Abstract
Background
The aim of this study was to assess the association of high-sensitivity cardiac troponin (hs-cTnT) and other cardiac, kidney, hyperglycemia, and inflammatory biomarkers with peripheral neuropathy (PN) in a community-based population.
Methods
We conducted a cross-sectional analysis of 3056 black and white participants in the Atherosclerosis Risk in Communities (ARIC) study who underwent standardized monofilament PN testing and had measures of cardiac function (hs-cTnT, N-terminal pro–B-type natriuretic peptide [NT-proBNP], and growth differentiation factor 15 [GDF15]), kidney function (serum creatinine, cystatin C, β-2 microglobulin, urine albumin-to-creatinine ratio), hyperglycemia (fasting glucose, hemoglobin A1c [Hb A1c], fructosamine, glycated albumin, 1,5-anhydroglucitol), and inflammation (C-reactive protein) assessed at visit 6 (2016–2017; age 71–94 years). We used logistic regression to assess the associations of these biomarkers (modeled in diabetes-specific tertiles) with PN in older adults with and without diabetes after adjusting for traditional risk factors.
Results
In total, 33.5% of participants had PN (37.3% with diabetes and 31.9% without diabetes). There was an independent association of hs-cTnT with PN regardless of diabetes status (diabetes T3 vs. T1: odds ratio [OR], 2.15 [95% CI, 1.44–3.22]; no diabetes: OR, 2.31 [95%CI, 1.76–3.03]; P = 0.72 for interaction). Among participants without diabetes, there were also significant associations of NT-proBNP (OR, 1.40 [95% CI, 1.08–1.81]) and urine albumin-to-creatinine ratio (OR, 1.55 [95% CI, 1.22–1.97]) with PN. Associations of hyperglycemia biomarkers including Hb A1c (OR, 1.76 [95% CI, 1.22–2.54]), fructosamine (OR, 1.71 [95% CI, 1.19–2.46]), and glycated albumin (OR, 1.45 [95% CI, 1.03–2.03]) with PN were significant only among participants with diabetes.
Conclusions
Overall, hs-cTnT appears to be a global marker of end organ damage, including PN. Laboratory biomarkers may be able to help us identify those individuals with PN.
Keywords: biomarker, peripheral neuropathy, hs-cTnT, NT-proBNP, ARIC, diabetes
Introduction
Peripheral neuropathy (PN) is estimated to affect between 11% and 19% of the general population (1) and is substantially more common in older than younger adults (2). We recently found that the prevalence of PN is 27% among older US adults aged ≥70 years. The prevalence is higher among older adults with diabetes, ranging from 33% to 42% depending on diabetes duration. Despite its high burden, risk factors for PN are poorly characterized.
Traditional and novel cardiac, kidney, and hyperglycemia biomarkers allow us to readily and rigorously characterize subclinical disease status and improve risk prediction (3). Novel cardiac biomarkers including cardiac troponin T measured with a high-sensitivity assay (hs-cTnT), N-terminal pro–B-type natriuretic peptide (NT-proBNP), and growth differentiation factor 15 (GDF15) reflect cumulative damage to the heart (4–7), are strongly associated with diabetes and its complications (8–10), and are of growing interest for risk prediction and stratification (4, 6, 8). Biomarkers of kidney filtration and kidney damage are strongly linked to diabetes, cardiovascular disease, and related complications (11–14) but have not been investigated in relation to PN. Prior studies have established hyperglycemia in diabetes as a major risk factor for PN (15), but few studies have investigated the association of measures of hyperglycemia with PN outside of diagnosed diabetes, nor have prior studies examined nontraditional hyperglycemia biomarkers including fructosamine, glycated albumin, and 1,5-anhydroglucitol (1,5-AG). Ultimately, data are limited on the association of major traditional and nontraditional cardiac, kidney, and diabetes biomarkers with PN in adults with and without diabetes in the community.
The aim of this study was to assess the association of a panel of cardiac, kidney, and diabetes (hyperglycemia) biomarkers and a marker of inflammation (C-reactive protein [CRP]) with PN in a community-based population of older adults. We hypothesized that these biomarkers, previously shown to be associated with microvascular disease, would be associated with PN.
Materials and Methods
Study Design
The Atherosclerosis Risk in Communities (ARIC) study is a prospective community-based cohort initially comprising 15792 adults from Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and suburbs of Minneapolis, Minnesota. Participants were originally recruited and examined from 1987 to 1989 (visit 1) at ages 45 to 64 and have been followed prospectively thereafter with serial in-person visits, annual or semiannual (after 2012) telephone calls, and continuous surveillance for cardiovascular events and other outcomes. The ARIC study was originally designed to characterize the risk factors associated with atherosclerotic disease in the general population.
In this study, we conducted a cross-sectional analysis of black and white participants in the ARIC study who underwent monofilament PN testing at ARIC visit 6 (2016–2017). A total of 4003 participants attended visit 6. We used a single study population for all analyses. Participants with missing monofilament testing or visit 6 biomarker data (n = 674), those who were nonfasting for their biomarker testing (n = 198), and those missing other covariates of interest (n = 57) were excluded from the study.
All participants in the study provided written informed consent. The institutional review boards for all participating institutions approved the study protocol.
Covariates
We collected data on participant sociodemographics (age, race and center, sex, education), physical information (body mass index), lifestyle factors (smoking status, alcohol consumption), and comorbidities (diabetes, hypertension, hypercholesterolemia, history of cardiovascular disease, and peripheral artery disease). All data were collected at visit 6 except for education (visit 1).
Diabetes was defined as a self-reported physician diagnosis or diabetes medication use at visit 6. Hypertension was defined as a mean systolic blood pressure ≥140 mmHg, a mean diastolic blood pressure ≥90 mmHg (based on the mean of the second and third seated resting oscillometric blood pressure measurements), or self-reported antihypertensive medication use. Hypercholesterolemia was defined as total cholesterol ≥240 mg/dL or taking cholesterol-lowering medication. Cardiovascular disease was defined as a history of heart failure, stroke, or coronary heart disease before visit 6. Details pertaining to cardiovascular surveillance and adjudication of cardiovascular diagnoses in ARIC have been described previously (16, 17). Peripheral artery disease was defined according to the International Classification of Diseases, Ninth Revision, Clinical Modification codes for atherosclerosis of the native arteries of the extremities (440.20, 440.21, 440.22, 440.23, 440.24, 440.29, 440.3, 440.8) or prior leg artery revascularization (38.18, 39.25, 39.29, 39.50) documented in any hospitalization before visit 6 (18).
Biomarkers Of Interest
Markers hs-cTnT, NT-proBNP, and GDF15 were measured in EDTA plasma using electrochemiluminescence immunoassays on a Roche Cobas e411 analyzer (Roche Diagnostics). Serum creatinine concentrations were measured by the Roche enzymatic method (Roche Diagnostics), cystatin C was measured using Gentian Cystatin C reagent (Gentian AS), and β-2 microglobulin concentrations were measured immunoturbidimetrically, all using a Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics). Creatinine-based, cystatin C-based, and creatinine–cystatin C–based estimated glomerular filtration rate were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations (19, 20). Urine albumin and creatinine concentrations were measured from spot urine samples on a Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics). Glucose was measured in serum using the Roche hexokinase method on a Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics). Hemoglobin A1c (Hb A1c) was measured in EDTA whole blood on the Tosoh HPLC Glycohemoglobin Analyzer (Tosoh Medics) using an automated high-performance liquid chromatography method. This method was calibrated utilizing standard values derived by the National Glycohemoglobin Standardization Program. Fructosamine (Roche Diagnostics), glycated albumin (Asahi Kasei Corp), 1,5-AG (Glycomark), and CRP (Roche Diagnostics) levels were all measured in serum on the Roche Cobas 6000 Chemistry Analyzer (Roche Diagnostics).
Blood was shipped to the central laboratory every 2 weeks. The interassay coefficients of variation were <10% for all assays studied.
Peripheral Neuropathy
PN data were collected at ARIC visit 6 via Semmes-Weinstein 10-g monofilament testing of 3 sites on each foot: the plantar–hallux, the plantar–first metatarsal head, and the plantar–fifth metatarsal head. Each site was tested 3 times by certified technicians following a protocol adapted from the National Health and Nutrition Examination Survey (NHANES) (21). If 2 of 3 responses for a site were incorrect or indeterminate, the response was considered insensate at that site. PN was defined as having at least 1 insensate site.
Statistical Analysis
We compared median levels of each biomarker for participants at ARIC visit 6 according to PN and diabetes status using Wilcoxon rank sum tests. We used logistic regression to evaluate the associations of each biomarker, modeled in diabetes-specific tertiles, with PN, stratified by diabetes. We evaluated 2 models: model 1 included demographic variables (age, sex, race and center, education), and model 2 included all variables in model 1 plus cardiovascular risk factors (body mass index, smoking status, alcohol status, hypertension, hyperlipidemia), history of cardiovascular disease, and peripheral artery disease. We also modeled each biomarker using restricted cubic splines with 4 knots placed at the 5th, 35th, 65th, and 95th percentiles to characterize the shape of the continuous associations of PN in the overall population.
We performed all analyses using Stata version 15.1 (StataCorp) with P < 0.05 denoting statistical significance.
Results
Of the 3056 study participants, 1024 (33.5%) had PN: 37.3% of participants with diabetes (332 of 890) and 31.9% (692 of 2166) of participants without diabetes. Participants with PN were older, were predominantly male, and had higher body mass index than participants without PN (Table 1). Prevalent cardiovascular disease was significantly more common in participants with PN. No significant difference was noted in the prevalence of peripheral arterial disease by PN status (P = 0.27).
Table 1.
Characteristics of study population according to diagnosed diabetes and peripheral neuropathy status, Atherosclerosis Risk in Communities (ARIC) visit 6 (2016–2017).
| No diabetes |
Diagnosed diabetes |
|||
|---|---|---|---|---|
| No PN | PN | No PN | PN | |
| n | 1474 | 692 | 558 | 332 |
| Age, y, mean (SD) | 78.7 (4.3) | 80.7 (5.0)a | 78.6 (4.3) | 79.8 (4.6)a |
| Female, % | 67.1 | 40.2a | 64.3 | 42.5a |
| Black, % | 17.4 | 16.2 | 24.9 | 28.0 |
| Education, %a | ||||
| Less than high school | 8.1 | 11.4 | 11.8 | 18.7 |
| High school or vocational school | 41.0 | 36.4 | 45.2 | 42.2 |
| College and above | 50.8 | 52.2 | 43.0 | 39.2 |
| Body mass index, kg/m2, %a | ||||
| <25 | 33.7 | 32.2 | 19.4 | 11.4 |
| 25 to <30 | 40.9 | 37.3 | 38.5 | 39.8 |
| ≥30 | 25.4 | 30.5 | 42.1 | 48.8 |
| Current smoker, % | 6.4 | 7.1 | 5.6 | 7.2 |
| Former smoker, % | 48.4 | 50.6 | 47.8 | 50.3 |
| Drinking status, % | ||||
| Never | 18.8 | 19.5 | 23.1 | 19.3 |
| Former | 24.5 | 26.6 | 32.4 | 38.6 |
| Current | 56.7 | 53.9 | 44.4 | 42.2 |
| Hypertension, % | 78.6 | 83.1a | 91.0 | 94.3 |
| Hypercholesterolemia, % | 55.8 | 53.8 | 70.4 | 72.9 |
| Cardiovascular disease, % | 15.6 | 21.4a | 19.7 | 29.2a |
| Peripheral artery disease, % | 3.3 | 3.0 | 5.7 | 3.0 |
P < 0.05, with vs without peripheral neuropathy.
There were significant differences in cardiac, kidney, and hyperglycemia biomarkers by PN status in participants with and without diabetes (Table 2). There were higher median levels of hs-cTnT, NT-proBNP, GDF15, creatinine, cystatin C, β-2 microglobulin, urine albumin-to-creatinine ratio, fasting glucose, Hb A1c, fructosamine, and glycated albumin in participants with PN compared with those without (all, P < 0.05). There were no significant differences in 1,5-anhydroglucitol and CRP levels in participants with vs without PN regardless of diabetes status (all, P > 0.05).
Table 2.
Median (25th, 75th percentiles) cardiac, kidney, glycemic, and inflammatory biomarkers according to diagnosed diabetes and peripheral neuropathy status, Atherosclerosis Risk in Communities (ARIC) visit 6 (2016–2017).
| Marker | No diabetes |
Diagnosed diabetes |
||
|---|---|---|---|---|
| No PN | PN | No PN | PN | |
| Cardiac | ||||
| hs-Troponin T, ng/L | 10.0 (8.0, 14.0) | 15.0 (10.0, 22.0)a | 12.0 (8.0, 18.0) | 17.0 (11.0, 24.0)a |
| NT-proBNP, pg/mL | 140.6 (72.7, 273.3) | 179.9 (90.2, 397.0)a | 135.0 (65.2, 304.0) | 172.5 (66.8, 421.4)a |
| GDF15, pg/mL | 1470.5 (1150.0, 1947.0) | 1703.5 (1309.0, 2269.0)a | 1966.5 (1436.0, 2866.0) | 2349.5 (1616.5, 3298.5)a |
| Kidney | ||||
| Serum creatinine, mg/dLb | 0.9 (0.8, 1.0) | 1.0 (0.8, 1.1)a | 0.9 (0.8, 1.1) | 1.0 (0.9, 1.3)a |
| Cystatin C, mg/L | 1.1 (1.0, 1.3) | 1.2 (1.0, 1.4)a | 1.2 (1.0, 1.4) | 1.3 (1.1, 1.5)a |
| β-2 microglobulin, mg/L | 2.2 (1.9, 2.7) | 2.4 (2.0, 3.0)a | 2.5 (2.0, 3.1) | 2.7 (2.2, 3.3)a |
| Urine albumin: creatinine ratio, mg/g | 5.9 (3.1, 12.3) | 7.8 (3.7, 22.4)a | 9.2 (4.1, 28.0) | 11.6 (4.3, 35.0)a |
| Glycemic | ||||
| Fasting glucose, mg/dLc | 95.0 (90.0, 102.0) | 97.0 (91.0, 104.0)a | 114.0 (99.0, 139.0) | 123.5 (101.0, 152.0)a |
| Hb A1c, % | 5.6 (5.4, 5.9) | 5.7 (5.5, 5.9)a | 6.3 (5.9, 7.0) | 6.7 (6.0, 7.3)a |
| Fructosamine, µmol/L | 238.0 (225.0, 254.0) | 241.0 (227.0, 256.0)a | 261.0 (239.0, 288.0) | 270.0 (248.5, 304.0)a |
| Glycated albumin, % | 13.0 (12.0, 14.0) | 13.0 (12.0, 14.0)a | 14.0 (13.0, 17.0) | 15.0 (14.0, 17.5)a |
| 1,5-anhydroglucitol, ug/mL | 16.8 (12.4, 20.7) | 17.2 (12.3, 21.4) | 13.6 (7.9, 18.5) | 12.6 (7.5, 17.6) |
| Inflammation | ||||
| hs-CRP, mg/L | 1.6 (0.7, 3.3) | 1.8 (0.8, 3.4) | 1.9 (0.9, 4.1) | 2.5 (1.0, 4.8) |
P < 0.05, with vs without PN.
To convert mg/dL to mmol/L, multiply by 0.088 for creatinine.
To convert mg/dL to mmol/L, multiply by 5.55 for glucose.
Based on logistic regression analysis using diabetes-specific tertiles for each biomarker, there were significant associations of hs-cTnT, GDF15, and β-2 microglobulin with PN in participants with and without diabetes after adjusting for demographic factors (model 1, Table 3). There were also associations of NT-proBNP and urine albumin-to-creatinine ratio with PN in participants without diabetes, although these associations were not significant among participants with diabetes. In contrast, there were significant associations of fasting glucose, Hb A1c, fructosamine, and glycated albumin with PN in participants with diabetes but not in those without. Serum creatinine, cystatin C, estimated glomerular filtration rate, 1,5-AG, and CRP were not associated with PN in participants with or without diabetes after risk adjustment (Table 3, Supplemental Table 1).
Table 3.
Associations (odds ratios and 95% CIs) of biomarker categories (diabetes-specific tertiles) with peripheral neuropathy, Atherosclerosis Risk in Communities (ARIC) visit 6 (2016–2017).
| Cardiac markers | No diabetes |
Diagnosed diabetes |
||||
|---|---|---|---|---|---|---|
| Tertile value | Model 1a | Model 2b | Tertile value | Model 1a | Model 2b | |
| hs-Troponin, ng/L | ||||||
| T1 | ≤ 9 | 1 (reference) | 1 (reference) | ≤ 10.0 | 1 (reference) | 1 (reference) |
| T2 | 10.0–14.0 | 1.37 (1.06–1.77) | 1.33 (1.02–1.73) | 11.0–18.0 | 1.55 (1.08–2.23) | 1.44 (1.00–2.09) |
| T3 | ≥ 15 | 2.36 (1.81–3.09) | 2.31 (1.76–3.03) | ≥ 19.0 | 2.43 (1.65–3.58) | 2.15 (1.44–3.22) |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| NT-proBNP, pg/mL | ||||||
| T1 | ≤ 99.2 | 1 (reference) | 1 (reference) | ≤ 86.3 | 1 (reference) | 1 (reference) |
| T2 | 99.3–241.3 | 0.95 (0.75–1.22) | 0.97 (0.76–1.24) | 86.7–254.1 | 0.84 (0.58–1.20) | 0.84 (0.58–1.21) |
| T3 | ≥ 241.7 | 1.33 (1.04–1.71) | 1.40 (1.08–1.81) | ≥ 255.9 | 1.22 (0.84–1.77) | 1.18 (0.79–1.75) |
| P for trend | 0.006 | 0.002 | 0.10 | 0.20 | ||
| GDF15, pg/mL | ||||||
| T1 | ≤ 1298.0 | 1 (reference) | 1 (reference) | ≤ 1665.0 | 1 (reference) | 1 (reference) |
| T2 | 1299.0–1835.0 | 1.18 (0.92–1.51) | 1.14 (0.89–1.46) | 1666.0–2682.0 | 1.22 (0.86–1.75) | 1.18 (0.82–1.69) |
| T3 | ≥ 1836.0 | 1.33 (1.031.71) | 1.28 (0.98–1.66) | ≥ 2683.0 | 1.47 (1.03–2.11) | 1.45 (1.00–2.09) |
| P for trend | 0.034 | 0.07 | 0.04 | 0.05 | ||
| Kidney markers | ||||||
| Serum creatinine, mg/dLc | ||||||
| T1 | ≤ 0.82 | 1 (reference) | 1 (reference) | ≤ 0.85 | 1 (reference) | 1 (reference) |
| T2 | 0.83–1.02 | 0.85 (0.66–1.10) | 0.82 (0.64–1.06) | 0.86–1.10 | 1.47 (1.02–2.13) | 1.44 (0.98–2.10) |
| T3 | ≥ 1.03 | 0.95 (0.73–1.24) | 0.91 (0.69–1.19) | ≥ 1.11 | 1.52 (1.04–2.24) | 1.39 (0.93–2.07) |
| P for trend | 0.89 | 0.66 | 0.06 | 0.18 | ||
| Cystatin C, mg/L | ||||||
| T1 | ≤ 1.02 | 1 (reference) | 1 (reference) | ≤ 1.09 | 1 (reference) | 1 (reference) |
| T2 | 1.03–1.23 | 1.19 (0.93–1.51) | 1.10 (0.86–1.41) | 1.10–1.36 | 1.08 (0.76–1.54) | 0.96 (0.67–1.38) |
| T3 | ≥ 1.24 | 1.26 (0.99–1.61) | 1.14 (0.88–1.47) | ≥ 1.37 | 1.31 (0.92–1.86) | 1.10 (0.76–1.60) |
| P for trend | 0.08 | 0.35 | 0.12 | 0.54 | ||
| β-2 microglobulin, mg/L | ||||||
| T1 | ≤2.05 | 1 (reference) | 1 (reference) | ≤ 2.21 | 1 (reference) | 1 (reference) |
| T2 | 2.06–2.56 | 1.18 (0.93–1.50) | 1.11 (0.87–1.42) | 2.22–2.90 | 1.32 (0.92–1.87) | 1.21 (0.85–1.74) |
| T3 | ≥2.57 | 1.35 (1.05–1.73) | 1.24 (0.96–1.60) | ≥ 2.92 | 1.43 (1.00–2.06) | 1.22 (0.84–1.78) |
| P for trend | 0.02 | 0.10 | 0.07 | 0.37 | ||
| Urine albumin:creatinine ratio, mg/g | ||||||
| T1 | ≤3.97 | 1 (reference) | 1 (reference) | ≤ 5.39 | 1 (reference) | 1 (reference) |
| T2 | 3.98–10.50 | 1.15 (0.91–1.47) | 1.17 (0.92–1.50) | 5.40–20.00 | 1.03 (0.72–1.46) | 0.99 (0.69–1.43) |
| T3 | ≥10.60 | 1.51 (1.19–1.92) | 1.55 (1.22–1.97) | ≥ 20.73 | 1.15 (0.81–1.64) | 1.04 (0.72–1.50) |
| P for trend | 0.001 | < 0.001 | 0.39 | 0.79 | ||
| Glycemic markers | ||||||
| Fasting glucose, mg/dLd | ||||||
| T1 | ≤92 | 1 (reference) | 1 (reference) | ≤ 104 | 1 (reference) | 1 (reference) |
| T2 | 93–100 | 0.90 (0.71–1.14) | 0.85 (0.67–1.09) | 105–132 | 0.99 (0.70–1.42) | 0.95 (0.66–1.37) |
| T3 | ≥101 | 1.21 (0.95–1.54) | 1.12 (0.88–1.44) | ≥ 133 | 1.47 (1.03–2.09) | 1.39 (0.97–2.00) |
| P for trend | 0.08 | 0.24 | 0.020 | 0.044 | ||
| Hb A1c, % | ||||||
| T1 | ≤5.5 | 1 (reference) | 1 (reference) | ≤ 6.0 | 1 (reference) | 1 (reference) |
| T2 | 5.6–5.8 | 1.06 (0.84–1.34) | 1.06 (0.84–1.33) | 6.1–6.9 | 1.41 (0.99–2.01) | 1.37 (0.95–1.97) |
| T3 | ≥ 5.9 | 1.24 (0.97–1.57) | 1.21 (0.95–1.55) | ≥ 7.0 | 1.84 (1.29–2.63) | 1.76 (1.22–2.54) |
| P for trend | 0.09 | 0.13 | 0.001 | 0.003 | ||
| Fructosamine, umol/L | ||||||
| T1 | ≤ 230.0 | 1 (reference) | 1 (reference) | ≤ 249.0 | 1 (reference) | 1 (reference) |
| T2 | 231.0–249.0 | 1.19 (0.94–1.51) | 1.25 (0.99–1.59) | 250.0–283.0 | 1.35 (0.95–1.93) | 1.44 (1.00–2.08) |
| T3 | ≥ 250.0 | 1.05 (0.82–1.34) | 1.15 (0.89–1.47) | ≥ 284.0 | 1.62 (1.14–2.31) | 1.71 (1.19–2.46) |
| P for trend | 0.75 | 0.32 | 0.009 | 0.006 | ||
| Glycated albumin, % | ||||||
| T1 | ≤ 12.0 | 1 (reference) | 1 (reference) | ≤ 14.0 | 1 (reference) | 1 (reference) |
| T2 | 13.0–13.0 | 0.87 (0.69–1.11) | 0.92 (0.72–1.17) | 15.0–16.0 | 1.29 (0.89–1.86) | 1.31 (0.90–1.91) |
| T3 | ≥ 14.0 | 1.03 (0.82–1.31) | 1.10 (0.87–1.40) | ≥ 17.0 | 1.46 (1.05–2.03) | 1.45 (1.03–2.03) |
| P for trend | 0.82 | 0.46 | 0.03 | 0.04 | ||
| 1,5-AG, ug/mL | ||||||
| T1 | ≤ 13.8 | 1 (reference) | 1 (reference) | ≤ 9.5 | 1 (reference) | 1 (reference) |
| T2 | 13.9–19.5 | 0.90 (0.71–1.14) | 0.90 (0.71–1.14) | 9.6–16.5 | 1.02 (0.73–1.44) | 1.02 (0.72–1.45) |
| T3 | ≥ 19.6 | 1.02 (0.80–1.29) | 0.98 (0.77–1.25) | ≥ 16.6 | 0.77 (0.54–1.10) | 0.75 (0.52–1.07) |
| P for trend | 0.90 | 0.87 | 0.17 | 0.13 | ||
| Inflammatory Marker | ||||||
| hs-CRP, mg/L | ||||||
| T1 | ≤0.988 | 1 (reference) | 1 (reference) | ≤ 1.194 | 1 (reference) | 1 (reference) |
| T2 | 0.991–2.611 | 1.13 (0.90–1.43) | 1.09 (0.86–1.38) | 1.196–3.323 | 1.17 (0.82–1.66) | 1.05 (0.73–1.51) |
| T3 | ≥ 2.613 | 1.20 (0.95–1.53) | 1.07 (0.84–1.38) | ≥ 3.338 | 1.37 (0.96–1.94) | 1.18 (0.82–1.72) |
| P for trend | 0.17 | 0.69 | 0.09 | 0.35 | ||
Model 1: adjusted for age, sex, race and center, and education.
Model 2: adjusted for variables in model 1 plus body mass index, smoking status, alcohol status, hypertension, hyperlipidemia, cardiovascular disease, and peripheral artery disease.
To convert mg/dL to mmol/L, multiply by 0.088 for creatinine.
To convert mg/dL to mmol/L, multiply by 5.55 for glucose.
After further adjusting for cardiovascular risk factors, only the association of hs-cTnT with PN remained significant in participants with and without diabetes (model 2, Table 3). The association of hs-cTnT with PN was similar regardless of diabetes status (P = 0.72 for interaction). There was also a weak association of GDF15 with PN in adults with and without diabetes, although this was no longer significant in model 2. There were persistent associations of NT-proBNP and urine albumin-to-creatinine ratio with PN in participants without diabetes and of Hb A1c, fructosamine, and glycated albumin with PN in participants with diabetes (all, P < 0.05).
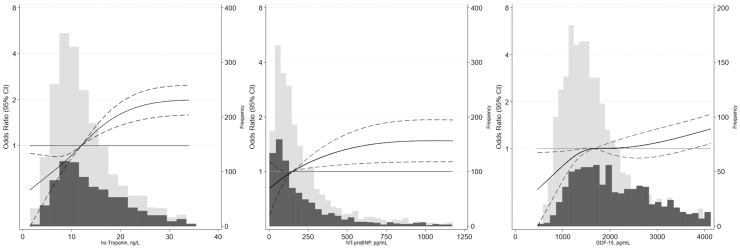
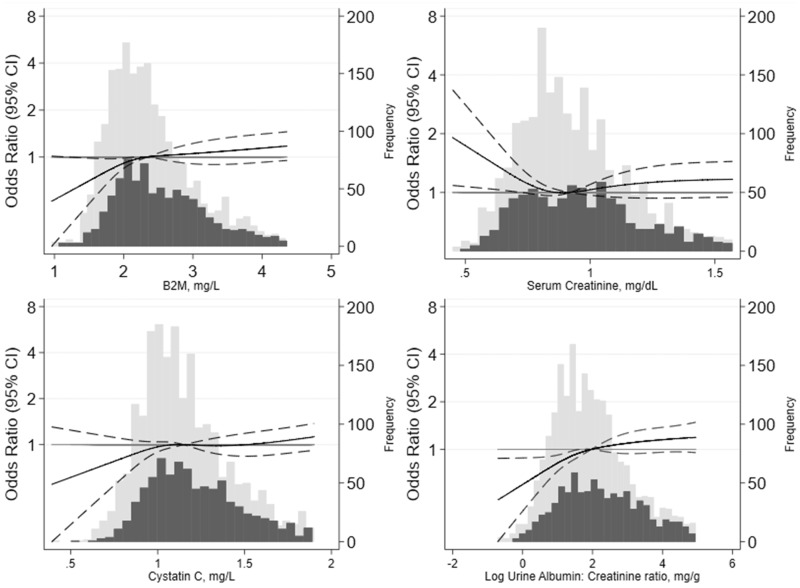
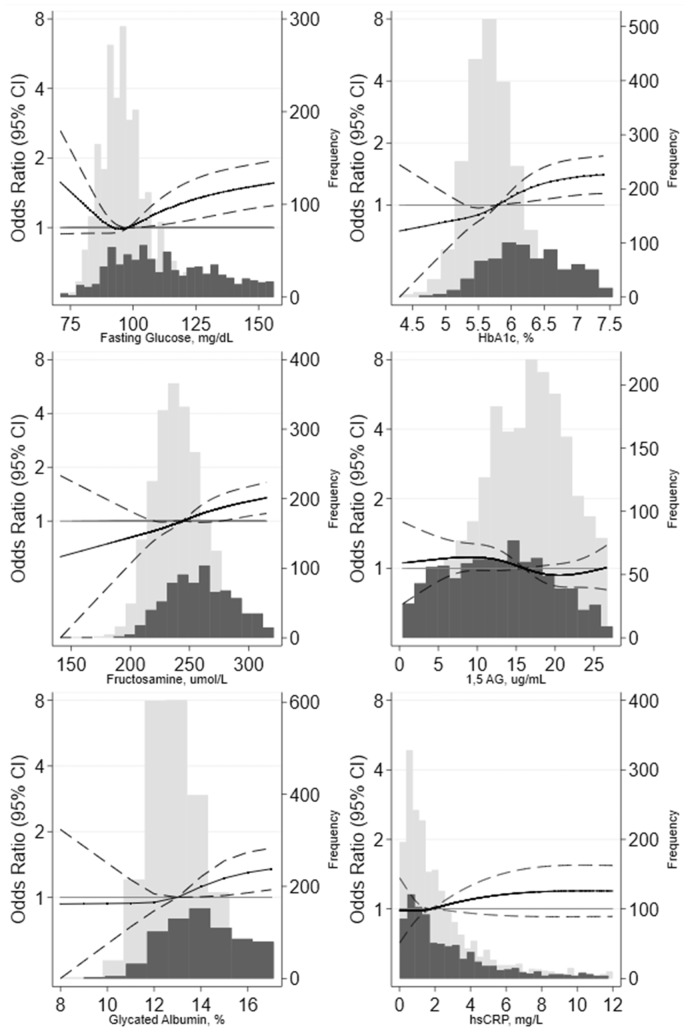
Our results were generally similar when the biomarkers were modeled more flexibly using splines. We observed robust associations of hs-cTnT and NT-proBNP and a weaker association of GDF15 with PN in the overall population (Fig. 1). Among the kidney biomarkers, urine albumin-to-creatinine ratio appeared to be moderately associated with PN, whereas β-2 microglobulin had a weaker association (Fig. 2). Associations of fasting glucose, Hb A1c, glycated albumin, and fructosamine with PN were similar (Fig. 3) but driven primarily by the higher levels in participants with diabetes (Table 3); we observed relatively flat associations of glycemic biomarkers with PN in the nondiabetic range. There was no association of 1,5-AG or CRP with PN regardless of diabetes status (Fig. 3; Table 3).
Fig. 1.
Adjusted odds ratios and 95% CIs for the continuous associations of cardiac markers with prevalent PN. Odds ratios are from logistic regression adjusted for age, sex, race and center, education, body mass index, smoking status, alcohol status, hypertension, hyperlipidemia, prevalent cardiovascular disease, and prevalent peripheral artery disease. Biomarkers were modeled using a restricted cubic spline with knots at the 5th, 35th, 65th and 95th percentiles. The models were centered at the 50th percentile, and the display of graphs was truncated at the 95th percentile. The light gray bars denote the distribution of participants without diabetes, and dark gray bars denote the distribution of participants with diabetes.
Fig. 2.
Adjusted odds ratios and 95% CIs for the continuous associations of kidney markers with prevalent PN. Odds ratios are from logistic regression adjusted for age, sex, race and center, education, body mass index, smoking status, alcohol status, hypertension, hyperlipidemia, prevalent cardiovascular disease, and prevalent peripheral artery disease. Biomarkers were modeled using a restricted cubic spline with knots at the 5th, 35th, 65th and 95th percentiles. The models were centered at the 50th percentile, and the display of graphs was truncated at the 95th percentile. The light gray bars denote the distribution of participants without diabetes, and the dark gray bars denote the distribution of participants with diabetes.
Fig. 3.
Adjusted odds ratios and 95% CIs for the continuous associations of glycemic markers and inflammation marker with prevalent peripheral neuropathy. Odds ratios are from logistic regression adjusted for age, sex, race and center, education, body mass index, smoking status, alcohol status, hypertension, hyperlipidemia, prevalent cardiovascular disease and prevalent peripheral artery disease. Biomarkers were modeled using a restricted cubic spline with knots at the 5th, 35th, 65th, and 95th percentiles. The models were centered at the 50th percentile, and the displays of graph were truncated at the 95th percentile. The light gray bars denote the distribution of participants without diabetes, and the dark gray bars denote the distribution of participants with diabetes.
We assessed the correlations of the biomarkers studied (Supplemental Table 2). There were strong linear associations between the glycemic markers and between kidney markers. The correlations of the cardiac markers with each other were weaker.
Discussion
There was a high prevalence of PN in both adults with and without diabetes in this community-based cohort of older adults. In these participants, we found crude and adjusted associations of cardiac and kidney biomarkers with PN. The association of hs-cTnT with PN was particularly robust, persisting in older adults with and without diabetes even after adjustment for cardiovascular risk factors. Among older adults with diabetes, Hb A1c and nontraditional measures of glycemic control were also associated with PN, but none of the glycemic markers were found to be significantly associated with PN in nondiabetic adults. Overall, our data provide insight into the possible pathogenic mechanisms of PN.
The association of hs-cTnT with PN supports the hypothesis that hs-cTnT is a global marker of end organ damage. Cardiac troponin T is a marker of primary ischemic myocardial injury (22) and has been recognized by the European Society of Cardiology and American College of Cardiology as one of the preferred markers in the diagnosis of acute coronary syndrome (23). However, increases in cardiac troponin T measured using high-sensitivity assays are strongly predictive of future coronary heart disease, heart failure, and death in the general population without known coronary heart disease or stroke (4). In addition, hs-cTnT has been associated with noncardiac outcomes including ischemic stroke (24), silent brain infarcts (25), end-stage renal disease (26), elevated liver enzymes (27), abdominal aortic aneurysms (28), and peripheral artery disease (29). The mechanism of these associations is not entirely clear but may involve subclinical microvascular ischemia, inflammation, and/or arterial wall stress (30). Myocardial microischemia resulting in increased hs-cTnT could be caused by insufficiency of small intramyocardial arterioles in the setting of small vessel disease (31). Similarly, PN may be caused by a combination of oxidative stress, inflammation, and microvascular disease (32) that ultimately leads to cell death and segmental axonal degeneration (33). The concept that hs-cTnT may be a marker of end organ damage in addition to myocardial ischemia may explain why increased concentrations of hs-cTnT were associated with PN in our study.
The associations of other cardiac biomarkers (GDF15 and NT-proBNP) with PN were weaker than for hs-cTnT, particularly among participants without diabetes. GDF15 is strongly associated with aging and is expressed in response to inflammation, oxidative stress, and hypoxia and has been associated previously with cardiovascular disease, chronic kidney disease, and cancer (5). Although the association of GDF15 with PN has not been documented previously, the factors that drive its expression are similar to those thought to contribute to PN (32). NT-proBNP is a marker of myocardial stretch that is strongly associated with incident heart failure (6) and has been shown to be predictive of both cardiovascular and noncardiovascular mortality (7). NT-proBNP levels have been previously associated with microvascular complications, including neuropathy, in adults with diabetes (10, 34). Interestingly, NT-proBNP was associated with PN in participants without diabetes, even after adjustment for cardiovascular risk factors, suggesting that the previously reported utility of this biomarker as a marker for microvascular complications extends to individuals without diabetes.
We found associations of kidney biomarkers β-2 microglobulin and urine albumin-to-creatinine ratio with PN after adjusting for demographic risk factors. However, only the association of urine albumin-to-creatinine ratio with PN remained significant in adults without diabetes after further adjusting for cardiovascular risk factors. Moreover, β-2 microglobulin is a marker of kidney filtration and has been associated with end-stage renal disease, cardiovascular disease, and all-cause mortality in the general population (35, 36). The attenuation of its association with PN after risk adjustment suggests that β-2 microglobulin expression may be mediated by cardiovascular factors. Urine albumin-to-creatinine ratio is a marker of kidney damage and has been shown to be an independent risk factor for subclinical atherosclerosis (37) and all-cause and cardiovascular mortality (38). Elevated urine albumin-to-creatinine ratio levels may reflect systemic transvascular “leakiness” that occurs in association with subclinical atherosclerotic disease (38), suggesting a possible shared microvascular etiology for chronic kidney disease and PN among nondiabetic adults.
Hyperglycemia is an established risk factor for PN (15). Consistent with the literature, we observed that Hb A1c was associated with PN in the setting of diabetes. Similar associations were observed for the nontraditional serum measures of hyperglycemia fructosamine and glycated albumin. This is consistent with our prior work in ARIC, demonstrating high correlations of fructosamine and glycated albumin with Hb A1c levels (27, 39) and similar associations with long-term complications of diabetes (40). The 1,5-AG marker of glycosuria was not an important risk factor for PN in our study.
The limitations of our study include the cross-sectional design and a lack of clinical neuropathy-related outcomes. We cannot determine the temporality of the observed associations or eliminate the possibilities of reverse causality or residual confounding. Consequently, the utility of this panel of biomarkers to predict the development of incident PN is unknown. We also cannot exclude possible false-positive findings, given the number of biomarkers examined.
In conclusion, we found that a panel of cardiac and kidney biomarkers was associated with PN in older adults. In particular, hs-cTnT was associated with PN regardless of diabetes status and independent of traditional risk factors. Associations with measures of hyperglycemia were specific to PN in adults with diabetes. Our findings support the hypothesis that cardiac and kidney biomarkers may be useful global measures of end organ damage and that laboratory biomarkers—specifically hs-cTnT—may help us identify individuals who have PN. Further research is necessary to understand whether these biomarkers can also help identify individuals at risk of developing PN.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Nonstandard Abbreviations
PN, peripheral neuropathy; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro–B-type natriuretic peptide; GDF15, growth differentiation factor 15; 1,5-AD, 1,5-anhydroglucitol; CRP, C-reactive protein; ARIC, Atherosclerosis Risk in Communities.
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
D. Wang and N. Daya, statistical analysis; E. Selvin, financial support, statistical analysis, administrative support, provision of study material or patients.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
C.M. Ballantyne, Abbott, Denka Seiken, Roche.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under Contract No. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Funding for biospecimen collection and laboratory testing at ARIC Visit 6 was supported by grant R01DK089174 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH. Reagents for the hs-cTnT, NT-proBNP, GDF15, β-2 microglobulin, C-reactive protein, and fructosamine assays were donated by the Roche Diagnostics Corporation. Reagents for the 1,5-anhydroglucitol assays were donated by GlycoMark, Inc. Reagents for the glycated albumin assays were donated by the Asahi Kasei Pharma Corporation. B. G. Windham received support from the NHLBI; E. Selvin received support from NIH/NIDDK grants K24DK106414 and R01DK089174, NIH/NHLBI R01HL134320; K. Matsushita received support from NHLBI grant R21HL133694; C. M. Ballantyne received support from NIH/NHLBI R01HL134320, Abbott, Roche.
Expert Testimony
None declared.
Patents
C.M. Ballantyne, 61721475.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
References
- 1. Gregg EW, Gu Q, Williams D, de Rekeneire N, Cheng YJ, Geiss L, Engelgau M.. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabetes Res Clin Pract 2007;77:485–8. [DOI] [PubMed] [Google Scholar]
- 2. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH.. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–4. [DOI] [PubMed] [Google Scholar]
- 3. Koenig W. Update on integrated biomarkers for assessment of long-term risk of cardiovascular complications in initially healthy subjects and patients with manifest atherosclerosis. Ann Med 2009;41:332–43. [DOI] [PubMed] [Google Scholar]
- 4. Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wollert KC, Kempf T, Wallentin L.. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem 2017;63:140–51. [DOI] [PubMed] [Google Scholar]
- 6. Nambi V, Liu X, Chambless LE, de Lemos JA, Virani SS, Agarwal S, et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk–the atherosclerosis risk in communities study. Clin Chem 2013;59:1802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oluleye OW, Folsom AR, Nambi V, Lutsey PL, Ballantyne CM, Investigators AS.. Troponin T, B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Ann Epidemiol 2013;23:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whelton SP, McEvoy JW, Lazo M, Coresh J, Ballantyne CM, Selvin E.. High-sensitivity cardiac troponin T (hs-cTnT) as a predictor of incident diabetes in the atherosclerosis risk in communities study. Diabetes Care 2017;40:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parrinello CM, Matsushita K, Woodward M, Wagenknecht LE, Coresh J, Selvin E.. Risk prediction of major complications in individuals with diabetes: the Atherosclerosis Risk in Communities study. Diabetes Obes Metab 2016;18:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamano K, Nakadaira I, Suzuki J, Gonai M.. N-terminal fragment of probrain natriuretic peptide is associated with diabetes microvascular complications in type 2 diabetes. Vasc Health Risk Manag 2014;10:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rebholz CM, Inker LA, Chen Y, Liang M, Foster MC, Eckfeldt JH, et al. Risk of ESRD and mortality associated with change in filtration markers. Am J Kidney Dis 2017;70:551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsushita K, Sang Y, Ballew SH, Astor BC, Hoogeveen RC, Solomon SD, et al. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol 2014;34:1770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, Coresh J.. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis 2012;59:653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsushita K, Ballew SH, Coresh J, Arima H, Ärnlöv J, Cirillo M, et al. Measures of chronic kidney disease and risk of incident peripheral artery disease: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2017;5:718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Callaghan BC, Little AA, Feldman EL, Hughes RA.. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012;6:CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol 1996;49:223–33. [DOI] [PubMed] [Google Scholar]
- 18. Vart P, Coresh J, Kwak L, Ballew SH, Heiss G, Matsushita K.. Socioeconomic status and incidence of hospitalization with lower-extremity peripheral artery disease: Atherosclerosis Risk in Communities study. J Am Heart Assoc 2017;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 2008;51:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 1999-2000 data documentation, codebook, and frequencies: lower extremity disease—peripheral neuropathy (LEXPN). https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/LEXPN.htm. 2002. (Accessed August 2019).
- 22. Park KC, Gaze DC, Collinson PO, Marber MS.. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res 2017;113:1708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation 2007;116:e148–304. [DOI] [PubMed] [Google Scholar]
- 24. Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the Atherosclerosis Risk in Communities study. Stroke 2013;44:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dadu RT, Fornage M, Virani SS, Nambi V, Hoogeveen RC, Boerwinkle E, et al. Cardiovascular biomarkers and subclinical brain disease in the Atherosclerosis Risk in Communities study. Stroke 2013;44:1803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim Y, Matsushita K, Sang Y, Grams ME, Skali H, Shah AM, et al. Association of high-sensitivity cardiac troponin T and natriuretic peptide with incident ESRD: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 2015;65:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lazo M, Rubin J, Clark JM, Coresh J, Schneider A, Ndumele C, et al. The association of liver enzymes with biomarkers of subclinical myocardial damage and structural heart disease. J Hepatol 2015;62:841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Folsom AR, Yao L, Alonso A, Lutsey PL, Missov E, Lederle FA, et al. Circulating biomarkers and abdominal aortic aneurysm incidence: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2015;132:578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsushita K, Kwak L, Yang C, Pang Y, Ballew SH, Sang Y, et al. High-sensitivity cardiac troponin and natriuretic peptide with risk of lower-extremity peripheral artery disease: the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J 2018;39:2412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beatty AL, Ku IA, Christenson RH, DeFilippi CR, Schiller NB, Whooley MA.. High-sensitivity cardiac troponin T levels and secondary events in outpatients with coronary heart disease from the Heart and Soul Study. JAMA Intern Med 2013;173:763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Selvin E, Lazo M, Chen Y, Shen L, Rubin J, McEvoy JW, et al. Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage. Circulation 2014;130:1374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feldman EL, Nave KA, Jensen TS, Bennett D.. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017;93:1296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, Boulton AJ.. Sural nerve fibre pathology in diabetic patients with mild neuropathy: relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology. Acta Neuropathol 2001;101:367–74. [DOI] [PubMed] [Google Scholar]
- 34. Grauslund J, Nybo M, Green A, Sjolie AK.. N-terminal pro brain natriuretic peptide reflects long-term complications in type 1 diabetes. Scand J Clin Lab Invest 2010;70:392–8. [DOI] [PubMed] [Google Scholar]
- 35. Foster MC, Inker LA, Levey AS, Selvin E, Eckfeldt J, Juraschek SP, et al. Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis 2013;62:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J.. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol 2009;20:2214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kong X, Jia X, Wei Y, Cui M, Wang Z, Tang L, et al. Association between microalbuminuria and subclinical atherosclerosis evaluated by carotid artery intima-media in elderly patients with normal renal function. BMC Nephrol 2012;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen G, Clausen P, Scharling H, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation 2004;110:32–5. [DOI] [PubMed] [Google Scholar]
- 39. Mo Y, Ma X, Li H, Ran X, Yang W, Li Q, et al. Relationship between glycated albumin and glycated hemoglobin according to glucose tolerance status: a multicenter study. Diabetes Res Clin Pract 2016;115:17–23. [DOI] [PubMed] [Google Scholar]
- 40. Selvin E, Rawlings AM, Grams M, Klein R, Sharrett AR, Steffes M, Coresh J.. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2014;2:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.