Abstract
Background
Progressive disseminated histoplasmosis (PDH) is a serious fungal infection that affects people living with HIV. The best way to treat the condition is unclear.
Objectives
We assessed evidence in three areas of equipoise.
1. Induction. To compare efficacy and safety of initial therapy with liposomal amphotericin B versus initial therapy with alternative antifungals.
2. Maintenance. To compare efficacy and safety of maintenance therapy with 12 months of oral antifungal treatment with shorter durations of maintenance therapy.
3. Antiretroviral therapy (ART). To compare the outcomes of early initiation versus delayed initiation of ART.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; Cochrane CENTRAL; MEDLINE (PubMed); Embase (Ovid); Science Citation Index Expanded, Conference Proceedings Citation Index‐Science, and BIOSIS Previews (all three in the Web of Science); the WHO International Clinical Trials Registry Platform, ClinicalTrials.gov, and the ISRCTN registry, all up to 20 March 2020.
Selection criteria
We evaluated studies assessing the use of liposomal amphotericin B and alternative antifungals for induction therapy; studies assessing the duration of antifungals for maintenance therapy; and studies assessing the timing of ART. We included randomized controlled trials (RCT), single‐arm trials, prospective cohort studies, and single‐arm cohort studies.
Data collection and analysis
Two review authors assessed eligibility and risk of bias, extracted data, and assessed certainty of evidence. We used the Cochrane 'Risk of bias' tool to assess risk of bias in randomized studies, and ROBINS‐I tool to assess risk of bias in non‐randomized studies. We summarized dichotomous outcomes using risk ratios (RRs), with 95% confidence intervals (CI).
Main results
We identified 17 individual studies. We judged eight studies to be at critical risk of bias, and removed these from the analysis.
1. Induction
We found one RCT which compared liposomal amphotericin B to deoxycholate amphotericin B. Compared to deoxycholate amphotericin B, liposomal amphotericin B may have higher clinical success rates (RR 1.46, 95% CI 1.01 to 2.11; 1 study, 80 participants; low‐certainty evidence). Compared to deoxycholate amphotericin B, liposomal amphotericin B has lower rates of nephrotoxicity (RR 0.25, 95% CI 0.09 to 0.67; 1 study, 77 participants; high‐certainty evidence). We found very low‐certainty evidence to inform comparisons between amphotericin B formulations and azoles for induction therapy.
2. Maintenance
We found no eligible study that compared less than 12 months of oral antifungal treatment to 12 months or greater for maintenance therapy.
For both induction and maintenance, fluconazole performed poorly in comparison to other azoles.
3. ART
We found one study, in which one out of seven participants in the 'early' arm and none of the three participants in the 'late' arm died.
Authors' conclusions
Liposomal amphotericin B appears to be a better choice compared to deoxycholate amphotericin B for treating PDH in people with HIV; and fluconazole performed poorly compared to other azoles. Other treatment choices for induction, maintenance, and when to start ART have no evidence, or very low certainty evidence. PDH needs prospective comparative trials to help inform clinical decisions.
Plain language summary
How best to treat progressive disseminated histoplasmosis in people with HIV
What was the aim of this review?
The aim of this Cochrane Review was to investigate some treatment dilemmas with progressive disseminated histoplasmosis in people living with HIV. We collected and analysed all relevant studies to answer this question and found 17 studies.
Key messages
Liposomal amphotericin B may improve clinical success compared to deoxycholate amphotericin B when starting treatment.
Liposomal amphotericin B results in less kidney damage compared to deoxycholate amphotericin B when starting treatment.
We are unsure how long people should stay on treatment after they have successfully completed the starting stage. We are unsure at what time during treatment of the fungal infection it is best to start treatment to fight the HIV virus.
What was studied in this review?
Histoplasmosis is an infection caused by inhaling a fungus called Histoplasma. The most severe form of histoplasmosis is called progressive disseminated histoplasmosis, in which the infection spreads from the lungs to other organs. It is life‐threatening for people with advanced HIV.
The treatment of progressive disseminated histoplasmosis starts with 'induction', in which medicines are started to rapidly attack the fungus. The next phase is called 'maintenance', in which medicines are used to prevent the fungus taking hold again. During treatment of the fungus, antiretroviral medicines are started to fight the HIV virus.
We wanted to find out the best induction treatment, if maintenance could be for less than one year, and when was the best time to start antiretroviral medicines.
What are the main results of the review?
We found 17 studies. We removed eight from the review as they did not include important measurements that might change results. These included how severe the HIV infection was, or if the patients had other infections at the same time.
One study compared two forms of the same medicine for starting treatment of histoplasmosis, liposomal amphotericin B and deoxycholate amphotericin B. It found that the more expensive liposomal form is less likely to cause kidney damage and may have higher clinical success rates than the deoxycholate form.
None of the studies looked at whether maintenance could be less than one year. Two studies looked a antiretroviral medicines, but we do not know when it is best to start them.
How up to date is the review?
We searched for studies that had been published up to 20 March 2020.
Summary of findings
Summary of findings 1. Induction: liposomal amphotericin compared with amphotericin deoxycholate.
| Liposomal amphotericin compared with amphotericin deoxycholate for induction therapy of progressive disseminated histoplasmosis | ||||||
|
Patient or population: adults with HIV and progressive disseminated histoplasmosis Settings: endemic areas Intervention: induction therapy with liposomal amphotericin B Comparison: amphotericin B deoxycholate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| dAmB | lAmB | |||||
| Clinical success | 560 per 1000 | 818 per 1000 (566 to 1000) | RR 1.46 (1.01 to 2.11) | 80 (1 study) | ⊕⊕⊝⊝ Lowa | Compared to dAmB, lAmB may have higher clinical success rates. |
| Death | 125 per 1000 | 19 per 1000 (3 to 173) | RR 0.15 (0.02 to 1.38) | 77 (1 study) | ⊕⊕⊝⊝ Lowb |
Treatment with lAmB may result in lower mortality than treatment with dAmB. |
| Safety outcomes: nephrotoxicity | 375 per 1000 | 94 per 1000 (34 to 251) | RR 0.25 (0.09 to 0.67) | 77 (1 study) | ⊕⊕⊕⊕ High |
Treatment with lAmB resulted in lower rates of nephrotoxicity compared to treatment with dAmB; this was supported by findings of a Cochrane Review which reported moderate‐certainty evidence (Botero Aguirre 2015). |
| Safety outcomes: drug discontinuation | 83 per 1000 | 19 per 1000 (2 to 198) | RR 0.23 (0.02 to 2.38) | 77 (1 study) | ⊕⊝⊝⊝ Very lowc | We do not know if treatment with lAmB leads to fewer treatment discontinuations than dAmB. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; dAmB: deoxycholate amphotericin B; lAmB: liposomal amphotericin B; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded two levels for very serious imprecision: the CI met the line of no effect and was based on very few events (73 participants, 1 randomized controlled trial). bDowngraded two levels for very serious imprecision: the CIs were wide and crossed the line of no effect. cDowngraded one level for serious risk of bias (due to unclear reporting criteria) and two levels for very serious imprecision (the CIs were wide and crossed the line of no effect).
Background
Description of the condition
Progressive disseminated histoplasmosis (PDH) is an important infectious disease among people living with HIV. PDH is one of the endemic mycoses, meaning a fungal infection localized to a specific region. It is caused by two human pathogens, Histoplasma capsulatum var. capsulatum (in the Americas) and Histoplasma capsulatum var. duboisii (in Africa). It causes severe morbidity and carries a risk of mortality of over 60% (Adenis 2014; Cano‐Torres 2019). H capsulatum var. capsulatum has historically been thought of as predominantly effecting the Americas, but there is evidence of a wider global distribution (Baker 2019).
The diagnosis of PDH in people living with HIV is usually made based on:
risk factors for the disease (advanced HIV);
clinical manifestations consistent with disseminated histoplasmosis, such as fever, fatigue, weight loss, and hepatosplenomegaly;
histoplasma antigen assays;
microscopic demonstration or isolation of Histoplasma from extrapulmonary sites; due to slow growth, isolation is likely to be too slow to allow diagnosis.
Description of the intervention
The current standard of care for PDH is typically based on Infectious Diseases Society of America 2007 guidelines (Wheat 2007). This guideline recommends:
for moderately severe to severe disease, liposomal amphotericin B (3.0 mg/kg daily for 1 to 2 weeks), followed by oral itraconazole (200 mg 3 times daily for 3 days and then 200 mg twice daily for a total of at least 12 months);
for mild‐to‐moderate disease, itraconazole (200 mg 3 times daily for 3 days), and then twice daily for at least 12 months.
Alongside treatment of PDH, HIV is treated with antiretroviral therapy (ART). Commencing ART might rapidly restore immune function. This may cause an excessive inflammatory response known as immune reconstitution inflammatory syndrome (IRIS) (Melzani 2020).
How the intervention might work
Azoles inhibit biosynthesis of ergosterol, which is essential in fungal cell membranes. Itraconazole, voriconazole, and posiconazole are thought to be fungicidal for histoplasma, but fluconazole is thought to have fungistatic activity only. Polyenes, such as amphotericin B, bind to fungal membrane sterols and disrupt cell membranes. They are thought to have fungicidal activity. Non‐randomized trial data from animal studies suggest that near maximal antifungal activity with amphotericin B occurs within three days, which has led to interest in shorter courses in treatment of other mycoses, such as cryptococcal meningitis (Tenforde 2018).
Why it is important to do this review
Currently available guidelines for management of PDH date from 2007. These were designed for use by clinicians in the USA, a high‐resource country. The advent of widespread availability of ART internationally has changed treatment paradigms for HIV. In resource‐limited settings, there is interest in revisiting the optimal treatment options for PDH. This review summarizes available evidence, and in particular we aimed to understand if new evidence could inform updated international guidelines on PDH.
Objectives
1. Induction. To compare efficacy and safety of initial therapy with liposomal amphotericin B versus initial therapy with alternative antifungals.
2. Maintenance. To compare efficacy and safety of maintenance therapy with 12 months of oral antifungal treatment with shorter durations of maintenance therapy. (Please note, itraconazole is a preferred oral antifungal agent, see results.)
3. Antiretroviral therapy (ART). To compare the outcomes of early initiation of ART versus delayed initiation of ART.
Methods
Criteria for considering studies for this review
Types of studies
We planned to synthesize the study types in order of priority. At each stage, if we found a sufficient number of studies to allow a high‐certainty synthesis, we did not intend to progress further. As we did not find sufficient evidence to allow high‐certainty synthesis, our review includes the following study types:
randomized controlled trials (RCTs);
quasi‐RCTs/non‐RCTs;
prospective cohort studies;
retrospective cohort studies;
single arm cohort studies.
We excluded case reports and case series.
Types of participants
HIV‐positive children, adolescents, and adults with a clinical diagnosis of PDH.
Types of interventions
We aimed to make the following comparisons.
| Objective | Intervention | Comparisons |
| 1. Induction | Liposomal amphotericin B (3.0 mg/kg daily) for 1–2 weeks | Lipid complex amphotericin B Deoxycholate amphotericin B Other antifungal agents |
| 2. Maintenance | Oral antifungal treatment for < 12 months | Oral antifungal treatment for ≥ 12 months |
| 3. ART | Early initiation (within 4 weeks of commencing antifungal therapy) | Delayed initiation (> 4 weeks after starting antifungal treatment) |
Types of outcome measures
We collected data on key outcomes, as summarized in the table below.
| Objective | Efficacy outcomes of interest | Safety outcomes of interest |
| 1. Induction | Clinical failure at or before study end Laboratory failure at or before study end |
Toxicity Early discontinuation |
| 2. Maintenance | Relapse of histoplasmosis at 12 months, or other clinically important time points All‐cause mortality at 12 months |
Toxicity Early discontinuation |
| 3. ART | Incidence of immune reconstitution inflammatory syndrome Viral failure |
Toxicity Early discontinuation |
Where possible, we collected dichotomous and time‐to‐event data for relevant outcomes. We also collected data on mortality, and severe adverse events, including type and frequency.
Search methods for identification of studies
Electronic searches
We developed our search strategy with the assistance of the Information Specialist, Vittoria Lutje. We searched the following databases on 20 March 2020 using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register; Central Register of Controlled Trials (CENTRAL; 2020, Issue 3, published in the Cochrane Library); MEDLINE (PubMed, from 1966); Embase (Ovid, from 1947); Science Citation Index Expanded (SCI‐EXPANDED, from 1900), Conference Proceedings Citation Index‐Science (CPCI‐S, from 1900), and BIOSIS Previews (from 1926) (all three using the Web of Science platform). We also searched the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/search/en/), ClinicalTrials.gov (clinicaltrials.gov/), and the ISRCTN registry (www.isrctn.com/) to identify ongoing studies.
Searching other resources
We examined reference lists of relevant studies and reviews.
Data collection and analysis
Selection of studies
Two review authors (MM and PH) screened the titles and abstracts of the search results to determine eligibility using Covidence (www.covidence.org/). We did not perform double screening as we prepared the review rapidly to inform a guidelines meeting. We each assessed a random sample of the other author's screening. There were no disagreements. Both review authors screened the full texts of potentially eligible studies, and resolved any disagreement by discussion. At the time of full‐text screening, we categorized the studies by study design.
Data extraction and management
One review author (PH) extracted data, and one review author (MM) reviewed all data extraction to ensure accuracy.
Assessment of risk of bias in included studies
For each included study, both review authors performed a risk of bias assessment resolving any disagreements through discussion. We used the Cochrane 'Risk of bias' tool for RCTs. For non‐randomized studies, we used the Risk of Bias In Non‐Randomized Studies of Interventions (ROBINS‐I) tool. We developed a theoretical target study and assessed each non‐randomized study across up to seven domains. Each assessment was discontinued if a domain was deemed to be at critical risk of bias. Each outcome was assessed. We identified relevant confounding factors through investigation of the literature and in discussion with expert clinicians. These a priori factors included severity of disease (histoplasmosis and CD4 count); time to treatment; and, for objectives 2 and 3, adherence to ART/maintenance therapy for histoplasmosis.
Data synthesis
Narrative synthesis
We followed narrative synthesis methodology (Popay 2006). Within this synthesis, we organized findings from included studies to describe patterns across the studies in terms of the:
direction of effects;
size of effects.
We calculated 95% confidence intervals (CI) for binomial proportions. We calculated 95% CIs for risk ratios (RR) using Review Manager 5 (Review Manager 2014). Studies assessed as at critical risk of bias were excluded from narrative synthesis.
Quantitative synthesis
We did not identify trials that were sufficiently similar in design or outcomes to allow a meaningful meta‐analysis of outcome data. Therefore, we have not performed quantitative synthesis.
Exploring relationships in the data
We planned to explore relationships to consider the factors that might explain any differences in direction and size of effect across the included studies. For data included in narrative synthesis, we explored relationships using textual descriptions of key study elements (see Characteristics of included studies table), groupings and clusters of similar studies, and presentation of findings in tabulated form.
Assessing the certainty of our conclusions
We planned to present adapted GRADE tables to summarize the certainty of our findings for each outcome. As we did not find good evidence to answer all objectives, we presented a GRADE table for outcomes relevant to 'Objective 1. Induction' detailing certainty of findings. We could not include any studies to answer 'Objective 2. Maintenance', so presented a narrative summary of indirect evidence in the body of the review only. We presented an additional summary table for 'Objective 3. ART'.
Results
Description of studies
Results of the search
We retrieved 1259 results from our electronic search. After title and abstract screening, we identified 206 reports for full‐text screening. Following full‐text screening, we identified 16 individual studies which were relevant to the review. These included:
two RCTs (ACTG‐A5164, 2009; Johnson 2002);
four single arm trials (ACTG084, 1992; ACTG120, 1992; ACTG174, 1994; McKinsey 1989);
four prospective cohort studies (Baddley 2008; Couppié 2004; Goldman 2004; Ramdial 2002);
six retrospective cohort studies (Luckett 2015; Mootsikapun 2006; Myint 2014; Negroni 2017; Norris 1994; Pietrobon 2004).
We have shown the results of our search in Figure 1.
1.
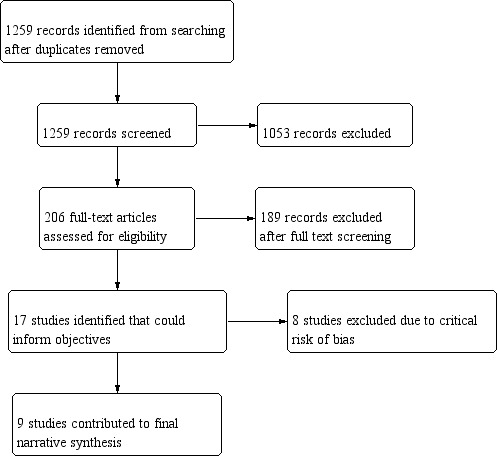
Review flow diagram.
We found one additional unpublished retrospective cohort study via correspondence with authors (Melzani 2020).
Therefore, there were 17 individual studies. We excluded eight of these studies from analysis as we assessed them to be at critical risk of bias using ROBINS‐I methodology (Table 2). We listed these below.
1. Table of studies at critical risk of bias overall (ROBINS‐I): disease‐related outcomes.
| Studies at critical risk of bias outcomes: death, relapse of histoplasmosis | |||
| Study | Review objective | Domain | Comment |
| McKinsey 1989 | 1 and 2 | Confounding | Confounding domains were not controlled for. No report of ART use, CD4 counts, or clinical condition of participants. Rationale for selection of treatment regimen not described. |
| Couppié 2004 | 1 | Participant selection | Selection of intervention was made by the treating physician. As more severely ill participants were more likely to get AmB than ITRA selection was strongly related to the outcome. |
| Ramdial 2002 | 1 | Confounding | Confounders not addressed with respect to treatment regimens. Descriptive account provided of management of participants without detail on severity of conditions, comedications, or comorbidities. No information provided on time to treatment or duration of treatment. |
| Baddley 2008 | 2 | Confounding | Logistic regression used to determine association of variables with mortality including potential confounders, age, and race. Antifungal treatment data were not included in regression or reported in detail per participant. Authors reported 32/41 participants received ITRA, 22/41 dAmB, and 7/41 lAmB. Switches between medications were not reported. Authors reported 29 participants received ITRA after AmB. Denominator not reported. 8/13 participants who died were receiving an AmB preparation and 5/13 receiving ITRA. Time of transition from AmB to azole not reported. |
| Myint 2014 | 2 | Confounding | Physician determined discontinuation of maintenance therapy may have been influenced by prognostic factors that were not controlled for. Multiple logistic regression used to determine variables associated with relapse; however, assignment to treatment arms were based on clinical assessment and viral load. 'Adherence to therapy' not defined. Unclear if this referred to ART, ITRA, or both. No evidence of adjustment for time‐varying confounding. |
| Negroni 2017 | 2 | Confounding | Comorbidity and comedications were not reported or controlled for. Outcome data were not linked to disease severity. Outcomes not reported by drug regimen. Treatment regimens varied by drug and duration. There were drug switches. |
| Norris 1994 | 2 | Confounding | Authors reported no specific criteria to select participants for intervention. Criteria included unavailability of ITRA and preference for oral therapy. Intervention group determined by treating physicians who also evaluated clinical evidence of relapse and side effects. Severity of HIV, comorbidities and comedication were not reported. |
| Pietrobon 2004 | 2 | Confounding |
For outcome 'relapse of histoplasmosis': follow‐up periods not reported. Duration of ITRA or FCN not reported. Switches between regimens not reported. Concurrent medication not reported. No statistical methods to control for confounding reported. For outcome 'death': this study can be considered to be at 'serious risk of bias'. No use of ART during treatment period. Comorbidities mentioned but unclear whether all relevant co morbidities studied. Severity of PDH not reported. No report of statistical methods to control for confounders. ≥ 1 known important domain was not appropriately measured or controlled for. Given the very small numbers, we have not reported further details in synthesis. |
For details of risk of bias assessments see Appendix 3.
AmB: amphotericin B; ART: antiretroviral therapy; dAmB: deoxycholate amphotericin B; FCN: fluconazole; ITRA: itraconazole; lAmB: liposomal amphotericin B.
Included studies
1. Induction
From our search, the studies that gave information about relevant outcomes for induction therapies included:
one RCT (Johnson 2002);
two single arm trials (ACTG120, 1992; ACTG174, 1994);
one retrospective cohort study (Luckett 2015).
The following studies were excluded from narrative synthesis as they were at critical risk of bias using ROBINS‐I methodology.
one single arm trial (McKinsey 1989);
two prospective cohort studies (Couppié 2004; Ramdial 2002).
2. Maintenance
From our search, the studies that gave information about relevant outcomes for maintenance therapies included:
three single arm trials (ACTG084, 1992; ACTG120, 1992; ACTG174, 1994);
one prospective cohort study (Goldman 2004);
one retrospective cohort study (Mootsikapun 2006).
The following studies were excluded from narrative synthesis as they were at critical risk of bias using ROBINS‐I methodology:
one single arm trial (McKinsey 1989);
one prospective cohort study (Baddley 2008);
four retrospective cohort studies (Myint 2014; Negroni 2017; Norris 1994; Pietrobon 2004).
3. ART
We found one RCT which helped inform decisions regarding ART (ACTG‐A5164, 2009). We included Melzani 2020 in a narrative synthesis as it provided evidence of baseline risk, but could not directly inform the objective.
Excluded studies
We excluded 186 studies (including an RCT, single arm trials, prospective cohort studies, and retrospective cohort studies) after full‐text review. In the majority of cases, we were unable to extract relevant data from the study reports. We reported reasons for exclusion for a sample of 34 references in the Characteristics of excluded studies table.
Risk of bias in included studies
For the two randomized studies, risk of bias was low (Johnson 2002 shown in Table 3; ACTG‐A5164, 2009 shown in Table 4). These are summarized in Figure 2.
2. Risk of bias Johnson 2002.
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Unclear risk | Authors reported randomizations in blocks. Details of method of randomization not provided |
| Allocation concealment | Low risk | Closed envelopes |
| Blinding of participants and personnel | Low risk | Authors reported that participants received the intervention and comparator by intravenous infusion "in a blinded fashion". It is possible that both participants and personnel were blinded. |
| Blinding of outcome assessment | Low risk | Clinical and mycological outcomes were predetermined. These included objective components including temperature and laboratory findings. |
| Incomplete outcome data | Low risk | Reasons reported for missing data. Proportion of data missing from each group was similar. |
| Selective reporting | Low risk | No protocol cited; however, reported outcomes are consistent with trial aims. |
3. Risk of bias ACTG‐A5164, 2009.
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation | Low | Random sequence was generated by central computer using permuted blocks within strata. Neither block size nor treatment assignments to other sites were public. |
| Allocation concealment | Unclear | No details provided in protocol or included study. |
| Blinding of participants and personnel | High | Protocol stated that for arm B (deferred ART), no study‐provided drugs were to be provided initially hence blinding of participants and personnel was not possible. |
| Blinding of outcome assessment | Low | Primary outcome was a composite endpoint of survival and viral load. Detection bias was unlikely. |
| Incomplete outcome data | Low | Equal numbers withdrew without primary endpoint data in each study arm. Details provided for these participants. |
| Selective reporting | Low | Reported outcomes were consistent with protocol. |
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included randomized study.
For the remaining 15 non‐randomized studies, we assessed eight to be at critical risk of bias using ROBINS‐I, and excluded these from synthesis as described above (Table 2). The remaining seven non‐randomized studies were at serious risk of bias using ROBINS‐I (Table 5). One study was at critical risk of bias for the relapse outcome, and serious risk of bias for the mortality outcome (Pietrobon 2004). We excluded this study from synthesis as the mortality outcome did not sufficiently inform the objective.
4. Table of studies at serious risk of bias overall (ROBINS‐I): disease‐related outcomes.
| Studies at serious risk of bias outcomes: death, relapse of histoplasmosis | |||
| Study | Review objective | Domain(s) | Comment |
| ACTG120, 1992 | 1 and 2 | Confounding; participant selection; intervention classification | Severity of HIV; severity of PDH and comorbidities were not controlled for using appropriate statistical methodology. ART use at baseline of an earlier phase of the trial reported: those who responded to the intervention (ITRA) in the induction phase were selected for the intervention in the maintenance phase: participants started intervention at various doses and had reductions in dose made at variable intervals. While this was likely to have been informed by ITRA blood levels that were being monitored, detailed data were not provided per participant. |
| ACTG174, 1994 | 1 and 2 | Confounding; participant selection | At 3 months, protocol was revised and treatment regimen amended. Analyses were performed on participants who received the revised protocol (higher doses of FCN). Severity and management of HIV was not reported or controlled with appropriate statistical methods: selection into the maintenance arm of the study was related to the effect of the intervention in the induction phase. |
| Luckett 2015 | 1 | Intervention classification; outcome measurement | No information about dose, frequency, and timing of interventions. Information was collected retrospectively. Treatment failure outcome was based on clinician judgement only. This was likely to favour switch from azole to amphotericin. |
| ACTG084, 1992 | 2 | Confounding | Severity of HIV infection and ART use were not controlled for with appropriate statistical methods. |
| Goldman 2004 | 2 | Participant selection | Start of intervention varied – participants enrolled after a range of 14–81 months of antifungal therapy. Unclear how many eligible people were not enrolled. |
| Mootsikapun 2006 | 2 | Confounding; participant selection | ≥ 1 known important domain was not appropriately measured or controlled for: details of disease severity, comedications and comorbidities not provided for 27 participants discharged from hospital: maintenance therapy was commenced in those who responded to initial treatment on amphotericin B. Timing of start of maintenance therapy was not reported. Selection into this part of the study was related to the intervention. |
| Melzani 2020 | 3 | Confounding | ART was discontinued in 2/22 participants at the physician's decision; 2/22 due to patient choice. In unmasking group (14 participants), 10/14 received lAmB and 4/14 received ITRA. Paradoxical group (8 participants) physicians continued ART and ITRA for 6/8. Rationale for treatment choices not reported. Appropriate statistical measures to control for confounding were not reported. ≥ 1 known important domain was not appropriately measured or controlled for. |
For details of risk of bias assessment see Appendix 3.
ART: antiretroviral therapy; FCN: fluconazole; ITRA: itraconazole; lAmB: liposomal amphotericin B; PDH: progressive disseminated histoplasmosis.
Risk of bias was low in both included randomized studies (Table 3; Table 4; Figure 2). Eight non‐randomized studies were at critical risk of bias and eight at serious risk of bias overall using ROBINS‐I. Details on assessment by outcome are provided in Table 2 and Table 5. Detailed domain assessments are available in Appendix 2.
Effects of interventions
See: Table 1
1. Induction therapy for progressive disseminated histoplasmosis
Liposomal amphotericin B compared to deoxycholate amphotericin B
One RCT compared liposomal amphotericin B and deoxycholate amphotericin B (Johnson 2002). There was greater treatment success with liposomal amphotericin B compared to deoxycholate amphotericin B (RR 1.46, 95% CI 1.01 to 2.11; 1 trial, 80 participants; Analysis 1.1). There were three deaths in the deoxycholate amphotericin B arm and one death in the liposomal amphotericin B arm (RR 0.15, 95% CI 0.02 to 1.38; 1 trial, 77 participants; Analysis 1.2). There were lower rates of nephrotoxicity (defined as creatinine greater than twice the upper limit of normal) with liposomal amphotericin B than with deoxycholate amphotericin B (RR 0.25, 95% CI 0.09 to 0.67; 1 trial, 77 participants; Analysis 1.3). The authors did not report other safety data, including frequencies of commonly reported toxicities such as anaemia.
1.1. Analysis.

Comparison 1: Liposomal amphotericin B (lAmB) versus deoxycholate amphotericin B (dAmB), Outcome 1: Clinical success
1.2. Analysis.

Comparison 1: Liposomal amphotericin B (lAmB) versus deoxycholate amphotericin B (dAmB), Outcome 2: Death
1.3. Analysis.

Comparison 1: Liposomal amphotericin B (lAmB) versus deoxycholate amphotericin B (dAmB), Outcome 3: Safety outcomes
Liposomal amphotericin B compared to other antifungals
No RCTs compared liposomal amphotericin B to other antifungals.
One retrospective cohort study compared all forms of amphotericin B to triazole therapy (including itraconazole, posaconazole, and voriconazole) (Luckett 2015). Treatment success for triazoles was 83% (95% CI 62% to 95%). The report did not disaggregate data by disease severity, but reported that across the study (which included people who were immunocompromised for reasons other than HIV infection), frequency of triazole failure was similar among people with severe infection compared with those with mild‐to‐moderate infection.
Deoxycholate amphotericin B compared to other antifungals
No study compared deoxycholate amphotericin B to other antifungals.
Treatment success rates for other antifungals
In the absence of comparative studies, we reported treatment success rates for antifungal agents (see 'Narrative results table 1').
Itraconazole
One single arm trial of itraconazole in mild‐to‐moderate PDH reported a treatment success rate of 85% (95% CI 73% to 93%; 1 study, 50/59 participants) at a dose of 300 mg twice daily for three days then 200 mg twice daily for 12 weeks (ACTG120, 1992).
Fluconazole
One single arm trial of fluconazole reported a treatment success rate of 74% (95% CI 59% to 85%; 1 study, 36 successes of 49 participants), at a dose of 1600 mg on day one, then 800 mg once daily for 12 weeks (ACTG174, 1994). This study initially reported a treatment success rate of 80% (95% CI 56% to 94%; 16 successes of 20 participants) for fluconazole 1600 mg on day one followed by 600 mg once daily for eight weeks. However, the protocol was intensified when relapse was subsequently observed in 6/16 participants (37.5%, 95% CI 15% to 64%).
Narrative results: table 1: induction therapy
| Study | Method | Participants | Interventions | Primary outcome(s) | Setting | Disease severity | Overall risk of bias | Narrative of efficacy findings (95% CIs) | Narrative of safety findings |
| ACTG120, 1992 | Single arm trial | 59 with PDH | ITRA 300 mg BD for 3 days then 200 mg BD for 12 weeks | "Response to therapy" Death |
USA | Mild to moderate | Serious |
Clinical success: 50/59 participants 85% (73% to 93%) Death: 1/59 |
2/59 participants withdrew due to adverse events, and responded to AmB. |
| ACTG174, 1994 | Single arm trial | 49 with PDH |
Initial protocol FCN 1200 mg, then 600 mg OD for 8 weeks Revised protocol FCN 1200 mg, then 80 mg OD for 8 weeks |
"Treatment response" | USA | Mild to moderate | Serious |
Clinical success: 36/49 participants. 74% (59% to 85%) Revised protocol |
2 discontinuations due to toxicity, unclear if induction or maintenance. |
| Johnson 2002 | RCT | 81 with PDH | lAmB (55 participants) dAmB (26 participants) |
Efficacy: "Clinical success" Safety: early discontinuation |
USA | Moderate to severe | Low |
Clinical success: lAmB: 82% (69% to 91%) 45/55 participants. dAmB: 56% (37% to 76%) 14/25 participants. RR 1.46 (1.01 to 2.11) Death: lAmB: 1/53 (2%) dAmB: 3/24 (13%) RR 0.15 (0.02 to 1.38) |
Early discontinuation: 1/53 (2%) with lAmB vs 2/24 (8%) with dAmB RR 0.23 (95% CI 0.02 to 2.38) Nephrotoxicity: 5/53 (9%) with lAmB vs 9/24 (37%) with dAmB RR 0.25 (95% CI 0.09 to 0.67) Of note, authors did not report specific data in relation to other commonly recognized adverse effects, including anaemia. |
| Luckett 2015 | Retrospective cohort study | 56 with HIV and PDH | ITRA/VORI/POSA AmB |
Death (90‐day histoplasmosis‐related) Triazole failure |
USA | Mild to severe | Serious |
Death 5/56 participants. Not reported by treatment regimen. Clinical success: triazole induction successful in 20/24 participants 83% (62% to 95%) |
No safety issues reported. |
| AmB: amphotericin B; BD: twice daily; CI: confidence interval; dAmB: deoxycholate amphotericin B; FCN: fluconazole; ITRA: itraconazole; IAmB: liposomal amphotericin B; n: number of participants; OD: once daily; PDH: progressive disseminated histoplasmosis; POSA: posaconazole; RCT: randomized controlled trial; RR: risk ratio; VORI: voriconazole. | |||||||||
2. Maintenance therapy
Less than 12 months of oral itraconazole compared to 12 months or greater of oral itraconazole
No included study compared less than 12 months of oral itraconazole and 12 months or greater of oral itraconazole.
Continuation of antifungals versus discontinuation of antifungals
This result is summarized in Table 6.
5. Additional summary 1: amphotericin B formulations versus azoles.
| Early ART compared with deferred ART for PDH in HIV | |||
|
Patient or population: adults with HIV and progressive disseminated histoplasmosis Settings: endemic areas Intervention: induction therapy with triazoles Comparison: induction therapy with amphotericin B formulations | |||
| Outcomes | Narrative summary | Certainty of the evidence (GRADE) | Comments |
| Treatment success | No RCTs make direct comparisons of amphotericin B and triazoles. Treatment success of triazoles (other than fluconazole) were 83% and 85% in the 2 studies which reported them. | ⊕⊝⊝⊝ Very lowa,b |
— |
| ART: antiretroviral therapy; PDH: progressive disseminated histoplasmosis; RCT: randomized controlled trial. | |||
aDowngraded two levels for serious imprecision. The CIs are wide due to small numbers of participants. bDowngraded one level for serious indirectness. No studies report direct comparisons.
One prospective single‐arm cohort study followed a cohort of participants who discontinued antifungal therapy after at least 12 months, providing the participant had received at least six months of ART and had achieved a CD4 count of 150 cells/μL or greater (Goldman 2004). There were no relapses in 32 participants who discontinued therapy after 12 months.
Treatment success rates for modalities of maintenance therapies
'Narrative results table 2' indicates crude treatment success rates for different maintenance therapies used in studies.
Itraconazole
Two single arm trials reported low relapse rates of approximately 0.5% with itraconazole (ACTG084, 1992; ACTG120, 1992).
Fluconazole
One single arm trial was discontinued early due to higher relapse rates (11/36 participants; 30%, 95% CI 16% to 48%) (ACTG174, 1994). This trial used 400 mg doses of fluconazole after induction with fluconazole.
Narrative results table 2: maintenance therapy
| Study | Method | Participants | Interventions | Primary outcomes | Setting | Disease severity | Overall risk of bias | Narrative of efficacy findings | Narrative of safety findings |
| Studies assessing discontinuation of oral antifungals | |||||||||
| Goldman 2004 | Prospective cohort study (single arm) | 32 | Discontinuation after > 12 months of ITRA/FCN/AmB | Relapse after 1 year | USA | ≥ 6 months of ART. CD4 count < 150 cells/μL |
Serious | No relapses after 12 months of discontinuation of antifungal therapy. | No safety issues reported. |
| Studies reporting outcomes of maintenance therapy regimens | |||||||||
| ACTG084, 1992 | Single arm trial | 42 (after dAmB induction) | ITRA 200 mg BD; continuous | Relapse Death |
USA | No ART Median CD4 count 47 cells/μL (35 participants) |
Serious |
Relapse: 2/42 participants (0.5%, 95% CI 0.05% to 16%) Median follow‐up 98 weeks Death: 1/42 deaths attributed to PDH |
Treatment discontinuation in 1/42 participants Severe or life‐threatening adverse events in 37/42 participants, attributed mainly to HIV infection, opportunistic infections, and adverse effects of other medications. |
| ACTG120, 1992 | Single arm trial | 46 (of 59 enrolled) | ITRA 200 mg (26 participants) ITRA 400 mg (18 participants) ITRA 600 mg (2 participants) 4 participants remained on doses > 200 mg. Median duration 64 weeks (range 7–120 weeks) |
Relapse | USA | No ART Median CD4 29 cells/µL |
Serious |
Relapse: 2/46 participants (0.4%, 95% CI 0.05% to 14%) Median follow‐up 87 weeks Death: 1/46 deaths due to relapse |
Treatment discontinuation in 8/46 participants. 24/46 participants enrolled known to have died, including discontinuations; pre‐ART. |
| ACTG174, 1994 | Single arm trial | 49 with PDH, 36 entered maintenance therapy | FCN 400 mg Continuous Median duration 30 weeks (early termination of study) |
Relapse | USA | No ART Mild to severe |
Serious |
Relapse: 11/36 participants (30%, 95% CI 16% to 48%) |
2 discontinuations due to toxicity, unclear if induction or maintenance. |
| AmB: amphotericin B; ART: antiretroviral therapy; BD: twice daily; dAmB: deoxycholate amphotericin B; FCN: fluconazole; ITRA: itraconazole; n: number of participants; PDH: progressive disseminated histoplasmosis. | |||||||||
3. ART initiation
One trial compared early ART initiation to late ART initiation in people with coexisting opportunistic infections (ACTG‐A5164, 2009). There were 10/282 participants with a presumptive or confirmed diagnosis of histoplasmosis. No diagnostic criteria were given for histoplasmosis. One participant with histoplasmosis in each trial arm developed IRIS. Both survived. One out of seven participants in the early ART died by day 30. None of the three participants with histoplasmosis in the delayed group died by day 30. This result is summarized in 'Narrative results table 3' and Table 7.
6. Additional summary 2: early versus deferred ART.
| Early ART compared with deferred ART for PDH in HIV | ||||||
|
Patient or population: adults with HIV and progressive disseminated histoplasmosis Settings: endemic areas Intervention: early ART (< 14 days) Comparison: late ART (> 14 days) | ||||||
| Outcomes | Illustrative risks | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| IRIS | 1/3 participantsa | 1/7 participantsa |
RR 0.43 (0.04 to 4.82) |
10 (1 study) | ⊕⊝⊝⊝ Very lowb,c |
We do not know if early ART increases the risk of IRIS in people with HIV and PDH. |
| ART: antiretroviral therapy; IRIS: immune reconstitution inflammatory syndrome; PDH: progressive disseminated histoplasmosis; RCT: randomized controlled trial; RR: risk ratio. | ||||||
a1/3 people with histoplasmosis in deferred ART arm developed histoplasma IRIS (day 47). 1/7 people with histoplasmosis in early ART arm developed hepatitis C IRIS (day 14). bDowngraded two levels for serious imprecision. The CI was wide. cDowngraded one level for serious indirectness. The trial only included 10 participants with histoplasmosis and the case definitions were not stated.
One additional study reported crude incidence rates of histoplasma IRIS including paradoxical IRIS (flare‐up of a previously treated histoplasmosis) (Melzani 2020). This indicated an overall incidence rate of 0.74 cases per 1000 person‐years. This study does not directly answer the objective of early versus deferred ART, but provides an indication of baseline risk.
Narrative results table 3: antiretroviral initiation
| Study | Method | Participants | Interventions | Outcomes | Setting | Disease severity | Overall risk of bias | Narrative of findings |
| ACTG‐A5164, 2009 | RCT | 282, AIDS‐related opportunist infections 10 people with histoplasmosis |
Early ART (n 7) Deferred ART (n 3) |
Primary: composite endpoint of death and HIV viral load. Safety: IRIS; lab adverse events Grades 2–4; clinical adverse events Grades 2‐4. |
USA, South Africa | Baseline median CD4 count 29 (IQR 10–55) cells/μL | Low | 1/7 participants with histoplasmosis dies in the early ART group. 0/3 participants with histoplasmosis died in the deferred ART group. 1/3 people with histoplasmosis in deferred ART arm developed histoplasma IRIS (day 47). IRIS aetiology: hepatitis C. Survived. 1/7 people with histoplasmosis in early ART arm developed hepatitis C IRIS (day 14). IRIS aetiology: histoplasmosis. Survived. |
| ART: antiretroviral therapy; IQR: interquartile range; IRIS: immune reconstitution inflammatory syndrome. | ||||||||
Discussion
Summary of main results
For 'Objective 1. Induction' comparing liposomal amphotericin B to other antifungals, four studies met the inclusion criteria: one RCT with 81 participants and three non‐randomized studies with 164 participants. We judged all three non‐randomized studies at serious risk of bias. Compared to deoxycholate amphotericin B, liposomal amphotericin B may have higher clinical success rates (low‐certainty evidence), and has lower rates of nephrotoxicity (high‐certainty evidence). We do not know if all amphotericin B formulations are more effective than azoles for the induction phase of management (very low‐certainty evidence).
For 'Objective 2. Maintenance', comparing less than 12 months of oral antifungal treatment to greater than 12 months, no studies met the inclusion criteria.
For both objectives 1 and 2, fluconazole performed poorly in comparison to other azoles in the single‐arm trial which studied this (ACTG174, 1994). No further trials were done as there was no longer sufficient equipoise to justify this.
'Objective 3: ART' sought to compare early and delayed initiation of ART. One RCT with 282 participants met the inclusion criteria. Only 10 participants had coexisting HIV and a presumptive or confirmed diagnosis of PDH. By day 30, one of seven participants in the 'early' arm and none of the three participants in the 'late' arm died. We do not know the efficacy and safety outcomes of early versus late initiation of ART (very low‐certainty evidence).
Overall completeness and applicability of evidence
The overall evidence was limited; therefore, we were unable to address all the objectives of our review. Most studies were in the USA and such findings may not be generalizable to low‐resource settings as availability of liposomal amphotericin B and management of HIV may differ. This affects the external validity of our review. There is insufficient evidence to be confident that azoles and other formulations of amphotericin are as effective and safe as liposomal amphotericin B in the induction phase of the management of PDH in people living with HIV. Clinical practice may be governed by availability. Current clinical practice would indicate that shorter courses of maintenance therapy may be acceptable; however, there is insufficient evidence available to corroborate or refute this practice. There is insufficient evidence to be confident of the safety of discontinuation of maintenance therapy before 12 months or the timing of ART. Overall, clinical practice tends to favour early ART in most situations. The low rates of IRIS in the single RCT do not present a signal that this practice is unsafe; however, there is insufficient evidence to confirm this. It seems that people in resource‐limited settings are doing what they are able to do.
Certainty of the evidence
Overall, the certainty of the evidence for most outcomes was low or very low. Non‐randomized study designs predominate in this area of research, many of which were critically compromised by uncontrolled confounding. For the comparison of liposomal amphotericin B to deoxycholate amphotericin B for induction therapy, there was high‐certainty evidence of lower rates of nephrotoxicity (RR 0.25, 95% CI 0.09 to 0.67; 1 study, 77 participants). Evidence for clinical success for this comparison was of low certainty due to very serious imprecision indicating that further research is very likely to have an important impact on our confidence in the estimate of effect (RR 1.46, 95% CI 1.01 to 2.11; 1 study, 80 participants). For maintenance regimens, all six studies were of a non‐randomized design, at serious risk of bias, and they could only provide low‐certainty evidence.
Potential biases in the review process
To minimize the effect of all domains of bias in the non‐randomized studies we presented only those at serious risk of bias. There is little evidence available from populations outside the USA.
Agreements and disagreements with other studies or reviews
Liposomal amphotericin B has previously been found to be significantly safer than conventional amphotericin B with respect to renal toxicity in PDH. One systematic review published in 2015 studying any invasive fungal infections reported an effect size of RR 0.49 (95% CI 0.40 to 0.59; 12 studies, 2298 participants; Botero Aguirre 2015).
There is insufficient evidence to confidently challenge or concur with current clinical guidelines on duration of maintenance therapy (Wheat 2007).
One systematic review that investigated the effects of early versus late ART in participants with coexisting HIV and cryptococcal meningitis found the RR for development of IRIS to be 3.56 (95% CI 0.51 to 25.02, 2 RCTs). The authors graded this evidence as very‐low certainty due to imprecision, risk of bias, and indirectness indicating that they had little confidence in the effect estimate. This is consistent with the certainty of our findings for this outcome although the effect size of our included study was considerably smaller (RR 0.43, 95% CI 0.04 to 4.82; 1 study, 10 participants). This study also concluded that the risk of death appeared to be higher with early ART, leading to World Health Organization recommendations that treatment should be deferred for four to six weeks (Eshun‐Wilson 2018).
Authors' conclusions
Implications for practice.
Liposomal amphotericin B appears to be a better choice compared to deoxycholate amphotericin B for treating progressive disseminated histoplasmosis in people with HIV. Fluconazole appears unsuitable for induction or maintenance.
Implications for research.
As there is very low‐certainty evidence to inform other treatment choices, we recommend further prospective research. A priority question is whether in people with a clinical and immunological response to therapy, maintenance antifungal therapy can be safely discontinued earlier than 12 months. A further question is when is the optimal time to start ART, and in what circumstances the risk of IRIS may be higher? The high and varying costs of appropriate oral antifungal agents make these questions more pertinent.
History
Review first published: Issue 4, 2020
Acknowledgements
The Academic Editor is Professor George Rutherford.
We acknowledge the contribution made by the clinical experts in the development of the research questions studied in this review. We thank Vittoria Lutje, Information Specialist at the Cochrane Infectious Diseases Group (CIDG) for conducting the searches. We thank all the library staff at Liverpool School of Tropical Medicine (LSTM), especially Cathy Booth, for their assistance in obtaining articles. We thank Paul Garner for his guidance throughout the review.
We thank the members of the Pan American Health Organization Guideline Development Group who helped develop the questions for the review.
MM, PH, and the CIDG editorial base are supported by the Research, Evidence and Development Initiative (READ‐It). READ‐It (project number 300342‐104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies.
Appendices
Appendix 1. Detailed search strategies
Cochrane Central Register of Controlled Trials Issue 3 of 12, March 2020
#1 MeSH descriptor: [HIV] explode all trees
#2 MeSH descriptor: [HIV Infections] explode all trees
#3 Hiv OR hiv‐1 OR hiv‐2* OR hiv1 OR hiv2 OR hiv infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR ((human immun*) AND (deficiency virus )) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR ((acquired immun*) AND (deficiency syndrome))
#4 #1 or #2 or #3
#5 MeSH descriptor: [Histoplasma] explode all trees
#6 MeSH descriptor: [Histoplasmosis] explode all trees
#7 histoplasm*
#8 #5 or #6 or #7
#9 #4 and #8
PubMed (MEDLINE)
| Search set | Search terms |
| 1 | ((HIV Infections[MeSH] OR HIV[MeSH] OR (Hiv OR hiv‐1 OR hiv‐2* OR hiv1 OR hiv2 OR hiv infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR ((human immun*) AND (deficiency virus )) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR ((acquired immun*) AND (deficiency syndrome)) Field: Title/Abstract |
| 2 | "Histoplasma"[Mesh] OR "Histoplasmosis"[Mesh] or Histoplasm* Field: Title/Abstract |
| 3 | 1 and 2 |
| 4 | "Antifungal Agents"[Mesh] |
| 5 | "Amphotericin B"[Mesh]) OR amphotericin Field: Title/Abstract |
| 6 | "Itraconazole"[Mesh]) OR itraconazole Field: Title/Abstract |
| 7 | 4 or 5or 6 |
| 8 | 3 and 7 |
| 9 | "Randomized Controlled Trial" [Publication Type] OR "Controlled Clinical Trial" [Publication Type] |
| 10 | randomized or placebo or randomly or trial or groups Field: Title/Abstract |
| 11 | drug therapy [Subheading] |
| 12 | "Interrupted Time Series Analysis"[Mesh] |
| 13 | "Controlled Before‐After Studies"[Mesh |
| 14 | “time series” Field: Title/Abstract |
| 15 | "cross‐over studies"[MeSH] or crossover or cross‐over Field: Title/Abstract |
| 16 | "longitudinal studies"[MeSH] |
| 17 | Longitudinal or cohort* Field: Title/Abstract |
| 18 | "Systematic Review" [Publication Type] |
| 19 | metaanalysis or meta‐analysis or “systematic review” Field: Title/Abstract |
| 20 | 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 |
| 21 | 8 and 20 |
Embase 1947‐Present, updated daily
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 HIV infection.mp. or HIV Infections/
2 exp HIV/ or Acquired Immunodeficiency Syndrome/
3 (Hiv or hiv‐1 or hiv‐2* or hiv1 or hiv2 or hiv infect* or human immunodeficiency virus or human immunedeficiency virus or human immuno‐deficiency virus or human immune‐deficiency virus or (human immun* and deficiency virus) or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or (acquired immun* and deficiency syndrome)).mp.
4 aids.mp. or acquired immune deficiency syndrome/
5 1 or 2 or 3 or 4
6 histoplasma.mp. or Histoplasma/
7 histoplasmosis.mp. or histoplasmosis/
8 6 or 7
9 5 and 8
10 antifungal agents.mp. or antifungal agent/
11 itraconazole.mp. or itraconazole/
12 amphotericin B/ or Amphotericin B.mp.
13 10 or 11 or 12
14 9 and 13
15 randomized controlled trial/ or controlled clinical trial/
16 (randomized or placebo or randomly or trial or groups).mp
17 "time series".mp. or time series analysis/
18 (controlled before and after).mp.
19 crossover procedure/
20 cohort analysis/ or prospective study/ or cohort.mp.
21 longitudinal study.mp. or longitudinal study/
22 systematic review.mp. or "systematic review"/
23 (metaanalysis or meta‐analysis).mp.
24 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25 14 and 24
26 9 and 24
27 25 or 26
Science Citation Index Expanded (SCI‐EXPANDED) Conference Proceedings Citation Index‐Science (CPCI‐S) and BIOSIS Previews (all from Web of Science)
| # | 9 | #8 AND #5 |
| # | 8 | #7 OR #6 |
| # | 7 | TOPIC: (antifungal*) |
| # | 6 | TOPIC: (itraconazole or amphotericin) |
| # | 5 | #4 AND #3 |
| # | 4 | TOPIC: (histoplasma or histoplasmosis) |
| # | 3 | #2 OR #1 |
| # | 2 | TOPIC: (Hiv OR hiv‐1 OR hiv‐2* OR hiv1 OR hiv2 OR hiv infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR ((human immun*) AND (deficiency virus )) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR ((acquired immun*) AND (deficiency syndrome))) |
| # 1 | TOPIC: (HIV OR AIDS OR "human immunodeficiency virus" or "acquired immunodeficiency syndrome" or AIDS) |
WHO International Clinical Trials Registry Platform (WHO ICTRP), Clinicaltrials.gov, and ISRCTN registry
histoplasm* and HIV*
Appendix 2. ROBINS‐I methodology
Risk of bias: ROBINS‐I
Target trial(s)
To determine bias, as defined by systematic differences between a non‐randomized study and a hypothetical pragmatic randomized trial, we formulated the following target trial (Sterne 2016).
Design: individually randomized
Participants: HIV‐positive children, adolescents, and adults with a clinical diagnosis of progressive disseminated histoplasmosis.
| Objective | Intervention | Comparisons |
| 1. Induction | Liposomal amphotericin B (3.0 mg/kg daily) for 1–2 weeks | Lipid complex amphotericin B Deoxycholate amphotericin B Other antifungal agents |
| 2. Maintenance | Oral antifungal treatment for < 12 months | Oral antifungal treatment for ≥ 12 months |
| 3. ART | Early initiation (within 4 weeks of commencing antifungal therapy) | Delayed initiation (> 4 weeks after starting antifungal treatment |
The aim was to assess the effect of assignment to the intervention, that is, we assessed studies on the basis of intention to treat. Judgements were guided by the use of signalling questions at domain level using ROBINS‐I methodology (Sterne 2016).
Confounding domains relevant to all or most studies that were determined a priori informed by current literature and clinical expertise.
Table A a priori confounding domains
| Confounding domains relevant to all or most studies | Cointerventions that could be different between intervention groups and that could impact on outcomes |
| Severity of PDH | ART at time of PDH diagnosis |
| Severity of HIV (CD4 count) | Supportive therapy |
| Comorbidities and comedications | — |
| ART: antiretroviral therapy; PDH: progressive disseminated histoplasmosis | |
Severity of progressive disseminated histoplasmosis (PDH) and HIV were considered likely to influence clinicians to favour intravenous therapy including liposomal amphotericin B. Comorbidities and comedications were also determined to influence medical management. In particular, use of medications which may interact with azoles may cause a clinician to favour use of amphotericin during induction therapy.
Overall risk of bias judgements were determined using the following table from detailed guidance on the use of ROBINS‐I. Sterne JAC, Higgins JPT, Elbers RG, Reeves BC and the development group for ROBINS‐I. Risk Of Bias In Non‐randomized Studies of Interventions (ROBINS‐I): detailed guidance, updated 12 October 2016. Available from www.riskofbias.info (accessed 4 June 2019).
Table B guidance on ROBINS‐I judgements
| Response option | Criteria |
| Low risk of bias (the study is comparable to a well‐performed randomized trial). | The study is at low risk of bias for all domains. |
| Moderate risk of bias (the study appears to provide sound evidence for a non‐randomized study but cannot be considered comparable to a well‐performed randomized trial). | The study is at low or moderate risk of bias for all domains. |
| Serious risk of bias (the study has some important problems). | The study is at serious risk of bias in ≥ 1 domain, but not at critical risk of bias in any domain. |
| Critical risk of bias (the study is too problematic to provide any useful evidence and should not be included in any synthesis). | The study is at critical risk of bias in ≥ 1 domain. |
| No information on which to base a judgement about risk of bias. | There is no clear indication that the study is at serious or critical risk of bias and there is a lack of information in ≥ 1 key domains of bias. |
| Sterne 2016. | |
Table C ROBINS‐I. Assessments of non‐randomized studies
| Study:Luckett 2015. Outcome: treatment success | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Severe, moderate, and mild illness were defined | No | Yes | — |
| Severity of HIV | CD4 count and viral load reported | No | Yes | — |
| Comorbidities and comedications | Not reported | No | Not measured | Comorbidities likely to influence clinicians to favour liposomal amphotericin. |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No, remains important cointervention. 27% on ART prior to study. 1/3 adherent. | Favour intervention | ||
| Supportive therapy | Reported level of clinical care (e.g. ICU care) | Favour intervention | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Authors described severity of PDH, and reported median and range of CD4 count. Authors did not report any comorbidities or comedications. Baseline characteristics of participants in azole and amphotericin groups not reported. Authors did not report use of appropriate statistical methods to control for important baseline confounding. Rationale for treatment choice in azole and amphotericin groups not reported. There was insufficient information to judge bias due to confounding. | No information | |
| Bias in participant selection | 2.1–2.5 | No evidence that selection into the study based on participant characteristics observed after intervention. Authors did not report the time between start of follow‐up and start of intervention. | Moderate | |
| Bias in classification of intervention | 3.1–3.3 | No information about dose, frequency, and timing of interventions. Information was collected retrospectively. | Serious | |
| Bias due to deviations from intended intervention | 4.1–4.6 | No evidence of deviations from interventions. No comment on whether patients not on ART were commenced on ART. No measure of adherence to antifungal therapy. | No information | |
| Bias due to missing data | 5.1–5.5 | No information regarding loss to follow‐up. | No information | |
| Bias in measurement of outcomes | 6.1–6.4 | For mortality outcome, unlikely to display measurement bias. For treatment failure outcome, based on clinician judgement only, likely to favour switch from azole to amphotericin. | Serious | |
| Bias in selection of reported result | 7.1–7.3 | Limited analysis. | No information | |
| Overall bias | SERIOUS | |||
| Study:Mootsikapun 2006.Outcome 1: inhospital mortality | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Not reported | No | No information provided | — |
| Severity of HIV | Not reported | No | No information provided | — |
| Comorbidities and comedications | None reported | No | No information provided | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | ≥ 1 known important domain was not appropriately measured or controlled for. Details of disease severity, comedications, and comorbidities not provided for the 3 inhospital deaths. | Serious | |
| Bias in participant selection | 2.1–2.5 | Authors reported data on 29 participants who received AmB 0.7 mg/kg/day. Data not provided on alternate treatment regimens or time to treatment; however, since all participants received the same treatment, it is probable that selection into the study was not related to the outcome. | Moderate | |
| Bias in classification of intervention | 3.1–3.3 | Comparison was between disease populations not intervention groups. Intervention status was well defined. | Moderate | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Deviations were not reported. Important cointerventions were not reported. | No information | |
| Bias due to missing data | 5.1–5.5 | Data reported on inhospital deaths likely to be reasonably complete. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | The outcome measure was unlikely to be influenced by knowledge of the intervention. | Low | |
| Bias in selection of reported result | 7.1–7.3 | There is too little information to make a judgement on bias in reporting in this retrospective review of medical records. | No information | |
| Overall bias | SERIOUS | |||
| Study:Mootsikapun 2006. Outcome 2: relapse on maintenance itraconazole. Median follow‐up 22 months (1–75 months) | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Not reported | No | — | — |
| Severity of HIV | Not reported | No | — | — |
| Comorbidities and comedications | None reported | No | — | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | ≥ 1 known important domain was not appropriately measured or controlled for: details of disease severity, comedications, and comorbidities not provided for 27 participants discharged from hospital. | Serious | |
| Bias in participant selection | 2.1–2.5 | Maintenance therapy was commenced in those who responded to initial treatment on AmB. Timing of start of maintenance therapy was not reported. Selection into this part of the study was related to the intervention. | Serious | |
| Bias in classification of intervention | 3.1–3.3 | Authors reported that participants who responded to treatment with AmB received oral itraconazole 400 mg/day for 3 months then 200 mg/day afterwards. Median follow‐up for participants was 22 (range 1–75) months. Although response to treatment was likely to have been a clinical decision bias intervention, status appeared to be adequately defined. Range of duration of follow‐up was wide. | Moderate | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Cointerventions were not reported. Deviations from practice not reported. | Moderate | |
| Bias due to missing data | 5.1–5.5 | Data obtained from medical records from a 7‐year period. Range of follow‐up was 1–75 months. Outcome data not provided on individual participants. There was insufficient information to base a judgement about risk of bias for this domain. | No information | |
| Bias in measurement of outcomes | 6.1–6.4 | Retrospective data collection. Unlikely to be assessor bias in participant eligibility for maintenance therapy. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Authors reported no relapse in participants who had itraconazole as long‐term therapy; however, range of duration of follow‐up was wide. | Moderate | |
| Overall bias | SERIOUS | |||
| Study:Myint 2014.Outcome: relapse of histoplasmosis | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Defined and reported | No | — | — |
| Severity of HIV | Defined and reported | No | — | — |
| Comorbidities and comedications | ART adherence monitored with CD4 count and HIV RNA load | No | — | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | 34% in each group (12/35, 19/56) | — | ||
| Supportive therapy | Reported | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Physician determined discontinuation of maintenance therapy may have been influenced by prognostic factors that were not controlled for. Multiple logistic regression used to determine variables associated with relapse; however, assignment to treatment arms was based on clinical assessment and viral load. 'Adherence to therapy' not defined. Unclear if this referred to ART, ITRA, or both. No evidence of adjustment for time‐varying confounding. | Critical | |
| Bias in participant selection | 2.1–2.5 | — | — | |
| Bias in classification of intervention | 3.1–3.3 | — | — | |
| Bias due to deviations from intended intervention | 4.1–4.6 | — | — | |
| Bias due to missing data | 5.1–5.5 | — | — | |
| Bias in measurement of outcomes | 6.1–6.4 | — | — | |
| Bias in selection of reported result | 7.1–7.3 | — | — | |
| Overall bias | CRITICAL | |||
| Study:Myint 2014.Outcome: death | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Defined and reported | No | Yes | — |
| Severity of HIV | Defined and reported | No | Yes | — |
| Comorbidities and comedications | ART adherence monitored with CD4 count and HIV RNA load | No | Yes | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Participants in the physician continued therapy group are likely to have been more ill and therefore at higher risk of death. | Critical | |
| Bias in participant selection | 2.1–2.5 | |||
| Bias in classification of intervention | 3.1–3.3 | |||
| Bias due to deviations from intended intervention | 4.1–4.6 | |||
| Bias due to missing data | 5.1–5.5 | |||
| Bias in measurement of outcomes | 6.1–6.4 | |||
| Bias in selection of reported result | 7.1–7.3 | |||
| Overall bias | CRITICAL | |||
| Study:Negroni 2017.Outcome: response to maintenance therapy | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Not defined | No | ||
| Severity of HIV | CD4 count | No | ||
| Comorbidities and comedications | 17.5% on ART at baseline 41% of participants discharged from hospital continued with follow‐up of ART and maintenance therapy. Authors reported use of AmB in participants who were more ill and in those on medication likely to interact with itraconazole such as rifampicin suggesting there could have been comorbid TB. |
No | — | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Comorbidity and comedications were not reported or controlled for. Outcome data were not linked to disease severity. Outcomes not reported by drug regimen. Treatment regimens varied by drug and duration. There were drug switches. | Critical | |
| Bias in participant selection | 2.1–2.5 | — | — | |
| Bias in classification of intervention | 3.1–3.3 | — | — | |
| Bias due to deviations from intended intervention | 4.1–4.6 | — | — | |
| Bias due to missing data | 5.1–5.5 | — | — | |
| Bias in measurement of outcomes | 6.1–6.4 | — | — | |
| Bias in selection of reported result | 7.1–7.3 | — | — | |
| Overall bias | CRITICAL | |||
| Study:Norris 1994.Outcome: relapse of histoplasmosis | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Not reported. All participants had completed induction treatment for histoplasmosis before study started. Successful induction determined clinically. Fungal cultures not performed in all patients to confirm success of induction. | — | — | — |
| Severity of HIV | No measured variable reported. | — | — | — |
| Comorbidities and comedications | Multiple drug regimens used prior to intervention. HIV management not reported. |
— | — | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Authors reported no specific criteria to select participants for intervention. Criteria included unavailability of ITRA and preference for oral therapy. Intervention group determined by treating physicians who also evaluated clinical evidence of relapse and adverse effects. Severity of HIV, comorbidities, and comedication were not reported. | Critical | |
| Bias in participant selection | 2.1–2.5 | — | — | |
| Bias in classification of intervention | 3.1–3.3 | — | — | |
| Bias due to deviations from intended intervention | 4.1–4.6 | — | — | |
| Bias due to missing data | 5.1–5.5 | — | — | |
| Bias in measurement of outcomes | 6.1–6.4 | — | — | |
| Bias in selection of reported result | 7.1–7.3 | — | — | |
| Overall bias | CRITICAL | |||
| Study:Pietrobon 2004. Outcome: mortality | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Not defined or reported. | No | No | — |
| Severity of HIV | Authors reported 11/16 participants with histoplasmosis had CD4 count < 50 cells/μL | No | Yes | — |
| Comorbidities and comedications | Authors reported none of participants were receiving ART. Study population was 16. Comorbidities mentioned but not systemically. | No | No | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | No use of ART during treatment period. Comorbidities mentioned but unclear whether all relevant comorbidities studied. Severity of PDH not reported. No report of statistical methods to control for confounders. ≥ 1 known important domain was not appropriately measured or controlled for. | Serious | |
| Bias in participant selection | 2.1–2.5 | Drug treatment for the initial phase provided for all participants – all received AmB. Time to commencement of treatment not reported. Data reported were retrospective analyses of medical records of patients with HIV and opportunistic infections. | No information | |
| Bias in classification of intervention | 3.1–3.3 | Intervention for management of acute phase was well defined. | Low | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Deviations not reported. | No information | |
| Bias due to missing data | 5.1–5.5 | Data were reasonably complete. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | Measurement of mortality outcome unlikely to be influenced by knowledge of intervention received. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Descriptive retrospective study. No protocol; however, there was no indication of selection of the cohort for analysis and reporting on the basis of the result. | Moderate | |
| Overall bias | Serious | |||
| Study:Pietrobon 2004.Outcome: relapse of histoplasmosis | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Not defined or reported. | No | No | — |
| Severity of HIV | Authors reported 8/12 participants with histoplasmosis had CD4 count < 50 cells/μL | No | Yes | — |
| Comorbidities and comedications | Authors reported none of participants were receiving ART. Study population was 16. This included participants with various opportunistic infections. Coinfection with Cryptococcus neoformans reported in 1 participant; however, authors did not specify if this participant also had histoplasmosis. | No | No | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Follow‐up periods not reported. Duration of ITRA or FCN not reported. Switches between regimens not reported. Concurrent medication not reported. No statistical methods to control for confounding reported. | Critical | |
| Bias in participant selection | 2.1–2.5 | Participants treated with maintenance therapy would have responded to initial treatment. Time to commencement of maintenance therapy not reported. Insufficient information to judge if start of follow‐up and start of intervention coincided for most participants. | No information | |
| Bias in classification of intervention | 3.1–3.3 | Commencement of maintenance therapy was dependent on response to initial therapy with AmB. Intervention status was not well defined. | Serious | |
| Bias due to deviations from intended intervention | 4.1–4.6 | No reported deviations from intended intervention. | No information | |
| Bias due to missing data | 5.1–5.5 | Outcome data available for all participants. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | Relapse was not clearly defined. Time periods were not reported. The outcome measure was subjective and assessed by assessors aware of the intervention. | Serious | |
| Bias in selection of reported result | 7.1–7.3 | Descriptive retrospective study. No protocol; however, there was no indication of selection of the cohort for analysis and reporting on the basis of the result. | Moderate | |
| Overall bias | Critical | |||
| Study:Baddley 2008. Outcome: all‐cause mortality at 3 months postdiagnosis | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Clinical parameters were defined and laboratory confirmation criteria were specified. | No | — | — |
| Severity of HIV | CD4 count and HIV viral load | No | — | — |
| Comorbidities and comedications | Among those with previously diagnosed HIV 21.7% reported ART use. | No | — | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Logistic regression used to determine association of variables with mortality including potential confounders, age, and race. Antifungal treatment data were not included in regression or reported in detail per participant. Authors reported 32/41 participants received ITRA, 22/41 received dAmB, 7/41 received lAmB formulation. Switches between medications were not reported. Authors reported 29 participants received ITRA after AmB. Denominator not reported. 8/13 participants who died received an AmB preparation and 5/13 received ITRA. Time of transition from AmB to azole not reported. | Critical | |
| Bias in participant selection | 2.1–2.5 | — | — | |
| Bias in classification of intervention | 3.1–3.3 | — | — | |
| Bias due to deviations from intended intervention | 4.1–4.6 | — | — | |
| Bias due to missing data | 5.1–5.5 | — | — | |
| Bias in measurement of outcomes | 6.1–6.4 | — | — | |
| Bias in selection of reported result | 7.1–7.3 | — | — | |
| Overall bias | Critical | |||
| Study:Couppié 2004.Outcome: death within 1 month of starting antifungal treatment | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Severe was defined as either shock that required treatment with vasopressors or respiratory failure that required mechanical ventilation. Other cases were defined as 'non severe'. | No | 'Non‐severe' included a wide range of severity. | — |
| Severity of HIV | CD4 count | No | Yes | — |
| Comorbidities and comedications | 17.1% of participants were taking antiretroviral medication. | No | Not enough information. | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Choice of first‐line antifungal treatment was made by the treating physician based on severity of histoplasmosis and renal function. Authors reported higher rate of mortality in participants treated with AmB than those treated with ITRA in univariate analysis. However, as participants who were more clinically ill were more likely to have been commenced on AmB by the treating physician, authors recognized that this was a confounding factor. Appropriate methods to control for this were not reported. | Severe | |
| Bias in participant selection | 2.1–2.5 | Selection of intervention was made by the treating physician. As more severely ill participants were more likely to get AmB than ITRA, selection was strongly related to the outcome. | Critical | |
| Bias in classification of intervention | 3.1–3.3 | Classification of intervention status could have been affected by risk of the outcome. | Moderate | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Details of treatment regimens were not provided. | No information | |
| Bias due to missing data | 5.1–5.5 | Participants lost at follow‐up in the first 30 days were excluded. No information on proportion missing with respect to the outcome. | No information | |
| Bias in measurement of outcomes | 6.1–6.4 | The outcome measure was unlikely to have been influenced by knowledge of the intervention. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Prospective study that determined a priori to report death as dichotomous variable: death within 30 days of starting antifungal treatment and no death during the first 30 days of treatment. Data presented for both outcomes. | Low | |
| Overall bias | Critical | |||
| Study:Goldman 2004. Outcome: relapse of histoplasmosis | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Evidence of histoplasmosis infection AND remission with antigen concentrations < 4.1 units and negative blood culture. | No | Yes | — |
| Severity of HIV | CD4 – required to have > 150 cells/μL for study entry. | No | Yes | — |
| Comorbidities and comedications | Required to have received both antifungal and ART treatment for ≥ 12 months prior to starting trial. | No | Yes | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | All participants were required to be on ART at time of initiation into trial. | — | ||
| Supportive therapy | Not reported | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Protocol stipulated eligibility criteria for all the confounding domains cited above. | Low | |
| Bias in participant selection | 2.1–2.5 | Start of intervention varied – participants enrolled after a range of 14–81 months of antifungal therapy. Unclear how many eligible patients not enrolled. | Serious | |
| Bias in classification of intervention | 3.1–3.3 | Intervention was well‐defined – discontinuation of antifungal treatment | Low | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Regular monitoring of participants ensured that intervention was delivered as intended. | Low | |
| Bias due to missing data | 5.1–5.5 | Details on missing data were reported. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | Relapse of histoplasmosis was clearly defined in the protocol. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Outcome of interest was determined in the protocol and reported. | Low | |
| Overall bias | Serious | |||
| Study:Ramdial 2002.Outcome: death at 32 months | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Organ involvement | No | Not according to standard classification | — |
| Severity of HIV | CD4 count | No | Yes | — |
| Comorbidities and comedications | Not reported | No | — | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No. ART use was not reported. | — | ||
| Supportive therapy | No. Not reported. | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Confounders not addressed with respect to treatment regimens. Descriptive account provided of management of participants without detail on severity of conditions, comedications, or comorbidities. No information provided on time to treatment or duration of treatment. | Critical | |
| Bias in participant selection | 2.1–2.5 | Some of these cases are likely to have been Emergomyces. | — | |
| Bias in classification of intervention | 3.1–3.3 | — | — | |
| Bias due to deviations from intended intervention | 4.1–4.6 | — | — | |
| Bias due to missing data | 5.1–5.5 | — | — | |
| Bias in measurement of outcomes | 6.1–6.4 | — | — | |
| Bias in selection of reported result | 7.1–7.3 | — | — | |
| Overall bias | Critical | |||
| Study:McKinsey 1989. Outcome: 2‐year survival | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Not defined or reported | No | No | — |
| Severity of HIV | Not defined or reported | No | No | — |
| Comorbidities and comedications | Not reported | No | No | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No. No report of ART use. | — | ||
| Supportive therapy | No. Not described. | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Confounding domains were not controlled for. Rationale for selection of treatment regimen not described. No report of ART use, CD4 counts, or clinical condition of participants. | Critical | |
| Bias in participant selection | 2.1–2.5 | — | — | |
| Bias in classification of intervention | 3.1–3.3 | — | — | |
| Bias due to deviations from intended intervention | 4.1–4.6 | — | — | |
| Bias due to missing data | 5.1–5.5 | — | — | |
| Bias in measurement of outcomes | 6.1–6.4 | — | — | |
| Bias in selection of reported result | 7.1–7.3 | — | — | |
| Overall bias | CRITICAL | |||
| Study:McKinsey 1989. Outcome: relapse of histoplasmosis | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Not defined or reported | No | No | — |
| Severity of HIV | Not defined or reported | No | No | — |
| Comorbidities and comedications | Not reported | No | No | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No. No report of ART use. | — | ||
| Supportive therapy | No. Not reported. | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Confounding domains were not controlled for. Rationale for selection of treatment regimen not described. No report of ART use, CD4 counts or clinical condition of participants | Critical | |
| Bias in participant selection | 2.1–2.5 | — | — | |
| Bias in classification of intervention | 3.1–3.3 | — | — | |
| Bias due to deviations from intended intervention | 4.1–4.6 | — | — | |
| Bias due to missing data | 5.1–5.5 | — | — | |
| Bias in measurement of outcomes | 6.1–6.4 | — | — | |
| Bias in selection of reported result | 7.1–7.3 | — | — | |
| Overall bias | Critical | |||
| Study:ACTG084, 1992.Outcome: response to therapy (prevention of relapse) | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Participants required to have been treated successfully for confirmed disseminated histoplasmosis within 6 weeks of enrolment. | No | Unsure | — |
| Severity of HIV | CD4 | No | Yes | — |
| Comorbidities and comedications | Protocol stipulated parameters on comorbidities and comedications. | No | Probably | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No. Zidovudine use reported. | — | ||
| Supportive therapy | — | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Severity of HIV infection and ART use were not controlled for with appropriate statistical methods. | Serious | |
| Bias in participant selection | 2.1–2.5 | Selection into the study may have been related to the intervention and outcome. Start of follow‐up and start of intervention coincided for all participants. | Moderate | |
| Bias in classification of intervention | 3.1–3.3 | Intervention status was clearly defined. | Low | |
| Bias due to deviations from intended intervention | 4.1–4.6 | No reported deviations from usual practice. Management of HIV not reported. | No information | |
| Bias due to missing data | 5.1–5.5 | Outcome data available for all participants. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | Relapse was determined by clinical assessment, Histoplasma c. antigen levels in urine and serum and blood cultures at predetermined intervals. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Reported results are consistent with preregistered protocol. | Low | |
| Overall bias | Serious | |||
| Study:ACTG174, 1994. Outcome: response to therapy | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Defined with clinical and laboratory parameters including Histoplasma capsulatum antigen in blood or urine. | No | Yes | — |
| Severity of HIV | CD4 | No | Yes | — |
| Comorbidities and comedications | Comorbidities and comedications not clearly reported. Exclusions included various medications, allergies, adrenal insufficiency, and pregnancy. | No | — | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No. Number of participants on ART at baseline was reported (33%). | — | ||
| Supportive therapy | No. | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | At 3 months, protocol was revised and treatment regimen amended. Analyses were performed on participants who received the revised protocol (higher doses of FCN). Severity and management of HIV was not reported or controlled with appropriate statistical methods. | Serious | |
| Bias in participant selection | 2.1–2.5 | Selection into the maintenance arm of the study was related to the effect of the intervention in the induction phase. | Serious | |
| Bias in classification of intervention | 3.1–3.3 | Intervention was well defined based on information collected at the time of intervention. | Low | |
| Bias due to deviations from intended intervention | 4.1–4.6 | The possibility of a requirement to modify the original protocol was raised a priori. Some deviations from intervention were reported such as 3 participants who relapsed when receiving FCN 400 mg being given 800 mg prior to successful treatment with AmB; however, there was insufficient information reported on deviation from the intended intervention to make a judgement in this domain. | No Information | |
| Bias due to missing data | 5.1–5.5 | Some missing data in outcome measurement. | No information | |
| Bias in measurement of outcomes | 6.1–6.4 | Definition and proposed measurement of relapse were clearly defined a priori. Some cultures were missing or not done in the induction phase; however, 6/7 non‐responders to induction had cultures taken. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Results correspond to intended outcomes. Analysis restriction to those treated per the revised protocol was not predetermined. | Moderate | |
| Overall bias | Serious | |||
| Study:ACTG120, 1992. Outcome: relapse of histoplasmosis – maintenance phase | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Defined – severe disease excluded. | No | Yes | — |
| Severity of HIV | CD4 | No | No. Not reported after baseline of induction phase. | — |
| Comorbidities and comedications | Participants receiving concurrent treatment with drugs that interact with ITRA including rifampin were excluded. | No | No | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No. ART use not reported. Median CD4 at baseline ofinduction phase was 29 (range 2–346) cells/μL. CD4 count at baseline of maintenance phase was not reported. | A2— | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Severity of HIV; severity of PDH and comorbidities were not controlled for using appropriate statistical methodology. ART use at baseline of an earlier phase of the trial reported. | Serious | |
| Bias in participant selection | 2.1–2.5 | Those who responded to the intervention (ITRA) in the induction phase were selected for the intervention in the maintenance phase. | Serious | |
| Bias in classification of intervention | 3.1–3.3 | Participants started intervention at various doses and had reductions in dose made at variable intervals. While this is likely to have been informed by ITRA blood levels that were being monitored detailed data is not provided per participant. | Serious | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Data reported indicates that deviations from the intended intervention were not beyond that expected in usual practice. | Low | |
| Bias due to missing data | 5.1–5.5 | 12/46 participants withdrew from the study. Reasons reported for all. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | Definition and proposed measurement of relapse were clearly defined a priori. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Reported results correspond to intended outcomes. | Low | |
| Overall bias | Serious | |||
| Study:ACTG120, 1992. Outcome: relapse of histoplasmosis –induction phase | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Defined – severe disease excluded. | No | Yes | — |
| Severity of HIV | CD4 | No | No. Only baseline reported | — |
| Comorbidities and comedications | Participants receiving concurrent treatment with drugs that interact with ITRA including rifampin were excluded. | No | No. Limited data reported | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No. Report 35/59 using ART at baseline. No further data provided on HIV management. | — | ||
| Supportive therapy | Those requiring intensive supportive therapy were excluded. | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Severity of HIV; severity of PDH and comorbidities were not controlled for using appropriate statistical methodology. | Serious | |
| Bias in participant selection | 2.1–2.5 | Selection into the study may have been related to the intervention and outcome as those with less severe histoplasmosis were more likely to be selected; however, start of follow‐up and intervention appear to coincide. | Moderate | |
| Bias in classification of intervention | 3.1–3.3 | Data provided per participant for ITRA levels in non‐responders. Detailed data on ITRA levels not reported for remaining participants. ITRA levels determined dose of intervention. | Serious | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Deviations from intended intervention were consistent with usual practice. Data reported for toxicity and clinical reasons for discontinuation of intervention. | Low | |
| Bias due to missing data | 5.1–5.5 | Data were reasonably complete. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | Outcome measures were confirmed by laboratory assessments such as blood culture. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Reported results correspond to intended outcomes. | Low | |
| Overall bias | Serious | |||
| Study:ACTG120, 1992.Outcome: mortality –maintenance phase | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Defined – severe disease excluded. | No | Yes | — |
| Severity of HIV | CD4 | No | No. Not reported after baseline of induction phase. | — |
| Comorbidities and comedications | Participants receiving concurrent treatment with drugs that interact with ITRA including rifampin were excluded. | No | No | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No. ART use not reported. Median CD4 at baseline ofinduction phase was 29 (range 2–346) cells/dL. CD4 count at baseline of maintenance phase was not reported. | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Severity of HIV; severity of PDH and comorbidities were not controlled for using appropriate statistical methodology. ART use at baseline of an earlier phase of the trial reported. | Serious | |
| Bias in participant selection | 2.1–2.5 | Participants who responded to the intervention (ITRA) in the induction phase were selected for the intervention in the maintenance phase. | Serious | |
| Bias in classification of intervention | 3.1–3.3 | Participants started intervention at various doses and had reductions in dose made at variable intervals. While this is likely to have been informed by ITRA blood levels that were being monitored, detailed data were not provided per participant. | Serious | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Deviations from intended intervention were consistent with usual practice. Data reported for toxicity and clinical reasons for discontinuation of intervention. | Low | |
| Bias due to missing data | 5.1–5.5 | 12/46 participants withdrew from the study. Reasons reported for all. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | The outcome measure was unlikely to be influenced by knowledge of the intervention received. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Reported results correspond to intended outcomes. | Low | |
| Overall bias | Serious | |||
| Study:ACTG120, 1992.Outcome: mortality –induction | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Defined – severe disease excluded | No | Yes | — |
| Severity of HIV | CD4 | No | No. Only baseline data reported. | — |
| Comorbidities and comedications | Participants receiving concurrent treatment with drugs that interact with ITRA including rifampin were excluded. | No | No. Limited data reported. | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | Severity of HIV; severity of PDH and comorbidities were not controlled for using appropriate statistical methodology. | Serious | |
| Bias in participant selection | 2.1–2.5 | Selection into the study may have been related to the intervention and outcome as those with less severe histoplasmosis were more likely to be selected; however, start of follow‐up and intervention appear to coincide. | Moderate | |
| Bias in classification of intervention | 3.1–3.3 | 1 of the 2 deaths was reported to have died after 1 week of AmB. | Serious | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Data reported indicates that deviations from the intended intervention were not beyond that expected in usual practice. | Low | |
| Bias due to missing data | 5.1–5.5 | Detailed data provided for non‐responders. 1/9 lost to follow‐up. | Low | |
| Bias in measurement of outcomes | 6.1–6.4 | The outcome measure was unlikely to be influenced by knowledge of the intervention received. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Reported results correspond to intended outcomes. | Low | |
| Overall bias | Serious | |||
| Study:Melzani 2020.Outcome: incidence paradoxical and unmasking IRIS | ||||
| Confounding domains | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably? | OPTIONAL Is failure to adjust for this variable expected to favour intervention or comparator |
| Severity of PDH | Severity of IRIS defined. lAmB considered as proxy for histoplasmosis‐related IRIS severity. Severity of PDH not reported. | — | — | — |
| Severity of HIV | CD4 and HIV viral load | No | Yes | — |
| Comorbidities and comedications | TB excluded. No information reported on comorbidities. ART, antifungal, and steroid use described. 2/22 participants received steroids. | No | Yes. Taken from medical records. | — |
| Cointerventions | Is there evidence that controlling for this cointervention was unnecessary? | Is presence of this cointervention likely to favour outcomes in the intervention or comparator? | ||
| ART at time of PDH diagnosis | No | — | ||
| Supportive therapy | No. Clinical management was reported. | — | ||
| Bias domain | Signalling questions | Comments | Risk of bias judgement | |
| Bias due to confounding | 1.1–1.8 | ART was discontinued in 2/22 participants at the physician's decision; 2/22 due to patient choice. In unmasking group, 10/14 participants received lAmB and 4/14 received ITRA. Paradoxical group physicians continued ART and ITRA for 6/8. Rationale for treatment choices not reported. Appropriate statistical measures to control for confounding were not reported. ≥ 1 known important domain was not appropriately measured or controlled for. | Serious | |
| Bias in participant selection | 2.1–2.5 | Information on timelines not provided. | No information | |
| Bias in classification of intervention | 3.1–3.3 | Detailed information on type, dose, and timing of interventions not reported. | No information | |
| Bias due to deviations from intended intervention | 4.1–4.6 | Deviations are likely to be consistent with usual practice. | Low | |
| Bias due to missing data | 5.1–5.5 | Authors reported that data were missing and files were difficult to review due to poor storage conditions. Insufficient information to make an informed judgement in this domain. | No Information | |
| Bias in measurement of outcomes | 6.1–6.4 | Outcome measure was unlikely to be influenced by knowledge of the intervention. | Low | |
| Bias in selection of reported result | 7.1–7.3 | Reported results correspond to all intended outcomes. | Low | |
| Overall bias | Serious | |||
AmB: amphotericin B; ART: antiretroviral therapy; dAmB; deoxycholate amphotericin B; FCN: fluconazole; ICU: intensive care unit; IRIS: immune reconstitution inflammatory syndrome; ITRA: itraconazole; lAmB: liposomal amphotericin B; PDH: progressive disseminated histoplasmosis; RNA: ribonucleic acid; TB: tuberculosis.
Notes
Appropriate methods to control for measured confounders: stratification; regression; matching; standardization; g‐estimation; and inverse probability weighting.
Time‐varying confounding – when intervention received can change over time. The effect of interest is 'starting and adhering' (per‐protocol) NOT 'assignment to intervention' (intention to treat).
Appendix 3. Excluded studies: not eligible due to study design, but may inform PICO
Review papers (0)
We excluded reviews from our protocol, but present them in this report for completeness.
| Review | Commentary on methods | Commentary on outcome |
| Botero Aguirre 2015 | Cochrane review, robust methods. | lAmB less nephrotoxic. |
| Cano‐Torres 2019 | No protocol, registration with PROSPERO or quality assessment. | Aimed to provide estimate of frequency and mortality of histoplasmosis in people living with HIV on HAART in Latin America but heterogeneity precluded aggregated estimates. |
| Hamill 2013 | Single author, narrative drug review. No protocol, registration with PROSPERO, or quality assessment. Limited search strategy. | lAmB safer than conventional AmB with at least equivalent efficacy. |
| Hughes 2010 | Details of methodology not provided. No protocol, registration with PROSPERO, or quality assessment reported. Limited search strategy. | Azoles (except FCN) posed greatest risk of interactions with ART. There was limited evidence that risk was lower with echinocandins. Tenofovir should be used with caution with AmB with close monitoring of renal function advised. |
| Karimzadeh 2013 | 5 databases searched. Details of screening not provided. Language restriction. No protocol, registration with PROSPERO, or quality assessment reported. | Coadministration of mannitol did not show any clinically significant benefit in preventing AmB‐induced nephrotoxicity. Lipid formulations are clinically effective and safe at preventing AmB‐induced nephrotoxicity. |
| Keating 2005 | Single author drug profile. | Posaconazole was associated with 100% success rate in histoplasmosis. |
| Moen 2009 | Methodology not reported in detail. Databases searched from 1980 to 2009.No protocol, registration with PROSPERO, or quality assessment reported. | For the treatment of confirmed invasive fungal infections, liposomal AmB was more effective than AmB and remained first‐line option for empirical therapy in people with disseminated histoplasmosis. |
| Pan 2013 | 3 databases (English and Chinese) searched. Papers independently reviewed by 2 authors. No protocol or quality assessment reported. | 300 cases of histoplasmosis were reported in China from 1990 to 2011, of which 257 had PDH. Cases had a prominent geographical distribution, mainly in vicinity of Yangtze river. |
| Siberry 2013 | Guideline for the prevention and treatment of opportunistic infections in HIV‐exposed and HIV‐infected children. Specialists reviewed the literature for new information since publication of the last guidelines (2009). | AmB is preferred for initial treatment of moderately severe‐to‐severe infections. |
| Slain 2001 | Therapeutic review. Included data from in vitro and preclinical studies as well as Phase 2 and 3 clinical trials. 1 database searched. | Intravenous ITRA is less toxic alternative to AmB for people with pulmonary and extrapulmonary histoplasmosis. |
| AmB: amphotericin B; ART: antiretroviral therapy; FCN: fluconazole; HAART: highly active antiretroviral therapy; ITRA: itraconazole; lAmB: liposomal amphotericin B; PDH: progressive disseminated histoplasmosis. | ||
Case series or unclear study design (6–7)
We present a simple list of excluded case reports and case series which may inform PICO, but are not included in the main review due to their design.
| Study | Commentary |
| Armstrong 1988 | Overview of the treatment of opportunistic infections in people with AIDS. Highlights lack of evidence for maintenance regimens. |
| Assi 2006 | Case series of gastrointestinal histoplasmosis. None of the patients identified had received HAART. |
| Barlows 1996 | Case study of hypothermia following IV AmB. |
| Benson 2005 | Guidelines for treatment of OI in HIV‐infected adults and children; CDC, NIH, and HIVMA. |
| Bernard 1989 | Case series of treatment with FCN. 1 person with histoplasmosis. Urine still positive at day 75. 20 participants. 10 people living with HIV. Did not report HIV status of the person with histoplasmosis. |
| Bonifaz 2009 | Case series of PDH. Authors did not report any use of ART. |
| BSAC 1992 | Treatment recommendations from British Society for Antimicrobial Chemotherapy. |
| Caplivski 2005 | Case series. 4 participants had histoplasmosis and AIDS. |
| Carme 1993 | Case series. 14 participants with Histoplasma duboisii seen in Congo. |
| Chastain 2017 | Review update on epidemiology, diagnosis, and management of OIs in people living with HIV. |
| Del 1990 | Case series; authors do not report outcomes by treatment regimen. |
| Ferguson‐Paul 2018 | Case series of disseminated histoplasmosis in paediatric kidney transplant recipients. |
| Ferreira 2002 | Case series of participants with oral manifestations of histoplasmosis. 8/10 people living with HIV. |
| Gustafson 1985 | Case report in letter to editor of Archives of Internal Medicine detailing failure of ketoconazole as maintenance therapy. |
| Hage 2011 | Antigen clearance study. |
| Hajjeh 2001 | Case‐control study to identify risk factors for histoplasmosis among people living with HIV. |
| Harrison 1990 | Case report of 2 children. Participant 1 had AIDS and was treated successfully with AmB for induction and maintenance. Participant 2 was HIV positive and died 6 months after diagnosis. |
| Hostetler 1991 | Review of use of ITRA in treatment of systemic fungal infections. 8 participants had histoplasmosis and AIDS. Authors did not report outcomes by treatment regimen or detail ART. |
| Hung 2004 | Prospective single arm. 1 or 2 participants with histoplasmosis. No data on management. |
| Johnson 1989 | Clinical review. Comparison of case series of 64 participants with PDH and AIDS with summaries of 61 participants in published literature. |
| Johnson 1986 | Case series. Comparison of case series of 12 cases with summaries of 20 previously reported cases. |
| Johnson 1988 | Case series. 48 participants with PDH and AIDS. Concluded that because of the permanence of immunodeficiency, PDH was resistant to treatment in this population. |
| Kassamali 2012 | Case series, some of whom may have had PDH, but insufficient data reported. |
| LeMonte 2000 | MICs determined to 10 clinical isolates to investigate efficacy of combined treatment with FCN and AmB. Caution against use of FCN+AmB for treatment of histoplasmosis. |
| Lewin 1995 | Case report. |
| Machado 1991 | Case series of 6 people living with HIV with cutaneous‐mucosal involvement of histoplasmosis. |
| Majluf‐Cruz 1993 | Case series of 3 cases with haemophagocytic syndrome associated with histoplasmosis and AIDS. |
| Marianelli 2014 | Case report of IRIS in people living with HIV with histoplasmosis osteomyelitis. |
| Mashayekhi 2016 | Renal transplant recipients with histoplasmosis. |
| Mazumder 2007 | Retrospective case‐control study of people living with HIV with CD4 count < 50 cells/μL and PDH. 26 cases, 42 controls. On multivariate analysis high alkaline phosphatase and weight loss were independent predictors of PDH. |
| Moazeni 2009 | Clinical overview of OIs in people living with HIV and AIDS. |
| Murphy 2015 | Case series of 3 participants highlighting difficulties managing PDH in resource‐limited settings where lAmB and ITRA are not readily available. |
| Negroni 1990 | Case series. Provided information on outcomes by treatment regimen. |
| Negroni 1992 | Case series of 27 patients with AIDS and PDH. Treated with ITRA 200 mg or 400 mg for 6 months. 23/27 patients assessed as responders given ITRA 100 mg. Mean survival 7.8 months. |
| Neubauer 1992 | Case series of 23 patients with AIDS and PDH. 21/23 patients treated with AmB therapy; formulation not specified. |
| Oliveira 2007 | Case series of 21 patients with PDH and AIDS. |
| Pamnani 2009 | Case series of 4 patients with PDH. 2 were people living with HIV. |
| Restrepo 2007 | Case series. 6 patients. 3 were people living with HIV and PDH. |
| Reyes 2003 | Case series. 3 patients with AIDS and cutaneous manifestations of histoplasmosis. |
| Scharfstein 1997 | Cost‐effectiveness modelling for FCN used as prophylaxis for AIDS‐related systemic fungal infections. |
| Townsend 2015 | Case series of histoplasmosis‐induced haemophagocytic syndrome. |
| Vantilcke 2014 | Case series. PDH found to be the most common febrile OI in Western French Guiana. |
| Wheat 2006 | Susceptibility testing on paired isolates from patients with AIDS who failed on treatment with FCN for histoplasmosis. |
| Wheat 2007 | Guidelines for the management of people with histoplasmosis: 2007 update by the Infection Diseases Society of America. |
AmB: amphotericin B; ART: antiretroviral therapy; CDC: Centers for Disease Control and Prevention; FCN: fluconazole; HAART: highly active antiretroviral therapy; HIVMA: HIV Medicine Association; IRIS: immune reconstitution inflammatory syndrome; ITRA: itraconazole; IV: intravenous; lAmB: liposomal amphotericin B; MIC: minimal inhibitory concentration; NIH: National Institutes of Health; OI: opportunist infection; PDH: progressive disseminated histoplasmosis.
Data and analyses
Comparison 1. Liposomal amphotericin B (lAmB) versus deoxycholate amphotericin B (dAmB).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Clinical success | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.01, 2.11] |
| 1.2 Death | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.38] |
| 1.3 Safety outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Infusion‐related toxicity | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.22, 0.69] |
| 1.3.2 Nephrotoxicity | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.09, 0.67] |
| 1.3.3 Drug discontinuation | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.02, 2.38] |
Comparison 2. Early antiretroviral therapy (ART) versus deferred ART.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Immune reconstitution inflammatory syndrome | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.82] |
2.1. Analysis.

Comparison 2: Early antiretroviral therapy (ART) versus deferred ART, Outcome 1: Immune reconstitution inflammatory syndrome
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACTG‐A5164, 2009.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 282 people with AIDS‐related OIs (TB excluded), 85% male 78 African‐American; 91 Hispanic; 18 from South Africa 10 with HIV + PDH 241 ART‐naive at study entry |
|
| Interventions | Early ART (< 14 days, median 12 days) Deferred ART (≥ 28 days, median 45 days) after start of OI treatment |
|
| Outcomes |
Primary: composite: 3 ordered categories:
For ALL participants there was no statistically significant difference in the composite outcome between early and deferred ART groups. Secondary: AIDS progression/death; CD4 count at 24/48 weeks; HIV VL < 50% at 48 weeks, safety parameters including IRIS. Death/AIDS progression in ALL participants Favours early treatment Deaths in people with histoplasmosis in early ART group: 1/7 Deaths in people with histoplasmosis in deferred ART group: 0/3 Safety: IRIS; lab adverse events Grades 2–4; clinical adverse events Grades 2–4 |
|
| Age | Median 38 years | |
| Setting | USA including Puerto Rico, ZAF | |
| Disease severity | Median CD4+ T cell count 29 cells/µL | |
| Notes | NCT00055120, ACTG A5164 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by central computer using permuted blocks within Strata. Neither block size nor treatment assignments to other sites were public. |
| Allocation concealment (selection bias) | Unclear risk | No details provided in protocol or included study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Protocol stated that for arm B (deferred ART), no study‐provided drugs were to be provided initially, hence blinding of participants and personnel was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was a composite endpoint of survival and VL. Detection bias was unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Equal numbers withdrew without primary endpoint data in each study arm. Details provided. |
| Selective reporting (reporting bias) | Low risk | Reported outcomes were consistent with protocol. |
ACTG084, 1992.
| Study characteristics | ||
| Methods | Single arm trial | |
| Participants | 42 (after dAmB induction) participants enrolled; 1 excluded after enrolment as diagnosis not confirmed. | |
| Interventions | ITRA 200 mg BD for prevention of relapse (maintenance) | |
| Outcomes | Relapse (clinical evaluation) Death Follow‐up 109 weeks (range 4–134 weeks) |
|
| Age | Mean 33 (range 16–50) years | |
| Setting | USA | |
| Disease severity | Not stated | |
| Notes | 2/42 participants relapsed | |
ACTG120, 1992.
| Study characteristics | ||
| Methods | Single arm trial | |
| Participants | 59 people with PDH in induction phase 46 people in maintenance phase 96% male 65% white and non‐Hispanic people Diagnosis based on clinical findings and laboratory evidence, including stains of tissues or body fluids, positive cultures, or detection of Histoplasma capsulatum antigen in blood or urine. People receiving rifampicin therapy were excluded. Participants were allowed to receive amphotericin 1.5 mg/kg prior to induction with ITRA. |
|
| Interventions | Induction phase: ITRA 300 mg BD for 3 days then 200 mg BD for 12 weeks Maintenance phase: ITRA dose reduced to 200 mg OD if induction serum levels were at least 4 µg/mL at week 8 or 200 mg BD if blood concentrations were lower. |
|
| Outcomes | Death Response to therapy: defined as resolution of clinical signs and symptoms of histoplasmosis. Adverse events. |
|
| Age | Mean 33 (range 16–68) years | |
| Setting | USA | |
| Disease severity | People with severe disease excluded. Defined as PO2 < 60 mmHg, SBP < 90 mmHg, or CNS histoplasmosis | |
| Notes | Response to therapy: 50/59 induction phase. Of 9 failures, 2 died; 6 responded to AmB; 1 lost to follow‐up. Relapse of histoplasmosis infection: 2/46 participants at median follow‐up of 87 weeks. 1 due to poor adherence and 1 to concurrent use of rifampicin. Toxicity: 5/46 participants discontinued treatment Mortality: 24/46 (included participants who discontinued treatment before death). Median survival time from start of maintenance estimated at 79 weeks. 1‐year survival rate estimated at 73%. |
|
ACTG174, 1994.
| Study characteristics | ||
| Methods | Single arm trial | |
| Participants | 49 people with PDH according to the revised protocol | |
| Interventions | Induction: FCN 1200 mg on first day, then 600 mg OD for 8 weeks Maintenance: FCN 200 mg OD for ≥ 1 year Following revision of protocol due to high failure rate (10/20) Induction: FCN 1600 mg on first day, then 800 mg OD for 12 weeks Maintenance: 400 mg OD for 1 year |
|
| Outcomes | "Treatment response" Induction: 36/49 (73.5%) participants responded at 12 weeks; 28 of these had resolution of signs/symptoms and negative cultures; 8 had clinical response but cultures missing/not done 7/49 failed treatment: 1 died histoplasmosis and pneumocystis around day 3 Maintenance: 11/36 participants relapsed. Median time on maintenance was 30 weeks. 10/11 had blood cultures, 8/10 were positive 1/36 participants withdrew due to drug toxicity |
|
| Age | Mean 36 (range not stated) years | |
| Setting | USA | |
| Disease severity | Mild to moderate | |
| Notes | Study terminated early due to relatively high relapse rate (compared to earlier ITRA trial) | |
Baddley 2008.
| Study characteristics | ||
| Methods | Prospective cohort study | |
| Participants | 46, 43 people with PDH | |
| Interventions | ITRA (dosing not stated) (32/41 participants) dAmB (22/41 participants) lAmB (7/41 participants) |
|
| Outcomes | All‐cause mortality at 3 months postdiagnosis of histoplasmosis | |
| Age | Mean 38 (range not stated) years | |
| Setting | USA | |
| Disease severity | Mild to severe | |
| Notes | All‐cause mortality at 3 months postdiagnosis of histoplasmosis was 18/46 participants. Mortality data not reported by treatment regimen. |
|
Couppié 2004.
| Study characteristics | ||
| Methods | Prospective cohort study | |
| Participants | 82 people with PDH | |
| Interventions | ITRA 400 mg OD (60 participants) AmB 0.7 mg/kg/day (22 participants) |
|
| Outcomes | Mortality | |
| Age | Mean 38 (range 19–68) years | |
| Setting | GUF | |
| Disease severity | Mild to severe | |
| Notes | Early death: defined as death within 30 days of initiation of antifungal treatment Severe histoplasmosis: 12/15 participants died; dAmB 8/12, ITRA 4/12 Non‐severe histoplasmosis: 6/67 participants died; dAmB 2/6, ITRA 4/6 First episode histoplasmosis; histoplasmosis confirmed by ≥ 1/3 methods; HIV confirmed; on antifungal treatment for histoplasmosis; > 15 years 17% receiving ART. Severe histoplasmosis was defined as either shock that required treatment with vasopressors or respiratory failure that required mechanical ventilation. |
|
Goldman 2004.
| Study characteristics | ||
| Methods | Prospective cohort study | |
| Participants | 32 people living with HIV with documented histoplasmosis and ≥ 12 months of antifungal maintenance therapy 97% male |
|
| Interventions | Discontinuation of antifungal therapy for PDH | |
| Outcomes | Relapse after 1 year: 0/32 | |
| Age | Mean 40 (range 22–68) years | |
| Setting | USA | |
| Disease severity | Median CD4 count at baseline 289 cells/m3 | |
| Notes | Aim: to assess the safety of stopping maintenance therapy for disseminated histoplasmosis among people living with HIV after response to ART. At study entry, participants discontinued maintenance therapy for disseminated histoplasmosis. The median duration of antifungal maintenance therapy before study enrolment was 34 months. Median follow‐up 24 months |
|
Johnson 2002.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 81 people with PDH, 88% male 52% African American, 15% Hispanic |
|
| Interventions | lAmB (55 participants) dAmB (26 participants) |
|
| Outcomes |
Efficacy: "Clinical success" (defined as a maximum daily temperature < 37.8 °C for 72 hours; no increase in severity of signs, symptoms, or laboratory abnormalities attributable to histoplasmosis; and the resolution of ≥ 1 of the signs or symptoms of histoplasmosis that qualified the patient for enrolment in the trial). 73 participants evaluated for efficacy in ITT analysis Clinical success in 45/51 lAmB vs 14/22 dAmB Safety: early discontinuation 77 participants evaluated for safety in ITT analysis Early discontinuation: 1/53 (2%) lAmB vs 2/24 (8%) dAmB Nephrotoxicity: 5/53 (9%) lAmB vs 9/24 (37%) dAmB Death: 1/53 (2%) lAmB (Staphylococcus aureus) vs 3/24 (12%) dAmB (progression of histoplasmosis) |
|
| Age | Mean 33 (range 16–68) years | |
| Setting | USA | |
| Disease severity | Moderate to severe | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors reported randomization in blocks. Details of method of randomization not provided. |
| Allocation concealment (selection bias) | Low risk | Closed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors reported that participants received the intervention and comparator by intravenous infusion "in a blinded fashion". It is possible that both participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinical and mycological outcomes were predetermined. These included objective components including temperature and laboratory findings. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons reported for missing data. Proportion of data missing from each group was similar. |
| Selective reporting (reporting bias) | Low risk | No protocol cited; however, reported outcomes were consistent with trial aims. |
Luckett 2015.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | 56 people with HIV and PDH | |
| Interventions | ITRA/VORI/POSA (24 participants) AmB (32 participants) |
|
| Outcomes | Overall and histoplasmosis‐related mortality, relapse, and treatment failure | |
| Age | Mean 42 (range 26–74) years | |
| Setting | USA | |
| Disease severity | Mild to severe | |
| Notes | Death not reported by treatment regimen Triazole failure: 4/24 participants |
|
McKinsey 1989.
| Study characteristics | ||
| Methods | Single arm trial (pilot) | |
| Participants | 22 people with PDH; 17 received maintenance treatment 95% male |
|
| Interventions |
Induction: all received dAmB 0.5–1.0 mg/kg (22 participants) Initial intensive/maintenance: Group 1 dAmB 1 g (7 participants) then weekly infusions 50–80 mg to 2000 mg cumulatively then indefinite twice weekly infusions of 50–80 mg Group 2 dAmB 2g (9 participants) then weekly infusions of 80 mg dAmB 2g (1 participant) course then ketoconazole. |
|
| Outcomes |
Induction: dAmB < 1 g, 5 participants: 2 participants died before treatment; 1 died early in treatment; 2 died from other causes. dAmB > 1 g, 17 participants: 13 survived → maintenance phase dAmB; 1 survived → maintenance ketoconazole; 1 died histoplasmosis relapse; 2 died from other causes. Initial intensive/maintenance: Group 1: 6/7 survived at study end without clinical or laboratory evidence of relapse; 1/7 died of unrelated cause. Group 2: 7/9 survived at study end; 1 died of histoplasmosis relapse; 1/9 died of unrelated cause. Median follow‐up 14 months (range 2–23 months) |
|
| Age | Mean 35 (range 22–57) years | |
| Setting | USA | |
| Disease severity | Not stated | |
| Notes | Findings suggested that, although immediate treatment of histoplasmosis in people living with HIV favoured higher dose of dAmB, during the remainder of induction there appeared to be little difference between 1 g and 2 g regimens. | |
Melzani 2020.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | People living with HIV within a cohort formed from 3 major hospitals in French Guiana from 1 January 1997 to 30 September 2017. | |
| Interventions | ART initiation | |
| Outcomes | IRIS cases were classified as: 1. certain, case‐definition fulfilled; 2. probable, relevant case with ≥ 1 case‐definition criteria missing; or 3. non‐IRIS, data missing, or not sufficient to conclude. | |
| Age | Mean 40.5 (SD 7.0) years | |
| Setting | GUF | |
| Disease severity | Not stated, lAmB used as proxy indicator for severe histoplasmosis. | |
| Notes | All episodes of histoplasmosis within 6 months of ART initiation. | |
Mootsikapun 2006.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | 68 participants; 32 with PDH, 36 with penicilliosis 29 with HIV + PDH |
|
| Interventions | AmB 0.7 mg/kg/day for 30 participants with PDH, duration not specified ITRA 400 mg/day for 3 months; 200 mg/day thereafter for 27/32 participants with PDH |
|
| Outcomes | Death: 3/32 participants Relapse: 0/27 participants (median follow‐up 9.5 months) |
|
| Age | 33 (SD 7) years | |
| Setting | THA | |
| Disease severity | Not stated | |
| Notes | Outcomes not disaggregated by HIV status. | |
Myint 2014.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | 97 people with PDH 38/97 participants in discontinued group (ITRA < 1 year) (A) 59/97 participants in continued group (ITRA > 1 year) (B) |
|
| Interventions | ITRA for < 1 year ITRA for > 1 year |
|
| Outcomes | Relapse | |
| Age | Mean: 37 years in group A, 40 years in group B (range not stated) | |
| Setting | USA | |
| Disease severity | Mild to severe | |
| Notes | Relapsed: 0/38 participants in group A; 21/59 participants in group B. Relapse defined as clinical and laboratory confirmation > 3 months after initial therapy. Adherence to antifungal and ART was determined by the physicians' assessment and HIV RNA levels. Adherence: 87% in group A vs 39% in group B; P ≤ 0.0001 Follow‐up: median 49 (range 12–170) months |
|
Negroni 2017.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | 26 people with PDH who were followed up after discharge (from 80 hospitalized participants) | |
| Interventions | ITRA 200 mg OD (20 participants) dAmB twice/week (6 participants) Duration: until CD4 count > 150 cells/μL |
|
| Outcomes | Not stated | |
| Age | Mean 36 (range 18–60) years | |
| Setting | ARG | |
| Disease severity | Not stated | |
| Notes | ||
Norris 1994.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | 82 people with PDH | |
| Interventions | FCN at physician determined doses | |
| Outcomes | Relapse | |
| Age | Not stated | |
| Setting | USA | |
| Disease severity | Not stated | |
| Notes | ||
Pietrobon 2004.
| Study characteristics | ||
| Methods | Retrospective cohort study | |
| Participants | 16 people with PDH. 14 men | |
| Interventions | AmB 1 mg/kg/day up to 1 g followed by oral itraconazole 400 mg/day or FCN 200 mg/day | |
| Outcomes | Death, clinical relapse | |
| Age | Mean 28 (range 20–36) years | |
| Setting | Argentina | |
| Disease severity | Baseline CD4 count < 50 cells/µL (11 participants) | |
| Notes | ||
Ramdial 2002.
| Study characteristics | ||
| Methods | Prospective cohort study | |
| Participants | 14 people living with HIV with disseminated cutaneous histoplasmosis | |
| Interventions | ITRA 200 mg BD (4 participants) AmB 0.5–1 mg/kg/day (7 participants) |
|
| Outcomes | Death Clinical success |
|
| Age | Mean 28 (range 3–41) years | |
| Setting | ZAF | |
| Disease severity | Not stated | |
| Notes | Follow‐up: 32 months Death: 5/14; 3 died before treatment started; 1 died day 1 in ITRA group; 1 died day 2 in AmB group Clinical success: 9/14 Induction: 6/9 ITRA; 3/9 dAmB Maintenance: 7/9 ITRA; 2/9 dAmB |
|
AmB: amphotericin B; ART: antiretroviral therapy; BD: twice daily; CNS: central nervous system; dAmB: deoxycholate amphotericin B; FCN: fluconazole; IRIS: immune reconstitution inflammatory syndrome; ITRA: itraconazole; ITT: intention to treat; lAmB: liposomal amphotericin B; OD: once daily; OI: opportunist infection; PDH: progressive disseminated histoplasmosis; PO2: partial pressure of oxygen; POSA: posaconazole; RCT: randomized controlled trial; RNA: ribonucleic acid; SBP: systolic blood pressure; SD: standard deviation; TB: tuberculosis; VL: viral load; VORI: voriconazole.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Assi 2007 | Retrospective cohort study, unable to extract data to inform our objectives. |
| Borges 1997 | Retrospective cohort study, only 9 participants received antifungal therapy. |
| Boulougoura 2019 | Conference abstract reporting incidence |
| Brilhante 2012 | Retrospective cohort study, unable to extract data to inform our objectives: authors indicated high failure rate with AmB, but did not elaborate on formulation, or upon disease severity. |
| Casariego 1997 | Retrospective cohort study, unable to extract data to inform our objectives: authors stated similar efficacy for ITRA and AmB, but no numerators or denominators given. |
| Chaiwarith 2013 | Randomized controlled trial. Unable to extract data to inform our objectives. Authors reported 2 participants with histoplasmosis in secondary prophylaxis group but no details given on their medication type or duration. |
| Crabtree‐Ramírez 2016 | Prospective cohort study, unable to extract data specific to PDH to inform our objectives. |
| Cunha 2007 | Retrospective cohort study, unable to extract data to inform our objectives: authors stated that 3 participants were treated with oral ITRA, and switched to AmB due to poor response. |
| Damasceno 2013 | Retrospective cohort study. Authors did not report drug doses, course durations, or disease severity. |
| Damasceno‐Escoura 2020 | Retrospective cohort study. Reports percentages of patients treated with different approaches, but not outcomes. |
| Dismukes 1992 | Prospective trial, included only 1 participant with HIV and histoplasmosis. |
| Falci 2015 | Retrospective cohort study of haematological toxicities associated with AmB formulation. Unable to extract data specific to HIV and PDH. |
| Gerber 1995 | Retrospective cohort study. Unable to extract data to inform our objectives as authors did not report HIV subgroup outcomes. |
| Gopalakrishnan 2012 | Retrospective cohort study, only included 4 participants with PDH and HIV. Unable to extract data to inform our objectives as authors did not report HIV subgroup outcomes. |
| Gutierrez 2005 | Retrospective cohort study. Authors did not report drug doses, course durations, or disease severity. |
| Huber 2008 | Retrospective cohort study. Authors did not report outcomes by type of therapy. |
| Karimi 2002 | Retrospective cohort study. Authors did not report outcomes by drug doses, course durations, or disease severity. |
| Lopez Daneri 2016 | Retrospective cohort study. Authors did not report outcomes by drug doses, course durations, or disease severity. |
| Mata‐Essayag 2008 | Retrospective cohort study. Authors were able to collect data on treatment in only 72/158 participants. |
| McKinsey 1996 | Single arm trial of FCN for histoplasmosis. People with HIV were excluded. |
| Meng 2016 | Retrospective cohort study. Conference abstract only, unable to extract data to inform our objectives. |
| Messina 2018 | Retrospective cohort study. Authors did not report outcomes by treatment regimen. |
| Mora 2008 | Retrospective cohort study. Authors did not report outcomes by treatment regimen. |
| Nacher 2014 | Prospective cohort study. Authors did not report outcomes by treatment regimen. |
| Negroni 1997 | Retrospective cohort study. Authors did not report outcomes by treatment regimen. |
| Negroni 2004 | Retrospective cohort study reporting on discontinuation of secondary prophylaxis after restoration of CD4 count. Authors did not report treatment durations, therefore, unable to include. |
| Nightingale 1990 | Retrospective cohort study, reporting survival following a stated regimen of AmB in the pre‐ART era. Unable to extract outcomes to inform our objectives. |
| Salzman 1988 | Retrospective cohort study. Details on treatment regimens for PDH not provided. Details on HIV status not reported. Participants were described as at risk of AIDS. |
| Samayoa 2017 | Prospective cohort study. Authors did not report outcomes by treatment regimen. |
| Santos 1998 | Retrospective cohort study. Authors reported good initial response to treatment but no data provided on follow‐up. 1 person with histoplasmosis. |
| Silva 2017 | Retrospective cohort study. Authors did not report outcomes by treatment regimen. |
| Subramanian 2005 | Retrospective cohort study, included only 4 participants with HIV and PDH. |
| Thompson 2016 | Single arm prospective trial of isavuconazole. Included 4 participants with PDH. They did not appear to have HIV/AIDS. |
| Tobon 2005 | Retrospective cohort study. Authors did not report outcomes by treatment regimen. |
| Wheat 1992 | Retrospective cohort study. Outcome was surrogate marker rather than clinical response. |
| Wheat 2018 | Retrospective cohort study. Outcome data not available for HIV subgroup, or by treatment arm. Reported similar mortality between induction therapy with azoles and induction therapy with AmB formulations. |
AmB: amphotericin B; ART: antiretroviral therapy; FCN: fluconazole; ITRA: itraconazole; PDH: progressive disseminated histoplasmosis.
Characteristics of studies awaiting classification [ordered by study ID]
NCT00002159.
| Methods | Randomized open comparative multicentre study |
| Participants | People with blastomycosis or histoplasmosis |
| Interventions | Intravenous itraconazole vs amphotericin B |
| Outcomes | Not reported |
| Notes | Information sought unsuccessfully from databases, registries, citation searching, and clinical experts. |
Characteristics of ongoing studies [ordered by study ID]
Pasqualotto 2019.
| Study name | Randomized Trial of Liposomal Amphotericin B for Histoplasmosis in AIDS Patients |
| Methods | Prospective randomized non‐comparative multicenter open label trial of induction therapy with LAmB for DH in AIDS patients |
| Participants | The sample size planned is 99 patients of both sexes, older than 18 years |
| Interventions | three study arms: (i) single IV dose of 10 mg/kg of L‐AmB; (ii) single IV dose of 10 mg/kg of L‐AmB on day 1, followed by 5 mg/kg of L‐AmB on day 3; (iii) IV dose of 3 mg/kg of L‐AmB for 2 weeks. |
| Outcomes | Primary Outcome Measures
|
| Starting date | Not yet recruiting |
| Contact information | Alessandro C. Pasqualotto. acpasqualotto@hotmail.com |
| Notes | ‐ |
Differences between protocol and review
We initially prepared this review as a rapid review for a Pan American Health Organization/World Health Organization guidelines development group meeting. We registered the protocol on the PROSPERO International prospective register of systematic reviews (CRD42019126075). We used a modified risk of bias assessment in the rapid review. Following completion of the rapid review, the protocol was approved by CIDG Editors, and we performed a further iteration of the review using the methodology described under Methods.
Contributions of authors
MM and PH drafted the protocol, extracted data, and assessed risk of bias.
PH analysed results.
MM and PH drafted the final review and approved the final version.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
External sources
-
Department for International Development, UK
Project number 300342‐104
Declarations of interest
MM was previously employed by the CIDG.
PH was previously employed full‐time by the CIDG, and currently works full‐time within the UK National Health Service (NHS). He received a Registration Scholarship to attend the 23rd Annual British HIV Association Conference 2017 from ViiV Healthcare. ViiV had no involvement in the selection of recipients of the scholarship. In 2018, he attended a continuing professional development‐certified clinical research training programme organized and funded by Gilead Sciences Europe Ltd. To the best of his knowledge, neither financial nor non‐financial conflicts of interests have influenced the current submitted work.
New
References
References to studies included in this review
ACTG084, 1992 {published data only}
- NCT00000992. A study of itraconazole in preventing the return of histoplasmosis, a fungal infection, in patients with AIDS [Pilot study to determine the feasibility of itraconazole for suppression of relapse of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome]. clinicaltrials.gov/show/NCT00000992 (first received 1 August 2001).
- Wheat J, Hafner R, Wulfsohn M, Spencer P, Squires K, Powderly W, et al. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Annals of Internal Medicine 1993;118(8):610-6. [DOI] [PubMed] [Google Scholar]
ACTG120, 1992 {published data only}
- Hecht F, Korzun A, Wheat J, Hafner R. Itraconazole maintenance treatment for histoplasmosis in AIDS: prospective multi-center trial. Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy 1995;35:241. [Google Scholar]
- Hecht FM, Wheat J, Korzun AH, Hafner R, Skahan KJ, Larsen R, et al. Itraconazole maintenance treatment for histoplasmosis in AIDS: a prospective, multicenter trial. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1997;16(2):100-7. [DOI] [PubMed] [Google Scholar]
- NCT00000975. A study of itraconazole in the treatment and prevention of histoplasmosis, a fungal infection, in patients with AIDS [Pilot study to determine the feasibility of itraconazole for primary treatment and suppression of relapse of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome]. clinicaltrials.gov/show/NCT00000975 (first received 31 August 2001).
- Wheat J, Hafner R, Korzun AH, Limjoco MT, Spencer P, Larsen RA, et al. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trial Group. American Journal of Medicine 1995;98(4):336-42. [DOI] [PubMed] [Google Scholar]
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrobial Agents and Chemotherapy 2002;46(1):248-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTG174, 1994 {published data only}
- NCT00000627. Pilot study to determine the feasibility of fluconazole for induction treatment and suppression of relapse of histoplasmosis in patients with the acquired immunodeficiency syndrome. clinicaltrials.gov/show/NCT00000627 (first received 31 August 2001).
- Wheat J, MaWhinney S, Hafner R, McKinsey D, Chen D, Korzun A, et al. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Acquired Immunodeficiency Syndrome Clinical Trials Group and Mycoses Study Group. American Journal of Medicine 1997;103(3):223-32. [DOI] [PubMed] [Google Scholar]
- Wheat LJ, Connolly P, Haddad N, Le Monte A, Brizendine E, Hafner R. Antigen clearance during treatment of disseminated histoplasmosis with itraconazole versus fluconazole in patients with AIDS. Antimicrobial Agents and Chemotherapy 2002;46(1):248-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheat LJ, Connolly P, Smedema M, Brizendine E, Hafner R. Emergence of resistance to fluconazole as a cause of failure during treatment of histoplasmosis in patients with acquired immunodeficiency disease syndrome. Clinical Infectious Diseases 2001;33(11):1910-3. [DOI] [PubMed] [Google Scholar]
ACTG‐A5164, 2009 {published data only}
- Grant PM, Komarow L, Andersen J, Sereti I, Pahwa S, Lederman MM, et al. Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PloS One 2010;5(7):e11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PloS One 2009;4(5):e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baddley 2008 {published data only}
- Baddley JW, Sankara IR, Rodriquez JM, Pappas PG, Many WJ Jr. Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagnostic Microbiology and Infectious Disease 2008;62(2):151-6. [DOI] [PubMed] [Google Scholar]
Couppié 2004 {published data only}
- Couppié P, Sobesky M, Aznar C, Bichat S, Clyti E, Bissuel F, et al. Histoplasmosis and acquired immunodeficiency syndrome: a study of prognostic factors. Clinical Infectious Diseases 2004;38(1):134-8. [DOI] [PubMed] [Google Scholar]
Goldman 2004 {published data only}
- Goldman M, Zackin R, Fichtenbaum CJ, Skiest DJ, Koletar SL, Hafner R, et al. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clinical Infectious Diseases 2004;38(10):1485-9. [DOI] [PubMed] [Google Scholar]
- NCT00006316. Withdrawal of antifungal treatment for histoplasmosis in patients after improved immune response to anti-HIV drugs [Discontinuation of antifungal therapy for histoplasmosis following immunologic response to antiretroviral therapy]. clinicaltrials.gov/show/NCT00006316 (first received 31 August 2001).
Johnson 2002 {published data only}
- Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Annals of Internal Medicine 2002;137(2):105-9. [DOI] [PubMed] [Google Scholar]
- Wheat LJ, Cloud G, Johnson PC, Connolly P, Goldman M, Le Monte A, et al. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrobials Agents and Chemotherapy 2001;45(8):2354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luckett 2015 {published data only}
- Luckett K, Dummer JS, Miller G, Hester S, Thomas L. Histoplasmosis in patients with cell-mediated immunodeficiency: human immunodeficiency virus infection, organ transplantation, and tumor necrosis factor-alpha inhibition. Open Forum Infectious Diseases 2015;2(1):ofu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKinsey 1989 {published data only}
- McKinsey DS, Gupta MR, Riddler SA, Driks MR, Smith DL, Kurtin PJ. Long-term amphotericin B therapy for disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome (AIDS). Annals of Internal Medicine 1989;111(8):655-9. [DOI] [PubMed] [Google Scholar]
Melzani 2020 {unpublished data only}
- Melzani A, Reynal de Saint Michel R, Ntab B, Djossou F, Epelboin L, Nacher M, et al. Incidence and trends in immune reconstitution inflammatory syndrome associated with Histoplasma capsulatum among people living with HIV: a 20-year case series and literature review. Clinical Infectious Diseases 2020;70(4):643-52. [DOI] [PubMed] [Google Scholar]
Mootsikapun 2006 {published data only}
- Mootsikapun P, Srikulbutr S. Histoplasmosis and penicilliosis: comparison of clinical features, laboratory findings and outcome. International Journal of Infectious Diseases 2006;10(1):66-71. [DOI] [PubMed] [Google Scholar]
Myint 2014 {published data only}
- Myint T, Anderson AM, Sanchez A, Farabi A, Hage C, Baddley JW, et al. Histoplasmosis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): multicenter study of outcomes and factors associated with relapse. Medicine (Baltimore) 2014;93(1):11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negroni 2017 {published data only}
- Negroni R, Messina F, Arechavala A, Santiso G, Bianchi M. Efficacy of the treatment and secondary antifungal prophylaxis in AIDS-related histoplasmosis. Experience at the Francisco J. Muñiz Infectious Diseases Hospital in Buenos Aires. Revista Iberoamericana de Micologia 2017;34(2):94-8. [DOI] [PubMed] [Google Scholar]
Norris 1994 {published data only}
- Norris S, Wheat J, McKinsey D, Lancaster D, Katz B, Black J, et al. Prevention of relapse of histoplasmosis with fluconazole in patients with the acquired immunodeficiency syndrome. American Journal of Medicine 1994;96(6):504-8. [DOI] [PubMed] [Google Scholar]
Pietrobon 2004 {published data only}
- Pietrobon D, Negro-Marquínez L, Kilstein J, Galíndez J, Greca A, Battagliotti C. Disseminated histoplasmosis and AIDS in an Argentine hospital: clinical manifestations, diagnosis and treatment. Enfermedades Infecciosas y Microbiologia Clinica 2004;22(3):156-9. [DOI] [PubMed] [Google Scholar]
Ramdial 2002 {published data only}
- Ramdial PK, Mosam A, Dlova NC, Satar NB, Aboobaker J, Singh SM. Disseminated cutaneous histoplasmosis in patients infected with human immunodeficiency virus. Journal of Cutaneous Pathology 2002;29(4):215-25. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Assi 2007 {published data only}
- Assi MA, Sandid MS, Baddour LM, Roberts GD, Walker RC. Systemic histoplasmosis: a 15-year retrospective institutional review of 111 patients. Medicine (Baltimore) 2007;86(3):162-9. [DOI] [PubMed] [Google Scholar]
Borges 1997 {published data only}
- Borges AS, Ferreira MS, Silvestre MT, Nishioka Sde A, Rocha A. Histoplasmosis in immunodepressed patients: study of 18 cases seen in Uberlandia, MG. Revista da Sociedade Brasileira Medicina Tropical 1997;30(2):119-24. [DOI] [PubMed] [Google Scholar]
Boulougoura 2019 {published data only}
- Boulougoura A, Laidlaw E, Roby G, Mejia Y, Pau A, Sheikh V, Sereti I, Manion, M. Immune reconstitution inflammatory syndrome in patients with HIV/aids and histoplasmosis: A case series. In: Open Forum Infectious Diseases. Vol. 6. 2019:S195.
Brilhante 2012 {published data only}
- Brilhante RS, Fechine MA, Mesquita JR, Cordeiro RA, Rocha MF, Monteiro AJ, et al. Histoplasmosis in HIV-positive patients in Ceará, Brazil: clinical-laboratory aspects and in vitro antifungal susceptibility of Histoplasma capsulatum isolates. Transactions of the Royal Society of Tropical Medicine and Hygiene 2012;106(8):484-8. [DOI] [PubMed] [Google Scholar]
Casariego 1997 {published data only}
- Casariego Z, Kelly GR, Perez H, Cahn P, Guelfan L, Kaufman S, et al. Disseminated histoplasmosis with orofacial involvement in HIV-I-infected patients with AIDS: manifestations and treatment. Oral Diseases 1997;3(3):184-7. [DOI] [PubMed] [Google Scholar]
Chaiwarith 2013 {published data only}
- Chaiwarith R, Praparattanapan J, Nuntachit N, Kotarathitithum W, Supparatpinyo K. Discontinuation of primary and secondary prophylaxis for opportunistic infections in HIV-infected patients who had CD4+ cell count <200 cells/mm3 but undetectable plasma HIV-1 RNA: an open-label randomized controlled trial. AIDS Patient Care and STDs 2013;27(2):71-6. [DOI] [PubMed] [Google Scholar]
Crabtree‐Ramírez 2016 {published data only}
- Crabtree-Ramírez B, Caro-Vega Y, Shepherd BE, Grinsztejn B, Wolff M, Cortes CP, et al. Time to HAART initiation after diagnosis and treatment of opportunistic infections in patients with AIDS in Latin America. PloS One 2016;11(6):e0153921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunha 2007 {published data only}
- Cunha VS, Zampese MS, Aquino VR, Cestari TF, Goldani LZ. Mucocutaneous manifestations of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome: particular aspects in a Latin-American population. Clinical and Experimental Dermatology 2007;32(3):250-5. [DOI] [PubMed] [Google Scholar]
Damasceno 2013 {published data only}
- Damasceno LS, Ramos AN Jr, Alencar CH, Goncalves MV, Mesquita JR, Soares AT, et al. Disseminated histoplasmosis in HIV-infected patients: determinants of relapse and mortality in a north-eastern area of Brazil. Mycoses 2014;57(7):406-13. [DOI] [PubMed] [Google Scholar]
- Damasceno LS, Ramos AN, Alencar CH, Lima DT, Sidrim JJ, Goncalves MV, et al. Disseminated histoplasmosis and aids: relapse and late mortality in endemic area in north-eastern Brazil. Mycoses 2013;56(5):520-6. [DOI] [PubMed] [Google Scholar]
Damasceno‐Escoura 2020 {published data only}
- Damasceno-Escoura AH, Mora DJ, Cardeal AC, Berto-Nascimento JC, Etchebehere RM, Meneses ACO, Adad SJ, Micheletti AMR, Silva-Vergara ML. Histoplasmosis in HIV-Infected Patients: Epidemiological,Clinical and Necropsy Data from a Brazilian TeachingHospital. Mycopathologia 2020;Feb 20:[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Dismukes 1992 {published data only}
- Dismukes WE, Bradsher RW Jr, Cloud GC, Kauffman CA, Chapman SW, George RB, et al. Itraconazole therapy for blastomycosis and histoplasmosis. American Journal of Medicine 1992;93(5):489-97. [DOI] [PubMed] [Google Scholar]
Falci 2015 {published data only}
- Falci DR, Da Rosa FB, Pasqualotto AC. Hematological toxicities associated with amphotericin B formulations. Leukemia and Lymphoma 2015;56(10):2889-94. [DOI] [PubMed] [Google Scholar]
Gerber 1995 {published data only}
- Gerber ME, Rosdeutscher JD, Seiden AM, Tami TA. Histoplasmosis: the otolaryngologist's perspective. Laryngoscope 1995;105(9 Pt 1):919-23. [DOI] [PubMed] [Google Scholar]
Gopalakrishnan 2012 {published data only}
- Gopalakrishnan R, Nambi PS, Ramasubramanian V, Abdul Ghafur K, Parameswaran A. Histoplasmosis in India: truly uncommon or uncommonly recognised? Journal of the Association of Physicians of India 2012;60:25-8. [PubMed] [Google Scholar]
Gutierrez 2005 {published data only}
- Gutierrez ME, Canton A, Sosa N, Puga E, Talavera L. Disseminated histoplasmosis in patients with AIDS in Panama: a review of 104 cases. Clinical Infectious Diseases 2005;40(8):1199-202. [DOI] [PubMed] [Google Scholar]
Huber 2008 {published data only}
- Huber F, Nacher M, Aznar C, Pierre-Demar M, El Guedj M, Vaz T, et al. AIDS-related Histoplasma capsulatum var. capsulatum infection: 25 years experience of French Guiana. AIDS 2008;22(9):1047-53. [DOI] [PubMed] [Google Scholar]
Karimi 2002 {published data only}
- Karimi K, Wheat LJ, Connolly P, Cloud G, Hajjeh R, Wheat E, et al. Differences in histoplasmosis in patients with acquired immunodeficiency syndrome in the United States and Brazil. Journal of Infectious Diseases 2002;186(11):1655-60. [DOI] [PubMed] [Google Scholar]
Lopez Daneri 2016 {published data only}
- Lopez Daneri AG, Arechavala A, Iovannitti CA, Mujica MT. Disseminated histoplasmosis in patients with AIDS. Buenos Aires, 2009-2014. Medicina 2016;76(6):332-7. [PubMed] [Google Scholar]
Mata‐Essayag 2008 {published data only}
- Mata-Essayag S, Colella MT, Rosello A, Capriles CH, Landaeta ME, Salazar CP, et al. Histoplasmosis: a study of 158 cases in Venezuela, 2000-2005. Medicine (Baltimore) 2008;87(4):193-202. [DOI] [PubMed] [Google Scholar]
McKinsey 1996 {published data only}
- McKinsey DS, Kauffman CA, Pappas PG, Cloud GA, Girard WM, Sharkey PK, et al. Fluconazole therapy for histoplasmosis. Clinical Infectious Diseases 1996;23(5):996-1001. [DOI] [PubMed] [Google Scholar]
Meng 2016 {published data only}
- Meng J, Lv X. A clinical review of 34 cases with progressive disseminated histoplasmosis. Respirology 2016;Volume 21(Suppl 3):63. [Google Scholar]
Messina 2018 {published data only}
- Messina FA, Corti M, Negroni R, Arechavala A, Bianchi M, Santiso G. Histoplasmosis in AIDS patients without tegumentary manifestations. Revista Chilena de Infectologia 2018;35(5):560-5. [DOI] [PubMed] [Google Scholar]
Mora 2008 {published data only}
- Mora DJ, dos Santos CT, Silva-Vergara ML. Disseminated histoplasmosis in acquired immunodeficiency syndrome patients in Uberaba, MG, Brazil. Mycoses 2008;51(2):136-40. [DOI] [PubMed] [Google Scholar]
Nacher 2014 {published data only}
- Nacher M, Adenis A, Blanchet D, Vantilcke V, Demar M, Basurko C, et al. Risk factors for disseminated histoplasmosis in a cohort of HIV-infected patients in French Guiana. PLoS Neglected Tropical Diseases 2014;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negroni 1997 {published data only}
- Negroni R, Robles AM, Arechavala AI, Bianchi M, Helou S. Histoplasmosis related to AIDS: present status in Argentina. Prensa Medica Argentina 1997;84(7):696-700. [Google Scholar]
Negroni 2004 {published data only}
- Negroni R, Helou SH, Lopez-Daneri G, Robles AM, Arechavala AI, Bianchi MH. Interruption of antifungal secondary prophylaxis in AIDS-related histoplasmosis. Revista Iberoamericana de Micologia 2004;21(2):75-8. [PubMed] [Google Scholar]
Nightingale 1990 {published data only}
- Nightingale SD, Parks JM, Pounders SM, Burns DK, Reynolds J, Hernandez JA. Disseminated histoplasmosis in patients with AIDS. Southern Medical Journal 1990;83(6):624-30. [DOI] [PubMed] [Google Scholar]
Salzman 1988 {published data only}
- Salzman SH, Smith RL, Aranda CP. Histoplasmosis in patients at risk for the acquired immunodeficiency syndrome in a nonendemic setting. Chest 1988;93(5):916-21. [DOI] [PubMed] [Google Scholar]
Samayoa 2017 {published data only}
- Samayoa B, Roy M, Cleveland AA, Medina N, Lau-Bonilla D, Scheel CM, et al. High mortality and coinfection in a prospective cohort of human immunodeficiency virus/acquired immune deficiency syndrome patients with histoplasmosis in Guatemala. American Journal of Tropical Medicine and Hygiene 2017;97(1):42-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 1998 {published data only}
- Santos J, Palacios R, Esteve A, Garcia V, Rivero A, Marquez M. Fungemia in patients with HIV infection. Annales de Medecine Interne 1998;15(10):523-7. [PubMed] [Google Scholar]
Silva 2017 {published data only}
- Silva TC, Tremea CM, Zara AL, Mendonca AF, Godoy CS, Costa CR, et al. Prevalence and lethality among patients with histoplasmosis and AIDS in the Midwest Region of Brazil. Mycoses 2017;60(1):59-65. [DOI] [PubMed] [Google Scholar]
Subramanian 2005 {published data only}
- Subramanian S, Abraham OC, Rupali P, Zachariah A, Mathews MS, Mathai D. Disseminated histoplasmosis. Journal of the Association of Physicians of India 2005;53:185-9. [PubMed] [Google Scholar]
Thompson 2016 {published data only}
- Basilea Pharmaceutica 2014. Open-label study of isavuconazole in the treatment of patients with aspergillosis and renal impairment or of patients with invasive fungal disease caused by rare moulds, yeasts or dimorphic fungi. Available from astellasclinicalstudyresults.com/study.aspx?ID=51 (accessed prior to 3 March 2020).
- Thompson GR, Rendon A, Ribeiro dos Santos R, Queiroz-Telles F, Ostrosky-Zeichner L, Azie N, et al. Isavuconazole treatment of cryptococcosis and dimorphic mycoses. Reviews of Infectious Diseases 2016;63(3):356-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tobon 2005 {published data only}
- Tobon AM, Agudelo CA, Rosero DS, Ochoa JE, De Bedout C, Zuluaga A, et al. Disseminated histoplasmosis: a comparative study between patients with acquired immunodeficiency syndrome and non-human immunodeficiency virus-infected individuals. American Journal of Tropical Medicine and Hygiene 2005;73(3):576-82. [PubMed] [Google Scholar]
Wheat 1992 {published data only}
- Wheat LJ, Connollystringfield P, Blair R, Connolly K, Garringer T, Katz BP, et al. Effect of successful treatment with amphotericin-B on histoplasma-capsulatum variety capsulatum polysaccharide antigen levels in patients with aids and histoplasmosis. American Journal of Medicine 1992;92(2):153-60. [DOI] [PubMed] [Google Scholar]
Wheat 2018 {published data only}
- Wheat J, Myint T, Guo Y, Kemmer P, Hage C, Terry C, et al. Central nervous system histoplasmosis: multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine (Baltimore) 2018;97(13):e0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00002159 {published data only}
- NCT00002159. A randomized, open, comparative multicenter study of initial treatment with intravenous itraconazole versus amphotericin B followed by consolidation treatment with itraconazole capsules in patients with blastomycosis or histoplasmosis. clinicaltrials.gov/ct2/show/NCT00002159 (first received 31 August 2001).
References to ongoing studies
Pasqualotto 2019 {unpublished data only}
- Pasqualotto A. Randomized Trial of Liposomal Amphotericin B for Histoplasmosis in AIDS Patients. ClinicalTrials.gov Identifier: NCT04059770 .
Additional references
Adenis 2014
- Adenis A, Nacher M, Hanf M, Vantilcke V, Boukhari R, Blachet D, et al. HIV-associated histoplasmosis early mortality and incidence trends: from neglect to priority. PLoS Neglected Tropical Diseases 2014;8(8):e3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 1988
- Armstrong D. Life-threatening opportunistic fungal infection in patients with the acquired immunodeficiency syndrome. Annals of the New York Academy of Sciences 1988;544:443-50. [DOI] [PubMed] [Google Scholar]
Assi 2006
- Assi M, McKinsey DS, Driks MR, O'Connor MC, Bonacini M, Graham B, et al. Gastrointestinal histoplasmosis in the acquired immunodeficiency syndrome: report of 18 cases and literature review. Diagnostic Microbiology and Infectious Disease 2006;55(3):195-201. [DOI] [PubMed] [Google Scholar]
Baker 2019
- Baker J, Setianingrum F, Wahyuningsih R, Denning D. Mapping histoplasmosis in South East Asia – implications for diagnosis in AIDS. Emerging Microbes and Infections 2019;8(1):1139-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlows 1996
- Barlows TG 3rd, Luber AD, Jacobs RA, Guglielmo BJ. Hypothermia following the intravenous administration of amphotericin B. Clinical Infectious Diseases 1996;23(5):1187-8. [DOI] [PubMed] [Google Scholar]
Benson 2005
- Benson CA, Kaplan JE, Masur H, Pau A, Holmes KK. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. Clinical Infectious Diseases 2005;40(Suppl 3):S131-235. [PubMed] [Google Scholar]
Bernard 1989
- Bernard E, Carles M, Toussaint-Gari M, Fournier J P, Dellamonica P. Value of fluconazole in the treatment of systemic yeast infection. Pathologie et Biologie 1989;37(5 Pt 2):690-3. [PubMed] [Google Scholar]
Bonifaz 2009
- Bonifaz A, Chang P, Moreno K, Fernandez-Fernandez V, Montes de Oca G, Araiza J, et al. Disseminated cutaneous histoplasmosis in acquired immunodeficiency syndrome: report of 23 cases. Clinical and Experimental Dermatology 2009;34(4):481-6. [DOI] [PubMed] [Google Scholar]
Botero Aguirre 2015
- Botero Aguirre JP, Restrepo Hamid AM. Amphotericin B deoxycholate versus liposomal amphotericin B: effects on kidney function. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD010481] [DOI] [PMC free article] [PubMed] [Google Scholar]
BSAC 1992
- British Society for Antimicrobial Chemotherapy. Antifungal chemotherapy in patients with acquired immunodeficiency syndrome. British Society for Antimicrobial Chemotherapy Working Party. Lancet 1992;340(8820):648-51. [PubMed] [Google Scholar]
Cano‐Torres 2019
- Cano-Torres JO, Olmedo-Reneaum A, Esquivel-Sanchez JM, Camiro-Zuniga A, Perez-Carrisoza A, Madrigal-Iberri C, et al. Progressive disseminated histoplasmosis in Latin America and the Caribbean in people receiving highly active antiretroviral therapy for HIV infection: a systematic review. Medical Mycology 2019;18:18. [DOI] [PubMed] [Google Scholar]
Caplivski 2005
- Caplivski D, Salama C, Huprikar S, Bottone EJ. Disseminated histoplasmosis in five immunosuppressed patients: clinical, diagnostic, and therapeutic perspectives. Reviews in Medical Microbiology 2005;16(1):1-7. [Google Scholar]
Carme 1993
- Carme B, Hayette MP, Ngaporo AI, Ngolet A, Darozzin F, Moyikoua A, et al. African histoplasmosis due to Histoplasma duboisii (Histoplasma capsulatum var. duboisii): fourteen cases observed in Congo during 10 years (1981-1990). Journal de Mycologie Medicale 1993;3(2):67-73. [Google Scholar]
Chastain 2017
- Chastain DB, Henao-Martinez AF, Franco-Paredes C. Opportunistic invasive mycoses in AIDS: cryptococcosis, histoplasmosis, coccidiodomycosis, and talaromycosis. Current Infectious Disease Reports 2017;19(10):36. [DOI] [PubMed] [Google Scholar]
Del 1990
- Del Bianco R, Neves MA, Neves FD, Mendonca M, Prado SP, Stocco MJ, et al. Study of clinic-therapeutic pathology of 10 cases of disseminated histoplasmosis and AIDS in Sao Paulo Brazil. Sixth International Conference on AIDS; June 20-24 1990; San Francisco (CA) 1990.
Eshun‐Wilson 2018
- Eshun-Wilson I, Okwen MP, Richardson M, Bicanic T. Early versus delayed antiretroviral treatment in HIV-positive people with cryptococcal meningitis. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD009012.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferguson‐Paul 2018
- Ferguson-Paul K, Park C, Childress S, Arnold S, Ault B, Bagga B. Disseminated histoplasmosis in pediatric kidney transplant recipients – a report of six cases and review of the literature. Pediatric Transplantation 2018;22(7):e13274. [DOI] [PubMed] [Google Scholar]
Ferreira 2002
- Ferreira OG, Cardoso SV, Borges AS, Ferreira MS, Loyola AM. Oral histoplasmosis in Brazil. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2002;93(6):654-9. [DOI] [PubMed] [Google Scholar]
Gustafson 1985
- Gustafson PR, Henson A. Ketoconazole therapy for AIDS patients with disseminated histoplasmosis. Archives of Internal Medicine 1985;145(12):2272. [PubMed] [Google Scholar]
Hage 2011
- Hage CA, Kirsch EJ, Stump TE, Kauffman CA, Goldman M, Connolly P, et al. Histoplasma antigen clearance during treatment of histoplasmosis in patients with AIDS determined by a quantitative antigen enzyme immunoassay. Clinical and Vaccine Immunology 2011;18(4):661-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajjeh 2001
- Hajjeh RA, Pappas PG, Henderson H, Lancaster D, Bamberger DM, Skahan KJ, et al. Multicenter case-control study of risk factors for histoplasmosis in human immunodeficiency virus-infected persons. Clinical Infectious Diseases 2001;32(8):1215-20. [DOI] [PubMed] [Google Scholar]
Hamill 2013
- Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 2013;73(9):919-34. [DOI] [PubMed] [Google Scholar]
Harrison 1990
- Harrison LF, Hanson CG, Rosenblatt HM, Blomquist MD, Taber LH, Shearer WT. Lymphocyte function and follow-up of pediatric aids patients presenting with disseminated histoplasmosis. Journal of Allergy and Clinical Immunology 1990;85(1 Part 2):240. [Google Scholar]
Hostetler 1991
- Hostetler JS, Stevens DA. The treatment of aspergillosis, cryptococcosis and histoplasmosis in immunocompromised patients. Report of experience in the United States. Medizinische Klinik (Munich, Germany : 1983) 1991;86 Suppl 1:8-10. [PubMed] [Google Scholar]
Hughes 2010
- Hughes CA, Foisy M, Tseng A. Interactions between antifungal and antiretroviral agents. Expert Opinion on Drug Safety 2010;9(5):723-42. [DOI] [PubMed] [Google Scholar]
Hung 2004
- Hung CC, Chang SC. Impact of highly active antiretroviral therapy on incidence and management of human immunodeficiency virus-related opportunistic infections. Journal of Antimicrobial Chemotherapy 2004;54(5):849-53. [DOI] [PubMed] [Google Scholar]
Johnson 1986
- Johnson PC, Sarosi GA, Septimus EJ, Satterwhite TK. Progressive disseminated histoplasmosis in patients with the acquired immune deficiency syndrome: a report of 12 cases and a literature review. Seminars in Respiratory Infections 1986;1(1):1-8. [PubMed] [Google Scholar]
Johnson 1988
- Johnson PC, Khardori N, Najjar AF, Butt F, Mansell PW, Sarosi GA. Progressive disseminated histoplasmosis in patients with acquired immunodeficiency syndrome. American Journal of Medicine 1988;85(2):152-8. [DOI] [PubMed] [Google Scholar]
Johnson 1989
- Johnson PC, Hamill RJ, Sarosi GA. Clinical review: progressive disseminated histoplasmosis in the AIDS patient. Seminars in Respiratory Infections 1989;4(2):139-46. [PubMed] [Google Scholar]
Karimzadeh 2013
- Karimzadeh I, Khalili H, Farsaei S, Dashti-Khavidaki S, Sagheb MM. Role of diuretics and lipid formulations in the prevention of amphotericin B-induced nephrotoxicity. European Journal of Clinical Pharmacology 2013;69(7):1351-68. [DOI] [PubMed] [Google Scholar]
Kassamali 2012
- Kassamali Z, Danziger L, Glowacki R, Schwartz D. How low can you go? Use of low- and standard-dose liposomal amphotericin B for treatment of invasive fungal infections at a USA public hospital. Clinical Microbiology and Infection 2012;3:178-9. [DOI] [PubMed] [Google Scholar]
Keating 2005
- Keating GM. Posaconazole. Drugs 2005;65(11):1553-67; discussion 1568. [DOI] [PubMed] [Google Scholar]
LeMonte 2000
- LeMonte AM, Washum KE, Smedema ML, Schnizlein-Bick C, Kohler SM, Wheat LJ. Amphotericin B combined with itraconazole or fluconazole for treatment of histoplasmosis. Journal of Infectious Diseases 2000;182(2):545-50. [DOI] [PubMed] [Google Scholar]
Lewin 1995
- Lewin SR, Street AC. Disseminated histoplasmosis: successful maintenance therapy with oral fluconazole. Australian and New Zealand Journal of Medicine 1995;25(1):56. [DOI] [PubMed] [Google Scholar]
Machado 1991
- Machado AA, Coelho IC, Roselino AM, Trad ES, Figueiredo JF, Martinez R, et al. Histoplasmosis in individuals with acquired immunodeficiency syndrome (AIDS): report of six cases with cutaneous-mucosal involvement. Mycopathologia 1991;115(1):13-8. [DOI] [PubMed] [Google Scholar]
Majluf‐Cruz 1993
- Majluf-Cruz AS, Hurtado Monroy R, Souto-Meirino C, Rio Chiriboga C, Simon J. Hemophagocytic syndrome associated with histoplasmosis in the acquired immunodeficiency syndrome: description of 3 cases and review of the literature. Sangre 1993;38(1):51-5. [PubMed] [Google Scholar]
Marianelli 2014
- Marianelli LG, Frassone N, Marino M, Debes J. Immune reconstitution inflammatory syndrome as histoplasmosis osteomyelitis in South America. AIDS 2014;28(12):1848-50. [DOI] [PubMed] [Google Scholar]
Mashayekhi 2016
- Mashayekhi M, Langone A. Analysis of a large single-center cohort of renal transplant recipients with histoplasmosis. American Journal of Transplantation 2016;16 (Suppl 3):732. [Google Scholar]
Mazumder 2007
- Mazumder S, Hota B, Pulvirenti J, Muppidi U, Adeyemi O. Clinical predictors of disseminated histoplasmosis (DH) in hospitalized HIV plus patients. Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy 2007;47:308. [Google Scholar]
Moazeni 2009
- Moazeni M, Haghmorad D, Mirshafiey A. Opportunistic fungal infections in patients with HIV and AIDS. Journal of Chinese Clinical Medicine 2009;4(2):106-20. [Google Scholar]
Moen 2009
- Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs 2009;69(3):361-92. [DOI] [PubMed] [Google Scholar]
Murphy 2015
- Murphy RA, Gounder L, Manzini TC, Ramdial PK, Castilla C, Moosa MY. Challenges in the management of disseminated progressive histoplasmosis in human immunodeficiency virus-infected patients in resource-limited settings. Open Forum Infectious Diseases 2015;2(1):ofv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Negroni 1990
- Negroni R, Taborda A, Benetucci J, Da Bouza J, Macias J. Mucocutaneous manifestations of histoplasmosis in AIDS patients. Revista Argentina de Dermatologia 1990;71(2):71-8. [Google Scholar]
Negroni 1992
- Negroni R, Taborda A, Robies AM, Archevala A. Itraconazole in the treatment of histoplasmosis associated with AIDS. Mycoses 1992;35(11-12):281-7. [DOI] [PubMed] [Google Scholar]
Neubauer 1992
- Neubauer MA, Bodensteiner DC. Disseminated histoplasmosis in patients with AIDS. Southern Medical Journal 1992;85(12):1166-70. [DOI] [PubMed] [Google Scholar]
Oliveira 2007
- Oliveira FM, Fernandes SS, Severo CB, Guazzelli LS, Severo LC. Histoplasma capsulatum fungemia in patients with acquired immunodeficiency syndrome: Detection by lysis-centrifugation blood-culturing technique. Revista do Instituto de Medicina Tropical de Sao Paulo 2007;49(3):135-8. [DOI] [PubMed] [Google Scholar]
Pamnani 2009
- Pamnani R, Rajab JA, Githang'a J, Kasmani R. Disseminated histoplasmosis diagnosed on bone marrow aspirate cytology: report of four cases. East African Medical Journal 2009;86(12 Suppl):S102-5. [DOI] [PubMed] [Google Scholar]
Pan 2013
- Pan B, Chen M, Pan W, Liao W. Histoplasmosis: a new endemic fungal infection in China? Review and analysis of cases. Mycoses 2013;56(3):212-21. [DOI] [PubMed] [Google Scholar]
Popay 2006
- Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme 2006.
Restrepo 2007
- Restrepo A, Tobon A, Clark B, Graham DR, Corcoran G, Bradsher RW, et al. Salvage treatment of histoplasmosis with posaconazole. Journal of Infection 2007;54(4):319-27. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 2003
- Reyes M, Arenas LR, Pichardo P, Vick R, Torres A, Zacarias R. Cutaneous histoplasmosis and AIDS. Gaceta Medica de Mexico 2003;139(3):270-5. [PubMed] [Google Scholar]
Scharfstein 1997
- Scharfstein JA, David Paltiel A, Freedberg KA. The cost-effectiveness of fluconazole prophylaxis against primary systemic fungal infections in AIDS patients. Medical Decision Making 1997;17(4):373-81. [DOI] [PubMed] [Google Scholar]
Siberry 2013
- Siberry GK, Abzug MJ, Nachman S, Steinbach WJ, Kleiman MB, Gaur A, et al. Executive summary: guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, the Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Journal of the Pediatric Infectious Diseases Society 2013;2(4):293-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slain 2001
- Slain D, Rogers PD, Cleary JD, Chapman SW. Intravenous itraconazole. Annals of Pharmacotherapy 2001;35(6):720-9. [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tenforde 2018
- Tenforde MW, Shapiro AE, Rouse B, Jarvis JN, Li T, Eshun-Wilson I, et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database of Systematic Reviews 2018, Issue 7. [DOI: 10.1002/14651858.CD005647] [DOI] [PMC free article] [PubMed] [Google Scholar]
Townsend 2015
- Townsend JL, Shanbhag S, Hancock J, Bowman K, Nijhawan AE. Histoplasmosis-induced hemophagocytic syndrome: a case series and review of the literature. Open Forum Infectious Diseases 2015;2(2):ofv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vantilcke 2014
- Vantilcke V, Boukhari R, Jolivet A, Vautrin C, Misslin C, Adenis A, et al. Fever in hospitalized HIV-infected patients in Western French Guiana: first think histoplasmosis. International Journal of STD & AIDS 2014;25(9):656-61. [DOI] [PubMed] [Google Scholar]
Wheat 2006
- Wheat LJ, Connolly P, Smedema M, Durkin M, Brizendine E, Mann P, et al. Activity of newer triazoles against Histoplasma capsulatum from patients with AIDS who failed fluconazole. Journal of Antimicrobial Chemotherapy 2006;57(6):1235-9. [DOI] [PubMed] [Google Scholar]
Wheat 2007
- Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2007;45(7):807-25. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
CRD42019126075
- Murray M, Hine P, Garner P. Treatment regimens for progressive disseminated histoplasmosis in people living with HIV. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019126075 (accessed prior to 13 September 2019).


