Abstract
Diagnostic testing and antibiotics are not routinely recommended for young children with community-acquired pneumonia. In a national sample of >6 million outpatient 1- to 6-year-olds with community-acquired pneumonia between 2008 and 2015, a complete blood count was obtained for 8.6% (95% confidence interval [CI], 6.1%–11.1%), radiography was performed for 43% (95% CI, 36%–50%), and antibiotics were given for 73.9% (95% CI, 67.1%–80.7%). There were no changes in testing or antibiotic use over time.
Keywords: antibiotics, children, diagnostic testing, pneumonia, radiography
Community-acquired pneumonia (CAP) is one of the most common infections in children. CAP is the largest contributor to total days of antibiotic use in children’s hospitals and leads to ~1.5 million pediatric healthcare visits each year in the United States [1, 2]. Substantial variation in the management of children with CAP exist, particularly in the use of diagnostic testing and antibiotics (including those that do not improve outcomes and might cause harm) [3]. In addition, CAP in most young children is caused by viral infections, for which antibiotics do not provide benefit [4]. As such, the 2011 Pediatric Infectious Diseases Society (PIDS)/Infectious Diseases Society of America (IDSA) pediatric CAP guideline recommends against routinely performing chest radiography (CXR), obtaining complete blood count (CBC) or blood cultures, and providing antimicrobial therapy in preschool-aged children treated as outpatients [4]. When antibiotics are indicated, narrow-spectrum aminopenicillins are recommended as the first-line therapy. Unnecessary testing and antibiotic use have important drawbacks, including contributing to the spread of antimicrobial resistance, antibiotic-associated adverse events, avoidable hospitalizations, discomfort, and cost. Our objective was to evaluate the use of diagnostic testing and antibiotics in young outpatient children with CAP in the United States before and after publication of the PIDS/IDSA 2011 guideline to determine the frequency of testing and therapy not routinely recommended by this guideline.
METHODS
We performed a cross-sectional study using data from the 2008–2015 National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS), nationally representative surveys of visits to outpatient clinics and emergency departments (EDs). Children aged 1 to <6 years with CAP were identified using a validated algorithm based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes [5]. Children were defined as having CAP if they had a primary or secondary diagnosis of pneumonia (according to ICD-9-CM code 480.0–480.2, 480.8–480.9, 481, 482.0, 482.30–482.32, 482.41–482.42, 482.83, 482.89–482.90, 483.8, 484.3, 485, 486, or 487.0). Children younger than 1 year were excluded to minimize inclusion of infants with bronchiolitis, and we performed a sensitivity analysis excluding patients aged 1 to 2 years for the same reason. Hospitalized children and those with a diagnostic code(s) for a chronic medical condition (according to the Feudtner complex chronic diseases classification) or concurrent bacterial infection were excluded [6].
Outcomes included weighted proportion of visits during which CBC, CXR, blood culture and antibiotics were ordered. Antibiotic prescribing was defined as all new antimicrobial agents prescribed during the encounter. Antibiotics were categorized according to the following classes: aminopenicillins (eg, penicillin, amoxicillin, ampicillin), macrolides (eg, azithromycin, erythromycin, clarithromycin), cephalosporins, fluoroquinolones, clindamycin, and sulfonamides. Broad-spectrum antibiotics were defined as any nonpenicillin antibiotic.
Sample weights were applied to provide national estimates. SAS 9.4 survey procedures for complex sampling design were used to conduct χ2 tests to make comparisons before and after guideline publication, in addition to tests for trends over time. An interrupted time series analysis was performed for CBC, CXR, and antibiotic orders, and we found no significant changes over time; however, small sample sizes and large relative standard errors (beyond those recommended by the NAMCS/NHAMCS) preclude reporting of these results. Statistical tests were 2-sided, and a P value of <.01 indicated significance (per NAMCS/NHAMCS recommendations) [7].
RESULTS
Over the 8-year period, 601 children were included, representing an estimated 6.3 million (95% confidence interval [CI], 5.3–7.4 million) pediatric visits for CAP. The median age was 1.7 years (interquartile range, 1.0–3.2 years), 55.1% (95% CI, 47.8%–62.4%) were male, 68.9% (95% CI, 62.1%–75.7%) were white, 41.1% (95% CI, 33.3%–48.9%) were publicly insured. Of the included visits, 34.9% (95% CI, 28.8%–41%) were ED visits, and 65.1% (95% CI, 59%–71.2%) were clinic visits. We found no significant demographic differences in the study populations before and after guideline publication (data not shown).
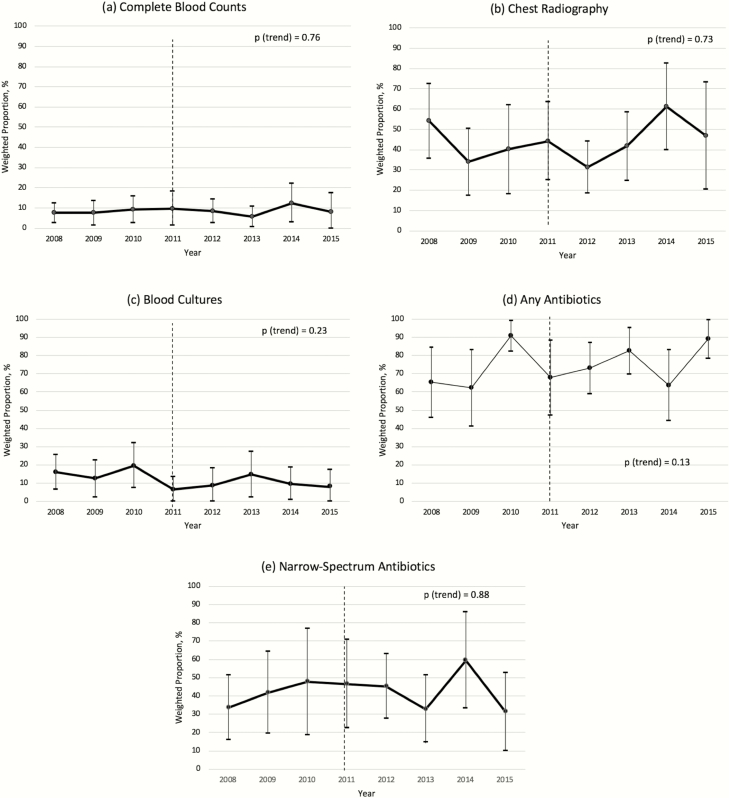
Overall, at these visits, a CBC was ordered in 8.6% (95% CI, 6.1%–11.1%), CXR in 43% (95% CI, 36%–50%), and blood culture in 11.1% (95% CI, 7.4%–14.8%), and antibiotics were prescribed in 73.9% (95% CI, 67.1%–80.7%). Second-line broad-spectrum antibiotics (ie, cephalosporins, macrolides) were prescribed at most visits (Table 1). We found no differences in CBC, CXR, blood culture, and antibiotic orders overall or according to antibiotic class between the preguideline and postguideline periods. CBCs and CXR were ordered more frequently in EDs than in clinics. No significant differences were found in antibiotic use or class according to setting. We found no change in the proportions of children with CAP for whom a CBC (P trend = .76), CXR (P trend = .73), blood culture (P trend = .23), any antibiotic (P trend = 0.13), or a narrow-spectrum antibiotic (P trend = .88) was ordered according to year over the course of the study (Figure 1).
Table 1.
Diagnostic Testing and Antibiotic Use in Outpatient Young Children With Community-Acquired Pneumonia in the United States, 2008–2015a
| Diagnostic Test or Treatment | Overall | Practice Setting | Guideline Publication Timing | ||||
|---|---|---|---|---|---|---|---|
| ED | Clinic | P | Before (2008–2011) | After (2012–2015) | P | ||
| CBC obtained | 8.6 (6.1–11.1) | 20.0 (15.1–24.9) | 2.5 (0.0–5.0)b | ˂.0001 | 9.0 (5.6–12.4) | 8.2 (4.5–11.8) | .75 |
| CXR obtained | 43.0 (36.0–50.0) | 83.1 (77.5–88.6) | 21.5 (12.1–30.8) | ˂.0001 | 42.4 (33.1–51.8) | 43.6 (33.2–54.0) | .86 |
| Blood culture obtained | 11.1 (7.4–14.8) | 12.8 (9.0–16.6) | 5.7 (0.0–14.5)b | .72 | 14.1 (9.1–19.1) | 9.3 (4.2–14.4) | .19 |
| Received antibiotics | 73.9 (67.1–80.7) | 80.9 (75.2–86.6) | 70.2 (60.4–80.0) | .06 | 69.9 (59.7–80.2) | 78.7 (71.0–86.3) | .17 |
| Antibiotic class | |||||||
| Aminopenicillins | 42.1 (33.0–51.2) | 43.6 (35.5–51.7) | 41.2 (27.4–54.9) | .76 | 42.9 (29.4–56.4) | 41.3 (29.7–52.9) | .86 |
| Cephalosporins | 28.1 (20.4–35.7) | 35.2 (27.5–42.8) | 23.7 (12.0–35.3) | .02 | 27.2 (17.5–36.9) | 29.0 (17.0–41.0) | .82 |
| Macrolides | 38.7 (30.3–47.1) | 34.7 (27.8–41.6) | 41.2 (28.2–54.3) | .39 | 37.3 (25.2–49.4) | 40.2 (28.1–52.2) | .75 |
| Fluoroquinolones | 0.6 (0.0–1.8) | 0.0(0.0–0.0) | 1.0 (0.0–2.9) | NA | 1.2 (0.0–3.5)b | 0.0 (0.0–0.0) | NA |
| Clindamycin | 1.0 (0.0–2.7) | 0.2 (0.0–0.7)b | 1.4 (0.0–4.2)b | .41 | 1.7 (0.0–5.1) | 0.2 (0.0–0.5)b | .37 |
| Sulfonamides | 0.6 (0.0–1.5) | 0.3 (0.0–0.7) | 0.7 (0.0–2.1) | .58 | 0.1 (0.0–0.2)b | 1.1 (0.0–2.9)b | .29 |
Abbreviations: CBC, complete blood count; CXR, chest radiograph; ED, emergency department; NA, not applicable.
aAll values are weighted percentages (95% confidence interval) unless otherwise noted.
bEstimate for which the relative standard error was >0.3.
Figure 1.
Weighted proportions of complete blood count, chest radiography, and antibiotic use for outpatient young children with community-acquired pneumonia in the United States according to year. (a) Complete blood count; (b) chest radiography; (c) blood cultures; (d) any antibiotics; (e) narrow-spectrum antibiotics. Error bars, 95% confidence interval; dotted line, year of Pediatric Infectious Diseases Society/Infectious Diseases Society of America pediatric CAP guideline publication (2011).
We found no substantive changes in the results when we performed sensitivity analyses that excluded all children younger than 2 years or when amoxicillin-clavulanate was removed from the aminopenicillin class (to account for the broader spectrum of activity of amoxicillin-clavulanate).
DISCUSSION
Despite evidence that suggests the limited benefits of a CBC, CXR, blood culture, and antibiotics in young outpatient children with CAP and the 2011 PIDS/IDSA guideline recommending against their routine use, diagnostic testing and antibiotic use remain high in outpatient settings, including EDs, across the United States. When antibiotics were prescribed, most of them were macrolides or cephalosporins, which is also inconsistent with PIDS/IDSA guideline recommendations to use narrow-spectrum penicillins as first-line agents. High use of nonrecommended tests and antibiotics, particularly CXR and broad-spectrum antibiotics, has persisted over time, and publication of the PIDS/IDSA guideline has had no discernable effect on overall use. Patients evaluated in an ED were more likely to undergo testing than were patients in other outpatient settings, but antibiotic prescribing and antibiotic class did not differ according to setting (clinic vs ED).
These results expand on previous nationally representative studies performed before guideline publication, which found CBC use in 30% and CXR in 83% of ED visits for pediatric CAP and antibiotic use in 68% of office visits and 86% of ED visits (broad-spectrum antibiotics were the most common type prescribed) [8, 9]. One study of ED visits and hospitalizations for CAP at 32 children’s hospitals examined the effect of the guideline on testing and found that for patients discharged from the ED, the use of CBCs and CXR was higher than expected after guideline publication [10]. Also consistent with our results are the results of another study of antibiotic prescribing in more than 10 000 children with CAP in an outpatient primary care network, which revealed that most children were prescribed nonpenicillin antibiotics [11]. To our knowledge, our study is the first nationally representative study to have evaluated the use of these resources in the outpatient setting after publication of the PIDS/IDSA guideline.
Testing and treatment rates remain high despite the PIDS/IDSA recommendations for several potential reasons. Overlapping clinical phenotypes of pneumonia and other common respiratory conditions in young children result in diagnostic uncertainty. Although the PIDS/IDSA guideline recommends making a diagnosis of CAP without radiography (ie, based on clinical signs and symptoms), the lack of reliability of clinical signs and symptoms commonly used to diagnose CAP is problematic [12] and might force clinicians to obtain radiographs to confirm their suspicion or clarify an ambiguous clinical presentation. Antibiotic use likely remains high despite recommendations because of the lack of objective easily obtained tests to determine CAP etiology. Although surveillance data using molecular diagnostic testing suggest that the etiology of most instances of CAP in preschool-aged children is viral, some of these tests are not widely available and also might not be indicative of infection in the lower respiratory tract [13]. Nevertheless, it is highly unlikely that 70% to 80% of CAP cases in this age group are bacterial in origin, particularly in well-appearing immunized outpatients, which suggests that interventions are required to decrease antimicrobial overuse.
Most previous work that evaluated the effect of the 2011 PIDS/IDSA pediatric CAP guideline focused on hospitalized children. One study of 32 children’s hospitals found that inpatient diagnostic testing for CAP decreased after guideline publication [10]. Another study performed with data from 38 children’s hospitals found that the use of ampicillin and amoxicillin increased in hospitalized children with CAP after guideline publication, yet most children continued to be prescribed a macrolide or cephalosporin [14]. In another study, guideline-recommended penicillin prescribing increased across 28 children’s hospitals; local implementation efforts were seemingly important in guideline adoption [15]. It is well established that guideline publication alone does not alter practice patterns, and success often lies in local implementation efforts [16]. In children with CAP, studies have emphasized the successful use of local quality-improvement initiatives to increase guideline-recommended testing and treatment [17, 18]. Additional quality-improvement efforts are needed to both maintain guideline adherence and promote adherence by other health care providers and systems.
In addition to improving clinical outcomes and decreasing costs of care, adherence to the IDSA/PIDS CAP guideline recommendations for testing and treatment has important implications for diagnostic and antimicrobial stewardship. Diagnostic testing in children with CAP is associated with increased resource use, including hospitalization, after adjusting for severity [3]. Although white blood cell count is not specific for the diagnosis of bacterial pneumonia and the degree of elevation does not distinguish between bacterial and viral infections, a CBC was ordered at 20% of ED visits for outpatient CAP in our study, contrary to PIDS/IDSA recommendations [4]. Similarly, CXR cannot reliably determine the etiology of CAP and does not have a substantial effect on clinical outcomes, particularly when clinical suspicion for CAP is high; yet, in our study, 43% of children cared for in an outpatient setting and 83% of those cared for in an ED underwent CXR [4]. The rate of positive blood culture results in well-appearing children with CAP cared for as an outpatient was low (<2%); therefore, it is concerning that a blood culture was performed for 11% of the children in our study [4]. The benefits of avoiding unnecessary antibiotics and using the narrowest-spectrum antibiotic possible are well established. These benefits include reducing the spread of antimicrobial resistance, antibiotic-associated adverse effects, and costs of care.
This study has limitations. Although the algorithm we used to identify children with CAP has been validated, reliance on diagnostic codes can result in occasional misclassification. In addition, the lack of clinical data, including microbiologic data, prevented an examination of factors that might have influenced test or antibiotic use. This lack of data was mitigated by excluding children with a chronic condition or coexisting bacterial infection and those who needed hospitalization. However, it might be that no decrease in resource use was seen because use was already at a reasonable level, particularly for testing, because we were unable to determine motivations behind the clinical decisions made using these data.
Despite the high prevalence of viral infection in young children with CAP, the use of antibiotics (mostly broad spectrum) remained high, inconsistent with national guideline recommendations. In addition, testing that does not routinely change outcomes is used frequently. Effective interventions are needed to decrease potentially unnecessary diagnostic testing and treatment.
Notes
Financial support. This work was supported, in part, by the National Institute for Allergy and Infectious Diseases, National Institutes of Health (grant K23 AI121325 to T. A. F.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Keren R, Luan X, Localio R, et al. ; Pediatric Research in Inpatient Settings Network Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med 2012; 166:1155–64. [DOI] [PubMed] [Google Scholar]
- 2. Gerber JS, Kronman MP, Ross RK, et al. . Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol 2013; 34:1252–8. [DOI] [PubMed] [Google Scholar]
- 3. Florin TA, French B, Zorc JJ, et al. . Variation in emergency department diagnostic testing and disposition outcomes in pneumonia. Pediatrics 2013; 132:237–44. [DOI] [PubMed] [Google Scholar]
- 4. Bradley JS, Byington CL, Shah SS, et al. ; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53:e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams DJ, Shah SS, Myers A, et al. . Identifying pediatric community-acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr 2013; 167:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics 2000; 106:205–9. [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Survey methods and analytic guidelines. Available at: https://www.cdc.gov/nchs/ahcd/survey_methods.htm; Accessed October 30, 2018.
- 8. Neuman MI, Shah SS, Shapiro DJ, Hersh AL. Emergency department management of childhood pneumonia in the United States prior to publication of national guidelines. Acad Emerg Med 2013; 20:240–6. [DOI] [PubMed] [Google Scholar]
- 9. Kronman MP, Hersh AL, Feng R, et al. . Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994–2007. Pediatrics 2011; 127:411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parikh K, Hall M, Blaschke AJ, et al. . Aggregate and hospital-level impact of national guidelines on diagnostic resource utilization for children with pneumonia at children’s hospitals. J Hosp Med 2016; 11:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Handy LK, Bryan M, Gerber JS, et al. . Variability in antibiotic prescribing for community-acquired pneumonia. Pediatrics 2017; 139:e20162331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Florin TA, Ambroggio L, Brokamp C, et al. . Reliability of examination findings in suspected community-acquired pneumonia. Pediatrics 2017; 140:e20170310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ross RK, Hersh AL, Kronman MP, et al. . Impact of Infectious Diseases Society of America/Pediatric Infectious Diseases Society guidelines on treatment of community-acquired pneumonia in hospitalized children. Clin Infect Dis 2014; 58:834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams DJ, Hall M, Gerber JS, et al. . Impact of a national guideline on antibiotic selection for hospitalized pneumonia. Pediatrics 2017; 139: e20163231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lomas J, Anderson GM, Domnick-Pierre K, et al. . Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med 1989; 321:1306–11. [DOI] [PubMed] [Google Scholar]
- 17. Ambroggio L, Thomson J, Murtagh Kurowski E, et al. . Quality improvement methods increase appropriate antibiotic prescribing for childhood pneumonia. Pediatrics 2013; 131:e1623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murtagh Kurowski E, Shah SS, Thomson J, et al. . Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics 2015; 135:e1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]