Abstract
STUDY QUESTION
Does interleukin-1β (IL-1β) play a role in promoting nerve growth factor expression, neurogenesis and deep dyspareunia in endometriosis?
SUMMARY ANSWER
IL-1β directly stimulates nerve growth factor (NGF) expression in endometriosis and is associated with local neurogenesis around endometriosis and more severe deep dyspareunia.
WHAT IS KNOWN ALREADY
Local nerve density around endometriosis (using the pan-neuronal marker PGP9.5) is associated with deep dyspareunia in endometriosis, mediated in part by NGF expression.
STUDY DESIGN, SIZE, DURATION
This in vitro study included endometriotic tissue samples from 45 patients.
PARTICIPANTS/MATERIALS, SETTING, METHODS
This study was conducted in a university hospital affiliated research institute and included 45 women with surgically excised deep uterosacral/rectovaginal endometriosis (DIE, n = 12), ovarian endometriomas (OMA, n = 14) or superficial peritoneal uterosacral/cul-de-sac endometriosis (SUP, n = 19). Immunolocalisation of IL-1β, IL-1 receptor type 1 (IL-1R1), NGF and PGP9.5 in endometriotic tissues was examined by immunohistochemistry (IHC), and the intensity of IHC staining in the endometriotic epithelium and stroma was semi-quantitatively evaluated using the Histoscore method (H-score). For each case, deep dyspareunia was pre-operatively rated by the patient on an 11-point numeric rating scale (0–10). In addition, primary endometriosis stromal cells were isolated and cultured from surgically excised endometriosis. These cells were treated with IL-1β alone or in combination of Anakinra (an inhibitor of IL-1R1), small inference RNA (siRNA) against IL-1R1, siRNA against c-FOS or NGF neutralising antibody. The mRNA and protein levels of target genes (NGF and c-FOS) were assessed by reverse-transcription qPCR and western blot/ELISA, respectively. Furthermore, immunofluorescent microscopy was used to examine the neurite growth of rat pheochromocytoma PC-12 cells, as an in vitro model of neurogenesis.
MAIN RESULTS AND THE ROLE OF CHANCE
For IHC, IL-1β expression in the endometriosis epithelium was significantly associated with more severe deep dyspareunia (r = 0.37, P = 0.02), higher nerve fibre bundle density around endometriosis (r = 0.42, P = 0.01) and greater NGF expression by the endometriosis epithelium (r = 0.42, P = 0.01) and stroma (r = 0.45, P = 0.01). In primary endometriosis stromal cells, treatment with exogenous IL-1β significantly increased the mRNA and protein levels of NGF and c-FOS. Pre-treatment with Anakinra, siRNA against IL-1R1, or siRNA against c-FOS, each attenuated IL-1 β-induced increases of NGF expression. In addition, supernatants from IL-1β treated endometriosis stromal cells significantly stimulated PC-12 neurite growth compared to controls, and these effects could be attenuated by pre-treatment with NGF neutralising antibody or Anakinra.
LARGE-SCALE DATA
N/A
LIMITATIONS, REASONS FOR CAUTION
We did not have data from cultures of endometriosis glandular epithelium, due to the known difficulties with primary cultures of this cell type.
WIDER IMPLICATIONS OF THE FINDINGS
Our study revealed a mechanism for deep dyspareunia in endometriosis, whereby IL-1β stimulates NGF expression, promoting local neurogenesis around endometriosis, which in turn leads to tender pelvic anatomic sites and thus deep-hitting dyspareunia. There may also be potential for drug targeting of IL-1β and/or NGF in the management of endometriosis-associated pain.
STUDY FUNDING/COMPETING INTEREST(S)
This study was funded by grants from the Canadian Institutes of Health Research (MOP-142273 and PJT-156084). P.Y. is also supported by a Health Professional Investigator Award from the Michael Smith Foundation for Health Research. MB has financial affiliations with Abbvie and Allergan. Otherwise, there are no conflicts of interest to declare.
Keywords: IL-1β, NGF, c-FOS, neurogenesis, nerve growth factor, endometriosis, dyspareunia
Introduction
Endometriosis is associated with sexual pain, specifically pain with deep penetration (i.e. deep dyspareunia). The etiology of deep dyspareunia in endometriosis is multifactorial (Yong, 2017). In addition to nervous system and myofascial contributors to deep dyspareunia (manifesting as bladder or pelvic floor tenderness), endometriosis-specific factors including depth of disease may be important. As well, there may be a role for local neurogenesis around endometriosis (Fraser 2010) in the pathophysiology of deep dyspareunia. In a previous study, we focused on sample of patients with endometriosis who reported deep dyspareunia that was reproducible on palpation of the posterior pelvic compartment (i.e. uterosacrals/cul-de-sac) (Williams et al., 2016). This patient sample was found to have a higher density of nerve fibre bundles around the endometriosis epithelium/stroma, compared to patients without deep dyspareunia. In subsequent work using this patient sample, endometriosis epithelium/stroma had a higher expression of nerve growth factor (NGF) and its TrkA receptor, and NGF expression in stroma in turn was correlated with local nerve fibre bundle density around endometriosis and deep dyspareunia (Peng et al., 2017).
Interleukin-1β (IL-1β) is a potent pro-inflammatory cytokine that was found to be increased in peritoneal fluid from women with endometriosis and pelvic pain when compared to women without the disease (Akoum et al., 2008). Recently, it was reported that IL-1β enhances the expression of brain-derived neurotrophic factor (BDNF) in primary eutopic endometriosis stromal cells (Yu et al., 2018). IL-1β has also been found to upregulate NGF in different cell types (Frossard et al., 2005; Jauneau et al., 2006). IL-1β activity is tightly regulated by a naturally produced antagonist, IL1Ra (Palomo et al., 2015). A recombinant form of IL1Ra, Anakinra, is non-glycosylated with a methionine residue added to the amino terminus and is currently in use for rheumatoid arthritis (Mertens and Singh, 2009).
In this study, we performed both immunohistochemistry on patient surgical samples and functional studies on primary cultures of endometriosis stromal cells, in order to elucidate the relationship between IL-1 β, NGF, local neurogenesis and deep dyspareunia in endometriosis. First, we used immunohistochemistry to determine whether IL-1 β expression in the endometriosis epithelium/stroma was associated with NGF expression, local nerve fibre bundle density and more severe deep dyspareunia. Second, we treated primary cultures of endometriosis stromal cells with IL-1 β to determine whether there is an increase in NGF expression and whether this in turn promotes neurogenesis in a PC12 cell in vitro model. We also studied whether Anakinra can inhibit this IL-1β-induced NGF promotion of neurogenesis.
Materials and Methods
Study setting
Endometriosis specimens were obtained from excisional surgeries at the BC Women’s Centre for Pelvic Pain and Endometriosis from 2010–2017. The specimens were obtained from a tissue bank with prospective consent or from a pathology archive with a waiver of consent. Clinical data, including deep dyspareunia pain score (on an 11-point numeric rating scale), were obtained from pre-operative patient questionnaires and medical chart review.
Ethical approval
Approval for this study was from University of British Columbia ethics #H14-03040 and #H17-00329.
Antibodies and reagents
Antibodies against human IL-1β (ab 2105, Abcam, Toronto, Canada), IL-1R1 (sc-688, Santa Cruz Biotechnology, Dallas, USA), NGF (#2046, Cell Signaling Technology, Danvers, MA, USA), c-FOS (Clone 9F6, #2250, Cell Signaling Technology), phosphorylated (p)-c-FOS (Clone D82C12, #5348, Cell Signaling Technology), PGP9.5 (760-4434, Ventana, Roche Diagnostics, Basel, Switzerland), Substance P (SP) (ab14184, Abcam, Toronto, Canada) and CD10 (MCA1806T, Bio-Rad, Mississauga, Canada) were utilised for this study. Recombinant human IL-1β protein was obtained from Cell Signaling Technology, and Anakinra (Kineret™, an IL-1R1 inhibiting antibody) was obtained from Sobi Inc. (Waltham, MA, USA) through our hospital pharmacy.
Sample for immunohistochemistry
There were 45 cases included in the immunohistochemistry component of the study. This included patients with samples of deep uterosacral/rectovaginal endometriosis (DIE) (n = 12), ovarian endometriomas (OMA) (n = 14) and superficial peritoneal uterosacral/cul-de-sac endometriosis (SUP) (n = 19). It should be noted that 6 DIE, 7 OMA and 19 SUP cases were used for experiments in previous work (Williams et al., 2016; Peng et al., 2017; AlKusayer et al., 2018).
Immunohistochemistry
Standard immunohistochemistry (IHC) was carried out as published (Alotaibi et al., 2019). Briefly, IHC using the EnVision+ Dual Link system (Dako) and 3,3′-diaminobenzidine (DAB) was performed (Alotaibi et al., 2019), using dilutions of IL-1β antibody (1:200), IL-1R1 antibody (1:100) and NGF antibody (1:20), which were semi-quantitatively evaluated in endometriotic epithelium and stroma using the Histoscore calculation (Alotaibi et al., 2019).
For nerve fibre bundle density, the PGP9.5 scoring method has been described previously (Williams et al., 2016). Briefly, high-powered fields (HPFs) were systematically scanned from top to bottom and then from left to right, using a ×20 objective and a ×10 ocular. The number of PGP9.5 positive nerve fibre bundles, defined as PGP9.5 positive nerve fibres on IHC surrounded by a perineurium, was counted, as was the number of HPFs examined. To calculate the PGP9.5-positive nerve fibre bundle density in each case, the total number of PGP9.5-positive nerve fibre bundles counted was divided by the total number of HPFs within the slide (fibre bundles/HPF). Five random PGP9.5 slides were scored by another blinded observer to measure intraclass correlation: the value was 0.83, indicating good inter-observer reliability. We also used Substance P (SP) (1/100) to identify nerve fibres involved in nociception (e.g. C-fibres).
Statistical analysis for IHC
The Spearman or Pearson correlation (depending on whether assumptions were met) was used to investigate the associations between IL-1β, IL-1R1, NGF and PGP9.5 scores between each other, and with severity of deep dyspareunia. We employed SPSS 22.0 software (IBM Corporation, Armonk, NY, USA), with P < 0.05, and means are presented with standard deviations.
Sample for primary endometrial stromal cell cultures
Primary endometrial stromal cell (ESC) isolation and culture from an additional eight (ectopic) endometriosis lesions were performed based on the method employed by our group (Peng et al., 2017). Briefly, endometriotic lesions were collected from excisional surgeries and minced into small pieces, digested with 1 mg/ml type I collagenase (Thermo Fisher Scientific, Waltham, MA, USA) for 60–90 min; non-stromal cells were filtered out with 100- and 40-μm sieves, and the remaining primary ESCs were cultured in phenol red-free Dulbecco’s modified Eagle medium/F12 medium 1:1 with 10% FBS and antibiotics. We have performed CD10 immunocytochemistry as an endometriosis stromal marker on primary cultures from this methodology and found that these cells were > 90% CD10-positive (see Supplementary Fig. S1).
ESC cultures
Primary ESCs (2 × 105 cells) were seeded before serum starvation. All primary ESCs were incubated in the medium containing 0.1% FBS 18 h before treatments. ESCs were then incubated with vehicle control (water) or human IL-1β (20 ng/ml) for different durations (1–24 h) and were treated with different concentrations of IL-1β (0, 0.2, 2 or 20 ng/ml) for 1 h for mRNA collection, 24 h for protein collection or 48 h for supernatant collection. Different concentrations of IL-1R1 inhibitor Anakinra (0, 20, 100, 500, 2000 ng/ml) were added 1 h prior to treatment with IL-1β (20 ng/ml).
Small interfering RNA transfection
Primary ESCs (105 cells) were seeded, and small interfering RNA (siRNA) (Dharmacon, Lafayette, CO, USA) targeting human IL-1R1 or FOS genes (25 nM) or control siRNAs (Dharmacon) were transfected via Lipofectamine® RNAiMAX Reagent (Thermo Fisher Scientific) for 48 h before 20 ng/ml IL-1β was added in the culture.
Reverse transcription quantitative real-time PCR
RNA was extracted and reverse-transcribed, and quantitative real-time PCR (qPCR) was performed; this was repeated in duplicate for three different ESC cultures as described (Peng et al., 2017). The primers used were NGF, 5′-TGA AGC TGC AGA CAC TCA GG-3′ (forward) and 5′-AGA ATT CGC CCC TGT GGA AG-3′ (reverse); FOS, 5′-CGT CTC CAG TGC CAA CTT CA-3′ (forward) and 5′-GGT CCG GAC TGG TCG AGA T-3′ (reverse); and GAPDH, 5′-ATG GAA ATC CCA TCA CCA TCTT-3′ (forward) and 5′-CGC CCC ACT TGA TTT TGG-3′ (reverse). mRNA levels were determined using 2−∆∆Cq with GAPDH reference (Peng et al., 2017).
Western blots
Protein was extracted, SDS gel electrophoresis was performed, and chemiluminescence was quantified as described (Peng et al., 2017). Primary antibodies were against NGF (1:1000, #2046), c-FOS (1: 1000, #2250) and phosphorylated-c-FOS (1: 1000, #5348) or β-actin (Clone C4, sc-47 778, 0.2 μg/ml, Santa Cruz Biotechnologies, Santa Cruz, CA). GeneTools software (Syngene, Frederick, MD) was used for densitometry with β-actin normalisation.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was performed in standard fashion on culture supernatants (conditioned medium) from ESCs, done in duplicate (Peng et al., 2017). NGF sandwich ELISA was performed with the human β-NGF DuoSet ELISA Kit (R&D Systems, Minneapolis, MN).
Immunofluorescence
ESCs were seeded on coverslips and starved using medium containing 0.1% FBS 18 h prior to IL-1β (20 ng/ml) treatment. Cells were treated for 30 min, fixed in 4% formaldehyde for 20 min, and permeabilised with NP-40 (0.1% in PBS) before staining with specific rabbit anti phosphorylated-c-FOS (1:100) and mouse anti-β-actin (1:100) antibodies overnight. After incubation for 1 h with Alexa Fluor® 488 goat anti-mouse IgG or Alexa Fluor® 594 goat anti-rabbit IgG (Thermo Fisher Scientific), as well as DAPI staining, cells were examined with a DM 4000 B epifluorescence microscope (Leica).
PC-12 neurite growth assay
The method of assessing the neurite growth of a rat pheochromocytoma cell line PC-12 has been previously reported (Radio et al., 2008) and modified in our research laboratory. Briefly, PC-12 cells were maintained in an RPMI 1640 medium supplement with 10% FBS, 1% L-glutamine and 1% penicillin/streptomycin. Two experimental procedures were performed: (i) ESCs were untreated or treated with IL-1β (20 ng/ml) for 48 h before culture supernatant were collected, and Ctrl IgG or NGF neutralising antibody (1000 ng/ml; ALM-006, Alomone Lab, Jerusalem, Israel) were added to the supernatant for 1 h to generate conditioned medium, and (2) ESCs were pre-treated with Ctrl IgG or Anakinra (2000 ng/ml) for 1 h before IL-1β (20 ng/ml) or no treatment, then subjected to the culture for 48 h and supernatant were collected as conditioned medium. PC-12 cells were seeded at relative low density (103 cells/ml) in 12 well plates pre-coated with rat tail collagen type I (Thermo Fisher Scientific). At 24 h after seeding, 1 ml of conditioned medium from ESCs was added into PC-12 cell culture. At 48 h after conditioned medium treatment, PC-12 cells were fixed with 4% formaldehyde, permeabilised with 0.1% NP-40 and stained with a mouse anti-α-tubulin antibody (1:100, Clone B-7, sc-5286, Santa Cruz Biotechnologies) overnight. Cells were incubated with Alexa Fluor® 594 goat anti-mouse IgG (Thermo Fisher Scientific) for 1 h. The morphology of PC-12 cells was observed under an SP8X confocal microscope with inverted scopes (Leica, Wetzlar, Germany). The differentiation status of PC-12 cells was quantified by neurite numbers per branching point and mean neurite lengths using Wimasis image analysis (Munich, Germany). Data from three representative images per well were pooled as one experiment, and results from three independent experiments were averaged for statistical analysis.
Statistical analysis for ESCs
ANOVA was performed followed by post hoc pair-wise comparisons using Tukey test, with P value <0.05, using GraphPad Prism 5 software (GraphPad software, San Diego, CA, USA).
Results
IL-1β associations with nerve fibre bundle density, NGF and deep dyspareunia
There were 45 cases included in the immunohistochemistry studies, with average age 34.5 ± 6.4 years (range 22–49), 46.5% (20/43) on hormonal suppression and 55.6% (25/45) Stage I/II and 44.4% (20/45) Stage III/IV.
Immunohistochemistry results are summarised in Figure 1 and Table I. IL-1β expression in the endometriosis epithelium was associated with NGF expression in the endometriosis epithelium and stroma (r = 0.42, P = 0.01; r = 0.45, P = 0.01), nerve fibre bundle density around endometriosis (r = 0.42, P = 0.01) and severity of deep dyspareunia (r = 0.37, P = 0.02). IL-1β expression in endometriosis stroma was also associated with nerve fibre bundle density (r = 0.45, P < 0.01) and severity of deep dyspareunia (r = 0.37, P = 0.02).
Figure 1.
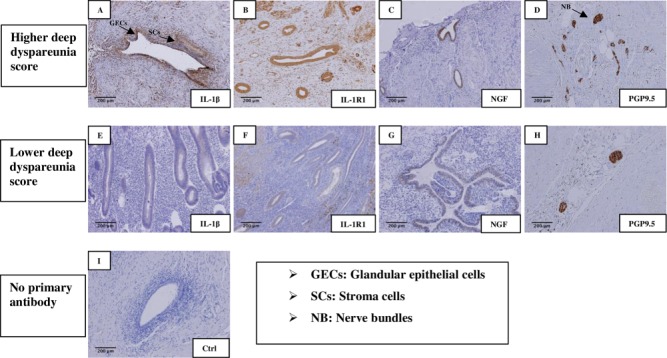
Immunohistochemical staining in endometriotic tissues. IHC staining of IL-1β (A,E), IL-1R1 (B,F), NGF (C,G) and PGP9.5 (D,H) No staining was observed in control sections incubated without primary antibody (I). The top row (A–D) shows sample staining in patients with more severe deep dyspareunia. The middle row (E–H) shows sample staining in patients with milder/absent deep dyspareunia. Abbreviations used: GEC, glandular epithelial cells; SC, stromal cells; NB, nerve bundles. Original magnification, all images, ×10; Bar = 200 μm.
Table I.
Correlations with IL-1β expression.
| Spearman’s/Pearson’s correlationa | IL-1β Histoscore in endometriotic epithelium | IL-1β Histoscore in endometriotic stroma | ||||
|---|---|---|---|---|---|---|
| Correlation coefficient | Significance (two-tailed) | N | Correlation coefficient | Significance (two-tailed) | N | |
| Deep dyspareunia (0–10) | 0.37b | 0.02 | 40 | 0.37b | 0.02 | 40 |
| Nerve fibre bundle density (bundles/high-powered field) | 0.42b | 0.01 | 42 | 0.45c | <0.01 | 42 |
| NGF Histoscore in endometriotic epithelium | 0.42b | 0.01 | 37 | 0.29 | 0.09 | 37 |
| NGF Histoscore in endometriotic stroma | 0.45b | 0.01 | 37 | 0.32 | 0.05 | 37 |
| IL-1RI Histoscore in endometriotic epithelium | 0.37b | 0.02 | 41 | 0.29 | 0.07 | 41 |
| IL-1RI Histoscore in endometriotic stroma | 0.32 | 0.05 | 41 | 0.29 | 0.07 | 41 |
aSpearman correlation (for non-normality) and Pearson correlation (for normality) were used, with the correlation significant at the 0.05 levelb or at the 0.01 levelc. *Sample sizes 37–41, due to missing data for a given pairwise analysis.
Scatterplots for associations with IL-1β expression deep dyspareunia score are shown in Supplementary Figure S2. IHC for IL-1β, IL-1R1, NGF and PGP9.5 on adjacent sections for a single case is shown in Supplementary Figure S3. We also performed IHC for Substance P from a subset of 12 cases and found a correlation between SP nerve density and PGP9.5 nerve density on adjacent sections (r = 0.59, P = 0.04) (Supplementary Figure S4 and Supplementary Table SI).
There was no statistical correlation between IL-1β and other parameters including age, stage or hormonal suppression (data not shown). In particular, there was no association between IL-1β expression and severity of dysmenorrhea (0–10) (epithelium r = −0.05, P = 0.75; stroma r = −0.13, P = 0.43), in contrast to the findings for deep dyspareunia.
IL-1β induces NGF expression in primary ESCs
Our immunohistochemistry data supported an association between IL-1β and NGF expression in endometriotic tissues; therefore, we further explored the regulatory function of IL-1β for NGF expression using an in vitro primary cell model. Treatment of primary ESCs with IL-1β at a concentration of 20 ng/ml induced a rapid increase in NGF mRNA levels at 1, 3 and 6 h (Fig. 2A), stimulated the elevation of NGF protein level at 24 h (Fig. 2B) and increased the concentration of NGF protein in the culture supernatant of primary ESCs at 48 h (Fig. 2C). In addition, IL-1β treatment at increasing concentrations (0.2 ng/ml, 2 ng/ml, 20 ng/ml) significantly increased NGF mRNA levels at 1 h (Fig. 2D), while the higher concentration (20 mg/ml) of IL-1β significantly increased NGF protein levels at 24 h (Fig. 2E).
Figure 2.
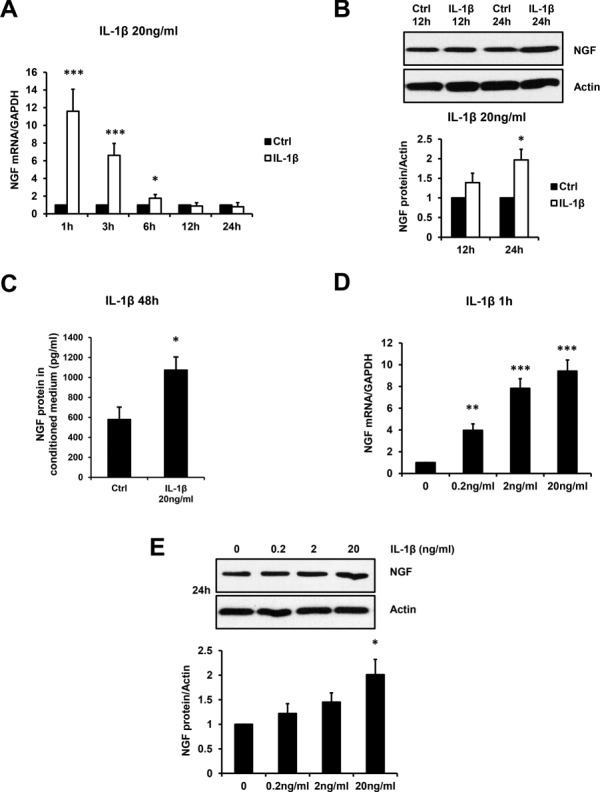
IL-1β stimulates NGF expression in primary endometriotic stromal cells (ESCs). Primary ESCs were treated with or without 20 ng/ml IL-1β for 1, 3, 6, 12, 24 and 48 h. The NGF mRNA (A) and protein (B) levels in primary ESCs as well as secreted NGF protein in conditioned medium (C) are presented. Additionally, primary ESCs were treated with increasing concentrations (0, 0.2, 2 or 20 ng/ml) of IL-1β, and the NGF mRNA levels at 1 h (D) and protein levels at 24 h (E) are presented in the bar graphs. Results are presented as the mean ± SEM of four independent experiments (n = 4, *P < 0.05, **P < 0.01, ***P < 0.001 vs. individual controls).
IL-1R1 siRNA and IL-1R1 antibody Anakinra inhibit IL-1β-induced NGF expression
Next, we sought to examine the involvement of IL-1 receptor type 1 (IL-1R1) in IL-1β-induced NGF expression. After knockdown of IL-1R1 via siRNA in primary ESCs for 48 h, IL-1β-induced (20 ng/ml) mRNA expression (Fig. 3A), protein expression (Fig. 3B) and supernatant concentrations (Fig. 3C) of NGF were attenuated at time points 1, 24 and 48 h, respectively. Similarly, pre-treatment of Anakinra at 500 and 2000 ng/ml significantly inhibited IL-1β-induced increases of NGF mRNA levels (Fig. 3D) at 1 h. Moreover, Anakinra at 2000 ng/ml further inhibited IL-1β-induced NGF protein levels (Fig. 3E) at 24 h and supernatant concentration (Fig. 3F) at 48 h in primary ESCs.
Figure 3.
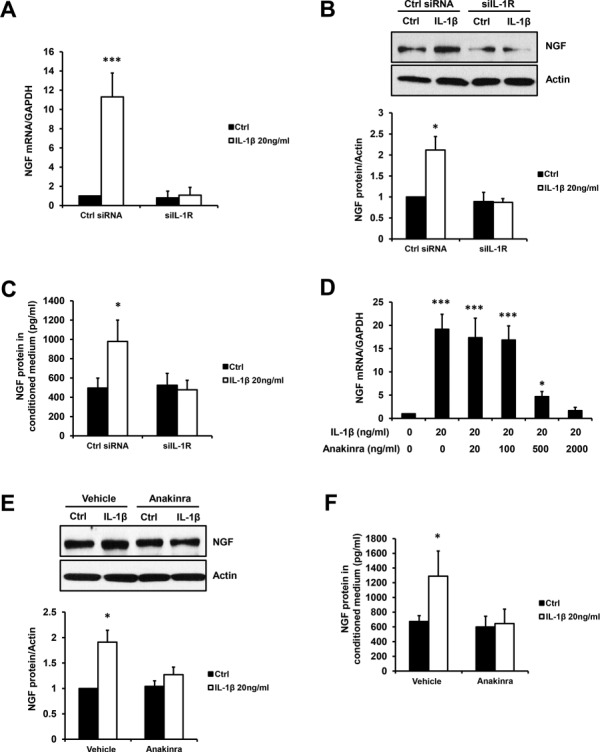
Inhibiting IL-1R1 by siRNA or antagonist Anakinra attenuates IL-1β-induced NGF expression. Primary ESCs were transfected by Ctrl or IL-1R1 siRNA (siIL-1R, 50 ng) for 48 h before incubation with or without 20 ng/ml IL-1β, and the NGF mRNA levels at 1 h (A) and protein levels at 24 h (B) as well as secreted NGF protein in conditioned medium at 48 h (C) are shown. In addition, primary ESCs were treated with 20 ng/ml IL-1β in combination of either increasing concentration (0, 20, 100, 500 or 2000 ng/ml) (D) or a fixed concentration (2000 ng/ml) (E and F) of Anakinra (IL-1R1 inhibiting antagonist). The NGF mRNA levels at 1 h (D), protein levels at 24 h (E) and secreted NGF protein at 48 h (F) are presented in the bar graphs. Results are presented as the mean ± SEM of four independent experiments (n = 4, *P < 0.05, ***P < 0.001 vs. individual controls)
c-FOS mediates IL-1β induction of NGF expression
AP-1 transcription factor c-FOS is known to be downstream of IL-1β; therefore, we investigated the role of c-FOS in IL-1β-induced NGF expression. As shown in Figure 4, IL-1 β (20 ng/ml) significantly increased c-FOS mRNA levels (Fig. 4A) at 1, 3 and 6 h. as well as its protein levels (Fig. 4B) at 3 and 6 h. Additionally, treatment of primary ESCs with IL-1 β increased the levels of phosphorylation of c-FOS protein (Fig. 4C) as well as the nucleus accumulation of phosphorylated (p)-c-FOS (Fig. 4D). Moreover, knockdown of c-FOS via siRNA in ESCs resulted in reduction in both basal and IL-1β-induced NGF mRNA expression (Fig. 4E), protein expression (Fig. 4F) and supernatant concentration (Fig. 4G).
Figure 4.
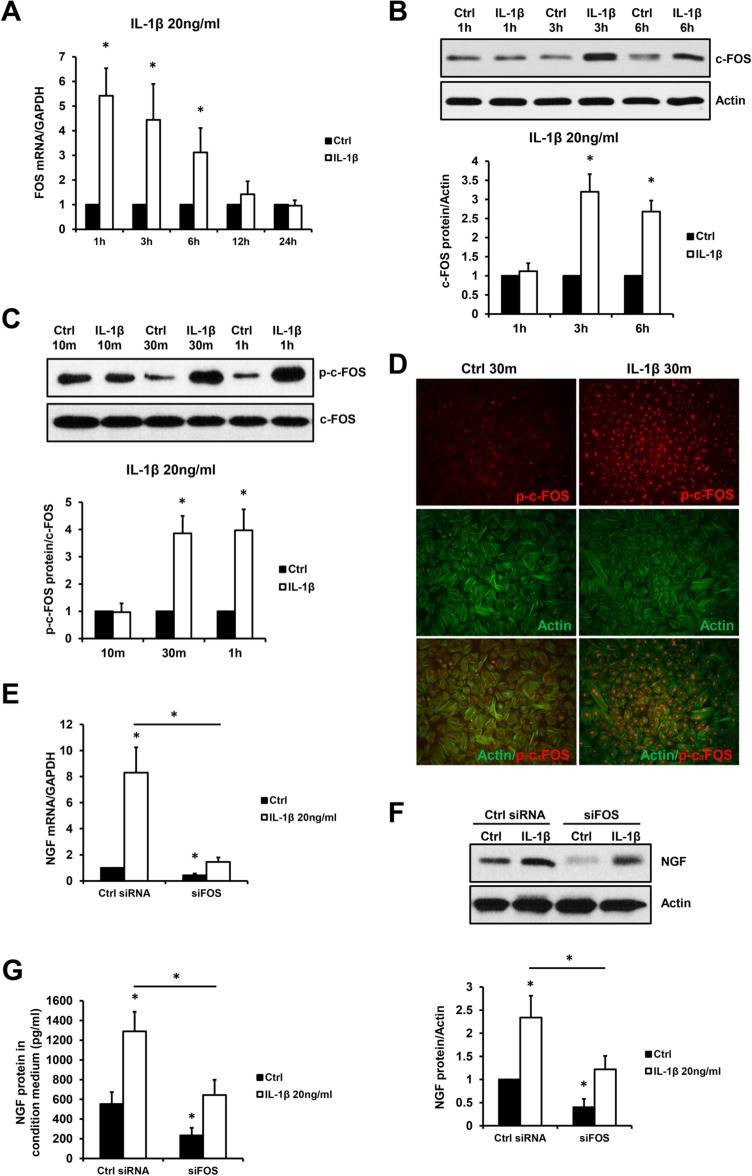
c-FOS mediates IL-1β-induced NGF expression. Primary ESCs were treated with or without 20 ng/ml IL-1β for 10 m, 30 m, 1 h, 3 h, 6 h, 12 h and 24 h. The FOS mRNA levels (A), c-FOS protein levels (B) and phosphorylated (p)-c-FOS protein levels (C) are presented in the bar graphs. The cellular localisations of p-c-FOS and actin proteins (D) are shown in the micrographs (×100). Additionally, primary ESCs were transfected by Ctrl or FOS siRNA (siFOS, 50 ng) for 48 h before incubation with or without 20 ng/ml IL-1β, and the NGF mRNA levels at 1 h (E) and protein levels at 24 h (F) as well as secreted NGF protein in the conditioned medium at 48 h (G) are shown. Results are presented as the mean ± SEM of four independent experiments (n = 4, *P < 0.05 vs. individual controls).
NGF-neutralising antibody and Anakinra inhibit IL-1β induction of PC12 neurite outgrowth
Based on our finding that IL-1β is capable of stimulating NGF expression in primary ESCs, we further investigated whether IL-1β-induced NGF could promote neurite outgrowth using an in vitro PC-12 model. The supernatant (conditioned medium) from IL-1β-treated ESCs successfully induced PC-12 cell neurite outgrowth (Fig. 5A), and addition of the NGF-neutralising antibody to the conditioned medium inhibited both basal and IL-1β-induced PC12 cell neuronal differentiation (Fig. 5A) by reducing the number of neurites per branching point (Fig. 5B, upper panel) and mean neurite length (Fig. 5B, lower panel). Similarly, addition of Anakinra inhibited both basal and IL-1β-induced PC-12 cell neurite outgrowth (Fig. 5C) by attenuating the number of neurites per branching point (Fig. 5D, upper panel) and mean neurite length (Fig. 5D, lower panel). These results suggest that the IL-1β-induced PC-12 neurite outgrowth is likely mediated by NGF secreted by primary ESCs.
Figure 5.
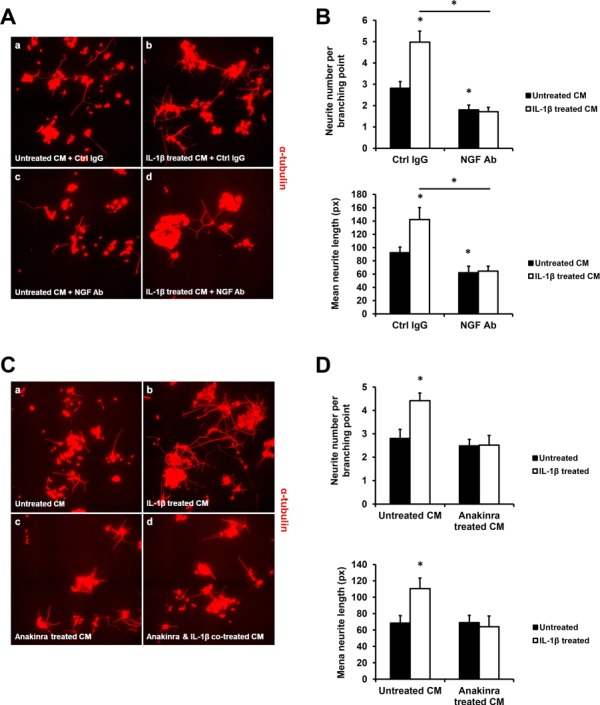
NGF neutralising antibody and Anakinra attenuate IL-1β-induced PC-12 neurite outgrowth. Primary ESCs were treated with or without 20 ng/ml IL-1β for 48 h, then NGF neutralising antibody or Ctrl IgG was added to the conditioned medium for 1 h before incubation with PC-12 for another 48 h. The micrograph showing neurite outgrowth of PC-12 cells (A) and quantifications of neurite numbers per branching point (B, upper panel) and mean neurite growth (B, lower panel) are shown. In addition, primary ESCs were pre-treated with or without 2000 ng/ml Anakinra for 1 h before 20 ng/ml IL-1β or no treatment, and the conditioned medium was added to PC-12 for another 48 h. The micrograph showing neurite outgrowth of PC-12 cells (C), and quantifications of neurite numbers per branching point (D, upper panel) and mean neurite growth (D, lower panel) are shown. Results are presented as the mean ± SEM of four independent experiments (n = 4, *P < 0.05 vs. individual controls).
Discussion
We found that IL-1β expression in endometriosis was associated with NGF expression in the endometriosis stroma/epithelium, local nerve density around endometriosis and the symptom of deep dyspareunia. In primary cultures of endometriosis stroma, IL-1β induced NGF expression in endometriosis stromal cells, mediated in part by c-FOS, and the conditioned medium from these endometriosis stromal cells was able to induce PC12 neuronal differentiation. Notably, the effect of this conditioned medium on PC12 neuronal differentiation was inhibited by the NGF-neutralising antibody and IL-1R1 antagonist (Anakinra).
Therefore, we propose a model (Fig. 6) whereby IL-1β expression by endometriosis induces NGF expression in endometriosis stroma, through autocrine and paracrine signalling and via c-FOS, which in turn promotes local neurogenesis around endometriosis (Fraser 2010). This local neurogenesis is associated with more severe deep dyspareunia, through production of tender anatomic sites that are hit with deep penetration.
Figure 6.
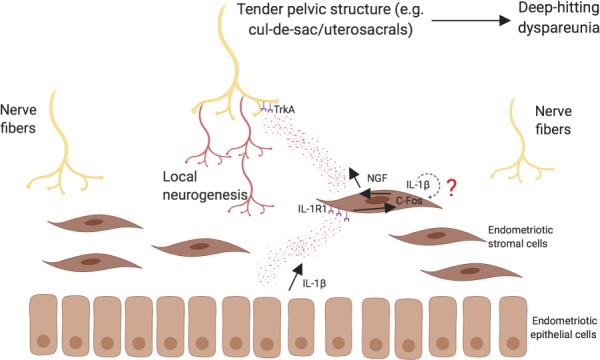
Model of IL-1β induced NGF expression in local neurogenesis and deep dyspareunia in endometriosis. Based on the current study’s results and previous work (Peng et al., 2017), we proposed a mechanism for deep dyspareunia in endometriosis. IL-1β expression from endometriosis (in particular from epithelium; see Table I) induces NGF expression from endometriosis stroma in a paracrine fashion, via its receptor (IL-1R1) and C-FOS. IL-1β autocrine signalling from the stroma itself is also possible (?). We have previously found that NGF expression from stroma was most strongly associated with local nerve bundle density (Peng et al., 2017). Thus, we propose that NGF is secreted from the stroma, which induces local neurogenesis around endometriosis via its main receptor TrkA. This local neurogenesis results in a tender pelvic anatomic site (e.g. the uterosacrals/cul-de-sac), which is hit with deep penetration and results in deep dyspareunia.
The strengths of this study include the combination of both immunohistochemistry and functional studies using primary endometriotic stromal cells, and the use of PC12 cells as an in vitro model of neurogenesis. The main limitation of this study is the lack of data on primary cultures of the endometriosis glandular epithelium, due to the known difficulties with primary cultures of this cell type, and thus we treated ESCs with exogenous IL-1β. Furthermore, there was insufficient power with the current sample to determine whether IL-1β expression differed by anatomic subtype (deep, ovarian or superficial endometriosis).
IL-1β, an important pro-inflammatory mediator, is known to have neuroinflammatory stimulating effects and has been reported to upregulate the expression of several neurotropins in vertebral disc cells from the annulus fibrosus including derived neurotrophic factors, neurotrophic factor 3, neurotrophic factor 4 and NGF (Gruber et al., 2012). Utilising primary eutopic endometriosis stromal cells, it was found that IL-1β upregulated BDNF, mediated in part by the NF-κB and JNK pathways, and induced fibroblast morphology in the cells (Yu et al., 2018). Our findings are further supported by previous studies of the disease progression of osteoarthritis, where treatment of synovial joint cells by IL-1β significantly promotes the synthesis and secretion of NGF (Manni et al., 2003). Interestingly, in women with adenomyosis, IL-1β and NGF were found to be co-expressed in proliferative and invasive endometrial cells within the myometrium (Carrarelli et al., 2017), providing further evidence for the importance of the IL-1β-NGF pathway in the pathogenesis of aberrant growing endometrial cells.
Surprisingly, the molecular mechanism(s) of IL-1β-induced NGF expression has yet to be intensively studied. An early study found that NF-κB signalling may mediate IL-1β-induced NGF expression in rat astrocytes, as inhibition of endogenous NF-κB-1 via antisense oligonucleotide attenuated IL-1β-stimulated increase of NGF mRNA levels (Friedman et al., 1996). Our results suggested a new mechanism with involvement of AP-1 transcription factor c-FOS, since we observed that knockdown of the FOS gene by specific siRNA inhibited both basal and IL-1β-induced NGF mRNA and protein expression. This finding is in line with previous research showing that c-FOS is a downstream transcription factor of IL-1β (Goehler et al., 1998) and that c-FOS mediates NGF induction by external stimulation such as trauma (Hengerer et al., 1990). Moreover, the AP-1-binding sequence is known to be present in the NGF promoter, supporting the role of c-FOS in potentiation of NGF by IL-1β (Cartwright et al., 1992).
Endometriosis animal models have also been utilised to study local neurogenesis. In a rat model where segments of uterine horn (ectopic implants) were removed from donor rats and sewn around the mesenteric arteries of recipient rats (Chen et al., 2014), NGF siRNA treatment of ectopic implants resulted in lower nerve fibre bundle density compared to mock plasmid treatment. In future research, such models could be utilised to block NGF via siRNA or neutralising antibodies, to assess not only nerve fibre bundle density around ectopic implants but also impact on behavioural pain measurements.
We are currently conducting a prospective validation study of the immunohistochemistry data, in a highly phenotyped sample that includes standardised assessment of deep dyspareunia and other pelvic pain symptoms in endometriosis and sufficient power to look at anatomic subtype. Additionally, it would be of interest to explore NGF or IL-1β inhibition as a novel treatment strategy in endometriosis. Tanezumab is a monoclonal antibody against NGF that has been shown to reduce pain in clinical trials of osteoarthritis, although patients on tanezumab have had more discontinuations compared to placebo including for neuropathic symptoms (Kan et al., 2016) (Chen et al., 2016). Tanezumab has also shown efficacy for bladder pain, particularly in women (Nickel et al., 2016). Anakinra is an IL-1R1-inhibiting antibody currently in use for managing pain and inflammation in rheumatoid arthritis (Mertens and Singh, 2009); we found that Anakinra could successfully abolish IL-1β-induced NGF expression and neurogenesis in our in vitro model. Thus, Anakinra may have potential as a drug for inhibition for endometriosis-associated neurogenesis, although its limitations include requirement for subcutaneous injection and also potential for immunosuppression although opportunistic infections are rare (Cavalli and Dinarello, 2018). A TNF-α monoclonal antibody, infliximab, has been examined for the treatment of pain associated with deep endometriosis but did not show a difference compared to placebo (Koninckx et al., 2008). More research into non-hormonal drug targeting of endometriosis-associated inflammation is needed, with potential value in patients who have pain non-responsive to hormonal suppression or in patients who cannot take hormonal suppression due to side-effects or a wish to conceive.
Authors’ roles
B.P.: substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the article and final approval of the version to be published. F.T.A.: substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the article and final approval of the version to be published. S.S.: substantial contributions to acquisition of data, revising the article critically for important intellectual content and final approval of the version to be published. M.A.B.: substantial contributions to conception and design, interpretation of data, revising the article critically for important intellectual content and final approval of the version to be published. P.J.Y.: substantial contributions to conception and design, analysis and interpretation of data, drafting the article and final approval of the version to be published.
Funding
Canadian Institutes of Health Research (MOP-142273 and PJT-156084); Health Professional Investigator Award from the Michael Smith Foundation for Health Research (to P.Y.).
Conflict of interest
M.B. has a financial affiliation with Abbvie and Allergan. Otherwise, there are no conflicts of interest to declare.
Supplementary Material
References
- Akoum A, Al-Akoum M, Lemay A, Maheux R, Leboeuf M. Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil Steril 2008;89:1618–1624. [DOI] [PubMed] [Google Scholar]
- AlKusayer GM, Pon JR, Peng B, Klausen C, Lisonkova S, Kinloch M, Yong P, Muhammad EMS, Leung PCK, Bedaiwy MA. HOXB4 immunoreactivity in endometrial tissues from women with or without endometriosis. Reprod Sci 2018;25:950–957. [DOI] [PubMed] [Google Scholar]
- Alotaibi FT, Peng B, Klausen C, Lee AF, Abdelkareem AO, Orr NL, Noga H, Bedaiwy MA, Yong PJ. Plasminogen activator inhibitor-1 (PAI-1) expression in endometriosis. PLoS One 2019;14:e0219064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrarelli P, Yen CF, Funghi L, Arcuri F, Tosti C, Bifulco G, Luddi A, Lee CL, Petraglia F. Expression of inflammatory and neurogenic mediators in adenomyosis. Reprod Sci 2017;24:369–375. [DOI] [PubMed] [Google Scholar]
- Cartwright M, Martin S, D'mello S, Heinrich G. The human nerve growth factor gene: structure of the promoter region and expression in L929 fibroblasts. Brain Res Mol Brain Res 1992;15:67–75. [DOI] [PubMed] [Google Scholar]
- Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol 2018;9:1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li J, Li R, Wang H, Yang J, Xu J, Zha Z. Efficacy and safety of tanezumab on osteoarthritis knee and hip pains: a meta-analysis of randomized controlled trials. Pain Med 2016;18:374–385. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li D, Zhang Z, Takushige N, Kong BH, Wang GY. Effect of siRNA against β-NGF on nerve fibers of a rat model with endometriosis. Reprod Sci 2014;21:329–339. [DOI] [PubMed] [Google Scholar]
- Fraser IS. Mysteries of endometriosis pain: Chien-Tien Hsu Memorial Lecture 2009. J Obstet Gynaecol Res 2010;36:1–10. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Thakur S, Seidman L, Rabson AB. Regulation of nerve growth factor mRNA by interleukin-1 in rat hippocampal astrocytes is mediated by NFkappaB. J Biol Chem 1996;271:31115–31120. [DOI] [PubMed] [Google Scholar]
- Frossard N, Naline E, OLgart Höglund C, Georges O, Advenier C. Nerve growth factor is released by IL-1 and induces hyperresponsiveness of the human isolated bronchus. Eur Respir J 2005;26:15–20. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res 1998;804:306–310. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Hoelscher GL, Bethea S, Hanley EN Jr. Interleukin 1-beta upregulates brain-derived neurotrophic factor, neurotrophin 3 and neuropilin 2 gene expression and NGF production in annulus cells. Biotech Histochem 2012;87:506–511. [DOI] [PubMed] [Google Scholar]
- Hengerer B, Lindholm D, Heümann R, Ruther U, Wagner EF, Thoenen H. Lesion-induced increase in nerve growth factor mRNA is mediated by c-fos. Proc Natl Acad Sci U S A 1990;87:3899–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauneau A, Ischenko A, Chatagner A, Benard M, Chan P, Schouft M, Patte C, Vaudry HF Interleukin-1beta and anaphylatoxins exert a synergistic effect on NGF expression by astrocytes. J Neuroinflammation 2006;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan SL, Li Y, Ning GZ, Yuan ZF, Chen LX, Bi MC, Sun JC, Feng SQ. Tanezumab for patients with osteoarthritis of the knee: a meta-analysis. PLOS ONE 2016;11:e0157105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koninckx PR, Craessaerts M, Timmerman D, Cornillie F, Kennedy S. Anti-TNF-α treatment for deep endometriosis-associated pain: a randomized placebo-controlled trial. Hum Reprod 2008;23:2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni L, Lundeberg T, Fiorito S, Bonini S, Vigneti E, Aloe L. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-alpha, interleukin-1 beta and cholecystokinin-8: the possible role of NGF in the inflammatory response. Clin Exp Rheumatol 2003;21:617–624. [PubMed] [Google Scholar]
- Mertens M, Singh J. Anakinra for rheumatoid arthritis: a systematic review. J Rheumatol 2009;36:1118–1125. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Mills IW, Crook TJ, Jorga A, Smith MD, Atkinson G, Krieger JN. Tanezumab reduces pain in women with interstitial cystitis/bladder pain syndrome and patients with nonurological associated somatic syndromes. J Urol 2016;195:942–948. [DOI] [PubMed] [Google Scholar]
- Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family – balance between agonists and antagonists in inflammatory diseases. Cytokine 2015;76:25–37. [DOI] [PubMed] [Google Scholar]
- Peng B, Klausen C, Campbell L, Leung PC, Horne AW, Bedaiwy MA. Gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor are expressed at tubal ectopic pregnancy implantation sites. Fertil Steril 2016;105:1620–1627. [DOI] [PubMed] [Google Scholar]
- Peng B, Zhan H, Alotaibi F, Alkusayer GM, Bedaiwy MA, Yong PJ. Nerve growth factor is associated with sexual pain in women with endometriosis. Reprod Sci 2017;25:540–549. [DOI] [PubMed] [Google Scholar]
- Radio NM, Breier JM, Shafer TJ, Mundy WR. Assessment of chemical effects on neurite outgrowth in PC12 cells using high content screening. Toxicol Sci 2008;105:106–118. [DOI] [PubMed] [Google Scholar]
- Williams C, Hoang L, Yosef A, Alotaibi F, Allaire C, Brotto L, Fraser IS, Bedaiwy MA, Ng TL Lee AF et al. Nerve bundles and deep dyspareunia in endometriosis. Reprod Sci 2016;23:892–901. [DOI] [PubMed] [Google Scholar]
- Yong PJ. Deep dyspareunia in endometriosis: a proposed framework based on pain mechanisms and genito-pelvic pain penetration disorder. Sex Med Rev 2017;5:495–507. [DOI] [PubMed] [Google Scholar]
- Yu J, Francisco AMC, Patel BG, Cline JM, Zou E, Berga SL, Taylor RN. IL-1β stimulates brain-derived neurotrophic factor production in eutopic endometriosis stromal cell cultures: a model for cytokine regulation of neuroangiogenesis. Am J Pathol 2018;188:2281–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


