify up to 12 MCGs among 459 S. rolfsii isolates originating from a wide geographic range but a single host crop. This is apparently novel work in S. rolfsii because previously published work has involved S. rolfsii isolates from: (i) widely distant geographic areas and diverse host sources (Punja & Grogan, 1983; Harlton et al., 1995; Punja & Sun, 2001); (ii) a restricted region and diverse hosts (2000, 2002; Almeida et al., 2001; Sarma et al., 2002); and (iii) a single region and host plant (Nalim et al., 1995; Okabe & Matsumoto, 2000; Adandonon et al., 2005). Two abundant and prevalent MCGs (MCGs i and iii) were found. Some other MCGs were: (a) less prevalent but present in various regions of different countries (MCG v) or regions within a country (MCGs ii, vi and ix); (b) restricted to specific localities (MCGs viii, xi and xii); or (c) single‐isolate member groups (MCGs iv, vii and x).
The results agree with most previous reports of a single S. rolfsii MCG comprising isolates from widely distant geographic areas of Brazil (Almeida et al., 2001), India (Sarma et al., 2002), South Africa (Cilliers et al., 2000) and the USA (Harlton et al., 1995; Punja & Sun, 2001). In this same context, the existence of MCGs at high frequencies with wide geographic distribution was also reported for S. sclerotiorum infecting canola (Brassica napus) through Canada, where a single MCG representing 18% of the isolates was found in all three distant provinces sampled (Kohli et al., 1992).
In southern Spain, S. rolfsii was reported infecting sugar beet crops in some localities in Córdoba and Seville provinces in the centre of Andalusia in the early 1940s (Benlloch, 1943). By 1944, the pathogen had also been reported in Cáceres province in the Extremadura region of southwest Spain, close to the Portuguese border (Domínguez García‐Tejero, 1951). Interestingly, this coincided with when the crop sowing date was brought forward from spring to early winter (Morillo‐Velarde et al., 2003), which appears more favourable for disease development (Aycock, 1966; Punja, 1985). In autumn sowings, several important changes were implemented in the husbandry of sugar beet, which included complete mechanization of harvesting using heavy equipment. Thus, movement of equipment across fields, together with transportation of harvested roots from fields to distant processing factories, may have facilitated dispersal of the pathogen in soil particles and could be responsible for the rapid spread of MCGs i and iii through Andalusia (Fig. 2). On the other hand, sugar beet has been extensively grown in Portugal since the late 1990s (FAOSTAT, 2010), and sclerotium root rot was of great concern in the major growing areas of central and southern Portugal in 2003, at which time a disease prevalence of 21% of fields accounted for 16·6% of the cultivated area being affected (M. Paim, Associação para Desenvolvimento da Beterraba, Portugal, personal communication). In Chile, sugar beet has been grown since the early 1950s and S. rolfsii was first reported in the central regions affecting diverse crops in 1981 (Esterio & Auger, 1982) and on sugar beet in the Metropolitan region and V region in 1983 (Acuña, 1985). Thereafter, the pathogen spread to the southern regions reaching the VIII region in 1999 (R. Paillalef‐Monnrad, IANSAGRO, Chile, personal communication). MCG iii was the most prevalent group in the current study, being present in two of four, three of eight and all five locations sampled in Chile, Spain and Portugal, respectively. This suggests that MCG iii may have been spread extensively within and among cultivated areas by cultural practices, vehicle movements, planting material, containers, etc. The existence of S. rolfsii isolates of the same MCG in different distant countries was also reported by Harlton et al. (1995), who identified MCG 8 in the USA (Alabama, California and Maryland) and Pakistan; and MCG 1 in the USA (California, Georgia and North Carolina) and Mexico. Similarly, Kull et al. (2004) reported MCG 8 of S. sclerotiorum in soyabean fields in several states in the USA, on soyabean in Switzerland and on canola in Canada.
The present results are also consistent with those of other authors, suggesting a correlation between MCG and geographical source of isolates, as S. rolfsii isolates originating from the same locality tended to form a distinct MCG (Punja & Grogan, 1983; Harlton et al., 1995; 2000, 2002; Punja & Sun, 2001; Sarma et al., 2002). In fact, three of the 12 MCGs identified (MCGs ii, vi and ix) were found only at two close localities and six were present at a single locality. This was the case of MCG iv, which was identified exclusively in Bari (Italy), MCGs vii, viii, x and xii, which were present in each of four different localities in Chile, and MCG xi, which was identified only in Huelva (Spain) (Fig. 2). In a comparable study, Nalim et al. (1995) found that a rather small number of MCGs existed within single peanut fields in Texas. Localization of unique S. sclerotiorum MCGs was also observed in vegetable‐growing regions in New Zealand (Carpenter et al., 1999) and on winter canola in Ontario, Canada (Kohn et al., 1991). Explanations for unique low‐frequency MCGs in a sampling area may include recent MCG introductions or random mutation events. The emergence of new genotypes localized in single fields may be an indication of MCGs or clones becoming adapted to specific field microclimates or hosts, and are less likely to be the result of genetic exchange and recombination (Ben‐Yephet & Bitton, 1985; Hambleton et al., 2002). The existence of single‐member MCGs was also reported with variable frequency in different geographic areas and/or host sources (Punja & Grogan, 1983; Harlton et al., 1995; Nalim et al., 1995; Cilliers et al., 2000; Okabe & Matsumoto, 2000; Almeida et al., 2001; Punja & Sun, 2001; Sarma et al., 2002).
The population of the pathogen in Chile was particularly diverse. The area sampled for the study in that country was similar in size to that in southern Spain. However, MCGs identified in Chile accounted for half of the 12 MCGs identified in the study, of which five were found only in this country. Moreover, a single location in Chile could contain up to three different MCGs, as occurred in Linares (MCGs viii, ix and xii) and Chillán (MCGs iii, ix and x). The existence of considerable genetic variability among S. rolfsii isolates was also reported in Brazil, where 13 compatibility groups were identified using 30 isolates from 14 states and 11 hosts (Almeida et al., 2001).
At field‐plot level, a relatively low number of MCGs was found within each of the eight sugar beet fields sampled in the Guadalquivir Valley in southern Spain. In these fields, three, two and one MCGs were identified in one, five and two fields, respectively. MCG i was always present, being unique and predominant in two and four fields, respectively (2, 3). Interestingly, the number of MCGs in a field was not related to the number of S. rolfsii isolates sampled in it. Thus, for a similar sampled area the number of isolates ranged from 28 to 80, the field harbouring three MCGs being represented by 31 isolates. These results do not fully agree with those reported by Nalim et al. (1995), who studied four peanut fields in Texas and found one, three and five MCGs in one, two and one field, respectively. However, none of the MCGs reported by Nalim et al. (1995) were prevalent across fields, as only one MCG was present in two fields, while the others were specific to each field. In contrast, the results of the present study are closer to those of Okabe & Matsumoto (2000) in peanut fields in Japan. These authors analysed seven peanut fields and identified four MCGs, with up to three MCGs occurring in a single field and one MCG being dominant in most of the fields sampled. In similar studies on S. sclerotiorum, Kohn et al. (1991) found much higher variability within two canola fields in Ontario, Canada, where they identified 26 and six MCGs from 30 and 33 isolates, respectively, with two MCGs being predominant in one field, and only one MCG being common to the two fields. Similar results were described for the MCG population structure of S. sclerotiorum on soyabean in Argentina and Illinois (USA) (Kull et al., 2004). The present observations and those of Nalim et al. (1995) and Okabe & Matsumoto (2000) are coherent with a ‘founder effect’ and may reflect the mode of reproduction of S. rolfsii, which does not produce conidia and spreads by mycelial growth. Whilst S. rolfsii does have a perfect stage, its role in nature is not fully understood. In contrast, sexual reproduction in S. sclerotiorum and a greater selection for specific MCGs in populations of this fungus compared with that in S. rolfsii may account for the smaller number of S. rolfsii MCGs found per field in this study. Thus, in the six sugar beet fields in southern Spain comprising more than one S. rolfsii MCG, the MCGs appeared scattered within distinct disease foci with no discernible spatial pattern. This agrees with reports on S. rolfsii in peanut fields in Texas (Nalim et al., 1995) or S. sclerotiorum on soyabean in Illinois (Kull et al., 2004).
The results demonstrate that high pathogenic variability occurs among isolates representative of S. rolfsii MCGs as well as in the degree of susceptibility among the tested plant species. To our knowledge this is the first genetic and virulence characterization of S. rolfsii populations. Overall, MCGs vi and ii were highly virulent, MCGs i, iii, ix and x moderately virulent, and MCGs iv, v, vii, viii, xi and xii were mildly virulent. However, that range in virulence was not directly related to the pathogenic spectrum of a given MCG.
The results also showed a low level of pathogenic variability among S. rolfsii isolates within a MCG. In fact, for five of the most abundant MCGs identified in this study, isolates within a MCG were similarly virulent to three plant species. Only one out of 12 MCG i isolates tested caused a disease reaction that differed significantly from that induced by the MCG i type isolate, Sr078, being more virulent to all three plant species, sunflower, tomato and watermelon (Fig. 7). These results suggest that a correlation may exist between MCG typing and virulence trait.
Among plant species, chickpea and sunflower were highly susceptible; cotton, pepper, tomato and watermelon moderately susceptible; broccoli and sugar beet mildly susceptible; and corn and wheat were resistant. A degree of variability in host plant reaction to a S. rolfsii isolate from sugar beet in Pakistan was reported by Yaqub & Shahzad (2005): mungbean, sugar beet and sunflower were highly susceptible; cabbage, lentil, tomato and sweet pumpkin were mildly susceptible; and cauliflower was completely resistant. To some extent, these results agree with those of Flores‐Moctezuma et al. (2006) who evaluated the reactions of 12 plant species to a set of 20 non‐MCG‐typed S. rolfsii isolates collected from different agricultural and ecological areas in Mexico. These authors found that gooseberry, lentil, pumpkin, radish and tomato were susceptible to all tested isolates, whereas the degree of susceptibility of the remaining seven species varied widely with the isolate. In the present study, sugar beet cv. Markus was susceptible, moderately susceptible and resistant to isolates from one, eight and three MCGs, respectively. Similarly, Sharma et al. (1991) found that only one of five S. rolfsii isolates from different sugar beet‐growing areas in northwest India was highly virulent to sugar beet in artificial inoculations.
The results of this study have important implications for the management of sclerotium root rot of sugar beet in Mediterranean‐type climates, particularly in relation to crop rotation and choice of appropriate crops for use in infested soils previously cropped to sugar beet. Crop rotations are essential to maintain crop productivity and reduce the build‐up of soilborne plant pathogens and diseases, which can devastate crops grown in multiple consecutive years (Cook, 2000). Generally, the degree of control is based on the level of susceptibility or resistance of crops involved and the sequences of cropping. In the present study, monocotyledonous species (wheat and corn) were resistant to all MCGs tested and thus are suitable for rotation with susceptible crops. These results agree with those of Boyle (1967), who indicated a reduction in the incidence of sclerotium root rot in peanut fields following a monocotyledonous crop, as well as with those of Minton et al. (1991), who reported that disease incidence in a wheat–peanut rotation was lower than that in a fallow–peanut rotation. Similarly, disease incidence was the lowest in peanut following 2 years of bahiagrass, intermediate following 2 years of corn or cotton, and highest in continuous peanut (Johnson et al., 1999). Nevertheless, results of this present study suggest that S. rolfsii isolates in any of the identified MCGs were able to cause different levels of plant mortality in at least six of the nine susceptible plant species tested. This, together with the occurrence of up to three different MCGs within a field plot or locality differing in pathogenic and virulence profiles, make it difficult to recommend crop rotation as a sole control measure. It would be of interest to evaluate the risk of occurrence of severe epidemics on a given crop based on the prevalence of a given MCG of the pathogen. This would be of importance for southern Spain and Portugal, where vegetable crops such as carrots, peppers, tomato, etc. are becoming alternative crops for sugar beet as a result of the sugar beet production area being drastically reduced or nearly eliminated, respectively. Consequently, sclerotium root rot diseases can become an important threat for vegetable crop production in these areas, as was recently reported for pepper fields in southern Spain (Remesal et al., 2010).
Studies are in progress to assess the molecular diversity existing within S. rolfsii MCGs and to determine any genetic relationships that might exist among them.
Acknowledgements
Financial support for this research was provided by grants AGL2002‐01418 and AGL2005‐00751 from the ‘Ministerio de Educación y Ciencia’ of Spain and the European Social Fund. ER is recipient of a FPI fellowship BES‐2006‐13693 from the ‘Ministerio de Educación y Ciencia’ of Spain. We are grateful to R. Paillalef‐Monnrad (IANSAGRO, Chile), M. Paim (ADB, Portugal) and Dr R. Morillo‐Velarde (AIMCRA, Spain) for providing sclerotium‐root‐rot‐infected root samples for isolation of the pathogen from Chile, Portugal and Italy, respectively. Also, we thank Dr M. Gutierrez‐Sosa (AIMCRA) for helping in the selection of field plots in southern Spain, J. M. Gallego del Estal (IAS‐CSIC) for excellent technical assistance, Drs B. B. Landa and P. Castillo for critically reading the manuscript prior to submission, and anonymous reviewers of a previous version of the manuscript for their comments and helpful suggestions.
References
- Acuña R, 1985. Pudrición radicular causada por Sclerotium rolfsii Sacc., en cultivos de remolacha en la región central del país. Simiente 55, 34–5. [Google Scholar]
- Adandonon A, Aveling TAS, van der Merwe NA, Sanders G, 2005. Genetic variation among Sclerotium isolates from Benin and South Africa, determined using mycelial compatibility and ITS rDNA sequence data. Australasian Plant Pathology 34, 19–25. [Google Scholar]
- Agresti A, 2002. Categorical Data Analysis, 2nd edn Hoboken, NJ, USA: John Wiley & Sons, Inc. [Google Scholar]
- AIMCRA , 2005. Memoria de los Trabajos Efectuados en la Campaña 2003/04. Siembra Otoño 2002 Zona Sur. Valladolid, Spain: Asociación para la Mejora del Cultivo de la Remolacha Azucarera (AIMCRA). [Google Scholar]
- Akram A, Iqbal SM, Rauf CA, Aleem R, 2008. Detection of resistant sources for collar rot disease in chickpea germplasm. Pakistan Journal of Botany 40, 2211–5. [Google Scholar]
- Almeida AMR, Abdelnoor RV, Calvo ES, Tessnman D, Yorinori JT, 2001. Genotypic diversity among Brazilian isolates of Sclerotium rolfsii . Journal of Phytopathology 149, 493–502. [Google Scholar]
- Aycock R, 1966. Stem rot and other diseases caused by Sclerotium rolfsii or the status of Rolf’s fungus after 70 years. North Carolina Agricultural Experiment Station Technical Bulletin No. 174.
- Bayraktar H, Türkkan M, Dolar FS, 2010. Characterization of Fusarium oxysporum f. sp. cepae from onion in Turkey based on vegetative compatibility and rDNA RFLP analysis. Journal of Phytopathology 158, 691–7. [Google Scholar]
- Benlloch M, 1943. El “mal del esclerocio” en los remolachares de algunas vegas andaluzas. Boletín de Patología Vegetal y Entomología Agrícola 12, 221–8. [Google Scholar]
- Ben‐Yephet Y, Bitton S, 1985. Use of a selective medium to study the dispersal of ascospores of Sclerotinia sclerotiorum . Phytoparasitica 13, 33–40. [Google Scholar]
- Borcard D, Gillet F, Legendre P, 2011. Numerical Ecology with R. New York, USA: Springer. [Google Scholar]
- Boyle LW, 1967. A factorial study of certain schemes of peanut culture. University of Georgia Agriculture Experimental Station Research Bulletin 18, 11–2. [Google Scholar]
- Branch WD, Brenneman TB, 1999. Stem rot disease evaluation of mass‐selected peanut populations. Crop Protection 18, 127–30. [Google Scholar]
- Carling DE, Kuninaga S, Brainard KA, 2002. Hyphal anastomosis reactions, rDNA‐internal transcribed spacer sequences, and virulence levels among subsets of Rhizoctonia solani anastomosis group‐2 (AG‐2) and AG‐BI. Phytopathology 92, 43–50. [DOI] [PubMed] [Google Scholar]
- Carpenter MA, Frampton C, Stewart A, 1999. Genetic variation in New Zealand populations of the plant pathogen Sclerotinia sclerotiorum . New Zealand Journal of Crop and Horticultural Science 27, 13–21. [Google Scholar]
- Caten CE, 1972. Vegetative compatibility and cytoplasmic infection in fungi. Journal of General Microbiology 72, 221–9. [DOI] [PubMed] [Google Scholar]
- Chulze SN, Ramirez ML, Torres A, Leslie JF, 2000. Genetic variation in Fusarium section Liseola from no‐till maize in Argentina. Applied and Environmental Microbiology 66, 5312–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi MB, Meyer MC, Costa MJN, Zala M, McDonald BA, Ceresini PC, 2008. Genetic structure of populations of Rhizoctonia solani anastomosis group‐1 IA from soybean in Brazil. Phytopathology 98, 932–41. [DOI] [PubMed] [Google Scholar]
- Cilliers AJ, Herselman L, Pretorius ZA, 2000. Genetic variability within and among mycelial compatibility groups of Sclerotium rolfsii in South Africa. Phytopathology 90, 1026–31. [DOI] [PubMed] [Google Scholar]
- Cilliers AJ, Pretorius ZA, van Wyk PS, 2002. Mycelial compatibility groups of Sclerotium rolfsii in South Africa. South African Journal of Botany 68, 389–92. [DOI] [PubMed] [Google Scholar]
- Collado‐Romero M, Mercado‐Blanco J, Olivares‐García C, Valverde‐Corredor A, Jiménez‐Díaz RM, 2006. Molecular variability within and among Verticillium dahliae vegetative compatibility groups determined by fluorescent amplified fragment length polymorphism and polymerase chain reaction markers. Phytopathology 96, 485–95. [DOI] [PubMed] [Google Scholar]
- Cook RJ, 2000. Advances in plant health management in the twentieth century. Annual Review of Phytopathology 38, 95–116. [DOI] [PubMed] [Google Scholar]
- Domínguez García‐Tejero F, 1951. Distribución en España de las plagas y enfermedades de la remolacha. Boletín de Patología Vegetal y Entomología Agrícola 18, 181–204. [Google Scholar]
- Dukes PD, Ferry RL, Jones A, 1983. Evaluating peppers, cowpeas, sweet potato, and tomatoes for resistance to southern blight incited by Sclerotium rolfsii Sacc. Phytopathology 73, 785–6. [Google Scholar]
- Earnshaw DM, McDonald MR, Boland GJ, 2000. Interactions among isolates and mycelial compatibility groups of Sclerotium cepivorum and cultivars of onion (Allium cepa). Canadian Journal of Plant Patholology 22, 387–91. [Google Scholar]
- Elias KS, Zamir D, Lichtman‐Pleban T, Katan T, 1993. Population structure of Fusarium oxysporum f. sp. lycopersici: restriction fragment length polymorphisms provide genetic evidence that vegetative compatibility group is an indicator of evolutionary origin. Molecular Plant-Microbe Interactions 6, 565–72. [Google Scholar]
- Esterio M, Auger J, 1982. Presencia de Pellicularia rolfsii (Curzi) West (Sclerotium rolfsii Sacc.) en la zona central del país. Simiente 52, 32. [Google Scholar]
- FAOSTAT , 2010. FAOSTAT production statistics of crops. [Accessed September 01 2011: http://faostat.fao.org/site/567/default.aspx#ancor].
- Fery RL, Dukes PD Sr, 2002. Southern blight (Sclerotium rolfsii Sacc.) of cowpea: yield‐loss estimates and sources of resistance. Crop Protection 21, 403–8. [Google Scholar]
- Flores‐Moctezuma HE, Montes‐Belmont R, Jiménez‐Pérez A, Nava‐Juárez R, 2006. Pathogenic diversity of Sclerotium rolfsii isolates from Mexico, and potential control of southern blight through solarization and organic amendments. Crop Protection 25, 195–201. [Google Scholar]
- Hambleton S, Walker C, Kohn LM, 2002. Clonal lineages of Sclerotinia sclerotiorum previously known from other crops predominate in 1999–2000 samples from Ontario and Quebec soybean. Canadian Journal of Plant Pathology 24, 309–15. [Google Scholar]
- Harlton CE, Uvesque CA, Punja ZK, 1995. Genetic diversity in Sclerotium (Athelia) rolfsii and related species. Phytopathology 85, 1269–81. [Google Scholar]
- van Heerden SW, Wingfield MJ, 2001. Genetic diversity of Cryphonectria cubensis isolates in South Africa. Mycological Research 105, 94–9. [Google Scholar]
- Jenkins SF, Averre CW, 1986. Problems and progress in integrated control of southern blight of vegetables. Plant Disease 70, 614–9. [Google Scholar]
- Johnson AW, Minton NA, Brenneman TB et al. , 1999. Bahiagrass, corn, cotton rotations, and pesticides for managing nematodes, diseases, and insects on peanut. Journal of Nematology 31, 191–200. [PMC free article] [PubMed] [Google Scholar]
- Kohli Y, Morrall RAA, Anderson JB, Kohn LM, 1992. Local and trans‐Canadian clonal distribution of Sclerotinia sclerotiorum on canola. Phytopathology 82, 875–80. [Google Scholar]
- Kohn LM, Stasovski E, Carbone I, Royer J, Anderson JB, 1991. Mycelial incompatibility and molecular markers identify genetic variability in field populations of Sclerotinia sclerotiorum . Phytopathology 81, 480–5. [Google Scholar]
- Korolev N, Katan J, Katan T, 2000. Vegetative compatibility groups of Verticillium dahliae in Israel: their distribution and association with pathogenicity. Phytopathology 90, 529–36. [DOI] [PubMed] [Google Scholar]
- Kull LS, Pedersen WL, Palmquist D, Hartman GL, 2004. Mycelial compatibility grouping and aggressiveness of Sclerotinia sclerotiorum . Plant Disease 88, 325–32. [DOI] [PubMed] [Google Scholar]
- Lal RJ, Srivastava SN, Agnihotri VP, 1997. Epidemiology and management of Sclerotium root rot of sugarbeet: a threatening disease In: Agnihotri VP, Sarbhoy AK, Singh DV, eds. Management of Threatening Plant Diseases of National Importance. New Delhi, India: Malhotra Publishing House, 161–78. [Google Scholar]
- Leslie JF, 1993. Fungal vegetative compatibility. Annual Review of Phytopathology 31, 127–50. [DOI] [PubMed] [Google Scholar]
- Maechler M, Rousseeuw P, Struyf A, Hubert M, 2005. Cluster Analysis: Basics and Extensions: An R Library . [Accessed September 01 2011: http://cran.r-project.org/web/packages/cluster/].
- Minton NA, Csinos AS, Lynch RE, Brenneman TB, 1991. Effects of two cropping and two tillage systems and pesticides on peanut pest management. Peanut Science 18, 41–6. [Google Scholar]
- Morillo‐Velarde R, Bermejo JL, Ayala J, Moreno A, Gutiérrez M, Márquez L, 2003. Remolacha Azucarera de Siembra Otoñal. Normas Técnicas de Cultivo. Sevilla, Spain: Junta de Andalucía, Consejería de Agricultura y Pesca. [Google Scholar]
- Nalim FA, Starr JL, Woodard KE, Segner S, Keller NP, 1995. Mycelial compatibility groups in Texas peanut field populations of Sclerotium rolfsii . Phytopathology 85, 1507–12. [Google Scholar]
- Okabe I, Matsumoto N, 2000. Population structure of Sclerotium rolfsii in peanut fields. Mycoscience 41, 145–8. [Google Scholar]
- Okabe I, Morikawa C, Matsumoto N, Yokoyama K, 1998. Variation in Sclerotium rolfsii isolates in Japan. Mycoscience 39, 399–407. [Google Scholar]
- Oksanen J, Guillaume Blanchet F, Kindt R et al. , 2011. Vegan: community ecology package. R package version 1.17‐11. [Accessed September 01 2011: http://vegan.r-forge.r-project.org/].
- Piepho HP, 2004. An algorithm for a letter‐based representation of all‐pairwise comparisons. Journal of Computational and Graphical Statistics 13, 456–66. [Google Scholar]
- Pratt RG, Rowe DE, 2002. Enhanced resistance to Sclerotium rolfsii in populations of alfalfa selected for quantitative resistance to Sclerotinia trifoliorum . Phytopathology 92, 204–9. [DOI] [PubMed] [Google Scholar]
- Puhalla JE, 1985. Classification of strains of Fusarium oxysporum on the basis of vegetative compatibility. Canadian Journal of Botany 63, 179–83. [Google Scholar]
- Punja ZK, 1985. The biology, ecology and control of Sclerotium rolfsii . Annual Review of Phytopathology 23, 97–127. [Google Scholar]
- Punja ZK, Grogan RG, 1983. Hyphal interactions and antagonism among field isolates and single‐basidiospore strains of Athelia (Sclerotium) rolfsii . Phytopathology 73, 1279–84. [Google Scholar]
- Punja ZK, Sun LJ, 2001. Genetic diversity among mycelial compatibility groups of Sclerotiun rolfsii (teleomorph Athelia rolfsii) and Sclerotium delphinii . Mycological Research 105, 537–46. [Google Scholar]
- Remesal E, Lucena C, Azpilicueta A, Landa BB, Navas‐Cortes JA, 2010. First report of southern blight of pepper caused by Sclerotium rolfsii in Southern Spain. Plant Disease 94, 280. [DOI] [PubMed] [Google Scholar]
- Sarma BK, Singh UP, Singh KP, 2002. Variability in Indian isolates of Sclerotium rolfsii . Mycologia 94, 1051–8. [PubMed] [Google Scholar]
- Schneider CL, Whitney ED, 1996. Sclerotium root rot In: Whitney ED, Duffus JE, eds. Compendium of Beet Diseases and Insects. St Paul, MN, USA: APS Press, 22–3. [Google Scholar]
- Shannon CE, Weaver W, 1963. The Mathematical Theory of Communication. Urbana, IL, USA: University of Illinois Press. [Google Scholar]
- Sharma BS, Pathak VN, Bhatnagar K, 1991. Morphological, cultural and pathogenic variations in Sclerotium rolfsii Sacc. causing root rot of sugarbeet. Cryptogamie Mycologie 12, 71–9. [Google Scholar]
- Yaqub F, Shahzad S, 2005. Pathogenicity of Sclerotium rolfsii on different crops and effect of inoculum density on colonization of mungbean and sunflower roots. Pakistan Journal of Botany 37, 175–80. [Google Scholar]


