Abstract
STUDY QUESTION
Has the number of preimplantation genetic testing (PGT) cycles in the UK and USA changed between 2014 and 2016?
SUMMARY ANSWER
From 2014 to 2016, the number of PGT cycles in the UK has remained the same at just under 2% but in the USA has increased from 13% to 27%.
WHAT IS KNOWN ALREADY
PGT was introduced as a treatment option for couples at risk of transmitting a known genetic or chromosomal abnormality to their child. This technology has also been applied as an embryo selection tool in the hope of increasing live birth rates per transfer. ART cycles are monitored in the UK by the Human Fertilisation and Embryology Authority (HFEA) and in the USA by the Society for Assisted Reproductive Technology (SART). Globally, data are monitored via the ESHRE PGT Consortium.
STUDY DESIGN, SIZE, DURATION
This cross-sectional study used the HFEA and SART databases to analyse PGT cycle data and make comparisons with IVF data to examine the success of and changes in patient treatment pathways. Both data sets were analysed from 2014 to 2016. The UK data included 3385 PGT cycles and the USA data included 94 935 PGT cycles.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Following an extensive review of both databases, filters were applied to analyse the data. An assessment of limitations of each database was also undertaken, taking into account data collection by the ESHRE PGT Consortium. In the UK and USA, the publicly available information from these datasets cannot be separated into different indications.
MAIN RESULTS AND THE ROLE OF CHANCE
The proportion of PGT cycles as a total of ART procedures has remained the same in the UK but increased annually in the USA from 13% to 27%. Between 2014 and 2016 inclusive, 3385 PGT cycles have been performed in the UK, resulting in 1074 PGT babies being born. In the USA 94 935 PGT cycles have been performed, resulting in 26 822 babies being born. This gave a success rate per egg collection for PGT of 32% for the UK and 28% for the USA. Analysis of the data by maternal age shows very different patient populations between the UK and USA. These differences may be related to the way PGT is funded in the UK and USA and the lack of HFEA support for PGT for aneuploidy.
LIMITATIONS, REASONS FOR CAUTION
Data reported by the HFEA and SART have different limitations. As undertaken by the ESHRE PGT Consortium, both data sets should separate PGT data by indication. Although the HFEA collects data from all IVF clinics in the UK, SART data only represent 83% of clinics in the USA.
WIDER IMPLICATIONS OF THE FINDINGS
Worldwide, a consistent reporting scheme is required in which success rates can convey the effectiveness of PGT approaches for all indications.
STUDY FUNDING/COMPETING INTEREST(S)
No specific funding was obtained and there are no competing interests to declare that are directly related to this project. Joyce Harper is the director of the Embryology and PGD Academy, which offers education in these fields.
Keywords: preimplantation genetic testing, PGS, PGD, ART, reproductive genetics, UK, USA
Introduction
Since the birth of Louise Brown, the world’s first ‘test-tube baby’ in 1978 (Steptoe and Edwards, 1978), the use of ART has increased dramatically. With over 6 million births worldwide, this global industry is now estimated to be worth £20–30 billion (Weinerman, 2018).
Preimplantation genetic testing (PGT) is an ART procedure that is used to investigate the genetic make-up of embryos produced by IVF (Zhang et al., 2016). PGT-M (monogenic disease) and PGT-SR (structural rearrangements) are treatment options for couples whose children are at high risk of inheriting a known genetic or chromosomal abnormality (Harper and SenGupta, 2012). Although most couples undergoing PGT-M tend to be fertile, success rates are similar to those undergoing routine IVF (De Rycke et al., 2017). Patients undergoing PGT-SR are generally phenotypically normal, but often their infertility issues make them aware they are balanced carriers of chromosomal rearrangements.
PGT-A (aneuploidy) is an IVF adjunct and its use is controversial (Harper et al., 2017; Wilkinson et al., 2019). PGT-A is carried out for selection of a euploid embryo, which will be used for transfer in the hope of increasing live birth rates (LBRs) (Brezina and Kutteh, 2015). PGT-A has been applied to women of advanced maternal age, couples who have had repeated miscarriages but have a normal karyotype, those with repeated implantation failure, egg donors and good prognosis patients (Mastenbroek et al., 2011).
The first reports of PGT world data were published by Harper and Handyside (1994) and Harper (1996). This led to establishment of the ESHRE PGT Consortium, which has been reporting annual PGT data since 1997 (Harper et al., 2012). The Consortium reports the IVF and biopsy method, analysis details, embryology and clinical outcomes per egg collection and per embryo transfer (ET) procedure and collects detailed data on individual PGT cycles separated into PGT-A, PGT-M, PGT-SR, PGT using sexing only and PGT for social sexing. The outcome of individual embryos following an ART treatment can be tracked from start to finish in the newer databases (De Rycke et al., 2017). This gives a very rich picture of the use of PGT but the main limitation is that the majority of clinics worldwide do not report data to the Consortium. It is therefore not possible to work out the global use of PGT.
Since 1991 the Human Fertilisation and Embryology Authority (HFEA), an independent regulator of fertility treatments in the UK, has provided information to the public about licensed fertility treatments (HFEA, 2017). All UK clinics have a statutory duty to record and submit data to the HFEA. The HFEA data are reported as LBR per treatment cycle (PTC) and per embryo transferred (PET) for fresh and frozen cycles. In their annual report, the HFEA gives detailed data of PGT-M and PGT-SR cycles, but since the HFEA does not recognize PGT-A as an evidence-based procedure and it is considered an IVF add-on, PGT-A cycles are not reported separately but instead are included in the overall IVF data.
In the USA, the American Society for Assisted Reproductive Technology (SART) provides reports on IVF and PGT success rates. Unlike the HFEA, SART only requires data from SART-registered clinics, which includes 375 clinics, accounting for 83% of all American clinics (Toner et al., 2016). SART has continued to review the data, with detailed reports being completed annually since 1988. PGT data are reported per cycle-started, per egg collection and per embryo transfer procedure. PGT-A, PGT-M and SR data cannot be separated.
Data reported by the ESHRE PGT Consortium, HFEA and SART all have limitations. In this study we have analysed the HFEA and SART IVF and PGT data in detail to determine if PGT has increased, identify key information regarding PGT (including LBR) and suggest ways of improving PGT data collection.
Materials and Methods
Data collection
The two databases were obtained from the HFEA and SART and data analysed from 2014 to 2016. Both databases were in an electronic format and allowed a treatment cycle for IVF and PGT to be followed up to a live birth occurrence. Only cycles where the patients used their own eggs were analysed.
The fields that were available for both databases were assessed, and the appropriate filters were chosen. Filters were applied to both sets of data to undertake the analysis. In cases where there were spurious results, data were excluded from analysis and highlighted where necessary.
HFEA data collection
The HFEA presents treatment data as cycles. PGT-A, PGT-M/SR and IVF treatment cycles could be separated to allow a better understanding of the demographics of each ART technique. These treatments could be further separated into fresh and frozen cycles. The HFEA data include patient age at treatment, the number of treatment cycles and outcomes such as LBR PET and LBR PTC.
The HFEA PGT databases are fixed and closed. The full database that was used is not currently publicly available. In the HFEA reports, they separate out the PGT-M/SR data but leave the PGT-A data with the IVF data. For this study, the PGT-A data had to be extracted from the IVF data. The HFEA sent the PGT-M/SR data to the authors on 6 January 2018 and the PGT-A data on 22 January 2018.
In order to collect relevant data from the HFEA database, the ‘Year of Treatment’ field was filtered to separate out the individual years in which treatment cycle data were available. Once separated, data collection for each year was grouped into the following categories: ‘Fresh IVF’, ‘Frozen IVF’, ‘Fresh PGT’ and ‘Frozen PGT’.
Throughout this report the success rates are reported in different ways (Table I). The LBRs were measured in line with the HFEA annual report by using LBR PTC and LBR PET.
Table I.
Live birth rate calculations used to assess the HFEA IVF and PGT data.
| Live birth rate calculations | |
| Live birth rate per treatment cycle (LBR PTC) | The number of live birth occurrences (a multiple birth is counted as a single birth occurrence) divided by the sum of treatment cycles multiplied by 100. |
| Live birth rate (LBR) per cycle that reached a successful egg collection (PEC) | The number of live birth occurrences (a multiple birth is counted as a single birth occurrence) divided by the number of cycles that reached a successful egg collection multiplied by 100. |
| Live birth rate (LBR) per cycle that reached warming | The number of live birth occurrences (a multiple birth is counted as a single birth occurrence) divided by the number of cycles that reached warming for frozen cycles. |
| Live birth rate per cycle to ET procedure (LBR PCET) | The number of live birth occurrences (a multiple birth is counted as a single birth occurrence) divided by cycles to embryo transfer (ET) procedure multiplied by 100. |
| Live birth rate per embryo transferred (LBR PET) | The number of live birth occurrences (a multiple birth is counted as a single birth occurrence) divided by the sum of embryos transferred multiplied by 100. |
HFEA: Human Fertilisation and Embryology Authority, PGT: preimplantation genetic testing
SART data collection
The SART data showed the outcome from one egg collection. A cycle was counted when a woman started fertility drugs for the purpose of an ART procedure (SART, 2018), or, for a natural cycle, the first day of the woman’s menstrual cycle for a procedure that month. Following an egg collection, most patients underwent an ET procedure (fresh or frozen) within 1 year. The outcome of this first ET was the ‘primary outcome’. The ‘subsequent outcome’ was the thawing of eggs/embryos after the primary outcome had been determined. This also included ET procedures that occurred 1 year after the egg collection date. In the case of multiple egg collection cycles being performed, each collection was counted in the denominator when calculating outcomes (see below).
A filter on the ‘Reporting year’ field was used to separate out the individual years. Data collection was grouped into the following categories: ‘IVF primary outcome’, ‘IVF subsequent outcome’, ‘PGT primary outcome’, and ‘PGT subsequent outcome’.
Statistics
Significance was calculated using repeated measures ANOVA when assessing two factors of interest, or two-way repeated ANOVA when there were three factors of interest. This enabled comparisons to be drawn between two or more groups for a dependent variable over the years. Assumptions of normality and constant variance were checked by a study of residuals. If there was concern regarding assumptions, a square root transformation was taken for counts or the function ‘Arsin(SQRT(percentage/100))’ was taken for percentages. These transformations did not appear to improve the constant variance assumption so the analysis was performed on the raw data, recognizing the fact the P values may have been incorrect. However, as P values were extreme, the conclusions drawn were probably correct. Results were classed as significant if the P value was less than 0.05. Significant differences in groups with more than three categories allowed post-hoc tests to be performed for each dependent variable so further comparisons could be made. Specifically the Bonferroni’s test was used to adjust the P value to avoid spuriously significant results arising from multiple comparisons. Regression analysis was also performed to infer a linear relationship between variables of interest. After checking normality assumptions, the raw data were used. SPSS Statistics for Windows, Version 24.0. Released 2016 (IBM Corp., Armonk, NY, USA) was used for all statistical analysis.
Results
Table II summarizes the number of cycles and babies born from the UK and USA data.
Table II.
A summary of the IVF and PGT cycles performed in the UK and USA between 2014 and 2016 inclusive.
| UK | USA | |
|---|---|---|
| Total IVF cycles | 199 498 | 458 541 |
| Total PGT cycles | 3385 | 94 935 |
| PGT cycles as a % of IVF cycles | 2% | 21% |
| Number PGT babies | 1074 | 26 822 |
| PGT LBR per egg collection | 32% | 28% |
HFEA
For the HFEA data, a total of 281 IVF cycles and 18 PGT-M/SR cycles had to be excluded due to the inability to distinguish between fresh and frozen cycles.
Over the 3 years, there were a total of 199 498 IVF cycles and 3385 PGT cycles (2%). A total of 1074 babies were born (32% LBR per egg collection) (Table II).
Table III shows the HFEA data for the proportion of IVF and PGT cycles of a total of ART procedures for 2014–2016. The number of PGT cycles has not increased. PGT-M/SR account for more cycles that PGT-A.
Table III.
HFEA data—the proportion of IVF and PGT cycles of total of ART procedures.
| Year | Number of IVF and PGT treatment cycles | Number of IVF treatment cycles (%) | Number of PGT-M/SR treatment cycles (%) | Number of PGT-A treatment cycles (%) | Total PGT (%) |
|---|---|---|---|---|---|
| 2014 | 64 395 | 63 404–98.5% | 608–0.9% | 383–0.6% | 991 (1.5%) |
| 2015 | 66 183 | 64 903–98.1% | 687–1% | 593–0.9% | 1280 (1.9%) |
| 2016 | 68 920 | 67 806–98.4% | 708–1% | 406–0.6% | 1114 (1.6%) |
PGT-A: PGT-aneuploidy, PGT-M/SR: PGT-monogenic defects/chromosomal structural rearrangements
LBR
The LBRs for fresh PGT-A cycles decreased from 2014 to 2016 (Fig. 1A). From 2015, the LBRs for frozen cycles exceeded those of fresh cycles.
Figure 1.
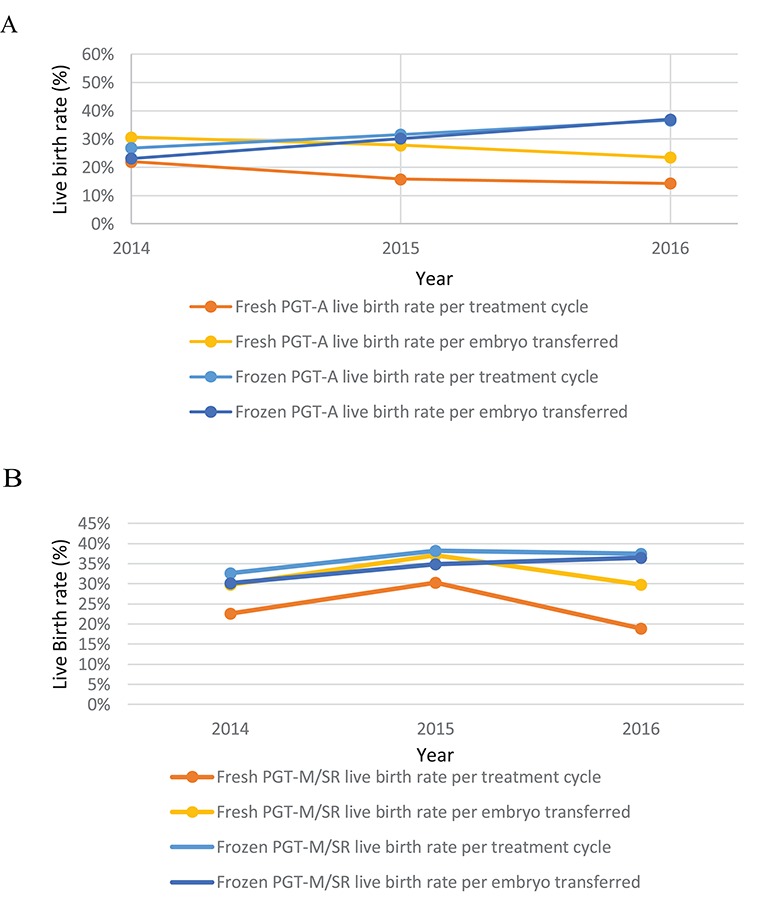
Data from the HFEA on the use of PGT for the years 2014–2016. (A) PGT-A live birth rates per year. For fresh PGT-A the live birth rate (LBR) per treatment cycle for the years 2014, 2015 and 2016 was 22.0% (72/327), 15.8% (63/400) and 14.3% (19/133) respectively. For fresh PGT-A the LBR per embryo transferred for the years 2014, 2015, 2016 was 30.6% (72/235), 27.8% (63/227) and 23.5% (9/81), respectively. For frozen PGT-A the LBR per treatment cycle for the years 2014, 2015, 2016 was 26.8% (15/56), 31.6% (61/193) and 36.6% (100/273), respectively. For frozen PGT-A the LBR per embryo transferred for the years 2014, 2015, 2016 was 23.1% (15/65), 30.2% (61/202) and 37.0% (100/270), respectively. (B) PGT-M/SR LBRss per year. For fresh PGT-M/SR the LBR per treatment cycle for the years 2014, 2015 and 2016 was 22.7% (58/256), 30.3% (46/152) and 18.9% (25/132), respectively. For fresh PGT-M/SR the LBR per embryo transferred for the years 2014, 2015 and 2016 was 29.7% (58/195), 37.1% (46/124) and 29.8% (25/84), respectively. For frozen PGT-M/SR the LBR per treatment cycle for the years 2014, 2015 and 2016 was 32.7% (115/352), 38.2% (204/535) and 37.5% (216/576), respectively. For the PGT-M/SR the LBR per embryo transferred was 30.2% (115/381), 34.9% (204/584) and 36.5% (216/592), respectively. HFEA: Human Fertilisation and Embryology Authority, PGT-A: preimplantation genetic testing aneuploidy, PTC: per treatment cycle, PET: per embryo transferred, PGT-M/SR monogenic defects/chromosomal structural rearrangements.
In 2016, for PGT M/SR, LBRs for frozen treatments were higher than LBRs for fresh cycles (Fig. 1B).
Age profile
The maternal age profile for PGT-A treatment has remained broadly the same over time (P > 0.999) (Fig. 2A). The 40–42 years age category was significantly higher than all other age categories (P < 0.001). The over 44 years age group made up the lowest percentage of PGT-A cycles (P < 0.05).
Figure 2.
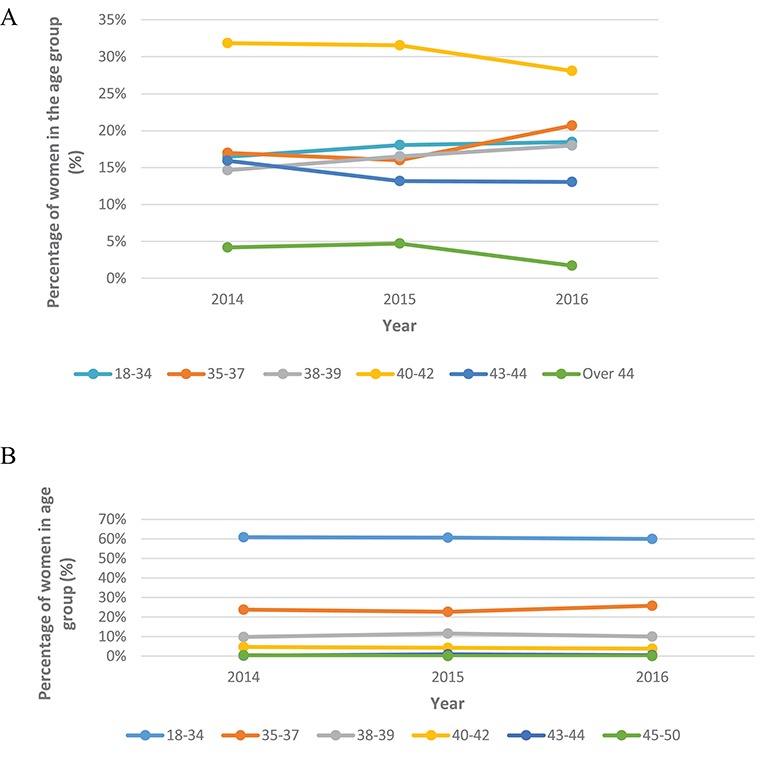
Data from the HFEA on PGT treatment cycles for the years 2014–2016. (A) PGT-A treatment cycles by age, per year. For 18–34 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 16.5% (63/383), 18.0% (107/593) and 18.5% (75/406), respectively. For 35–37 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 17.0% (65/383), 16.0% (95/593) and 20.7% (84/406), respectively. For 38–39 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 14.6% (56/383), 16.5% (98/593) and 18.0% (73/406), respectively. For 40–42 years olds this was 31.9% (122/383), 31.5% (187/593), 28.1% (114/406), respectively. For 43–44 year olds this was 15.9% (61/383), 13.2% (78/593) and 13.1% (53/406), respectively. For the over 44 year olds for the percentage of women in the age group from 2014, 2015 and 2016 this was 4.2% (16/383), 4.7% (28/593) and 1.7% (7/406), respectively. Post-hoc tests were performed to show the 40–42 years age category was significantly higher than all other age categories (P < 0.001). The over 44 years age group made up the lowest percentage of PGT-A cycles (P < 0.05). (B) PGT-M/SR treatment cycles by age, per year. For 18–34 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 60.9% (371/609), 60.7% (417/687) and 59.9% (424/708), respectively. For 35–37 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 23.8% (145/609), 22.7% (156/687) and 25.7% (182/708), respectively. For 38–39 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 9.9% (60/609), 11.5% (79/687) and 10.0% (71/708), respectively. For 40–42 years olds this was 4.8% (29/609), 4.2% (29/687), 3.8% (27/708), respectively. For 43–44 year olds this was 0.2% (1/609), 0.9% (6/687) and 0.6% (4/708), respectively. For the over 44 year olds for the percentage of women in the age group from 2014, 2015 and 2016 this was 0.5% (3/609), 0% (0/687) and 0% (0/708), respectively. Post-hoc tests showed the percentages of women in the 18–34, 35–37 and 38–39 year age groups were significantly higher than all other age groups (P < 0.001).
In 2016, 60% (424/708) of those receiving PGT-M/SR were aged18–34 years (Fig. 2B). Post-hoc tests showed the percentages of women in the 18–34, 35–37 and 38–39 year age groups were significantly higher than all other age groups (P < 0.001).
PGT-A LBRs were assessed by patient age (Fig. 3A). For the first time in 2016, frozen cycles resulted in higher LBRs for patients in both age categories (less than or over 38 years) when compared to fresh cycles. Statistical analysis showed no evidence for differences between LBR PTC and PET in terms of age groups (P = 0.146 and P = 0.823, respectively) or frozen cycles (P = 0.555) but there was a significant difference in fresh cycles (P = 0.023).
Figure 3.
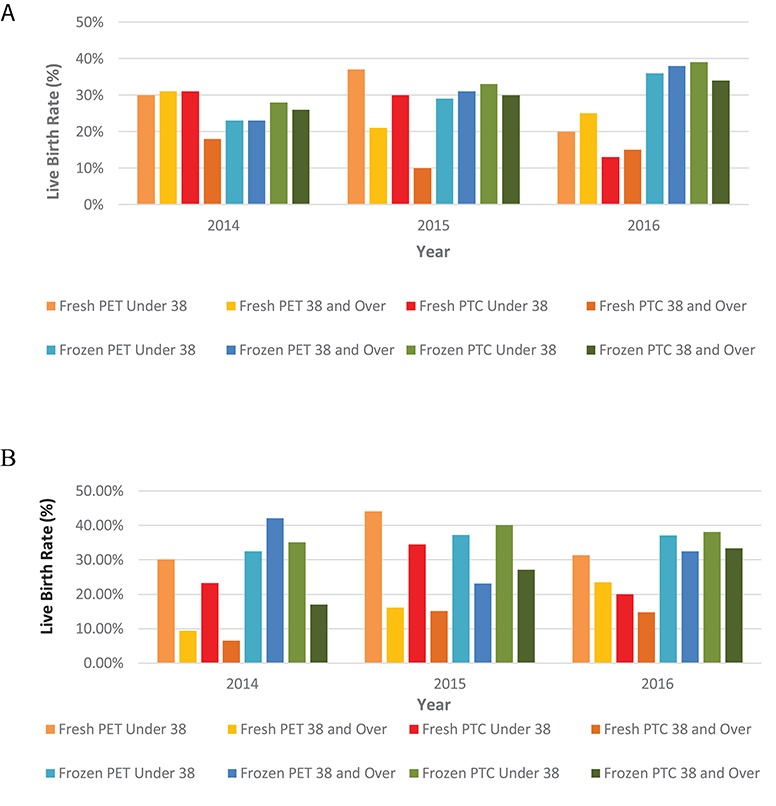
Data from the HFEA on the use of PGT, by age group, for the years 2014–2016. (A) PGT-A LBRs per year by age. The fresh PET LBR for under 38 s for the years 2014, 2015 and 2016 was 29.9% (32/107), 36.8% (35/95) and 20.0% (5/25), respectively. The fresh PET LBR for 38 and over for the years 2014, 2015 and 2016 was 31.3% (40/128), 21.2% (28/132) and 25.0% (14/56), respectively. The fresh PTC LBR for under 38 s for the years 2014, 2015 and 2016 was 31.1% (32/103), 30.4% (35/115) and 12.5% (5/40), respectively. The fresh PTC LBR over 38 and over for the year 2014, 2015 and 2016 was 17.9% (40/224), 9.8% (28/285) and 15.1% (14/93), respectively. The frozen PET LBR for under 38 s for the years 2014, 2015 and 2016 was 23.3% (7/30), 29.0% (29/100) and 35.9% (47/131). The frozen PET LBR for ages 38 and over for the years 2014, 2015 and 2016 was 22.8% (8/35), 31.4% (32/102), and 38.1% (53/139), respectively. The frozen PTC LBR for ages under 38 for the years 2014, 2015 and 2016 was 28.0% (7/25), 33.3% (29/87) and 39.5% (47/119), respectively, and the frozen PTC LBR for ages 38 and over for the years 2014, 2015 and 2016 was 25.8% (8/31), 30.2% (32/106) and 34.4% (53/154), respectively. Statistical analysis via an ANOVA test showed no evidence for differences between LBR PTC and PET in terms of age groups (P = 0.146 and P = 0.823, respectfully) or frozen cycles (P = 0.555) but there was a significant difference in fresh cycles (P = 0.023). (B) PGT-M/SR LBRs per year by age. The LBR for fresh PET for under 38 s for the years 2014, 2015 and 2016 were 30.1% (49/163), 44.1% (41/93) and 31.4% (21/67), respectively. The LBR for fresh PET for ages 38 and over for the years 2014, 2015 and 2016 were 9.8% (3/32), 16.1% (5/31) and 23.5% (4/17), respectively. The LBR for fresh PTC under 38 for the years 2014, 2015 and 2016 was 23.2% (49/211), 34.5% (41/119) and 20.0% (21/105), respectively. The LBR for fresh PTC ages 38 and over for the years 2014, 2015, and 2016 was 6.5% (3/46), 15.2% (5/33) and 14.8% (4/27), respectively. The LBR for frozen PET for under 38 s for the years 2014, 2015 and 2016 was 32.5% (107/329), 37.2% (182/489) and 27.2% (191/514). The LBR for frozen PET ages 38 and over for the years 2014, 2015 and 2016 was 42.1%, (8/19), 23.2% (22/95), 32.5% (25/77), respectively. The LBR for frozen PTC for ages under 38 for the years 2014, 2015 and 2016 was 35.1% (107/305), 40.1% (182/454), and 38.1% (191/501), respectively. The LBR for frozen PTC for ages 38 and over for the years 2014, 2015 and 2016 was 17.0% (8/47), 27.2% (22/81) and 33.3% (25/75), respectively. ANOVA analysis was carried out and showed the LBR PTC for both fresh and frozen cycles was higher in patients under 38 years than over 38 years (P = 0.002), whereas the LBR PET did not differ by age (P = 0.077). The LBR PTC and PET did not differ for frozen cycles (P = 0.158) but there was a difference between LBR PTC and PET in fresh cycles (P−0.002).
For PGT-M/SR, for all years the LBR PTC for both fresh and frozen cycles was higher in patients under 38 years than over 38 years (P = 0.002), whereas the LBR PET did not differ by age (P = 0.077) (Fig. 3B). The LBR PTC and PET did not differ for frozen cycles (P = 0.158) but there was a difference between LBR PTC and PET in fresh cycles (P−0.002).
SART
Over the 3 years, there were a total of 458 541 IVF cycles and 94 935 PGT cycles (21%) (Table IV). A total of 26 822 PGT babies were born (28% LBR per egg collection) (Table II).
Table IV.
SART data—the proportion of IVF and PGT cycles of total of ART procedures.
| Year | Number of IVF and PGT treatment cycles (cumulative) | Number of IVF treatment cycles (cumulative) (%) | Number of PGT treatment cycles (cumulative) (%) |
|---|---|---|---|
| 2014 | 140 561 | 121 786–87% | 18 775–13% |
| 2015 | 154 001 | 121 494–79% | 32 507–21% |
| 2016 | 163 979 | 120 326–73% | 43 653–27% |
SART: Society for Assisted Reproductive Technology
From 2014 to 2016, the number of cryopreserved PGT treatment cycles (after the primary ET had been determined) increased by 237% (6986/2949). Across the 3 years, an average of 61 thaw procedures had no embryos suitable for transfer.
The number of cryopreserved cycles started for PGT increased over the 3 years (P = 0.007). The number of thaw procedures did not differ between years (P = 0.091).
LBR
The LBR per egg collection and per transfer procedure for PGT remained unchanged in 2014–2016 (Fig. 4).
Figure 4.
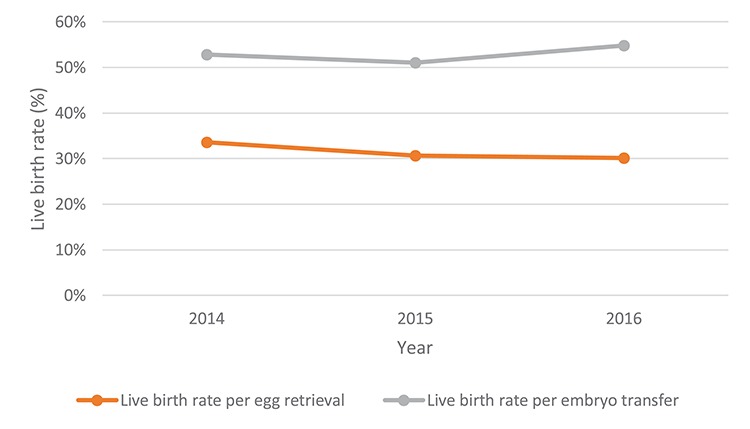
SART data—PGT LBRs per year (fresh and frozen cycles). The LBR per egg collection for the years 2014, 2015 and 2016 was 33.6% (5311/15 826), 30.7% (8102/26 393) and 30.1% (10 158/33 718) and the LBR per embryo transfer procedure for the years 2014, 2015 and 2016 was 52.8% (5311/10 058), 51.1% (8102/15 862) and 54.9% (10 158/18 512), respectively. SART: Society for Assisted Reproductive Technology.
Age profiles
The PGT treatment age profile has remained constant from 2014 to 2016 (Fig. 5). Cycles are most commonly performed in women under 35 years of age. The over 42 years age group makes up the lowest percentage, accounting for just 7% (2391/33 718) of treatment cycles in 2016.
Figure 5.
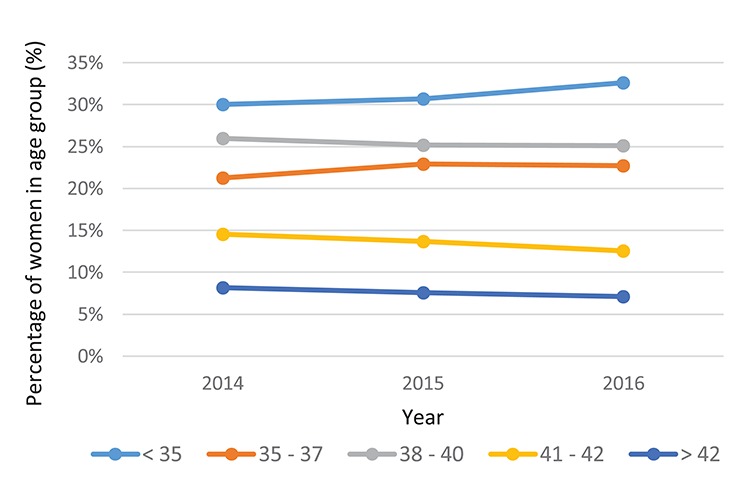
SART data—PGT treatment cycles (fresh and frozen) by age, per year. For under 35 s the percentage of women in the age group from 2014, 2015 and 2016 was 30.1% (4754/15 826), 30.7% (8099/26 393) and 32.6% (10 991/33 718), respectively. For 35–37 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 21.3% (3365/15 826), 22.9% (6041/26 393) and 22.7% (7655/33 718), respectively. For 38–40 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 26.0% (4111/15 826), 25.2% (6643/26 393) and 25.1% (8458/33 718), respectively. For 41–42 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 14.6% (2304/15 826), 13.7% (3604/26 393) and 12.5% (4223/33 718), respectively. For over 42 year olds the percentage of women in the age group from 2014, 2015 and 2016 was 8.2% (1292/15 826), 7.6% (2006/26 393) and 7.1% (2391/33 718), respectively.
The LBRs were shown to be higher in patients under 38 years of age than over 38 years for PGT (Fig. 6) (P < 0.001 for LBR per cycle started and per collection, and P = 0.008 per transfer).
Figure 6.
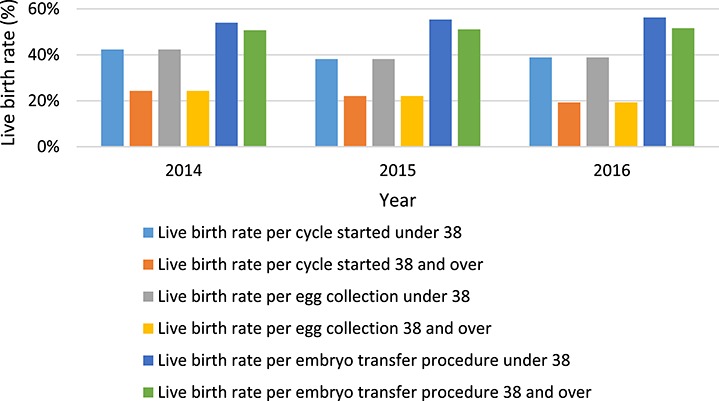
SART data—PGT LBRs per year by age group. The LBR per cycle started under age 38 for the years 2014, 2015 and 2016 were 42.3% (3436/8119), 38.2% (5400/14 140) and 38.9% (7253/18 646), respectively. The LBR per cycle started 38 years and over for the years 2014, 2015 and 2015 and 2016 was 24.3% (1874/7707), 22.1% (2702/12 253) and 19.3% (2905/15 072) respectively. The LBR per egg collection under 38 for the years 2014, 2015 and 2016 was 42.3% (3436/8119), 38.2% (5400/14 140) and 38.9% (7253/18 646), respectively. The LBR per egg collection age 38 and over for the years 2014, 2015 and 2016 was 24.3% (1874/7707), 22.1% (2702/12 253) and 19.3% (2905/15 072), respectively. The LBR per embryo transfer procedure for ages under 38 for the years 2014, 2015 and 2016 was 54.0% (3435/6364), 55.4% (5855/10 576) and 56.3% (72 481/12 887), respectively. The LBR per embryo transfer procedure for ages 38 and over for the years 2014, 2015 and 2016 was 50.7% (1874/3694), 51.1% (2702/5286) and 51.0% (2902/5625), respectively.
Discussion
Currently, it is unknown how many PGT-A, PGT-M and PGT-SR cycles are performed globally each year. The latest report from the ESHRE PGT Consortium shows that from data I-XV (1997–2013) a total of 56 093 PGT cycles have been reported to the Consortium, which includes 32 832 PGT-A, 14 340 PGT-M (12 712 single gene disorders, 1628 PGT for sexing only for X linked disease), and 8921 PGT-SR (De Rycke et al., 2017). The latest ESHRE PGT Consortium paper contains data from five UK and two US clinics (De Rycke et al., 2017).
In this paper we have presented a unique analysis of the UK and USA PGT data for 2014–2016, including 1382 PGT-A and 2003 PGT-M/SR cycles from the UK and 94 935 PGT cycles from the USA. Therefore, the USA has performed more cycles in 3 years than all of the ESHRE Consortium data collected since 1997. In the UK, PGT-A and PGT-M/SR make up less than 2% of the total ART cycles performed each year. In the USA, PGT makes up 21% of ART cycles (27% in 2016). Even though the USA data cannot be separated into PGT-A, PGT-M and PGT-SR, it is likely that the majority of cycles performed are for PGT-A as the ESHRE PGT Consortium data have consistently shown that PGT cycles account for the majority of cycles (De Rycke et al., 2017).
One key question is why is there such a difference in the use of PGT in the UK and USA?
Approximately 40% of ART in the UK is funded by the National Health Service and they do not fund PGT-A. In the UK, the additional cost of PGT-A can be over £3000 but in the USA it can be as high as $12 000. The HFEA does not report the PGT-A data in their annual report as they consider it an unproven add-on. They have produced a patient web page called IVF add-ons, which uses a traffic light system to rate the treatments (HFEA, 2018a). A green signal indicates that there is more than one good quality randomized controlled trial (RCT) that shows the procedure is effective and safe. An amber signal indicates that there is a very small or conflicting body of evidence, which means further research is still required and the technique cannot be recommended for routine use. A red signal indicates that there is no evidence to show that the technique is effective or safe: this may be because no studies have been conducted, or because studies have been conducted but they show no evidence of an improvement in success rates. PGT-A using cleavage stage biopsy and using blastocyst biopsy are both rated red.
A group of stakeholders, including the HFEA, ESHRE, British Fertility Society and the Association of Clinical Embryologists, has published a consensus document, urging clinics to think carefully before using non-evidence-based add-ons and to ensure that the information they give to the patients is accurate (HFEA et al., 2018b).
In the USA, the Practice Committee of the American Society of Reproductive Medicine and the Society for Assisted Reproductive Technology has recently stated ‘The value of preimplantation genetic testing for aneuploidy (PGT-A) as a screening test for in vitro fertilization (IVF) patients has yet to be determined’ (Penzias et al., 2018). However, ART is offered under very different principles with little regulation of the use of add-ons. There is a more commercial ART market in the USA as all treatments are self-funded so women may be more willing to use IVF add-ons, such as PGT-A, on the basis that they are already paying for fertility treatment.
The evidence for the use of PGT-A to increase LBR for any particular group of patients is still conflicting. Eleven RCTs carried out up to 2010 using PGT-A version 1 (mainly cleavage stage biopsy and FISH, which is no longer recommended for PGT) did not show a significant difference in LBR (Harper et al., 2010). This procedure was limited by the analysis of a single cell from a cleavage stage embryo and by the use of an inefficient technique (FISH). Since 2010, four small RCTs have been published using comprehensive chromosome testing for good and poor prognosis patients and have shown a benefit of the so called PGT-A version 2 (Yang et al., 2012; Scott et al., 2013; Forman et al., 2013; Rubio et al., 2017). Rubio (2019) has reported that when adding in the cumulative LBR the difference seen by PGT-A is reduced. A systematic analysis evaluated the three RCTs performed on good prognosis patients and concluded that it is yet to be determined if the data on good prognosis patients can be extrapolated to poor prognosis patients (Dahdouh et al., 2015).
However, the two multicentre RCTs on PGT-A published in 2018 have shown no improvement in LBR/ongoing pregnancy rate (Verpoest et al., 2018; Munne et al., 2019). The positive outcomes of the ESHRE multicentre RCT (ESTEEM study) are that PGT-A patients had fewer transfers, fewer miscarriages, fewer cryopreserved embryos but the same LBR as the control group (Verpoest et al., 2018). The results of the multicentre STAR trial showed that there was no overall improvement in ongoing pregnancy rates at 20 weeks. Subgroup analysis of the women aged 35–40 years did show an increase in ongoing pregnancy rate if two or more embryos were biopsied, but these data were not significant when analysed by intention-to-treat and there was no effect on miscarriage rates (Munne et al., 2019). For both the ESHRE and STAR trials, the additional births from frozen embryos have not been included and may result in a lower live birth rate in the PGT-A group. Some authors argue that PGT will not increase LBR as it does not change the embryos but it will decrease miscarriages, is a reduced cost compared to multiple ART cycles and reduces the time to pregnancy (Rubio et al., 2017). Only the ESHRE study on polar body biopsy has shown an effect on miscarriages (Verpoest et al., 2018); the STAR trial did not (Munne et al., 2019). Clinics need to think carefully what they tell their patients regarding the benefit of PGT-A in relation to live birth rate, cumulative live birth rate and miscarriage rates.
Patient characteristics
For the HFEA data, the majority of patients receiving PGT-A treatment were aged 40–42 years and those aged over 44 years were the smallest group receiving PGT-A (Fig. 2A). The majority of those undergoing PGT-M/SR treatment was aged 18–34 years (Fig. 2B). These age demographics were similar to those found in other studies (Chang et al., 2016; De Rycke et al., 2017).
In the USA, for PGT treatment cycles the majority of women undergoing treatment were under 35 years of age and the smallest group was over 42 years (Fig. 5). It should be noted that a higher percentage of younger women had a subsequent cryopreservation in comparison to a primary cycle. This was expected as older women are less likely to have embryos frozen.
LBR
Over the 3 years from the HFEA data for PGT-A there was no difference in LBR PTC and PET between age groups (Fig. 3A). This finding agrees with the hypothesis that PGT-A is used to balance out LBRs across age groups (Munné and Cohen, 2017) but the same result was not seen for the SART data.
For the HFEA data on PGT-M/SR, the LBR PTC for both fresh and frozen cycles was significantly higher in patients under 38 years of age than over 38 years (Fig. 3B). The HFEA LBR PET and PTC were calculated in line with the HFEA annual report (HFEA, 2017). PET gives patients an estimate of the number of transferable embryos required to achieve a live birth. This may lead patients to want multiple embryos transferred per transfer procedure. Patients should be made aware of the risks associated with such procedures, such as multiple pregnancies, so informed consent can be given regarding single embryo transfer (Forman et al., 2013).
In the SART data, the LBR per cycle started, per egg collection and per embryo transfer procedure was significantly higher in the under 38-year olds for all 3 years compared to older women (Fig. 6). The LBR for the subsequent PGT cycles was also significantly higher for the under 35 years age group than for patients over 41 years old.
ESHRE and SART success measures focus on transfer procedures in which at least one embryo is transferred (De Rycke et al., 2017). This gives a patient who reaches the ET stage a success rate that could be easier to understand. IVF has created new challenges for reporting outcomes, in which it is difficult for one success rate alone to convey both the effectiveness and safety of a procedure (Braakhekke et al., 2015). However, LBRs of PGT could be inflated due to the exclusion of embryo banking cycles, a factor highlighted in other studies (Kushnir et al., 2016, 2017).
Globally, the IVF LBR is decreasing in some countries, with Canada and Japan seeing the most dramatic effect (Gleicher et al., 2019). Gleicher et al. (2019) suggest that IVF add-ons, including PGT-A, have been partly to blame for this. In the USA, the decrease in IVF LBR started in 2011 and has continued. In 2010, the LBR was 30%, and by 2016 it had dropped to just over 20%.
Frozen embryos are used for PGT as it is almost essential for blastocyst biopsy. Blastocyst vitrification provides an unconstrained time frame for genetic diagnosis to be completed. Blastocyst biopsy means there are more cells for testing, reducing the chance of a ‘no result’ (Chang et al., 2011). The high proportion of frozen PGT cycles is also likely to reflect the trends towards next generation sequencing (Rodriguez-Purata et al., 2016; Coates et al., 2017). Owing to resource and finance limitations, most clinics performing PGT do not carry out diagnostics in-house; it is therefore easier and cheaper to batch frozen samples (Penzias et al., 2018). The use of vitrified embryos may lead to improved health of patients and babies, a reduced risk of ovarian hyperstimulation syndrome and a reduced number of multiple pregnancies (Li et al., 2014; Rienzi et al., 2017). Additionally, policies set in the UK requiring a lower ET order to reduce the multiple birth rate have subsequently led to an increase in embryos available for cryopreservation, which has gradually become the trend since 2009 (Rienzi et al., 2017).
Limitations
This was a retrospective report looking at data, and its major limitations arose from the data itself. Overall, the HFEA data were poorly collated. A total of 299 cases were excluded from analysis as it was unclear whether they were classified as a fresh or frozen cycle. This calls for consistent reporting on what is classified as a fresh or frozen cycle.
Additionally, it was unclear whether a cycle-started had been recorded if they did not reach egg collection. This can be related to the early Consortium reports in which cycle-started was abandoned, as clinics rarely reported accurate data (Geraedts et al., 2000). If today’s clinics are not submitting these data to the HFEA, this report may not provide an accurate view on the total number of ART procedures. Furthermore, by excluding cancelled and failed cycles, the chances of success for prospective patients are exaggerated by assuming that stimulation, fertilization, thawing etc. will be successful (Wilkinson et al., 2017). In comparison, the SART data include all intended collections when assessing outcomes. Any cancelled or failed cycles are hence taken into account when assessing treatment numbers or outcomes per cycle-started (Wilkinson et al., 2017).
The HFEA reporting system does not adequately capture embryo cryopreservation and genetic testing. For PGT treatments, patients may go through multiple egg collections in order to cryopreserve and bank embryos (Chamayou et al., 2017). However, the data for these cases are not currently linked, although the HFEA are developing this. Depending on how clinics fill out HFEA treatment forms, those egg collection procedures could appear as ‘for embryo storage’ or for ‘treatment now and embryo storage’. Therefore, the egg collection cannot be followed to the final outcome. When reporting such data, it is unclear whether embryologists are mindful that these data are part of a person’s full treatment, or if a form is filled in as an individual cycle. However, as per the SART data, all collections should be counted when calculating the outcomes. This allows the wider picture of fertility treatments to be more accurately captured (Wilkinson et al., 2017). This inaccuracy would easily be corrected by amending the form that clinics submit to the HFEA to include a section for the number of collections performed and allowing cycles to be linked. LBRs per egg collection, as per SART and Consortium data, could then be calculated.
Likewise, the number of HFEA PGT cycles that were due to be fresh but had no normal embryos available for transfer could not be identified. This is a problem specifically for many PGT-M/SR treatment cycles as the probability of creating an affected embryo that is not suitable for transfer is high (Lewis et al., 2001). If these cycles were not recorded, the number of PGT cycles would be much larger than those reported in this study.
Owing to the possibility of cycle inflation, only those with an indicated ‘treatment now’ in the ‘main reason for producing embryos storing eggs’ were included in this analysis. These ensured patients were not counted twice for the same cycle. This filter excluded patients going through an ART procedure for egg sharing or donation, which would have reduced the chance of pregnancy and affected the overall outcomes.
Unlike the HFEA, SART PGT data could not be separated into PGT-M/SR and PGT-A. Therefore, age profile and LBRs were not adequately captured. In addition, as the primary and subsequent cycles were linked it was difficult to infer whether the age at a subsequent cycle was the age of the women at egg collection or the age at the transfer procedure. If age was only recorded at the ET procedure date, the success rates of older groups may be artificially raised, especially in cases of embryos/oocytes being cryopreserved several years earlier. It would therefore be valuable for clinics to report on both, especially for patients using IVF for fertility preservation.
Although the Consortium is not able to collect data from many of the clinics that SART and the HFEA report on (De Rycke et al., 2017), the Consortium has an important role in collecting data. A much better follow-through of an embryo is reported. The effect of such a technology-driven approach is the impact of accuracy and later complications with increasing ART procedures. Unlike the Consortium, both the HFEA and SART data do not separate PGT-M and PGT-SR data, making it difficult to show the impact of new technologies. It could be that the PGT-SR cycle numbers have stayed static over time, if older technologies were used. In contrast, PGT-M cycle numbers may have increased, as most diseases can now be tested by new technologies. Therefore, it would have been interesting to see if technological advancements had increased the proportion of PGT-M over PGT-SR or vice versa. The ESHRE data can validate these hypotheses, which has been useful for future planning of the patient pathway and determining the types of treatments required for patients. Like the Consortium, the HFEA and SART should separate PGT-M, PGT-SR and PGT-A to identify the type of technologies used and the resulting impact on the delivery of the service (De Rycke et al., 2015). Recording these nuances in LBR PTC and PET will allow assessment and thereby improvement in approaches to PGT.
Conclusion
This report has demonstrated that there is an increasing number of patients using PGT in the USA at a time when the USA LBR has decreased. The data collection limitations in both the USA and UK have been highlighted. By amending HFEA clinic forms, a wider set of data could be collected to allow reporting on important data measures. Although anonymous, cycles should be linked in order to assess treatment burden to patients, which can subsequently be taken into account when calculating outcomes. Clinics should be educated on optimal data reporting methods. To improve data presentation, the HFEA should conform to the standards of SART. Although SART produces an excellent template for reporting UK data, after comparing databases to the ESHRE PGT Consortium, it is clear that other impactful factors have been missed. PGT-M, PGT-SR and PGT-A must be separated to allow prospective patients to view a more accurate breakdown of patient demographics and outcomes. Furthermore, separation into techniques used, in line with ESHRE, will allow the impact of new technologies on treatment to be understood. Further consideration of better ways of reporting the data should be extended worldwide.
The need for a consistent reporting of success rates has been highlighted by this report. As with increasing PGT use there is a growing need to monitor its practice and report upon accurate and useful information. The availability of such data allows objective quality assessment of PGT services.
Acknowledgements
We would like to thank Caylin Joski-Jethi (then Head of Intelligence at the HFEA) for her help with the HFEA data analysis.
Authors’ roles
Joyce Harper and Rachel Theobald designed the methods. Rachel Theobald carried out the data collection and wrote the initial draft of the paper. All three authors edited the final paper.
Funding
No specific funding was used.
Conflict of interest
None relating to this project. Joyce Harper is the director of the Embryology and PGD Academy, which offers education in these fields.
References
- Braakhekke M, Kamphuis EI, Mol F, Norman RJ, Bhattacharya S, Van Der Veen F, Mol BW. Effectiveness and safety as outcome measures in reproductive medicine. Hum Reprod 2015;30:2249–2251. [DOI] [PubMed] [Google Scholar]
- Brezina PR, Kutteh WH. Clinical applications of preimplantation genetic testing. BMJ 2015;350:g7611. [DOI] [PubMed] [Google Scholar]
- Chamayou S, Sicali M, Alecci C, Ragolia C, Liprino A, Nibali D, Storaci G, Cardea A, Guglielmino A. The accumulation of vitrified oocytes is a strategy to increase the number of euploid available blastocysts for transfer after preimplantation genetic testing. J Assist Reprod Genet 2017;34:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EM, Han JE, Kim YS, Lyu SW, Lee WS, Yoon TK. Use of the natural cycle and vitrification thawed blastocyst transfer results in better in-vitro fertilization outcomes: cycle regimens of vitrification thawed blastocyst transfer. J Assist Reprod Genet 2011;28:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Boulet SL, Jeng G, Flowers L, Kissin DM. Outcomes of in vitro fertilization with preimplantation genetic diagnosis: an analysis of the United States assisted reproductive technology surveillance data, 2011–2012. Fertil Steril 2016;105:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, Ata B, Cohen J, Munne S. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril 2017;107:723–730.e3. [DOI] [PubMed] [Google Scholar]
- De Rycke M, Belva F, Goossens V, Moutou C, Sengupta SB, Traeger-Synodinos J, Coonen E. ESHRE PGT consortium data collection XIII: cycles from January to December 2010 with pregnancy follow-up to October 2011. Hum Reprod 2015;30:1763–1789. [DOI] [PubMed] [Google Scholar]
- De Rycke M, Goossens V, Kokkali G, Meijer-Hoogeveen M, Coonen E, Moutou C. ESHRE PGT consortium data collection XIV–XV: cycles from January 2011 to December 2012 with pregnancy follow-up to October 2013†. Hum Reprod 2017;32:1974–1994. [DOI] [PubMed] [Google Scholar]
- Dahdouh EM, Balayla J, García-Velasco JA. (2015) impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reprod Biomed Online 2015;30:281–289. [DOI] [PubMed] [Google Scholar]
- Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, Treff N, Scott RT. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril 2013;100:100–107.e1. [DOI] [PubMed] [Google Scholar]
- Geraedts J, Handyside A, Harper J, Liebaers I, Sermon K, Staessen C, Thornhill A, Viville S, Wilton L. ESHRE preimplantation genetic diagnosis (PGD) consortium: data collection II (May 2000). Hum Reprod 2000;15:2673–2683. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Kushnir VA, Barad DH. Worldwide decline of IVF birth rates and its probable causes. Hum Reprod Open 2019. doi: 10.1093/hropen/hoz017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JC, Handyside AH. The current status of preimplantation diagnosis. Curr Obstet Gynaecol 1994;4:143–149. [Google Scholar]
- Harper JC. Preimplantation diagnosis of inherited disease by embryo biopsy: an update of the world figures. J Assist Reprod Genet 1996;13:90–95. [DOI] [PubMed] [Google Scholar]
- Harper J, Coonen E, De Rycke M, Fiorentino F, Geraedts J, Goossens V, Harton G, Pehlivan Budak T, Renwick P, Sengupta S et al. What next for preimplantation genetic screening (PGS)? A position statement from the ESHRE PGT consortium steering committee. Hum Reprod 2010;25:821–823. [DOI] [PubMed] [Google Scholar]
- Harper JC, Sengupta SB. Preimplantation genetic diagnosis: state of the ART 2011. Hum Genet 2012;131:175–186. [DOI] [PubMed] [Google Scholar]
- Harper JC, Wilton L, Traeger-Synodinos J, Goossens V, Moutou C, Sengupta SB, Pehlivan Budak T, Renwick P, De Rycke M, Geraedts JPM et al. The ESHRE PGT consortium: 10 years of data collection. Hum Reprod Update 2012;18:234–247. [DOI] [PubMed] [Google Scholar]
- Harper J, Jackson E, Sermon K, Aitken RJ, Harbottle S, Mocanu E, Hardarson T, Mathur R, Viville S, Vail A et al. Adjuncts in the IVF laboratory: where is the evidence for ‘add-on’ interventions? Hum Reprod 2017;32:485–491. [DOI] [PubMed] [Google Scholar]
- HFEA Fertility treatment 2014–2016 trends and figures [online]. 2017; https://www.hfea.gov.uk/media/2563/hfea-fertility-trends-and-figures-2017-v2.pdf.
- HFEA https://www.hfea.gov.uk/treatments/explore-all-treatments/treatment-add-ons/ [accessed 31 August 2018]. 2018a.
- HFEA. et al. The responsible use of treatment add-ons in fertility services: a consensus statement. 2018b; https://www.hfea.gov.uk/media/2792/treatment-add-ons-consensus-statement-final.pdf.
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Effect of embryo banking on U.S. National Assisted Reproductive Technology Live Birth Rates. PLoS One 2016;11:e0154620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004–2013. Reprod Biol Endocrinol 2017;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, PinêL T, Whittaker JC, Handyside AH. Controlling misdiagnosis errors in preimplantation genetic diagnosis: a comprehensive model encompassing extrinsic and intrinsic sources of error. Hum Reprod 2001;16:43–50. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod 2014;29:2794–2801. [DOI] [PubMed] [Google Scholar]
- Mastenbroek S, Twisk M, Van Der F, Repping S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update 2011;17:454–466. [DOI] [PubMed] [Google Scholar]
- Munné S, Cohen J. Advanced maternal age patients benefit from preimplantation genetic diagnosis of aneuploidy. Fertil Steril 2017;107:1145–1146. [DOI] [PubMed] [Google Scholar]
- Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, Silverberg K, Kalista T, Handyside AH, Katz-Jaffe M et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112:1071–1079.e7. [DOI] [PubMed] [Google Scholar]
- Penzias A, Bendikson K, Butts S, Coutifaris C, Falcone T, Fossum G, Gitlin S, Gracia C, Hansen K, La Barbera A et al. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril 2018;109:429–436. [DOI] [PubMed] [Google Scholar]
- Rienzi L, Gracia C, Maggiulli R, Labarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Purata J, Lee J, Whitehouse M, Duke M, Grunfeld L, Sandler B, Copperman A, Mukherjee T. Reproductive outcome is optimized by genomic embryo screening, vitrification, and subsequent transfer into a prepared synchronous endometrium. J Assist Reprod Genet 2016;33:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohí J et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril 2017;107:1122–1129. [DOI] [PubMed] [Google Scholar]
- Rubio C. PGT-A and RCT proof in AMA and SMF couples. Fertil Steril 2019;38:e8. [Google Scholar]
- SART Clinic Summary Report [online]. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?ClinicPKID=2071, 2018.
- Scott RTJ, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, Tao X, Treff NR. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril 2013;100:697–703. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet 1978;2:366. [DOI] [PubMed] [Google Scholar]
- Toner JP, Coddington CC, Doody K, Van Voorhis B, Seifer DB, Ball GD, Luke B, Wantman E. Society for Assisted Reproductive Technology and assisted reproductive technology in the United States: a 2016 update. Fertil Steril 2016;106:541–546. [DOI] [PubMed] [Google Scholar]
- Verpoest W, Staessen C, Bossuyt PM, Goossens V, Altarescu G, Bonduelle M, Devesa M, Eldar-Geva T, Gianaroli L, Griesinger G et al. Preimplantation genetic testing for aneuploidy by microarray analysis of polar bodies in advanced maternal age: a randomized clinical trial. Hum Reprod 2018;33:1767–1776. [DOI] [PubMed] [Google Scholar]
- Weinerman R. In vitro fertilization (IVF): where are we now? Birth Defects Res 2018;110:623–624. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Roberts SA, Vail A. Developments in IVF warrant the adoption of new performance indicators for ART clinics, but do not justify the abandonment of patient-centred measures. Hum Reprod 2017;32:1155–1159. [DOI] [PubMed] [Google Scholar]
- Wilkinson J, Malpas P, Hammarberg K, Mahoney Tsigdinos P, Lensen S, Jackson E, Harper J, Mol B. Do à la carte menus serve infertility patients? The ethics and regulation of IVF add-ons. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills ES, Salem RD. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet 2012;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li N, Wang L, Sun H, Ma M, Wang H, Xu X, Zhang W, Liu Y, Cram DS et al. Molecular analysis of DNA in blastocoele fluid using next-generation sequencing. J Assist Reprod Genet 2016;33:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]


