Abstract
Background
P1093 is an ongoing phase I/II multicenter open-label study of dolutegravir plus an optimized background regimen in age-defined pediatric cohorts; here we report the long-term safety and virologic efficacy outcomes for the oldest cohort.
Methods
The study enrolled human immunodeficiency virus type 1 (HIV-1)–infected treatment-experienced adolescents aged 12 to <18 years, with an HIV-1 RNA level ≥1000 copies/mL . Cumulative safety and HIV-1 RNA outcomes were assessed once the last enrolled participant reached 144 weeks of follow-up.
Results
Among 23 adolescents enrolled, 16 remained in the study at least 144 weeks; the median follow-up was 153 weeks (range, 55–193 weeks). Dolutegravir was well tolerated, with grade 3 clinical adverse events in 5 participants, grade 3 laboratory abnormalities in 3, and grade 4 laboratory abnormalities in 1; none of the adverse events or abnormalities were judged to be treatment related. In an-intent-to-treat analysis, an HIV-1 RNA level <400 copies/mL at week 144 was achieved in 43% (10 of 23 participants; 95% confidence interval, 23.2%–65.5%); in addition, 35% (8 of 23; 16.4%–57.3%) had an HIV-1 RNA level <50 copies/mL. Nine participants (39%) discontinued study treatment before 144 weeks, but none because of adverse events or drug intolerance. All participants with sustained virologic control had excellent adherence; most who experienced virologic failure had adherence levels <90%. HIV-1 genotypic drug resistance testing was available at time of failure from 6 participants; 1 had evolution in integrase resistance with E138T, S147G, and R263K mutations at week 192 and phenotypic dolutegravir resistance of a 5.1-fold change.
Conclusions
Dolutegravir plus an optimized background regimen seemed safe, well tolerated, and efficacious in this cohort of treatment-experienced HIV-1-infected adolescents. Adherence remains problematic in this population.
Clinical Trials Registration
Keywords: Antiretroviral agents, Dolutegravir, HIV-1 integrase inhibitors, Adolescent, long-term follow-up
The phase I/II P1093 study enrolled treatment-experienced human immunodeficiency virus–infected adolescents, followed up for a median of 153 weeks. Dolutegravir plus an optimized background regimen was safe, well tolerated, and efficacious for those who adhered to their antiretroviral regimen.
Effective combination antiretroviral (ARV) therapy (ART) leading to suppression of viral replication with improvements in immune status is associated with declining morbidity, hospitalization, and mortality rates in children infected with human immunodeficiency virus (HIV) type 1 (HIV-1) [1–5]. However, short and long-term toxic effects, poor adherence to ARV regimens, and lack of treatment alternatives due to HIV-1 resistance resulting from prior failing ARV regimens, local drug availability, and the paucity of appropriate pediatric fixed-dose combination tablets further complicates HIV-1 management and contributes to the development of additional drug resistance mutations [6–9]. The development of safe, palatable, and potent ARV regimens, particularly those that block alternative targets in the HIV-1 life cycle that have a high barrier to resistance, are critically important for all children and adolescents, especially those who have treatment failure or are unable to tolerate currently available ARVs.
Dolutegravir is a second-generation potent inhibitor of the HIV-1 integrase that has shown good safety, tolerability, predictable pharmacokinetics, and efficacy in treatment-naive and treatment-experienced adults in phase III trials [10–12]. Its pharmacologic properties allow for once-daily dosing, preclude the need for pharmacokinetic boosting, and include a low potential for drug-drug interactions [13, 14]. The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1093 is a phase I/II multicenter, open-label study designed to evaluate the safety, tolerability, pharmacokinetic and antiviral efficacy of dolutegravir in combination with an optimized background regimen in HIV-1-infected, treatment-experienced adolescents, children, and infants, from 4 weeks to <18 years of age [15].
Based on data from P1093, dolutegravir is approved by regulatory authorities for children in Europe from 6 years down to a weight of 15 kg, and in the United States for children and adolescents down to a weight of 30 kg [14–19]. As reported elsewhere, dolutegravir at a dose of approximately 1 mg/kg once daily in treatment-experienced adolescents achieved pharmacokinetic exposures comparable to those in adults, seemed safe and well tolerated, and showed efficacy comparable to that in adults at 48-week follow-up [15]. In the current article, we report on the cumulative long-term safety and efficacy of dolutegravir when administered as part of a combination regimen in this treatment-experienced adolescent cohort of P1093.
METHODS
Study Design and Participants
P1093 is an open-label, nonrandomized, multicenter phase I/II study of HIV-1-infected, treatment-experienced children aged 4 weeks to <18 years in age-defined cohorts. Adolescents between 12 and <18 years old constitute cohort I and are the subjects of this report. Selected entry criteria included plasma HIV-1 RNA level >1000 copies/mL, being ART-experienced but naive to integrase inhibitors, having a screening HIV-1 genotype showing sensitivity to ≥1 other active ARV agent added to an optimized treatment regimen, laboratory values at grade ≤2 toxicity criteria, and absence of active opportunistic infection or current cancer. The study was conducted at the IMPAACT Network sites, after approvals were obtained from local institutional review boards and in-country ethics committees responsible for oversight of the study. All parents and legal guardians gave written permission for participation. Adolescents were informed about the study and gave assent, as per local guidelines.
The study was conducted with 2 sequential stages. Stage I evaluated intensive pharmacokinetic and short-term safety in 10 participants and led to cohort dose selection (1 mg/kg/d), as described elsewhere [15]. An additional 13 participants were then enrolled into stage II to assess long-term safety and efficacy when treated with the stage I dolutegravir dose. Stage I and II participants are combined for reporting the long-term results of dolutegravir treatment in adolescents. The dolutegravir dose was prescribed using the weight band approach, as follows: 50 mg for children weighing ≥40 kg and 35 mg for children 30–40 kg, given as 2 tablets (25 mg + 10 mg). Dolutegravir was administered with an optimized background regimen that was chosen by the clinician and then approved by study chairs. After the initial 48-week study period, participants were evaluated every 12 weeks.
Sites were required to report all grade ≥3 toxicity events, serious adverse events [20], cancers, and pregnancies to the P1093 team and study sponsor, the Division of AIDS, National Institute of Allergy and Infectious Disease. Adherence was assessed at each study visit, with participants completing an adherence questionnaire (3-day recall) and site personnel collecting and counting the returned dolutegravir tablets to determine missed doses at each visit. Plasma HIV-1 RNA concentrations were determined at entry and at regular intervals using the RealTime HIV-1 assay (Abbott Molecular). Emergence of resistant virus was monitored by isolating viral RNA from plasma specimens from participants with persistent viremia, defined as not achieving a >1.0 log decrease at week 12 or later unless the HIV-1 RNA level was <400 copies/mL or, after week 24, having 2 consecutive viral load measurements >400 copies/mL, with the confirmatory RNA measurement performed 1–4 weeks later.
Protease and reverse-transcriptase (RT) resistance was assessed with the TRUGENE HIV-1 Genotyping Assay (Siemens), following the manufacturer’s instructions. Genotypic resistance to integrase inhibitors was evaluated using a CLIA-approved, VQA-certified assay that amplifies HIV-1 pol encoding integrase codons 1–286 for bidirectional Sanger sequencing. Plasma specimens from enrollment and virologic failure visits were compared to identify newly selected resistance mutations. Drug resistance was defined by the Stanford University HIV-1 Drug Resistance Database (http://hivdb.stanford.edu). Phenotypic dolutegravir susceptibility was determined using the PhenoSense HIV-1 assay (Monogram Biosciences). In this assay, drug susceptibility is expressed as the fold change of the drug concentration required to inhibit viral replication by 50%. Participants were allowed to continue receiving study drug if the site and protocol team agreed that the participant was actively addressing adherence issues and in his or her best interest.
Participants were discontinued from the study if they became pregnant, failed to comply with study procedures in a manner that risked harm, or required treatment with a disallowed medication. Documented nonadherence to study drug and/or persistent viremia were also criteria for discontinuation, but continued participation was allowed if the site and study team believed it was in the child’s best interest. Participants who met criteria for discontinuation were followed up for ≥4 weeks after study drug cessation for safety follow-up before discontinuation.
Analysis
All available safety and laboratory data were included for participants from enrollment until the date that the last enrolled participant in this cohort reached 144 weeks of study and the database was locked (14 February 2015) for a regulatory submission. This analysis therefore included data from participants who enrolled either in stage I or stage II. Virologic efficacy was a prespecified secondary study objective and was assessed using the intent-to-treat (ITT) analysis, snapshot algorithm defined by the US Food and Drug Administration [21]; virologic success was defined as having a plasma HIV-1 RNA level <400 copies/mL at week 144. The proportion achieving HIV-1 RNA levels <50 copies/mL at week 144 was also assessed. For each participant, an average of 3-day recall reports from all visits was determined to assess treatment adherence.
RESULTS
Baseline Characteristics
Twenty-three perinatally infected adolescents were enrolled between April 2011 and April 2012. Participants were mostly female (78%), with a median age of 15 years (range, 12–17 years) and a median weight of 52 kg (33–91 kg), from multiple sites in the United States and Thailand [15] (Table 1). All participants were nucleoside RT inhibitor experienced, and a majority were protease inhibitor and/or nonnucleoside RT inhibitor-experienced; 35% were triple class experienced. In addition, 2 of the 23 (9%) were entry inhibitor (enfuvirtide) experienced. The median exposure to ART before enrollment was 12.5 years (interquartile range, 10.8–14.0 years).
Table 1.
Participant Demographics and Baseline Characteristics (N = 23)
| Characteristic | Participants, No. (%)a |
|---|---|
| Age, median (IQR), y | 15 (12–16) |
| Female sex | 18 (78) |
| Race | |
| Black or African American | 12 (52) |
| White | 8 (35) |
| Asian | 3 (13) |
| Hispanic ethnicity | 6 (26) |
| Study site | |
| United States | 20 (87) |
| Thailand | 3 (13) |
| Plasma HIV-1 RNA, median (IQR), log10 copies/mL | 4.3 (3.9–4.6) |
| CD4+ cell count, median (IQR), cells/µL | 466 (297–771) |
| CD4+ proportion, median (IQR), % | 22 (1–39) |
| CDC HIV-1 classification | |
| Stage 1 | 6 (26) |
| Stage 2 | 5 (22) |
| Stage 3 | 12 (52) |
| Prior ART experience | |
| NRTI | 23 (100) |
| PI | 18 (78) |
| NNRTI | 12 (52) |
| Triple class | 8 (35) |
| Enfuvirtide | 2 (9) |
| Duration of prior ART, median (IQR), y | 12.5 (10.8–14.0) |
Abbreviations: ART, antiretroviral therapy; CDC, US Centers for Disease Control and Prevention; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
aData represent No. (%) of participants unless otherwise specified.
Optimized Background Regimen
The most common chosen optimized background regimen was tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and ritonavir-boosted darunavir (DRV/r), used in 7 of the 23 participants (30%). The following regimens were used in 3 participants (13%) each: abacavir, lamivudine (3TC), and DRV/r; TDF, 3TC, and ritonavir-boosted lopinavir; and TDF, FTC, and efavirenz (EFV). TDF and FTC was used in 2 participants (9%), and the following regimens in 1 participant (4%) each: TDF, FTC, DRV/r, and etravirine; TDF and ritonavir-boosted atazanavir; TDF, FTC, and ritonavir-boosted atazanavir; abacavir, 3TC, and atazanavir; and zidovudine, 3TC, and DRV/r. Detailed information is provided in Supplementary Table 1.
Outcomes, Safety, and Adverse Events
With 16 of 23 participants completing ≥144 weeks in the study, the median follow-up was 153 weeks (range, 55–93 weeks). Seven discontinued study participation before 144 weeks: 2 were lost to follow-up (at weeks 60 and 84), 1 stopped after meeting the protocol-specified virologic failure end point (at week 84), 1 left when the local clinical research site was defunded (at week 120), 1 left when the family relocated distant from the study clinic (at week 96), and 2 were discontinued owing to their noncompliance with taking prescribed medications (at weeks 60 and 108). The study drug was received by participants for a median of 147 weeks (range, 40–193 weeks), with 9 discontinuing the study drug before week 144. In addition to the above 7 study discontinuations, 1 participant became pregnant (week 156) and another chose to switch to commercially available dolutegravir to be included in prepared packaging to facilitate overall adherence (week 108).
Dolutegravir was well tolerated, and no adverse events were judged to be “probably” or “definitely” related to the study drug. All participants experienced ≥1 grade 1 or 2 transient clinical event; the most frequently reported involved the respiratory, gastrointestinal, and musculoskeletal systems and/or findings consistent with common clinical events (ie, fever) experienced by an adolescent population (Table 2). There were 5 serious clinical adverse events: gastritis, depression with a suicide attempt in a participant with preexisting mental illness, deep vein thrombosis, pelvic inflammatory disease, and non-Hodgkin lymphoma (Table 3). All participants experiencing a serious adverse event continued dolutegravir therapy except the participant with non-Hodgkin lymphoma, who had to discontinue study participation to receive chemotherapy at a nonparticipating institution.
Table 2.
Clinical Adverse Events
| Adverse Event | Participants, No. (%) (N = 23) |
|---|---|
| Respiratory system | |
| Cough | 13 (56) |
| Pharyngeal pain | 8 (35) |
| Nasal congestion | 7 (30) |
| Sinus congestion | 4 (17) |
| Gastrointestinal system | |
| Diarrhea | 8 (35) |
| Decreased appetite | 7 (30) |
| Abdominal pain | 5 (21) |
| Nausea | 3 (13) |
| General | |
| Fever | 7 (30) |
| Lymphadenopathy | 6 (26) |
| Headache | 6 (26) |
| Dizziness | 4 (17) |
| Musculoskeletal system | |
| Extremity pain | 6 (26) |
| Arthralgia | 3 (13) |
| Back pain | 3 (13) |
Table 3.
Grade 3 or 4 Clinical and Laboratory Adverse Events
| Clinical or Laboratory Adverse Event | Participants, No. (%) (N = 23) | |
|---|---|---|
| Grade 3 Event | Grade 4 Event | |
| Clinical adverse events | ||
| Blood and lymphatic system disorders | ||
| Lymphadenopathy | 1 (4.3) | 0 (0) |
| Gastrointestinal disorders | 0 (0) | |
| Abdominal pain | 1 (4.3) | 0 (0) |
| Gastritis | 1 (4.3) | 0 (0) |
| Infection or infestation | ||
| Pelvic inflammatory disease | 1 (4.3) | 0 (0) |
| Psychiatric disorders | ||
| Depression | 0 (0) | 1 (4.3) |
| Reproductive system and breast disorders | ||
| Pelvic pain | 1 (4.3) | 0 (0) |
| Skin and subcutaneous tissue disorders | ||
| Alopecia | 1 (4.3) | 0 (0) |
| Vascular disorders | ||
| Deep vein thrombosis | 1 (4.3) | 0 (0) |
| Laboratory adverse events | ||
| Increased blood bilirubin | 1 (4.3) | 0 (0) |
| Increased lipase | 1 (4.3) | 0 (0) |
| Decreased neutrophil count | 0 (0) | 1 (4.3) |
| Decreased white blood cell count | 1 (4.3) | 0 (0) |
Three participants experienced grade 3 laboratory abnormalities; 1 experienced leukopenia, 1 had unconjugated bilirubin elevation while taking concomitant atazanavir, and 1 had transient asymptomatic lipase elevation, which was deemed unrelated to treatment and resolved spontaneously. Persistent grade 4 neutropenia developed in 1 participant with a long history of neutropenia (Table 3). There were no grade 4 adverse events, serious adverse events, or discontinuations owing to adverse events. Of note, 1 female participant discontinued study drug during the first trimester of her pregnancy at study week 156, and later delivered a full-term healthy newborn.
Efficacy
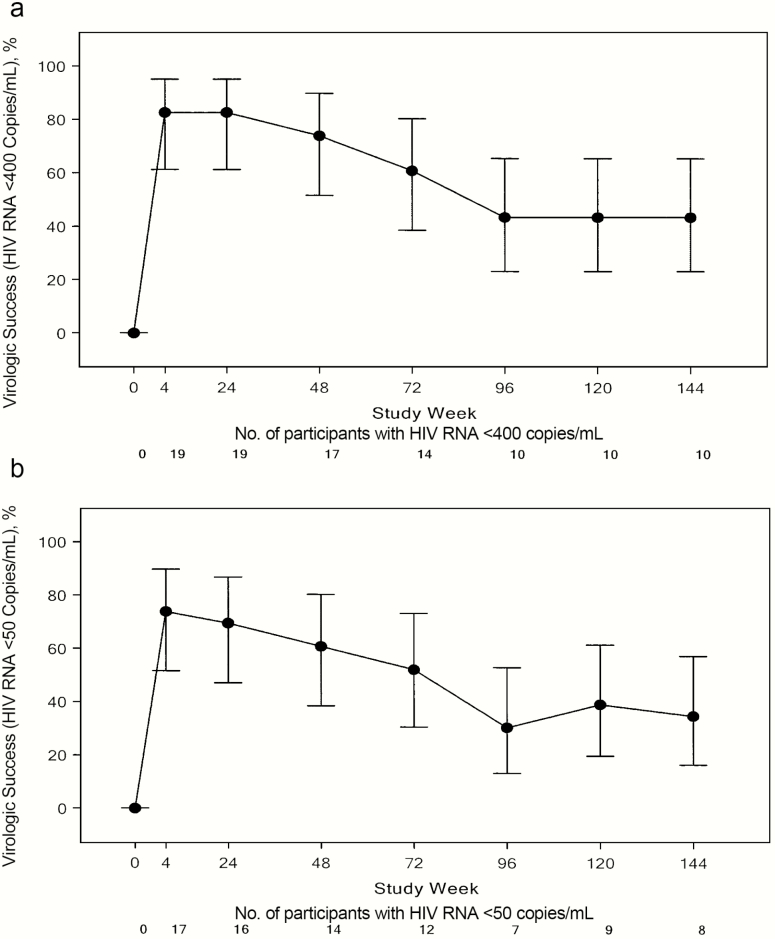
At week 4, of 23 participants, 19 (83%) had a plasma HIV-1 RNA level <400 copies/mL, although there was a progressive decrease in participants with sustained virologic control over time. In the ITT analysis, virologic success (viral load <400 copies/mL at week 144) was achieved in 10 of 23 participants (43%; 95% confidence interval, 23.2%–65.5%), and 8 of 23 (35%; 16.4%–57.3%) had an HIV-1 RNA level <50 copies/mL at week 144 (Figure 1).
Figure 1.
Proportion of participants with human immunodeficiency virus type 1 (HIV-1) RNA level <400 (A) or <50 (B) copies/mL, in the intent-to-treat analysis (N = 23). Vertical bars represent 95% confidence intervals.
ARV Drug Resistance
Of the 13 participants who had virologic failure per the ITT analysis, a resistance testing sample was available and testing performed in 6. Four of these 6 participants experienced their first incidence of virologic failure before or at week 48 but remained in the study. One of them participants (participant 3; Table 4) had a treatment-emergent R263R/K mutation with additional integrase mutations at positions 138 and 147 observed at week 132, which was sustained at week 162. This participant reported only partial adherence to dolutegravir and almost no adherence to the other optimized background regimen components, remained engaged in care, and continued the failing optimized background regimen as chosen by the local physician.
Table 4.
Drug Resistance Mutations in 6 Participants With Confirmed VF (Viral Load >400 Copies/mL) at Week 48 and Beyond
| Participant | Baseline HIV-1 RNA, Copies/mL (wk 0) |
HIV-1 RNA at VF, Copies/mL (wk) |
OBR ART |
Coding Region |
Baseline Genotype |
VF Genotype |
VF Genotype |
VF Genotype |
|---|---|---|---|---|---|---|---|---|
| wk 0 | wk 36 | wk 132 | wk 162 | |||||
| 3 | 7739 | 13 722 (36) | TDF, FTC | RT | WT | WT | WT | WT |
| 1367 (132) | PR | I84V | M36I | WT | WT | |||
| 1996 (162) | IN | L74L/M | R263R/K | E138A/E/K/T | E138T | |||
| S147S/G | S147G | |||||||
| R263K | R263K | |||||||
| wk 0 | wk 24 | wk 84 | ||||||
| 6 | 184 871 | 82 432 (24) | TDF, FTC, DRV/r | RT | WT | WT | WT | |
| 61 781 (84) | PR | D30N, A71T | D30N, A71T | D30N, A71T | ||||
| N88D | N88D | N88D | ||||||
| IN | WT | WT | WT | |||||
| 0 | 154 | |||||||
| 8 | 1168 | 12 875 (154) | TDF, FTC, DRV/r |
RT | K103N | K103N | ||
| PR | WT | WT | ||||||
| IN | WT | WT | ||||||
| wk 0 | wk 48 | wk 96 | ||||||
| 15 | 23 499 | 3002 (48) | TDF, 3TC, LPV/r | RT | WT | WT | WT | |
| 18 849 (96) | PR | WT | M36I, L89M | M36I, L89M | ||||
| IN | WT | WT | WT | |||||
| wk 0 | wk 192 | |||||||
| 18 | 16 305 | 1758 (192) | EFV, TDF, FTC | RT | WT | M41L, T215F/L | ||
| PR | WT | WT | ||||||
| IN | WT | G118R, L74M | ||||||
| wk 0 | wk 24 | wk 144 | ||||||
| 21 | 18 228 | 45 986 (24) | ABC, 3TC, ATV | RT | M184V, K103N | M184V, K103N | K103K/N | |
| 64 139 (144) | PR | WT | WT | WT | ||||
| IN | WT | WT | WT |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV, atazanavir; DRV/r, ritonavir-boosted darunavir; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; IN, integrase; OBR, optimized background regimen; LPV/r, ritonavir-boosted lopinavir; PR, protease; RT, reverse-transcriptase; TDF, tenofovir disoproxil fumarate; VF, virologic failure; WT, wild type.
Dolutegravir phenotypic resistance studies after virologic failure showed a progressive decrease in dolutegravir susceptibility, from a 1.2-fold change at week 36 to a 5.1-fold change at weeks 132 and 162. There was no emergence of protease mutations conferring drug resistance in participants 6, 8, 15, or 21, while they were receiving a protease inhibitor–containing regimen (Table 4). There was no evolution in RT-associated mutations in any participants except participant 18, who, while taking TDF, FTC, and EFV, had emergent multiple nucleoside RT inhibitor–associated mutations M41L and T215F/L but no EFV-associated mutation at week 192 (Table 4). This participant, aside from the RT-associated mutations, showed minor integrase-associated resistance mutations G118R and L74M. The reported adherence was 73%, but this participant had not been taking dolutegravir for 5 months before genotypic testing (Table 4).
Adherence
Adherence was measured by the 3-day recall adherence questionnaire for the participants who remained on a dolutegravir regimen at most scheduled visits through week 144. Most participants (15 of 23) reported >95% mean adherence with study medication. Among the 10 of 23 participants with virologic control, dolutegravir adherence was 97%–100%. Two of the participants with failure due to premature study discontinuation (site closure or participant choice) had controlled viremia and 100% adherence. Of the remaining 11 participants with virologic failure, the mean overall adherence was 85% (range, 53%–100%). Two participants who met virologic failure criteria continued taking study medication, had HIV-1 RNA levels <50 copies/mL at week 144 (by polymerase chain reaction), and had overall adherence rates of 97% and 98%.
DISCUSSION
This article describes the safety and efficacy of dolutegravir in ART-experienced adolescents through a median of 153 weeks of follow-up, representing the longest reported longitudinal study of the safety and efficacy of an integrase inhibitor–based therapy in an adolescent population with perinatally acquired HIV-1 infection. In this study, dolutegravir was found to be safe and well tolerated, with no discontinuations due to adverse events. The proportion of participants in an ITT analysis with HIV-1 RNA values <50 copies/mL continued to progressively diminish beyond week 48, from 61% to 35%, at a median of 153 weeks of follow-up. The high virologic failure rate we report in P1093 reflects numerous challenges, including many psychosocial and behavioral, affecting sustained drug adherence and continued study participation in a population of perinatally infected adolescents with a median of 12.5 years of prior ARV exposure.
All 10 adolescents with virologic success reported ≥97% medication adherence based on 3-day recall, whereas the mean reported adherence rate among those with virologic failure was 85%. Two adolescents with controlled viremia prematurely terminated their study participation owing to extenuating circumstances and were included as failures in the ITT analysis. Two other participants who met virologic failure criteria elected to continue their dolutegravir regimen with enhanced adherence counseling and then had virologic suppression, which was sustained through week 144. This finding supports the long-term potency of a dolutegravir-based regimen in the presence of sustained adherence and suggests that, in adolescents, efforts to improve and sustain adherence or longer acting ARV preparations are still needed.
Dolutegravir has been extensively studied in HIV-1-infected adults. The SINGLE study, a randomized double-blind noninferiority study, evaluated the safety and efficacy of dolutegravir, abacavir, and 3TC versus EFV, tenofovir, and FTC in 833 ART-naive adults [22]. Dolutegravir showed superior efficacy (71% vs 63%) in maintaining HIV-1 RNA levels <50 copies/mL at week 144, with fewer discontinuations due to adverse events in the dolutegravir arm [22]. Another phase III study (FLAMINGO) in treatment-naive adults randomized to dolutegravir versus DRV/r found higher efficacy among the dolutegravir arm at week 96 [23]. The higher efficacy of dolutegravir was driven by a better response among participants with high baseline HIV-1 RNA levels [23]. The long-term safety profile of dolutegravir was similar to that of DRV/r, with gastrointestinal effects and headache the most frequent adverse events, effects commonly seen in our adolescent study. In this study, dolutegravir had a safety profile similar to that in adult trials, whereas efficacy in adolescents was lower than among treatment-naive adults, probably owing to challenges associated with sustained adherence to study drugs in heavily treatment-experienced perinatally infected adolescents.
Owing to the lack of treatment options available to our pediatric participants experiencing virologic failure, many continued on a failing regimen without accumulating additional RT-associated mutations. In some cases, addressing adherence challenges resulted in subsequent suppression of virus, which was sustained through week 144. However, in 1 participant who continued treatment with TDF, FTC, and dolutegravir through week 162, additional integrase resistance mutations accumulated, in the absence of developing nucleotide or nucleoside analogue resistant–associated mutations, possibly because of poor adherence with the nucleotide/nucleoside analogue backbone and functional dolutegravir monotherapy. This participant’s integrase inhibitor genotype evolved from a mixed R263R/K mutation at week 36, with emergence of E138T, S147G, and R263K mutations at week 162. The corresponding HIV-1 phenotypic dolutegravir resistance increased over time from 1.2-fold to 5.1-fold in the drug concentration required to inhibit viral replication by 50%. The emergence of the dolutegravir resistance mutation R263K has been observed in vitro and occasionally in vivo among participants with incomplete adherence failing dolutegravir [12, 24]. This R263K mutation confers some fitness cost and therefore has not been associated with high-level phenotypic resistance [25].
In our population of heavily treatment-experienced adolescents with perinatally acquired HIV-1 infection, dolutegravir was safe, well tolerated, and efficacious among the adolescents who remained adherent to their ART regimen and to study visits after a median of 153 weeks. Sustained medication adherence in perinatally infected populations, especially adolescents, owing to multiple psychosocial and behavioral issues, remains a challenge and affects efficacy over extended periods of time. Study P1093 continues to evaluate the pharmacokinetics, safety, and efficacy of dolutegravir in pediatric populations as young as 1 month.
Supplementary Material
Notes
Acknowledgments. We thank all the participants and their caregivers participating in the P1093 study.
P1093 study team. Study team members include Barbara Heckman, Stephanie Popson, Thucuma Sise, Katelyn Hergott, and Kathryn Myers. Participant sites and site personnel include 5018 University of South Florida–Tampa Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) CRS (Carina A. Rodriguez, MD, Patricia J. Emmanuel, MD, and Denise Casey, RN); 5091 University of California, San Francisco, NICHD CRS (Diane Wara, MD, and Nicole Tilton, PNP); 5083 Rush University Cook County Hospital Chicago NICHD CRS (Mariam Aziz, MD, Maureen McNichols, RN, MSN, CCRC, and Latania Logan, MD); 31784 Chiang Mai University HIV Treatment CRS (Virat Sirisanthana, MD, Linda Aurpibul, MD, MPH, and Nataporn Kosachunhanan, BPharm); 4001 Chicago Children’s CRS (Jennifer Jensen, PNP, Ruth Williams, RN, and Tarannum Qureshi, PharmD); 5013 Jacobi Medical Center Bronx NICHD CRS (Joanna Dobroszycki, MD, Heesun Huh, PharmD, and Francisco Reinoso, RN); 5044 Howard University Washington DC NICHD CRS (Sohail Rana, MD, Patricia Houston, MS, and Mulu Mengistab, PharmD); 5009 Children’s Hospital of Boston NICHD CRS (Sandra K. Burchett, MD, MS, Nancy Karthas, RN, MN, CPNP, and Catherine Kneut, RN, MN, CPNP).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants UM1AI068632 [IMPAACT LOC], UM1AI068616 [IMPAACT SDMC], and UM1AI106716 [IMPAACT LC]), the NICHD, the National Institute of Mental Health, and ViiV Healthcare.
Potential conflict of interest. R. M. V. owns stock in AbbVie and in Cidara Therapeutics. A. M. B. and C. V. are ViiV Healthcare employees and own stock and/or stock options in GlaxoSmithKline (GSK). Rajendra Singh is a GSK employee and owns stock and/or stock options in GSK. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: ID Week, San Diego, California, 7–11 October 2015. Presentation 468.
Contributor Information
The P1093 Study Team:
Barbara Heckman, Stephanie Popson, Thucuma Sise, Katelyn Hergott, Kathryn Myers, Carina A Rodriguez, Patricia J Emmanuel, Denise Casey, Diane Wara, Nicole Tilton, Mariam Aziz, Maureen McNichols, Latania Logan, Virat Sirisanthana, Linda Aurpibul, Nataporn Kosachunhanan, Jennifer Jensen, Ruth Williams, Tarannum Qureshi, Joanna Dobroszycki, Heesun Huh, Francisco Reinoso, Sohail Rana, Patricia Houston, Mulu Mengistab, Sandra K Burchett, Nancy Karthas, and Catherine Kneut
References
- 1. Mocroft A, Ledergerber B, Katlama C, et al. ; EuroSIDA study group Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet 2003; 362:22–9. [DOI] [PubMed] [Google Scholar]
- 2. Gortmaker SL, Hughes M, Cervia J, et al. ; Pediatric AIDS Clinical Trials Group Protocol 219 Team Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med 2001; 345:1522–8. [DOI] [PubMed] [Google Scholar]
- 3. Viani RM, Araneta MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis 2004; 39:725–31. [DOI] [PubMed] [Google Scholar]
- 4. Resino S, Resino R, Maria Bellón J, et al. ; Spanish Group of Pediatric HIV Infection Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV type-1-infected children. Clin Infect Dis 2006; 43:243–52. [DOI] [PubMed] [Google Scholar]
- 5. Kourtis AP, Bansil P, Posner SF, et al. . Trends in hospitalizations of HIV-infected children and adolescents in the United States: analysis of data from the 1994–2003 Nationwide Inpatient Sample. Pediatrics 2007; 120:e236-43. [DOI] [PubMed] [Google Scholar]
- 6. van Rossum AM, Fraaij PL, de Groot R. Efficacy of highly active antiretroviral therapy in HIV-1 infected children. Lancet Infect Dis 2002; 2:93–102. [DOI] [PubMed] [Google Scholar]
- 7. Mocroft A, Lundgren JD. Starting highly active antiretroviral therapy: why, when and response to HAART. J Antimicrob Chemother 2004; 54:10–3. [DOI] [PubMed] [Google Scholar]
- 8. Delaugerre C, Warszawski J, Chaix ML, et al. . Prevalence and risk factors associated with antiretroviral resistance in HIV-1-infected children. J Med Virol 2007; 79:1261–9. [DOI] [PubMed] [Google Scholar]
- 9. Dehority W, Deville JG, Lujan-Zilbermann J, et al. . Effect of HIV genotypic drug resistance testing on the management and clinical course of HIV-infected children and adolescents. Int J STD AIDS 2013; 24:549–53. [DOI] [PubMed] [Google Scholar]
- 10. Raffi F, Jaeger H, Quiros-Roldan E, et al. . extended SPRING-2 Study Group Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013; 13:927–35. [DOI] [PubMed] [Google Scholar]
- 11. Walmsley SL, Antela A, Clumeck N, et al. . SINGLE Investigators Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 12. Cahn P, Pozniak AL, Mingrone H, et al. . extended SAILING Study Team Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382:700–8. [DOI] [PubMed] [Google Scholar]
- 13. Cottrell ML, Hadzic T, Kashuba AD. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 2013; 52:981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Min S, Song I, Borland J, et al. . Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother 2010; 54:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viani RM, Alvero C, Fenton T, et al. . P1093 Study Team Safety, pharmacokinetics and efficacy of dolutegravir in treatment-experienced HIV-1 infected adolescents: forty-eight-week results from IMPAACT P1093. Pediatr Infect Dis J 2015; 34:1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tivicay®. Dolutegravir (prescribing information). ViiV Healthcare Available at: https://accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf. Accessed 14 September 2018.
- 17. European Medicines Agency. Tivicay®, ViiV Healthcare. INN-dolutegravir-European Medicines Agency-Europa EU Available at: www.ema.europa.eu/docs/en_GB/document_library/WC500160680.pdf. Accessed 14 September 2018.
- 18. Viani R, Zheng N, Alvero C, et al. . Safety and efficacy of dolutegravir (DTG; GSK1349572) in treatment-experienced HIV-1 infected adolescents: 24-week results from IMPAACT P1093. Abstract 172. In: ID Week, 2–6 October 2013, San Francisco CA, 2013.
- 19. Wiznia A, Alvero C, Fenton T, et al. . IMPAACT 1093: Dolutegravir in 6–12 year old HIV infected children: 48-week results. In: Conference on Retroviruses and Opportunistic Infections, 22–25 February 2016. Boston, MA, 2016.
- 20.Division of AIDS, National Institute of Allergy and Infectious Diseases. Manual for expedited reporting of adverse events to DAIDS. Version 2.0. January 2010. Available at: https://rsc.niaid.nih.gov/sites/default/files/manual-exped-aes-v2_0.pdf. Accessed 3 January 2011.
- 21. Smith F, Hammerstrom T, Soon G, et al. . A meta-analysis to assess the FDA DAVP’s TLOVR algorithm in HIV submissions. Drug Inf J 2011; 45:291–300. [Google Scholar]
- 22. Walmsley S, Baumgarten A, Berenguer J, et al. . Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70:515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Molina JM, Clotet B, van Lunzen J, et al. . FLAMINGO study team Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV 2015; 2:e127–36. [DOI] [PubMed] [Google Scholar]
- 24. Quashie PK, Sloan RD, Wainberg MA. Novel therapeutic strategies targeting HIV integrase. BMC Med 2012; 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wainberg M, Anstett K, Mesplede T, et al. . The R263K mutation in HIV integrase that is selected by dolutegravir may actually prevent clinically relevant resistance to this compound. J Int AIDS Soc 2014; 17:19518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.