Abstract
The actin cytoskeleton plays a central role in establishing cell polarity and shape during embryonic morphogenesis. Daam1, a member of the Formin family of actin cytoskeleton regulators, is a Dvl2-binding protein that functions in the Wnt/Planar Cell Polarity (PCP) pathway. To examine the role of the Daam proteins in mammalian development, we generated Daam-deficient mice by gene targeting and found that Daam1, but not Daam2, is necessary for fetal survival. Embryonic development of Daam1 mutants was delayed most likely due to functional defects in the labyrinthine layer of the placenta. Examination of Daam2 and Daam1/2 double mutants revealed that Daam1 and Daam2 are functionally redundant during placental development. Of note, neural tube closure defects (NTD), which are observed in several mammalian PCP mutants, are not observed in Wnt5a or Daam1 single mutants, but arise in Daam1;Wnt5a double mutants. These findings demonstrate a unique function for Daam genes in placental development and are consistent with a role for Daam1 in the Wnt/PCP pathway in mammals.
Introduction
The actin cytoskeleton plays a central role in the morphogenesis of the mammalian embryo by controlling cell shape, division, polarity and movement. Previous studies revealed that Rho-GTPases are important regulators of the actin cytoskeleton [1, 2] and are essential for embryogenesis because loss of the Rho family members Rac1 or Cdc42 leads to early embryonic lethality [3, 4]. One signaling pathway known to control the activity of Rho family proteins during mammalian development is the Wnt/Planar Cell Polarity (PCP) pathway [5]. Dishevelled2 (Dvl2) is a cytoplasmic phosphoprotein that has common roles in transducing Wnt signals. Dvl2 possesses three conserved functional domains, the N-terminal DIX domain, a central PDZ domain, and a C-terminal DEP domain (reviewed in [6, 7]). The DIX domain is essential for transducing canonical Wnt signals through the Wnt/βcatenin pathway, whereas the PDZ and DEP domains function in the Wnt/PCP pathway [8–12].
Core components of the PCP pathway, which include the aforementioned Dvl, as well as the frizzled Wnt receptors, the transmembrane protein Van Gogh, the LIM-domain containing protein prickle, the G-protein coupled protein flamingo, and the ankyrin repeat-containing protein diego, were first identified in Drosophila where they were shown to control the direction of cells in the wing epidermis and eye. Establishment of PCP depends on the asymmetric localization of the PCP core components in these cells (Reviewed in [13]). Rho and Rac function downstream of these PCP core genes to control tissue polarity. In Xenopus and zebrafish, RhoA, Rac1 and Cdc42, along with the PCP pathway, control the convergence and extension (CE) movements responsible for the cell intercalations in the midline that drive the extension of the embryonic anterior-posterior (A-P) axis (reviewed in [5, 14–17]). Asymmetric localization of PCP core proteins was also observed in the cells undergoing the CE movements [18, 19].
Mice with mutations in core PCP genes exhibit a common set of phenotypes, including defects in the cochlea, polarity of multiciliated cells, Left-Right (LR) axis formation, A-P axis elongation, neural tube closure, and cardiac outflow tract septation [20–36]. Asymmetric localization of core PCP proteins in hair cells of the cochlea, multiciliated cells, and node cells has been reported. Disruption of these PCP proteins perturbed the orientation of the cells in the inner cochlea and multiciliated cells, or cilia position in the node in a fashion that is analogous to the PCP defects observed in the fly wing [22, 23, 27–29, 31–33, 37–39]. On the other hand, the mammalian A-P axis and neural tube closure defects are more similar to the CE movement defects observed when the PCP pathway is perturbed in Xenopus and zebrafish. These data suggest that the Wnt/PCP pathway has adapted to function in several different developmental contexts in diverse organisms.
These functional adaptations may have arisen from the regulation of different Rho GTPase effectors in a given cell type or tissue (reviewed in [40, 41]). Daam1 is a member of the Formin subfamily of Rho GTPase effectors that was originally identified as a Dvl2 binding protein [42]. Formins contain a GTPase binding domain (GBD) at the N-terminus, and the Formin homology domains (FH) 1 and 2 at the C-terminus of the protein. Formins are auto-inhibited by interactions between the N-terminus diaphanous inhibitory domain (DID) and the C-terminus diaphanous autoregulatory domain (DAD). Binding of Rho GTPases to the GBD domain alleviates auto-inhibition, exposing the FH2 functional domain to increase nucleation of the actin cytoskeleton (reviewed in [43–45]). The N-terminal region of the Daam1 protein containing the GBD and DID domains has dominant negative activity, whereas the C-terminal region of Daam1, which contains the FH2 domain, is constitutively active [42, 46]. In mammals, a second member of this highly conserved family, Daam2, exhibits both distinct and overlapping expression patterns with Daam1 during early mouse development [47]. Daam1 interacts with the PDZ and DEP domains of Dvl2 to control CE in Xenopus and zebrafish, consistent with a role of Daam1 in the Wnt/PCP pathway [42, 46, 48–50].
It is unclear whether Drosophila DAAM participates in the establishment of PCP in the wing or eye, but it was demonstrated that DAAM regulates the actin cytoskeleton in the tracheal cuticle and the axonal growth cone [51, 52]. Drosophila DAAM interacts genetically with RhoA and Rac GTPases [51, 52], suggesting that Daam proteins have conserved functions as effectors of Rho GTPases. To determine whether Daam proteins function in the mammalian Wnt/PCP pathway, we generated loss-of-function (LOF) mutations in Daam1 and 2. We found that neither Daam1 nor Daam2 mutants display phenotypes characteristic of PCP mutants. However, genetic interactions between Daam1 and Wnt5a or Vangl2 are consistent with Daam1 having a role in the noncanonical Wnt pathway. Of note, the Daam1 mutant had abnormal connections between maternal blood sinuses and fetal blood vessels in the placenta, and Daam1/2 double mutants had a more severe phenotype. Our results demonstrate that Daam genes are essential genes with redundantly functions in placental development.
Materials and methods
Mice
Daam1Flox and Daam2LacZ mice were reported previously [53]. Daam1 FloxNeo/+heterozygotes were crossed with ACTB-flpe to remove the PGK-neo cassette. The resultant Daam1Flox/+ mice were crossed with ACTB-cre to generate the Daam1 mutant allele (Daam1Δ/+). The Daam1 mutant allele with PGK-neo cassette (Daam1ΔNeo/+) was generated by Daam1FloxNeo/+ heterozygotes crossed with ACTB-cre to remove the Exon6.
Vangl2Lp/+, ACT-flpe and Meox2-Cre mice were purchased from Jackson Labs. C57BL6 (Ly5.1 and Ly5.2) mice were provided by Charles River Laboratories. The Wnt5a knockout [54] and ACNB-cre [55] mice were obtained from the originating labs.
This study was carried out in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by Frederick National Lab Animal Care and Use Committee (Proposal #17–408). Rodents were euthanized by CO2 inhalation in accordance with the most recent AVMA Guidelines on Euthanasia. For the harvesting of viable embryonic tissue, the embryos were harvested, placed in cold PBS, cooled down on ice and decapitated before harvesting of fetal livers.
Antibodies and immunofluorescent reagents
The following antibodies and reagents were used: mouse anti-Daam1 C monoclonal antibodies were raised against mouse Daam1 peptide a.a.1057-1068 following standard procedures. Cloned lines were selected by ELISA and Western blotting and anti-Daam1 C monoclonal antibodies were purified from identified cell lines using a protein A column. Additional antibodies and reagents: mouse anti-human Daam1 N (a.a. 1–111) monoclonal antibody (M05) (Abnova), mouse anti-Actin monoclonal antibody (Chemicon), mouse anti-Myc (9E10) (Santa Cruz Biotechnology), rat anti-TER119-PE and rat anti-CD71-FITC (BD-Pharmingen), anti-PECAM-1 (MEC13.3)(BD-Pharmingen), goat anti-mouse Ig Horseradish Peroxidase-linked (GE healthcare), goat anti-rat Ig Horseradish Peroxidase-linked (GE healthcare), Rhodamine-phalloidin, Texas Red-X phalloidin, BODIPY488-phalloidin, and 4’,6-diamidini-2-phenylindole, dihydrochloride (DAPI) (BD Bioscience).
Western blot
Whole embryonic day 10.5 (E10.5) embryos were dissected in cold PBS. Placentas were dissected and separated from the maternal portion. These samples were subsequently homogenized on ice in TNE (50 mM Tris-HCl (pH 7.5), 0.15 M NaCl, 1 mM EDTA, and 1% NP-40 with protease inhibitors Complete Mini (Roche)), centrifuged, and the supernatants were used. Protein concentrations were measured using the Bio-Rad Protein Assay (Bio-Rad) according to the manufacturer’s protocol. Proteins were separated using 4–15% Ready Gel (Bio-Rad), transferred to Immobilon transfer membrane (MILLIPORE), incubated with antibodies, and detected using the ECL Plus Western Blotting Detection System (GE Healthcare).
Immunocytochemistry
Immunocytochemistry and stress fiber quantification were performed as previously described [46]. Briefly, the plasmids were transfected into NIH3T3 cells using Polyfect reagent (Qiagen). The cells were fixed, permeabilized, and stained with anti-Myc antibody and phalloidin. For quantification of effects on actin fibers, a baseline of ten fibers/cell was used. Cells containing greater or less than ten fibers were scored as a normal or depleted stress fiber, respectively.
Xenopus embryo manipulation
Microinjection and manipulation of Xenopus embryos were performed as described previously [46]. Embryos with a curved and shortened axis were scored as mild defective embryos and embryos with an open neural tube and curved shortened axis were scored as severely defective embryos. The collective total number of injected embryos from all experiments is indicated below each bar.
Flow cytometry, BFU-E colony formation assay and transplantation
Single-cell suspensions of fetal liver cells were collected from E12.5 embryos. For flow cytometry, cells were suspended in sort buffer (PBS (Gibco), penicillin/streptomycin (Gibco), and 0.2% de-ionized BSA (Sigma)), blocked with the non-specific FcR (CD16/32) with 2.4G2 (Purified anti-CD16/32: BD Phamingen), stained with antibodies, and analyzed with LSRI (Becton Dickinson).
For the BFU-E colony formation assay, 3 × 104 cells were cultured for 10 days in IMDM (Gibco) with 1.1% Methylcellulose, 25% FCS, 30ng/ml IL-3, 100ng/ml SCF, 5 units/ml of Erythropoietin, 50 μM 2-Mercaptoethanol (SIGMA), and Penicillin/Streptomycin (Gibco). For transplantation, Ly5.2-positive C57BL6 mice were irradiated with 950rads, and 8× 105 cells were injected intravenously. Transplanted mice were observed for two months, followed by harvesting of their thymus, spleen, and bone marrow for flow cytometry.
Whole-mount immunohistochemistry
Embryos were fixed with 4%PFA/PBS and stained as previously described [56].
Isolation of primary mouse embryonic fibroblasts (MEFs)
MEFs were prepared from E12.5 embryos by an established procedure [57]. Cell proliferation was measured using <4-passage cells in 96 well plates using the CellTiter96 Aqueous One Solution Cell proliferation Assay (MTS assay) (Promega) according to the manufacturer’s protocol.
Reverse Transcription and quantitative PCR (qPCR)
Total RNA was purified using TRIzol (Invitrogen) according to the manufacturer’s recommendations. One microgram of RNA was treated with Dnase1 and first strand cDNA was synthesized using oligo dT and superscript III reverse transcriptase (Invitrogen). cDNA was quantitated by qPCR using the CFX96 real-Time PCR Detection System (Bio-Rad) and Fast Start Universal SYBR Green Master (Roche). The specificity of all primers was monitored by electrophoresis of the amplicons on agarose gels. The mean expression values obtained for each gene were normalized to GAPDH (ΔΔC (t) method) and to the expression of Daam1 in the lowest expressing sample. The following gene specific oligos were used.
Daam1-RT exon6 FW-CCAgAAgTATgCCAgCgAgAgAA
Daam1-RT exon7 RV-ACgCCCAgTgCTTTTATCCAAgT
GAPDH-RT.FW- AATgTgTCCgTCgTggATCTg;
GAPDH-RT.RV- CTgCTTCACCACCTTCTTgATgT.
In situ hybridization
Section in situ hybridization was performed as previously described [47, 58]. Unless indicated otherwise, at least 4 mutant embryos were examined for each probe, and all yielded similar results.
Histology
Embryos and placentas were dissected in ice-cold PBS and fixed with 4% PFA/PBS overnight at 4°C and transferred to 70% ethanol/saline for paraffin sections or 20% sucrose/PBS for frozen sections. Paraffin and frozen sections were cut at 8-μm and 10-μm thickness, respectively. Sections were stained with Hematoxylin and Eosin (H&E) following standard protocols.
Plasmids
Daam1 vectors were constructed by standard restriction digestion or PCR amplification cloning techniques, and subcloned into the pCS2+MT vector. The plasmids designated as Daam1 FL, Daam1 N, and Daam1 Δ contain cDNA fragments that correspond to a.a.1-1077, 1–420, and 1–148 of the mouse Daam1 protein, respectively.
GST pull-down assay
Myc-tagged proteins used in GST pull-down assays were generated by TNT Quick-Coupled Transcription/Translation systems (Promega). GST pull-down assays were performed as described previously [46].
Imaging
Embryos and sections were photographed with a Leica MZFLIII stereoscope (Leica) or a Zeiss AxioPlan2, equipped with a Zeiss Axiocam HR digital camera, and Zeiss Axiovision version 4.5 imaging software (Zeiss).
Statistical analysis
Student t-test and chi-square test were performed using excel software (Microsoft). Wilcoxon Rank-sum test was performed using R software (The R Foundation).
Results
Characterization of Daam1 mutant mice
To address Daam1 function in vivo, we generated the Daam1 null allele (Daam1Δ) from Daam1 conditional knockout mice. Heterozygous Daam1Δ/+ mice are viable, fertile, and have no obvious phenotypes. Homozygous Daam1Δ/Δ offspring arising from Daam1Δ/+ intercrosses were not found at birth (Table 1). Western blot analysis of whole embryo lysates using an antibody against the C-terminus of Daam1 confirmed that Daam1Δ/Δ embryos did not express the full-length Daam1 protein, and that heterozygotes expressed roughly half the amount (S1A Fig). Further analysis of mutant embryos with an antibody that recognizes the N-terminus of Daam1 revealed a weakly expressed 16-kDa protein in Daam1Δ/Δ lysates, which is the expected size when exon 6 of Daam1 is removed from the locus (S1B Fig). Functional analysis of this 16-kDa Daam1Δ protein expressed in NIH3T3 and Xenopus embryos revealed it to be non-functional (S2 Fig). We therefore concluded that the Daam1Δ allele is functionally null.
Table 1. Number of Daam1+/+, Daam1Δ/+, and Daam1Δ/Δ for each developmental stage.
| Daam1+/+ | Daam1Δ/+ | Daam1Δ/Δ | Empty decidua | |
|---|---|---|---|---|
| E10.5 | 9 (1) | 29 (3) | 18 (5) | 4 |
| E12.5 | 55 (1) | 105 (2) | 39 (10) | 22 |
| E13.5 | 25 (1) | 60 (0) | 14 (5) | 17 |
| E14.5 | 17 (1) | 27 (2) | 11 (6) | 19 |
| E16.5 | 15 (0) | 22 (1) | 2 (2) | 1 |
| Postnatal | 29 | 89 | 0 |
Numbers of dead embryos are indicated in parentheses.
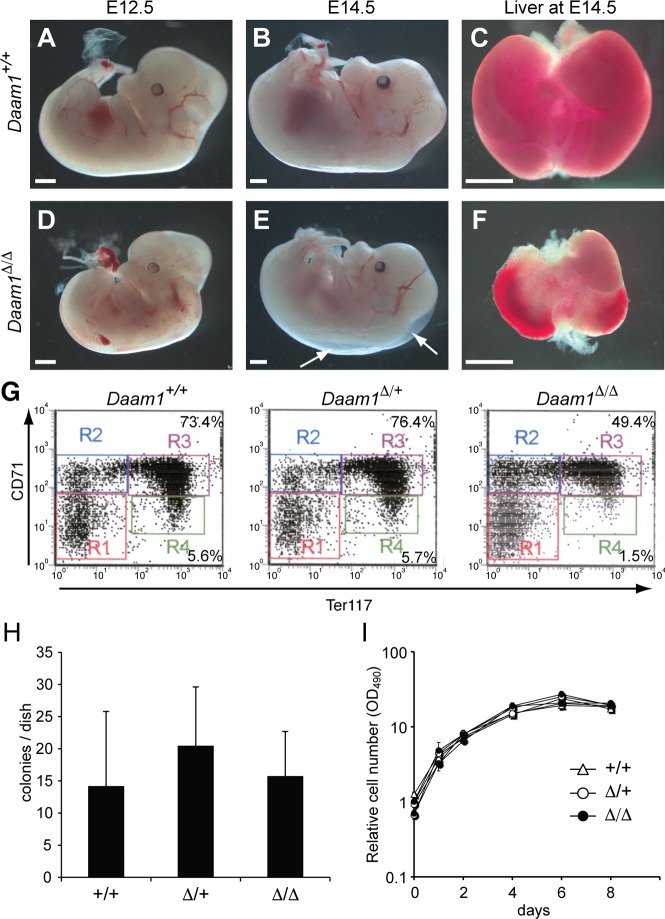
Analysis of Daam1Δ/Δ embryos demonstrated that embryo viability declined after E12.5, and live embryos were no longer observed by E16.5 (Table 1). E12.5–14.5 Daam1Δ/Δ embryos were small and pale compared with wild-type and Daam1Δ/+ littermates (Fig 1A, 1B, 1D and 1E). Reduced liver mass was apparent in whole Daam1Δ/Δ embryos by E14.5 (Fig 1C and 1F), but reduced numbers of total fetal liver cells were quantified as early as E12.5 (2.2-fold reduction) and E13.5 (4-fold reduction) compared with wild-type and Daam1Δ/+ littermates. The differentiation status of hematopoietic cells of the mutant fetal liver was assessed by flow cytometry. Daam1Δ/Δ embryos exhibited normal differentiation of the T cell, B cell, and myeloid cell lineages, however erythrocyte differentiation was perturbed. Using CD71 and Ter119 expression as markers of erythrocyte differentiation [59], we found that Daam1Δ/Δ embryos had fewer differentiated, mature erythrocyte cells, than wild-type and Daam1Δ/+ embryos at E12.5 (Fig 1G). The reduction of mature erythrocytes may have been caused by fewer erythrocyte progenitors or an inability to differentiate. To examine these possibilities, we performed an erythroid burst-forming units (BFU-E) colony formation assay. Daam1Δ/Δ and control embryos had similar numbers of erythrocyte progenitors (Fig 1H). To examine their differentiation capacity in vivo, fetal liver cells from wild-type and mutant embryos were transplanted into lethally irradiated wild-type hosts. Engraftment of Daam1Δ/Δ fetal liver cells leading to the viability of recipient hosts was observed (S1 Table), demonstrating that Daam1Δ/Δ hematopoietic progenitor cells retain the ability to differentiate into functional erythrocytes. These results suggest that the reduced viability of Daam1Δ/Δ embryos was not due to intrinsic defects in erythropoiesis or fetal liver function.
Fig 1. Observation of Daam1-deficient embryos.
(A-E) Daam1+/+ (A-C) and Daam1Δ /Δ (D-F) embryos at E12.5 (A, D), E14.5 (B, E) and livers at E14.5 (C, F). Edema found in Daam1Δ /Δ embryo is indicated by arrows (E). (G) Representative results of fetal liver-derived erythrocyte differentiation analyzed by flow cytometry. Percentages of the cells gated in R3 and R4 were as follows: Daam1+/+: R3 (CD71hi;Ter119hi) = 76.5 ± 2.4%, R4 (CD71lo;Ter119hi) = 6.4 ± 0.8%; n = 5, Daam1Δ/+ R3 = 73.8 ± 6.8%, R4 = 6.3 ± 1.7%; n = 4, and DaamΔ /Δ:R3 = 64.2 ± 9.0%, R4 = 3.3 ± 1.6%; n = 5. Daam1Δ /Δ erythrocytes were significantly reduced compared with Daam1+/+ and Daam1Δ/+: t-test R3 p<0.01, R4 p<0.002. (H) BFU-E colony formation assay using fetal liver cells. Daam1+/+: n = 5, Daam1Δ/+: n = 5, and Daam1Δ/Δ: n = 4. Standard deviations are shown as error bars. No significant difference was observed. (I) MEFs proliferation assay. Relative cell numbers were measured by MTS assay. Results were normalized by day 0 as 1. Results represent analyses of 5 or more embryos of each genotype. No significant difference was observed.
Delayed development of Daam1Δ/Δ mutants due to placental defects
As Daam1Δ/Δ embryos were smaller than their littermates, we considered the possibility that Daam1 might regulates cell proliferation. MEFs were established from E12.5 embryos, and cell proliferation was analyzed by MTS assay. No difference was observed in the number of Daam1Δ/Δ MEFs compared with wild-type and Daam1Δ/+ MEFs (Fig 1I), indicating that Daam1 does not play a major role in cell proliferation.
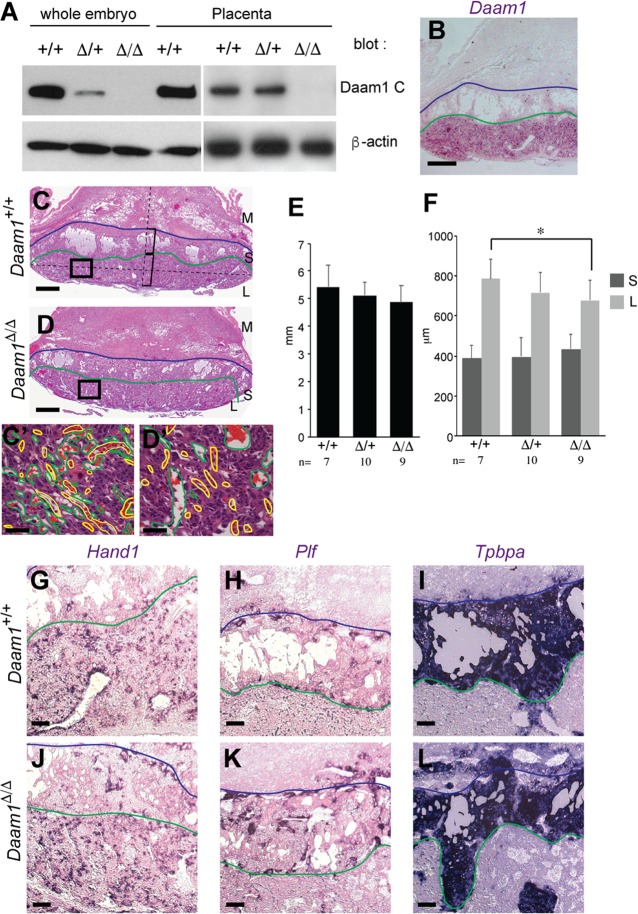
In addition to the reduced size of Daam1Δ/Δ embryos, we also observed edema, which is indicative of a circulatory defect (Fig 1E). Circulatory defects can arise from defects in the vasculature, the heart, or the placenta. As the vasculature had developed normally in Daam1ΔNeo/ΔNeo embryos (S3 Fig), we considered a role of Daam1 in heart and placental function. As reported in a previous paper [53], myocardial-specific Daam1 conditional KO exhibits myocardial maturation defects, but does not cause embryonic lethality. Analysis of Daam1 protein expression in the placenta by Western blotting confirmed that Daam1 was indeed expressed in the placenta, at a similar level to that observed in embryos, and was undetectable in the Daam1Δ/Δ placenta (Fig 2A). In situ hybridization of sectioned placenta revealed that Daam1 mRNA was highly expressed in the placenta, although restricted to the labyrinthine layer (Fig 2B). The labyrinthine layer plays an important role in placental function, because it is where the direct exchange of nutrition and gases between the maternal and fetal blood supplies occurs [60]. Defects in the function of the labyrinthine layer lead to placental insufficiency, which may account for the fetal growth retardation and death. Histological analyses revealed that the spongiotrophoblast layer in Daam1Δ/Δ placentas were of a similar size and thickness as controls, but the labyrinthine layer in Daam1Δ/Δ placentas was slightly thinner than in wildtype. (Fig 2C–2F).
Fig 2. Placental developmental defects in Daam1-deficient mice.
(A) Western blot analysis for Daam1 (top) and β-actin (bottom). Whole embryo lysates and the embryonic part of the placenta from indicated genotypes at E10.5 were used.(B) In situ hybridization of wild-type placenta sections using the Daam1 probe. (C) Daam1+/+ and (D) Daam1Δ/Δ placentas at E12.5. High magnification images’ (C’, D’) positions are indicated as boxes on C and D. Maternal blood sinuses (green) and fetal blood vessels (yellow) are labeled with lines on C’ and D’. (E) Size of the placenta of each genotype which was measured as the distance between edges of the boundary between the spongiotrophoblast and labyrinthine layers. (F) Thickness of the spongiotrophoblast layer (S) and labyrinthine layer (L) in each genotype. The thickness of each layers was measured on the line that connected the center of the boundary between spongiotrophoblast and labyrinthine layers and the thickest part of placenta as shown in C. t-test *: p<0.05. (G-L) In situ hybridization of Daam1+/+ (G-I) and Daam1Δ/Δ (J-L) placenta sections using Hand1 (G, J), Plf (H, K) and Tpbpa (I, L) probes. Blue lines depict the boundary between the maternal decidua (M) and spongiotrophoblast layer (S), and green lines depict the boundary between spongiotrophoblast and labyrinthine layers (L). Scale bar = 200 μm in B, and G-L. 500 μm in C and D. 50 μm in C’ and D’.
Marker analyses using the trophoblast giant cell marker Hand1, the endothelial trophoblast marker Plf, and the spongiotrophoblast marker Tpbpa, revealed no differences in their expression between control and Daam1Δ/Δ placentas (Fig 2G–2L) suggesting that cell fates were unchanged in Daam1Δ/Δ placentas. A detailed analysis of the labyrinthine layers revealed thin and refined maternal blood sinuses adjacent to fetal blood vessels in control placentas, however, maternal blood sinuses were enlarged and not entwined with fetal blood vessels in the Daam1Δ/Δ labyrinthine layer (Fig 2C’ and 2D’). These results suggest that nutritional deficiency caused by reduced placental function was responsible for the delayed development of Daam1 mutants.
To assess whether Daam1 has an intrinsic function in the embryo, we used Meox2-cre to inactivate Daam1 specifically in the epiblast, thereby enabling wild-type function in the placenta. As Daam1 and Meox2 are located on the same chromosome, mutant alleles (Daam1Flox/Δ; Meox2-cre) on different sister chromosomes were rarely inherited together (Table 2). Notably, Daam1Flox/Δ; Meox2-cre mice were viable for >1 year and lacked overt phenotypes (Table 2). These results are consistent with the absence of intrinsic function by Daam1 during embryonic development, however, the mosaic expression of Meox2-cre in the epiblast [61] remains a caveat. Indeed, Daam1 RNA and protein remain detectable in varying amounts in adult organs, depending on the tissue (ex. 1.3–29.6% of Daam1 RNA level observed in control tissues (S4A and S4B Fig)). Nevertheless, these results together suggest that the embryo lethality of Daam1Δ/Δ mutants is due to the requirement of Daam1 in the placenta.
Table 2. Number of embryos or mice from crosses between Daam1flox/flox and Daam1Δ/+; Meox2-cre mice.
| Daam1Flox/+ | Daam1Flox/+; Meox2-cre | Daam1Flox/Δ | Daam1Flox/Δ; Meox2-cre | |
|---|---|---|---|---|
| E16.5 | 4 | 18 | 12 | 3 |
| Postnatal | 3 | 36 | 40 | 3 |
Daam1 and Daam2 are functionally redundant in the placenta
Despite the broad pattern of embryonic expression [47], removal of Daam1 only resulted in placental defects. To address the possibility that Daam2 functionally compensates for the loss of Daam1 in the embryo, we used Daam2LacZ mice. Examination of β-galactosidase activity in Daam2 heterozygous (Daam2LacZ/+) embryos revealed a pattern similar to that of endogenous Daam2 mRNA expression (S5A Fig, and [47]). Daam2LacZ/LacZmice were also viable and fertile and had no obvious phenotype.
On examination of Daam1Δ/Δ;Daam2LacZ/LacZ embryos at E10.5, double mutants were observed to be smaller than their litter mates (n = 8/16) (Fig 3A–3D) and had enlarged pericardial cavities (n = 3/16) (Fig 3D). All the Daam1Δ/Δ;Daam2 LacZ/LacZ embryos dissected at E11.5 (n = 4) and E12.5 (n = 2), and the Daam1Δ/Δ;Daam2 LacZ/+ embryos dissected at E12.5 (n = 5) were dead and had started to be resorbed (Table 3). Daam2 expression in placenta was examined by β-gal staining. Daam2 was highly expressed in embryonic mesoderm-derived tissue, and expressed at low levels in the labyrinthine layer, but not in the spongiotrophoblast (S5B and S5B’ Fig). Examination of Daam1Δ/Δ;Daam2 LacZ/LacZ placentas at E10.5, revealed normal fusion of the chorioallantoic mesoderm in controls and Daam1Δ/Δ;Daam2 LacZ/LacZ placentas (Fig 3E–3G and 3E’–3G’), but vascularization of the labyrinthine layer was markedly impaired in Daam1Δ/Δ;Daam2 LacZ/LacZ placentas (Fig 3G and 3G’). As these placental defects were more severe and occured earlier (E10.5) in the double mutants than in the single Daam1Δ/Δ placentas (E12.5) (Figs 2C’ and 2D’ and 3E‘–3G’), we conclude that Daam1 and Daam2 have redundant functions in placental development.
Fig 3. Embryonic developmental delay and placental developmental defects in Daam1/2-deficient mice.
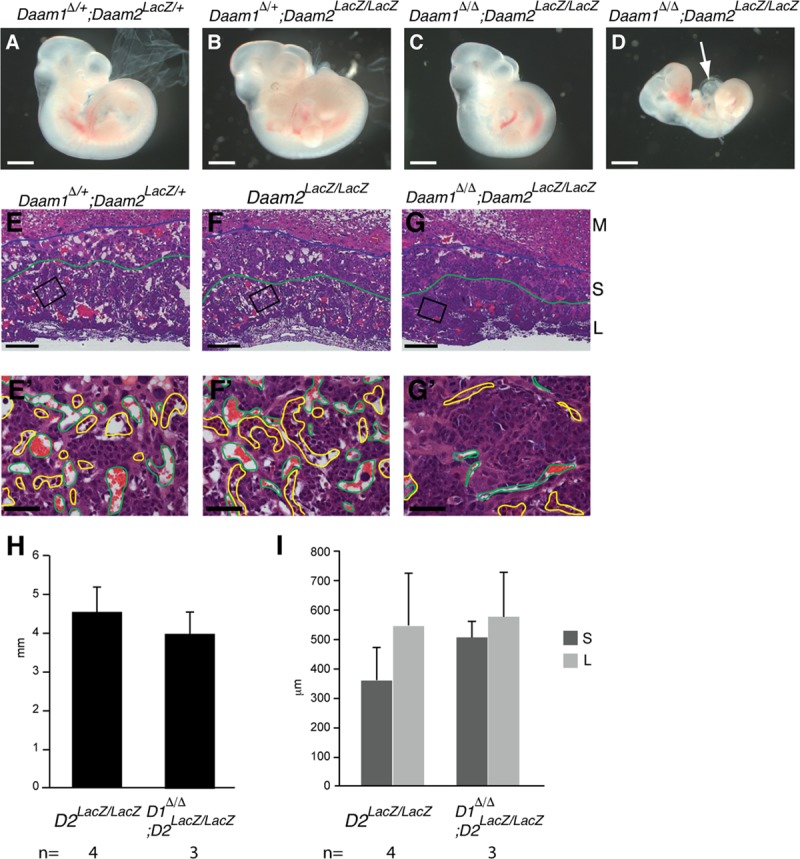
(A-C) Daam1Δ/+, Daam2LacZ/+ (A), Daam1Δ/+, Daam2 LacZ/LacZ (B), and Daam1Δ/Δ, Daam2LacZ/LacZ (C, D), embryos at E10.5. Some of Daam1Δ/Δ, Daam2 LacZ/LacZ embryos were found dead with an enlarged pericardial cavity (arrow, D). (E-G, E’-G’) H&E staining of E10.5 placenta sections of Daam1Δ/+, Daam2 LacZ/+ (E), Daam2 LacZ/LacZ (F), and Daam1Δ/Δ, Daam2 LacZ/LacZ (G). High magnification images’ (E’-G’) positions are indicated as boxes on E-G. (H) The size of the placenta of Daam2 LacZ/LacZ and Daam1Δ/Δ, Daam2 LacZ/LacZ was measured as described for Fig 2E. (I) The thickness of the spongiotrophoblast layer (S) and labyrinthine layer (L) in Daam2 LacZ/LacZ and Daam1Δ/Δ, Daam2 LacZ/LacZ, measured as described in Fig 2F. Blue lines depict the boundary between the maternal decidua (M) and spongiotrophoblast layer (S), and green lines depict the boundary between the spongiotrophoblast and labyrinthine layers (L). Maternal blood sinuses and fetal blood vessels are outlined in green and yellow, respectively (E’-G’). Scale bars = 1 mm in A-D, 200 μm in E-G, 50 μm in E’- G’.
Table 3. Number of Daam1 and Daam2 mutants for each developmental stage.
| Daam1+/+;Daam2+/+ | Daam1+/+; Daam2LacZ/+ | Daam1+/+; Daam2LacZ/LacZ | Daam1Δ/+; Daam2+/+ | Daam1Δ/+; Daam2LacZ/+ | Daam1Δ/+; Daam2LacZ/LacZ | Daam1Δ/Δ; Daam2+/+ | Daam1Δ/Δ; Daam2LacZ/+ | Daam1Δ/Δ; Daam2LacZ/LacZ | |
|---|---|---|---|---|---|---|---|---|---|
| E10.5 | 1 | 8 | 20 | 9 | 18 | 33 (1) | 1 | 12 (2) | 16 (2) |
| E11.5 | 4 | 11 | 4 (4) | ||||||
| E12.5 | 2 | 2 | 12 | 2 | 10 | 9 | 0 | 5 (5) | 2 (2) |
E10.5 and E12.5 embryos were obtained from inter se crosses of Daam1Δ/+, Daam2 LacZ/+ or Daam1Δ/+, Daam2 LacZ/LacZ mice.
E11.5 embryos were obtained from inter se crosses of Daam1Δ/+, Daam2 LacZ/LacZ mice.
Numbers of dead embryos are indicated in parentheses.
Genetic interaction between Daam1 and PCP pathway components
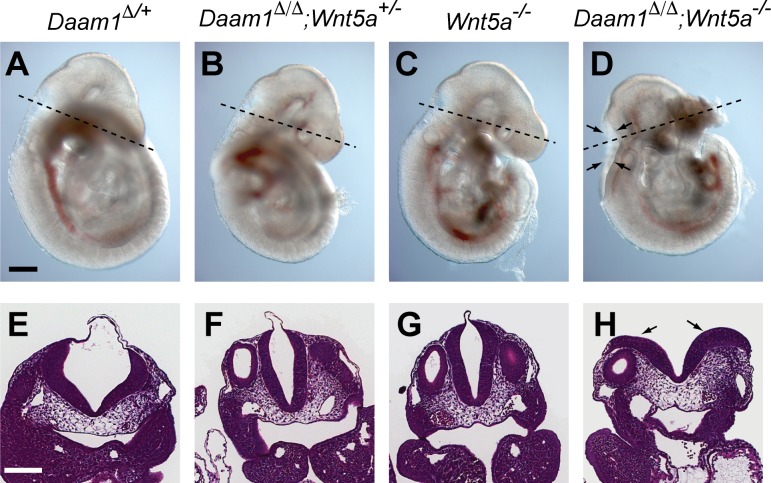
Although Daam1 was originally identified as a regulator of Wnt/PCP signaling [42], phenotypes characteristic of mammalian PCP mutants such as Vangl2Lpt such as neural tube closure defects and convergent-extension defects, were not observed in Daam mutants. To investigate whether Daam1 has a role in the mammalian PCP pathway, we looked for genetic interactions between Daam1 and Vangl2 or Wnt5a mutants, both of which are components of the noncanonical PCP pathway [24, 54, 62, 63]. Vaginal atresia or imperforate vagina is a little-discussed but frequently observed phenotype of Vangl2Lpt mice [63], including our colony of Vangl2Lpt/+ females (7 of 15 mice). This frequency increased when crossed with the Daam1Δ/+ background (13 of 15 Daam1Δ/+;Vangl2Lpt/+ females). However, vaginal atresia in Daam1Δ/+;Vangl2Lpt/+ female mice precluded the generation of Daam1Δ/Δ;Vangl2Lpt/Lpt embryos. Examination of Daam1 and Wnt5a interactions at E9.5 (Table 4) revealed that Daam1Δ/ Δ;Wnt5a+/- embryos were indistinguishable from wild-type, Daam1Δ/+, or Daam1Δ/ Δ embryos (Fig 4A, 4B, 4E and 4F). Although Daam1Δ/ Δ;Wnt5a-/- embryos exhibited a short AP axis and small somite phenotype similar to Wnt5a-/- embryos (Fig 4C and 4D) [54], double mutants frequently exhibited an open neural tube (5 of 8 Daam1Δ/ Δ;Wnt5a-/-embryos) at the position of the fourth ventricle (Fig 4D and 4H). This phenotype was never observed in Daam1Δ/ Δ or Wnt5a-/- single mutants.
Table 4. Number of Daam1 and Wnt5a mutants at E9.5 stage.
| Daam1+/+; Wnt5a+/+ | Daam1+/+; Wnt5a+/- | Daam1+/+; Wnt5a-/- | Daam1Δ/+; Wnt5a+/+ | Daam1Δ/+; Daam2LacZ/+ | Daam1Δ/+; Daam2LacZ/LacZ | Daam1Δ/Δ; Wnt5a+/+ | Daam1Δ/Δ; Wnt5a+/- | Daam1Δ/Δ; Wnt5a-/- |
|---|---|---|---|---|---|---|---|---|
| 13 | 27 | 10 | 22 | 46 | 11 | 7 | 11 | 8 |
These embryos were obtained from 15 litters of inter se crosses of Daam1Δ/+, Wnt5a+/-, 1 litter of Daam1Δ/+, Wnt5a+/- male crossed with Wnt5a+/- female, and 4 litter of Daam1Δ/+, Wnt5a+/- male crossed with Daam1Δ/+ females.
Fig 4. Neural tube closure defects in Daam1;Wnt5a double mutants.
(A-D) Lateral view of (A) Daam1Δ/+, (B) Daam1Δ/Δ, Wnt5a+/-, (C) Wnt5a-/- and (D) Daam1Δ/Δ, Wnt5a-/- embryos at E9.5. H&E staining of cross-sections of the embryos shown in A-D through the fourth ventricle (E-H) Section positions are indicated by dotted lines in A-D. Arrows indicate the open neural tube. Scale bars = 200 μm.
Discussion
We investigated the role of Daam1 in mammalian development and discovered that Daam1 plays an essential role in murine placentation. The placenta functions as a fetomaternal organ during gestation, enabling nutrient uptake, waste elimination, and gas exchange between the mother and developing conceptus. Most rodents and primates (including humans) have hemochorial or labyrinthine placentas, in which maternal blood directly contacts the fetal chorionic trophoblast. The close apposition of the vascularized uterus and trophoblast in the labyrinth constitutes the major site of maternal and fetal exchange. We demonstrated that Daam1 is expressed in the trophoblast cells of the labyrinthine layer, but not in the embryonic mesoderm-derived vascular cells, and that Daam1Δ/Δ placentas undergo normal trophoblast cell differentiation but exhibit structural defects in the labyrinthine layer. As gas and nutrient exchange relies upon diffusion across the placental membrane (reviewed in [64, 65]), we suggest that the increased separation between maternal blood sinuses and fetal blood vessels observed in the Daam1Δ/Δ labyrinthine layer (Fig 2D’) is sufficient to impair the efficiency of the maternal/fetal exchange, and is therefore likely to result in fetal death. The observation that the embryo-specific deletion of Daam1 did not impair fetal development is consistent with a requisite role for Daam1 in placental trophoblast cells. How does Daam1 control the maternal blood sinus and fetal blood vessel connection? One possible explanation is that Daam1 controls trophoblast branching morphogenesis in the labyrinthine layer which is essential for the vascularization of the labyrinth, as the Wnt/PCP genes have been reported to be involved in branching morphogenesis of other epithelial tissues [66–69]. Another possibility is that Daam1 controls the maternal blood sinus and fetal blood vessel connection by stabilizing cell-cell junctions. Daam1 interacts with the E-cadherin–β-catenin–α-catenin complex at the lateral membrane contact sites to stabilize the interactions between epithelial cells [70]. Similar molecular mechanisms might operate at the maternal blood sinus and fetal blood vessel connection. Indeed, the absence of E-cadherin or β-catenin in the placenta also causes vascularization defects in the labyrinthine layers [71, 72].
Embryos lacking both Daam1 and Daam2 died earlier than single Daam1 mutants, but they did not have additional embryonic phenotypes. Daam1Δ/Δ, Daam2 LacZ/LacZ placentas had more marked vascularization defects than Daam1Δ/Δ placentas suggesting that the earlier embryo lethality arises from the more severe placental defects. mDia1, a paralog of Daam1/2, was reported to interact with RhoA, B, and/or C to control actin assembly, similar to Daam1 [73, 74]: reviewed in [45, 75]. mDia1 deficient mice also had no developmental defects [76], raising the possibility that these family members have redundant functions. Future studies are needed to address whether mDia1, Daam1 and Daam2 compensate for one another.
Our data demonstrating that the reduced Daam1 gene dosage worsened the vaginal atresia phenotype in Vangl2Lpt/+ mice and caused NTDs in Wnt5a-/- embryos. Most of the NTDs reported in the PCP core factors mutant mice were craniorachischisis, complete open neural tube [20, 21, 28, 29, 34–36, 62], however the NTD observed in Daam1Δ/ Δ;Wnt5a-/- embryos was opened only at the hindbrain. Similar partial NTDs were observed compound heterozygous of PCP core factor mutants [35] and Dvl2 single null mice [21] with low penetrance. It is likely that the partial NTDs are mild phenotype of NTDs caused by the PCP pathway aberration. The NTD observed in Daam1Δ/ Δ;Wnt5a-/- embryos suggested that Daam1 functioning, at least in part, in the Wnt/PCP pathway. This is supported by a report demonstrating a key role for Daam1 in the Wnt/PCP pathway during chick neural tube closure [77]. However, neither Daam1Δ/Δ nor Daam1Δ/Δ, Daam2 LacZ/LacZ mouse embryos exhibited neural tube closure defects and the early embryonic lethality observed in Daam1Δ/Δ, Daam2 LacZ/LacZ embryos precludes our ability to fully evaluate the role of the Daam1 gene in the PCP pathway. A report of hypomorphic Daam1 mutants, which survive to later developmental stages, described cardiac outflow tract septation defects that are commonly found in PCP mutants [78]. As severe circulation defects from placental abnormality in Daam1Δ/Δ and Daam1Δ/Δ, Daam2 LacZ/LacZ mouse embryos caused midgestational lethality, we were unable to assess heart developmental defects in Daam1 and Daam2 null mutants. Our previous study of cardiomyocyte-specific conditional Daam1 knockouts revealed indispensable roles of Daam1 in cardiac development [53]. We did not observe heart defects in Daam1Flox/Δ; Meox2-cre mice presumably due to leaky expression of Daam1 caused by the mosaic expression of Meox2-cre. The low frequency of surviving Daam1Flox/Δ; Meox2-cre adult mice (3 out of 83 mice) compared with the frequency of Daam1Flox/Δ; Meox2-cre embryos (3 out of 37 embryos) suggest that some mutants did not survive to adulthood.
In addition to the neural tube and cardiac outflow tract, the Wnt/PCP pathway was reported to regulate tube formation in the kidney, gut, and female reproductive tract [25, 79–84]. Tissue-specific inactivation of Daam1 and Daam2 in these organs will provide an opportunity to analyze the role of Daam proteins in these PCP-dependent tissues at later developmental stages.
A role for the PCP pathway in placental development has not been previously described. However, mice deficient in Wnt2 or the Wnt receptor Fz5 were found to have vascularization defects in the labyrinthine layer of the placenta similar to the Daam1/2 mutant phenotype [85, 86]. Wnt2 is expressed in embryo-derived allantoic mesoderm cells, whereas Fz5, like Daam1, is expressed in the extraembryonic labyrinth layer. During vascularization, interactions between trophoblast and embryonic mesoderm cells are important for forming capillary structures. We speculate that Wnt ligands provided by the allantois stimulate trophoblast cells in a paracrine manner to regulate capillary formation. Of note, Wnt2 is known to regulate the Wnt/β-catenin pathway in the developing lung [87], and Daam2 participates in the dorsal patterning of the spinal cord via the Wnt/β-catenin pathway [88]. Together, these results suggest that Daam1 and Daam2 could function in different Wnt pathways in a tissue-dependent manner. Another possibility is that Daam1 and Daam2 function in different signaling pathways to control placental development. Studies on Daam1 and Daam2 functions in cardiomyocytes and endothelial cells [53, 89] suggested that Daam1 has roles in the activation of the Src-Akt-Gsk3β pathway. Indeed, mice deficient in Akt1 also exhibit vascularization defects in the labyrinthine layer of the placenta that are similar to the Daam1/2 mutant phenotype [90].
We demonstrated that Daam1 and Daam2 play essential roles in placental vascularization and the establishment of the maternal/fetal blood supply. Feto-placental problems are proposed to be one of the major causes of pre-eclampsia, intrauterine growth restriction and miscarriage [91]. Further analysis of the mechanisms through which Daam functions in the placenta may improve our understanding of placental pathologies.
Supporting information
Western blot analysis to confirm Daam1 expression. (A) The top panel shows the results using an antibody against the Daam1 C-terminus antigen. (B) Top panels show the results using an antibody against the Daam1 N-terminus antigen. The right panel is longer exposure of the left panel. The arrow and arrowhead indicate full length and truncated Daam1 protein, respectively. The asterisk indicates non-specific bands. The bands labeled by double asterisks are likely degraded or splicing variants of Daam1 protein. Bottom panels show actin as loading controls.
(TIF)
(A) GST-pulldown assay of Daam1 truncated proteins with GST-RhoA. Top panels showed mouse Daam1 Δ, Daam1 N (dominant-negative form), and Daam1 FL (full length) proteins from left to right, detected by anti-Myc antibody. Bottom panels show GST or GST-RhoA proteins detected by Coomassie staining. Daam1 Δ transfected cells (B-D) and Daam1 N transfected cells (E-G) are shown. (B, E) Daam1 truncated proteins were detected by anti-Myc antibody. (C, F) Phalloidin stained cells. (C’, F’) High-magnification image of the inset is shown on the side. (D. G) Merged images of B, C and E, F are shown. (H) Quantification of effects by overexpression of Daam1 deletion proteins on stress fibers. Examined cell numbers are indicated below the graph. Chi-square test *: p<0.001 (I) Xenopus embryos were injected with mRNA transcribed from indicated plasmids, and were scored at stage 35. Scoring was performed following previously described criteria [46] Examined embryo numbers are indicated below the graph. Wilcoxon Rank-sum test *: p<0.001 (J) Representative embryos injected with each mRNA are shown.
(TIF)
PECAM-1 staining of Daam1+/+(A), Daam1ΔNeo/+(B), and Daam1ΔNeo/ΔNeo embryos at E10.5 stage are shown. No gross abnormalities in vasculature development were observed in these embryos nor in the Daam1Δ/Δembryos.
(TIF)
(A) Expression of Daam1 was examined by qPCR. Relative Daam1 expression in each tissue is shown. Mut-4(Daam1F/Δ;Meox2-cre), and cont-1 (Daam1F/Δ), mut-5 (Daam1F/Δ;Meox2-cre), and cont-2 (Daam1F/+), mut-6 (Daam1F/Δ;Meox2-cre), and cont-3 (Daam1F/+;Meox2-cre) are litter mates, respectively. SEM is shown as error bar. Western blot analysis for Daam1 protein (top) and β-actin (bottom). Pedigree number and tissues are shown above and below the panels, respectively.
(TIF)
(A) X-gal staining of E10.5 Daam2LacZ/+ embryo. (B) X-gal staining of E10.5 placental section. High magnification image (B’) positions are indicated as boxes on E. Arrow indicates embryo-derived mesodermal tissue. Blue and green lines depict the boundary between the maternal decidua (M) and spongiotrophoblast layer (S), and the spongiotrophoblast and labyrinthine layers (L), respectively. Scale bars = 500 μm in D, 200 μm in E, and 50 μm in E.
(TIF)
(TIF)
(TIF)
(TIFF)
(DOCX)
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This project has been funded with federal funds from the National Institutes of Health, Z01(ZIA BC 010345) to TPY, (HHSN26120080001E) to JRK, (GM078172) to RH, and by JSPS KAKENHI grant (18K06324) and The Japan Spina Bifida & Hydrocephalus Research Foundation to RA.
References
- 1.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–35. Epub 2002/12/13. 10.1038/nature01148 nature01148 [pii]. . [DOI] [PubMed] [Google Scholar]
- 2.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33(Pt 5):891–5. Epub 2005/10/26. BST20050891 [pii] 10.1042/BST20050891 . [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Ma L, Parrini MC, Mao X, Lopez M, Wu C, et al. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr Biol. 2000;10(13):758–65. Epub 2000/07/19. S0960-9822(00)00571-6 [pii]. 10.1016/s0960-9822(00)00571-6 . [DOI] [PubMed] [Google Scholar]
- 4.Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, et al. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1998;17(26):3427–33. Epub 1999/02/25. 10.1038/sj.onc.1202595 . [DOI] [PubMed] [Google Scholar]
- 5.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23(3):265–77. Epub 2009/02/11. 23/3/265 [pii] 10.1101/gad.1760809 . [DOI] [PubMed] [Google Scholar]
- 6.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22(5):717–27. Epub 2009/12/17. S0898-6568(09)00368-4 [pii] 10.1016/j.cellsig.2009.11.021 . [DOI] [PubMed] [Google Scholar]
- 7.Wharton KA Jr. Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253(1):1–17. Epub 2002/12/20. S0012160602908699 [pii]. 10.1006/dbio.2002.0869 . [DOI] [PubMed] [Google Scholar]
- 8.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12(16):2610–22. Epub 1998/08/26. 10.1101/gad.12.16.2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83(1–2):27–37. Epub 1999/10/03. 10.1016/s0925-4773(99)00046-5 . [DOI] [PubMed] [Google Scholar]
- 10.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405(6782):76–81. Epub 2000/05/16. 10.1038/35011068 . [DOI] [PubMed] [Google Scholar]
- 11.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127(10):2227–38. Epub 2000/04/19. . [DOI] [PubMed] [Google Scholar]
- 12.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405(6782):81–5. Epub 2000/05/16. 10.1038/35011077 . [DOI] [PubMed] [Google Scholar]
- 13.Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011;12(6):385–91. 10.1038/nrg2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roszko I, Sawada A, Solnica-Krezel L. Regulation of convergence and extension movements during vertebrate gastrulation by the Wnt/PCP pathway. Semin Cell Dev Biol. 2009;20(8):986–97. Epub 2009/09/19. S1084-9521(09)00169-4 [pii] 10.1016/j.semcdb.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8(2):126–38. Epub 2007/01/19. nrg2042 [pii] 10.1038/nrg2042 . [DOI] [PubMed] [Google Scholar]
- 16.Tada M, Heisenberg CP. Convergent extension: using collective cell migration and cell intercalation to shape embryos. Development. 2012;139(21):3897–904. Epub 2012/10/11. 139/21/3897 [pii] 10.1242/dev.073007 . [DOI] [PubMed] [Google Scholar]
- 17.Shindo A. Models of convergent extension during morphogenesis. Wiley Interdiscip Rev Dev Biol. 2018;7(1). Epub 2017/09/15. 10.1002/wdev.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439(7073):220–4. Epub 2006/01/13. 10.1038/nature04375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180(1):221–32. Epub 2008/01/16. 10.1083/jcb.200704150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008;4(11):e1000259 Epub 2008/11/15. 10.1371/journal.pgen.1000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129(24):5827–38. Epub 2002/11/08. 10.1242/dev.00164 . [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12(2):170–6. Epub 2010/01/26. ncb2020 [pii] 10.1038/ncb2020 . [DOI] [PubMed] [Google Scholar]
- 23.Minegishi K, Hashimoto M, Ajima R, Takaoka K, Shinohara K, Ikawa Y, et al. A Wnt5 Activity Asymmetry and Intercellular Signaling via PCP Proteins Polarize Node Cells for Left-Right Symmetry Breaking. Dev Cell. 2017;40(5):439–52 e4. 10.1016/j.devcel.2017.02.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423(6936):173–7. Epub 2003/05/02. 10.1038/nature01618 nature01618 [pii]. . [DOI] [PubMed] [Google Scholar]
- 25.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96(3):292–9. Epub 2005/01/08. 01.RES.0000154912.08695.88 [pii] 10.1161/01.RES.0000154912.08695.88 . [DOI] [PubMed] [Google Scholar]
- 26.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306(1):121–33. Epub 2007/04/17. S0012-1606(07)00194-7 [pii] 10.1016/j.ydbio.2007.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, et al. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466(7304):378–82. Epub 2010/06/22. nature09129 [pii] 10.1038/nature09129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133(9):1767–78. Epub 2006/03/31. dev.02347 [pii] 10.1242/dev.02347 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26(8):2147–56. Epub 2006/02/24. 26/8/2147 [pii] 10.1523/JNEUROSCI.4698-05.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, Faux CH, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134(4):789–99. Epub 2007/01/19. dev.000380 [pii] 10.1242/dev.000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohata S, Nakatani J, Herranz-Perez V, Cheng J, Belinson H, Inubushi T, et al. Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014;83(3):558–71. Epub 2014/07/22. 10.1016/j.neuron.2014.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi D, Komatsu K, Hirao M, Toyooka Y, Koyama H, Tissir F, et al. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development. 2014;141(23):4558–68. Epub 2014/11/20. 10.1242/dev.115659 . [DOI] [PubMed] [Google Scholar]
- 33.Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13(6):700–7. Epub 2010/05/18. 10.1038/nn.2555 . [DOI] [PubMed] [Google Scholar]
- 34.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13(13):1129–33. Epub 2003/07/05. 10.1016/s0960-9822(03)00374-9 . [DOI] [PubMed] [Google Scholar]
- 35.Murdoch JN, Damrau C, Paudyal A, Bogani D, Wells S, Greene ND, et al. Genetic interactions between planar cell polarity genes cause diverse neural tube defects in mice. Dis Model Mech. 2014;7(10):1153–63. Epub 2014/08/17. 10.1242/dmm.016758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10(22):2593–601. Epub 2001/11/16. 10.1093/hmg/10.22.2593 . [DOI] [PubMed] [Google Scholar]
- 37.Deans MR, Antic D, Suyama K, Scott MP, Axelrod JD, Goodrich LV. Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. J Neurosci. 2007;27(12):3139–47. Epub 2007/03/23. 27/12/3139 [pii] 10.1523/JNEUROSCI.5151-06.2007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci. 2006;26(19):5265–75. Epub 2006/05/12. 26/19/5265 [pii] 10.1523/JNEUROSCI.4680-05.2006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37(9):980–5. Epub 2005/08/24. ng1622 [pii] 10.1038/ng1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29(4):356–70. Epub 2007/03/22. 10.1002/bies.20558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9(9):690–701. Epub 2008/08/23. nrm2476 [pii] 10.1038/nrm2476 . [DOI] [PubMed] [Google Scholar]
- 42.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–54. Epub 2002/01/10. S0092-8674(01)00614-6 [pii]. 10.1016/s0092-8674(01)00614-6 . [DOI] [PubMed] [Google Scholar]
- 43.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. Epub 2007/03/22. 10.1146/annurev.biochem.75.103004.142647 . [DOI] [PubMed] [Google Scholar]
- 44.Wallar BJ, Alberts AS. The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13(8):435–46. Epub 2003/07/31. S0962892403001533 [pii]. 10.1016/s0962-8924(03)00153-3 . [DOI] [PubMed] [Google Scholar]
- 45.Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta. 2010;1803(2):183–90. Epub 2008/11/04. S0167-4889(08)00342-X [pii] 10.1016/j.bbamcr.2008.09.017 . [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, et al. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105(1):210–5. Epub 2007/12/29. 0707277105 [pii] 10.1073/pnas.0707277105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakaya MA, Habas R, Biris K, Dunty WC Jr., Kato Y, He X, et al. Identification and comparative expression analyses of Daam genes in mouse and Xenopus. Gene Expr Patterns. 2004;5(1):97–105. Epub 2004/11/10. S1567133X04000857 [pii] 10.1016/j.modgep.2004.06.001 . [DOI] [PubMed] [Google Scholar]
- 48.Ju R, Cirone P, Lin S, Griesbach H, Slusarski DC, Crews CM. Activation of the planar cell polarity formin DAAM1 leads to inhibition of endothelial cell proliferation, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2010;107(15):6906–11. Epub 2010/03/31. 1001075107 [pii] 10.1073/pnas.1001075107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kida YS, Sato T, Miyasaka KY, Suto A, Ogura T. Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc Natl Acad Sci U S A. 2007;104(16):6708–13. Epub 2007/04/07. 0608946104 [pii] 10.1073/pnas.0608946104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, et al. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133(21):4219–31. Epub 2006/10/06. dev.02590 [pii] 10.1242/dev.02590 . [DOI] [PubMed] [Google Scholar]
- 51.Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133(5):957–66. Epub 2006/02/14. 133/5/957 [pii] 10.1242/dev.02266 . [DOI] [PubMed] [Google Scholar]
- 52.Matusek T, Gombos R, Szecsenyi A, Sanchez-Soriano N, Czibula A, Pataki C, et al. Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008;28(49):13310–9. Epub 2008/12/05. 28/49/13310 [pii] 10.1523/JNEUROSCI.2727-08.2008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ajima R, Bisson JA, Helt JC, Nakaya MA, Habas R, Tessarollo L, et al. DAAM1 and DAAM2 are co-required for myocardial maturation and sarcomere assembly. Dev Biol. 2015;408(1):126–39. 10.1016/j.ydbio.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126(6):1211–23. Epub 1999/02/18. . [DOI] [PubMed] [Google Scholar]
- 55.Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62:159–68. Epub 1997/01/01. . [PubMed] [Google Scholar]
- 56.Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, et al. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25(1):29–41. Epub 2000/03/09. S0896-6273(00)80869-7 [pii]. 10.1016/s0896-6273(00)80869-7 . [DOI] [PubMed] [Google Scholar]
- 57.Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. Epub 1963/05/01. 10.1083/jcb.17.2.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biris KK, Dunty WC Jr., Yamaguchi TP. Mouse Ripply2 is downstream of Wnt3a and is dynamically expressed during somitogenesis. Dev Dyn. 2007;236(11):3167–72. Epub 2007/10/17. 10.1002/dvdy.21342 . [DOI] [PubMed] [Google Scholar]
- 59.Pop R, Shearstone JR, Shen Q, Liu Y, Hallstrom K, Koulnis M, et al. A key commitment step in erythropoiesis is synchronized with the cell cycle clock through mutual inhibition between PU.1 and S-phase progression. PLoS Biol. 2010;8(9). 10.1371/journal.pbio.1000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cross JC. How to make a placenta: mechanisms of trophoblast cell differentiation in mice—a review. Placenta. 2005;26 Suppl A:S3–9. Epub 2005/04/20. S0143-4004(05)00047-0 [pii] 10.1016/j.placenta.2005.01.015 . [DOI] [PubMed] [Google Scholar]
- 61.Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119 Suppl 1:S97–S101. 10.1016/s0925-4773(03)00099-6 . [DOI] [PubMed] [Google Scholar]
- 62.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28(3):251–5. Epub 2001/06/30. 10.1038/90081 [pii]. . [DOI] [PubMed] [Google Scholar]
- 63.Strong LC, Hollander WF. Hereditary loop-tail in the house mouse accompanied by imperforate vagina and craniorachischisis when homozygous. J Hered. 1949;40:329–34. [Google Scholar]
- 64.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2(7):538–48. Epub 2001/07/04. 10.1038/35080570 [pii]. . [DOI] [PubMed] [Google Scholar]
- 65.Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284(1):12–24. Epub 2005/06/21. S0012-1606(05)00302-7 [pii] 10.1016/j.ydbio.2005.05.010 . [DOI] [PubMed] [Google Scholar]
- 66.Abdelhamed ZA, Natarajan S, Wheway G, Inglehearn CF, Toomes C, Johnson CA, et al. The Meckel-Gruber syndrome protein TMEM67 controls basal body positioning and epithelial branching morphogenesis in mice via the non-canonical Wnt pathway. Dis Model Mech. 2015;8(6):527–41. Epub 2015/06/04. 10.1242/dmm.019083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brzoska HL, d'Esposito AM, Kolatsi-Joannou M, Patel V, Igarashi P, Lei Y, et al. Planar cell polarity genes Celsr1 and Vangl2 are necessary for kidney growth, differentiation, and rostrocaudal patterning. Kidney Int. 2016;90(6):1274–84. Epub 2016/09/07. 10.1016/j.kint.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, et al. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet. 2010;19(23):4663–76. Epub 2010/09/17. 10.1093/hmg/ddq397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yates LL, Schnatwinkel C, Murdoch JN, Bogani D, Formstone CJ, Townsend S, et al. The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet. 2010;19(11):2251–67. Epub 2010/03/13. 10.1093/hmg/ddq104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nishimura T, Ito S, Saito H, Hiver S, Shigetomi K, Ikenouchi J, et al. DAAM1 stabilizes epithelial junctions by restraining WAVE complex-dependent lateral membrane motility. J Cell Biol. 2016;215(4):559–73. Epub 2016/11/04. 10.1083/jcb.201603107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162(6):1111–22. Epub 2003/09/17. 10.1083/jcb.200212157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stemmler MP, Bedzhov I. A Cdh1HA knock-in allele rescues the Cdh1-/- phenotype but shows essential Cdh1 function during placentation. Dev Dyn. 2010;239(9):2330–44. Epub 2010/07/24. 10.1002/dvdy.22375 . [DOI] [PubMed] [Google Scholar]
- 73.Higashi T, Ikeda T, Murakami T, Shirakawa R, Kawato M, Okawa K, et al. Flightless-I (Fli-I) regulates the actin assembly activity of diaphanous-related formins (DRFs) Daam1 and mDia1 in cooperation with active Rho GTPase. J Biol Chem. 2010;285(21):16231–8. Epub 2010/03/13. M109.079236 [pii] 10.1074/jbc.M109.079236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higashi T, Ikeda T, Shirakawa R, Kondo H, Kawato M, Horiguchi M, et al. Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets. J Biol Chem. 2008;283(13):8746–55. Epub 2008/01/26. M707839200 [pii] 10.1074/jbc.M707839200 . [DOI] [PubMed] [Google Scholar]
- 75.Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell. 2005;16(1):1–13. Epub 2004/10/29. E04-07-0565 [pii] 10.1091/mbc.E04-07-0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, et al. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204(9):2031–8. Epub 2007/08/08. jem.20062647 [pii] 10.1084/jem.20062647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149(5):1084–97. Epub 2012/05/29. S0092-8674(12)00525-9 [pii] 10.1016/j.cell.2012.04.021 . [DOI] [PubMed] [Google Scholar]
- 78.Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138(2):303–15. Epub 2010/12/24. 138/2/303 [pii] 10.1242/dev.055566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Dev Biol. 2009;326(2):285–94. Epub 2008/12/23. S0012-1606(08)01389-4 [pii] 10.1016/j.ydbio.2008.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41(7):793–9. Epub 2009/06/23. ng.400 [pii] 10.1038/ng.400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kibar Z, Capra V, Gros P. Toward understanding the genetic basis of neural tube defects. Clin Genet. 2007;71(4):295–310. Epub 2007/05/02. CGE793 [pii] 10.1111/j.1399-0004.2007.00793.x . [DOI] [PubMed] [Google Scholar]
- 82.Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125(16):3201–11. Epub 1998/07/22. . [DOI] [PubMed] [Google Scholar]
- 83.Vandenberg AL, Sassoon DA. Non-canonical Wnt signaling regulates cell polarity in female reproductive tract development via van gogh-like 2. Development. 2009;136(9):1559–70. Epub 2009/04/14. 136/9/1559 [pii] 10.1242/dev.034066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yun K, Ajima R, Sharma N, Costantini F, Mackem S, Lewandoski M, et al. Non-canonical Wnt5a/Ror2 signaling regulates kidney morphogenesis by controlling intermediate mesoderm extension. Hum Mol Genet. 2014;23(25):6807–14. Epub 2014/08/02. ddu397 [pii] 10.1093/hmg/ddu397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, et al. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128(1):25–33. Epub 2000/11/28. . [DOI] [PubMed] [Google Scholar]
- 86.Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122(11):3343–53. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 87.Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17(2):290–8. 10.1016/j.devcel.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee HK, Deneen B. Daam2 is required for dorsal patterning via modulation of canonical Wnt signaling in the developing spinal cord. Dev Cell. 2012;22(1):183–96. 10.1016/j.devcel.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aspenstrom P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res. 2006;312(12):2180–94. Epub 2006/04/25. S0014-4827(06)00108-X [pii] 10.1016/j.yexcr.2006.03.013 . [DOI] [PubMed] [Google Scholar]
- 90.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278(34):32124–31. Epub 2003/06/05. 10.1074/jbc.M302847200 M302847200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 91.Cross JC. The genetics of pre-eclampsia: a feto-placental or maternal problem? Clin Genet. 2003;64(2):96–103. Epub 2003/07/16. 127 [pii]. 10.1034/j.1399-0004.2003.00127.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot analysis to confirm Daam1 expression. (A) The top panel shows the results using an antibody against the Daam1 C-terminus antigen. (B) Top panels show the results using an antibody against the Daam1 N-terminus antigen. The right panel is longer exposure of the left panel. The arrow and arrowhead indicate full length and truncated Daam1 protein, respectively. The asterisk indicates non-specific bands. The bands labeled by double asterisks are likely degraded or splicing variants of Daam1 protein. Bottom panels show actin as loading controls.
(TIF)
(A) GST-pulldown assay of Daam1 truncated proteins with GST-RhoA. Top panels showed mouse Daam1 Δ, Daam1 N (dominant-negative form), and Daam1 FL (full length) proteins from left to right, detected by anti-Myc antibody. Bottom panels show GST or GST-RhoA proteins detected by Coomassie staining. Daam1 Δ transfected cells (B-D) and Daam1 N transfected cells (E-G) are shown. (B, E) Daam1 truncated proteins were detected by anti-Myc antibody. (C, F) Phalloidin stained cells. (C’, F’) High-magnification image of the inset is shown on the side. (D. G) Merged images of B, C and E, F are shown. (H) Quantification of effects by overexpression of Daam1 deletion proteins on stress fibers. Examined cell numbers are indicated below the graph. Chi-square test *: p<0.001 (I) Xenopus embryos were injected with mRNA transcribed from indicated plasmids, and were scored at stage 35. Scoring was performed following previously described criteria [46] Examined embryo numbers are indicated below the graph. Wilcoxon Rank-sum test *: p<0.001 (J) Representative embryos injected with each mRNA are shown.
(TIF)
PECAM-1 staining of Daam1+/+(A), Daam1ΔNeo/+(B), and Daam1ΔNeo/ΔNeo embryos at E10.5 stage are shown. No gross abnormalities in vasculature development were observed in these embryos nor in the Daam1Δ/Δembryos.
(TIF)
(A) Expression of Daam1 was examined by qPCR. Relative Daam1 expression in each tissue is shown. Mut-4(Daam1F/Δ;Meox2-cre), and cont-1 (Daam1F/Δ), mut-5 (Daam1F/Δ;Meox2-cre), and cont-2 (Daam1F/+), mut-6 (Daam1F/Δ;Meox2-cre), and cont-3 (Daam1F/+;Meox2-cre) are litter mates, respectively. SEM is shown as error bar. Western blot analysis for Daam1 protein (top) and β-actin (bottom). Pedigree number and tissues are shown above and below the panels, respectively.
(TIF)
(A) X-gal staining of E10.5 Daam2LacZ/+ embryo. (B) X-gal staining of E10.5 placental section. High magnification image (B’) positions are indicated as boxes on E. Arrow indicates embryo-derived mesodermal tissue. Blue and green lines depict the boundary between the maternal decidua (M) and spongiotrophoblast layer (S), and the spongiotrophoblast and labyrinthine layers (L), respectively. Scale bars = 500 μm in D, 200 μm in E, and 50 μm in E.
(TIF)
(TIF)
(TIF)
(TIFF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.