Abstract
Background
Recent increases in pertussis morbidity and mortality rates among young infants have led to a recommendation in some countries for vaccination against pertussis during pregnancy. Having data on the burden of pediatric pertussis in a large population over time is important for establishing the true burden of disease in the acellular pertussis (aP) vaccine era. Here, we describe age-specific epidemiology and morbidity and mortality rates in children hospitalized with pertussis over 17 years across Canada in the aP vaccine era.
Methods
Patients aged ≤16 years who were admitted to 1 of 12 pediatric tertiary-care hospitals across Canada between 1999 and 2015 with confirmed (laboratory-confirmed or epidemiologically linked) or probable (clinically diagnosed) pertussis were included.
Results
Overall, 1402 patients with pertussis were included. Infants aged <2 months had the highest mean annual incidences of pertussis hospitalization and intensive care unit (ICU) admission (116.40 [95% confidence interval (CI), 85.32–147.49] and 33.48 [95% CI, 26.35–40.62] per 100 000 population, respectively). The overall proportion of children who required ICU admission was 25.46%, and the proportion was highest in infants aged <2 months (37.90%). Over the span of this study, 21 deaths occurred. Age of <16 weeks, prematurity, encephalopathy, and a confirmed pertussis diagnosis were independent risk factors for ICU admission. Age of <4 weeks, prematurity, and female sex were independent risk factors for death.
Conclusions
In the aP vaccine era, endemic pertussis still contributes considerably to childhood morbidity and death, particularly in infants aged <2 months. Vaccination against pertussis during pregnancy has the potential to reduce this disease burden.
Keywords: Bordetella pertussis, infant pertussis, whooping cough, vaccination in pregnancy
Infants aged <2 months remain the major contributor to pediatric pertussis hospitalization. Independent risk factors for intensive care unit admission were age of <16 weeks, prematurity, encephalopathy, and confirmed pertussis diagnosis. Independent risk factors for death were age of <4 weeks, prematurity, and female sex.
Pertussis remains a major global public health concern, with a global estimate of 24.1 million cases of and 160700 deaths caused by pertussis in children aged <5 years in 2014 [1]. Most industrialized countries use diphtheria-tetanus-acellular pertussis vaccines for primary and/or booster immunization against pertussis [2, 3]. Despite the high rate of vaccination coverage with acellular pertussis (aP) vaccine, pertussis outbreaks that result in substantial morbidity and death continue to occur [4, 5].
Previous reports on the burden of pertussis disease in the aP vaccine era described incidence rates and clinical outcomes in small populations, a single center, a single region, a specific epidemic period, or another limited time period or years during which both whole-cell pertussis (wP) and aP vaccines were used [4, 6–11]. Moreover, estimates of the burden of severe pertussis disease that requires intensive care unit (ICU) admission were made from studies that spanned over a limited time period [10, 12]. Having data on the burden of pediatric pertussis from a large population over time is important for establishing the true burden of disease in the aP vaccine era, not only in an epidemic or outbreak setting but also to inform cost-effectiveness analyses of different immunization strategies. This data is especially important, because several countries have recommended universal vaccination against pertussis during pregnancy in response to a recent rise in pertussis morbidity and mortality rates among young infants [13–15].
Between 1997 and 1998, all Canadian provinces and territories changed from wP to the aP vaccine, given at 2, 4, 6, and 18 months and 4 to 6 years of age [16]. The burden of pertussis disease among children in Canada during the wP and wP–aP vaccine transition periods has been described [16, 17]. In this study, we report the age-specific epidemiology and morbidity and mortality rates in children hospitalized with pertussis disease in Canada in the aP vaccine era. We also identify risk factors associated with poor outcomes (morbidity and death) among pediatric patients hospitalized with pertussis.
METHODS
Study Locations
Patients with pertussis who were admitted to a hospital that is part of the Immunization Monitoring Program, Active (IMPACT) were included. IMPACT is an active surveillance network that has collected data from 12 pediatric tertiary-care hospitals across Canada since 1991 and accounts for approximately 90% of the pediatric tertiary-care beds in the country [16].
Study Subjects
Patients aged ≤16 years with pertussis who were admitted to an IMPACT hospital between January 1, 1999, and December 31, 2015 were included. A clinical case of pertussis was defined as a cough illness that lasted for ≥2 weeks with paroxysmal coughing. Posttussive vomiting, whoop, cyanosis during coughing, or apnea episodes were supportive evidence of a pertussis case. Consistent with the Canadian national pertussis case definitions [18], confirmed cases were laboratory confirmed (at least 1 positive microbiological test result for Bordetella pertussis [from culture, polymerase chain reaction, direct fluorescent assay, or serology]) or epidemiologically linked (ie, the patient met our clinical case definition and had contact with another patient with laboratory-confirmed pertussis). A probable case was defined as illness that met the clinical case definition but none of the confirmed-case criteria. Compatible illnesses shown to be attributable to another cause were excluded, but coinfections with B pertussis or another Bordetella species were included. Cases confirmed to be caused only by Bordetella species other than B pertussis were not included.
Data Collection and Management
Standardized case-report forms were used at all IMPACT hospitals. Pertussis cases were identified via microbiology laboratories, ward and ICU admission lists, infection-control practitioners, and/or a search of hospital records for International Classification of Diseases, Ninth or Tenth Revision, discharge codes that included terms for pertussis. Clinical data were collected from patient health records. Prematurity was defined as birth before 37 weeks’ gestation. Encephalopathy was defined as a decreased level of consciousness not associated with the postictal period. For each patient admitted with pertussis, vaccination records in the hospital record were reviewed. Data in the hospital records were confirmed from the relevant vaccine provider (public health or family physician), which varied according to region. When a discrepancy was found, the vaccine provider’s record was considered accurate. All data were reviewed at the IMPACT data center in Vancouver before being entered into an electronic database by using a dual-entry system with preprogrammed consistency checks.
Pertussis Vaccination Status
A valid vaccine dose was considered any dose administered ≥28 days before hospital admission with pertussis, and this information was used to classify children aged ≥3 months with laboratory-confirmed pertussis disease (see Supplementary Table 1).
Statistical Analysis
We used Pearson’s χ2 test to compare categorical variables, the Student t test for normally distributed continuous variables, and the Mann Whitney U test for nonnormally distributed continuous variables.
Annual age-specific pertussis hospitalization incidences were calculated using population estimates of each study hospital’s catchment area obtained from the 2006 Canadian census [19]. Each IMPACT hospital defined its estimated local population catchment area. Pertussis cases from the IMPACT hospital were matched to this catchment area using the first 3 characters of the postal code. The first 3 characters of the postal code form the forward sortation area, which represents a specific area within a major geographic region or province. Cases from outside the hospital catchment areas and 1 hospital with a large referral population and an undefined catchment area (The Hospital for Sick Children, Toronto) were not included in these hospitalization incidence calculations (Supplementary Table 2). Annual age-specific pertussis ICU admission incidences were calculated using relevant provincial population estimates obtained from the 2006 Canadian census [19]. Outside the province of Ontario, IMPACT hospitals are the only pediatric centers that admit to an ICU. Age-specific population estimates of the study population areas included the respective province. Patients with pertussis admitted to an IMPACT hospital ICU were matched to their respective province. Case-patients who were admitted to an IMPACT hospital ICU but resided in the Canadian territories were excluded from the ICU incidence analysis. Ontario also was excluded from the ICU incidence analysis, because patients also might have been admitted to another ICU not included in the IMPACT network (Supplementary Table 2). The overall mean annual pertussis hospitalization and ICU admission incidence rates and their 95% confidence intervals (CIs) were calculated for the 17-year period using the annual hospitalization and ICU admission incidence rates, respectively.
The variability of the median hospital lengths of stay (LOS), the proportion of children who required ICU admission during the study period, and the proportions of children who required ICU admission according to their gestational age at birth were analyzed using the Kruskal-Wallis test and the χ2 test for trend for continuous and categorical variables, respectively.
Univariate logistic regression analysis was used to identify risk factors for ICU admission and death. To identify the most appropriate age cutoff as a risk factor for ICU admission and death, we generated regression models using all ages between 0 and 16 years in 1-week intervals. The model with the lowest Akaike information criterion (AIC) was used as the final model. Forward stepwise multivariable logistic regression models were developed to identify independent risk factors for ICU admission and death. These models included all variables identified in the univariate regression model with a P value of ≤.25 and the age cutoff with the lowest AIC. Variables with a P value of <.05 were retained in the final model. All P values of <.05 were considered statistically significant for all tests. R 3.4.0 (R Project, Vienna, Austria) was used for all analyses.
RESULTS
Study Population
Overall, we included 1402 children, 1157 (82.52%) of whom had a confirmed case of pertussis (1145 [81.67%] laboratory confirmed, 12 [0.85%] epidemiologically linked) and 245 (17.48%) of whom had a probable case. The majority (810 [70.74%] of 1145) of laboratory-confirmed cases were diagnosed by polymerase chain reaction alone (Supplementary Figure 1). Patients with a confirmed case of pertussis were significantly younger, had longer hospital and ICU LOS, and were more likely to be admitted to the ICU than those who had a probable case (Table 1). The median hospital LOS among infants aged <6 months was 8 days (Table 1). We found significant year-to-year variation in the median hospital LOS (range, 4–10 days) over the study period (P = .0016).
Table 1.
Characteristics of Patients with Pertussis Admitted to IMPACT Hospitals in Canada, 1999–2015
| Characteristic | Pertussis Cases | P d | ||
|---|---|---|---|---|
| All (n = 1402)a | Confirmed (n = 1157)b | Probable (n = 245)c | ||
| Demographics | ||||
| Sex, male (n [% of total]) | 655 (46.71) | 525 (45.37) | 130 (53.06) | .0340 |
| Age (median [overall range (IQR)]) (wk) | 10 (1–886 [6–17]) | 9 (1–884 [6–16]) | 11 (1–886 [7–22]) | .0027 |
| Age group (n [% of total]) | ||||
| <1 year | 1265 (90.23) | 1056 (91.27) | 209 (85.30) | |
| 0–1 mo | 612 (43.65) | 523 (45.20) | 89 (36.32) | |
| 2–3 mo | 460 (32.81) | 381 (32.92) | 79 (32.24) | |
| 4–5 mo | 119 (8.48) | 91 (7.86) | 28 (11.42) | |
| 6–11 mo | 74 (5.27) | 61 (5.27) | 13 (5.30) | |
| 1–4 years | 72 (5.14) | 53 (4.58) | 19 (7.75) | |
| 5–9 years | 24 (1.71) | 15 (1.29) | 9 (3.67) | |
| 10–16 years | 41 (2.92) | 33 (2.85) | 8 (3.26) | |
| Clinical features | ||||
| Comorbidity (n [% of total])e | ||||
| Underlying condition(s) | 178 (12.69) | 152 (13.13) | 26 (10.61) | .5002 |
| Prematurityf | 50 (3.56) | 44 (3.80) | 6 (2.44) | |
| Pulmonary | 31 (2.21) | 23 (1.98) | 8 (3.26) | |
| Neurologic | 27 (1.92) | 24 (2.07) | 3 (1.22) | |
| Congenital cardiac | 23 (1.64) | 20 (1.72) | 3 (1.22) | |
| Gastrointestinal | 20 (1.42) | 16 (1.38) | 4 (1.63) | |
| Genetic-metabolic | 13 (0.92) | 10 (0.86) | 3 (1.22) | |
| Failure to thrive | 12 (0.85) | 11 (0.95) | 1 (0.40) | |
| Immunocompromised | 10 (0.71) | 10 (0.86) | 0 (0.00) | |
| Other | 34 (2.42) | 30 (2.59) | 4 (1.63) | |
| Seizures (n [% of total]) | .1204 | |||
| New seizures | 30 (2.13) | 28 (2.42) | 2 (0.81) | |
| Exacerbation of an existing seizure disorder | 8 (0.57) | 8 (0.69) | 0 (0.00) | |
| Encephalopathy (n [% of total]) | 8g (0.57) | 8 (0.69) | 0 (0.00) | .3443 |
| Outcome | ||||
| Hospitalization | ||||
| Median (overall range [IQR]) (days) | 7 (1–185 [3–13]) | 8 (1–185 [4–14]) | 4 (1–45 [2–7]) | <.0001 |
| Age-specific median LOS (overall range [IQR]) (days) | ||||
| <1 year | 8 (1–185 [3–14]) | 9 (1–185 [4–15]) | 4 (1–45 [2–8]) | <.0001 |
| 0–1 mo | 10 (1–87 [5–16]) | 11 (1–87 [6–17]) | 5 (1–45 [2–9]) | <.0001 |
| 2–3 mo | 6 (1–185 [3–12]) | 7 (1–185 [3–12]) | 4 (1–37 [2–9]) | .0003 |
| 4–5 mo | 4 (1–59 [2–8]) | 5 (1–59 [3–9.50]) | 3 (1–15 [2–4]) | .0002 |
| 6–11 mo | 4 (1–38 [2–9.75]) | 4 (1–38 [2–10]) | 3 (1–8 [2–5]) | .0507 |
| 1–4 years | 3 (1–60 [1–5]) | 3 (1–60 [2–5]) | 2 (1–13 [1–4.50]) | .2307 |
| 5–9 years | 2 (1–8 [1–5]) | 2 (1–68 [1–4.5]) | 3 (1–5 [2–5]) | .5019 |
| 10–16 years | 3 (1–14 [1–5]) | 1 (1–14 [2–5]) | 1 (1–5 [1–5]) | .1244 |
| ICU admission (n [% of total]) | 357 (25.46) | 316 (27.31) | 41 (16.73) | .0007 |
| Median ICU LOS (overall range [IQR]) (days) | 4 (1–79 [2–9]) | 5 (1–79 [2–10]) | 3 (1–23 [2–7]) | .0268 |
| Age-specific median ICU LOS (overall range [IQR]) (days) | ||||
| <1 year | 5 (1–79 [2-10]) | 5 (1–79 [2.5-10]) | 3 (1–23 [2–7]) | .0333 |
| 0–1 mo | 5 (1–79 [3–9]) | 5 (1–79 [3-9.75]) | 3 (1–23 [2–6]) | .0314 |
| 2–3 mo | 4 (1–62 [2–10]) | 4 (1–62 [2-9]) | 3 (1–13 [3–11]) | .8883 |
| 4–5 mo | 7 (1–42 [2–12]) | 7 (1–42 [2.50-12.25]) | 7 (1–8 [4–7.50]) | .5595 |
| 6–11 mo | 2.5 (1–16 [1.75–9]) | 5 (1–16 [2.25–13]) | 1.5 (1–2 [1.25–1.75]) | .2377 |
| 1–4 years | 2 (1–29 [1–4]) | 2.5 (1–29 [1.75–5.25]) | 1 (1–1 [1–1]) | .3226 |
| Death (n [% of total]) | 21 (1.49) | 21 (1.81) | 0 (0.00) | .0664 |
Abbreviations: IMPACT, Canadian Immunization Monitoring Program, Active; IQR, interquartile range; LOS, length of stay.
aIncludes confirmed and probable pertussis cases.
bLaboratory-confirmed (n = 1145) or epidemiologically linked (n = 12) pertussis cases.
cClinical pertussis cases.
dFor the comparison of confirmed versus probable pertussis cases.
eAs clinically denoted in the records; patients could have >1 comorbidity.
fPrematurity was defined as birth at <37 weeks’ gestation.
gFive case-patients had both new seizures and encephalopathy.
Incidence of Pertussis Hospitalization
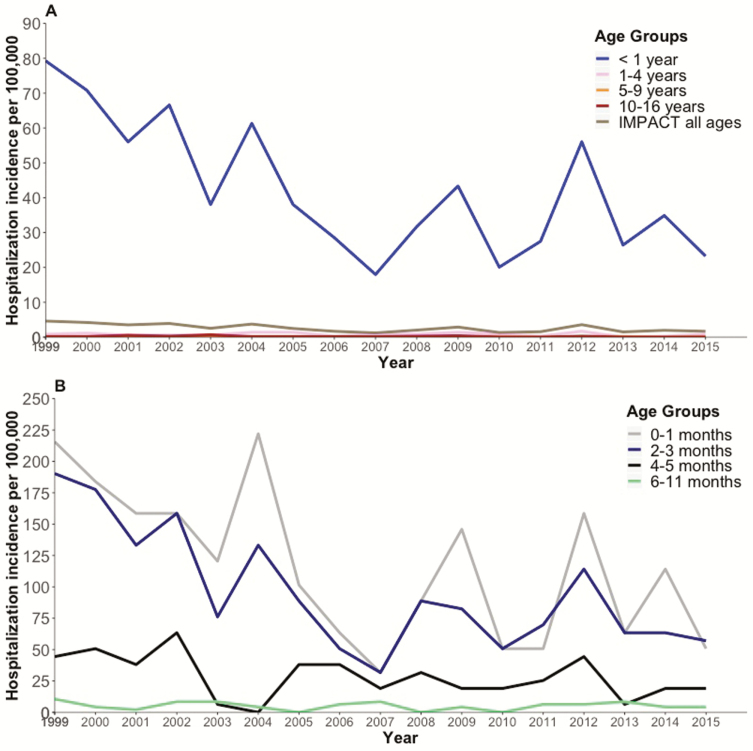
In total, 747 (58.81%) of 1270 pertussis cases occurred in the defined catchment areas for the study hospitals and were included in the hospitalization incidence calculations (Figure 1). The case-patients used for the hospitalization incidence analysis had similar baseline characteristics (age, sex, and comorbidities) as those not included in this analysis (data not shown). The overall mean annual pertussis hospitalization incidence was highest among infants aged <2 months (Table 2). Pertussis hospitalization incidence rates in young infants fluctuated over time, and peaks occurred every 2 to 5 years (Figure 1B).
Figure 1.
Hospitalization incidences among case-patients with pertussis at Canadian Immunization Monitoring Program, Active (IMPACT) hospitals, 1999–2015. Shown are age-specific population-based pertussis hospitalization incidences in children aged ≤16 years (A) and children aged <1 year (B).
Table 2.
Mean Annual Pertussis Hospitalization and Intensive-Care Unit Admission Incidence at IMPACT According to Age Groups, 1999-2015
| Age | Hospitalization Incidence (per 100000 population [(95% CI])a | ICU Admission Incidence (per 100000 population [(95% CI])b |
|---|---|---|
| <1 year | 42.34 (32.53–52.15) | 8.64 (6.96–10.31) |
| <2 mo | 116.40 (85.32–147.49) | 33.48 (26.35–40.62) |
| 2–3 mo | 95.88 (71.56–120.20) | 14.58 (10.55–18.61) |
| 4–5 mo | 28.35 (19.49–37.22) | 2.52 (1.13–3.90) |
| 6–11 mo | 5.09 (3.42–6.77) | 0.42 (0.15–0.68) |
| 1–4 years | 0.81 (0.56–1.05) | 0.06 (0.02–0.09) |
| 5–9 years | 0.16 (0.10–0.22) | 0.005 (0–0.017) |
| 10–16 years | 0.19 (0.09–0.29) | 0.004 (0–0.012) |
| All age groups | 2.61 (2.03–3.18) | 0.50 (0.40–0.60) |
Abbreviations: CI, confidence interval; IMPACT, Canadian Immunization Monitoring Program, Active.
aThe analysis included 11 of 12 IMPACT hospitals (excluding The Hospital for Sick Children, Toronto)
bThe analysis included 10 of 12 IMPACT hospitals and their 7 respective provinces, excluding 2 hospitals from the province of Ontario (The Hospital for Sick Children and Children’s Hospital of Eastern Ontario [Ottawa]).
ICU Admission
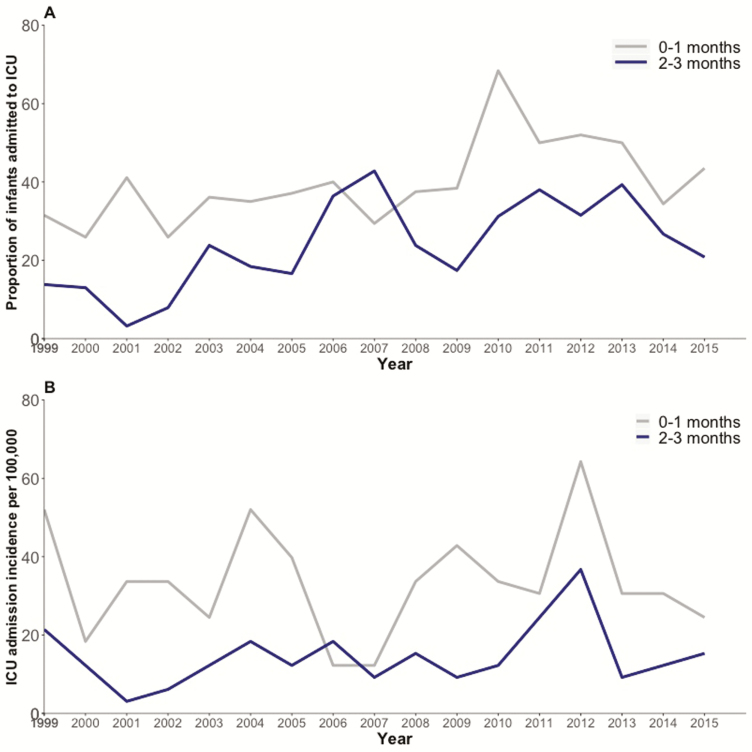
In total, 357 (25.46%) of the 1402 children were admitted to an ICU. We found significant year-to-year variation between years in the proportions of children admitted to an ICU over the 17 years (from 14.03% to 41.66%; P < .0001). Of the case-patients admitted to the ICU, 316 (88.51%) of 357 had a confirmed pertussis case. Case-patients who required admission to the ICU were younger, had a higher rate of neurological complications, had longer hospital LOS, and were more likely to have a comorbidity than those who were not admitted to an ICU (Table 3). The proportion of case-patients admitted to an ICU was 37.90% (232 of 612) for infants aged <2 months, 19.78% (91 of 460) for those aged 2 to 3 months, 12.60% (15 of 119) for those aged 4 to 5 months, 10.81% (8 of 74) for those aged 6 to 11 months, and 27.35% (346 of 1265) for those aged <1 year. The proportion of case-patients admitted to an ICU decreased as gestational age at birth increased (P < .0001) (Supplementary Figure 2). With the exception of 2007, the proportion of children admitted to an ICU was higher for infants aged <2 months than for those aged 2 to 3 months (Figure 2A).
Table 3.
Characteristics of Patients with Pertussis Admitted to IMPACT Hospitals ICU in Canada, 1999–2015
| Characteristic | Admitted to the ICU (n = 357) |
Not Admitted to the ICU (n = 1045) | P a |
|---|---|---|---|
| Demographics | |||
| Sex, male (n [% of total]) | 171 (47.89) | 484 (46.31) | .6482 |
| Age (median [overall range (IQR)]) (wk) | 7 (2–872 [4–10]) | 11 (1–886 [7–19]) | <.0001 |
| Age groups (n [% of total]) | |||
| <1 year | 346 (96.91) | 919 (87.94) | |
| 0–1 mo | 232 (64.98) | 380 (36.36) | |
| 2–3 mo | 91 (25.49) | 369 (35.31) | |
| 4–5 mo | 15 (4.20) | 104 (9.95) | |
| 6–11 mo | 8 (2.24) | 66 (6.31) | |
| 1–4 years | 9 (2.52) | 63 (6.02) | |
| 5–9 years | 1 (0.28) | 23 (2.20) | |
| 10–16 years | 1 (0.28) | 40 (3.82) | |
| Clinical features | |||
| Comorbidity (n [% of total])b | |||
| Underlying conditions (n [% of total]) | 61 (17.08) | 117 (11.19) | .0133 |
| Prematurityc | 30 (8.40)d | 20 (1.91)e | <.0001 |
| Congenital cardiac | 8 (2.24) | 10 (0.95) | |
| Pulmonary | 3 (0.84) | 24 (2.29) | |
| Genetic-metabolic | 3 (0.84) | 5 (0.47) | |
| Gastrointestinal | 3 (0.84) | 8 (0.76) | |
| Failure to thrive | 2 (0.56) | 5 (0.47) | |
| Neurologic | 1 (0.28) | 14 (1.33) | |
| Immunocompromised | 0 (0.00) | 9 (0.86) | |
| Other | 11 (3.08) | 22 (2.10) | |
| Evidence of diagnosis (n [% of total]) | .0007 | ||
| Confirmedf | 316 (88.51) | 841 (80.47) | |
| Probableg | 41 (11.48) | 204 (19.52) | |
| Seizures (n [% of total]) | <.0001 | ||
| New seizures | 17 (4.76) | 13 (1.24) | |
| Exacerbation of an existing seizure disorder | 4 (1.12) | 4 (0.38) | |
| Encephalopathy (n [% of total]) | 6 (1.68) | 2 (0.19) | .0046 |
| Outcome | |||
| Hospitalization | |||
| Median LOS (overall range [IQR]) (days) | 13 (1–185 [7–22]) | 5 (1–60 [3–10]) | <.0001 |
| Death (n [% of total]) | 21 (5.88) | 0 (0.00) | <.0001 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; IMPACT, Canadian Immunization Monitoring Program, Active; LOS, length of stay.
aFor the comparison of case-patients admitted to an ICU versus those not admitted to an ICU.
bAs clinically denoted in the records; patients could have >1 comorbidity.
cPrematurity was defined as birth at <37 weeks’ gestation.
dData on gestational age at birth were available for 28 of 30 prematurely born case-patients admitted to the ICU; 5 were born at 24 to 26 weeks’ gestation, 7 were born at 27 to 31 weeks’ gestation, and 16 were born at 32 to 36 weeks’ gestation.
eData on gestational age at birth were available for 19 of 20 prematurely born case-patients not admitted to the ICU; 1 was born at 25 weeks’ gestation, 4 were born at 27 to 31 weeks’ gestation, and 14 were born at 32 to 36 weeks’ gestation.
fLaboratory-confirmed or epidemiology linked pertussis cases.
gClinical pertussis cases.
Figure 2.
Intensive care unit (ICU) admission rates and admission incidences at Canadian Immunization Monitoring Program, Active (IMPACT) hospitals, 1999–2015. Shown are age-specific (<4 months) population-based ICU admission incidences (per 100000 population) (A) and ICU admission rates (percentages) (B) among infants hospitalized with pertussis in IMPACT hospitals, 1999–2015. The small numbers of patients older than 4 months who were admitted to an ICU precluded description of the incidence rates here.
In total, 298 (83.47%) of 357 case-patients with pertussis who were admitted to the ICU were from provinces in which almost all ICU admissions are captured by IMPACT hospitals (excluding Ontario and the Canadian territories) and were included in the ICU incidence calculations. The overall mean annual incidence of ICU admission over the 17-year period was highest among infants <2 months of age (Table 2). With the exception of 2006, the incidence of ICU admission was higher for case-patients aged <2 months than for those aged 2 to 3 months; a peak occurred in 2004, 2009, and 2012 (Figure 2B).
Death
Twenty-one children (aged 2 to 14 weeks at admission) died during the study period (Table 4). None of the infants who died had received a valid dose of vaccine, although 17 of these infants were aged <3 months and therefore were too young to have received a valid pertussis vaccine dose. Death occurred 1 to 47 days after admission; 61.90% (13 of 21) of these children died within the first 3 days after admission (Supplementary Figure 3). The overall case-fatality rate (CFR) was 1.49% (21 of 1402). The CFRs for infants aged <2 months, those aged 2 to 3 months, and those aged <1 year were 2.28% (14 of 612), 1.52% (7 of 460), and 1.66% (21 of 1265), respectively.
Table 4.
Characteristics of Patients with Fatalities From Pertussis at IMPACT Hospitals, 1999–2015
| Characteristic | Deaths (n = 21) | No Deaths (n = 1381) | P |
|---|---|---|---|
| Sex, male (n [% of total]) | 4 (19.04) | 651 (47.13) | .0192 |
| Age | |||
| Median (overall range [IQR]) (wk) | 5 (2–14 [3–9]) | 10 (1–886 [6 [minus]17]) | <.0001 |
| Age groups (n [% of total]) | |||
| <1 year | 21 (100) | 1244 (90.07) | |
| 0–1 mo | 14 (66.66) | 598 (43.30) | |
| 2–3 mo | 7 (33.33) | 453 (32.80) | |
| 4–5 mo | 0 (0.00) | 119 (8.61) | |
| 6–11 mo | 0 (0.00) | 74 (5.35) | |
| 1–4 years | 0 (0.00) | 72 (5.21) | |
| 5–9 years | 0 (0.00) | 24 (1.73) | |
| 10–16 years | 0 (0.00) | 41 (2.96) | |
| Evidence of diagnosis | .0569 | ||
| Confirmed | 21 (100) | 1124 (81.39) | |
| Probable | 0 (0.00) | 257 (18.60) | |
| Morbidity | |||
| Seizures (n [% of total]) | .665 | ||
| No seizures | 20 (95.23) | 1344 (97.32) | |
| New seizures | 1 (4.76) | 29 (2.09) | |
| Exacerbation of an existing seizure disorder | 0 (0.00) | 8 (0.57) | |
| Encephalopathy (n [% of total])a | <.0001 | ||
| Not present | 19 (90.47) | 1373 (99.42) | |
| Present | 0 (0.00) | 8 (0.57) | |
| Hospitalization | |||
| Median LOS (overall range [IQR]) (days) | 3 (1–79 [2–14]) | 7 (1 185 [2–14]) | .0501 |
| Comorbidity (n [% of total])b | |||
| Underlying conditions | 5 (23.80) | 173 (12.52) | .3031 |
| Prematurityc | 4 (19.04) | 46 (3.33) | .0011 |
Abbreviations: IMPACT, Canadian Immunization Monitoring Program, Active; IQR, interquartile range; LOS, length of stay.
aTwo cases of fatality with no data on encephalopathy.
bAs clinically denoted in the records; patients could have >1 comorbidity.
cPrematurity was defined as birth at <37 weeks’ gestation. One case-patient was born at 29 weeks’ gestation, 2 were born at 34 weeks’ gestation, and 1 was born at 35 weeks’ gestation.
Risk Factors for Admission to ICU and Death
Independent risk factors for ICU admission were age of <16 weeks, prematurity, encephalopathy, confirmed pertussis diagnosis, and a more recent year of admission (Table 5). Independent risk factors for death were age of <4 weeks, prematurity, female sex, and a more recent year of admission (Table 5).
Table 5.
Risk Factors for Admission to ICU and Death in Patients With Pertussis (n = 1392)
| Variable (N) | Univariate Analysis | Multivariable Analysisa | ||
|---|---|---|---|---|
| P | OR (95% CI) | P | aOR (95% CI) | |
| Risk factors for admission to intensive care unit | ||||
| Age | ||||
| ≥16 wk (380) | <.0001 | Reference | <.0001 | Reference |
| <16 wk (1012) | 4.47 (3.12–6.59) | 4.83 (3.30–7.27) | ||
| Sex | ||||
| Male (648) | .569 | Reference | Not included | |
| Female (744) | 0.93 (0.3–1.18) | |||
| Seizures | ||||
| No new seizures (1363) | .0004 | Reference | Not included | |
| New seizures (29) | 3.77 (1.80–8.06) | |||
| Encephalopathy | ||||
| No (1385) | .0074 | Reference | .0073 | Reference |
| Yes (7) | 18.08 (3.07–342.22) | 21.13 (3.18–425.21) | ||
| Prematurityb | ||||
| No (1343) | <.0001 | Reference | <.0001 | Reference |
| Yes (49) | 4.59 (2.58–8.35) | 5.81 (3.04–11.37) | ||
| Comorbidity (other than prematurity) | ||||
| No (1252) | .58 | Reference | Not included | |
| Yes (140) | 1.11 (0.75–1.64) | |||
| Evidence for diagnosis | ||||
| Probable (245) | .0008 | Reference | .0405 | Reference |
| Confirmed (1147) | 1.84 (1.29–2.67) | 1.51 (1.03-2.27) | ||
| Admission date (year) | <.0001 | 1.05 (1.03-1.08) | .0005 | 1.05 (1.02–1.07) |
| Vaccination status (3–4 mo) (n = 184)c | ||||
| Unvaccinated (102) | .665 | Reference | Not included | |
| Age-appropriately vaccinated (n = 82) | 0.83 (0.35-1.89) | |||
| Risk factors for death | ||||
| Age | ||||
| ≥4 wk (1280) | <.0001 | Reference | .0002 | Reference |
| <4 wk (112) | 7.04 (2.57–17.89) | 6.73 (2.39-17.88) | ||
| Sex | ||||
| Male (648) | .0341 | Reference | .0318 | Reference |
| Female (744) | 3.31 (1.19-11.66) | 3.46 (1.21-12.47) | ||
| Prematurity b | ||||
| No (1343) | .0090 | Reference | .0147 | Reference |
| Yes (49) | 5.40 (1.23–16.95) | 5.36 (1.15–18.61) | ||
| Comorbidity other than prematurity | ||||
| No (1252) | .493 | Reference | Not included | |
| Yes (140) | 0.49 (0.03–2.41) | |||
| Admission date (year) | .0217 | 1.11 (1.01–1.22) | .0143 | 1.13 (1.03–1.25) |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
aThe multivariable ICU analysis was adjusted for patient age, occurrence of encephalopathy, existence of prematurity, admission year, Canadian Immunization Monitoring Program, Active (IMPACT) hospital, and evidence for diagnosis. The multivariable death analysis was adjusted for patient age, admission year, IMPACT hospital, and sex.
bPrematurity was defined as birth at <37 weeks’ gestation.
cThe subanalysis included case-patients aged 3 to 4 months with laboratory-confirmed pertussis who received >1 vaccine dose or were unvaccinated.
Vaccination Status of Case-Patients With Laboratory-Confirmed Pertussis
Among hospitalized patients aged 3 months to 16 years with laboratory-confirmed pertussis (n = 355), 31.83% (113 of 355) had received an age-appropriate number of pertussis vaccine doses, and 51.54% (183 of 355) were unvaccinated (Supplementary Figure 4 and Supplementary Results).
DISCUSSION
In this study, infants aged <2 months had the highest hospitalization incidence rate and were the major contributor to the burden of children hospitalized with pertussis disease. Almost 25% of all case-patients were admitted to an ICU, and the majority of these admissions were of infants aged <2 months. Age of <16 weeks was independently associated with a 5-fold increase in the risk for ICU admission over that in older children. Encephalopathy, prematurity, and confirmed pertussis diagnosis were independently associated with 21-fold, 6-fold, and 1.5-fold increased risk for ICU admission, respectively. Age of <4 weeks was the most significant independent risk factor for death; this age was associated with a 7-fold increased risk for death over that of older children. Prematurity and female sex were significantly associated with increased risk for death (5-fold and 3.5-fold increase, respectively). Our data have important implications in establishing the true burden of endemic pertussis disease and thus can assist public health policy makers in reaching evidence-based conclusions regarding the optimal cost-effective preventive approach. The identification of risk factors for poor outcome aids appropriate prioritization in management of young infants with pertussis and counseling for families during hospitalization.
In our study, the majority of children hospitalized with pertussis were <4 months old, which is consistent with previous data from Canada, the United States, and Australia in the aP vaccine era [4, 5, 7, 16]. Although almost 21% of global pertussis cases occur in infants aged <1 year [1], 90% of hospitalized patients with pertussis in the aP era and 92% of hospitalized patients with pertussis in the wP vaccine era in Canada were in this age group [17]. In addition, 85% of hospitalized patients with pertussis in this study and 79.1% of hospitalized patients with pertussis in the wP vaccine era in Canada were infants aged <6 months, which emphasizes the point that young infants are at a disproportionately high risk for severe pertussis. The incidence of hospitalization for pertussis among infants aged <2 months (116.40 of 100000) was lower than that reported in the wP vaccine era in England from 1995 to 1997 (164 of 100000 among infants aged <3 months) [20] and in Australia 4 years after the introduction of aP vaccine (~200 of 100000 among infants aged <2 months) [7]. Thus, although the incidence of pertussis that requires hospitalization among infants aged <2 months in the aP era is lower than that in the wP era, this age group accounts for a high proportion of pertussis-related hospital admissions. In addition, the incidence of hospitalization for pertussis among infants <1 year of age (42.3 of 100000) is lower than that reported in the wP vaccine era in Canada (136 of 100000) [16].
Children with pertussis had a median LOS of 7 days, which is notably higher than the median LOS of 4 days among patients hospitalized with pertussis (median age, 2.6 months) reported during the 2010 California pertussis epidemic [5]. This difference might stem from the fact that IMPACT hospitals are pediatric tertiary-care centers that admit children with more severe cases of pertussis. Moreover, the median LOS of 8 days among infants aged <6 months in this study is comparable to the median LOS of 9.3 days among infants aged <6 months with pertussis who were admitted to an IMPACT center during the wP era, and both studies used the same clinical case definition of pertussis [17]. In this study, 77.2% of patients aged 7 to 18 months had received fewer than 3 vaccine doses, whereas 44.9% of the patients aged 6 to 24 months who were admitted to an IMPACT center with pertussis during the wP era received fewer than 3 vaccine doses. This result emphasizes the fact that in the aP era, undervaccination is an important contributor to hospitalization for pertussis. Neurological manifestations (new-onset seizures and/or encephalopathy) were observed in 2.6% of patients admitted with pertussis, consistent with data from IMPACT centers in the wP era (2.4% among patients aged <2 years). Moreover, the CFR for all age groups in this study was 1.5% (2.3% for infants aged <2 months), which is higher than the CFR reported in Canada in the wP era (0.9% among patients aged <2 years). In our study, two-thirds of deaths were among infants aged <2 months, which is comparable with the data from IMPACT centers during the wP era, in which 80% of deaths that resulted from pertussis were among infants aged <2 months.
In our study, patients hospitalized with pertussis had frequent ICU admissions, and the highest proportion was among infants aged <2 months. The proportion of ICU admissions among infants aged <1 year was comparable to that reported for an epidemic in California (33% among hospitalized infants aged <1 year) [6] and higher than the rate reported during the wP era in Canada (16% among children aged <2 years and 19.2% among infants aged <6 months) [17]. In addition, the median ICU LOS found in our study (4 days) was comparable to that in reports from Australia and New Zealand (3.6 and 3.9 days, respectively) [21, 22].
To our knowledge, no data on risk factors for admission to the ICU exist, and data on the risk factors for death attributable to pertussis are scarce. Identifying infants at higher risk can help physicians in their clinical management decisions; thus, close monitoring and early consideration of the need for ICU admission are required for infants who have these risk factors. Infants with confirmed pertussis had longer hospital and ICU LOS than those with probable pertussis, which indicates that the clinical severity of confirmed pertussis disease is higher than that of clinically diagnosed pertussis disease, which might be because some case-patients with probable pertussis disease did not have it. A case-control study performed during a period in which both wP and aP vaccine were used in Canada found that the white blood cell (WBC) count was a risk factor for death caused by pertussis [23], which is consistent with recent data from California showing that patients with pertussis who had died had higher WBC counts than did those who did not die [24]. Lower birth weight, higher peak WBC count, pulmonary hypertension, seizures [25], and female sex [26] were independent risk factors for death among infants with pertussis. In the United States in the aP vaccine era, prematurity was a risk factor for death caused by pertussis in univariate analyses only [25, 26]. Premature infants were overrepresented (12 of 20) in a cohort of US patients with fatal pertussis in the wP vaccine era [27]. Our study found prematurity to be an independent risk factor for death caused by pertussis in the aP era.
Our study has a number of strengths. This report provides a detailed characterization of hospitalized pediatric patients with pertussis in the aP vaccine era. The study is unique for its inclusion of a national population, long duration, and extensive active case finding. IMPACT reporting is active, prospective, standardized, and performed by trained nurse monitors, and as such, the accuracy and completeness of the data are high. The 17-year time period enabled an evaluation of the burden and characteristics of pertussis disease that spanned over a prolonged period. IMPACT hospitals constitute 90% of Canada’s pediatric tertiary-care beds and thus provide good estimates of severe pediatric pertussis cases that necessitate hospitalization in a tertiary-care center. Moreover, the IMPACT hospitals’ catchment area covers 57% of the Canadian population aged <16 years.
Our study also has some limitations. Patients with less severe pertussis would have been admitted to a smaller hospital that is not part of the IMPACT network or diagnosed and treated in the community. This limitation is less significant in an assessment of severe pertussis that necessitated ICU admission, because IMPACT centers comprise most of the pediatric tertiary-care beds in Canada. Misclassification of probable cases is possible, although probable cases were minority of cases. Our data did not capture readmissions. However, readmissions are expected to be uncommon, because pertussis is a monophasic disease. A possibility exists that some of the patients included in this study were primed with wP vaccine; however, the proportion of such patients is expected to be low, because 90% of the patients were <1 year of age and admitted after 1999 (aP was introduced in Canada in 1997–1998). Our data did not capture the onset of cough, an important variable in defining a vaccine dose as valid. However, the 4-week interval between recent vaccine dose and admission, used in this study, minimizes this misclassification.
Vaccination against pertussis in pregnancy has proven effective in preventing pertussis disease among infants aged <3 months [13, 28, 29] and to decrease the risk of hospitalization, risk of ICU admission, and hospital LOS [30]. Given the significant morbidity from endemic pertussis disease among infants during the first months of life, as shown in this study, vaccination against pertussis in pregnancy has the potential to control the burden of pertussis among young infants in countries with long-standing use of aP vaccine. Vaccination against pertussis in pregnancy has proved to be highly (nearly 90%) effective in preventing pertussis disease and hospitalization among infants aged <3 months in the United Kingdom and United States [13, 29]. Assuming that vaccination against pertussis in pregnancy is 90% effective in the prevention of pertussis hospitalization among infants aged <3 months, approximately 825 cases of pertussis that necessitated hospitalization could have been prevented via maternal immunization in the study hospitals during this 17-year period.
Universal vaccination against pertussis in pregnancy is now recommended in an increasing number of countries and most recently in Canada [15]. Although the optimal timing of vaccination against pertussis in pregnancy is being researched, our finding that prematurity is an independent risk factor for ICU admission and death caused by pertussis might support vaccination earlier in pregnancy.
Supplementary Data
Supplementary materials are available at Journal of the Pediatric Infectious Diseases Society online.
Notes
Acknowledgments . We gratefully acknowledge the expert assistance provided by the staff of the data center at the Vaccine Evaluation Center, BC Children’s Hospital Research Institute (University of British Columbia, Vancouver, Canada) (K. Marty, W. Zhang, E. Grove, S. Liu, and E. Rousseau), the nurse monitors at all of the IMPACT centers, and S. Squires (Vaccine Preventable Diseases, Public Health Agency of Canada, Ottawa, Ontario, Canada). J. A. B. is a Michael Smith Foundation for Health Research Scholar.
Financial support. The Canadian IMPACT is a national surveillance initiative managed by the Canadian Pediatric Society (CPS) and conducted by the IMPACT network of pediatric investigators. IMPACT pertussis surveillance is funded by the Centre for Immunization and Respiratory Infectious Diseases of the Public Health Agency of Canada.
Potential conflicts of interest. M. S. is and has been an investigator on research grants received from Pfizer, Merck, and Variation Biotechnologies, Inc; all monies have been paid to his institution, and he has received no personal payments. S. A. H. has received grants and contracts for research from Sanofi Pasteur and GlaxoSmithKline, both producers of aP vaccine. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Canadian IMPACT investigators. The Canadian IMPACT investigators are N. Bridger and R. Morris (Eastern Health Janeway Children’s Health and Rehabilitation Centre, St. John’s, Canada), K. Top and S. Halperin (IWK Health Center, Halifax, Canada), P. Déry and R. Thibeault (Centre Mère-Enfant Soleil, Québec City, Canada), D. Moore and M. A. Lefebvre (Montreal Children’s Hospital of the McGill University Health Centre, Montreal, Canada), M. Lebel (CHU Sainte-Justine, Montreal, Canada), N. Le Saux (Children’s Hospital of Eastern Ontario, Ottawa, Canada), D. Tran, L. Ford-Jones, and S. Morris (The Hospital for Sick Children, Toronto, Canada), J. Embree and B. Law (Winnipeg Children’s Hospital, Winnipeg, Canada), B. Tan and A. McConnell (Royal University Hospital, Saskatoon, Canada), T. Jadavji, R. Chawla, O. Vanderkooi, and J. Kellner (Alberta Children’s Hospital, Calgary, Canada), W. Vaudry (Stollery Children’s Hospital, Edmonton, Canada), and D. Scheifele, J. Bettinger, M. Sadarangani, and L. Sauvé (BC Children’s Hospital, Vancouver, Canada).
Contributor Information
Members of the Canadian Immunization Monitoring Program, Active (IMPACT):
N Bridger, R Morris, K Top, S Halperin, P Déry, R Thibeault, D Moore, M A . Lefebvre, M Lebel, N Le Saux, D Tran, L Ford-Jones, S Morris, J Embree, B Law, B Tan, A McConnell, T Jadavji, R Chawla, O Vanderkooi, J Kellner, W Vaudry, D Scheifele, J Bettinger, M Sadarangani, and L Sauvé
References
- 1. Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis 2017; 17:974–80. [DOI] [PubMed] [Google Scholar]
- 2. Tan T, Halperin S, Cherry JD, et al. . Pertussis immunization in the global pertussis initiative North American region: recommended strategies and implementation considerations. Pediatr Infect Dis J 2005; 24:S83–6. [DOI] [PubMed] [Google Scholar]
- 3. Wirsing von König CH, Campins-Marti M, Finn A, et al. . Pertussis immunization in the global pertussis initiative European region: recommended strategies and implementation considerations. Pediatr Infect Dis J 2005; 24:S87–92. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Pertussis epidemic—Washington, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:517–22. [PubMed] [Google Scholar]
- 5. Winter K, Harriman K, Zipprich J, et al. . California pertussis epidemic, 2010. J Pediatr 2012; 161:1091–6. [DOI] [PubMed] [Google Scholar]
- 6. Winter K, Glaser C, Watt J, Harriman K; Centers for Disease Control and Prevention Pertussis epidemic—California, 2014. MMWR Morb Mortal Wkly Rep 2014; 63:1129–32. [PMC free article] [PubMed] [Google Scholar]
- 7. Wood N, Quinn HE, McIntyre P, Elliott E. Pertussis in infants: preventing deaths and hospitalisations in the very young. J Paediatr Child Health 2008; 44:161–5. [DOI] [PubMed] [Google Scholar]
- 8. Celentano LP, Massari M, Paramatti D, et al. ; EUVAC-NET Group Resurgence of pertussis in Europe. Pediatr Infect Dis J 2005; 24:761–5. [DOI] [PubMed] [Google Scholar]
- 9. Castagnini LA, Munoz FM. Clinical characteristics and outcomes of neonatal pertussis: a comparative study. J Pediatr 2010; 156:498–500. [DOI] [PubMed] [Google Scholar]
- 10. Murray EL, Nieves D, Bradley JS, et al. . Characteristics of severe Bordetella pertussis infection among infants ≤90 days of age admitted to pediatric intensive care units—Southern California, September 2009–June 2011. J Pediatric Infect Dis Soc 2013; 2:1–6. [DOI] [PubMed] [Google Scholar]
- 11. Elliott E, McIntyre P, Ridley G, et al. . National study of infants hospitalized with pertussis in the acellular vaccine era. Pediatr Infect Dis J 2004; 23:246–52. [DOI] [PubMed] [Google Scholar]
- 12. Namachivayam P, Shimizu K, Butt W. Pertussis: severe clinical presentation in pediatric intensive care and its relation to outcome. Pediatr Crit Care Med 2007; 8:207–11. [DOI] [PubMed] [Google Scholar]
- 13. Amirthalingam G, Andrews N, Campbell H, et al. . Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet 2014; 384:1521–8. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep 2013; 62:131–5. [PMC free article] [PubMed] [Google Scholar]
- 15. Brophy J, Baclic O, Tunis MC on behalf of the National Advisory Committee on Immunization (NACI). Summary of the NACI Update on Immunization in Pregnancy with Tetanus Toxoid, Reduced Diphtheria Toxoid and Reduced Acellular Pertussis (Tdap) Vaccine. Can Commun Dis Rep. 2018;44(3/4):91-4. https://doi.org/10.14745/ccdr.v44i34a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bettinger JA, Halperin SA, De Serres G, et al. . The effect of changing from whole-cell to acellular pertussis vaccine on the epidemiology of hospitalized children with pertussis in Canada. Pediatr Infect Dis J 2007; 26:31–5. [DOI] [PubMed] [Google Scholar]
- 17. Halperin SA, Wang EE, Law B, et al. . Epidemiological features of pertussis in hospitalized patients in Canada, 1991–1997: report of the immunization monitoring program–active (IMPACT). Clin Infect Dis 1999; 28:1238–43. [DOI] [PubMed] [Google Scholar]
- 18. Public Health Agency of Canada. Case definitions for diseases under national surveillance. Can Commun Dis Rep 2000;28:1–122. [PubMed] [Google Scholar]
- 19. Statistics Canada. 2006 census of population. Accessed July 1, 2017 http://www12.statcan.gc.ca/census-recensement/2006/dp-pd/tbt/Rp-eng.cfm?LANG=E&APATH=3&DETAIL=0&DIM=0&FL=A&FREE=0&GC=0&GID=0&GK=0&GRP=1&PID=88988&PRID=0&PTYPE=88971,97154&S=0&SHOWALL=0&SUB=0&Temporal=2006&THEME=66&VID=0&VNAMEE=&VNAMEF=. [Google Scholar]
- 20. Van Buynder PG, Owen D, Vurdien JE, et al. . Bordetella pertussis surveillance in England and Wales: 1995–7. Epidemiol Infect 1999; 123:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Straney L, Schibler A, Ganeshalingham A, et al. ; Australian and New Zealand Intensive Care Society Centre for Outcomes and Resource Evaluation and the Australian and New Zealand Intensive Care Society Paediatric Study Group Burden and outcomes of severe pertussis infection in critically ill infants. Pediatr Crit Care Med 2016; 17:735–42. [DOI] [PubMed] [Google Scholar]
- 22. Kaczmarek MC, Ware RS, McEniery JA, et al. . Epidemiology of pertussis-related paediatric intensive care unit (ICU) admissions in Australia, 1997–2013: an observational study. BMJ Open 2016; 6:e010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mikelova LK, Halperin SA, Scheifele D, et al. ; Members of the Immunization Monitoring Program, Active (IMPACT) Predictors of death in infants hospitalized with pertussis: a case-control study of 16 pertussis deaths in Canada. J Pediatr 2003; 143:576–81. [DOI] [PubMed] [Google Scholar]
- 24. Cherry JD, Wendorf K, Bregman B, et al. . An observational study of severe pertussis in 100 infants ≤120 days of age. Pediatr Infect Dis J 2018; 37:202–5. [DOI] [PubMed] [Google Scholar]
- 25. Winter K, Zipprich J, Harriman K, et al. . Risk factors associated with infant deaths from pertussis: a case-control study. Clin Infect Dis 2015; 61:1099–106. [DOI] [PubMed] [Google Scholar]
- 26. Haberling DL, Holman RC, Paddock CD, Murphy TV. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediatr Infect Dis J 2009; 28:194–8. [DOI] [PubMed] [Google Scholar]
- 27. Wortis N, Strebel PM, Wharton M, et al. . Pertussis deaths: report of 23 cases in the United States, 1992 and 1993. Pediatrics 1996; 97:607–12. [PubMed] [Google Scholar]
- 28. Dabrera G, Amirthalingam G, Andrews N, et al. . A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis 2015; 60:333–7. [DOI] [PubMed] [Google Scholar]
- 29. Winter K, Nickell S, Powell M, Harriman K. Effectiveness of prenatal versus postpartum tetanus, diphtheria, and acellular pertussis vaccination in preventing infant pertussis. Clin Infect Dis 2017; 64:3–8. [DOI] [PubMed] [Google Scholar]
- 30. Winter K, Cherry JD, Harriman K. Effectiveness of prenatal tetanus, diphtheria, and acellular pertussis vaccination on pertussis severity in infants. Clin Infect Dis 2017; 64:9–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.