Abstract
Scope
Perturbation of gut epithelial barrier function induces inflammation and other health problems that originate from the gut. Purple potato contains a high content of beneficial polyphenolic compounds. The objective of this study was to evaluate the effect of purple potato extract (PPE) on intestinal differentiation and barrier function, and explore its underlying mechanism using Caco-2 cells and ex vivo cultured gut tissues.
Methods and results
PPE increased transepithelial electrical resistance and decreased FITC-dextran paracellular flux in Caco-2 cells, which were associated with strengthened intestinal epithelial differentiation in both Caco-2 cells and ex vivo guts. Furthermore, PPE treatment enhanced AMP-activated Protein Kinase (AMPK) activity, concomitant with the increased expression of CDX2, a key transcriptional factor regulating intestinal epithelial differentiation. Knocking out AMPK using CRISPR/Cas9 system abolished the positive effects of PPE on intestinal epithelial differentiation and barrier function, in junction with the reduced expression of CDX2.
Conclusion
PPE improved gut epithelial differentiation and barrier function via activating AMPK, indicating that PPE, as well as associated purple potato consumption, could be used as a supportive dietary therapeutic strategy for improving gut epithelial health.
Keywords: AMPK, Barrier function, Epithelial differentiation, Gut, Purple potato extract
1. Introduction
Intestinal epithelium (IE) is a single layer of cells along the mucosal surface that absorb nutrients and secrete fluid. IE also functions as a frontier barrier protecting the mucosal integrity, which physically inhibits the penetration of harmful substances from the external environment [1]. The abnormal epithelial barrier function, also called leaky gut, is associated with many diseases and complications [2], including inflammatory bowel disease (IBD) [3], metabolic syndrome [4] and autoimmune disorders [5]. Thus, a proper intestinal barrier is essential for health. The major determinant of epithelial permeability is the tight junctions between paracellular enterocytes [6]. Tight junctions are developed during intestinal stem cells differentiating into epithelial cells [7]. Nutraceutical factors or therapeutic strategies that enhance intestinal epithelial differentiation can improve gut epithelial barrier function and health [8].
Accumulating evidence supports the beneficial effects of dietary phenolics in preventing leaky gut [9, 10]. Dietary intake of phenolic-rich food, such as green tea, grapes, and berries, strengthens intestinal barrier integrity and the formation of tight junctions [11] in an energy-dependent manner [12]. Furthermore, polyphenol extracts are known to activate adenosine monophosphate-activated protein kinase (AMPK) [9], an energy sensor that regulates tight junctions and epithelial permeability [13].
Purple potato is a source of polyphenols [14]. Purple potato extract (PPE) has health beneficial effects, such as antioxidative [15], anti-tumor [16], and antimicrobial [17] activity. However, the effects of PPE on gut barrier function and intestinal epithelial differentiation remain untested, and the mediatory role of AMPK in the improvement of epithelial barrier function by polyphenolics has only been sparsely explored [9]. It is also undefined whether AMPK mediates the beneficial health effects of phenolic-rich PPE. The objective of this study was to evaluate whether PPE has a beneficial effect on intestinal barrier function, and to further unveil the role of AMPK in linking PPE to gut epithelial differentiation and barrier function.
2. Materials and Methods
2.1. Purple potato extract
The purple potato extract (PPE) was prepared as described in the Supporting Information. Concentrated PPE was stored at −20°C until further use.
2.2. Total phenolic compounds determination
Total phenolic content was tested using Folin-Ciocalteu assay in a 96-well plate as described previously [18]. The brief method was described in the Supporting Information.
2.3. Cell culture
Caco-2 cells were seeded at a density of 2×105 per well on 12-well plates, and treated with PPE at various concentrations, as described in the Supporting Information.
2.4. MTT assay
Ten thousands of Caco-2 cells were seeded in each well of 96-well plates. Treated with 0, 5, 10, 20 or 40 μg/ml PPE for 48 hours after seeding, cells were incubated with 5mg/ml 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, Sigma, St. Louis, MO) for 4h. Formazan was resuspended in 100 μl DMSO, and the absorbance at 540 nm was measured using Synergy H1 microplate reader (BioTek, Winooski, VT)
2.5. In vitro epithelial barrier function assessment
Transepithelial electrical resistance (TEER) assay and the permeability of 4 kDa FITC-dextran (FD4) across the Caco-2 cell monolayer was measured [19], as described in the Supporting Information.
2.6. Alkaline phosphatase assay
Alkaline phosphatase (AP) assay was performed as previously described [19], which is briefly described in the Supporting Information.
2.7. Immunoblotting analysis
Immunoblotting analysis was performed according to the procedures as previously described [20]. Membranes were visualized using an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE). Band density was normalized to β-actin. Antibody information is described in the Supporting Information.
2.8. Calcium switch assay
The analysis was conducted with calcium-free DMEM [19], as described in the Supporting Information.
2.9. ex vivo gut culture
C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). All mice studies were performed in an approved protocol by the Institutional Animal Care and Use Committee (IACUC) at Washington State University. Ex vivo guts were isolated and cultured as described in the Supporting Information. Immunofluorescent staining and RT-qPCR were conducted as described in the Supporting Information.
2.10. AMPK knockout in Caco-2 cells
The pX330 CRISPR/Cas9 plasmids with PRKAA1 sgRNA (AMPK KO) and corresponding scramble control (SC) sequences were designed and purchased from GeneCopoeia (Rockville, MD). Caco-2 cells were transfected with AMPK KO or SC plasmids using Lipofectamine 3000 (Life Technologies) per manufacturer’s instructions as briefly described in the Supporting Information. Then the transfected cells were seeded onto 12-well plates at 2×105 cells per well, treated with 0 or 10 μg/ml PPE for 4 days for immunoblotting analysis, AP assay, and in vitro epithelial barrier function assessment.
2.11. Statistical analysis
Statistical analyses were conducted as previously described [21]. For cell studies, at least three independent experiments were carried out. For ex vivo studies, each cultured gut was considered as an experimental unit for immunofluorescent staining. For RT-qPCR assays, five cultured guts collected together were considered as an experimental unit. Data are presented as means ± standard error of the means (SEM). Comparisons of multiple means were analyzed using one-way ANOVA, followed by Duncan’s multiple comparison test. Data related to epithelial barrier function assessment were analyzed using repeated measures two-way ANOVA with post hoc Tukey’s test. Differing letters denote statistical differences in multiple comparison tests. All other data were analyzed using one-way ANOVA and two-tailed Student’s t-test. P < = 0.05 was considered to be statistically significant.
3. Results
3.1. PPE enhances intestinal barrier function
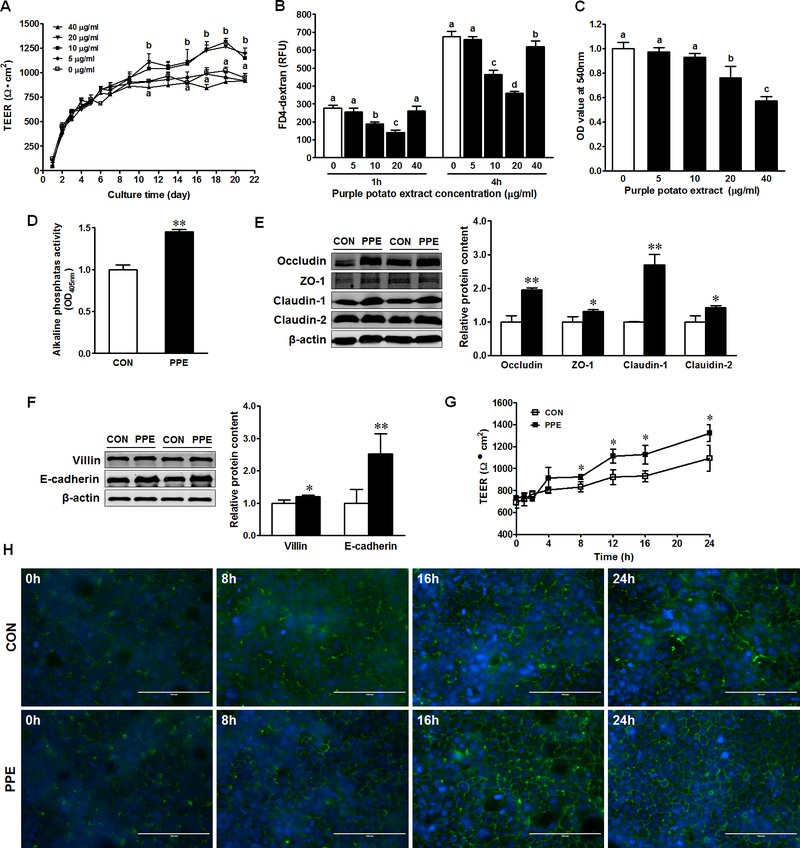
TEER was monitored during 21 days of differentiation. TEER increased with culture time, and its value increased faster in the treatment with 10 and 20 μg/ml PPE (Fig. 1A). FITC flux was reduced in Caco-2 cells treated with 10 and 20 μg/ml PPE (Fig. 1B). The permeability did not change with 40 μg/ml PPE at 1h, but decreased at 4h (Fig. 1B). 5 μg/ml PPE treatment had no significant effects on both TEER and FITC flux (Fig. 1A–B). PPE at 20 and 40 μg/ml caused a reduction in cell viability (Fig. 1C) that might explain the no difference of barrier function observed between Caco-2 cells without PPE treatment and Caco-2 cells treated with 40 μg/ml PPE (Fig. 1A). Since 10 μg/ml PPE increased intestinal barrier function, this concentration was selected to use in subsequent experiments.
Figure 1. PPE enhances intestinal epithelial differentiation, tight junction assembly and barrier function in Caco-2 cells.
(A) Transepithelial electrical resistance (TEER) of Caco-2 cells treated with 0, 5, 10, 20 and 40 μg/ml PPE. (B) FD4-dextran paracellular intestinal epithelial permeability at 21 days post PPE treatment. (C) MTT analysis for cell survival in CON (□) and PPE (■) treated cells. (D) Activity of alkaline phosphatase. (E) Protein contents of occludin, ZO-1, claudin-1, and claudin-2. (F) Protein contents of E-cadherin and villin. (G) TEER of Caco-2 cells after calcium switch. (H) Immunofluorescent staining of tight junction protein ZO-1 pre- and post- calcium switch assay. Caco-2 cells were grown to confluence and subjected to a calcium switch assay. Cells were fixed at various time points (0, 8, 16 and 24 h) after the restoration of Ca2+ containing medium. Scale bar is 100 μm. Mean ± SEM, n = 3, *: P < 0.05; **: P < 0.01. Differing letters denote statistical differences in multiple comparison tests.
3.2. PPE strengthens intestinal differentiation and tight junction assembly
In response to PPE, the epithelial differentiation markers, alkaline phosphatase (AP) activity as well as the contents of villin and E-cadherin, were enhanced (Fig. 1D, 1F). Additionally, PPE enhanced the protein content of occludin, zonula occludens-1 (ZO-1), claudin-1 and claudin-2 (Fig. 1E). Consistently, PPE enhanced the TEER post-calcium switch (Fig. 1G); fragmented strands of ZO-1 staining at cell borders were formed much faster in response to PPE treatment (Fig. 1H).
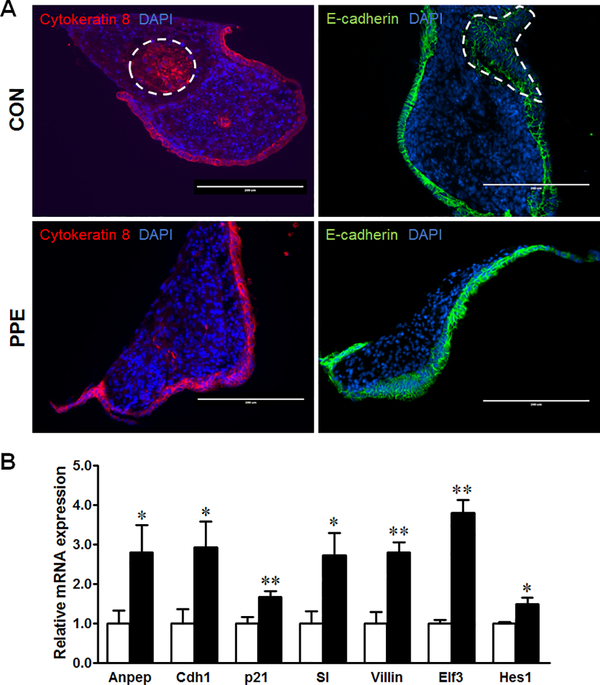
We further examined intestinal epithelial differentiation by immunofluorescence staining of ex vivo cultured guts. The outer epithelial layer was covered completely with E-Cadherin and cytokeratin-8 in ex vivo cultured guts treated with PPE, while a small number of the epithelial cells were still concentrated in CON gut lumen (Fig. 2A). Furthermore, PPE upregulated the mRNA expression of brush border enzymes (Anpep and SI), epithelial polarity markers (Cdh1 and Villin), intestinal differentiation transcription factors (Elf3 and Hes1) and the epithelial differentiation regulator (p21) in ex vivo cultured guts (Fig. 2B).
Figure 2. PPE promotes intestinal differentiation in ex vivo guts.
Intestine from E13.5 embryos was isolated, dissected, cultured to form ex vivo guts, and treated with 0 or 10 μg/ml PPE. (A) Immunofluorescent staining of cytokeratin 8 and E-cadherin. Scale bar is 200 μm. Dash lines indicate ex vivo gut lumen. (B) mRNA expression of Anpep, Cdh1, p21, SI, Villin, Elf3 and Hes1 in CON (□) and PPE (■) treated cells. Mean ± SEM, n = 4, *: P < 0.05; **: P < 0.01.
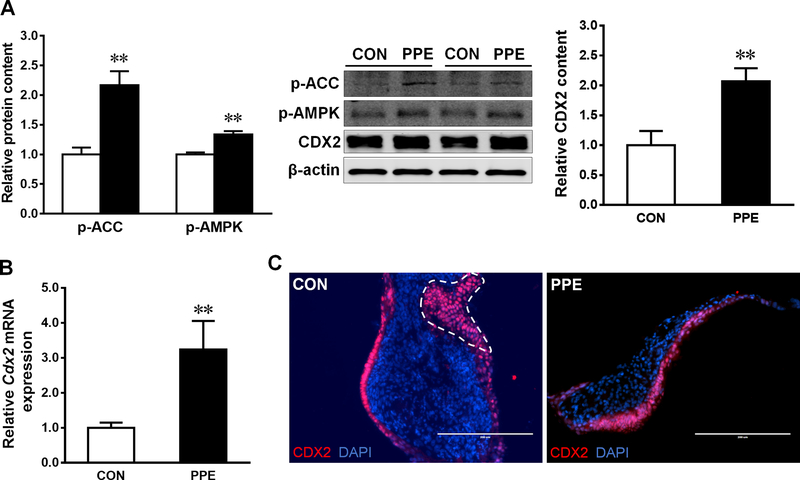
PPE treatment increased the protein content of CDX2 (Fig. 3A), a key transcription factor governing epithelial cell differentiation [22], in Caco-2 cells associated with enhanced phosphorylation of AMPK and ACC (Fig. 3A). Consistently, mRNA level of Cdx2 was increased in ex vivo gut with PPE treatment (Fig. 3B); the IF staining and distribution of CDX2 (Fig. 3C) was in accordance with the differentiation markers, cytokeratin 8 and E-cadherin (Fig. 2A).
Figure 3. PPE activates AMPK and CDX2 expression.
(A) Protein contents of p-AMPK, p-ACC and CDX2 in CON (□) and PPE (■) treated Caco-2 cells. (B) mRNA expression of Cdx2 in ex vivo guts. (C) Immunofluorescent staining of CDX2 in ex vivo guts. Scale bar is 200 μm. Dash line indicates ex vivo gut lumen. Mean ± SEM, n = 3, *: P < 0.05; **: P < 0.01.
3.3. AMPK mediates PPE-induced intestinal differentiation
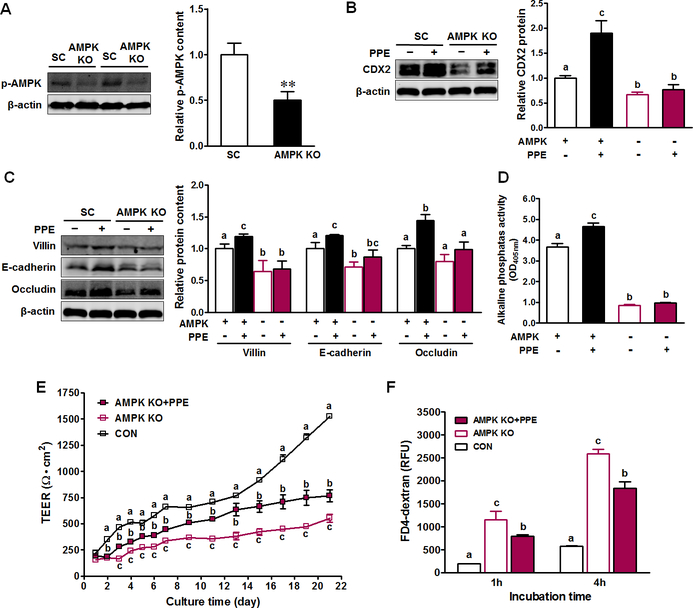
To investigate the regulatory role of AMPK in mediating PPE and its beneficial effects on gut health, we used the CRISPR/Cas9 system to knockout (KO) AMPK in Caco-2 cells, as indicated by the inactivation of p-AMPK (Fig. 4A). AMPK KO reduced CDX2 expression, which was unable to be elevated by PPE (Fig. 4B). Similarly, AMPK KO abolished the stimulatory effect of PPE on intestinal differentiation (Fig. 4C–D). AMPK KO had deleterious effects on TEER and FITC flux in Caco-2 cells; PPE treatment slightly improved barrier function in Caco-2 cells with AMPK KO (Fig. 4E–F).
Figure 4. AMPK mediates PPE-enhanced intestinal epithelial differentiation and barrier function.
Caco-2 cells were transfected with CRISPR/Cas9 plasmids with PRKAA1 sgRNA (AMPK KO) or corresponding scramble control (SC) sequences to delete AMPK (−) or not (+), then treated with 0 (−) or 10 μg/ml PPE (+). (A) Protein content of p-AMPK. (B) Protein contents of CDX2. (C) Protein contents of villin, E-cadherin and occludin. (D) Activity of alkaline phosphatase. (E) Transepithelial electrical resistance of Caco-2 cells. (F) FD4-dextran intestinal epithelial permeability at 21-day post incubation. CON: Caco-2 cells without PPE treatment; AMPK KO: Caco-2 cells with AMPK KO; AMPK KO + PPE: Caco-2 cells with AMPK KO treated with 10 μg/ml PPE. Mean ± SEM, n = 3. Differing letters denote statistical differences in multiple comparison tests.
4. Discussion
Caco-2 cells differentiate after confluence, resembling epithelial differentiation in vivo, which has been widely used for studying epithelial differentiation and barrier function [23]. 10 μg/ml PPE significantly strengthened barrier function, while 40 μg/ml PPE had no effects, which could be due to cell damage or apoptosis, as indicated by impaired cell viability. In support of our findings, 17 μM chicory phenol extracts upregulated TEER in Caco-2 cells, while 34 μM downregulated [24]. 1 mg/ml baobab fruit extract enhanced barrier function in Caco-2 cells, while 5 mg/ml baobab fruit extract induced cellular damage [25]. Our results are further supported by other studies on the beneficial roles of polyphenolic compounds, such as quercetin [26], kaempferol [27] and luteolin [28], in the barrier integrity of Caco-2 cells. Furthermore, anthocyanins from Meoru fruit extracts increased TEER in HCT-116 cells [29], and dietary chlorogenic acid decreased intestinal permeability in rat [30].
The integrity of the intestinal barrier is attributed to the expression and distribution pattern of junctional proteins during intestinal differentiation [31]. Retinoic acid enhances AP activity associated with upregulated mRNA of claudin-2 in Caco-2 cells [32]. The mRNA expression of claudin-2 and claudin-4, and TEER increased significantly in corneal epithelial RCE1(5T5) cells once differentiation initiated [33]. PPE stimulated intestinal differentiation, and enhanced tight junction assembly during intestinal development. Consistently, quercetin enhanced the distribution of claudin-1 and claudin-4 in Caco-2 cells [34]. Kaempferol treatment increased the intensity of claudin-3 and occludin at the intercellular junctions of Caco-2 cells [27]. Polyphenol-rich propolis extract increased the mRNA expression of ZO-1 and occludin, and improved tight junction structure in Caco-2 cells [9]. Claudin-1, occludin and ZO-1 are positively correlated to barrier function, while claudin-2 is inversely proportional to TEER [35]. Claudin-2 is the target of CDX2 transcription factor in Caco-2 cells [36] and HIEC cells [37]. Claudin-2 protein was enhanced by the expression of CDX2 facilitated by a retroviral vector in Colo 205 cells that do not express endogenous Cdx2 genes [38]. This indicates that PPE increased claudin-2 protein content possibly due to the activation of CDX2. However, the overall effect of PPE resulted in strengthened intestinal barrier function.
As a metabolic regulator, AMPK favors the maintenance and formation of epithelial barrier and differentiation [39]. AMPK promoted intestinal barrier function and differentiation through promoting the expression of CDX2, a critical transcription factor for intestinal differentiation [19]. Both AMPK activation and CDX2 expression were enhanced in response to PPE treatment. AMPK deletion notably abated the favorable function of PPE on epithelial differentiation and barrier function, confirming the regulatory roles of AMPK in PPE-induced gut differentiation. Consistently, a theaflavin derivative activated AMPK associated with augmented claudin-1 and ZO-1 and improved barrier in Caco-2 cells, while compound C, an AMPK inhibitor, treatment diminished this beneficial effects [40].
Purple-flesh potato contains high levels of polyphenolic compounds, chlorogenic acid and anthocyanins [41]. Oral administration of chlorogenic acid enhances tight junctions and intestinal barrier in rats [30]. Chlorogenic acid treatment phosphorylated AMPK in muscular cells associated with the stimulated glucose transportation, while an AMPK mutation abrogated the effects of chlorogenic acid [42]. Anthocyanins strengthen tight junctions and increase TEER in HCT-116 cells [29]; anthocyanins from meoru activate AMPK in HT-29 cells [43]. These data suggest that the beneficial effects of PPE on intestinal differentiation and barrier function might result from synergistic effects of anthocyanins and chlorogenic acid.
In summary, PPE strengthens intestinal differentiation and barrier function associated with the enhanced expression of CDX2 through a mechanism mediated by AMPK. Our data deepen the current understanding about the link between polyphenolic compounds and intestinal epithelial differentiation, and provide new insights into the molecular mechanisms underlying the beneficial role of polyphenolic compounds in intestinal barrier function. Our finding is important because intestinal barrier function is altered in a number of pathophysiological conditions such as obesity, diabetes, and inflammation, indicating that PPE, as well as associated purple potato consumption, could be used as a supportive dietary strategy for improving gut epithelial health.
Supplementary Material
Acknowledgements
This work was financially supported partially by National Institutes of Health (NIH) (R15HD073864) and Northwest Potato Research Consortium.
Abbreviations
- AMPK
AMP-activated protein kinase
- AP
alkaline phosphatase
- IBD
inflammatory bowel disease
- IE
intestinal epithelial
- PPE
purple potato extract
- SC
scramble control
- TEER
transepithelial electrical resistance
- ZO-1
zonula occludens-1
Footnotes
Competing interests
The authors declare no competing or financial interests.
5 References
- [1].Moore KA, Lemischka IR, Stem cells and their niches. Science 2006, 311, 1880–1885. [DOI] [PubMed] [Google Scholar]
- [2].Bischoff SC, Barbara G, Buurman W, Ockhuizen T, et al. , Intestinal permeability–a new target for disease prevention and therapy. BMC gastroenterology 2014, 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buhner S, Buning C, Genschel J, Kling K, et al. , Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut 2006, 55, 342–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brun P, Castagliuolo I, Di Leo V, Buda A, et al. , Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 2007, 292, G518–G525. [DOI] [PubMed] [Google Scholar]
- [5].Jenkins R, Rooney P, Jones D, Bienenstock J, Goodacre R, Increased intestinal permeability in patients with rheumatoid arthritis: a side-effect of oral nonsteroidal anti-inflammatory drug therapy? Rheumatology 1987, 26, 103–107. [DOI] [PubMed] [Google Scholar]
- [6].Hollander D, Intestinal permeability, leaky gut, and intestinal disorders. Current gastroenterology reports 1999, 1, 410–416. [DOI] [PubMed] [Google Scholar]
- [7].Ichikawa-Tomikawa N, Sugimoto K, Satohisa S, Nishiura K, Chiba H, Possible involvement of tight junctions, extracellular matrix and nuclear receptors in epithelial differentiation. BioMed Research International 2011, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sun X, Zhu M-J, AMP-activated protein kinase: a therapeutic target in intestinal diseases. Open Biology 2017, 7, 170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang K, Jin X, Chen Y, Song Z, et al. , Polyphenol-rich propolis extracts strengthen intestinal barrier function by activating AMPK and ERK signaling. Nutrients 2016, 8, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shigeshiro M, Tanabe S, Suzuki T, Dietary polyphenols modulate intestinal barrier defects and inflammation in a murine model of colitis. Journal of Functional Foods 2013, 5, 949–955. [Google Scholar]
- [11].Kosińska A, Andlauer W, Modulation of tight junction integrity by food components. Food research international 2013, 54, 951–960. [Google Scholar]
- [12].Shen L, Weber CR, Turner JR, The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. The Journal of cell biology 2008, 181, 683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zheng B, Cantley LC, Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proceedings of the National Academy of Sciences 2007, 104, 819–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lewis CE, Walker JR, Lancaster JE, Sutton KH, Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. Journal of the Science of Food and Agriculture 1998, 77, 45–57. [Google Scholar]
- [15].Han K-H, Sekikawa M, Shimada K. i., Hashimoto M, et al. , Anthocyanin-rich purple potato flake extract has antioxidant capacity and improves antioxidant potential in rats. British Journal of Nutrition 2006, 96, 1125–1134. [DOI] [PubMed] [Google Scholar]
- [16].Charepalli V, Reddivari L, Radhakrishnan S, Vadde R, et al. , Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. The Journal of nutritional biochemistry 2015, 26, 1641–1649. [DOI] [PubMed] [Google Scholar]
- [17].Ombra MN, Fratianni F, Granese T, Cardinale F, et al. , In vitro antioxidant, antimicrobial and anti-proliferative activities of purple potato extracts (Solanum tuberosum cv Vitelotte noire) following simulated gastro-intestinal digestion. Natural product research 2015, 29, 1087–1091. [DOI] [PubMed] [Google Scholar]
- [18].Zhang S, Zhu MJ, Characterization of polyphenolics in grape pomace extracts using ESI Q-TOF MS/MS. J Food Sci Nutr 2015, 1, 001. [Google Scholar]
- [19].Sun X, Yang Q, Rogers CJ, Du M, Zhu M-J, AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death & Differentiation 2017, 24, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhu MJ, Du M, Nathanielsz PW, Hess BW, Ford SP, Maternal nutrient restriction upregulates growth signaling in the cotyledonary artery of the cow placentome. J Soc Gynecol Investig 2006, 13, 77a–78a. [DOI] [PubMed] [Google Scholar]
- [21].Zhu MJ, Du M, Nathanielsz PW, Ford SP, Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta 2010, 31, 387–391. [DOI] [PubMed] [Google Scholar]
- [22].Beck F, Chawengsaksophak K, Waring P, Playford RJ, Furness JB, Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proceedings of the National Academy of Sciences 1999, 96, 7318–7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Peng L, Li Z-R, Green RS, Holzman IR, Lin J, Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. The Journal of nutrition 2009, 139, 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Azzini E, Maiani G, Garaguso I, Polito A, et al. , The Potential Health Benefits of Polyphenol-Rich Extracts from Cichorium intybus L. Studied on Caco-2 Cells Model. Oxidative medicine and cellular longevity 2016, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vreeburg RA, van Wezel EE, Ocaña‐Calahorro F, Mes JJ, Apple extract induces increased epithelial resistance and claudin 4 expression in Caco‐2 cells. Journal of the Science of Food and Agriculture 2012, 92, 439–444. [DOI] [PubMed] [Google Scholar]
- [26].Amasheh M, Schlichter S, Amasheh S, Mankertz J, et al. , Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. The Journal of nutrition 2008, 138, 1067–1073. [DOI] [PubMed] [Google Scholar]
- [27].Suzuki T, Tanabe S, Hara H, Kaempferol enhances intestinal barrier function through the cytoskeletal association and expression of tight junction proteins in Caco-2 cells. The Journal of nutrition 2011, 141, 87–94. [DOI] [PubMed] [Google Scholar]
- [28].Noda S, Tanabe S, Suzuki T, Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. Journal of agricultural and food chemistry 2012, 60, 4628–4633. [DOI] [PubMed] [Google Scholar]
- [29].Shin DY, Lu JN, Kim G-Y, Jung JM, et al. , Anti-invasive activities of anthocyanins through modulation of tight junctions and suppression of matrix metalloproteinase activities in HCT-116 human colon carcinoma cells. Oncology reports 2011, 25, 567. [DOI] [PubMed] [Google Scholar]
- [30].Ruan Z, Liu S, Zhou Y, Mi S, et al. , Chlorogenic acid decreases intestinal permeability and increases expression of intestinal tight junction proteins in weaned rats challenged with LPS. PLoS One 2014, 9, e97815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Groschwitz KR, Hogan SP, Intestinal barrier function: molecular regulation and disease pathogenesis. Journal of Allergy and Clinical Immunology 2009, 124, 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baltes S, Nau H, Lampen A, All‐trans retinoic acid enhances differentiation and influences permeability of intestinal Caco‐2 cells under serum‐free conditions. Development, growth & differentiation 2004, 46, 503–514. [DOI] [PubMed] [Google Scholar]
- [33].Ortiz-Melo MT, Sánchez-Guzmán E, González-Robles A, Valdés J, et al. , Expression of claudins-2 and-4 and cingulin is coordinated with the start of stratification and differentiation in corneal epithelial cells: retinoic acid reversibly disrupts epithelial barrier. Biology open 2012, BIO20123145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Suzuki T, Hara H, Quercetin enhances intestinal barrier function through the assembly of zonnula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. The Journal of nutrition 2009, 139, 965–974. [DOI] [PubMed] [Google Scholar]
- [35].Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, et al. , Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proceedings of the National Academy of Sciences 2010, 107, 4200–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sakaguchi T, Gu X, Golden HM, Suh E, et al. , Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1α. Journal of Biological Chemistry 2002, 277, 21361–21370. [DOI] [PubMed] [Google Scholar]
- [37].Escaffit F, Boudreau F, Beaulieu JF, Differential expression of claudin‐2 along the human intestine: implication of GATA‐4 in the maintenance of claudin‐2 in differentiating cells. Journal of cellular physiology 2005, 203, 15–26. [DOI] [PubMed] [Google Scholar]
- [38].Ezaki T, Guo R-J, Li H, Reynolds AB, Lynch JP, The homeodomain transcription factors Cdx1 and Cdx2 induce E-cadherin adhesion activity by reducing β-and p120-catenin tyrosine phosphorylation. American Journal of Physiology-Gastrointestinal and Liver Physiology 2007, 293, G54–G65. [DOI] [PubMed] [Google Scholar]
- [39].Novak EA, Mollen KP, Mitochondrial dysfunction in inflammatory bowel disease. Frontiers in cell and developmental biology 2015, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Park H-Y, Kunitake Y, Hirasaki N, Tanaka M, Matsui T, Theaflavins enhance intestinal barrier of Caco-2 Cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1. Bioscience, biotechnology, and biochemistry 2015, 79, 130–137. [DOI] [PubMed] [Google Scholar]
- [41].Lachman J, Hamouz K, Red and purple coloured potatoes as a significant antioxidant source in human nutrition-a review. Plant Soil and Environment 2005, 51, 477. [Google Scholar]
- [42].Ong KW, Hsu A, Tan BKH, Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PloS one 2012, 7, e32718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee Y-K, Lee WS, Kim GS, Park OJ, Anthocyanins are novel AMPKα1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncology reports 2010, 24, 1471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.