Abstract
Ginkgolic acids (GAs) are found in the leaves, nuts, and testa of Ginkgo biloba and have been reported to exhibit antitumor, antibacterial, and pro-apoptotic activities. However, their role in mitochondrial function is still unclear. Our previous study showed that genes related to the mitochondria present significant changes in GA-treated mouse bone marrow stromal cells. We hypothesize that GAs may regulate mitochondrial function. Here, we found that GA treatment induced mitochondrial fragmentation, reduced mtDNA copy numbers and mitochondrial protein levels, and impaired mitochondrial adenosine 5′-triphosphate production and oxygen consumption. The GA-induced mitochondrial mass loss may be due to decreased mitochondrial biogenesis. In addition, abolishing autophagy by Atg7 knockout or the administration of an autophagy inhibitor can restore the GA-induced decrease in mitochondrial mass. Furthermore, FUNDC1 knockdown restored the GA-induced changes in mitochondrial mass reduction and mitochondrial membrane potential loss. Together, our studies demonstrated that GAs impaired mitochondrial function by decreasing mitochondrial biogenesis and promoting FUNDC1-dependent mitophagy.
Keywords: ginkgolic acids, mitochondria, mitophagy, FUNDC1
INTRODUCTION
Mitochondria are highly dynamic and the main energyproducing organelles in mammalian cells, but they also play an important role in cell injury and death by releasing prodeath molecules and generating toxic reactive oxygen species.1 Mitophagy, a selective type of autophagy-dependent degradation of mitochondria, is the major pathway for the removal of damaged or no-longer-needed mitochondria in eukaryotic cells in various physiological and pathological conditions.2 For example, paternal mitochondria elimination is executed by mitophagy, which is necessary for the development in C. elegans and Drosophila.3,4 Defects in mitophagy contribute to neurodegenerative diseases, inflammation activation, cancer, and decreased lifespan.5–8 The PINK/Parkin pathway is one of the most well-known mitophagy pathways. Under normal conditions, the mitochondrial kinase PINK1 is constantly degraded through a proteasome pathway. While the mitochondria are damaged, PINK1 is stabilized and then recruits the ubiquitin E3 ligase Parkin, leading to the ubiquitination of the outer mitochondrial membrane proteins, thus triggering mitophagy.9 In addition to PINK-/Parkin-dependent mitophagy, BNIP3- and FUNDC1-dependent mitophagy pathways were also found in multiple contexts. BNIP3 regulates mitophagy in response to hypoxia and is critical for erythroid maturation.10 BNIP3 has been reported to bind to and preserve PINK1 from degradation, thus promoting mitophagy by recruiting Parkin to the mitochondrial membrane.11 FUNDC1 is a highly conserved mitochondrial outer-membrane protein identified as a mitophagy receptor that directly binds LC3 under hypoxic conditions.12,13 At present, relative to the well-established PINK-/Parkin-pathway-mediated mitophagy, the mechanisms of the other mitophagy pathways need to be further defined.
Ginkgo biloba, known as a “living gymnosperms fossil”, has been used to treat memory and cognitive impairment in Chinese medicine since 2000 years ago.14 In addition, the ginkgo leaf extract is used in cosmetics for its functions for skin benefits, such as potent antioxidant protection, skin-soothing effects, and reduced signs of aging.15 Moreover, the ginkgo nut is an edible delicacy in China, Japan, and Korean Peninsula.16 Ginkgolic acids (GAs) are a natural component extracted from the leaves, nuts, and testa of Ginkgo biloba and show a wide range of biological activities, including anti-tumor, anti-HIV, anti-bacterial, neurotoxic, pro-apoptosis, and pro-autophagy effects.17–20 GA has been reported to increase resistance against oxidative stress in lens epithelial cells21 and to participate in cancer cell migration and invasion.22 GA also regulates glucose metabolism, inflammation, and cell death in multiple cell lines.23–25 Furthermore, GA can directly bind to and inhibit sumoylation E1 (SAE1/SAE2) enzyme activity.26 In addition, GA has been reported to reduce mitochondrial membrane potential (MMP) and promote mitochondrial damage.27,28
Our previous study showed that genes related to the mitochondria present significant changes in GA-treated mouse bone marrow stromal cells,29 suggesting that GA may play an important role in mitochondria. However, the exact role and mechanism of GA regulation of mitochondrial function are still unclear. Here, we studied the mitochondrial morphology, mitochondrial mass, and mitochondrial function, as well as the underlying mechanisms upon GA (15:1) treatment in HeLa cells.
MATERIALS AND METHODS
Cell Culture and Treatment
HeLa cells were purchased from Shanghai Cell Bank, Type Culture Collection Committee, Chinese Academy of Sciences. ATG7 KO, GFP/Parkin-overexpressing, and control HeLa (gifts from Dr. Quan Chen, Nankai University) cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, New York, USA, C11995500BT) supplemented with 10% fetal bovine serum (HyClone, Logan, USA, SV30160.03) and 1% penicillin and streptomycin (Gibco, New York, USA, 15140–122). All cells were incubated at 37 °C under 5% CO2. In the treatment groups, the cells were treated with 25 or 50 μM GA (Calbiochem, Darmstadt, Germany, 345887) for 24 h.
Autophagy Measurement
For autophagy analysis, HeLa cells were transfected with GFP-LC3 for 48 h. Following the indicated treatment, the cells were fixed with 4% paraformaldehyde and imaged with a Leica TCS SP8 confocal microscope. GFP-LC3 puncta were counted in each cell. At least, 200 cells were analyzed per treatment. A diffuse distribution of GFP-LC3 was considered to represent nonautophagic puncta. For inhibition autophagy, cells were treated with 50 μM CQ (Sigma, St. Louis, USA, C6628) for 6 h.
BNIP3/FUNDC1 Knockdown
BNIP3 and FUNDC1 shRNA (shBNIP3: 5′ GCCTCGGTTTCTATTTATAAT 3′/shFUNDC: 5′ AAGTGATGACGACTCTTATGA 3′) were inserted into the pLKO.1 vector (a gift from Dr. Song Z. Y., Wuhan University), which was then transfected into 293T cells. After 48 h of transfection, the virus was collected and used to infect HeLa cells. Stable BNIP3 or FUNDC1 knockdown cell lines were selected using puromycin (Amresco, Pennsylvania, USA, J593) at a concentration of 1 μg/mL.
Immunofluorescent Staining
Cells were fixed with 4% formaldehyde and blocked with 2% bovine serum albumin (Amresco, Pennsylvania, USA, E588). Primary antibodies against HSP60 (Proteintech, Chicago, USA, 15282–1-AP; 1:500 dilution), cytochrome c (CST, Boston, USA, 4280; 1:500 dilution), LC3B (Sigma, St. Louis, USA, L7543; 1:500), and LAMP1 (CST, Boston, USA, 9091; 1:500 dilution) were applied overnight. The cells were then incubated with an anti-rabbit or anti-mouse antibody conjugated with Alexa 488 or Alexa 594 (Jackson ImmunoResearch, Lancaster, USA, AB_2337249 and AB_2307325). After DAPI costaining, the cells were imaged with a Leica TCS SP8 confocal microscope. For colocalization quantification, images were preprocessed by subtraction of a median filter-processed image and background, and then two images were proceeded with ImageJ plugin JACOP (National Institutes of Health).30
Western Blotting
Proteins were isolated in ice-cold RIPA buffer (Beyotime, Shanghai, China, P0013B) with proteinase inhibitors, and protein concentrations were determined in bicinchoninic acid assay (BCA). Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroblotted onto the poly-(vinylidene difluoride) membrane (Millipore, Darmstadt, Germany, IPVH00010), and probed with primary antibodies (Table S1). The protein bands detected by the antibodies were visualized by enhanced chemiluminescence (Beyotime, Shanghai, China, P0018) and evaluated using Quantity One 1D Analysis Software (Bio-Rad, Hercules, CA).
mtDNA Measurements
Total DNA was isolated by using the Gentra Puregene Cell Kit (Qiagen, New York, USA, 158388) according to the manufacturer’s instructions. For the measurement of mtDNA copy numbers, the quantitative polymerase chain reaction (qPCR) primers employed for mitochondrial tRNALeu (UUR) and nuclear β−2-microglobulin (Table S2) were used for the qPCR assay. Data analysis of mitochondrial contents was performed according to a previously described method.31
RNA Isolation and qPCR Analysis
Total RNA was isolated from cultured cells using RNA Iso Plus (TAKARA, Tokyo, Japan, 9109) as we previously reported.32 Total RNA was reverse-transcribed into cDNA by using the ABScript II cDNA First Strand Synthesis Kit (ABclonal, Wuhan, China, RK20400) following the manufacturers’ protocol. mRNA levels were quantified in a SYBR Green Select Master Mix (ABclonal, Wuhan, China, RK21203) on a CFX96 real-time system (Bio-Rad, Hercules, CA). The abundance of specific gene transcripts was assessed by qPCR (primers are listed in Table S2). Actb was used as the internal control. Relative gene expression was expressed as the fold change calculated using the 2−ΔΔCT method.
RNA-Seq Analysis and Gene Set Enrichment Analysis
Total RNA was extracted from the control and GA-treated HeLa cells. The RNA was then sequenced by the WuXi App Tec RNA-seq service (n = 2). GO analysis was performed using DAVID (http://david.abcc.ncifcrf.gov/). Gene expression clustering was analyzed using Cluster 3.0 and visualized using Java TreeView. For gene set enrichment analysis, we applied GSEA v2.2.0 to various functional and/or characteristic gene signatures. Gene sets were obtained from the MsigDB database v3.0. Statistical significance was assessed by comparing the enrichment score to enrichment results generated from 1000 random permutations of the gene set to obtain p values (nominal p value).
MMP Assay
The JC-1 probe was used to measure the MMP. Cells cultured in 12-well plates following the indicated treatments were incubated with 500 μL of JC-1 (Beyotime, Shanghai, China, C2006) at 37 °C for 20 min. The cells were then washed three times with JC-1 staining buffer and imaged with a Nikon Ti2-U microscope.
Oxygen Consumption Rate Measurements
Oxygen consumption rates (OCRs) were measured using Seahorse XF96 equipment (Seahorse Bioscience Inc., Santa Clara, USA). Briefly, cells were seeded at 8000 cells per well and treated with normal medium or medium containing 50 μM GA for 24 h in 80 μL of medium. The cell plates were incubated in a CO2-free incubator at 37 °C for 1 h before the measurements. Analysis was performed using 1 μM oligomycin, 0.5 μM carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone (FCCP), and 1 μM rotenone as indicated. The data were normalized to the protein levels. Adenosine 5′-triphosphate (ATP)-dependent respiration (or oligomycin-sensitive respiration) was calculated as the difference in the OCRs before and after the addition of oligomycin.
Analysis of ATP Levels
The ATP levels were determined using an ATP assay kit from Beyotime according to the manufacturer’s instructions. Briefly, after cell adherence for 12 h, the culture medium was changed to DMEM [no glucose, 10% fetal bovine serum (FBS)], and GA (50 μM) or dimethyl sulfoxide (DMSO) was added for 12 or 24 h, respectively. Cells were collected in lysis buffer. After centrifugation (12 000g for 5 min) to remove the cell debris, the pellets were used to determine the protein concentrations via BCA, and the supernatant was added to the substrate solution for the luciferase assay. Luminescence was recorded using an illuminometer with an integration time of 10 s per well. ATP levels were normalized to the protein contents of the samples.
Staistical Analysis
All experiments were carried out in triplicates. The results are expressed as the mean ± standard deviation (SD). The level of statistical significance was set at p < 0.05 using an unpaired two-tailed Student’s t-test. All statistical analyses were performed using GraphPad Prism software.
RESULTS
GA Treatment Regulated Mitochondrion-Related Cellular Processes
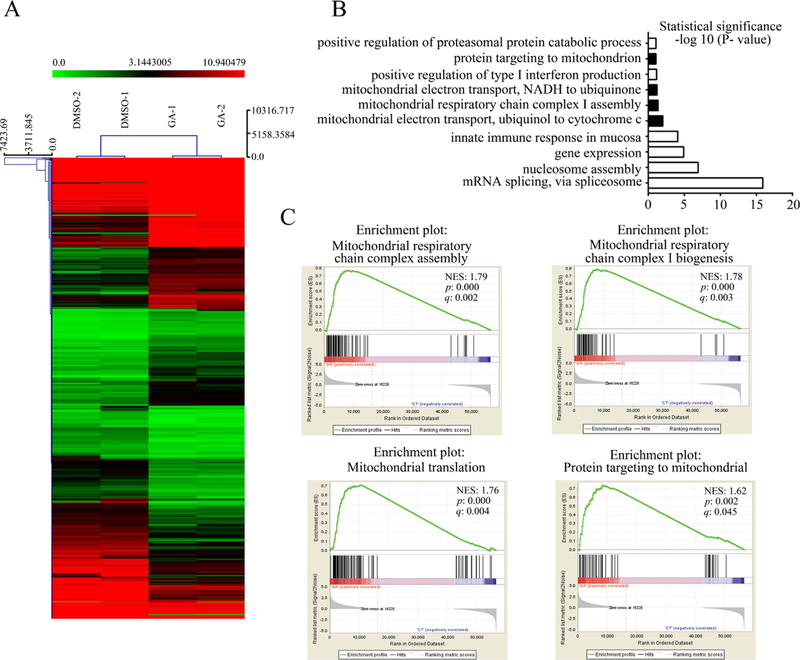
To examine whether GA regulates mitochondrion-related gene expression in a cell-type-specific manner, we performed RNA sequencing (RNA-Seq) analysis in GA- and DMSO-treated HeLa cells. Gene ontology (GO) analysis showed that mitochondrion-related biological processes were significantly changed in the GA-treated group (Figure 1A,B); the identified terms included mitochondrial electron transport of ubiquinol to cytochrome c, mitochondrial respirator chain complex I assembly, mitochondrial electron transport of NADH to ubiquinone, and protein targeting to mitochondrion. Accordingly, gene set enrichment analysis showed that mitochondrial respiratory chain complex assembly (NES = 1.79, p = 0.000, q = 0.002), mitochondrial respiratory chain complex I biogenesis (NES = 1.78, p = 0.000, q = 0.003), mitochondrial translation (NES = 1.76, p = 0.000, q = 0.004), and protein targeting to mitochondria (NES = 1.62, p = 0.002, q = 0.045) were significantly enriched in the GA group (Figure 1C). These data suggest that GA may regulate the mitochondrial function.
Figure 1.
GO and gene sets enrich analysis of gene expression profiling in GA- and DMSO-treated HeLa cells. (A) Heat map of differential gene expression in GA- and DMSO-treated HeLa cells (n = 2). (B) Most upregulated biological processes by DAVID GO analyses. (C) GSEA shows the biological processes enriched in GA-treated cells. (NES: normalized enrichment score; p: nominal p-value; q: false discovery rate q-value.)
GA-Induced Mitochondrial Fragmentation and Reduced Mitochondrial Mass
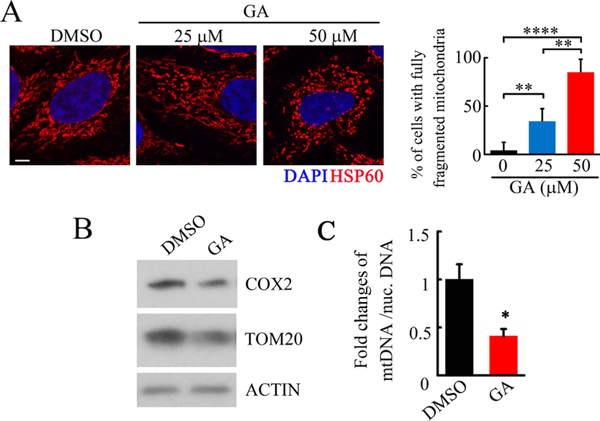
To verify the role of GA in mitochondria, we first examined the morphology of the mitochondria after GA treatment. The immunofluorescent staining of the mitochondrial marker HSP60 showed that GA dramatically induced mitochondrial fragmentation compared to the control group (Figure 2A). Moreover, we found that GA decreased the expression of a mitochondrion outer membrane protein (TOM20) and cytochrome c oxidase polypeptide II (COX2), as well as decreased SUMO1- and SUMO2-modified proteins (Figures 2B and S1). In addition, we detected significantly decreased relative mtDNA copy numbers in GA-treated HeLa cells compared with the DMSO treatment (Figure 2C). These data indicated that GA reduced both the morphology and the mass of mitochondria.
Figure 2.
GA treatment induced mitochondrial fragmentation and reduced mitochondrial mass. (A) Representative immunofluorescent staining of mitochondrial protein HSP60 followed by GA treatment for 24 h in HeLa cells, quantification of the percentage of the cells with fully fragmented mitochondria at the right (scale bar = 10 μm, **p < 0.01, ****p < 0.0001). (B) Western blots of COX2 and TOM20 upon DMSO and GA treatment for 24 h (50 μM GA), representative of three independent experiments. (C) Measurement of mitochondrial DNA (mtNDA) copy numbers in DMSO- and GA-treated HeLa cells by qPCR (50 μM GA for 24 h; n = 3, *p < 0.05 compared with the DMSO group).
GA-Impaired Mitochondrial Function
To obtain insight into the difference in the mitochondrial function between the GA- and DMSO-treated cells, we first mapped the mitochondrial function by quantifying the OCR using a Seahorse XF extracellular flux analyzer technology. The basal OCR showed no difference between the GA and DMSO treatments. Blockage of ATP synthase with oligomycin resulted in a decrease in the OCR to 60% of baseline under DMSO treatment but no changes under GA treatment. Addition of the mitochondrial membrane uncoupler FCCP resulted in an increased OCR in the control group but an unexpectedly decreased OCR in the GA group (Figure 3A). The individual parameter calculations showed significantly lower levels of ATP-linked, maximal respiration and spare capacity but dramatically higher proton leakage in GA-treated cells compared to DMSO-treated cells (Figure 3B). In addition, we measured the ATP levels of GA-treated and control cells using an ATP assay kit. Compared to the control group, the GA treatment significantly decreased the ATP levels generated by the mitochondrial oxidative phosphorylation system after complete inhibition of glycolysis (Figure 3C). These results suggested that GA impairs mitochondrial function.
Figure 3.
GA repressed mitochondrial oxygen consumption rate and ATP levels. (A) HeLa cell were seeded in the Seahorse Bioscience microplates (10 000 cells/well). After adherence for 12 h, GA (50 μM) or DMSO was added to the microplates for coincubation with cells for 12 h, and 1 μM oligomycin, 0.5 μM FCCP, and 0.5 μM rotenone/antimycin A were subsequently added for mitochondrial OCR measurement. (B) Individual parameter for respiration, including basal respiration, proton leak, ATP-linked oxygen consumption, maximal respiration, and spare respiration capacity in HeLa cells (n = 3, **p < 0.01; ***p < 0.001, compared with DMSO group). (C) Mitochondrial oxidative phosphorylation system graded ATP level measurement in HeLa cells. After cell adherence for 12 h, the culture medium was changed to DMEM (no glucose, 10% FBS), and GA (50 μM) or DMSO were added for 12 or 24 h, respectively. The ATP levels were measured by an illuminometer and normalized to the protein contents (n = 3, *p < 0.05; **p < 0.01).
GA Decreased Mitochondrial Biogenesis
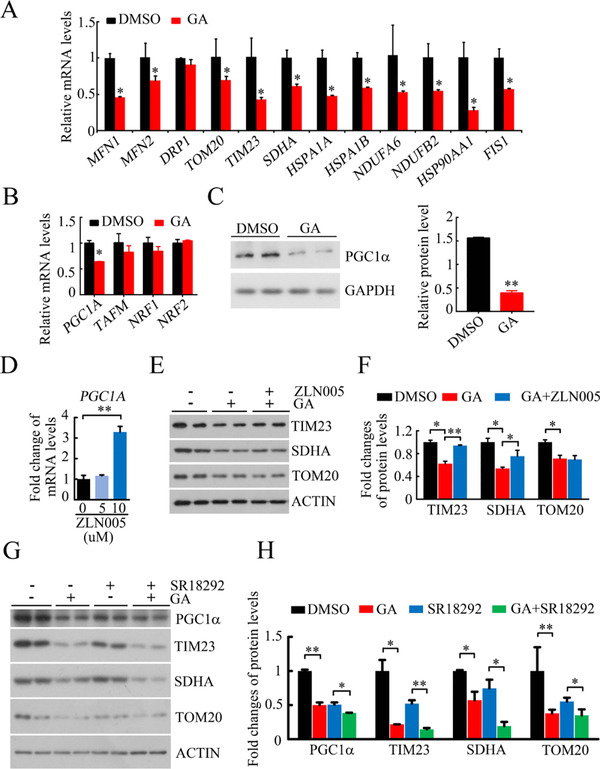
Next, we investigated whether the reduced mitochondrial mass was caused by the suppression of mitochondrial biogenesis. As shown in Figure 4A, the GA treatment significantly decreased the mRNA levels of mitochondrial fission (FIS1) and fusion proteins (MFN1 and MFN2), mitochondrial membrane proteins (TOM20 and TIM23), mitochondrial electron transport chain proteins (SDHA, NDUFA6, and NDUFB2), and molecular chaperones associated with mitochondrial function (HSPA1A and HSPA1B). Moreover, we examined the expression levels of PGC-1α, the key transcription factor involved in mitochondrial biogenesis in response to changes in the cellular environment or the physiological or pathological status of mammals.33 We found that the GA treatment dramatically decreased both the mRNA and protein expression of PGC-1α (Figure 4B,C). Next, we treated the HeLa cells with the PGC-1α agonist (ZLN005) or an inhibitor (SR18292) and examined the GA effect on mitochondrial proteins. We found that further treatment with PGC-1α agonist (ZLN005) could partially restore the GA-mediated mitochondrial protein loss (TIMM23 and SDHA) (Figure 4D–F). Furthermore, we treated the cells with the PGC-1α inhibitor (SR18292) for 24 h and then with the GA. As shown in Figure 4G,H, SR18292 treatment decreased mitochondrial proteins, and further treatment with GA also decreased mitochondrial proteins. Together, these data suggested that the GA treatment reduced mitochondrial mass partially by blocking PGC-1α-mediated mitochondria biogenesis.
Figure 4.
GA repressed mitochondrial biogenesis. (A) qPCR analysis of the mRNA expression levels of mitochondria-related genes, including mitochondrial fission and fusion proteins, mitochondrial membrane proteins, mitochondrial electron transport chain proteins, and molecular chaperones associated with mitochondrial function (n = 3,*p < 0.05, compared to DMSO treatment, GA: 50 μM for 24 h). (B) qPCR analysis of transcription factors to mitochondrial biogenesis (n = 3,*p < 0.05, compared to DMSO treatment, GA: 50 μM for 24 h). (C) Representative western blots of PGC1a upon GA or DMSO treatment, quantification at the right. (n = 3,*p < 0.05; GA: 50 μM for 24 h). (D) mRNA level of PGC1A in HeLa cells upon ZLN005 treatment (ZLN005 for 24 h, n = 3,**p < 0.01). (E,F) Representative western blots (E) and quantification (F) of mitochondrial proteins upon GA and ZLN005 treatment in HeLa cells (GA: 50 μM for 24 h; ZLN005: 10 μM for 24 h; *p < 0.05; **p < 0.01). (G,H) Representative western blots (G) and quantification (H) of mitochondrial proteins upon GA and SR18292 treatment (GA: 50 μM for 24 h; SR18292: 20 μM for 24 h; *p < 0.05; **p < 0.01).
GA-Induced Mitophagy
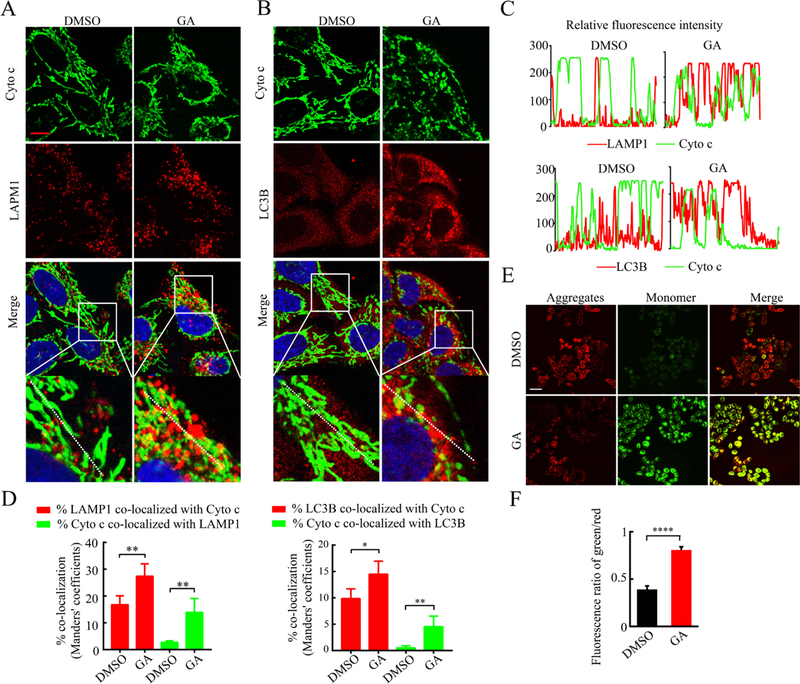
Another possible reason for the decrease in mitochondrial mass is an increase in mitochondrial elimination, and mitophagy is a major way to eliminate damaged or no-longer-needed mitochondria. We detected a significant increase in LC3B-II lipidation and in the number of LC3 puncta (an indicator of autophagosome formation) from the GA-treated cells (Figure S2), suggesting that GA activates autophagy. Next, the colocalization of mitochondria (green cytochrome c signal) and lysosomes (red LAMP1 signal, which can mark the ongoing autophagolysis of mitochondria) was examined by confocal microscopy. As shown in Figure 5A–D, the green and red fluorescence signals overlapped in most GA-treated cells. Similarly, increased colocalization of mitochondria and autophagosomes (red LC3 puncta) was found upon GA treatment (Figure 5B–D). Furthermore, we detected a significantly reduced MMP, as indicated by increased JC1 green fluorescence under GA treatment, while most of the DMSO-treated cells exhibited JC1 red staining (Figure 5E,F).
Figure 5.
GA treatment promoted the colocalization of mitochondria and lysosome/autophagosome. (A) Representative images of costaining of cyto c (anti-cytochrome c, green, labeled mitochondria) and LAMP1 (red, labeled lysosomes) in GA- or DMSO-treated HeLa cells (GA: 50 μM for 24 h, scale bar = 10 μm). (B) Representative images of costaining Cyto c and LC3B (red, labeled autophagosomes). (C) Trace outline is used for line scan (white dashed line) analysis of relative fluorescence intensity of Cyto c and LAMP1/LC3B in (A,B). (D) Quantitative analysis for the colocalization of mitochondria (Cyto c) with lysosomes (LAMP1) and autophagosomes (LC3B) in (A,B) by ImageJ plugin JACOP (*p < 0.05; **p < 0.01). (E,F) Representative images (E) and quantification (F) of the JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbo-cyanine iodide) staining in GA- or DMSO-treated HeLa cells (red, J-aggregates, high mitochondrial membrane potential; green, JC-1 monomer, low mitochondrial membrane potential; scale bar = 10 μm, ***p < 0.001).
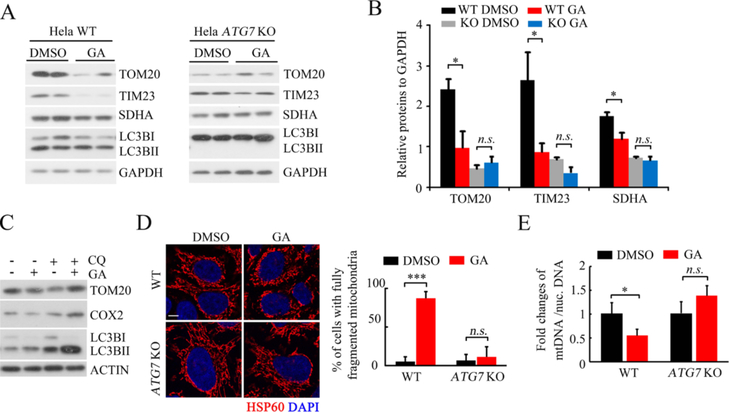
To confirm that GA activates mitophagy, we investigated whether autophagy inhibition would rescue the GA-induced mitochondrial mass loss. ATG7 knockout significantly rescued the GA-induced decrease in the expression of mitochondrial proteins including TOM20, TIM23, and SDHA, as well as induced increase in LC3B-II lipidation (Figures 6A,B, and S3). Similarly, the downregulation effect of the GA on mitochondrial TOM20 and COX2 was abolished by chloroquine (CQ)-mediated autophagy repression (Figure 6C). Moreover, ATG7 knockout abolished GA-induced mitochondrial fragmentation and reduced the relative mtDNA copy numbers (Figure 6D,E). Taken together, these data demonstrated that GA induces mitophagy.
Figure 6.
Autophagy defect abolished GA-induced mitochondrial mass loss and fragmentation. (A,B) Representative western blots of mitochondrial proteins in wild-type and ATG7 KO HeLa cells following the GA or DMSO treatment, quantification at (B) (GA: 50 μM for 24 h, *p < 0.05 compared with the DMSO group). (C) Representative western blots of indicated proteins in CQ- or GA-treated HeLa cells (CQ, 50 μM 24 h, GA: 50 μM 24 h). (D) Representative immunofluorescent staining of mitochondrial protein HSP60 following GA treatment for 24 h in WT or ATG7 knockout HeLa cells, quantification of the percentage of the cells with fully fragmented mitochondria at the right (scale bar = 10 μm, **p < 0.01). (E) Measurement of mtNDA copy numbers in DMSO- and GA-treated WT or ATG7 knockout HeLa cells by qPCR (50 μM GA for 24 h; n = 3, *p < 0.05 compared with the DMSO group, n.s.: no significance).
GA-Induced FUNDC1-Mediated Mitophagy
Next, we investigated which signaling pathway mediates GA-induced mitophagy. PINK/Parkin, BNIP3, and FUNDC1 pathways are the three common ones to mediate mitophagy. As HeLa cells lack endogenous Parkin,34 we ectopically expressed Parkin in HeLa cells. We found that the GA treatment significantly decreased the mitochondrial proteins (TIM23, TOM20, and SDHA) in both wild-type and Parkin-positive cells (Figure S4), suggesting that the GA induced a PINK/Parkin-independent type of mitophagy. Therefore, we evaluated the GA function in BNIP3- and FUNDC1 knockdown HeLa cells, where FUNDC1 knockdown abolished the GA-induced repression of mitochondrial proteins (TOM20, SDHA, and TIM23) compared to the control cells (pLKO.1 group) but BNIP3 knockdown did not (Figure 7A,B). Moreover, a GA-induced mtDNA reduction appeared in the control and BNIP3 knockdown cells, which disappeared in FUNDC1 knockdown cells (Figure 7C). Furthermore, knockdown of FUNDC1, but not BNIP3, partially restored the MMP of GA-treated cells (Figure 7D,E). In addition, FUNDC1 knockdown restored the reduced mRNA levels of some mitochondrial proteins, including MFN2, TOM20, SDHA, HSPA1A, and NDUFB2, upon GA treatment (Figures S5 and 4A). Furthermore, GA was shown to have activity as a sumoylation or histone acetyltransferase (HAT) inhibitor,26 so we tested whether FUNDC1 can be regulated by sumoylation or acetylation. We performed sumoylation or acetylation site prediction by using the GPS website of the CUCKOO Workgroup (http://www.biocuckoo.org/) and found that there are no conserved sumoylation sites in FUNDC1, but a sumoylation interaction motif and two lysine acetylation sites (lysines 114 and 115) were present (Figure S6). These data indicated that the GA-induced mitochondrial mass loss is also due to increased mitophagy and that FUNDC1 is required for GA-induced mitophagy.
Figure 7.
GA-induced mitophagy is FUNDC1-dependent. (A,B) Representative western blots (A) and quantification (B) of mitochondrial proteins in BNIP3 knockdown (shBNIP3), FUNDC1 knockdown (sh FUNDC1), and control (pLKO.1) HeLa cells following the GA or DMSO treatment (GA: 50 μM for 24; *p < 0.05, **p < 0.01, n.s.: no significance). (C) Measurement of mtNDA copy numbers in BNIP3 knockdown (shBNIP3), FUNDC1 knockdown (sh FUNDC1), and control (pLKO.1) HeLa cells by qPCR (50 μM GA for 24 h; n = 3, *p < 0.05 compared with the DMSO group, n.s.: no significance). (D,E) Representative images and quantification of the JC-1 staining in BNIP3 knockdown (shBNIP3), FUNDC1 knockdown (sh FUNDC1), and control (pLKO.1) HeLa cells.
DISCUSSION
Previously, we revealed that GA (15:1) promoted adipocyte commitment but suppressed adipocyte terminal differentiation in mouse bone marrow stromal cells, possibly through its activity as a sumoylation inhibitor, but not its activity as a HAT inhibitor.29 In addition, we observed the elevated expression of mitochondrial genes in GA-treated murine bone marrow mesenchymal stem cells.29 In the present study, we investigated the role of GA in regulating mitochondrial morphology and function. We found that the GA treatment induced mitochondrial fragmentation, reduced mtDNA copy numbers and mitochondrial proteins, and reduced ATP levels and OCRs (Figures 2 and 3). In addition, the GA treatment significantly reduced the MMP (Figure 5E,F), which is consistent with a previous study performed in MDCK and HepG2 cells in which the GA treatment resulted in loss of cell MMP and cell cycle arrest, which may contribute to cell death.17,27 Consistently, we also detected that the GA treatment significantly decreased the cell viability (Figure S7). GA has been used as a new molluscicide agent for its significant mitochondrial damage and is found to decrease the expression of mitochondrial enzymes in snails.35
Mitochondria are highly dynamic organelles that undergo constant fusion and fission, and their mass may be balanced by mitochondrial biogenesis and mitophagy.36 Mitochondrial fusion and fission are both mediated by the large GTPases of the dynamin family, which are conserved in different species.37 Mitofusins (Mfn1 and Mfn2) mediate mitochondrial outer-membrane fusion, while optic atrophy 1 mediates the inner-membrane fusion in mammals.38 Mitochondrial fission is mediated by several proteins, including GTPase dynamin-related protein1 (Drp1), which is most well studied.39 Drp1 is recruited to the mitochondrial outer membrane via a collection of receptor proteins (Mff, Fis1, MiD49, and MiD50), and then, it assembles around the tubule and constricts mitochondria in a GTP-dependent manner to mediate scission.40 Drp1 is regulated by multiple post-translational modifications, including phosphorylation, ubiquitination, S-nitrosylation, and sumoylation.37 MAPL sumoylation of Drp1 stimulates mitochondrial fission and stabilizes an ER/mitochondrial platform required for cell death.41,42 However, SENP3-mediated deSUMOylation of Drp1 increasing its association with mitochondria, cytochrome c release, and facilitates interaction with Mff to promote cell death.43,44 Our data showed that the GA treatment significantly decreased the mRNA levels of mitochondrial fission (Fis1) and fusion proteins (Mfn1 and Mfn2) but did not change the Drp1 mRNA expression (Figure 4A). GA can directly bind to and inhibit sumoylation E1 (SAE1/SAE2) enzyme activity,26 suggesting that GA may also inhibit the sumoylation of Drp1 to increase their association with mitochondria.
We found that the GA-induced decrease in mitochondrial mass may be caused by decreased mitochondrial biogenesis by a peroxisome proliferator-activated receptor γ-coactivator-1α (PGC-1α) (Figure 4B,C). PGC-1α is a major regulator of mitochondrial biogenesis in response to changes in the cellular environment or the physiological or pathological status of mammals.45 It is regulated by many post-translational modifications, including acetylation, phosphorylation, methylation, and sumoylation.45 The histone acetyltransferase GCN5 has been reported to acetylate and inhibit PGC-1α activity in vitro and in vivo,46,47 whereas Sirt1-mediated deacetylation of PGC-1α leads to its activation.48,49 SUMO1 conjugated with lysine 183 located in the activation domain of PGC-1α does not have an apparent effect on the subcellular localization or stability of PGC-1α but does attenuate the transcriptional activity of the coactivator.50,51 GA has been reported to directly bind to and inhibit the sumoylation E1 enzyme (SAE1/SAE2),26 and it also inhibits PCAF-mediated histone acetylation.26,52 Our data showed that GA reduced both mRNA and protein levels of PGC-1α, so GA may regulate the upstream of PGC-1α through its sumoylation inhibitor or PCAF inhibitor activity.
Moreover, we detected significant increases in LC3B-II lipidation and the number of LC3 puncta in GA-treated cells (Figure S2). Additionally, the combination of GA and CQ (lysosomal inhibitors) further increased LC3B-II compared to GA treatment alone (Figure 6C), suggesting that GA increased the autophagy flux. Similarly, GA has been reported to suppress colon cancer cell proliferation, migration, and invasion and to inhibit the epithelial to mesenchymal transition in lung cancer cells through repression of the mTOR signaling pathway, which is the major negative regulator of autophagy.20,53 Furthermore, we observed increased colocalization of mitochondria and autophagosomes/lysosomes. Additionally, autophagy inhibition induced chemically or genetically can restore the GA-induced mitochondrial mass loss. These overall demonstrate the function of GA in inducing mitophagy.
Because HeLa cells lack expression of Parkin and GA treatment significantly decreased the mitochondrial proteins (TIM23, TOM20, and SDHA) in both wild-type and Parkin-positive cells (Figure S4), the GA-induced mitophage should act in a Parkin-independent manner. It was shown that FUNDC1 knockdown, but not BNIP3 knockdown, blocked the activity of GA in inducing mitochondrial fragmentation and reducing mtDNA copy numbers and mitochondrial protein levels (Figure 7A–C), suggesting that GA induced FUNDC1-dependent mitophagy. FUNDC1, a mitochondrial outer-membrane protein, is a hypoxia-induced mitophagy receptor that interacts with and recruits LC3 to mitochondria for mitophagy.13 FUNDC1 is phosphorylated at tyrosine 18 (Y18) and serine 13 (S13) by SRC kinase and CK2, reducing its affinity for LC3.13 Under hypoxic conditions, FUNDC1 is dephosphorylated by PGAM5 or other yet-to-be-identified phosphatases, which greatly increases its interaction with LC3 or other autophagy genes for the initiation of mitophagy.54 Thus, we also examined the effects of GA on regulating SRC kinase, CK2, and PGAM5. We found that the GA treatment decreased the mRNA levels of SRC and CK2, but not PGAM5 (Figure S8A). Moreover, the GA treatment significantly decreased the protein level of SRC and CK2 (Figure S8B,C). These data suggested that GA may decrease the phosphorylation of FUNDC1 to promote its interaction with LC3, leading to the initiation of mitophagy.
GA has been shown to inhibit both the sumoylation E1 enzymes and histone acetyltransferase, which suggests that FUNDC1 may also be regulated by sumoylation and histone acetyltransferase. The prediction results showed that there are no conserved sumoylation sites in FUNDC1, but the presence of a sumoylation interaction motif (Figure S6A) indicated that FUNDC1 might interact with sumoylated proteins to mediate GA-induced FUNDC1-dependent mitophagy. In addition, we predicted that lysines 114 and 115 of FUNDC1 may be acetylated; in particular, lysine 115 of FUNDC1 may be acetylated by the histone acetyltransferase KAT2A (GCN5) or KAT2B (PCAF) (Figure S6B). As GA inhibits PCAF-mediated histone acetylation, we hypothesize that GA may block PCAF-mediated FUNDC1 acetylation, which results in mitophagy. In the future, we will pursue these hypotheses and reveal the regulatory mechanism of FUNDC1, which may promote the understanding of the exact role of FUNDC1 in mitophagy.
In conclusion, we found that the GA treatment caused mitochondrial fragmentation, reduced the MMP and mtDNA levels, and repressed mitochondrial OCRs. Mechanistic studies further revealed that the GA-induced mitochondrial mass loss may result from decreased mitochondrial biogenesis and increased FUNDC1-dependent mitophagy. Our work may provide new mechanistic insights of GA into its future clinical applications for cancers or other diseases, as well as the side effects of the ginkgo leaf extract for cosmetics and ginkgo nuts for food.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr. Quan Chen (Nankai University, China) and Dr. Zhiyin Song (Wuhan University, China) for the ATG7 KO and control HeLa cells and thank Dr. Xuhui Lai for technical help on the qPCR procedure.
Funding
This work was financially supported by the National Natural Science Foundation of China (grants 81601299, 81800833, and 81802189), the 111 Project (B16021), the National Key R&D Program of China (2018YFC2002000), the China Postdoctoral Science Foundation (grant 2018M631054), and the Natural Science Foundation of Guangdong Province (grant 2018A0303131002).
ABBREVIATIONS
- GA
ginkgolic acids
- FUNDC1
FUN14 domain-containing protein 1
- BNIP3
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3
- CQ
chloroquine
- LC3B
microtubule-associated proteins 1A/1B light chain 3B
- LAPM1
lysosome-associated membrane glycoprotein 1
- OCR
oxygen consumption rate
- FCCP
carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone
- MMP
mitochondrial membrane potential
- HSP60
60 kDa heat-shock protein, mitochondrial
- COX2
cytochrome c oxidase polypeptide II
- FIS1
mitochondrial fission 1 protein
- MFN1
mitofusin-1
- MFN2
mitofusin-2
- TOM20
mitochondrial import receptor subunit TOM20 homolog
- TIM23
mitochondrial import inner membrane translocase subunit Tim23
- SDHA
succinate dehydrogenase-[ubiquinone]flavoprotein subunit, mitochondrial
- NDUFA6
NADH dehydrogenase [ubiquinone] 1 α subcomplex subunit 6
- NDUFB2
NADH dehydrogenase [ubiquinone] 1 β subcomplex subunit 2, mitochondrial
- HSPA1A
heat shock 70 kDa protein 1A
- HSPA1B
heat shock 70 kDa protein 1B
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-α
- JC1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolocarbo-cyanine iodide
- cyto
c, cytochrome c
- mTOR
mechanistic target of rapamycin
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jafc.9b04178.
Primary antibodies, sequences of primers, SUMO2 and SUMO2/3, autophagy, ATG7 KO, wild-type and Parkin-positive HeLa cells, qPCR analysis, SUMOylation or acetylation, cell viability, and SRC kinase and CK2 (PDF)
REFERENCES
- (1).Jahani-Asl A; Germain M; Slack RS Mitochondria: joining forces to thwart cell death. Biochim. Biophys. Acta 2010, 1802, 162–166. [DOI] [PubMed] [Google Scholar]
- (2).Williams JA; Ding W-X Mechanisms, pathophysiological roles and methods for analyzing mitophagy—recent insights. Biol. Chem 2018, 399, 147–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Zhou Q; Li H; Li H; Nakagawa A; Lin JLJ; Lee E-S; Harry BL; Skeen-Gaar RR; Suehiro Y; William D; Mitani S; Yuan HS; Kang B-H; Xue D Mitochondrial endonuclease G mediates breakdown of paternal mitochondria upon fertilization. Science 2016, 353, 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Politi Y; Gal L; Kalifa Y; Ravid L; Elazar Z; Arama E Paternal mitochondrial destruction after fertilization is mediated by a common endocytic and autophagic pathway in Drosophila. Dev. Cell 2014, 29, 305–320. [DOI] [PubMed] [Google Scholar]
- (5).Gustafsson ÅB; Dorn GW Evolving and Expanding the Roles of Mitophagy as a Homeostatic and Pathogenic Process. Physiol. Rev 2019, 99, 853–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Ryu D; Mouchiroud L; Andreux PA; Katsyuba E; Moullan N; Nicolet-dit-Felix AA; Williams EG; Jha P; Lo Sasso G; Huzard D; Aebischer P; Sandi C; Rinsch C; Auwerx J Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med 2016, 22, 879–888. [DOI] [PubMed] [Google Scholar]
- (7).Kim M-J; Yoon J-H; Ryu J-H Mitophagy: a balance regulator of NLRP3 inflammasome activation. BMB Rep. 2016, 49, 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Pavlides S; Vera I; Gandara R; Sneddon S; Pestell RG; Mercier I; Martinez-Outschoorn UE; Whitaker-Menezes D; Howell A; Sotgia F; Lisanti MP Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid. Redox Signaling 2012, 16, 1264–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bingol B; Sheng M Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radical Biol. Med. 2016, 100, 210–222. [DOI] [PubMed] [Google Scholar]
- (10).Sandoval H; Thiagarajan P; Dasgupta SK; Schumacher A; Prchal JT; Chen M; Wang J Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008, 454, 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zhang T; Xue L; Li L; Tang C; Wan Z; Wang R; Tan J; Tan Y; Han H; Tian R; Billiar TR; Tao WA; Zhang Z BNIP3 Protein Suppresses PINK1 Kinase Proteolytic Cleavage to Promote Mitophagy. J. Biol. Chem 2016, 291, 21616–21629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chen M; Chen Z; Wang Y; Tan Z; Zhu C; Li Y; Han Z; Chen L; Gao R; Liu L; Chen Q Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy 2016, 12, 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Liu L; Feng D; Chen G; Chen M; Zheng Q; Song P; Ma Q; Zhu C; Wang R; Qi W; Huang L; Xue P; Li B; Wang X; Jin H; Wang J; Yang F; Liu P; Zhu Y; Sui S; Chen Q Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol 2012, 14, 177–185. [DOI] [PubMed] [Google Scholar]
- (14).Jacobs BP; Browner WS Ginkgo biloba: a living fossil. Am. J. Med 2000, 108, 341–342. [DOI] [PubMed] [Google Scholar]
- (15).Parsad D; Pandhi R; Juneja A Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin. Exp. Dermatol 2003, 28, 285–287. [DOI] [PubMed] [Google Scholar]
- (16).Ginkgo Nuts Nutrition Facts.
- (17).Qi Q-M; Xue Y-C; Lv J; Sun D; Du JX; Cai SQ; Li YH; Gu TC; Wang MB Ginkgolic acids induce HepG2 cell death via a combination of apoptosis, autophagy and the mitochondrial pathway. Oncol. Lett 2018, 15, 6400–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hua Z; Wu C; Fan G; Tang Z; Cao F The antibacterial activity and mechanism of ginkgolic acid C15:1. BMC Biotechnol 2017, 17, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lü J-M; Yan S; Jamaluddin S; Weakley SM; Liang Z; Siwak EB; Yao Q; Chen C Ginkgolic acid inhibits HIV protease activity and HIV infection in vitro. Med. Sci. Monit 2012, 18, BR293–BR298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Baek SH; Ko J-H; Lee JH; Kim C; Lee H; Nam D; Lee J; Lee S-G; Yang WM; Um J-Y; Sethi G; Ahn KS Ginkgolic Acid Inhibits Invasion and Migration and TGF-β-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol 2017, 232, 346–354. [DOI] [PubMed] [Google Scholar]
- (21).Chhunchha B; Singh P; Singh DP; Kubo E Ginkgolic Acid Rescues Lens Epithelial Cells from Injury Caused by Redox Regulated-Aberrant Sumoylation Signaling by Reviving Prdx6 and Sp1 Expression and Activities. Int. J. Mol. Sci 2018, 19, 3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yao Q-Q; Li L; Xu M-C; Hu H-H; Zhou H; Yu L-S; Zeng S The metabolism and hepatotoxicity of ginkgolic acid (17 : 1) in vitro. Chin. J. Nat. Med 2018, 16, 829–837. [DOI] [PubMed] [Google Scholar]
- (23).Yoon S-Y; Lee JH; Kwon SJ; Kang HJ; Chung SJ Ginkgolic acid as a dual-targeting inhibitor for protein tyrosine phosphatases relevant to insulin resistance. Bioorg. Chem 2018, 81, 264–269. [DOI] [PubMed] [Google Scholar]
- (24).Liu J; Li Y; Yang X; Dong Y; Wu J; Chen M Effects of ginkgol C17:1 on cisplatin-induced autophagy and apoptosis in HepG2 cells. Oncol. Lett 2018, 15, 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Li J; Li A; Li M; Liu Y; Zhao W; Gao D Ginkgolic acid exerts an anti-inflammatory effect in human umbilical vein endothelial cells induced by ox-LDL. Die Pharmazie 2018, 73, 408–412. [DOI] [PubMed] [Google Scholar]
- (26).Fukuda I; Ito A; Hirai G; Nishimura S; Kawasaki H; Saitoh H; Kimura K.-i.; Sodeoka M; Yoshida M Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem. Biol 2009, 16, 133–140. [DOI] [PubMed] [Google Scholar]
- (27).Yao Q-Q; Liu Z-H; Xu M-C; Hu H-H; Zhou H; Jiang H-D; Yu L-S; Zeng S Mechanism for ginkgolic acid (15 : 1)-induced MDCK cell necrosis: Mitochondria and lysosomes damages and cell cycle arrest. Chin. J. Nat. Med 2017, 15, 375–383. [DOI] [PubMed] [Google Scholar]
- (28).Hecker H; Johannisson R; Koch E; Siegers C-P In vitro evaluation of the cytotoxic potential of alkylphenols from Ginkgo biloba L. Toxicology 2002, 177, 167–177. [DOI] [PubMed] [Google Scholar]
- (29).Liu H; Li J; Lu D; Li J; Liu M; He Y; Williams BO; Li J; Yang T Ginkgolic acid, a sumoylation inhibitor, promotes adipocyte commitment but suppresses adipocyte terminal differentiation of mouse bone marrow stromal cells. Sci. Rep 2018, 8, 2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Bolte S; Cordelieres FP A guided tour into subcellular̀ colocalization analysis in light microscopy. J. Microsc 2006, 224, 213–232. [DOI] [PubMed] [Google Scholar]
- (31).Venegas V; Halberg MC Measurement of mitochondrial DNA copy number. Methods Mol. Biol 2012, 837, 327–335. [DOI] [PubMed] [Google Scholar]
- (32).Wang W; Wang Q; Wan D; Sun Y; Wang L; Chen H; Liu C; Petersen RB; Li J; Xue W; Zheng L; Huang K Histone HIST1H1C/H1.2 regulates autophagy in the development of diabetic retinopathy. Autophagy 2017, 13, 941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Cannino G; Di Liegro CM; Rinaldi AM Nuclear-mitochondrial interaction. Mitochondrion 2007, 7, 359–366. [DOI] [PubMed] [Google Scholar]
- (34).Villa E; Marchetti S; Ricci J-E No Parkin Zone: Mitophagy without Parkin. Trends Cell Biol. 2018, 28, 882–895. [DOI] [PubMed] [Google Scholar]
- (35).Li X; Deng F; Shan X; Pan J; Yu P; Mao Z Effects of the molluscicidal agent GA-C13:0, a natural occurring ginkgolic acid, on snail mitochondria. Pestic. Biochem. Physiol 2012, 103, 115–120. [Google Scholar]
- (36).Pickles S; Vigie P; Youle RJ Mitophagy and Qualitý Control Mechanisms in Mitochondrial Maintenance. Curr. Biol 2018, 28, R170–R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).van der Bliek AM; Shen Q; Kawajiri S Mechanisms of mitochondrial fission and fusion. Cold Spring Harbor Perspect. Biol 2013, 5, a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Song Z; Ghochani M; McCaffery JM; Frey TG; Chan DC Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell 2009, 20, 3525–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Chan DC Fusion and fission: interlinked processes critical for mitochondrial health. Annu. Rev. Genet 2012, 46, 265–287. [DOI] [PubMed] [Google Scholar]
- (40).Mishra P; Chan DC Metabolic regulation of mitochondrial dynamics. J. Cell Biol 2016, 212, 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Braschi E; Zunino R; McBride HM MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009, 10, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Prudent J; Zunino R; Sugiura A; Mattie S; Shore GC; McBride HM MAPL SUMOylation of Drp1 Stabilizes an ER/Mitochondrial Platform Required for Cell Death. Mol. Cell 2015, 59, 941–955. [DOI] [PubMed] [Google Scholar]
- (43).Guo C; Hildick KL; Luo J; Dearden L; Wilkinson KA; Henley JM SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J. 2013, 32, 1514–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Guo C; Wilkinson KA; Evans AJ; Rubin PP; Henley JM SENP3-mediated deSUMOylation of Drp1 facilitates interaction with Mff to promote cell death. Sci. Rep 2017, 7, 43811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Fernandez-Marcos PJ; Auwerx J Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr 2011, 93, 884S–890S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Lerin C; Rodgers JT; Kalume DE; Kim S.-h.; Pandey A; Puigserver P GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1α. Cell Metabol. 2006, 3, 429–438. [DOI] [PubMed] [Google Scholar]
- (47).Kelly TJ; Lerin C; Haas W; Gygi SP; Puigserver P GCN5-mediated Transcriptional Control of the Metabolic Coactivator PGC-1β through Lysine Acetylation. J. Biol. Chem 2009, 284, 19945–19952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Gerhart-Hines Z; Rodgers JT; Bare O; Lerin C; Kim S-H; Mostoslavsky R; Alt FW; Wu Z; Puigserver P Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007, 26, 1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Canto C; Gerhart-Hines Z; Feige JN; Lagouge M; Noriega L; Milne JC; Elliott PJ; Puigserver P; Auwerx J AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Rytinki MM; Palvimo JJ SUMOylation Attenuates the Function of PGC-1α. J. Biol. Chem 2009, 284, 26184–26193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Cai R; Yu T; Huang C; Xia X; Liu X; Gu J; Xue S; Yeh ETH; Cheng J SUMO-specific Protease 1 Regulates Mitochondrial Biogenesis through PGC-1α. J. Biol. Chem 2012, 287, 44464–44470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Balasubramanyam K; Swaminathan V; Ranganathan A; Kundu TK Small molecule modulators of histone acetyltransferase p300. J. Biol. Chem 2003, 278, 19134–19140. [DOI] [PubMed] [Google Scholar]
- (53).Liu Y; Yang B; Zhang L; Cong X; Liu Z; Hu Y; Zhang J; Hu H Ginkgolic acid induces interplay between apoptosis and autophagy regulated by ROS generation in colon cancer. Biochem. Biophys. Res. Commun 2018, 498, 246–253. [DOI] [PubMed] [Google Scholar]
- (54).Zhang W; Siraj S; Zhang R; Chen Q Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy 2017, 13, 10800–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.