Abstract
Relatively little is known about the exposure of nail technicians to semivolatile organic compounds (SVOCs) in nail salons. We collected preshift and postshift urine samples and silicone wrist bands (SWBs) worn on lapels and wrists from 10 female nail technicians in the Boston area in 2016–17. We analyzed samples for phthalates, phthalate alternatives, and organophosphate esters (OPEs) or their metabolites. Postshift urine concentrations were generally higher than preshift concentrations for SVOC metabolites; the greatest change was for a metabolite of the phthalate alternative di(2-ethylhexyl) terephthalate (DEHTP): mono(2-ethyl-5-carboxypentyl) terephthalate (MECPTP) more than tripled from 11.7 to 36.6 μg/g creatinine. DEHTP biomarkers were higher in our study participants’ postshift urine compared to 2015–2016 National Health and Nutrition Examination Survey females. Urinary MECPTP and another DEHTP metabolite were moderately correlated (r = 0.37–0.60) with DEHTP on the SWBs, suggesting occupation as a source of exposure. Our results suggest that nail technicians are occupationally exposed to certain phthalates, phthalate alternatives, and OPEs, with metabolites of DEHTP showing the largest increase across a work day. The detection of several of these SVOCs on SWBs suggests that they can be used as a tool for examining potential occupational exposures to SVOCs among nail salon workers.
Graphical Abstract
INTRODUCTION
Nail salon workers encounter a variety of exposures from products they use at work, including semivolatile organic compounds (SVOCs), which are added to personal care products, including nail polish, to increase flexibility and longevity, improve fragrance, and help nail polish adhere to fingernails.1,2 The SVOC most frequently used in nail polish in the past was dibutyl phthalate (DBP),3 which is associated with birth defects and negative developmental and reproductive system effects.4,5 Because of health concerns, DBP and other phthalates have been replaced by compounds claimed to be less harmful to human health: organophosphate esters (OPEs) such as triphenyl phosphate (TPHP), terephthalates such as di(2-ethylhexyl) terephthalate (DEHTP), or other phthalate alternatives such as 1,2-cyclohexane dicarboxylic acid and diisononyl ester (DINCH).3,6,7 However, choosing safer products may be challenging as labels may not list all ingredients.3,6
SVOCs partition in the indoor environment between vapor, particles, and surfaces, including human skin.8 Exposure occurs through inhalation, ingestion (e.g., via dust), and dermal absorption.8 The latter can occur following contact with products containing SVOCs, indoor surfaces, or air-to-skin partitioning.9 Many SVOCs are metabolized into measurable urinary metabolites.6,10
Nail salon workers are likely chronically more exposed to many SVOCs found in nail products than the general public, however, few studies have been conducted on SVOC exposure of nail salon workers.11–13 Hines et al. examined occupational exposure to certain phthalates, including dimethyl phthalate (DMP), DBP, di-isobutyl phthalate (DiBP), benzylbutyl phthalate (BzBP), and di(2-ethylhexyl) phthalate (DEHP), and reported that nail salon workers had significantly higher (p < 0.05) postwork shift urinary metabolites of these chemicals than the U.S. adult population from the National Health and Nutrition Examination Survey (NHANES).11 Kwapniewski et al. examined preshift versus postshift DBP exposure among manicurists and found significantly higher (p < 0.05) urinary metabolites of DBP in postshift urine samples compared to preshift samples with glove use mitigating this effect.12 Tran and Kannan (2015) analyzed air samples for DMP, DEP, DiBP, DBP, BzBP, and DEHP from various indoor environments, and found that hair and nail salons had the highest total median concentrations of phthalates, an order of magnitude greater than the other indoor environments tested.13
We are not aware of any research on nail technician exposures to newer phthalate alternative compounds such as DEHTP and TPHP. Therefore, we conducted a pilot study using urinary biomarkers to characterize preshift versus postshift exposure to a wide range of SVOCs among nail salon workers in the Greater Boston Area. Silicone wrist bands (SWBs) can be used to estimate SVOC exposure, and may function partly as personal passive air samplers, and also sample particulates and surface films;14 to our knowledge, SWBs have not been previously used to measure exposure in nail salon technicians. A secondary goal of this study was to determine whether SWBs can be used as an exposure assessment tool to measure SVOCs encountered during a single work shift among nail salon workers.
MATERIALS AND METHODS
This study was approved by IRBs at Harvard T.H. Chan School of Public Health and Boston University School of Public Health. All participants provided informed consent in their native language prior to enrollment. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subject research.
Study Population.
For this pilot study, we recruited and enrolled 10 nail salon technicians from seven nail salons in the Greater Boston, Massachusetts area as previously described.15 Eligible nail salon workers were nonsmoking females greater than 18 years of age employed full time (≥35 h per week) in nail salons primarily offering nail salon services. Participants were compensated for their time.
We assessed exposure of nail salon workers to SVOCs—focusing on exposure to phthalates, phthalate alternatives (e.g., DINCH and DEHTP), and OPEs—over the course of one work shift between November 2016 and June 2017 (Table 1 shows analytes and abbreviations). Upon enrollment, we scheduled a sampling visit for a shift where the participant had worked the previous day. We employed a Vietnamese translator during sampling visits when necessary. Prior to our study visit, we asked participants not to apply nail polish to themselves within 72 h before sampling. We collected preshift and postshift spot urine samples on site, and asked participants to wear a SWB on their wrist and pinned to their lapel. Because of one participant opting not to wear one of the SWBs, and misplacement of another, we collected nine each of SWBs pinned to lapels and worn on wrists. At the end of the work shift, we administered a questionnaire including both work-related and nonwork-related questions on factors that potentially contribute to exposure.
Table 1.
Parent Compounds and Metabolitesa Examined in SWBs and Urine Among Nail Salon Workers (2016–2017) in the Greater Boston Area
| parent compound in SWB | urinary metabolite | ||||
|---|---|---|---|---|---|
| name | acronym | LODc | name | acronym | LODd |
| Phthalates | |||||
| butylbenzyl phthalate | BBzP | 6.7 | monobenzyl phthalate | MBzP | 0.3 |
| di-n-butyl phthalate | DBP | 1120 | mono-hydroxybutyl phthalate | MHBP | 0.4 |
| mono-n-butyl phthalate | MBP | 0.4 | |||
| di-iso-butyl phthalate | DiBP | 17.6 | mono-isobutyl phthalate | MiBP | 0.8 |
| mono-hydroxy-isobutyl phthalate | MHiBP | 0.4 | |||
| diethyl phthalate | DEP | 31.5 | monoethyl phthalate | MEP | 1.2 |
| dimethyl phthalate | DMP | 1.8 | not measured | ||
| di(2-ethylhexyl) phthalate | DEHP | 9.3 | mono-2-ethylhexyl phthalate | MEHP | 0.8 |
| mono-2-ethyl-S-hydroxyhexyl phthalate | MEHHP | 0.4 | |||
| mono-2-ethyl-5-oxohexyl phthalate | MEOHP | 0.2 | |||
| mono-2-ethyl-S-carboxypentyl phthalate | MECPP | 0.4 | |||
| di-isodecyl phthalateb | DiDPb | b | mono carboxyisononyl phthalate | MCNP | 0.2 |
| di-isononyl phthalateb | DiNP | 17.2 | mono-isononyl phthalate | MiNP | 0.9 |
| mono carboxyisooctyl phthalate | MCOP | 0.3 | |||
| monooxononyl phthalate | MONP | 0.4 | |||
| di-n-octyl phthalate | DOPb | b | mono-3-carboxypropyl phthalate | MCPP | 0.4 |
| Phthalate Alternatives | |||||
| 1,2-cyclohexane dicarboxylic acid, diisononyl esterb | DINCHb | b | cyclohexane-12-dicarboxylic acid monohydroxy isononyl ester | MHiNCH | 0.4 |
| cyclohexane-12-dicarboxylic acid monocarboxyisooctyl ester | MCOCH | 0.5 | |||
| diethylhexyl adipate | DEHA | 1.8 | not measured | ||
| trioctyltrimellitate | TOTM | 0.04 | not measured | ||
| di(2-ethylhexyl) terephthalate | DEHTP | 1.3 | mono-2-ethyl-S-carboxypentyl terephthalate | MECPTP | 0.2 |
| mono-2-ethyl-5-hydrohexyl terephthalate | MEHHTP | 0.4 | |||
| OPEs | |||||
| tris(l-chloro-2-propyl) phosphate | TCIPP | 6.7 | bis(l-chloro-2-propyl) phosphate | BCIPP | 0.1 |
| tris(2-chloroethyl) phosphate | TCEP | 30.6 | bis-2-chloroethyl phosphate | BCEP | 0.1 |
| tris(l,3-dichloro-2-propyl) phosphate | TDCIPP | 1.2 | bis(l,3-dichloro-2-propyl) phosphate | BDCIPP | 0.1 |
| tri-n-butyl phosphate | TBuP | b | dibutyl phosphate | DBuP | 0.1 |
| triphenyl phosphate | TPHP | 6.1 | diphenyl phosphate | DPHP | 0.1 |
| Brominated Flame Retardant | |||||
| 2-ethylhexyl-2,3/4,5-tetrabromobenzoateb | eh-tbbb | b | 2,3,4,5-tetrabromobenzoic acid | TBBA | 0.05 |
There may be additional parent compounds and metabolites
Not examined in SWB.
Units are ng/g.
Units are μg/g.
Urine Sampling.
Participants provided spot urine samples (~30 mL) in sterile polypropylene containers using nitrile gloves to prevent contamination. Samples were stored on ice and transported to the Beth Israel Deaconess Medical Center (BIDMC) laboratory. Samples were stored at −20 °C, thawed and aliquoted into 3 mL polypropylene microvials, and stored at −80 °C until shipped for analysis.
Urine samples were analyzed at the Division of Laboratory Services at the National Center for Environmental Health, CDC (Atlanta, GA) for creatinine and metabolites of SVOCs as previously described.7,16,17 Briefly, urinary conjugates of target analytes were first hydrolyzed by enzymatic hydrolysis. We then extracted the deconjugated urinary metabolites using solid phase extraction, separated the target analytes from each other and other compounds present in urine using high-performance liquid chromatography, and quantified the target biomarkers using isotope-dilution tandem mass spectrometry. During analysis, we included a duplicate urine aliquot from two separate participant samples for each SVOC compound for quality assurance/quality control. The relative percent difference between samples for SVOC compounds included in our analyses above the limit of detection (LOD) (Table 1) ranged from 0 to 13.1% for phthalates, 0.4–5.7% for phthalate alternatives, and 0.8–30.4% for OPEs.
Passive Air Sampling.
SWBs were prepared for sampling as previously described.14 Briefly, researchers purchased commercially available SWBs (www.24hourwristbands.com), solvent-cleaned and dried them in a fume hood, wrapped them in aluminum foil, and placed them in precleaned amber glass jars until use. We collected four field blank SWBs during the course of the study, which were transported to randomly selected study nail salons, removed from the amber jars and aluminum foil, rewrapped immediately, and placed on ice. At the end of each participant’s work shift, we collected and wrapped the SWBs in aluminum foil and placed them in amber jars in coolers on ice, and transported them to the BIDMC laboratory for storage at −20 °C until shipped for analysis. All SWBs were handled with nitrile gloves.
SWBs were analyzed at Duke University as previously described.14,20 Briefly, SWBs were weighed but not washed before analysis, spiked with internal standards dTDCPP and 13C TPP, extracted, concentrated, and analyzed by gas chromatography–mass spectrometry. Recoveries of dTDCPP and 13C TPP averaged 93.3 and 68.9%, respectively, for all samples. Since the field blanks account for residual background levels that may be present on all SWBs, the LOD (Table 1) was calculated as three times the standard deviation of the levels measured in the field blanks. SVOC concentrations in SWBs were blank corrected using the average concentrations measured in the field blanks. Results were expressed as ng/g SWB.
Since participants had different work-shift lengths, and thus SWBs were worn for different durations, we also controlled for work shift length in our analyses (Tables S2 and S3). However, we do not know whether the chemicals are in the linear or saturation phase of uptake on the SWBs during the nail technicians’ work shifts, as to our knowledge a calibration study of the uptake of these chemicals in SWBs has not been published. Because of this, and the fact that these work shift-duration-adjusted data were almost perfectly correlated with the unadjusted data (Table S1), we focus on the unadjusted data in our results section.
Statistical Analysis.
Where instrumental readings were unavailable, urine and SWB nondetected samples were imputed using NDExpo’s regression on order statistics application: a robust method to handle nondetected samples, described by Helsel.18,19 We conducted statistical analyses for compounds detected in at least 50% of the samples. We used the Shapiro–Wilk test to examine whether SVOC concentrations were normally distributed; as concentrations were approximately log-normally distributed, we log-transformed the data, and report geometric means (GM), geometric standard deviations (GSD), medians, and ranges. We used paired t-tests to compare: (1) SVOC levels on SWBs worn on the lapels versus wrists and (2) preshift and postshift creatinine-corrected SVOC urinary metabolite concentrations. Postshift urine concentrations of mono(2-ethyl-5-carboxypentyl) terephthalate (MECPTP), however, were not normally or log-normally distributed, and we used the nonparametric Wilcoxon Rank Sum test to compare the preshift and postshift samples. We computed Spearman correlations between parent SVOCs in SWBs (a measure of external exposure during the shift) and their metabolites in urine, using the difference between postshift and preshift creatinine-corrected concentrations (an estimate of total exposure during the work shift). Statistical significance was set at p < 0.05. We compared nail technician creatinine-corrected urinary SVOC metabolites to those found in the general U.S. female population aged 3 and older in the NHANES 2015–2016 presented in the National Report on Human Exposure to Environmental Chemicals updated tables, the first time such data were available for DEHTP; the exception was for OPE metabolites for which we used the NHANES 2013–2014 data of U.S. females aged 6 and older.10,21 We performed all statistical analyses using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC).
RESULTS
Participant characteristics are presented in Table 2. A detailed description of personal protective equipment utilized by participants was described previously.15
Table 2.
Characteristics of 10 Nail Salon Participants, Greater Boston Area (2016–2017)
| participant characteristics | N participants (n = 10) | median | range |
|---|---|---|---|
| current age | 45 | 21–64a | |
| Country of Origin (Primary Language Spoken) | |||
| USA (English) | 4 | ||
| Vietnam (Vietnamese) | 6 | ||
| Occupational Title | |||
| nail technician | 8 | ||
| nail salon owner | 2 | ||
| Employment History | |||
| full-time in Nail Salon | 10 | <l–23a | |
| part-time in Nail Salon | 6 | <l–33a | |
| Hours worked | |||
| per week | 40 | 20–50b | |
| day of sampling | 9 | 6–llb | |
| Number of Procedures | |||
| regular manicure | 8 | 1–5c | |
| acrylic manicure | 2 | 3–9c | |
| gel manicure | 3 | 1–4c | |
| refill | 1 | lc | |
| pedicure | 8 | 1–3c | |
Years.
Hours.
Number of procedures.
SVOCs in Urine.
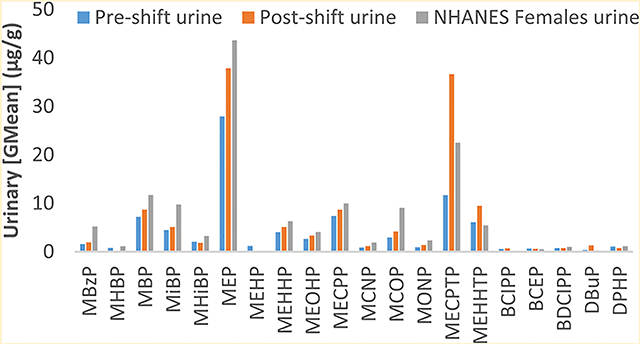
Descriptive statistics of the SVOC metabolites detected in participant urine, along with the postshift/preshift change in the SVOC concentrations are listed in Table 3. While the majority of postshift creatinine-corrected GM urinary SVOC metabolite concentrations were similar or higher than preshift urinary concentrations, none reached statistical significance. Despite this, the postshift GM concentration was more than triple that of the preshift samples for MECPTP. There was a general trend for higher urinary metabolite concentrations in postshift urine samples among many but not all participants (Figure S1, Supporting Information).
Table 3.
Descriptive Statistics for Creatinine-Corrected SVOC Metabolites Examined in Preshift and Postshift Urine Samples from Nail Salon Workers in the Greater Boston Area (2016–2017)a
| preshift urine concentrations (μg/g) (n = 10) | postshift urine concentrations (μg/g) (n = 10) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| biomarker | % >LOD | GM | GSD | median | range | % >LOD | GM | GSD | median | range | [postshift/preshift] difference | NHANESb GM |
| Phthalates | ||||||||||||
| MBzP | 70 | 1.6 | 2.7 | 1.2 | <0.3–14.0 | 70 | 1.9 | 2.7 | 1.9 | <0.3–12.2 | 0.3 | 5.2 |
| MHBP | SO | 0.79 | 2.0 | 0.7 | <0.4–2.5 | 40 | N/A | N/A | <0.4 | <0.4–3.0 | N/A | 1.13 |
| MBP | 90 | 7.2 | 2.0 | 7.6 | <0.4–24.0 | 90 | 8.7 | 2.5 | 9.1 | <0.4–50.3 | 1.5 | 11.7 |
| MiBP | 90 | 4.5 | 1.7 | 4.4 | <0.8–12.3 | 70 | 5.1 | 1.8 | 5.8 | <0.8–11.4 | 0.6 | 9.75 |
| MHiBP | 80 | 2.1 | 1.3 | 1.9 | <0.4–3.6 | 60 | 1.8 | 1.6 | 1.8 | <0.4–4.8 | −0.3 | 3.27 |
| MEP | 100 | 27.9 | 2.0 | 22.0 | 11.2–86.7 | 100 | 37.9 | 3.1 | 30.7 | 12.8–515 | 10.0 | 43.6 |
| MEHP | 50 | 1.2 | 2.8 | 1.2 | <0.8–9.8 | 40 | N/A | N/A | <0.8 | <0.8–4.4 | N/A | N/A |
| MEHHP | 90 | 4.0 | 2.4 | 3.1 | <0.4–15.9 | 90 | 5.1 | 2.4 | 7.4 | <0.4–15.3 | 1.1 | 6.29 |
| MEOHP | 90 | 2.6 | 2.5 | 2.3 | <0.2–11.1 | 90 | 3.3 | 2.7 | 3.8 | <0.2–11.2 | 0.7 | 4.08 |
| MECPP | 100 | 7.4 | 2.3 | 6.2 | 3.0–39.1 | 100 | 8.7 | 2.0 | 9.4 | 3.7–28.9 | 1.3 | 9.98 |
| MCNP | 70 | 0.9 | 3.0 | 0.7 | <0.2–6.2 | 70 | 1.2 | 2.6 | 0.9 | <0.2–4.8 | 0.3 | 1.90 |
| MiNP | 10 | N/A | N/A | <0.9 | <0.9–4.8 | 10 | N/A | N/A | <0.9 | <0.9–4.9 | N/A | N/A |
| MCOP | 90 | 2.9 | 2.0 | 3.4 | <0.3–7.4 | 90 | 4.2 | 2.2 | 4.4 | <0.3–17.2 | 1.3 | 9.06 |
| MONP | 50 | 0.9 | 2.1 | 0.9 | <0.4–2.7 | 60 | 1.4 | 2.7 | 1.1 | <0.4–6.7 | 0.5 | 2.32 |
| MCPP | 30 | N/A | N/A | <0.4 | <0.4–2.2 | 30 | N/A | N/A | <0.4 | <0.4–2.4 | N/A | 1.24 |
| Phthalate Alternatives | ||||||||||||
| MHiNCH | 20 | N/A | N/A | <0.4 | <0.4–2.3 | 20 | N/A | N/A | <0.4 | <0.4–2.0 | N/A | N/A |
| MCOCH | 20 | N/A | N/A | <0.5 | <0.5–2.7 | 20 | N/A | N/A | <0.5 | <0.5–2.7 | N/A | N/A |
| MECPTP | 100 | 11.7 | 3.3 | 17.4 | 1.0–50.1 | 100 | 36.6 | 4.3 | 26.9 | 11.5–1286 | 24.9 | 22.5 |
| MEHHTP | 100 | 6.1 | 2.1 | 6.4 | 1.5–22.7 | 100 | 9.5 | 3.4 | 7.4 | 2.1–166 | 3.4 | 5.45 |
| OPEs | ||||||||||||
| BCIPP | 90 | 0.5 | 2.3 | 0.6 | <0.1–1.6 | 90 | 0.7 | 2.3 | 0.8 | <0.1–2.4 | 0.2 | N/A |
| BCEP | 60 | 0.6 | 4.5 | 0.5 | <0.1–4.3 | 70 | 0.6 | 2.5 | 0.5 | <0.1–2.0 | 0.0 | 0.476 |
| BDCIPP | 80 | 0.8 | 2.2 | 0.8 | <0.1–2.4 | 90 | 0.8 | 2.0 | 0.7 | <0.1–2.4 | 0.0 | 0.993 |
| DBuP | SO | 0.4 | 4.4 | 0.3 | <0.1–7.6 | 20 | N/A | N/A | <0.1 | <0.1–9.1 | N/A | 0.227 |
| DPHP | 90 | 1.1 | 2.9 | 0.8 | <0.1–6.5 | 90 | 1.3 | 2.1 | 1.3 | <0.1–4.1 | 0.2 | 1.13 |
| Brominated Flame Retardant | ||||||||||||
| TBBA | 10 | N/A | N/A | <0.05 | <0.05–0.3 | 0 | N/A | N/A | <0.05 | <0.05 | N/A | N/A |
GM = geometric mean; GSD = geometric standard deviation; LOD = LOD; N/A not calculated: proportion of the results below the LOD was too high to provide a valid result.
NHANES phthalate and phthalate alternative data collected in 2015–2016, OPEs and brominated flame retardant data collected in 2013–2014.
Table 3 also presents a comparison of our results with females in NHANES. Most SVOC metabolites were similar or higher in the U.S. female population than in our study population’s postshift urine samples, however metabolites of DEHTP (MECPTP and MEHHTP) were higher in postshift urine samples from our study participants than in NHANES females.
SVOCs in Air.
A few of the participants’ SWB concentration results were estimated above the highest point on the calibration curve for individual SVOCs, including one each with high levels of DBP, DEHP, and DEHTP, and one with high levels of both DiNP and DEHTP; these values were included in the overall analyses. Descriptive statistics of the SVOC concentrations detected in lapel and wrist SWBs, along with the differences in SVOC concentrations and correlations between lapel/wrist are listed in Table 4. With the exception of TCIPP and TDCIPP, SVOC levels in lapel SWBs were higher compared with those worn on participants’ wrists; however, none of these differences reached statistical significance (Table 4, Figure S2). Correlations between the concentrations of SVOCs detected in lapel and wrist SWBs varied; only the phthalate alternative DEHA reached statistical significance (r = 0.81, p < 0.05).
Table 4.
Descriptive Statistics for SVOCs Examined in SWBs Worn During a Work Shift by Nail Salon Workers in the Greater Boston Area (2016–2017)a,d
| lapel SWB concentrations (ng/g) (n = 9) | wrist SWB concentrations (ng/g) (n = 9) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| compound | % >LOD | GM | GSD | median | range | % >LOD | GM | GSD | median | range | [lapel/wrist] ratio | wrist/lapel correlationb |
| Phthalates | ||||||||||||
| BBzP | 44.4 | N/A | N/A | <6.7 | <6.7–45.7 | 22.2 | N/A | N/A | <6.7 | <6.7–22.3 | N/A | N/A |
| DBP | 0.0 | N/A | N/A | <1120 | all <1120 | 11.1 | N/A | N/A | <1120 | <1120–1697 | N/A | N/A |
| DiBP | 33.3 | N/A | N/A | <17.6 | <17.6–56.1 | 33.3 | N/A | N/A | <17.6 | <17.6–143 | N/A | N/A |
| DEP | 22.2 | N/A | N/A | <31.5 | <31.5–576 | 22.2 | N/A | N/A | <31.5 | <31.5–967 | N/A | N/A |
| DMP | 33.3 | N/A | N/A | <1.8 | <1.8–3.8 | 33.3 | N/A | N/A | <1.8 | <1.8–5.8 | N/A | N/A |
| DEHP | 100 | 195 | 4.3 | 251 | 27.0–3572 | 55.6 | 29.6 | 22.5 | 42.4 | <9.3–2004 | 6.6 | 0.17 |
| DiNP | 88.9 | 182 | 4.1 | 200 | <17.2–1339 | 55.6 | 32.7 | 31.0 | 50.7 | <17.2–4719 | 5.6 | −0.21 |
| Phthalate Alternatives | ||||||||||||
| DEHA | 77.8 | 4.5 | 18.9 | 5.0 | <1.8–1364 | 55.6 | 1.8 | 25.6 | 2.3 | <1.8–260 | 2.5 | 0.81c |
| TOTM | 100 | 2.8 | 6.5 | 2.2 | 0.17–70.5 | 55.6 | 0.41 | 89.9 | 1.4 | <0.04–493 | 6.8 | 0.67 |
| DEHTP | 100 | 105 | 4.3 | 81.9 | 11.1–1636 | 77.8 | 21.8 | 29.1 | 20.8 | <1.3–5817 | 4.8 | 0.21 |
| OPEs | ||||||||||||
| TCIPP | 77.8 | 29.2 | 3.5 | 40.9 | <6.7–161 | 88.9 | 39.7 | 4.3 | 39.5 | <6.7–199 | 0.73 | 0.48 |
| TCEP | 11.1 | N/A | N/A | <30.6 | <30.6–56.2 | 0.0 | N/A | N/A | <30.6 | all <30.6 | N/A | N/A |
| TDCIPP | 77.8 | 3.5 | 4.1 | 3.3 | <1.2–27.4 | 88.9 | 8.8 | 3.8 | 15.7 | <1.2–35.0 | 0.39 | 0.14 |
| TPHP | 88.9 | 153 | 4.3 | 257 | <6.1–1006 | 100 | 95.8 | 3.4 | 132 | 11.8–368 | 1.6 | 0.07 |
GM = geometric mean; GSD = geometric standard deviation; and LOD = LOD.
Spearman correlation N/A not calculated: proportion of results below method detection limit was too high to provide a valid result.
Spearman correlation statistically significant (p < 0.05).
Note: not all compounds listed in Table 1 were measured in SWBs.
Air Versus Urine SVOCs.
The correlations for differences between preshift and postshift urinary SVOC metabolites and their parent compounds detected in SWBs are shown in Table 5. For DEHP, although not statistically significant, the difference in postshift urinary concentrations compared to preshift urinary concentrations was more correlated with SWBs worn on the lapel than on the wrist. The differences between preshift urinary metabolites and postshift urinary metabolites of DEHTP (MECPTP and MEHHTP) were moderately correlated with DEHTP on lapel SWBs and wrist SWBs.
Table 5.
Correlations between SVOCs in SWBs and Creatinine-Corrected Urinary Metabolites in Postshift Minus Preshift Urine Samples from Nail Salon Workers in the Greater Boston Area (2016–2017)
| Spearman correlation coefficients for difference of postshift and preshift urine | |||
|---|---|---|---|
| SWB parent compound | urinary metabolite | lapel SWB | Wrist SWB |
| Phthalates | |||
| DEHP | MEHP | 0.18 | 0.15 |
| MEHHP | 0.28 | 0.18 | |
| MEOHP | 0.17 | 0.07 | |
| MECPP | 0.63 | 0.22 | |
| DiNP | MCOP | 0.27 | 0.45 |
| MONP | −0.08 | 0.30 | |
| Phthalate Alternatives | |||
| DEHTP | MECPTP | 0.60 | 0.37 |
| MEHHTP | 0.38 | 0.57 | |
| OPEs | |||
| TCIPP | BCIPP | −0.40 | −0.30 |
| TDCIPP | BDCIPP | −0.10 | 0.20 |
| TPHP | DPHP | 0.13 | 0.28 |
DISCUSSION
To our knowledge, our pilot study is the first to characterize nail salon worker exposure to phthalates, phthalate alternatives, and OPEs, using both biomonitoring and SWBs, a relatively novel tool. This study provides evidence of exposure to many of these compounds and demonstrates the usefulness of SWBs.
Our most striking finding was evidence suggesting nail salon workers are occupationally exposed to the phthalate alternative DEHTP, with postshift urinary concentrations of a DEHTP metabolite (MECPTP) more than triple the level of preshift concentrations. This change was moderately correlated with DEHTP levels on SWBs, suggesting an occupational exposure source rather than primarily other exposure source such as diet that is unlikely to be picked up by SWBs. Increases of MECPTP during the day have previously been observed in general populations, however these increases were generally lower than what we observed for our preshift to postshift change (e.g. 1.6 times from NHANES compared to 3.1 times in our study, respectively), and further research is necessary to better understand this trend.21
Concentrations of DEHTP metabolites (MECPTP and MEHHTP) in nail technician postshift urine samples were also higher than what was observed in NHANES. This difference may be underestimated, as there is a significant downward trend in GM concentrations of MECPTP and MEHHTP (along with some other SVOCs) in NHANES with increasing age, and the NHANES female population includes children under age 18,21 while our study population only enrolled adult females over age 18. Although DEHTP is used as a replacement for the phthalate DEHP, the literature on DEHTP exposure is relatively sparse, but suggests increasing exposure in the U.S. and Europe.7,21,22 Because of the lack of studies examining the health consequences associated with DEHTP exposure in humans to date, we do not fully understand whether increasing human exposure or long-term exposure to low levels of it will negatively impact human health. Metabolites of DEHTP are generally higher in females than in males,21 suggesting exposure via personal care products or a similar source linked to behavioral differences between males and females. Interestingly, DEHTP is not a traditional SVOC in nail polish and we do not know the source of the DEHTP in nail salons. Although DEHTP may be present in some nail polishes, we found no evidence for this in a recent nail product study.3 DEHTP is likely present in other personal care products or materials used in nail salons, such as lotions, waxes, or skin scrubbing exfoliants.
Previous studies have found higher concentrations of certain phthalates (e.g., DEP and DBP) in nail salons compared to other indoor environments,13 and higher concentrations of certain SVOC urinary metabolites from nail salon workers compared to the U.S. general population (e.g. MBP and MEHP).11 While there was an upward trend for SVOC urinary metabolites from preshift to postshift samples in our study, none reached statistical significance, perhaps due to the small sample size. Exposure to many phthalates, phthalate alternatives, and OPEs is ubiquitous in the U.S. due to their common usage in personal care and other consumer products.10,17,21 Some SVOCs are also present in food or food packaging, a potential explanation for the lack of correlation between urine and SWBs for some compounds as SWBs do not capture dietary exposure sources.
Postshift urinary concentrations from our study participants were generally lower than concentrations from U.S. females from NHANES, with the exception of urinary metabolites of DEHTP, TCEP, and TPHP.10,21 As use of some of the more toxic phthalates such as DBP and DEHP have been reduced and replaced in the U.S. over time with alternatives such as DEHTP due to potential health concerns, concentrations of urinary metabolites such as MBP and MEHP have decreased, while metabolites from phthalate alternatives such as MECPTP and MEHHTP have increased.21,23,24 Factors that may partly explain the lower urinary metabolite concentrations from our participants include that NHANES includes females under 18, which our study excludes, and that the NHANES data were largely from an earlier time period (2013–2014 and 2015–2016) than our study (2016–2017).
Our pilot study demonstrated relatively high detection frequencies of a number of SVOCs on SWBs after having been worn by nail salon workers for only one shift (e.g., 6–11 h). Little is known about the kinetics of uptake of SVOCs by SWBs and we are aware of only one previously published occupational exposure study (of polycyclic aromatic hydro-carbons).25 The fact that SWBs not only function as passive air monitors, but can pick up material from contact with surfaces and skin was evident in SWBs utilized by our study participants, as many appeared dusty and/or had debris on the surface upon collection at the end of the work shift. Interestingly, TPHP concentrations on lapel and wrist SWBs were not correlated, perhaps suggesting that different exposure sources of these compounds are encountered based on contact with skin and surfaces (more likely with wrist SWBs) versus those in the air (more likely with lapel SWBs). More research is needed to understand this difference and its implications for exposure routes. The results for DEHTP also add to the small body of literature validating the use of SWBs with biomonitoring results.14,25,26
The replacement of phthalates with alternatives, and the corresponding increased exposure to the latter, is of concern since often we do not fully understand the toxicity of the alternatives.27 For example, DEHTP is thought of as a safer alternative to DEHP, a known male reproductive toxicant, since it is not known to cause reproductive toxicity.28 However, exposure to DEHTP may be associated with other health concerns. A dietary study of DEHTP fed to F-344 rats over 104 weeks found reduced weight gain and exacerbated geriatric retinal degeneration with chronic, high dietary exposure (6000 or 12 000 ppm).29 TPHP, another replacement chemical found in nail polish, has recently been identified as an endocrine disrupter that may be negatively associated with thyroid function and reproductive health.30–32 Future, larger biomonitoring studies of nail salon workers will help to verify and identify replacement chemicals of particular concern from changes in formulations to products used in nail salons.
Our study has a number of limitations, particularly the small sample size that limited statistical power. We used the difference between postshift and preshift metabolite concentrations in urine as a measure of exposure during the work day, but while this approach has several advantages (e.g. assessing exposure across inhalation, dermal, and other routes), it also has disadvantages. The appearance of metabolites in urine has a time lag due to pharmacokinetics (e.g., absorption and metabolism rate). As we were not able to collect urine later in the day after the shift (or the following first morning void), we may have missed some exposure. The human half-lives of many of these compounds is not known, but believed to be on the order of hours to days. Thus, if we collected participants’ urine for a longer time period (e.g. 24 or 48 h after their work shift) we would likely obtain a better understanding of these occupational exposures. As we only sampled participants on days when they had worked the previous day, preshift urine samples may partly reflect previous occupational exposure for the relatively more persistent SVOCs. A potential explanation of why we generally saw higher concentrations on SWBs pinned to participants’ lapels compared to the ones worn on their wrists is that those on the wrist may have been covered up by participants’ sleeves, thus in future studies it would be beneficial to ensure that all SWBs are exposed to salon air for the duration of participants’ work shifts. Additionally, asking participants to wear SWBs during working hours on multiple work days during a given work week may have captured more information on occupational SVOC exposures. We did not attempt to assess exposure at home, while commuting, or via diet.
Strengths of our study of nail salon workers include the use of biomonitoring, demonstration of the use of SWBs, collection of these samples in a sometimes difficult to reach population, and analysis for a wide spectrum of SVOCs. An additional strength is the paired use of biomonitoring and SWB data, which suggested that the increase of urinary DEHTP metabolites was due to occupational exposure rather than other sources such as diet.
Higher concentrations of SVOC urinary metabolites detected in postshift urine samples compared to preshift samples and the presence of parent compounds detected on SWBs worn during the work shift indicate that nail salon workers are occupationally exposed to SVOCs. The higher concentrations of DEHTP metabolites in our study population compared to the U.S. female population from the NHANES study, and more than tripling of preshift to postshift concentrations of MECPTP measured in urine suggest that nail salon workers are exposed to DEHTP during the work day. Finally, the detection of several phthalates, phthalate alternatives, and OPEs on SWBs worn during the work shift indicates that SWBs can be used as an exposure assessment tool for nail salon workers for future studies. Future, larger biomonitoring studies with more statistical power, and a longer sampling timeframe would help further clarify which SVOCs nail technicians are exposed to at work. Finally, it would be useful to compare the SVOCs detected on SWBs to active air samples collected during the work day to validate the effectiveness of SWBs as an occupational exposure assessment tool in nail salons.
Supplementary Material
ACKNOWLEDGMENTS
We express gratitude to the late Xiaoyun Ye, Nan Pham, Tammy Nguyen, Vy Ngygen, MyDzung Chu, Audrey Nathanson, and Wendy Hori for their critical contribution to this research in the laboratory and field. The authors are thankful for the salons and nail salon technicians for their participation in our study. This work was supported by pilot funding from Boston University School of Public Health and NIH/NIEHS T32ES014562. Dr. Webster was supported in part by NIH/NIEHS R01ES028800, R01ES016099, and USEPA grant 83564201. J.A.C. was supported in part by NIH R01 ES016099. Dr. Stapleton and S.H. were supported by NIH R01 ES016099 and USEPA grant 83564201. This work was also supported by funds contributed by Harvard NIEHS Research Center for Environmental Health pilot grant (NIH P30ES000002), NIH/NIEHS 2R25ES023635-04, NIOSH T42 OH008416, Harvard Catalyst NIH UL1 TR001102, Harvard JPB Environmental Health Fellowship, and Harvard Hoffman Program in Chemicals and Health.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.9b02474.
Comparison of creatinine-corrected preshift and postshift concentrations of phthalates, phthalate alternatives, and OPEs (ng/g) detected ≥66.7% in individual nail salon workers’ urine in the Greater Boston Area (2016–2017); concentrations of SVOCs detected at ≥66.7% on SWBs Worn on the lapel or wrist during a work shift by nail salon workers in the Greater Boston Area (2016–2017); correlations between SVOCs in SWBs measured in ng/g and SVOC concentrations measured in SWBs corrected for work shift length (ng/g)−hr from nail salon workers in the Greater Boston Area (2016–2017); descriptive statistics for SVOCs examined in SWBs worn during a work shift adjusted for work-shift duration by nail salon workers in the Greater Boston Area (2016–2017); and correlations between SVOCs in SWBs and creatinine-corrected urinary metabolites in postshift minus preshift urine samples adjusted for work-shift duration from nail salon workers in the Greater Boston Area (2016–2017) (PDF)
The authors declare no competing financial interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
REFERENCES
- 1.CosmeticsInfo. Triphenyl Phosphate. https://www.cosmeticsinfo.org/ingredient/triphenyl-phosphate (accessed Nov 1, 2018).
- 2.U.S. Food and Drug Administration. Phthalates. https://www.fda.gov/cosmetics/productsingredients/ingredients/ucm128250.htm#cos (accessed Nov 26, 2018).
- 3.Young AS; Allen JG; Kim U-J; Seller S; Webster TF; Kannan K; Ceballos DM Phthalate and Organophosphate Plasticizers in Nail Polish: Evaluation of Labels and Ingredients. Environ. Sci. Technol 2018, 52, 12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ATSDR, Agency for Toxic Substances and Disease Registry. Toxicological Profile for Di-n-butyl Phthalate; U.S. Department of Health and Human Services, 2001. [PubMed] [Google Scholar]
- 5.Hauser R; Calafat AM Phthalates and Human Health. Occup. Environ. Med 2005, 62, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendelsohn E; Hagopian A; Hoffman K; Butt CM; Lorenzo A; Congleton J; Webster TF; Stapleton HM Nail Polish as a Source of Exposure to Triphenyl Phosphate. Environ. Int 2016, 86, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva MJ; Wong L-Y; Samandar E; Preau JL; Calafat AM; Ye X Exposure to Di-2-Ethylhexyl Terephthalate in a Convenience Sample of U.S. Adults from 2000 to 2016. Arch. Toxicol 2017, 91, 3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little JC; Weschler CJ; Nazaroff WW; Liu Z; Cohen Hubal EA Rapid Methods to Estimate Potential Exposure to Semivolatile Organic Compounds in the Indoor Environment. Environ. Sci. Technol 2012, 46, 11171–11178. [DOI] [PubMed] [Google Scholar]
- 9.Weschler CJ; Nazaroff WW Semivolatile Organic Compounds in Indoor Environments. Atmos. Environ 2008, 42, 9018–9040. [Google Scholar]
- 10.CDC. Fourth National Report on Human Exposure to Environmental Chemicals, January 2019, Updated Tables; Centers for Disease Control and Prevention, 2019; Volume 1. [Google Scholar]
- 11.Hines CJ; Nilsen Hopf NB; Deddens JA; Calafat AM; Silva MJ; Grote AA; Sammons DL Urinary Phthalate Metabolite Concentrations among Workers in Selected Industries: A Pilot Biomonitoring Study. Ann. Occup. Hyg 2009, 53, 1–17. [DOI] [PubMed] [Google Scholar]
- 12.Kwapniewski R; Kozaczka S; Hauser R; Silva MJ; Calafat AM; Duty SM Occupational Exposure to Dibutyl Phthalate among Manicurists. J. Occup. Environ. Med 2008, 50, 705–711. [DOI] [PubMed] [Google Scholar]
- 13.Tran TM; Kannan K Occurrence of Phthalate Diesters in Particulate and Vapor Phases in Indoor Air and Implications for Human Exposure in Albany, New York, USA. Arch. Environ. Contam. Toxicol 2015, 68, 489–499. [DOI] [PubMed] [Google Scholar]
- 14.Hammel SC; Hoffman K; Webster TF; Anderson KA; Stapleton HM Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol 2016, 50, 4483–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceballos DM; Craig J; Fu X; Jia C; Chambers D; Chu MT; Fernandez AT; Fruh V; Petropoulos ZE; Allen JG; Vallarino J; Thornburg L; Webster TF Biological and Environmental Exposure Monitoring of Volatile Organic Compounds among Nail Technicians in the Greater Boston Area. - PubMed -NCBI. Indoor Air 2019, 29, 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayatilaka NK; Restrepo P; Williams L; Ospina M; Valentin-Blasini L; Calafat AM Quantification of Three Chlorinated Dialkyl Phosphates, Diphenyl Phosphate, 2,3,4,5-Tetrabromobenzoic Acid, and Four Other Organophosphates in Human Urine by Solid Phase Extraction-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Anal. Bioanal. Chem 2017, 409, 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva M; Samandar E; Preaujr J; Reidy J; Needham L; Calafat A Quantification of 22 Phthalate Metabolites in Human Urine. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 2007, 860, 106–112. [DOI] [PubMed] [Google Scholar]
- 18.Lavoue J NDExpo—Treatment of Non-detects in Industrial Hygiene Samples. http://expostats.ca/site/en/othertools.html (accessed July 15, 2019).
- 19.Helsel DR Nondetects and Data Analysis. Statistics for Censored Environmental Data; Wiley-Interscience, 2005. [Google Scholar]
- 20.Anderson KA; Points GL III; Donald CE; Dixon HM; Scott RP; Wilson G; Tidwell LG; Hoffman PD; Herbstman JB; O’Connell SG Preparation and Performance Features of Wristband Samplers and Considerations for Chemical Exposure Assessment. J. Expo. Sci. Environ. Epidemiol 2017, 27, 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva MJ; Wong L-Y; Samandar E; Preau JL; Jia LT; Calafat AM Exposure to Di-2-Ethylhexyl Terephthalate in the U.S. General Population from the 2015–2016 National Health and Nutrition Examination Survey. Environ. Int 2019, 123, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagorka R; Conrad A; Scheller C; Süßenbach B; Moriske H-J Diisononyl 1,2-Cyclohexanedicarboxylic Acid (DINCH) and Di(2-Ethylhexyl) Terephthalate (DEHT) in Indoor Dust Samples: Concentration and Analytical Problems. Int. J. Hyg Environ. Health 2011, 214, 26–35. [DOI] [PubMed] [Google Scholar]
- 23.Koch HM; Rüther M; Schütze A; Conrad A; Pälmke C; Apel P; Brüning T; Kolossa-Gehring M Phthalate Metabolites in 24-h Urine Samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a Comparison with US NHANES Data from 1999 to 2012. Int. J. Hyg Environ. Health 2017, 220, 130–141. [DOI] [PubMed] [Google Scholar]
- 24.Zota AR; Calafat AM; Woodruff TJ Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect 2014, 122, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connell SG; Kincl LD; Anderson KA Silicone Wristbands as Personal Passive Samplers. Environ. Sci. Technol 2014, 48, 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammel SC; Phillips AL; Hoffman K; Stapleton HM Evaluating the Use of Silicone Wristbands To Measure Personal Exposure to Brominated Flame Retardants. Environ. Sci. Technol 2018, 52, 11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakind JS; Birnbaum LS Out of the Frying Pan and out of the Fire: The Indispensable Role of Exposure Science in Avoiding Risks from Replacement Chemicals. J. Expo. Sci. Environ. Epidemiol 2010, 20, 115–116. [DOI] [PubMed] [Google Scholar]
- 28.Gray LE; Ostby J; Furr J; Price M; Veeramachaneni DN; Parks L Perinatal Exposure to the Phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, Alters Sexual Differentiation of the Male Rat. Toxicol. Sci 2000, 58, 350–365. [DOI] [PubMed] [Google Scholar]
- 29.Deyo JA Carcinogenicity and Chronic Toxicity of Di-2-Ethylhexyl Terephthalate (DEHT) Following a 2-Year Dietary Exposure in Fischer 344 Rats. Food Chem. Toxicol 2008, 46, 990–1005. [DOI] [PubMed] [Google Scholar]
- 30.Carignan CC; Mínguez-Alarcón L; Butt CM; Williams PL; Meeker JD; Stapleton HM; Toth TL; Ford JB; Hauser R Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environ. Health Perspect 2017, 125, 087018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lingua EN:Titlecase JD; Stapleton HM House Dust Concentrations of Organophosphate Flame Retardants in Relation to Hormone Levels and Semen Quality Parameters. Environ. Health Perspect 2010, 118, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preston EV; McClean MD; Claus Henn B; Stapleton HM; Braverman LE; Pearce EN; Makey CM; Webster TF Associations between Urinary Diphenyl Phosphate and Thyroid Function. Environ. Int 2017, 101, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


