Abstract
STUDY QUESTION
How efficacious and safe are the current approaches to controlled ovarian stimulation (COS) aimed at fertility preservation (FP) in women with breast cancer (BC)?
SUMMARY ANSWER
In women with BC undergoing COS aiming at egg/embryo cryopreservation, letrozole-based protocols and those randomly started were equally effective compared with conventional COS, and the overall survival was similar between the women that proceeded to FP and those who did not.
WHAT IS KNOWN ALREADY
Cryopreservation of oocytes and embryos is an established method for FP in women with BC. Recent improvements to COS protocols include concomitant use of letrozole, random-cycle start day of stimulation and the use of GnRHa for the egg maturation trigger. To date, limited sample size of the available studies has not allowed investigation of differences in the efficacy of the different approaches to COS for FP in this patient population.
STUDY DESIGN, SIZE, DURATION
A prospective multicenter study with national coverage including 610 women with BC counseled between 1 January 1995 and 30 June 2017 at six Swedish FP regional programs.
PARTICIPANTS/MATERIALS, SETTING, METHODS
After counseling, 401 women elected to undergo COS. Treatments differed in the use or not of concomitant letrozole, a conventional or random-cycle day COS initiation and the use of hCG versus GnRHa trigger for oocyte maturation. Numbers of cryopreserved oocytes and embryos were defined as primary outcome. Pregnancy attempts, reproductive outcomes and long-term survival, investigated by the linking of individuals of the cohort to the total population register of the Swedish Tax Agency (up to 25 November 2018), were evaluated.
MAIN RESULTS AND THE ROLE OF CHANCE
Using letrozole or not resulted in similar numbers of oocytes and embryos cryopreserved (meanoocytes = 9.7 versus 10 and meanembryos 4.0 versus 5.3, respectively), similar to COS with random versus conventional start (meanoocytes 9.0 versus 10.6 and meanembryos 4.8 versus 4.8). In COS with letrozole, a GnRHa trigger was associated with a higher number of oocytes retrieved (P < 0.05) and embryos cryopreserved (P < 0.005), compared with conventional hCG trigger. Of 99 women who returned to fertility clinics after cancer treatment, 32 proceeded to thawing of oocytes or embryos and 10 of them had live births. The all-cause survival between the women that underwent COS and those who did not was similar and did not differ between the two groups.
LIMITATIONS, REASONS FOR CAUTION
Data on tumor characteristics and estrogen receptor (ER) status were not known for all women at the time of FP counseling and planning of COS, thus protocols with letrozole have been used for both estrogen-sensitive and non-estrogen-sensitive BC. For the same reason, subsequent adjustment for ERs in the BC or tumor characteristics as potential confounders were not performed as these parameters were not available and did not influence the provision of FP through COS.
WIDER IMPLICATIONS OF THE FINDINGS
The results of our study support the premise that recently introduced potential improvements to COS protocols for FP in women with BC are efficacious and safe.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by research grants from the Swedish Cancer Society, the Stockholm County Council, the Percy Falk Stiftelsen, Radiumhemmets Forskningsfonder, The Swedish Breast Cancer Association and Karolinska Institutet to K.A.R.W. J.B. reports grants from Amgen, AstraZeneca, Pfizer, Roche, Sanofi-Aventis and Merck, outside the submitted work, and payment from UpToDate to Asklepios Medicine HB for a chapter on BC prediction and prognostication. All the other authors have no competing interests to report.
Keywords: breast cancer, fertility preservation, letrozole, antagonist protocol, controlled ovarian stimulation, random-start protocol, female, GnRHa trigger, cryopreservation, oocytes/embryos
Introduction
Breast cancer (BC) is the most common malignancy affecting women of childbearing age (Siegel et al., 2014). Survival rates have increased remarkably over the past few decades: ~88% of women diagnosed prior to age of 45 years survive at least 5 years (United States National Institutes of Health, 2019). About half of young BC patients wish to have children later in life (Letourneau et al., 2012). Still, pregnancy rate among women with previous BC is ~70% lower than expected in the general population (Stensheim et al., 2011; Anderson et al., 2018). This difference is thought to be secondary to gonadal toxicity of chemotherapy, longer periods of endocrine therapy, the practice of prophylactic oophorectomy in selected cases and a belief that pregnancy could cause cancer relapse (Peccatori et al., 2013). Fortunately, the negative effect of BC treatment on fertility appears to be reduced in the most recent treatment period, which possibly reflects a change not only in treatments but also in how patients are advised regarding postcancer pregnancy (Anderson et al., 2018).
The safety of fertility preservation (FP) in women with BC has been extensively discussed and scrutinized in previous studies (Azim et al., 2008; Kim et al., 2016; Moravek et al., 2018; Oktay et al., 2018; Rodriguez-Wallberg et al., 2018). Controlled ovarian stimulation (COS), a treatment that is required to obtain mature oocytes, increases systemic estradiol levels several-fold. Therefore, potentially safer COS protocols with the addition of tamoxifen or aromatase inhibitors have been proposed for women with estrogen-sensitive BC (Oktay et al., 2003, 2006). A previous prospective controlled study demonstrated superiority of letrozole over tamoxifen (Oktay et al., 2005). Thus, COS with letrozole has been implemented in many programs for FP worldwide. Additional COS improvements include the use of GnRHa for ovulation trigger, which further reduces estradiol levels after oocyte pick up, minimizing further the risk of ovarian hyperstimulation (Sonmezer et al., 2011), and the random-start initiation, which enables women with cancer to initiate promptly the COS treatment, irrespective of menstrual cycle phase (von Wolff et al., 2009; Sonmezer et al., 2011; Cakmak et al., 2013).
Most studies on the efficacy and safety of these novel approaches for FP in the population of women with BC have been small and retrospective (Oktay et al., 2010; Pereira et al., 2016; Rodgers et al., 2017), and no large prospective studies are yet available. Data on the reproductive outcome of women previously treated for cancer and the long-term outcomes of FP are also scarce (Alvarez et al., 2014; Donnez et al., 2015; Oktay et al., 2015; Rodriguez-Wallberg et al., 2019b), with all publications from single centers.
In Sweden, all university hospitals have currently established programs for FP, each covering a healthcare region. FP procedures are included in the public tax-funded healthcare system, ensuring an equal access to all citizens. In this study, data from a large cohort of women with BC that were counseled for FP at six Swedish reproductive centers covering healthcare regions, and together covering the whole country, were analyzed. Our primary aim was to examine the efficacy of COS protocols for oocyte and/or embryo banking and to investigate long-term outcomes including reproductive outcome and overall survival (OS).
Materials and Methods
Data source and patient population
For this study, we collected data on women with a diagnosis of BC referred to FP counseling from 1 January 1995 to 30 June 2017 to any of the programs for FP at the Swedish university hospitals: Karolinska University Hospital, Stockholm; Skåne University Hospital, Malmö; Sahlgrenska University Hospital, Gothenburg; Uppsala University Hospital, Uppsala; Linköping University Hospital, Linköping; and Örebro University Hospital, Örebro. Electronic medical records have been implemented at the Swedish university hospitals since 1997 and all data have been registered prospectively in the clinical treatment register of each hospital. Counseling on FP has followed international guidelines (ASCO, 2006; Oktay et al., 2018) and it has been adapted to the Swedish healthcare system through national collaborative work (Rodriguez-Wallberg et al., 2019a). At all reproductive medicine centers, emergency appointments for FP counseling were provided following a referral from the respective oncology center. In most cases, the women were referred after BC surgery, and thus BC stage and tumor receptor status were reported in the referral. However, in several cases, precise data on the staging or on receptor status were not reported or were not available. However, tumor characteristic or receptor status was not a determinant of provision of FP with or without hormonal stimulation. Clinical options including COS aiming at cryopreservation of embryos or oocytes have been recommended whenever time was sufficient before planned chemotherapy initiation. Due to legal concerns regarding the ownership of embryos, women in committed relationships could also choose to cryopreserve oocytes instead of embryos. Alternatives, such as egg retrieval in the natural cycle or cryopreservation of ovarian tissue were also discussed. Details of the specific counseling regarding FP to women at our center have been previously reported (Rodriguez-Wallberg et al., 2019b).
COS was performed in the vast majority of patients using an antagonist protocol, which allows shortening of the treatment cycle. The protocols have been adapted for FP of women with BC and have undergone improvements that have been reported over the years, such as the addition of letrozole (Oktay et al., 2006), a random-start day of stimulation (Cakmak et al., 2013) and use of GnRHa trigger (Oktay et al., 2010). Standard protocols for COS were in general applied to women without estrogen-sensitive BC until 2010, when protocols with the co-administration of letrozole were introduced for this patient population in Sweden (Rodriguez-Wallberg et al., 2019b). The choice of using a GnRHa versus hCG for maturation trigger was left at the discretion of the reproductive medicine specialist that planned the egg retrieval at the last monitoring appointment.
Detailed information about the COS protocols and specific procedures related to the retrieval of oocytes and cryopreservation of oocytes and embryos can be found in the Supplementary materials.
Ethics approval
This study was approved from the regional ethics committee in Stockholm, Sweden (Dnr 2011/1758-31/2, amendments Dnr 2014/470-32 and Dnr 2014/1825-32).
Study variables and outcomes
Baseline demographics and clinical data recorded included age at FP counseling, BMI (kg/m2), serum anti-Mullerian hormone (AMH) levels (ng/mL), total antral follicular counts (AFC), type of BC (estrogen receptor (ER), positive or negative), previous parity, partnership status, if the woman has chosen to proceed with FP or not, the reason for not proceeding with FP and the method elected for FP.
Regarding the efficacy of COS for FP, the primary outcome measure was the number of oocytes and embryos cryopreserved in treatments with versus without concurrent use of letrozole, in treatments with random versus conventional start and in letrozole treatments using hCG versus GnRHa egg maturation trigger. Twelve women underwent two COS cycles for FP before the start of chemotherapy. These additional consecutive cycles were not included in the analysis. Detailed data of COS treatments, medications and cycles outcomes were extracted from the clinical treatment database. For secondary reproductive outcomes, data on deliveries after cancer among the women who returned for fertility treatments were registered in each reproductive clinic’s database, whereas deliveries among those who had not come back for fertility counseling after treatment were retrieved from the medical obstetrical database of the respective hospital.
For the investigation of safety of COS in women with BC, OS was set as the main outcome. Data on survival of the women in the cohort were obtained by cross-linking the unique individual’s identification numbers assigned to the Swedish citizens with the Total Population Register of the Swedish Tax Agency as of 25 November 2018. A secondary outcome of safety of COS was the incidence of moderate or severe symptomatic ovarian hyperstimulation syndrome (OHSS) requiring either outpatient or inpatient management, respectively (Royal College of Obstetriacians and Gynaecologists, 2016).
Statistical analyses
Demographical (age, parity, partnership) and clinical (AMH, AFC, ER status of BC) characteristics of the patients were compared using Student’s t-tests and Mann–Whitney U-tests (for non-normally distributed variables) for continuous data and Chi square tests for categorical data.
For estimating the associations between FP treatment protocols and outcomes, complete-case linear regression models were used. The regression models were adjusted for age at FP (assuming a linear association for the effect of age). As a rule, all potential confounders included in the regression modeling in our study were selected a priori based on their known association with both the exposure (FP or not) and the outcome (number of oocytes and embryos cryopreserved).
Kaplan–Meier estimates of all-cause survival and log rank tests were used to compare survival for women who underwent FP versus those who did not and for women who underwent COS with or without letrozole, respectively. Survival was estimated from the date of FP counseling until the date of death or administrative censoring (24 November 2018), whichever came first. All statistical tests were two-sided with a significance level set to 0.05. The analyses were performed using STATA version 15.9 (StataCorp, 2017; Stata Statistical Software: Release 15. College Station).
Results
Patients’ characteristics and FP methods elected
A total of 610 women received FP counseling indicated by a BC diagnosis during the study period. Of 468 women who proceeded to FP treatment, 41% aimed at oocyte banking, 28% at embryo banking, 15% at combined oocyte and embryo banking and 14% have cryopreserved ovarian tissue (Fig. 1). In a few cases, combination of FP methods was practiced. Table I presents baseline characteristics of the women in the cohort. Women who underwent FP were significantly younger and had lower parity than those who did not proceed to FP treatments. FP treatment was not recommended or not offered after counseling to 42 women, most commonly because treatment initiation did not allow time for FP or the women were not eligible to tax-funded FP treatments (i.e. age over 40 years or already having two or more children). Additionally, 42 women were planned for FP treatment but finally decided not to undergo the procedures, as they found it to be additional pressure in an already difficult situation. For 29 cases, the reason for not pursuing FP was not recorded. Additionally, 29 women initiated stimulation cycles that were canceled (Fig. 1, upper branch).
Figure 1.
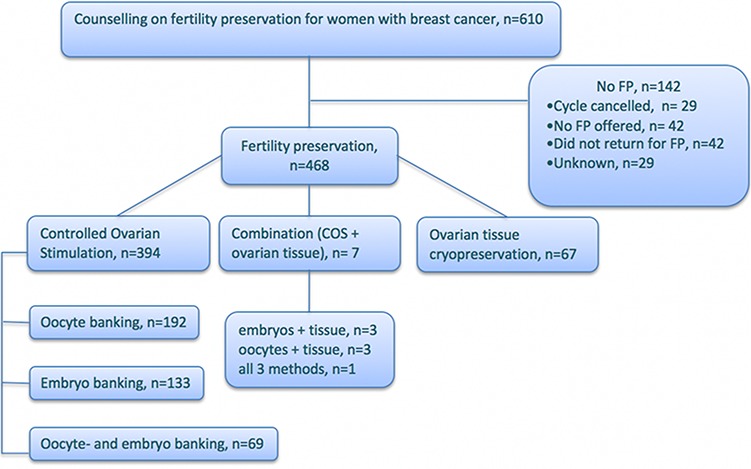
A flowchart of women with BC who were counseled on FP options at six Swedish university hospitals between 1 January 1995 and 30 June 2017.
Table I.
Baseline characteristics of 610 women counseled on FP.
| COS treatments in antagonist protocols. n = 380 | Letrozole-based protocols. n = 224 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | Underwent FP. n = 468 (%) | No FP. n = 142 (%) | P-value * | Letrozole (%) | No Letrozole (%) | P-value * | Conventional start (%) | Random start (%) | P-value * | GnRH-agonist trigger (%) | hCG trigger (%) | P-value * |
| Number of cycles | 224 | 156 | 179 | 201 | 96 | 128 | ||||||
| Age | 32.5 | 34.5 | <0.001 | 32.6 | 32.6 | 0.82 | 32.9 | 32.4 | 0.28 | 32.6 | 32.6 | 0.94 |
| Range | 21–42 | 21–43 | 21–42 | 21–42 | 21–42 | 22–42 | 23–40 | 21–42 | ||||
| Parity | 0.3 | 0.7 | 0.21 | 0.33 | 0.29 | 0.28 | 0.28 | 0.375 | ||||
| Range | 0–4 | 0–2 | <0.001 | 0–2 | 0–4 | 0.03 | 0–4 | 0–2 | 0.83 | 0–2 | 0–4 | 0.24 |
| Unknown | 2 | |||||||||||
| BMI | 23.7 | 24.8 | 23.7 | 23.9 | 23.8 | 23.8 | 24.0 | 23.4 | ||||
| Range | 16.2–46.5 | 19–47.2 | 0.136 | 16.2–42.8 | 17.4–46.5 | 0.79 | 16.2–46.5 | 16.4–42.8 | 0.92 | 17–42.8 | 16.2–41 | 0.3 |
| Unknown | 133 | 99 | 54 | 25 | 23 | 56 | 4 | 50 | ||||
| Partner | ||||||||||||
| Yes | 312 (67) | 67 (47) | 154 (69) | 112 (72) | 124 (69) | 142 (71) | 63 (66) | 91 (71) | ||||
| No | 154 (33) | 39 (27) | 0.46 | 69 (39) | 44 (28) | 0.007 | 55 (31) | 59 (29) | 0.60 | 32 (33) | 37 (29) | 0.38 |
| Unknown | 2 | 36 | 1 | 1 | 1 | |||||||
| AFC | 17.5 | 14.6 | 17.4 | 17.5 | 16.4 | 18.3 | 19.9 | 14.8 | ||||
| Range | 2–60 | 5–40 | 0.09 | 3–40 | 2–40 | 0.96 | 2–40 | 3–40 | 0.14 | 4–40 | 3–40 | 0.0004 |
| Unknown | 287 | 112 | 100 | 117 | 101 | 116 | 34 | 66 | ||||
| AMH | 2.8 | 2.4 | 2.9 | 2.8 | 2.7 | 3.0 | 3.4 | 2.4 | ||||
| Range | 0.1–18.8 | 0.1–13.2 | 0.42 | 0.1–18.8 | 0.2–5.1 | 0.93 | 0.23–18.8 | 0.1–15 | 0.58 | 0.16–18.8 | 0.1–15 | 0.03 |
| Unknown | 289 | 105 | 76 | 138 | 105 | 109 | 28 | 48 | ||||
| ER-status | ||||||||||||
| Pos | 246 (53) | 59 (41.5) | 122 (54) | 75 (48) | 112 (63) | 85 (42.3) | 75 (78) | 47 (37) | ||||
| Neg | 112 (24) | 24 (17) | 0.67 | 29 (13) | 54 (35) | <0.001 | 42 (23) | 41 (20.4) | 0.34 | 14 (15) | 15 (12) | 0.19 |
| Unknown | 110 (23) | 59 (41.5) | 73 (33) | 27 (17) | 25 (14) | 75 (37.3) | 7 (7) | 66 (51) | ||||
AFC indicates antral follicle count; AMH, Anti-Müllerian hormone (ng/mL); ER, estrogen receptor.
Data on women that underwent FP through COS using GnRH antagonists are summarized according to the protocol used.
*Students’ t-test, Mann–Whitney test or Chi square test.
Of 401 COS cycles performed, 21 did not use a GnRH antagonist and they were excluded.
In the remaining 380 COS cycles using a GnRH antagonist investigated, concurrent letrozole was used in 59% of the cases (n = 224) versus not (n = 156). Random start was practiced in 201 cycles compared with 179 cases of conventional start.
Letrozole versus no-letrozole protocols
The majority of women with ER-positive BC underwent COS with letrozole. Of 380 COS cycles included in the analysis, ER status was unknown in 100 cases at the time of counseling and 73% of these women were stimulated with letrozole. The total dose of gonadotropins used and the total number of days required for COS were significantly higher in letrozole cycles (P < 0.05), while the number of total and mature oocytes retrieved as well as the number of oocytes and embryos cryopreserved were similar between the groups (Table II).
Table II.
Comparison of outcomes of COS with and without letrozole.
| COS treatments in antagonist protocols, n = 380 | Letrozole-based protocols, n = 224 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cycle outcomes | Letrozole | No Letrozole | P-value* | Conventional start | Random start | P-value* | GnRH-agonist trigger | hCG trigger | P-value* |
| Number of cycles | 224 | 156 | 179 | 201 | 96 | 128 | |||
| Days of ovarian stimulation | 10.5 | 11.0 | 10.5 | 10.9 | 0.053 | 10.7 | 10.4 | ||
| Range | 5–18 | 7–17 | 0.03 | 5–17 | 5–18 | 6–18 | 5–18 | 0.34 | |
| Unknown | 2 | 0 | 1 | 1 | 0 | 2 | |||
| Total dose of gonadotropins | 2330 | 2064 | 0.004 | 2066 | 2359 | 0.001 | 2379 | 2294 | 0.51 |
| (IU) | 500–6750 | 800–5400 | 500–5550 | 750–6750 | 1050–6750 | 500–5850 | |||
| Range | |||||||||
| Oocytes retrieved | 12.32 | 12.21 | 0.917 | 12.3 | 12.2 | 0.79 | 13.66 | 11.32 | 0.027 |
| Range | 0–55 | 0–52 | 0–55 | 0–52 | 0–55 | 0–44 | |||
| Mature oocytes a | 8.45 | 8.52 | 0.941 | 9.1 | 7.9 | 0.197 | 9.3 | 7.6 | |
| Range | 0–46 | 0–24 | 0–44 | 0–23 | 0–44 | 0–26 | 0.14 | ||
| Unknown | 5 | 8 | 7 | 6 | 1 | 4 | |||
| Mature oocytes/total oocytes ratio a | 0.71 | 0.79 | 0.037 | 0.76 | 0.70 | 0.078 | 0.74 | 0.68 | 0.106 |
| OSI | 6.48 | 7.19 | 0.193 | 7.29 | 6.31 | 0.045 | 6.65 | 6.35 | 0.693 |
| Range | 0–45.8 | 0–31.1 | 0–45.83 | 0–22.88 | 0–26 | ||||
| Oocytes cryopreserved a | 9.7 | 10.0 | 0.81 | 10.6 | 8.97 | 0.067 | 10.4 | 9.1 | 0.252 |
| Range | 0–40 | 1–27 | 0–40 | 0–24 | 0–40 | 0–28 | |||
| Fertilization rate (%) b | 0.64 | 0.63 | 0.89 | 0.66 | 0.62 | 0.276 | 0.69 | 0.60 | 0.28 |
| Embryos cryopreserved b | 4.0 | 5.3 | 0.075 | 4.75 | 4.78 | 0.922 | 5.5 | 3.0 | 0.003 |
| Range | 0–11 | 0–29 | 0–16 | 0–29 | 0–11 | 0–9 | |||
In COS with letrozole, treatment outcomes were compared between those using GnRH agonist versus hCG trigger.
Note: data are presented as mean (range).
OSI indicates ovarian sensitivity index, the ration between oocyte yield and the dose of gonadotropins administered.
All comparisons were adjusted for age with linear regression models.
aIn total, 130 cycles in Letrozole group versus 58 cycles in No Letrozole group, 93 cycles in Conventional group versus 95 cycles in Random group and 62 cycles in GnRH agonist group versus 68 cycles in the hCG group were aimed for oocyte cryopreservation only.
bIn total, 53 cycles in Letrozole group versus 71 cycles in No Letrozole group, 59 cycles in Conventional group versus 65 cycles in Random group and 21 cycles in GnRH-agonist group versus 32 cycles in the hCG group were aimed for embryo cryopreservation only.
Final oocyte maturation was induced with GnRHa in 43% of letrozole cycles. Women in this group had significantly higher AFC and AMH than women in the group with hCG trigger. Maturation rate could be calculated only for the cycles that were aimed at oocyte cryopreservation (n = 188) and was somewhat lower in cycles with concurrent use of letrozole (P = 0.037). Use of GnRHa was associated with higher numbers of oocytes retrieved (P < 0.05) and embryos cryopreserved (P < 0.005; Table II). In a sub-analysis adjusted for AFC and AMH (complete data for 52 women in GnRHa and 57 women in hCG group), similar numbers of retrieved oocytes were found in both groups but a significantly higher number of cryopreserved embryos was obtained in the GnRHa group (P = 0.04).
There were no reported cases of moderate or severe OHSS requiring either outpatient or inpatient healthcare.
Random versus conventional start
Women undergoing random-start COS required higher total dose of gonadotropins (P < 0.001), while the number of retrieved oocytes and the number of cryopreserved oocytes and embryos were similar between the groups (Table II). The start of COS in relation to the menstrual phase was known in 105 of 201 random-start cycles: 46% started in late follicular and 54% in luteal phase. Among those who aimed at oocyte banking only, maturity rate was higher in luteal phase-start cycles (P < 0.05), while there was no difference in other documented outcomes.
Follow-up: survival
The mean follow-up time of the whole cohort was 6.3 years (median: 5.14 years; range: 3 months–23.6 years). The 5-year survival proportion was 0.94 (95% CI: 0.91–0.96) for women who had not undergone FP and 0.94 (95% CI: 0.87–0.97) for women in the FP group (Fig. 2). Across the entire follow-up, survival did not differ significantly between the women who pursued FP versus those who did not (P = 0.53). Comparing the group of women who underwent FP with COS to the non-COS group (i.e. women who did not undergo FP or had FP treatment without COS), the 5-year survival was 0.95 (95% CI: 0.92–0.97) and 0.92 (95% CI: 0.87–0.95), respectively, with no difference in survival across the entire follow-up (P = 0.16). Among women who underwent COS with versus without concurrent use of letrozole, the 5-year survival was 0.96 (95%CI: 0.91–0.98) versus 0.95 (95% CI: 0.90–0.97), without any significant difference across the entire follow-up (P = 0.4). As the majority of women in the letrozole group had ER-positive BC, we additionally estimated a series of Cox regression models adjusted for letrozole (Yes/No) alone and subsequently adjusted for ER-positivity (Yes/No) among those with complete information on ER status (280 of 380 cases). There was no significant difference in OS between women in the letrozole group (hazard ratio: 0.96; 95%CI: 0.27–3.34), compared with women who underwent COS without letrozole (reference).
Figure 2.
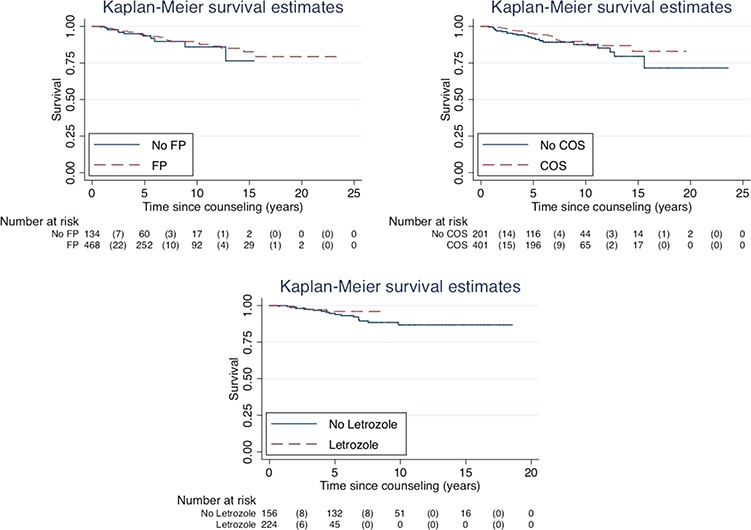
Overall survival in women with breast cancer who underwent FP at six Swedish hospitals from 1995 to 2017 and in control women unexposed to FP. Left upper panel: a comparison of overall survival with Kaplan–Meier estimates in women who underwent FP with or without hormonal stimulation and that in women unexposed to fertility preservation (n = 610). Right panel: a comparison of overall survival with Kaplan–Meier estimates in women who underwent FP with COS and in women who were unexposed top COS (n = 610). Left lower panel: a comparison of OS with Kaplan–Meier estimates in women who underwent COS with (Letrozole) or without (No Letrozole) concurrent use of letrozole (n = 380). The use of letrozole alongside gonadotropins in cycles aimed at FP was implemented in Sweden in 2010.
Follow-up: return rates and reproductive outcome
From the total FP cohort of 468 women, 21% (n = 99) returned after completed treatment for BC to use cryopreserved specimens or with a wish of new fertility counseling (Fig. 3). A total of 32 women proceeded to thawing of oocytes and/or embryos and 10 of them delivered at least one baby through FP. Use of gestational carriers is not allowed in Sweden; therefore, all the women have undergone embryo transfers to themselves. Two women proceeded to re-transplantation of ovarian tissue and one live birth has been achieved. Altogether, 26% of 99 women have delivered at least one baby by the time of this report, both spontaneous and ART pregnancies included. Among women who had undergone FP but have not returned for a new counseling or treatment, 5% (19 of 369) have delivered at least one baby. Similarly, 5% (n = 7) of 142 women who had been counseled but had not proceeded to FP, delivered at least one baby by the time of this report.
Figure 3.
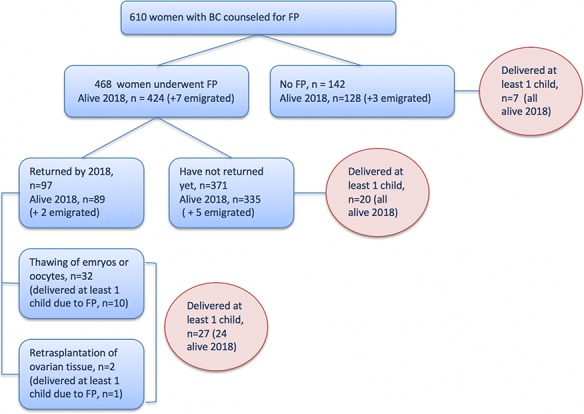
A flowchart of reproductive outcomes among women with BC who were counseled on FP options at six Swedish university hospitals between 1995 and June 2017.
Discussion
To the best of our knowledge, this study is the first prospective multicenter study with a population size large enough to allow comparisons of different approaches that have been proposed as improvements in COS for FP in women with BC and that reports on a long-time follow-up.
Our results indicate that concurrent use of letrozole, aiming to maintain low systemic ER levels during COS, results in a similar number of oocytes and embryos cryopreserved, compared with conventional COS protocols. Inconsistency in results of the previous studies indicating that addition of letrozole did not impact (Oktay et al., 2006; Checa Vizcaino et al., 2012), increased (Turan et al., 2018) or decreased the oocyte yields (Domingo et al., 2012; Revelli et al., 2013) may depend on their smaller size and differences in the COS protocols. A systematic review by Rodgers et al. (2017) concluded that the addition of letrozole does not seem to decrease the total oocyte yield but acknowledged the limitations of the existing literature on COS for FP in women with BC. Our findings support that conclusion.
Utilization of GnRHa trigger in women with cancer undergoing COS with letrozole has in the previous studies been associated with significantly higher yield of mature oocytes (Oktay et al., 2010; Reddy et al., 2014; Pereira et al., 2017) and embryos (Pereira et al., 2017), demonstrating additional benefits to the significant reduction of the risk of OHSS in women with cancer undergoing FP (Oktay et al., 2010). In our study, the use of GnRHa was not randomly allocated or systematic and the choice of GnRHa versus hCG trigger was left to the discretion of the physician planning the egg retrieval at the latest COS monitoring appointment. However, a significantly higher number of retrieved oocytes and cryopreserved embryos were found in the group that received oocyte maturation trigger with GnRHa after adjustment for age. Additionally, in a sub-analysis further adjusted for AFC and AMH (complete data on these parameters were not available for the whole cohort), the difference between the groups was still significant for the number of embryos obtained and cryopreserved (P = 0.04).
Random-start COS has been discussed as an option for FP in patients requiring urgent cancer treatment, as in contrast to the conventional start protocols it provides the possibility to initiate stimulation regardless of the menstrual cycle day. In a systematic review by Danis et al. (2017), random start of COS in the setting of FP indicated by cancer diagnosis was associated with a shorter interval between ovarian stimulation and oocyte retrieval, while the yield of mature oocytes and cryopreserved embryos was comparable with conventional stimulation protocols. Our results are consistent with the finding of comparable yields of oocytes and embryos, while the length of COS in our study was similar between the conventional and random-start groups.
Data on reproductive outcomes reported after FP to date have been limited by small patient numbers, relatively short duration of follow-up and its retrospective nature. Druckenmiller et al. (2016) reported return rate of 6% with 44% live birth rate among 176 women with cancer who underwent oocyte cryopreservation over a 9-year period. Oktay et al. (2015) found that of 131 women with BC who underwent embryo cryopreservation with concurrent use of letrozole, 25% returned to use embryos with 45% live birth rate per embryo and 5.25 years as the median time from cryopreservation to return. In a review, Moravek et al. (2018) reported that 10.3% of 204 FP patients returned to use cryopreserved specimen and 57.1% of them had live birth. In all the aforementioned studies, several women required gestational carriers—a procedure that is not allowed in Sweden. Our current study found that 7% (34/468) of women with a previous BC returned to use cryopreserved specimens, and of those, 32% (11/34) obtained at least one live birth. In a previous single center report including 852 females with all cancer diagnoses combined, we found a live birth rate of 21% by using cryopreserved embryos or oocytes after a mean follow-up of 3.9 years (Rodriguez-Wallberg et al., 2019b).
Knowledge on the oncologic safety of FP in women with BC is to date based on a limited number of observational studies. In a systemic review by Rodgers et al., (2017), including 464 women’s data on BC mortality and recurrence from four different studies, there was no evidence of decline of relapse-free survival among women who underwent COS with letrozole co-administration compared with women who did not undergo FP. In a study that used the Stockholm regional data from the Swedish National Breast Cancer Quality Register, no increased risk of BC recurrence was found in women that had undergone COS for FP when compared with age-matched controls that had not undergone FP, after a mean follow-up time of 6.6 years (Rodriguez-Wallberg et al., 2018).
In the current study, the data reported from six regional university hospitals providing standardized FP to women with BC did not reveal any statistically significant differences in survival neither among women who underwent FP versus no FP nor among those with COS versus no COS. Even though adjustment for potential confounders, such as tumor characteristics, year of diagnosis and treatment details, could not be performed, our findings support the thesis that COS for FP in women with BC is a safe procedure.
The fact that no patient has required hospitalization for OHSS, which could potentially delay the initiation of a planned chemotherapy treatment, is also reassuring. Our data indicate that the risk for severe OHSS might be kept low in the population of BC patients undergoing FP by COS, particularly when the use of GnRHa trigger is implemented in the clinical routine (Oktay et al., 2010).
To further test the theoretical model of improving long-term safety of ovarian stimulation in women with BC by reducing estrogen levels with co-administration of letrozole, a longer follow-up and investigation of BC relapse would be of interest (Oktay et al., 2006; Checa Vizcaino et al., 2012; Domingo et al., 2012; Revelli et al., 2013). In our study, similar survival was found among women who underwent COS with concurrent use of letrozole compared with standard COS.
The main strength of this study is its prospective design, national coverage and its large sample size. Due to the hormone responsiveness of BC in many cases, randomizing the women to letrozole versus non-letrozole protocol would be considered unethical. Additional strengths include the fact that FP in Sweden is performed via a healthcare program with full-population coverage and equal access to care, limiting the risk of selection bias.
A limitation of our study is that adjustment for potential confounders including tumor characteristics and specific cancer therapy could not be performed for the analysis of OS between women with and without a history of FP. For our primary outcome, defined as cryopreserved oocytes and embryos, these missing parameters were of less importance, as they could not affect the outcome, and therefore, by definition, would not confound the association under investigation (Rothman et al., 2008). Additional differences and similarities in clinical and demographic baseline characteristics were presented and, if applicable, adjusted for. Another limitation is the plausibility that data on pregnancy and delivery rates may be incomplete, as some patients might have moved from their healthcare region or may have searched fertility or pregnancy care abroad.
Aiming to increase the knowledge on safety of FP in this population, future studies should take these parameters in consideration and include recurrence rate of BC as an important outcome. Risk for OHSS and other short-term morbidities that might be associated with COS should also be further studied.
Conclusion
The results of this study support the practice of COS with co-administration of letrozole as safe for women with BC without affecting the number of cryopreserved oocytes and/or embryos obtained. The use of GnRHa trigger in letrozole cycles should be preferred to reduce the risk of OHSS and it may also improve the yield of oocytes and embryos. Random-start COS resulted in similar number of cryopreserved oocytes and embryos when compared with the conventional start and should be considered as a valid option for starting ovarian stimulation in urgent settings. The results of our study support the premise that FP in eligible women with BC is both safe and efficacious. Further research, including population-based data from nationwide registers with long-term oncologic and reproductive outcomes, would help to provide more evidence on this complex and important clinical area.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Acknowledgements
The authors thank all the personnel contributing to the fertility preservation programs of the Swedish university hospitals and the Swedish Consortium of Fertility Preservation. The authors are also grateful to Ferring Läkemedel AB, Sweden, for economical support to data analysis and statistics.
Authors’ roles
K.A.R.W, A.M. and S.E. designed the study. A.M., I.W., M.L.K., M.L., E.N., A.T.K., P.Z. and K.A.R.W. collected and assembled data with A.M. as the main responsible. A.M. drafted the first version of the manuscript. A.M., S.E., I.W., M.L.K., M.L., E.N, A.T.K., P.Z., J.B. and K.A.R.W. contributed to data analysis and interpretation, revised the manuscript and approved the final submitted version. K.A.R.W. provided financial and administrative support.
Funding
Swedish Cancer Society; Stockholm County Council; Percy Falk Stiftelsen; Radiumhemmets Forskningsfonder; The Swedish Breast Cancer Association and Karolinska Institutet to K.A.R.W. There are no competing interests to report.
Conflict of interest
J.B. reports grants from Amgen, AstraZeneca, Pfizer, Roche, Sanofi-Aventis and Merck, outside the submitted work, and payment from UpToDate to Asklepios Medicine HB for a chapter on breast cancer prediction and prognostication. All the other authors have no competing interests to report.
References
- Alvarez M, Sole M, Devesa M, Fabregas R, Boada M, Tur R, Coroleu B, Veiga A, Barri PN. Live birth using vitrified—warmed oocytes in invasive ovarian cancer: case report and literature review. Reprod Biomed Online 2014;28:663–668. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Brewster DH, Wood R, Nowell S, Fischbacher C, Kelsey TW, Wallace WHB. The impact of cancer on subsequent chance of pregnancy: a population-based analysis. Hum Reprod 2018;33:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASCO Recommendations on fertility preservation in cancer patients: guideline summary. J Oncol Pract 2006;2:143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol 2008;26:2630–2635. [DOI] [PubMed] [Google Scholar]
- Cakmak H, Katz A, Cedars MI, Rosen MP. Effective method for emergency fertility preservation: random-start controlled ovarian stimulation. Fertil Steril 2013;100:1673–1680. [DOI] [PubMed] [Google Scholar]
- Checa Vizcaino MA, Corchado AR, Cuadri ME, Comadran MG, Brassesco M, Carreras R. The effects of letrozole on ovarian stimulation for fertility preservation in cancer-affected women. Reprod Biomed Online 2012;24:606–610. [DOI] [PubMed] [Google Scholar]
- Danis RB, Pereira N, Elias RT. Random start ovarian stimulation for oocyte or embryo cryopreservation in women desiring fertility preservation prior to gonadotoxic cancer therapy. Curr Pharm Biotechnol 2017;18:609–613. [DOI] [PubMed] [Google Scholar]
- Domingo J, Guillen V, Ayllon Y, Martinez M, Munoz E, Pellicer A, Garcia-Velasco JA. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril 2012;97:930–934. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, Andersen CY. Fertility preservation for age-related fertility decline. Lancet 2015;385:506–507. [DOI] [PubMed] [Google Scholar]
- Druckenmiller S, Goldman KN, Labella PA, Fino ME, Bazzocchi A, Noyes N. Successful oocyte cryopreservation in reproductive-aged cancer survivors. Obstet Gynecol 2016;127:474–480. [DOI] [PubMed] [Google Scholar]
- Kim J, Turan V, Oktay K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab 2016;101:1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, Melisko ME, Cedars MI, Rosen MP. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer 2012;118:1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moravek MB, Confino R, Smith KN, Kazer RR, Klock SC, Lawson AK, Gradishar WJ, Pavone ME. Long-term outcomes in cancer patients who did or did not pursue fertility preservation. Fertil Steril 2018;109:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 2003;18:90–95. [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 2005;23:4347–4353. [DOI] [PubMed] [Google Scholar]
- Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, Wallace WH, Wang ET, Loren AW. Fertility preservation in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2018;36:1994–2001. [DOI] [PubMed] [Google Scholar]
- Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, Bang H. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab 2006;91:3885–3890. [DOI] [PubMed] [Google Scholar]
- Oktay K, Turan V, Bedoschi G, Pacheco FS, Moy F. Fertility preservation success subsequent to concurrent aromatase inhibitor treatment and ovarian stimulation in women with breast cancer. J Clin Oncol 2015;33:2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay K, Turkcuoglu I, Rodriguez-Wallberg KA. GnRH agonist trigger for women with breast cancer undergoing fertility preservation by aromatase inhibitor/FSH stimulation. Reprod Biomed Online 2010;20:783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccatori FA, Azim HA Jr, Orecchia R, Hoekstra HJ, Pavlidis N, Kesic V, Pentheroudakis G, ESMO Guidelines Working Group . Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi160–vi170. [DOI] [PubMed] [Google Scholar]
- Pereira N, Hancock K, Cordeiro CN, Lekovich JP, Schattman GL, Rosenwaks Z. Comparison of ovarian stimulation response in patients with breast cancer undergoing ovarian stimulation with letrozole and gonadotropins to patients undergoing ovarian stimulation with gonadotropins alone for elective cryopreservation of oocytes. Gynecol Endocrinol 2016;32:823–826. [DOI] [PubMed] [Google Scholar]
- Pereira N, Kelly AG, Stone LD, Witzke JD, Lekovich JP, Elias RT, Schattman GL, Rosenwaks Z. Gonadotropin-releasing hormone agonist trigger increases the number of oocytes and embryos available for cryopreservation in cancer patients undergoing ovarian stimulation for fertility preservation. Fertil Steril 2017;108:532–538. [DOI] [PubMed] [Google Scholar]
- Reddy J, Turan V, Bedoschi G, Moy F, Oktay K. Triggering final oocyte maturation with gonadotropin-releasing hormone agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation: an extended experience. J Assist Reprod Genet 2014;31:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelli A, Porcu E, Levi Setti PE, Delle Piane L, Merlo DF, Anserini P. Is letrozole needed for controlled ovarian stimulation in patients with estrogen receptor-positive breast cancer? Gynecol Endocrinol 2013;29:993–996. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Reid GD, Koch J, Deans R, Ledger WL, Friedlander M, Gilchrist RB, Walters KA, Abbott JA. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Hum Reprod 2017;32:1033–1045. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Eloranta S, Krawiec K, Lissmats A, Bergh J, Liljegren A. Safety of fertility preservation in breast cancer patients in a register-based matched cohort study. Breast Cancer Res Treat 2018;167:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Borgstrom B, Petersen C, Thurin-Kjellberg A, Morse H, Giwercman A, Jarfelt M, Work Group UNGA (YOUNG) for the Swedish Association of Local Authorities and Regions . National guidelines and multilingual age-adapted patient brochures and videos as decision aids for fertility preservation (FP) of children and teenagers with cancer—a multidisciplinary effort to improve children’s information and access to FP in Sweden. Acta Obstet Gynecol Scand 2019a;98:679–680. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Wallberg KA, Marklund A, Lundberg F, Wikander I, Milenkovic M, Anastacio A, Sergouniotis F, Wanggren K, Ekengren J, Lind T et al. A prospective study of women and girls undergoing fertility preservation due to oncologic and non-oncologic indications in Sweden-trends in patients’ choices and benefit of the chosen methods after long-term follow up. Acta Obstet Gynecol Scand 2019b;98:604–615. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd edn. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008. [Google Scholar]
- Royal College of Obstetriacians and Gynaecologists The management of Ovarian Hyperstimulation Syndrome. London: RCOG; 2016. (https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_5_ohss.pdf). Accessed 2019 Aug 1. [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- Sonmezer M, Turkcuoglu I, Coskun U, Oktay K. Random-start controlled ovarian hyperstimulation for emergency fertility preservation in letrozole cycles. Fertil Steril 2011;95:e2129–e2111. [DOI] [PubMed] [Google Scholar]
- StataCorp Stata statistical software: release 15. 2017. College Station TSL.
- Stensheim H, Cvancarova M, Moller B, Fossa SD. Pregnancy after adolescent and adult cancer: a population-based matched cohort study. Int J Cancer 2011;129:1225–1236. [DOI] [PubMed] [Google Scholar]
- Turan V, Bedoschi G, Emirdar V, Moy F, Oktay K. Ovarian stimulation in patients with cancer: impact of letrozole and BRCA mutations on fertility preservation cycle outcomes. Reprod Sci 2018;25:26–32. [DOI] [PubMed] [Google Scholar]
- United States National Institutes of Health SEER Cancer Stat Facts: Female Breast Cancer. National Cancer Institute. Bethesda, MD: Available at: https://seer.cancer.gov/statfacts/html/breast.html. Accessed 2019 July 31. [Google Scholar]
- von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, Strowitzki T. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertil Steril 2009;92:1360–1365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


