Abstract
Background
According to the Global Burden of Disease Study 2015, lower respiratory tract infection is the leading cause of infectious disease death, and the fifth most common cause of death overall. Vitamin C has a role in modulating resistance to infectious agents, therefore vitamin C supplementation may be important in preventing and treating pneumonia.
Objectives
To assess the impact of vitamin C supplementation to prevent and treat pneumonia in children and adults.
Search methods
We searched CENTRAL, MEDLINE, Embase, PubMed, CINAHL, LILACS, Web of Science, and two trials registers to 4 March 2020. We also checked references to identify additional studies. We did not apply any publication status or language filters.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs (studies using allocation methods that are not random, e.g. date of birth, medical record number) assessing the role of vitamin C supplementation in the prevention and treatment of pneumonia in children and adults compared to control or placebo.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included seven studies in the review and identified two ongoing studies. The seven included studies involved a total of 2774 participants; five studies were RCTs and two were quasi‐RCTs. The included studies were conducted in high‐income countries (UK, USA and Chile) and lower‐middle‐income countries (Bangladesh and Pakistan). Four studies were conducted in hospital inpatient settings, two in schools, and one in a military training centre. Three studies included children under five years of age, two school‐aged children, one adult participants, and one older participants aged 60 to 90 years. Two studies assessed the effect of vitamin C supplementation for pneumonia prevention; four studies assessed the effect of vitamin C supplementation as an adjunct to pneumonia treatment; and one study assessed the role of vitamin C for both prevention and treatment of pneumonia. For pneumonia prevention, the included studies provided supplementation in doses of 500 mg daily for 14 weeks, 2 g daily for 8 weeks, and 2 g daily for 12 weeks. For pneumonia treatment, the included studies provided vitamin C supplementation in doses of 125 mg daily (until discharge), 200 mg for 4 weeks, and 200 mg until discharge, as an adjunct to the pneumonia treatment. We assessed the included studies as at overall either high or unclear risk of bias for random sequence generation, allocation concealment, and blinding. We judged the quality of the evidence as very low.
Three studies assessed the effect of vitamin C supplementation for pneumonia prevention; we judged the quality of the evidence as very low. We are uncertain about the effect of vitamin C supplementation on pneumonia incidence (risk ratio (RR) 0.46, 95% confidence interval (CI) 0.06 to 3.61; 2 studies, 736 participants; I² = 75%; very low‐quality evidence) and adverse events (urticaria) (RR 3.11, 95% CI 0.13 to 76.03; 1 study, 674 participants; very low‐quality evidence). No included studies reported our other primary outcomes (pneumonia prevalence and mortality) or any of our secondary outcomes.
Five studies assessed the effect of vitamin C supplementation as an adjunct to pneumonia treatment; we judged the quality of the evidence as very low. One study reported a decrease in the duration of illness in the vitamin C supplementation group (3.4 days ± 2.54) compared to the control group (4.5 days ± 2.35), and one study reported a decrease in number of days required for improvement in oxygen saturation (1.03 days ± 0.16 versus 1.14 days ± 1.0) and respiratory rate (3.61 days ± 1.50 versus 4.04 days ± 1.62) in the vitamin C supplementation group compared to the control group. We are uncertain of the effect of vitamin C supplementation on mortality due to pneumonia (RR 0.21, 95% CI 0.03 to 1.66; 1 study, 57 participants; very low‐quality evidence). One study reported that the mean duration of hospital stay was 6.75 days amongst children in the vitamin C supplementation group and 7.75 days in the control group; another study reported a lower mean duration of hospital stay in the vitamin C supplementation group compared to the control group (109.55 hours ± 27.89 versus 130.64 hours ± 41.76).
Authors' conclusions
Due to the small number of included studies and very low quality of the existing evidence, we are uncertain of the effect of vitamin C supplementation for the prevention and treatment of pneumonia. Further good‐quality studies are required to assess the role of vitamin C supplementation in the prevention and treatment of pneumonia.
Plain language summary
Vitamin C supplementation for prevention and treatment of pneumonia
Review question
What is the role of vitamin C supplementation in the prevention and treatment of pneumonia in adults and children compared to no supplementation?
Background
Pneumonia is a chest infection caused by virus, bacteria, and fungi. Vitamin C has a role in the immune system, therefore supplementation could be important in preventing and treating pneumonia amongst children and adults. We assessed the role of vitamin C for the prevention and treatment of pneumonia.
Search date
We searched for evidence up to 4 March 2020.
Study characteristics
We included nine studies, of which two were classified as ongoing studies. The seven included studies involved a total of 2774 participants and were conducted in high‐income countries (UK, USA and Chile) and lower‐middle‐income countries (Bangladesh and Pakistan). Four studies were conducted in hospital settings, two in schools, and one at a military training centre. Three studies included children under five years of age, two school‐aged children, one adult participants, and one older participants aged 60 to 90 years. Two studies assessed the effect of vitamin C supplementation for pneumonia prevention; four studies assessed the effect of vitamin C supplementation in pneumonia treatment; and one study assessed the role of vitamin C for both prevention and treatment of pneumonia. The doses of vitamin C supplementation used were 125 mg, 200 mg, 500 mg, and 2 g.
Study funding sources
Four studies were funded by pharmaceutical companies. Three studies did not report funding sources.
Key results
We assessed the rate of pneumonia (incidence), how common pneumonia is (prevalence), numbers of deaths from pneumonia (mortality), and unintended and harmful outcomes (adverse effects) associated with vitamin C for preventing pneumonia. Only two studies (736 people) reported incidence, and one study reported one adverse effect (hives) associated with vitamin C for preventing pneumonia. No study reported on prevalence or mortality. Evidence was insufficient to determine the effect of vitamin C for preventing pneumonia.
We also assessed how long people were ill (duration of illness), how many people were cured, mortality, and adverse effects associated with the use of vitamin C as a treatment for pneumonia. Although two studies reported duration of illness, results could not be combined for analysis. One study reported mortality. No studies reported cure rates or adverse effects. Evidence was insufficient to determine the effect of vitamin C for treating pneumonia.
Quality of the evidence
We judged the included studies to be at overall high or unclear risk of bias. We rated the quality of the evidence as very low due to study limitations, variations amongst the studies, small sample sizes and uncertainty of estimates.
Summary of findings
Background
Description of the condition
Pneumonia is a lower respiratory tract infection characterised by cough, sputum, difficulty in breathing, sharp chest pain during deep breaths, fever, and lung inflammation (WHO 2014). Adults aged 65 years and over, and children aged up to two years, are at high risk of developing pneumonia. According to the Global Burden of Disease Study 2015, lower respiratory tract infection is the leading cause of infectious disease death, and the fifth most common cause of death overall (Troeger 2017). Approximately 2.74 million deaths and 103 million disability‐adjusted life‐years were attributed to lower respiratory tract infections in 2015. There was a disproportionate effect on children aged under five years, and 704,000 deaths occurred in this age group (Troeger 2017). Globally, disease burden has dramatically decreased over the last decade amongst children aged under five years, but in many regions disease burden has increased amongst people aged over 70 years (Whitney 2017).
Pneumonia is caused by viruses, bacteria, and fungi. Streptococcus pneumoniae and Haemophilus influenzae are the most common pathogens responsible for pneumonia in all age groups (Abubakar 2015). Pneumonia can be community acquired (occurs outside the hospital setting) or hospital acquired (occurs during hospital stay).
Pneumonia treatment guidelines recommend therapy according to pneumonia severity. Mild and moderate pneumonia can be treated with appropriate antibiotics and supportive care, including oxygen; severe pneumonia requires hospital treatment (Lim 2009; WHO 2014). In 2009, a Global Action Plan for the Prevention and Control of Pneumonia suggested integrated strategies for preventing and treating childhood pneumonia (WHO/UNICEF 2009). These included improved nutrition, immunisation, healthy environments, and increasing access to appropriate management. Strategies recommended for reducing pneumonia incidence in children include exclusive breastfeeding for the first six months, adequate complementary feeding, micronutrient intake, H influenzae type B (Hib) vaccination, pneumococcal conjugate vaccination, and controlling household air pollution (Niessen 2009; Theodoratou 2010; Webster 2011; WHO/UNICEF 2013). Although pneumonia is largely preventable and treatable, it remains a major cause of death.
Description of the intervention
Vitamin C is an essential nutrient that cannot be synthesised by the body and plays an important role in the body's immune‐modulating system. Vitamin C donates electrons that protect the body from oxidant damage generated through exposure to toxins and pollutants (Carr 1999; Figueroa‐Méndez 2015). Vitamin C works as a co‐factor for several enzymes involved in the biosynthesis of L‐carnitine, collagen, and neurotransmitters (Himmelreich 1998). Vitamin C stimulates non‐heme iron absorption from the intestine and modulates iron transport and storage, and consequently prevents anaemia (Iannotti 2006).
The recommended dietary intake of vitamin C is 90 mg/day for men, 75 mg/day for women, and 15 mg/day to 75 mg/day for children (aged 1 to 18 years) (IoM 2000). Serum concentration of vitamin C less than 11 µmol/L (or < 2 µg/mL) indicates deficiency, and 11 to 28 µmol/L (or 2 to 5 µg/mL) indicates depletion (Johnston 1998; Loria 2000). Although global epidemiological data on vitamin C deficiency are scarce, geographic‐specific epidemiological studies suggest that 7% of the USA population is vitamin C deficient (Schleicher 2009). In India, Malaysia, and China, between 14% and 17% of men and 0.7% and 11% of women are vitamin C deficient (Hughes 1998). Rates are higher in Mexico, where 23% of children and 39% of women are vitamin C deficient (Villalpando 2003).
Vitamin C supplementation has been evaluated for preventing and treating respiratory infections. A review assessing the impact of vitamin C on the incidence, duration, and severity of the common cold suggested no effect on the incidence of cold (Douglas 2005). Older studies in population subgroups have reported some positive effects of vitamin C supplementation on pneumonia, suggesting improved respiratory symptoms amongst hospitalised elderly patients (Hunt 1994), and reduced length of hospital stay (Mochalkin 1970).
How the intervention might work
Vitamin C is mostly present in the epithelial lining of the respiratory tract, where it functions as an immune‐stimulating agent, helping ameliorate symptoms of upper respiratory tract infections (Maggini 2017). Viral and bacterial infections can potentially decrease vitamin C levels because they generate reactive oxygen and nitrogen species through leukocyte activation that lead to oxidisation of extracellular vitamin C (Akaike 2001). Changes in vitamin C metabolism due to respiratory infections suggest that vitamin C may have a beneficial effect for people with pneumonia (Hemilä 2017).
Vitamin C antioxidant function limits damage from free radicals (oxygen and nitrogen) produced during normal cell metabolism and immune activation of neutrophils in response to bacteria, virus, and toxins (Carr 2017). Vitamin C stimulates neutrophil migration to the infection site in response to chemo‐attractants, enhances phagocytosis and oxidant generation, ultimately killing pathogens (Carr 2017). Phagocytes transfer oxidised vitamin C to cells, where it is converted by reactive oxygen species, altering the chain production of free radicals and preventing the host from cellular damage by products of lipid peroxidation (Nualart 2003). Production of reactive oxygen species during immune response of neutrophils by nicotinamide adenine dinucleotide phosphate helps to kill pathogens (Carr 2017; Winterbourn 2016). The products of lipid peroxidation produced by reactive oxygen species generate a chain reaction of free radicals by altering the structure and function of proteins, carbohydrates, and nucleic acid, which results in oxidative stress. Lipid peroxidation also decreases the immune response of lymphocytes by decreasing membrane fluidity (Ayala 2014). Vitamin C contributes to maintaining the redox integrity of cells and protects against reactive oxygen species (Hemilä 2017).
Vitamin C may have the potential to prevent and treat infections, but the impact of supplementation may differ according to baseline vitamin C deficiency status and other effect modifiers including other micronutrient deficiencies (Blumberg 2018; Smith 2017). The doses of supplementation might also have a potential effect and might differ for prevention and treatment. Prophylactic administration would require vitamin C intake that provides at least adequate intake levels in order to optimise cell and tissue levels. In contrast, treatment would require significantly higher doses to compensate for the increased metabolic demand (Carr 2017).
Why it is important to do this review
Vitamin C has a role in modulating resistance to an infectious agent, hence vitamin C deficiency may have a profound effect on the immune system and may increase the risk of respiratory infections. Vitamin C supplementation may therefore be important in preventing and treating pneumonia. This review evaluates the available literature to determine the role of vitamin C to prevent and treat pneumonia in children and adults.
Objectives
To assess the impact of vitamin C supplementation to prevent and treat pneumonia in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs evaluating the following:
role of vitamin C supplementation for the prevention of pneumonia; and
role of vitamin C supplementation as an adjunct to the treatment of pneumonia.
We included studies reported as full text, abstract only, and unpublished data.
Types of participants
We included studies involving:
healthy adults and children receiving vitamin C supplementation for the prevention of pneumonia; and
adults and children with confirmed pneumonia (as defined by study authors) receiving vitamin C supplementation as an adjunct to the treatment of pneumonia.
We excluded studies involving participants with immune suppression or with a primary diagnosis of meningitis, asthma, sickle cell anaemia, HIV/AIDS, and severe malnutrition. We excluded studies involving children whose births were preterm or whose birthweight was low. We also excluded studies involving participants with ventilator‐associated pneumonia or hospital‐acquired pneumonia.
Types of interventions
We included studies evaluating the impact of vitamin C supplementation via any route (oral or intravenous), frequency, dose, and duration given:
to prevent pneumonia compared to control or placebo; or
as an adjuvant to other treatment modalities for the treatment of pneumonia, compared to control or placebo.
We included interventional studies where the difference between control and intervention groups was vitamin C supplementation alone. We did not apply any restriction for route of administration, dose, duration, or frequency of vitamin C supplementation. We excluded studies evaluating the impact of food fortified with vitamin C.
Types of outcome measures
We did not consider evaluation of the outcomes listed below as criteria for inclusion in the review.
We included both incidence and prevalence as outcomes in pneumonia prevention because we anticipated that there might be potentially eligible studies that presented prevalence data at individual or population level rather than incidence, due to the unavailability of surveillance system.
Primary outcomes
Pneumonia prevention
Incidence of pneumonia (the incidence of pneumonia refers to the number of new cases occurring in a given population).
Prevalence of pneumonia (prevalence is defined as the number of people with pneumonia at a specified time divided by the population at risk at the specified time).
Mortality due to pneumonia.
Adverse effects.
Pneumonia treatment
Duration of illness.
Clinical cure rate (defined as clinical recovery by the end of treatment as defined by the study authors).
Mortality due to pneumonia.
Adverse effects.
Secondary outcomes
Pneumonia prevention
Hospital admission rate.
Cost‐effectiveness (as reported by the study authors).
Pneumonia treatment
Hospital admission rate.
Duration of hospitalisation (defined as the duration (in days) of total hospital stay from day of admission to discharge).
Relapse rate (defined as those declared clinically cured, but who experience pneumonia recurrence at follow‐up in a defined time period in each study).
Cost‐effectiveness (as reported by the study authors).
Search methods for identification of studies
Electronic searches
We identified trials from searches of the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 2) in the Cochrane Library, which includes the Cochrane Acute Respiratory Infections Group Trials Register (searched 4 March 2020);
MEDLINE Ovid (1946 to 4 March 2020);
Embase Elsevier (1974 to 4 March 2020);
PubMed National Library of Medicine (1946 to 4 March 2020);
LILACS (Latin American and Caribbean Health Sciences Literature) BIREME (1982 to 4 March 2020);
CINAHL (Cumulative Index of Nursing and Allied Health Literature) EBSCO (1937 to 4 March 2020); and
Web of Science (Clarivate Analytics) (1970 to 4 March 2020).
We also conducted searches of the USA National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov) (4 March 2020) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en) (4 March 2020). The detailed search strategy for all databases is shown in Appendix 1.
We did not impose any restrictions on language or publication status.
Searching other resources
We also checked reference lists of primary studies and reviews for additional references. We contacted study authors for information that was missing from the included studies.
Data collection and analysis
We conducted separate analyses to assess the impact of preventive and therapeutic supplementation of vitamin C and for child and adult populations.
Selection of studies
Four review authors (ZAP, ZM, AA, HB) independently screened titles and abstracts of all records identified as a result of the search strategy for potential inclusion in the review. We obtained the full texts of records deemed potentially relevant, and the review authors assessed each full text against the eligibility criteria. Any conflicts or disagreements were resolved through discussion with another review author (JKD). The excluded studies along with the reasons for their exclusion are provided in the Characteristics of excluded studies table. Decisions made during the screening process are recorded in the PRISMA flow diagram (see Figure 1) (Moher 2009).
1.
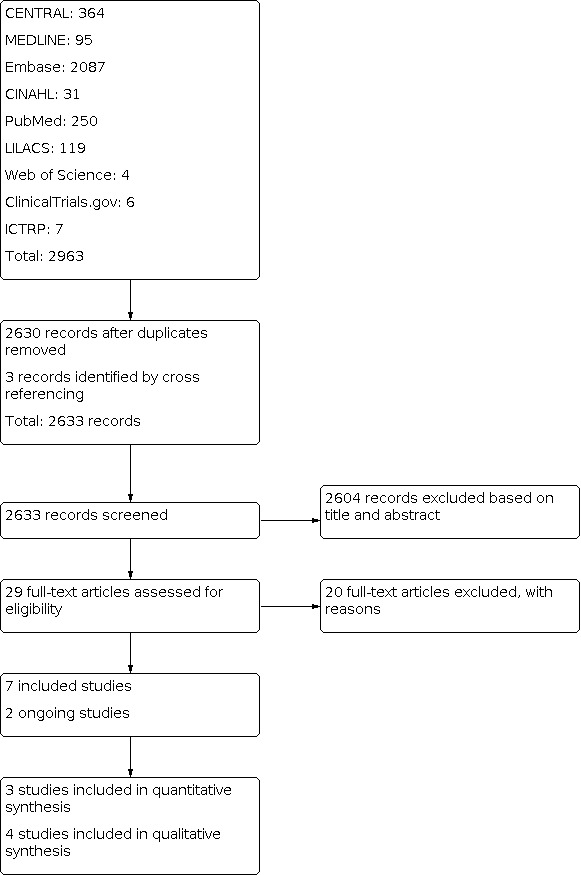
PRISMA flow diagram
Data extraction and management
We designed a data extraction form for data collection. Four review authors (ZAP, ZM, AA, HB) extracted the data using the agreed‐upon form. Any discrepancies were resolved through consensus or by consulting another review author (JKD or RAS) if required.
We entered data into Review Manager 5 (Review Manager 2014); a second review author (RAS) checked the data entry for accuracy against the trial report.
Assessment of risk of bias in included studies
Four review authors (ZAP, ZM, AA, HB) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We graded each potential source of bias as high, low, or unclear, and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table in the Characteristics of included studies. We summarised the 'Risk of bias' judgements across different studies for each of the listed domains. Any disagreements were resolved by discussion, and tables cross‐checked by another review author (RAS). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
Whilst assessing the treatment effects, we also considered risk of bias for the studies that contributed to that outcome. We also reported the source of funding to assess for potential bias related to funding.
Measures of treatment effect
We entered outcome data for each study into data tables in Review Manager 5 to calculate treatment effects (Review Manager 2014). We used risk ratio (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes with 95% confidence intervals (CIs). For trials that measured the same outcome but used different units of measurement, we used the standardised mean difference (SMD) to combine results.
We undertook meta‐analyses only where this was meaningful, that is if the treatments, participants, and the underlying clinical question were sufficiently similar for pooling to generate meaningful conclusions.
Unit of analysis issues
We conducted separate meta‐analyses for different study designs and for outcome subcategories. We planned to include cluster‐randomised trials, cross‐over trials, and individually randomised trials in the analyses. None of the included trials were cluster‐randomised or cross‐over trials.
Dealing with missing data
We contacted study authors to obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We assessed heterogeneity amongst studies in two ways: firstly by assessing heterogeneity at face value: heterogeneity in population, interventions, or outcomes; and secondly by using the Chi² test (P ≤ 0.10 was considered to be consistent with statistical heterogeneity) and the I² statistic to assess the presence of statistical heterogeneity (> 50% was considered to be substantial heterogeneity; > 75% was considerable heterogeneity) (Higgins 2011).
Assessment of reporting biases
We planned to assess reporting bias by constructing funnel plots. However, we did not pool more than 10 studies for a given outcome, therefore assessing reporting bias was not possible. For future updates, we plan to create and examine funnel plots to explore possible small‐study and publication biases for outcomes with more than 10 studies.
Data synthesis
We pooled data from studies judged to be clinically homogeneous using Review Manager 5 (Review Manager 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, that is where trials examined the same intervention, and the trials’ populations and methods were judged to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if statistical heterogeneity was detected (I² > 50%), we would use random‐effects meta‐analysis to produce an overall summary. The random‐effects summary was treated as the average of the range of possible treatment effects, and we have discussed the implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine the trials. We presented the findings narratively where it was not possible to extract and pool data in Review Manager 5 or as an Additional table.
GRADE and 'Summary of findings' table
We created two 'Summary of findings' tables to report the primary outcomes for prevention and treatment of pneumonia (Table 1; Table 2). For prevention, we reported the incidence and prevalence of pneumonia, mortality due to pneumonia, and adverse events. For treatment, we reported the duration of pneumonia, clinical cure rate, mortality due to pneumonia, and adverse events.
Summary of findings 1. Vitamin C compared to placebo for prevention of pneumonia.
| Vitamin C compared to placebo for prevention of pneumonia | ||||||
|
Patient or population: school‐aged children (10 to 15 years) and marine corps recruits Settings: Chile, USA, UK Intervention: vitamin C supplementation Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin C | |||||
|
Incidence of pneumonia Defined as patient admitted to hospital with a diagnosis of pneumonia Dose: 2 g per day Follow‐up at 8 and 12 weeks |
75 per 1000 | 61 per 1000 | RR 0.46 (0.06 to 3.61) |
736 (2 studies) |
⊕⊝⊝⊝ very low a, b, c, d | ‐ |
| Prevalance of pneumonia | ‐ | ‐ | ‐ | ‐ | ‐ | No included studies reported this outcome. |
| Mortality due to pneumonia | ‐ | ‐ | ‐ | ‐ | ‐ | No included studies reported this outcome. |
|
Adverse effects (urticaria) Defined as participants reporting urticaria (hives) Dose: 2 g per day Follow‐up at 8 weeks |
0 per 1000 | 3 per 1000 | RR 3.11 (0.13 to 76.03) |
674 (1 study) |
⊕⊝⊝⊝ very lowa, c, d | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: Any estimate of effect is very uncertain | ||||||
aDowngraded by one level due to study limitations (unclear sequence generation and allocation concealment and high risk of attrition bias). bDowngraded by one level due to high heterogeneity (I² = 75%). cDowngraded by one level due to small sample size. dDowngraded by one level due to imprecision (wide CI).
Summary of findings 2. Vitamin C compared to placebo for treatment of pneumonia.
| Vitamin C compared to placebo for treatment of pneumonia | ||||||
|
Patient or population: children under 5 years of age and adults Settings: UK and Pakistan Intervention: vitamin C supplementation Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin C | |||||
|
Duration of illness Defined as number of days until illness improved Dose: 200 mg daily and 2 g daily Follow‐up: 12 weeks |
Mean duration of illness was 4.5 days (± 2.35). | Mean duration of illness was 3.4 days (± 2.54). | Not pooled | 130 (1 study) | ⊕⊝⊝⊝ very lowa, b, c | |
| Mean number of days for improved oxygen saturation was 1.14 days (± 1.0). | Mean number of days for improved oxygen saturation was 1.03 days (± 0.16). | Not pooled | 222 (1 study) | |||
| Mean number of days for improved respiratory rate was 4.04 days (± 1.62). | Mean number of days for improved respiratory rate was 3.61 days (± 1.50). | Not pooled | 222 (1 study) | |||
| Clinical cure rate | ‐ | ‐ | ‐ | ‐ | ‐ | No included studies reported this outcome. |
|
Mortality due to pneumonia Defined as deaths due to pneumonia Dose: 200 mg Follow‐up: at 4 weeks |
72 per 1000 | 36 per 1000 | RR 0.21 (0.03 to 1.66) |
57 (1 study) | ⊕⊝⊝⊝ very lowa, b, c | |
| Adverse effects | ‐ | ‐ | ‐ | ‐ | ‐ | No included studies reported this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: Any estimate of effect is very uncertain | ||||||
aDowngraded by one level due to study limitations (high/unclear risk of bias for sequence generation and allocation concealment and unclear risk of bias for blinding). bDowngraded by one level due to small sample size. cDowngraded by one level due to imprecision (wide CI).
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2015). We also justified all decisions to downgrade the quality of studies using footnotes, and made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses by age group:
age: children younger than 5 years old; children and adolescents (aged 5 to 18 years); and adults (aged 18 years and over);
type of antibiotics used;
different doses and duration of vitamin C supplementation (as specified by the study authors);
inpatient versus outpatient treatment;
settings (community versus institutional settings); and
baseline vitamin status (deficient versus sufficient).
We also planned to use the Chi² test to test for subgroup interactions in Review Manager 5 (Review Manager 2014).
Sensitivity analysis
We planned to conduct sensitivity analysis to assess the impact of high risk of bias on the outcome by restricting the meta‐analysis to studies at low risk of bias and assessing if the conclusions were affected.
Results
Description of studies
For study details, see Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The searches from databases and other resources retrieved 2633 records after removal of duplicates. We excluded 2604 records based on title and abstract screening. We obtained the full text of the remaining 29 records and excluded 20 studies (see Characteristics of excluded studies table) after full‐text screening. We included seven studies and identified two ongoing studies (ACTRN12619000256178; NCT04264533).
Included studies
We included seven studies with a total of 2774 participants (Figure 1). Five studies were RCTs (Bancalari 1984; Coulehan 1974; Hunt 1994; Pitt 1979; Yaqub 2015), and two were quasi‐RCTs (Khan 2014; Wahed 2008).
Setting
The included studies were conducted in high‐income countries (UK, USA and Chile) and lower‐middle‐income countries (Bangladesh and Pakistan): USA (Coulehan 1974; Pitt 1979), UK (Hunt 1994), Chile (Bancalari 1984), Bangladesh (Wahed 2008), and Pakistan (Khan 2014; Yaqub 2015).
Four studies were conducted in inpatient hospital settings (Hunt 1994; Khan 2014; Wahed 2008; Yaqub 2015), two in school settings (Bancalari 1984; Coulehan 1974), and one at a military training centre (Pitt 1979).
Participants
The included studies involved participants of differing age groups. Three studies included children aged under five years (Khan 2014; Wahed 2008; Yaqub 2015); two studies included school‐aged children (10 to 15 years) (Bancalari 1984; Coulehan 1974); one study included adult participants (mean age 18.5 years) (Pitt 1979); and one study included older participants aged 60 to 90 years (Hunt 1994).
Intervention
Two studies assessed the effect of vitamin C supplementation for pneumonia prevention (Coulehan 1974; Pitt 1979); four studies assessed the effect of vitamin C supplementation as an adjunct to pneumonia treatment (Hunt 1994; Khan 2014; Wahed 2008; Yaqub 2015); and one study assessed the role of vitamin C for both prevention and treatment of pneumonia (Bancalari 1984).
Doses of vitamin C supplementation used were 125 mg (Wahed 2008), 200 mg (Hunt 1994; Khan 2014; Yaqub 2015), 500 mg (Coulehan 1974), and 2 g (Bancalari 1984; Pitt 1979).
Outcomes
Coulehan 1974 did not report on any of our prespecified primary or secondary outcomes.
Pneumonia prevention
The included studies reported two of our primary outcomes for pneumonia prevention: incidence of pneumonia and adverse effects. None of the included studies reported any of our secondary outcomes.
Pneumonia treatment
The included studies reported three of our primary outcomes for pneumonia treatment: duration of illness, clinical cure rate, and mortality due to pneumonia. Of our secondary outcomes, duration of hospitalisation was reported.
Support and sponsorship
Only four of the seven included studies reported details on support and sponsorship. Pitt 1979 was supported in part by the Naval Medical Research and Development Command Project M‐4305, Work Unit 5021. Vitamin C supplements were funded by pharmaceutical companies including La Roche, Coulehan 1974; Hunt 1994, and Merck (Bancalari 1984; Pitt 1979). Three included studies did not report study funding sources (Khan 2014; Wahed 2008; Yaqub 2015).
Excluded studies
We excluded 20 studies after full‐text screening (Alshami 2018; Arabi 2020; Beinert 2000; Carr 2017; Ceccato 2018; Glazebrook 1942; Hemilä 1997; Hemilä 2003; Hemilä 2007; Hemilä 2008; Hemilä 2011; Hunt 1984; Jain 2002; Kim 2018; Klenner 1948; Mahalanabis 2006a; Mahalanabis 2006b; NCT02186158; Ogal 2019; Socci 2012). The most common reason for exclusion was ineligible study design (n = 14) (Alshami 2018; Arabi 2020; Beinert 2000; Carr 2017; Ceccato 2018; Glazebrook 1942; Hemilä 1997; Hemilä 2003; Hemilä 2007; Hemilä 2008; Hemilä 2011; Kim 2018; Klenner 1948; Mahalanabis 2006b). Two studies were conducted in ineligible populations (Hunt 1984; NCT02186158), and four study interventions were not relevant to this review (Jain 2002; Mahalanabis 2006a; Ogal 2019; Socci 2012). See Characteristics of excluded studies table.
Ongoing studies
We identified two ongoing studies (ACTRN12619000256178; NCT04264533). For details see Characteristics of ongoing studies.
ACTRN12619000256178 is an individually randomised controlled trial conducted in New Zealand in people aged over 17 years diagnosed with community‐acquired pneumonia. The intervention in this study is intravenous infusion of 2.5 g of vitamin C every eight hours to be started as soon as possible, but not later than 72 hours after hospital admission, and within 24 hours of documentation of community‐acquired pneumonia. Intravenous vitamin C will be provided whilst the participant is receiving intravenous antimicrobial therapy, and will continue until the treatment is changed to oral antimicrobial therapy, or for a maximum of seven days. The study outcomes include all‐cause mortality in hospitalised patients with moderate/severe community‐acquired pneumonia, admission to intensive care unit (ICU), days until death, hospital mortality, length of hospital stay, quality of life, rate of recruitment of participants hospitalised with moderate/severe community‐acquired pneumonia, readmission to hospital, and resolution of symptoms.
NCT04264533 is an individually randomised controlled trial conducted in China in people diagnosed with severe 2019 novel coronavirus (2019‐nCoV) infected pneumonia. The intervention will be 24 g vitamin C with water for injection through an infusion pump for seven days compared to only water for injection. The study outcomes include ventilation‐free days, 28‐day mortality, ICU length of stay, demand for first aid measurements, vasopressor days respiratory indexes, ventilator parameters, Acute Physiology and Chronic Health Evaluation (APACHE II) scores, and Sequential Organ Failure Assessment (SOFA) scores.
Risk of bias in included studies
'Risk of bias' assessments for the included studies are summarised in Figure 2and Figure 3. We judged the included studies as overall at either high or unclear risk of bias for random sequence generation, allocation concealment, and blinding. See Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
We assessed two studies to be at low risk of bias for sequence generation (Pitt 1979; Yaqub 2015). Pitt 1979 randomly assigned participants to groups using a list of numbers in random pairs, and Yaqub 2015 employed a lottery method for randomisation. We judged three studies to be at high risk of bias for sequence generation. Coulehan 1974 and Wahed 2008 used alternative assignment techniques, and Khan 2014 did not randomise participants. We assessed two studies to be at unclear risk of bias due to insufficient information regarding the methods used for sequence generation (Bancalari 1984; Hunt 1994).
Allocation concealment
We judged two studies to be at high risk for allocation concealment due to inadequate methods to conceal allocation (Khan 2014; Wahed 2008). We assessed five studies to be at unclear risk of bias due to insufficient information regarding allocation concealment (Bancalari 1984; Coulehan 1974; Hunt 1994; Pitt 1979; Yaqub 2015).
Blinding
Blinding of participants and personnel
We judged five studies that reported applying adequate blinding techniques to be at low risk of bias for blinding of participants and personnel (Bancalari 1984; Coulehan 1974; Hunt 1994; Khan 2014; Pitt 1979). Wahed 2008 did not blind participants or personnel and was judged to be at high risk of bias for this domain. Yaqub 2015 was judged to be at unclear risk of bias due to insufficient information regarding blinding of participants and personnel.
Blinding of outcome assessors
We judged three studies that adequately blinded outcome assessors to be at low risk of bias for this domain (Bancalari 1984; Coulehan 1974; Pitt 1979). Wahed 2008 did not blind outcome assessors and was rated as at high risk of bias. Three studies did not provide sufficient data to permit judgement and were assessed as at unclear risk of bias (Hunt 1994; Khan 2014; Yaqub 2015).
Incomplete outcome data
We judged five studies to be at low risk for attrition bias as there were no reported losses to follow‐up (Bancalari 1984; Coulehan 1974; Hunt 1994; Khan 2014; Yaqub 2015). We assessed two studies as at high risk of attrition bias: Pitt 1979 reported 21.6% loss to follow‐up, and Wahed 2008 reported 30% loss to follow‐up.
Selective reporting
None of the studies provided any trial registration information or published protocols, therefore they were assessed as at unclear risk of reporting bias (Bancalari 1984; Coulehan 1974; Hunt 1994; Khan 2014; Pitt 1979; Wahed 2008; Yaqub 2015).
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
Comparison 1: vitamin C for pneumonia prevention
Two studies assessed the effect of vitamin C supplementation for pneumonia prevention (Coulehan 1974; Pitt 1979), and one study assessed the role of vitamin C for both the prevention and treatment of pneumonia (Bancalari 1984). Coulehan 1974 did not report on any of our prespecified primary or secondary outcomes. Bancalari 1984 and Coulehan 1974 were conducted amongst school‐aged children (10 to 15 years), whilst Pitt 1979 was conducted amongst adult participants (mean age 18.5 years). The dose of supplementation was 500 mg in Coulehan 1974 and 2 g in Bancalari 1984 and Pitt 1979. See Table 1. We could not conduct any of the planned subgroup and sensitivity analyses for this comparison due to the limited number of studies.
Primary outcomes
1. Incidence of pneumonia
Two studies reported incidence of pneumonia (Bancalari 1984; Pitt 1979). We are uncertain of the effect of vitamin C supplementation on pneumonia incidence (risk ratio (RR) 0.46, 95% confidence interval (CI) 0.06 to 3.61; 2 studies; 736 participants; I² = 75%; very low‐quality evidence; Analysis 1.1; Figure 4). We downgraded the evidence due to study limitations, heterogeneity, small sample size, and imprecision.
1.1. Analysis.

Comparison 1: Vitamin C for pneumonia prevention, Outcome 1: Incidence of pneumonia
4.

Forest plot of comparison: 2 Vitamin C for prevention of pneumonia versus placebo, outcome: 2.1 Incidence of pneumonia.
2. Prevalence of pneumonia
No included studies reported on this outcome.
3. Mortality due to pneumonia
No included studies reported on this outcome.
4. Adverse effects
Only Pitt 1979 reported urticaria as an adverse event. We are uncertain of the effect of vitamin C supplementation on adverse events (urticaria) (RR 3.11, 95% CI 0.13 to 76.03; 1 study, 674 participants; very low‐quality evidence; Analysis 1.2). We downgraded the evidence due to study limitations, small sample size, and imprecision.
1.2. Analysis.

Comparison 1: Vitamin C for pneumonia prevention, Outcome 2: Adverse effects (urticaria)
Secondary outcomes
1. Hospital admission rate
No included studies reported on this outcome.
2. Cost‐effectiveness
No included studies reported on this outcome.
Comparison 2: vitamin C as an adjunct to pneumonia treatment
Four studies assessed the effect of vitamin C supplementation as an adjunct to pneumonia treatment (Hunt 1994; Khan 2014; Wahed 2008; Yaqub 2015), and one study assessed the role of vitamin C for both the prevention and treatment of pneumonia (Bancalari 1984). Four studies included children under five years of age (Bancalari 1984; Khan 2014; Wahed 2008; Yaqub 2015), whilst one study included older participants aged 60 to 90 years (Hunt 1994). Doses of vitamin C supplementation were 125 mg (Wahed 2008), 200 mg (Hunt 1994; Khan 2014; Yaqub 2015), and to 2 g (Bancalari 1984). See Table 2. We could not conduct any of the planned subgroup and sensitivity analyses for this comparison due to the limited number of studies.
Primary outcomes
1. Duration of illness
Two studies reported duration of illness (Bancalari 1984; Khan 2014); however, we could not meta‐analyse the results because the studies reported different measures for duration of illness (Table 3).
1. Comparison 2: vitamin C as an adjunct to pneumonia treatment (outcomes not pooled).
| Study | Outcome | Intervention group | Control group |
| Primary outcomes | |||
| Bancalari 1984 | Duration of illness | 3.4 days ± 2.54 | 4.5 days ± 2.35 |
| Khan 2014 | Improvement in oxygen saturation | 1.03 days ± 0.16 | 1.14 days ± 1.0 |
| Improvement in respiratory rate | 3.61 days ± 1.50 | 4.04 days ± 1.62 | |
| Secondary outcomes | |||
| Wahed 2008 | Duration of hospitalisation | 6.75 days | 7.75 days |
| Yaqub 2015 | Duration of hospitalisation | 109.55 hours ± 27.89 | 130.64 hours ± 41.76 |
Bancalari 1984 reported a decrease in the duration of illness in the vitamin C supplementation group (3.4 days ± 2.54) compared to the control group (4.5 days ± 2.35).
Khan 2014 reported a decrease in number of days for improvement in oxygen saturation (1.03 days ± 0.16 versus 1.14 days ± 1.0) and respiratory rate (3.61 days ± 1.50 versus 4.04 days ± 1.62) in the vitamin C supplementation group compared to the control group. The number of days for improving chest indrawing did not differ between groups (estimates not reported).
We judged the quality of the evidence to be very low, downgrading due to study limitations, small sample size, and imprecision.
2. Clinical cure rate
No included studies reported on this outcome.
3. Mortality due to pneumonia
Hunt 1994 reported mortality due to pneumonia. We are uncertain of the effect of vitamin C supplementation on mortality due to pneumonia (RR 0.21, 95% CI 0.03 to 1.66; 1 study, 57 participants; very low‐quality evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Vitamin C as an adjunct to pneumonia treatment, Outcome 1: Mortality due to pneumonia
We downgraded the evidence due to study limitations, small sample size, and imprecision.
4. Adverse effects
No included studies reported on this outcome.
Secondary outcomes
1. Hospital admission rate
No included studies reported on this outcome.
2. Duration of hospitalisation
Two studies reported duration of hospitalisation (Wahed 2008; Yaqub 2015); however, we could not meta‐analyse the results because one of the studies, Wahed 2008, did not report standard deviation (Table 3).
Wahed 2008 reported a mean duration of hospital stay amongst children in the vitamin C supplementation group of 6.75 days compared to 7.75 days in the control group.
Yaqub 2015 reported that the mean duration of hospital stay in the vitamin C supplementation group was lower than in the control group (109.55 hours ± 27.89 versus 130.64 hours ± 41.76).
We judged the quality of the evidence to be very low, downgrading due to study limitations, small sample size, and imprecision.
3. Relapse rate
No included studies reported on this outcome.
4. Cost‐effectiveness
No included studies reported on this outcome.
Discussion
Summary of main results
We included seven studies involving 2774 participants in the review, and identified two ongoing studies. Two studies assessed the effect of vitamin C supplementation for pneumonia prevention; four studies assessed the effect of vitamin C supplementation as an adjunct to pneumonia treatment; and one study assessed the role of vitamin C for the prevention as well as treatment of pneumonia. We judged the evidence to be of very low quality.
For pneumonia prevention, the included studies reported two of our primary outcomes: incidence of pneumonia and adverse effects. None of the included studies reported any of our other primary outcomes (including pneumonia prevalence and mortality) or either of our prespecified secondary outcomes (hospital admission rate and cost‐effectiveness). We are uncertain of the effect of vitamin C supplementation on pneumonia incidence and adverse effects. There was significant heterogeneity in the analysis assessing the effect of vitamin C supplementation on pneumonia incidence (I² = 75%), possibly due to the different populations and settings in the two studies included in the analysis.
For pneumonia treatment, the included studies reported our primary outcomes of duration of illness and mortality due to pneumonia, and our secondary outcome of duration of hospitalisation. None of the included studies reported any of our other primary outcomes (clinical cure rate and adverse effects) or secondary outcomes (hospital admission rate, relapse rate, and cost‐effectiveness). We are uncertain of the effect of vitamin C supplementation on duration of illness, mortality due to pneumonia, and duration of hospitalisation.
Overall completeness and applicability of evidence
Overall, the evidence on the effect of vitamin C supplementation for the prevention and treatment of pneumonia is limited and of very low quality. Several of the included studies were published more than 25 years ago (Bancalari 1984; Coulehan 1974; Hunt 1994; Pitt 1979), and do not follow standard reporting guidelines (EQUATOR Network). Not all of our prespecified primary and secondary outcomes were reported in the included studies. Studies varied in terms of participants, settings, and doses and duration of vitamin C supplementation. For pneumonia prevention, two included studies recruited children from schools, whilst one study included marine corps. For pneumonia treatment, participants were children and adults. For pneumonia prevention, the included studies provided vitamin C supplementation in doses of 500 mg daily for 14 weeks, 2 g daily for 8 weeks, and 2 g daily for 12 weeks. For pneumonia treatment, the included studies provided vitamin C supplementation in doses of 125 mg daily (until discharge), 200 mg for four weeks, and 200 mg until discharge, as an adjunct to pneumonia treatment. Although we attempted to be as inclusive as possible in our searches, the literature we identified was predominantly published in English, so some studies published in other languages may have been missed by the searches.
We could not conduct our planned subgroup and sensitivity analyses due to the limited number of included studies.
Quality of the evidence
We assessed most studies as at either high or unclear risk of bias for random sequence generation, allocation concealment, and blinding. We judged the quality of the evidence for all outcomes as very low due to study limitations, heterogeneity, small sample size, and imprecision.
Potential biases in the review process
We were aware of the possibility of introducing bias at every stage of the review process. We rigorously followed Cochrane methods. We developed a comprehensive search strategy to capture eligible studies. We tried to minimise bias in a number of ways: two review authors assessed study eligibility, carried out data extraction, and assessed risk of bias. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements. Although we judged all included studies to be at low risk of reporting bias, we could not generate funnel plots to evaluate potential reporting bias amongst studies due to the small number of included studies. The review findings are largely based on the evidence available from the published studies, and may be prone to inherent within‐study biases including lack of true randomisation and reporting losses to follow‐up.
Agreements and disagreements with other studies or reviews
Hemilä 2004 assessed vitamin C supplementation for respiratory infections and included five small trials in military personnel and other participants living in conditions comparable to military recruits. Their findings suggest some positive impact of vitamin C supplementation on common cold incidence; however, the trials included by Hemilä 2004 were of short duration, and participants were under heavy levels of physical exertion during the trial.
A previous version of our review included three trials on preventive vitamin C supplementation and two trials on therapeutic vitamin C supplementation (Hemilä 2013). The findings of Hemilä 2013 suggest that the evidence is too weak to advocate prophylactic use of vitamin C to prevent pneumonia in the general population. Hemilä 2013 found that therapeutic vitamin C supplementation may be reasonable for people with pneumonia who have low vitamin C plasma levels. The cost and risks associated with vitamin C supplementation were found to be low (Hemilä 2013).
Our findings suggest that the evidence is too limited and low in quality to draw any firm conclusions on the role of vitamin C supplementation in either the prevention or treatment of pneumonia.
Authors' conclusions
Implications for practice.
We are uncertain of the role of vitamin C supplementation for the prevention of pneumonia and as an adjunct to the treatment of pneumonia due to limited and very low quality evidence. The population, settings, and dose of vitamin C supplementation varied widely amongst the included studies. The findings of this review thus have limited applicability and generalisability.
Implications for research.
We found very limited data on the effectiveness and safety of vitamin C supplementation for the prevention of pneumonia and as an adjunct to the treatment of pneumonia. Further good‐quality evidence is needed to evaluate the role of vitamin C supplementation. Future studies should explore the potential effect of vitamin C supplementation in varying doses and duration along with any potential adverse events associated with supplementation. Moreover, the effect of supplementation in various population groups should also be explored to rule out any potential variations in the effectiveness in deficient populations versus non‐deficient populations.
What's new
| Date | Event | Description |
|---|---|---|
| 9 November 2021 | Amended | We have reverted to the previous text, undoing the revisions made in relation to the studies Hunt 1994 and Bancalari 1994. This action is pending the imminent publication of a new version of the review that will incorporate these changes in a way that better retains the integrity of the scientific record. |
History
Protocol first published: Issue 9, 2018 Review first published: Issue 4, 2020
Acknowledgements
The Methods section of this review was based on a standard template developed by Cochrane Airways and adapted by the Cochrane Acute Respiratory Infections Group. We wish to thank the following people for commenting on the draft protocol: Robert Ware, Theresa Wrangham, and Mieke van Driel. We also thank the following people for commenting on the draft review: Ann Fonfa, Theresa Wrangham, Bisi Oduwole, Robert Ware, and Mieke van Driel.
Appendices
Appendix 1. Search strategies
MEDLINE (Ovid)
1. exp Pneumonia, Bacterial/ or exp Pneumonia, Lipid/ or exp Pneumonia, Necrotizing/ or exp Pneumonia, Pneumococcal/ or pneumonia.mp. or exp Pneumonia, Staphylococcal/ or exp Pneumonia, Mycoplasma/ or exp Pneumonia/ or exp Cryptogenic Organizing Pneumonia/ or exp Chlamydial Pneumonia/ or exp Pneumonia, Pneumocystis/ or exp Pneumonia, Viral/ or exp Pneumonia, Rickettsial/ or exp Pneumonia, Aspiration/
2. ascorbic acid.mp. or Ascorbic Acid/
3. 1 and 2
4. limit 3 to yr=‘1946 ‐Current’
PubMed (National Library of Medicine)
(‘Pneumonia’[Mesh] OR ‘Chlamydial Pneumonia’[Mesh] OR ‘Cryptogenic Organizing Pneumonia’[Mesh] OR ‘Pneumonia, Bacterial’[Mesh] OR ‘Pneumonia, Viral’[Mesh] OR ‘Pneumonia, Staphylococcal’[Mesh] OR ‘Pneumonia, Rickettsial’[Mesh] OR ‘Pneumonia, Pneumocystis’[Mesh] OR ‘Pneumonia, Mycoplasma’[Mesh] OR ‘Pneumonia, Pneumococcal’[Mesh] OR ‘Pneumonia, Lipid’[Mesh] OR ‘Pneumonia, Aspiration’[Mesh] OR ‘Pneumonia, Necrotizing’[Mesh] OR pneumonias OR pneumon* OR pneumothorax OR pneumonia infection bronchopneumon* OR ‘Idiopathic Interstitial Pneumonias’[Mesh] OR ‘Idiopathic Pulmonary Fibrosis’[Mesh] OR ‘Radiation Pneumonitis’[Mesh] OR ‘Bronchopneumonia’[Mesh] OR ‘Lymphoid Interstitial Pneumonia’ [Supplementary Concept] OR ‘Cholesterol pneumonia’ [Supplementary Concept]) OR ‘acute respiratory tract infection’ OR ‘ARI’ AND (‘Ascorbic Acid’[Mesh] OR (‘Vitamin C’) OR (‘Vit C’) OR ascorb* OR dehydroascorb*)
CINAHL (EBSCO)
((MH ‘Pneumonia+’) OR ‘ARI’ OR ‘Acute respiratory infection’ OR ‘pneumonia’ OR (MH ‘Pneumonia, Pneumocystis’) OR (MH ‘Cryptogenic Organizing Pneumonia’) OR (MH ‘Pneumonia, Mycoplasma’) OR (MH ‘Pulmonary Eosinophilia’) OR (MH ‘Idiopathic Interstitial Pneumonias+’) OR (MH ‘Pneumonia, Viral’) OR (MH ‘Pneumonia, Bacterial+’) OR (MH ‘Pneumonia, Aspiration’) OR (MH ‘Alveolitis, Extrinsic Allergic’) OR (MH ‘Radiation Pneumonitis’)) AND ((MH ‘Ascorbic Acid’) OR (‘Vitamin C’) OR (‘Vit C’))
LILACS (BIREME)
MH:’Pneumonia’ OR ‘pneumonias’ OR pneumon$ OR ‘acute respiratory infection’ OR ‘acute respiratory infections’ OR ‘ARI’ AND MH:’Ascorbic Acid’ OR dehydroascorb$ OR ‘Vit C’
Web of Science (Clarivate Analytics)
(‘Ascorbic Acid’ OR ‘Vitamin C’ OR ‘Vit C’ OR ascorb* OR dehydroascorb* OR vitamin ‘near’5 C OR vit ‘near’5 C OR ‘L‐Ascorbic Acid’ OR ‘Acid, L‐Ascorbic’ OR ‘L Ascorbic Acid’ OR ‘Hybrin’ OR ‘Magnorbin’ OR ‘Sodium Ascorbate’ OR ‘Ascorbate, Sodium’ OR ‘Ascorbic Acid, Monosodium Salt’ OR ‘Ferrous Ascorbate’ OR ‘Ascorbate, Ferrous’ OR ‘Magnesium Ascorbate’ OR ‘Ascorbate, Magnesium’ OR ‘Magnesium di‐L‐Ascorbate’ OR ‘Magnesium di L Ascorbate’ OR ‘di‐L‐Ascorbate, Magnesium’ OR ‘Magnesium Ascorbicum’) AND (‘Pneumonia’ OR ‘Chlamydial Pneumonia’ OR ‘Cryptogenic Organizing Pneumonia’ OR ‘Pneumonia, Bacterial’ OR ‘Pneumonia, Viral’ OR ‘Pneumonia, Staphylococcal’ OR ‘Pneumonia, Rickettsial’ OR ‘Pneumonia, Pneumocystis’ OR ‘Pneumonia, Mycoplasma’ OR ‘Pneumonia, Pneumococcal’ OR ‘Pneumonia, Lipid’ OR ‘Pneumonia, Aspiration’ OR ‘Pneumonia, Necrotizing’ OR ‘pneumonias’ OR pneumon* OR ‘pneumothorax’ OR pneumonia infection bronchopneumon* OR ‘Idopathic Interstitial Pneumonias’ OR Idiopathic Pulmonary Fibrosis OR ‘Radiation Pneumonitis’ OR ‘Bronchopneumonia’ OR ‘Lymphoid Interstitial Pneumonia’ OR ‘Cholesterol pneumonia’ OR ‘Pneumonias’ OR ‘Lobar Pneumonia’ OR Lobar Pneumonia* OR Experimental Lung Inflammation* OR ‘Pneumonitis’ OR ‘Pneumonitides’ OR ‘Pulmonary Inflammation’ OR ‘Inflammation, Pulmonary’ OR ‘Lung Inflammation’)
CENTRAL (Wiley)
MeSH descriptor: [Ascorbic Acid] explode all trees AND MeSH descriptor: [Pneumonia] explode all trees
Embase (Elsevier)
#4: #3 AND (1974:py OR 1975:py OR 1976:py OR 1977:py OR 1978:py OR 1979:py OR 1980:py OR 1981:py OR 1982:py OR 1983:py OR 1984:py OR 1985:py OR 1986:py OR 1987:py OR 1988:py OR 1989:py OR 1990:py OR 1991:py OR 1992:py OR 1993:py OR 1994:py OR 1995:py OR 1996:py OR 1997:py OR 1998:py OR 1999:py OR 2000:py OR 2001:py OR 2002:py OR 2003:py OR 2004:py OR 2005:py OR 2006:py OR 2007:py OR 2008:py OR 2009:py OR 2010:py OR 2011:py OR 2012:py OR 2013:py OR 2014:py OR 2015:py OR 2016:py OR 2017:py OR 2018:py OR 2019:py OR OR 2020:py)
#3: #1 AND #2
#2: 'ascorbic acid'/exp OR 'ascorbic acid' OR 'vitamin c'/exp OR 'vitamin c'
#1: 'pneumonia'/exp OR 'pneumonia' OR 'chlamydial pneumonia'/exp OR 'chlamydial pneumonia' OR 'cryptogenic organizing pneumonia'/exp OR 'cryptogenic organizing pneumonia' OR 'pneumonia, bacterial'/exp OR 'pneumonia, bacterial' OR 'pneumonia, viral'/exp OR 'pneumonia, viral' OR 'pneumonia, staphylococcal'/exp OR 'pneumonia, staphylococcal' OR 'pneumonia, rickettsial'/exp OR 'pneumonia, rickettsial' OR 'pneumonia, pneumocystis'/exp OR 'pneumonia, pneumocystis' OR 'pneumonia, mycoplasma'/exp OR 'pneumonia, mycoplasma' OR 'pneumonia, pneumococcal'/exp OR 'pneumonia, pneumococcal' OR 'pneumonia, lipid'/exp OR 'pneumonia, lipid' OR 'pneumonia, aspiration'/exp OR 'pneumonia, aspiration' OR 'pneumonia, necrotizing'/exp OR 'pneumonia, necrotizing' OR pneumonias OR pneumon* OR 'pneumothorax'/exp OR pneumothorax OR 'pneumonia infection' OR (('pneumonia'/exp OR pneumonia) AND ('infection'/exp OR infection) AND bronchopneumon*) OR 'idiopathic interstitial pneumonias'/exp OR 'idiopathic interstitial pneumonias' OR 'idiopathic pulmonary fibrosis'/exp OR 'idiopathic pulmonary fibrosis' OR 'radiation pneumonitis'/exp OR 'radiation pneumonitis' OR 'bronchopneumonia'/exp OR 'bronchopneumonia' OR 'lymphoid interstitial pneumonia'/exp OR 'lymphoid interstitial pneumonia' OR 'cholesterol pneumonia' OR 'acute respiratory tract infection'/exp OR 'acute respiratory tract infection' OR 'ari'
WHO ICTRP
Ascorbic Acid AND Pneumonia OR acute respiratory infection OR ARI
Clinicaltrials.gov
Vitamin C | Pneumonia
Data and analyses
Comparison 1. Vitamin C for pneumonia prevention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Incidence of pneumonia | 2 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.06, 3.61] |
| 1.2 Adverse effects (urticaria) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 2. Vitamin C as an adjunct to pneumonia treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mortality due to pneumonia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bancalari 1984.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomisation: individually randomised trial Type of study: preventive and therapeutic |
|
| Participants |
Location setting: study was carried out in Educational Unit D No. 675 in the city of Coronel, Chile Sample size: 62 children Dropouts/withdrawals: none Sex: male and female (63% female and 37% male) Mean age: 11.3 years in intervention arm and 11.8 years in control arm Diagnostic criteria: ARI examination took place 3 times a week amongst children. The specific criteria for ARI were not clearly defined. In the case of detection of any respiratory symptoms, temperature was noted orally. When a child was found absent from school, 1 of the authors visited the student’s home for investigation. If the child was sick, the illness was monitored until he/she was completely well. For the investigation of possible side effects, testing was done for plasma electrolytes, glucosuria, blood glucose, ketonuria, urinary pH, proteinuria, and haematuria. Moreover, medical histories were maintained in order to identify any gastrointestinal effects. Spectrophotometric analysis of ascorbic acid levels was conducted before, during (days 20, 50, and 80), and 48 hours after the conclusion of the treatment. Severity of condition: not specified |
|
| Interventions |
Intervention (sample size): 2 g oral vitamin C in tablet form twice weekly on Saturdays and Sundays for 12 weeks (N = 32) Concomitant interventions: none Control (sample size): placebo tablet (glucose) twice weekly on Saturdays and Sundays for 12 weeks (N = 30) Follow‐up: 3 times a week for 12 weeks |
|
| Outcomes |
Primary outcomes: incidence of pneumonia, adverse effects, duration of illness Secondary outcomes: not specified Timing of outcome assessment: 84 days |
|
| Notes |
Study start date: June 1981 Study end date: September 1981 Limitations: sample size not calculated Funding source: vitamin C tablets contributed by Merck Laboratories; placebo prepared by the Department of Applied Biochemistry, School of Pharmaceutical Chemistry and Biochemistry of the University of Concepción Conflict of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "The vitamin C tablets (2 g per day) and the placebo tablets were identical in colour, taste, size and consistency, and were marked with codes understood only by staff members... Like the children, those who collected the data did not know who was taking vitamin C and who was taking the placebo" (p. 3) Comment: adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "The vitamin C tablets (2 g per day) and the placebo tablets were identical in colour, taste, size and consistency, and were marked with codes understood only by staff members... Like the children, those who collected the data did not know who was taking vitamin C and who was taking the placebo." (p. 3) Comment: adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not specified. The outcomes specified in the methodology section have been reported in the results section. |
| Other bias | Low risk | No other source of potential bias reported. |
Coulehan 1974.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomisation: individually randomised trial Type of study: preventive study |
|
| Participants |
Location setting: Toyei Boarding School, Steamboat, Arizona, USA Sample size: 641 children Dropouts/withdrawals: 25 children dropped out of the school during the course of the study Sex: male and female Age range: 6 to 15 years Diagnostic criteria: firstly, clinical episodes of illness were observed, which included all illnesses for which children sought medical care through routine channels. Secondly, active surveillance was maintained to observe those respiratory illnesses for which no medical care was sought. Written diagnostic criteria were established for 5 respiratory syndromes (uncomplicated upper respiratory infection, pharyngitis, otitis media, bronchitis, and pneumonia). Diagnosis and duration of symptoms before day of diagnosis were recorded. The nurse followed each ill child daily until all symptoms were resolved, thus allowing for computation of total duration of illness. Each child's temperature was taken, each was examined for nasal discharge, and each was individually asked if symptoms were present on that day: runny nose, sore throat, earache, or cough. Only the presence or absence of these signs and symptoms was recorded. Temperatures of 37.5 °C or over were considered elevated. Severity of condition: not specified |
|
| Interventions |
Intervention (sample size): vitamin C supplements: children in grades 1 through 4 received 1 g daily, and children in grades 5 through 8 received 2 g daily (N = 321) Control (sample size): placebo tablet (citric acid) (N = 320) Concomitant interventions: none Follow‐up: the first blood drawing took place in January, before the study period, the second 7 weeks after the study had begun, and the final drawing in late May, 2 weeks after the study period had ended. |
|
| Outcomes |
Primary outcomes: incidence of pneumonia Secondary outcomes: not specified Timing of outcome assessment: 14 weeks |
|
| Notes |
Study start date: February 1973 Study end date: mid‐May 1973 Limitations: not specified Funding source: tablets were provided by Hoffmann‐La Roche Inc, Nutley, NJ, USA Conflict of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "All children were assigned alternately, from an alphabetical listing by classroom, to one of two study groups." (p. 7) Comment: not adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "Placebos were formulated from citric acid to be indistinguishable in taste and appearance from the vitamin C tablets...Tablets were distributed to school teachers in containers labelled only by code number...Persons involved in data collection were aware neither of which group received vitamin C nor of the group to which any given child belonged." (p. 7) Comment: adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Placebos were formulated from citric acid to be indistinguishable in taste and appearance from the vitamin C tablets...Tablets were distributed to school teachers in containers labelled only by code number...Persons involved in data collection were aware neither of which group received vitamin C nor of the group to which any given child belonged." (p. 7) Comment: adequately done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25/666 = 3.75% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not specified. The outcomes specified in the methodology section have been reported in the results section. |
| Other bias | Low risk | No other source of potential bias reported. |
Hunt 1994.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomisation: individually randomised trial Type of study: study on pneumonia treatment |
|
| Participants |
Location/setting: St. Luke's Hospital Huddersfield, UK Sample size: 57 Dropouts/withdrawals: 4 participants were excluded due to missing information, and there was no loss to follow‐up Sex: male and female (52% female and 48% male) Age range: intervention: male: 66 to 93 years, female: 76 to 88 years; control: male: 74 to 94 years, female: 72 to 90 years Diagnostic criteria: a "total respiratory score (TOTRESP)" was given to each participant on assessment at 0, 2, and 4 weeks. The main diagnostic features for respiratory condition included breathlessness, cough, and radiographic evidence for chest infection. Severity of condition: TOTRESP score |
|
| Interventions |
Intervention (sample size): 100 mg oral vitamin C was administered twice a day (total dose: 200 mg) for 4 weeks (N = 28) Concomitant interventions: participants were provided with their routine medications Control (sample size): placebo (lactose, cornstarch, talc, and magnesium stearate). Oral tablet given twice a day for 4 weeks (N = 29). Follow‐up: assessments were made on TOTRESP scores, and blood samples were taken for analysis of plasma, mononuclear cell, polymorphonuclear cell, and platelet vitamin C levels on admission (day 0), and at 2 weeks and 4 weeks |
|
| Outcomes |
Primary outcomes: mortality due to pneumonia Secondary outcomes: not specified Timing of outcome assessment: not specified |
|
| Notes |
Study start date: not specified Study end date: not specified Limitations: if participants were discharged from the hospital before 4 weeks they were labelled as well and were followed up further Funding source: Roche Pharmaceuticals Ltd Conflict of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "they were allocated on a randomised double blind basis" (p. 213) Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk |
Quote: "they were allocated on a randomised double blind basis" (p. 213) Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "The vitamin C and placebo tablets were indistinguishable from each other by look or taste." (p. 213) Comment: adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial registration information not provided. The outcomes specified in the methodology section have been reported in the results section. |
| Other bias | Low risk | No other potential bias detected. |
Khan 2014.
| Study characteristics | ||
| Methods |
Design: quasi‐RCT Unit of randomisation: not specified Type of study: study on pneumonia treatment |
|
| Participants |
Location/setting: study was carried out in Paediatric Department (ward), Islamic International Medical College‐Trust, (IIMC‐T), Railway Hospital, Rawalpindi, Pakistan Sample size: 222 children Dropout/withdrawals: none Sex: males and females (39% females and 61% males) Age range: 2 months to 5 years Diagnostic criteria: not specified Severity of condition: severe pneumonia |
|
| Interventions |
Intervention (sample size): 200 mg oral vitamin C drops once daily until symptoms of severe pneumonia were improved (N = 111) Control (sample size): placebo drops (sodium citrate with colouring agent mixed in water) were given once daily until severe pneumonia improved (N = 111) Concomitant interventions: IV amoxicillin and supportive treatment Follow‐up: clinical progress of both groups was taken thrice daily in terms of oxygen saturation, respiratory rate, and chest indrawing. The number of days to improvement of severe pneumonia was recorded. Improvements in respiratory rates was reported in less than 4 days, chest indrawing in less than 2 days, and oxygen saturation in less than 1 day. |
|
| Outcomes |
Primary outcomes: duration of Illness: mean number of days for improvement in respiratory rate, mean number of days for improvement in oxygen saturation, and mean number of days for improvement in chest indrawing Secondary outcomes: not specified Timing of outcome assessment: not specified |
|
| Notes |
Study start date: 1 April 2010 Study end date: 31 March 2011 Limitations: not specified Funding source: not specified Conflicts of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation not done. |
| Allocation concealment (selection bias) | High risk | Randomisation not done. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "In other group children received placebo drops (consisted of sodium citrate along with coloring agent mixed in water), which matched exactly with vitamin C drops in color and taste." (p. 56) Comment: adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "In other group children received placebo drops (consisted of sodium citrate along with colouring agent mixed in water), which matched exactly with vitamin C drops in colour and taste." (p. 56) Comment: insufficient information regarding outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | We did not find a study protocol. Outcomes specified in the methodology section have been reported in the results section. |
| Other bias | Low risk | No other potential bias detected. |
Pitt 1979.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomisation: individually randomised trial Type of study: preventive study |
|
| Participants |
Location/setting: US Marine Corps Recruit Depot, Parris Island, South Carolina, USA Sample size: 862 marine recruits Dropouts/withdrawals: 64 were removed for further training or discharged, plus 123 recruits (64 vitamin C; 59 placebo) were removed including 1 due to recurrent urticaria Sex: male Mean age: 18.5 years Diagnostic criteria: recruits were questioned about the duration, severity, and symptoms of cold, which were (1) fever/chills, (2) headache, (3) stuffy or runny nose, (4) sore throat, (5) dry or productive cough, (6) nausea or vomiting, (7) diarrhoea, and (8) stomach pain. The recruits were also asked to report on suspected side effects and any lapses in pill taking on a weekly basis. Pneumonia was confirmed with throat, sputum, and blood cultures; sputum Gram stains; WBC count; and acute and convalescent titres for influenza A and B, parainfluenza 1 to 3, adenovirus, rhinovirus, coxsackie B 1 to 6, respiratory syncytial virus, and Mycoplasma pneumoniae were performed. Severity of condition: each cold was rated by the recruits as being 'mild', 'average', 'bad', or the 'worst ever', and these 4 subjective classifications were given a numerical rating from 1 to 4. |
|
| Interventions |
Intervention (sample size): 500 mg oral ascorbic acid tablets in anhydrous form was given for 8 weeks. 2 tablets each morning and 2 tablets each evening (N = 331) Concomitant interventions: before the initiation of pill taking, each recruit received adenovirus‐4 and influenza vaccines and either intramuscular penicillin G benzathine or oral erythromycin estolate streptococcal prophylaxis Control (sample size): white plain tablet formulated from citric acid that was indistinguishable in appearance and taste from the vitamin C tablets, given for 8 weeks. 2 tablets each morning and 2 tablets each evening (N = 343) Follow‐up: weekly questionnaire and active surveillance of sick calls were taken for 8 weeks |
|
| Outcomes |
Primary outcomes: incidence of pneumonia and common cold, adverse effects (urticaria), duration of illness Secondary outcomes: not specified Timing of outcome assessment: not specified |
|
| Notes |
Study start date: October, November, and December 1974 Study end date: not specified Limitations: due to low incidence of pneumonia, no claim for the beneficial effect of vitamin C for pneumonia could be made Funding source: this study was supported in part by the Naval Medical Research and Development Command Project M‐4305, Work Unit 5021. Myron Brin, PhD and Hoffmann‐La Roche Inc supplied the tablets. Conflict of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Recruits were randomly assigned to either of the group from a list of number in random pairs." (p. 908) Comment: adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Quote: "Neither the recruits or drill instructors nor the physicians and corpsmen who treated the recruits were aware of which pill any individual recruit was taking." (p. 908) Comment: adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "Neither the recruits or drill instructors nor the physicians and corpsmen who treated the recruits were aware of which pill any individual recruit was taking." (p. 908) Comment: adequately done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 187/862: 21.6% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not specified. The outcomes specified in the methodology section have been reported in the results section. |
| Other bias | Low risk | No other potential sources of bias detected. |
Wahed 2008.
| Study characteristics | ||
| Methods |
Design: quasi‐RCT Unit of randomisation: not specified Type of study: study on pneumonia treatment |
|
| Participants |
Location/setting: Paediatrics Department of Rangpur Medical College Hospital, Bangladesh Sample size: 800 children Dropouts/withdrawals: 350 dropouts (8 children died, 100 children left the hospital on "Risk Bond"; 190 children were discharged on parents' request before cure; 8 children developed various complications; and 44 children were excluded from the study for other reasons) Sex: males and females (49% females and 51% males) Mean age: 6.5 months Diagnostic criteria: history of illness of the child was collected from the person who accompanied the child in the hospital. Clinical examination of the child was carried out on the day of admission, up to discharge. Children with clinical diagnosis of severe pneumonia and a radiological diagnosis of bronchopneumonia were included. Severity of condition: severe pneumonia |
|
| Interventions |
Intervention (sample size): total of 6 intervention arms:
Concomitant interventions: ampicillin (50 to 100 mg/kg/day) and gentamycin (5 to 7 mg/kg/day) in injection. 1 tablet daily of each intervention arm with antibiotics given intravenously from the day of admission and continued up to discharge. Control (sample size): only specific treatment administered, which consisted of ampicillin (50 to 100 mg/kg/day) and gentamycin (5 to 7 mg/kg/day) in injection for 6 days from the day of admission and continued up to discharge (N = 400) Follow‐up: follow‐up until discharge (discharge criterion was being free from clinical features of severe pneumonia for 2 consecutive days) |
|
| Outcomes |
Primary outcomes: not specified Secondary outcomes: duration of hospitalisation Timing of outcome assessment: not specified |
|
| Notes |
Study start date: 1 July 2004 Study end date: 30 June 2007 Limitations: outcomes not reported for vitamin C supplemented group Funding source: not specified Conflict of interest: not specified We contacted the study author to obtain information for the outcomes specific to the vitamin C supplementation group, but did not receive any response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk |
Quote: "The sampling method was systematic sampling and every 1st patient was given the intervention and 2nd patient was treated as control from a prepared register." Comment: not adequately done |
| Allocation concealment (selection bias) | High risk |
Quote: "The sampling method was systematic sampling and every 1st patient was given the intervention and 2nd patient was treated as control from a prepared register." Comment: not adequately done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Quote: "Blinding of the samples has not been done which could increase the quality of the study. " (p. 525) Comment: not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "Blinding of the samples has not been done which could increase the quality of the study. " (p. 525) Comment: not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 350/1150: 30% loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We did not find a study protocol. The outcomes specified in the methodology section have been reported in the results section. |
| Other bias | Low risk | No other potential sources of bias reported. |
Yaqub 2015.
| Study characteristics | ||
| Methods |
Design: RCT Unit of randomisation: individually randomised trial Type of study: study on pneumonia treatment |
|
| Participants |
Location/setting: Department of Paediatrics, Rawal Institute of Health Sciences (RIHS), Islamabad, Pakistan Sample size: 130 children Dropouts/withdrawals: none Sex: males and females (53% females and 47% males) Mean age: 19.93 months Diagnostic criteria: not specified Severity of condition: not specified |
|
| Interventions |
Intervention (sample size): vitamin C 200 mg in the form of Cecon drops, which contain 100 mg/mL, was given 8 hourly every day until discharge or until treatment end (N = 65) Control(sample size): water drops as placebo 8 hourly every day till discharge or until treatment end (N = 65) Concomitant interventions: both groups were given standard antibiotic therapy, i.e. intravenous ampicillin 100 mg/kg/day divided every 8 hours Follow‐up: both groups were followed daily to evaluate the outcome until discharge for change of antibiotics |
|
| Outcomes |
Primary outcome: length of hospital stay Secondary outcomes: not specified Timing of outcome assessment: until discharge |
|
| Notes |
Study start date: 1 December 2013 Study end date: 30 November 2014 Funding source: not specified Conflict of interest: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "All children with pneumonia were randomised based on lottery method into two groups. Lottery method was used to randomise participants." (p. 210) Comment: adequately done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote: "Group B was given only standard antibiotic therapy, i.e. intravenous ampicillin 100 mg/kg/day divided every 8 hourly during the hospital stay along with water drops as placebo." (p. 210) Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "Group B was given only standard antibiotic therapy, i.e. intravenous ampicillin 100 mg/kg/day divided every 8 hourly during the hospital stay along with water drops as placebo." (p. 210) Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Trial registration information not provided. However, the outcomes specified in the methodology section have been reported in the results section. |
| Other bias | Low risk | No other potential sources of bias reported. |
ARI: acute respiratory infections IU: international unit IV: intravenous N: number RCT: randomised controlled trial WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alshami 2018 | Wrong study design ‐ review article |
| Arabi 2020 | Wrong study design ‐ review article |
| Beinert 2000 | Wrong study design ‐ review article |
| Carr 2017 | Wrong study design ‐ review article |
| Ceccato 2018 | Wrong study design ‐ review article |
| Glazebrook 1942 | Wrong study design ‐ the study design was not appropriate since the study recruited new participants in the middle of the study |
| Hemilä 1997 | Wrong study design ‐ review article |
| Hemilä 2003 | Wrong study design ‐ correspondence |
| Hemilä 2007 | Wrong study design ‐ review article |
| Hemilä 2008 | Wrong study design ‐ correspondence |
| Hemilä 2011 | Wrong study design ‐ letter to the editor |
| Hunt 1984 | Wrong population ‐ the study included hospitalised geriatric patients with any diagnosis |
| Jain 2002 | Wrong intervention ‐ the study provided multiple micronutrient supplement |
| Kim 2018 | Wrong study design ‐ cohort study |
| Klenner 1948 | Wrong study design ‐ no comparison group |
| Mahalanabis 2006a | Wrong intervention ‐ intervention included co‐supplementation of alpha‐tocopherol and vitamin C |
| Mahalanabis 2006b | Wrong study design ‐ letter to the editor |
| NCT02186158 | Wrong population ‐ the study included participants with hospital‐acquired pneumonia |
| Ogal 2019 | Wrong intervention ‐ the intervention includes provision of echinacea |
| Socci 2012 | Wrong intervention ‐ the intervention included co‐administration of immunotherapy with oral bacterial lysates and vitamin C |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12619000256178.
| Study name | Vitamin C in community acquired pneumonia: a pilot study |
| Methods |
Design: randomised controlled trial Unit of randomisation: individually randomised |
| Participants |
Location setting: New Zealand Sample size: 140 participants Sex: both Age: 18 years Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: vitamin C: intravenous infusion of 2.5 g/8 hours will be started as soon as possible, but not later than 72 hours after hospital admission and within 24 hours of the documentation of a community‐acquired pneumonia CURB‐65 severity score > 1. Intravenous vitamin C will be provided whilst the participant is receiving IV antimicrobial therapy and will continue until the treatment is changed to oral antimicrobial therapy or for a maximum of 7 days. Following the cessation of IV vitamin C therapy, the participant will receive a further 7 days of oral vitamin C at a dose of 1 g (2 x 500 mg chewable tablets) 3 times per day. Participants will receive vitamin C for a minimum of 8 days and a maximum of 14 days. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 20 February 2019 |
| Contact information | Stephen Chambers, Professor University of Otago, Christchurch 2 Riccarton Avenue Christchurch Central Christchurch, 8011, New Zealand +64 3 3640649 steve.chambers@otago.ac.nz |
| Notes | Not yet recruiting |
NCT04264533.
| Study name | Vitamin C in vitamin C infusion for the treatment of severe 2019‐nCoV infected pneumonia |
| Methods |
Design: randomised controlled trial Unit of randomisation: individually randomised |
| Participants |
Location setting: Wuhan, China Sample size: 140 participants Sex: both Age: 18 years and older (adult, older adult) Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: experimental: vitamin C (24 g vitamin C + water for injection, total volume 50 mL. 7 mL/h; infusion pump) for 7 days Control: placebo comparator: water for injection (50 mL water for injection. 7 mL/h; infusion pump) for 7 days |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 10 February 2020 |
| Contact information | Zhiyong Peng, Professor +8618672396028 pengzy5@hotmail.com |
| Notes | Not yet recruiting |
APACHE: Acute Physiology and Chronic Health Evaluation CAP: community‐acquired pneumonia CURB‐65: confusion, urea > 7 mmol/L, respiratory rate ≥ 30 breaths/min, low blood pressure and age ≥ 65 years G6PD: glucose‐6‐phosphate dehydrogenase ICU: intensive care unit IV: intravenous SARI: severe acute respiratory infections severe 2019‐nCoV: severe 2019 novel coronavirus SOFA: Sequential Organ Failure Assessment
Differences between protocol and review
We could not conduct the planned subgroup and sensitivity analyses due to the limited number of studies included in the review.
We added a fourth outcome, that is adverse effects, to the review for both pneumonia prevention and pneumonia treatment.
We deleted text that was not applicable to this review from the Unit of analysis issues and Dealing with missing data sections.
Contributions of authors
Rehana A Salam (RAS), Jai K Das (JKD), and Zulfiqar A Bhutta (ZAB) designed the protocol for the review. RAS and JKD co‐ordinated the review. RAS and Zahra Ali Padhani (ZAP) designed the search strategy. ZAP, Zorays Moazzam (ZM), Alina Ashraf (AA), and Hasana Bilal (HB) undertook the searches, screened search results, organised retrieval of papers, and screened retrieved papers against eligibility criteria. RAS and JKD were consulted in the case of disagreements about study inclusion. ZAP, ZM, AA, and HB extracted data and appraised the quality of the included studies. ZAP, ZM, AA, and HB independently assessed risk of bias for each included study. ZAP and RAS performed GRADE assessment; disagreements were reviewed by JKD. RAS reviewed the final data extraction. ZAP attempted to contact authors for additional information. ZAP, RAS, and JKD entered data into Review Manager 5 and performed analyses. ZAP, RAS, and JKD interpreted the data. ZAP, ZM, and AA wrote the first draft of the review, and RAS and JKD finalised the final draft. ZAB provided oversight and advice throughout the review process.
All review authors reviewed and approved the final draft of the review. ZAB is the overall guarantor.
Sources of support
Internal sources
Aga Khan University, Pakistan
External sources
No sources of support provided
Declarations of interest
Zahra Ali Padhani: None known. Zorays Moazzam: None known. Alina Ashraf: None known. Hasana Bilal: None known. Rehana A Salam: None known. Jai K Das: None known. Zulfiqar A Bhutta: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bancalari 1984 {published data only}
- Bancalari A, Seguel C, Neira F, Ruíz I, Calvo C. Prophylactic value of vitamin C in acute respiratory tract infections in schoolchildren. Revista Médica de Chile 1984;12(9):871-6. [PubMed] [Google Scholar]
Coulehan 1974 {published data only}
- Coulehan JL, Reisinger KS, Rogers KD, Bradley DW. Vitamin C prophylaxis in a boarding school. New England Journal of Medicine 1974;290(1):6-10. [DOI] [PubMed] [Google Scholar]
Hunt 1994 {published data only}
- Hunt C, Chakravorty NK, Annan G, Habibzadeh N, Schorah CJ. The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. International Journal for Vitamin and Nutrition Research 1994;64(3):212-9. [PubMed] [Google Scholar]
Khan 2014 {published data only}
- Khan IM. Efficacy of vitamin C in reducing duration of severe pneumonia in children. Journal of Rawalpindi Medical College 2014;18(1):55-7. [Google Scholar]
Pitt 1979 {published data only}
- Pitt HA, Costrini AM. Vitamin C prophylaxis in marine recruits. JAMA 1979;241(9):908-11. [PubMed] [Google Scholar]
Wahed 2008 {published data only}
- Wahed MA, Islam MA, Khondakar P, Haque MA. Effect of micronutrients on morbidity and duration of hospital stay in childhood pneumonia. Mymensingh Medical Journal 2008;17(2):S77-83. [PubMed] [Google Scholar]
Yaqub 2015 {published data only}
- Yaqub A, Riaz N, Ghani Z, Gul S. Role of vitamin C in children having pneumonia. ISRA Medical Journal 2015;7(4):209-11. [Google Scholar]
References to studies excluded from this review
Alshami 2018 {published data only}
- Alshami A, Romero C, Varon J. Vitamin C in respiratory diseases. Current Respiratory Medicine Reviews 2018;14(2):62-3. [Google Scholar]
Arabi 2020 {published data only}
- Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Medicine 2020;46:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beinert 2000 {published data only}
- Beinert T, Binder D, Stuschke M, Oehm C, Jörres RA, Schweigert M, et al. Oxidative pulmonary stress under cytoreductive therapy. Pneumologie 2000;54(5):201-11. [DOI] [PubMed] [Google Scholar]
Carr 2017 {published data only}
- Carr AC, Maggini S. Vitamin C and immune function. Nutrients 2017;9(11):1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ceccato 2018 {published data only}
- Ceccato A, Ferrer M, Barbeta E, Torres A. Adjunctive therapies for community-acquired pneumonia. Clinics in Chest Medicine 2018;39(4):753-64. [DOI] [PubMed] [Google Scholar]
Glazebrook 1942 {published data only}
- Glazebrook AJ, Thomson S. The administration of vitamin C in a large institution and its effect on general health and resistance to infection. Journal of Hygiene 1942;42(1):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemilä 1997 {published data only}
- Hemilä H. Vitamin C intake and susceptibility to pneumonia. Pediatric Infectious Disease Journal 1997;16(9):836-7. [DOI] [PubMed] [Google Scholar]
Hemilä 2003 {published data only}
- Hemilä H. Vitamin C and SARS coronavirus. Journal of Antimicrobial Chemotherapy 2003;52(6):1049-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemilä 2007 {published data only}
- Hemilä H, Louhiala P. Vitamin C may affect lung infections. Journal of the Royal Society of Medicine 2007;100(11):495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemilä 2008 {published data only}
- Hemilä H. Vitamin C and sex differences in respiratory tract infections. Respiratory Medicine 2008;102(4):625-6. [DOI] [PubMed] [Google Scholar]
Hemilä 2011 {published data only}
- Hemilä H. Vitamin C and community-acquired pneumonia. American Journal of Respiratory and Critical Care Medicine 2011;184(5):621-2. [DOI] [PubMed] [Google Scholar]
Hunt 1984 {published data only}
- Hunt C, Chakravorty NK, Annan G. The clinical and biochemical effects of vitamin C supplementation in short-stay hospitalized geriatric patients. International Journal for Vitamin and Nutrition Research 1984;54(1):65-74. [PubMed] [Google Scholar]
Jain 2002 {published data only}
- Jain AL. Influence of vitamins and trace-elements on the incidence of respiratory infection in the elderly. Nutrition Research 2002;22(1-2):85-7. [DOI] [PubMed] [Google Scholar]
Kim 2018 {published data only}
- Kim WY, Jo EJ, Eom JS, Mok J, Kim MH, Kim KU, et al. Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: propensity score-based analysis of a before-after cohort study. Journal of Critical Care 2018 July 5 [Epub ahead of print]. [DOI: 10.1016/j.jcrc.2018.07.004] [DOI] [PubMed]
Klenner 1948 {published data only}
- Klenner FR. Virus pneumonia and its treatment with vitamin C. Southern Medicine and Surgery 1948;110(2):36-8. [PubMed] [Google Scholar]
Mahalanabis 2006a {published data only}
- Mahalanabis D, Basak M, Paul D, Gupta S, Shaikh S, Wahed M, et al. Antioxidant vitamins E and C as adjunct therapy of severe acute lower-respiratory infection in infants and young children: a randomized controlled trial. European Journal of Clinical Nutrition 2006;60(5):673-80. [DOI] [PubMed] [Google Scholar]
Mahalanabis 2006b {published data only}
- Mahalanabis D, Jana S, Shaikh S, Gupta S, Chakrabarti ML, Moitra P, et al. Vitamin E and vitamin C supplementation does not improve the clinical course of measles with pneumonia in children: a controlled trial. Journal of Tropical Pediatrics 2006;52(4):302-3. [DOI] [PubMed] [Google Scholar]
NCT02186158 {published data only}
- NCT02186158. Interest of ascorbic acid in the management of pneumonia in elderly people hospitalized (PNEUMO-VITA-C). clinicaltrials.gov/show/nct02186158 (first received 10 July 2014).
Ogal 2019 {published data only}
- Ogal M, Klein P, Suter A, Schoop R. Echinacea reduces antibiotics through prevention of respiratory tract infections in children: a randomized, blinded, controlled clinical trial. Planta Medica 2019;85(18):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Socci 2012 {published data only}
- Socci M, Slullitel P, Cortigiani L. Efficacy of immunotherapy with an oral bacterial lysate and vitamin C in the primary prevention of acute respiratory tract infections in children. World Allergy Organization Journal 2012;5:S69. [Google Scholar]
References to ongoing studies
ACTRN12619000256178 {published data only}
- ACTRN12619000256178. Vitamin C in community acquired pneumonia: a pilot study. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=376957&isReview=true (first received 14 February 2019).
NCT04264533 {published data only}
- NCT04264533. Vitamin C infusion for the treatment of severe 2019-nCoV infected pneumonia. clinicaltrials.gov/ct2/show/NCT04264533 (first received 11 February 2020).
Additional references
Abubakar 2015
- Abubakar II, Tillmann T, Banerjee A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385(9963):117-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akaike 2001
- Akaike T. Role of free radicals in viral pathogenesis and mutation. Reviews in Medical Virology 2001;11(2):87-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayala 2014
- Ayala A, Muñoz Mario F, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity 2014 May 8 [Epub ahead of print]. [DOI: 10.1155/2014/360438] [DOI] [PMC free article] [PubMed]
Blumberg 2018
- Blumberg JB, Bailey RL, Sesso HD, Ulrich CM. The evolving role of multivitamin/multimineral supplement use among adults in the age of personalized nutrition. Nutrients 2018;10(2):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carr 1999
- Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB Journal 1999;13(9):1007-24. [DOI] [PubMed] [Google Scholar]
Douglas 2005
- Douglas RM, Hemilä H. Vitamin C for preventing and treating the common cold. PLoS Medicine 2005;2(6):e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
EQUATOR Network
- Enhancing the quality and transparency of health research. www.equator-network.org (accessed 5 September 2019).
Figueroa‐Méndez 2015
- Figueroa-Méndez R, Rivas-Arancibia S. Vitamin C in health and disease: its role in the metabolism of cells and redox state in the brain. Frontiers in Physiology 2015;6:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 11 April 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hemilä 2004
- Hemilä H. Vitamin C supplementation and respiratory infections: a systematic review. Military Medicine 2004;169(11):920-5. [DOI] [PubMed] [Google Scholar]
Hemilä 2017
- Hemilä H. Vitamin C and infections. Nutrients 2017;9(4):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Himmelreich 1998
- Himmelreich U, Drew KN, Serianni AS, Kuchel PW. 13C NMR studies of vitamin C transport and its redox cycling in human erythrocytes. Biochemistry 1998;37(20):7578-88. [DOI] [PubMed] [Google Scholar]
Hughes 1998
- Hughes K, Ong CN. Vitamins, selenium, iron, and coronary heart disease risk in Indians, Malays, and Chinese in Singapore. Journal of Epidemiology and Community Health 1998;52(3):181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iannotti 2006
- Iannotti LL, Tielsch JM, Black MM, Black RE. Iron supplementation in early childhood: health benefits and risks. American Journal of Clinical Nutrition 2006;84(6):1261-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
IoM 2000
- Institute of Medicine (USA) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academies Press (USA), 2000. [PubMed] [Google Scholar]
Johnston 1998
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. Journal of the American College of Nutrition 1998;17(4):366-70. [DOI] [PubMed] [Google Scholar]
Lim 2009
- Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64(3):1-55. [DOI] [PubMed] [Google Scholar]
Loria 2000
- Loria CM, Klag MJ, Caulfield LE, Whelton PK. Vitamin C status and mortality in US adults. American Journal of Clinical Nutrition 2000;72(1):139-45. [DOI] [PubMed] [Google Scholar]
Maggini 2017
- Maggini S, Maldonado P, Cardim P, Fernandez Newball C, Sota Latino ER. Vitamins C, D and zinc: synergistic roles in immune function and infections. Vitamins & Minerals 2017;6(2):1-10. [Google Scholar]
Mochalkin 1970
- Mochalkin NI. Ascorbic acid in the complex therapy of acute pneumonia. Voenno-Meditsinskii Zhurnal 1970;9:17-21. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Niessen 2009
- Niessen L, ten Hove A, Hilderink H, Weber M, Mulholland K, Ezzati M. Comparative impact assessment of child pneumonia interventions. Bulletin of the World Health Organization 2009;87(6):472-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nualart 2003
- Nualart FJ, Rivas CI, Montecinos VP, Godoy AS, Guaiquil VH, Golde DW, et al. Recycling of vitamin C by a bystander effect. Journal of Biological Chemistry 2003;278(12):10128-33. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schleicher 2009
- Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). American Journal of Clinical Nutrition 2009;90(5):1252-63. [DOI] [PubMed] [Google Scholar]
Smith 2017
- Smith ER, Shankar AH, Wu LS, Aboud S, Adu-Afarwuah S, Ali H, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Global Health 2017;5(11):e1090-100. [DOI] [PubMed] [Google Scholar]
Theodoratou 2010
- Theodoratou E, Johnson S, Jhass A, Madhi Shabir A, Clark A, Boschi-Pinto C, et al. The effect of Haemophilus influenzae type b and pneumococcal conjugate vaccines on childhood pneumonia incidence, severe morbidity and mortality. International Journal of Epidemiology 2010;39(Suppl 1):i172-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Troeger 2017
- Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Swartz S, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infectious Diseases 2017;17(11):1133-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villalpando 2003
- Villalpando S, Montalvo-Velarde I, Zambrano N, García-Guerra A, Ramírez-Silva CI, Shamah-Levy T, et al. Vitamins A, and C and folate status in Mexican children under 12 years and women 12-49 years: a probabilistic national survey. Salud Publica de Mexico 2003;45(Suppl 4):S508-19. [DOI] [PubMed] [Google Scholar]
Webster 2011
- Webster J, Theodoratou E, Nair H, Seong AC, Zgaga L, Huda T, et al. An evaluation of emerging vaccines for childhood pneumococcal pneumonia. BMC Public Health 2011;11(3):S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitney 2017
- Whitney CG. Measuring progress on preventing pneumonia deaths: are we there yet? Lancet Infectious Diseases 2017;17(11):1100-1. [DOI] [PubMed] [Google Scholar]
WHO/UNICEF 2009
- World Health Organization and UNICEF . Global action plan for prevention and control of pneumonia (GAPP). www.who.int/maternal_child_adolescent/documents/fch_cah_nch_09_04/en/ (accessed 11 April 2018).
WHO/UNICEF 2013
- World Health Organization and UNICEF . Ending preventable child deaths from pneumonia and diarrhoea by 2025: the integrated global action plan for pneumonia and diarrhoea (GAPPD). apps.who.int/iris/bitstream/handle/10665/79200/9789241505239_eng.pdf (accessed 26 April 2018).
WHO 2014
- World Health Organization. Revised WHO classification and treatment of childhood pneumonia at health facilities: evidence summaries. apps.who.int/iris/bitstream/handle/ (accessed 11 April 2018).
Winterbourn 2016
- Winterbourn CC, Kettle AJ, Hampton MB. Reactive oxygen species and neutrophil function. Annual Review of Biochemistry 2016;85:765-92. [DOI: 10.1146/annurev-biochem-060815-014442] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Das 2018
- Das JK, Bilal H, Salam RA, Bhutta ZA. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD013134. [DOI: 10.1002/14651858.CD013134] [DOI] [Google Scholar]
Hemilä 2013
- Hemilä H, Chalker E. Vitamin C for preventing and treating pneumonia. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No: CD005532. [DOI: 10.1002/14651858.CD005532.pub3] [DOI] [PubMed] [Google Scholar]


