Abstract
STUDY QUESTION
Does male alcohol consumption affect fecundability?
SUMMARY ANSWER
In data pooled across Danish and North American preconception cohort studies, we found little evidence of an association between male alcohol consumption and reduced fecundability.
WHAT IS KNOWN ALREADY
Experimental and clinical studies have shown that alcohol affects male reproductive physiology, mainly by altering male reproductive hormones and spermatogenesis. However, few epidemiologic studies have examined the association between alcohol consumption and male fertility.
STUDY DESIGN, SIZE, DURATION
Data were collected from two ongoing prospective preconception cohort studies: the Danish ‘SnartForaeldre’ (SF) study (662 couples) and the North American ‘Pregnancy Study Online’ (PRESTO) (2017 couples). Participants included in the current analysis were enrolled from August 2011 through June 2019 (SF) and from June 2013 through June 2019 (PRESTO).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Eligible men were aged ≥18 years in SF and ≥21 years in PRESTO, in a stable relationship with a female partner and not using contraception or receiving fertility treatment. In both cohorts, alcohol consumption/serving size was self-reported as number of beers (330 mL/12 oz.), glasses of white or red wine (120 mL/4 oz. each), dessert wine (50 mL/2 oz.) and spirits (20 mL/1.5 oz.). Overall alcohol consumption was categorized as none, 1–5, 6–13 and ≥14 standard servings per week. Total menstrual cycles at risk were calculated using data from female partners’ follow-up questionnaires, which were completed every 8 weeks until self-reported pregnancy or 12 menstrual cycles, whichever came first. Analyses were restricted to couples that had been trying to conceive for ≤6 cycles at study entry. Proportional probability regression models were used to compute fecundability ratios (FRs) and 95% confidence interval (CIs). We adjusted for male and female age, female partner’s alcohol consumption, intercourse frequency, previous history of fathering a child, race/ethnicity, education, BMI, smoking and consumption of sugar-sweetened beverages and caffeine.
MAIN RESULTS AND THE ROLE OF CHANCE
The cumulative proportion of couples who conceived during 12 cycles of follow-up were 1727 (64.5%). The median (interquartile range) of total male alcohol consumption was 4.5 (2.0–7.8) and 4.1 (1.0–8.6) standard servings per week in the SF and PRESTO cohorts, respectively. In pooled analyses, adjusted FRs for male alcohol consumption of 1–5, 6–13 and ≥14 standard servings per week compared with no alcohol consumption were 1.02 (95% CI: 0.90–1.17), 1.10 (95% CI: 0.96–1.27) and 0.98 (95% CI: 0.81–1.18), respectively. For SF, adjusted FRs of 1–5, 6–13 and ≥14 standard servings per week compared with no alcohol consumption were 0.97 (95% CI: 0.73–1.28), 0.81 (95% CI: 0.60–1.10) and 0.82 (95% CI: 0.51–1.30), respectively. For PRESTO, adjusted FRs of 1–5, 6–13 and ≥14 standard servings per week compared with no alcohol consumption were 1.02 (95% CI: 0.88–1.18), 1.20 (95% CI: 1.03–1.40) and 1.03 (95% CI: 0.84–1.26), respectively.
LIMITATIONS, REASONS FOR CAUTION
Male alcohol consumption was ascertained at baseline only, and we did not distinguish between regular and binge drinking. In addition, we had insufficient numbers to study the effects of specific types of alcoholic beverages. As always, residual confounding by unmeasured factors, such as dietary factors and mental health, cannot be ruled out. Comorbidities thought to play a role in the reproductive setting (i.e. cancer, metabolic syndrome) were not considered in this study; however, the prevalence of cancer and diabetes was low in this age group. Findings for the highest categories of alcohol consumption (6–13 and ≥14 servings/week) were not consistent across the two cohorts.
WIDER IMPLICATIONS OF THE FINDINGS
Despite little evidence of an association between male alcohol consumption and reduced fecundability in the pooled analysis, data from the Danish cohort might indicate a weak association between reduced fecundability and consumption of six or more servings per week.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the National Institutes of Health (R01-HD060680, R01-HD086742, R21-HD050264, R21-HD072326, R03-HD090315), the Novo Nordisk Foundation, Oticon Fonden, Politimester J.P.N. Colind og hustru Asmine Colinds mindelegat and Erna og Peter Houtveds studielegat. PRESTO receives in-kind donations from FertilityFriend.com, Kindara.com, Swiss Precision Diagnostics and Sandstone Diagnostics for the collection of data pertaining to fertility. Dr Wise serves as a consultant on uterine leiomyomata for AbbVie.com. All other authors declare no conflict of interest.
Keywords: alcohol, fecundability, time to pregnancy, male fertility, infertility
Introduction
In developed countries, infertility affects up to 15% of couples (Slama et al., 2012, Thoma et al., 2013). The distress experienced by infertile couples and the increasing demand for ART (Kupka et al., 2014) have led to a greater focus on elucidating modifiable risk factors for infertility. In Western nations, a decline in sperm count has been observed over the last 40 years (Levine et al., 2017), and male factors contribute to infertility in approximately 50% of all cases (Irvine et al., 1998).
Potential modifiable risk factors for male infertility include lifestyle factors such as smoking (Soares and Melo, 2008; Harlev et al., 2015), obesity (Wise et al., 2010; Sundaram et al., 2017) and intake of sugar-sweetened beverages (Hatch et al., 2018); the impact of alcohol consumption is less clear. Alcohol consumption is a habitual part of daily life for a large proportion of males of reproductive age (WHO, 2014). In several countries, official guidelines recommend that men limit their consumption of alcohol to a maximum of 14 drinks per week, with no distinction made for men actively trying to conceive (Danish National Board of Health, 2015; The U.S. Department of Health and Human Services and the U.S. Department of Agriculture, 2015).
Previous studies have shown that alcohol affects the male reproductive system by altering the regulation of the hypothalamic–pituitary–gonadal (HPG) axis and spermatogenesis. Most studies of healthy young men have found higher alcohol consumption to be positively associated with testosterone levels and inversely associated with sex hormone-binding globulin levels (Shiels et al., 2009; Hansen et al., 2012; Jensen et al., 2014a; Jensen et al., 2014b). In contrast, decreased testosterone levels, and elevated levels of FSH and LH have been observed among alcoholic men, indicating that alcohol abuse may impair the HPG axis or cause Leydig-cell damage (Emanuele, 1998; Muthusami and Chinnaswamy, 2005; Maneesh et al., 2006).
Alcohol consumption also has been inversely associated with total sperm count, sperm concentration and percentage of morphologically normal sperm (Emanuele, 1998; Muthusami and Chinnaswamy, 2005; La Vignera et al., 2013; Jensen et al., 2014; Borges et al., 2018). Other studies have reported a positive association between moderate male alcohol consumption and semen quality (Ricci et al., 2018).
One cohort study found that male alcohol consumption was associated with shorter time to pregnancy (TTP) (Florack et al., 1994), whereas two other cohort studies found a weak association with longer TTP (Curtis et al., 1997; Olsen et al., 1997). Thus, the extent to which male alcohol consumption influences TTP is unclear. In the present study, we examined the association between male alcohol consumption and couples’ TTP in two prospective cohort studies of Danish and North American couples.
Materials and Methods
Study population
The SnartForaeldre (Soon Parents) study is an ongoing prospective cohort study of Danish pregnancy planners (Mikkelsen et al., 2009). The study was launched in August 2011. Participants are recruited primarily through web-based advertising (Christensen et al., 2017). Participants complete a screening questionnaire at the study website (http://snartforaeldre.dk), which confirms eligibility and ascertains how long they had tried to conceive before study entry. Eligible men and women are invited to complete a baseline questionnaire and are encouraged to invite their partner to take part in the study. Couples are linked by means of an email invitation sent to the partner or by their home address as registered in the Danish Civil Registration System on the date of study entry (Frank, 2000).
The Pregnancy Study Online (PRESTO) is similar in design to SnartForaeldre (Wise et al., 2015). It has recruited pregnancy planners from the USA and Canada since June 2013. Eligible women complete baseline and follow-up questionnaires at the study website (http://presto.bu.edu). After enrollment, female participants are given the option to invite their male partners to complete a one-time baseline questionnaire. PRESTO received non-financial support from Swiss Precision Technologies, Sandstone Diagnostics, FertilityFriend.com and Kindara.
In both cohorts, inclusion criteria are being in a stable relationship with a partner of the opposite sex and not using any contraception or receiving fertility treatment. SnartForaeldre recruits females aged 18–49 years and males aged ≥18 years, whereas PRESTO recruits females aged 21–45 years and males aged ≥21 years. Reasons for exclusion are shown in Fig. 1. Couples who had tried to conceive for >6 months at study entry are excluded to reduce bias related to changes in behavior due to subfertility. Male and female baseline questionnaires elicit socio-demographic data, information on behavioral and lifestyle factors and reproductive and medical history. Female follow-up questionnaires are completed bimonthly for up to 12 months or until reported pregnancy. These questionnaires update data on pregnancy status and lifestyle factors that vary over time.
Figure 1.
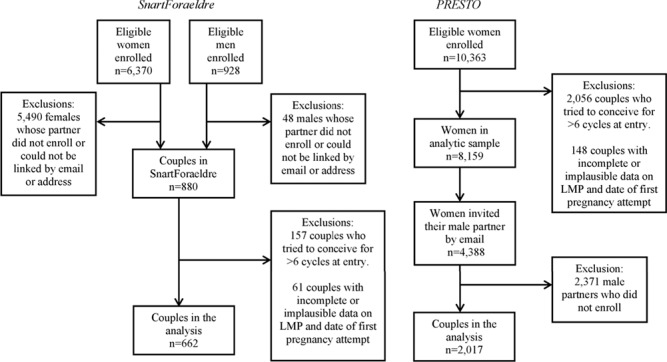
Flow chart of participants in SnartForaeldre and PRESTO. SnartForaeldre: the Danish study, PRESTO: the North American Pregnancy Study Online, LMP: last menstrual period.
Assessment of male alcohol exposure
On baseline questionnaires, men in both cohorts reported their average weekly alcohol consumption during the previous month as bottles of beer (330 mL/12 ounces), glasses of white and red wine (120 mL/4 ounces each), dessert wine (50 mL/2 ounces) and spirits (20 mL/1.5 ounces). Help buttons in the questionnaires instruct respondents on how to assess the number of average weekly servings and to report ‘no intake’ if they drank less than one drink per week. To standardize alcohol consumption across cohorts and types of beverages, we calculated total weekly alcohol consumption in standard servings (12 grams of alcohol per serving) by summing the amount of alcohol in grams from each type of beverage consumed and dividing by 12. In PRESTO, ounces were converted to milliliters (1 oz. = 29.57 mL). We assumed that a bottle of beer (330 mL), one glass of red wine or white wine (120 mL), dessert wine (50 mL) and spirits (20 mL) each contain 11.6, 12, 8 and 7 g of alcohol, respectively (National Food Institute, 2016).
Total weekly alcohol consumption was categorized as none, 1–5, 6–13 and ≥14 standard servings.
Assessment of pregnancies and cycles at risk
On each follow-up questionnaire, women reported the date of their last menstrual period (LMP) and their pregnancy status. TTP was estimated as the number of menstrual cycles during which a couple tried to achieve pregnancy and included the time trying to conceive, both before study entry and during follow-up. Total menstrual cycles at risk, rounded to the nearest whole number, were calculated as follows: cycles of attempt time at study entry + (((LMP date from most recent follow-up questionnaire − date of baseline questionnaire completion) / cycle length) + 1) (Wise et al., 2010).
Assessment of covariates
The male baseline questionnaire collected data on age, education, hours per week of employment, previous conception with a female partner, smoking, physical activity, height and weight, consumption of sugar-sweetened beverages, intake of multivitamins and caffeine and history of disease (migraine, asthma, hay fever, depression, anxiety, hypertension, diabetes, sexually transmitted infections and infections in the male reproductive organs). Female questionnaires elicited data on age, alcohol consumption, household income, pregnancy attempt time before study entry and timing and frequency of intercourse. We estimated total metabolic equivalents (METs) by multiplying the average number of physically active hours per week by METs for various activities. In SnartForaeldre, we estimated METs from walking, moderate exercise and vigorous exercise using the short-form International Physical Activity Questionnaire (Craig et al., 2003), whereas in PRESTO METs of various activities were estimated using the Compendium of Physical Activities (Ainsworth et al., 2000). We used baseline data on height and weight to calculate BMI as weight (kg)/height (m)2. Identical covariates were examined in both cohorts, except for race/ethnicity (obtained only in PRESTO) and education (ascertained differently in each cohort but harmonized into a single variable (Wise et al., 2017)).
Data analysis
We performed a pooled analysis with harmonized data and parallel analyses in each cohort for the August 2011–June 2019 (SnartForaeldre) and June 2013–June 2019 (PRESTO) study periods. We used proportional probability regression models to compute fecundability ratios (FRs) with 95% confidence intervals (CIs) (Weinberg et al., 1989). The FR represents the per-cycle probability of conception comparing exposed men with unexposed men; a FR below 1 indicates reduced fecundability. To account for left truncation (attempt time at study entry ranged from 0–6 cycles), we analyzed risk sets restricted to observed menstrual cycles (Schisterman et al., 2013). For example, if a couple had tried to conceive for 4 cycles at study entry and reported pregnancy after 8 cycles, they would have contributed Cycles 5 through 8 (four cycles) to the analysis (Wise et al., 2010). We right-censored couples who were lost to follow-up (9.6%), stopped trying to conceive (1.9%), started fertility treatment (7.6%) or reached 12 cycles of attempted pregnancy (12.6%). We compared baseline characteristics for those with and without complete follow-up (i.e. couples who responded only to the baseline questionnaire or who stopped responding to follow-up questionnaires before study completion). Most males were invited by their female partners. To evaluate the possibility of differential male participation, we compared characteristics (age, smoking status and BMI) between male participants and non-participants as reported by their female partners.
In the multivariate regression analysis, we adjusted for male and female age (continuous), female alcohol consumption in standard servings (continuous), frequency of intercourse (<1, 1, 2–3, ≥4 times/week), history of previously fathering a child (yes/no), education (≤12, 13–15, 16, ≥17 years), BMI (continuous), smoking (regular, occasional, former, never), sugar-sweetened beverage consumption (continuous), caffeine consumption (continuous) and study cohort (SnartForaeldre/PRESTO). PRESTO models were adjusted additionally for race/ethnicity (non-Hispanic white/other). We selected potential confounders based on the literature and on directed acyclic graphs. We used multiple imputations to impute missing exposure, covariate and outcome data. Couples who completed only the baseline questionnaire (6.7%) were assigned 1 cycle of follow-up, and their pregnancy status was imputed. We generated five imputed datasets, analyzed each dataset and subsequently combined the results across the imputed datasets (Sterne et al., 2009). We also used restricted cubic splines to assess the presence of a non-linear association between male alcohol intake and fecundability.
To assess whether reverse causation could explain our results, we stratified our analysis by pregnancy attempt time at enrollment (≤2 versus 3–6 cycles). Furthermore, we stratified results according to male BMI (<25 versus ≥25 kg/m2), history of previously fathering a child (yes versus no), female age (<30 versus ≥30 years) and gravidity (yes versus no), as those factors potentially modify the association between male alcohol intake and fecundability (Collins et al., 1995; Wang et al., 2008). In secondary analyses, we estimated FRs for alcohol consumption of 14–20 and ≥21 standard servings per week.
Ethics approvals
Study protocols were approved by the Danish Data Protection Agency (2016-051-000001, # 431) and the Institutional Review Board at Boston Medical Center. Participants provided informed consent online.
Results
Participant characteristics
Overall, 1727 (64.5%) of the 2679 participating couples conceived during follow-up. SnartForaeldre couples (N = 662) contributed 2475 menstrual cycles at risk and 450 pregnancies (68.0%), and PRESTO couples (N = 2017) contributed 7969 menstrual cycles at risk and 1277 pregnancies (63.3%). The median (interquartile range) of total male alcohol consumption was 4.5 (2.0–7.8) standard servings per week in SnartForaeldre and 4.1 (1.0–8.6) in PRESTO. The proportion of non-drinkers was 11.0% in SnartForaeldre and 20.4% in PRESTO. The proportions of male participants drinking ≥14 standard serving per week were 7.7% in SnartForaeldre and 13.9% in PRESTO. More men consumed beer (76.6%) than wine (48.7%) or spirits (43.7%). In total, 1598 (59.6%) men consumed a combination of two or more alcoholic beverages. Fewer men consumed only beer, wine or spirits (18.9, 2.6 and 3.4%, respectively).
Couples in SnartForaeldre and PRESTO had many similar characteristics, as shown in Table I. However, SnartForaeldre couples had a higher frequency of male physical activity, male sexually transmitted infections and infection in male reproductive organs than PRESTO couples. At the same time, men in PRESTO worked more hours per week, had a higher BMI and were more likely to consume soft drinks than men in SnartForaeldre. In both cohorts, caffeine consumption, regular smoking, female alcohol consumption and shorter attempt time at study entry were positively associated with male alcohol consumption, and men who previously had fathered a child were more likely to be non-drinkers. History of chronic disease ranged from 1.7% for diabetes to 12.3% for asthma and was not meaningfully associated with male alcohol consumption (data not shown). Overall, baseline characteristics were similar for couples with complete versus incomplete follow-up, except for couples lost to follow-up who were more likely to have shorter education, have lower income and be smokers (data not shown). Non-participating men were similar to participating men according to age and BMI, but more likely to be smokers (data not shown).
Table I.
Baseline characteristics of 2679 males, by level of alcohol intake in standard servings/week.
| Characteristics | Total | SNARTFORAELDRE (N = 662) | PRESTO (N = 2017) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| None a | 1–5 | 6–13 | ≥14 | None a | 1–5 | 6–13 | ≥14 | ||
| No. of men (%) | 2679 | 73 (11.0) | 303 (45.8) | 235 (35.5) | 51 (7.7) | 411 (20.4) | 712 (35.3) | 614 (30.4) | 280 (13.9) |
| Age, median years | 31 | 30 | 30 | 31 | 31 | 30 | 31 | 31 | 32 |
| Partner’s age, median years | 29 | 28 | 29 | 29 | 29 | 29 | 29 | 30 | 29.5 |
| Partner’s median alcohol intake, servings/week (IQR) | 2 (0–4) | 0 (0–2) | 1 (0–3) | 2.5 (1–4.5) | 4 (1–7) | 0 (0–1) | 1 (0–3) | 3 (2–5) | 5 (2–8.5) |
| Educational level <16 years, N (%) | 841 (31.4) | 17 (23.3) | 90 (29.7) | 60 (25.5) | 13 (25.5) | 182 (44.3) | 234 (32.9) | 141 (23.0) | 104 (37.1) |
| Median hours per week of employment | 40 | 37 | 37 | 37 | 37 | 40 | 40 | 42 | 41.5 |
| Household income (SF/PRESTO) <24 999 DKK monthly/<50 000 USD annual, N (%) |
444 (16.6) | 15 (20.5) | 47 (15.5) | 25 (10.6) | 8 (15.7) | 117 (28.5) | 127 (17.8) | 63 (10.3) | 42 (15.0) |
| BMI, median kg/m2 | 26.2 | 25.2 | 24.7 | 24.8 | 24.7 | 27.5 | 27.2 | 26.1 | 27.1 |
| Physical activity, median MET hours/week | 29 | 33 | 37.2 | 33 | 31.1 | 22.8 | 28.4 | 30.4 | 26.9 |
| Previously fathered a child, N (%) | 1170 (43.7) | 42 (57.5) | 107 (35.3) | 83 (35.3) | 21 (41.2) | 216 (52.6) | 322 (45.2) | 252 (41.0) | 127 (45.4) |
| Frequency of intercourse ≥2 times/week, N (%) | 1664 (62.1) | 42 (57.5) | 201 (66.3) | 148 (63.0) | 31 (60.8) | 242 (58.9) | 444 (62.4) | 378 (61.6) | 178 (63.6) |
| Timing of intercourseb, N (%) | 2044 (76.3) | 55 (75.3) | 227 (74.9) | 175 (74.5) | 32 (62.7) | 311 (75.7) | 539 (75.7) | 490 (79.8) | 215 (76.8) |
| Attempt time >2 months at study entry, N (%) | 825 (30.8) | 31 (42.5) | 103 (34.0) | 72 (30.6) | 14 (27.5) | 143 (34.8) | 213 (29.9) | 171 (27.9) | 78 (27.9) |
| Regular smoking, N (%) | 182 (6.8) | * | 18 (5.9) | 17 (7.2) | * | 29 (7.1) | 32 (4.5) | 33 (5.4) | 45 (16.1) |
| Multivitamin use, N (%) | 878 (32.8) | 25 (34.2) | 87 (28.7) | 67 (28.5) | 14 (27.5) | 138 (33.6) | 234 (32.9) | 213 (34.7) | 100 (35.7) |
| Softdrinksc >1 serving/week, N (%) | 1371 (51.2) | 28 (38.4) | 83 (27.4) | 77 (32.8) | 24 (47.1) | 269 (65.5) | 406 (57.0) | 311 (50.7) | 173 (61.8) |
| Caffeine consumption >150 mg/day, N (%) | 1397 (52.1) | 27 (37.0) | 181 (59.7) | 155 (66.0) | 43 (84.3) | 129 (31.4) | 327 (45.9) | 350 (57.0) | 185 (66.1) |
| History of sexually transmitted infection and/or infection in male reproductive organs, N (%) | 353 (13.2) | 24 (32.9) | 83 (27.4) | 77 (32.8) | 12 (23.5) | 27 (6.6) | 47 (6.6) | 58 (9.4) | 25 (8.9) |
| Asthma and/or hayfever, N (%) | 523 (19.5) | 17 (23.3) | 81 (26.7) | 56 (23.8) | 9 (17.6) | 67 (16.3) | 127 (17.8) | 125 (20.4) | 41 (14.6) |
| Depression and/or anxiety, N (%) | 380 (14.2) | * | 25 (8.3) | 19 (8.1) | * | 67 (16.3) | 109 (15.3) | 92 (15.0) | 50 (17.9) |
| Non-Hispanic white, N (%) | - | - | - | - | - | 344 (83.7) | 582 (81.7) | 542 (88.3) | 257 (91.8) |
aIncludes drinkers who reported drinking <1 drink per week.
bHaving intercourse at the time of highest probability of conception.
cIncludes sugar-sweetened soda intake and juice.
*Low numbers. The precise numbers cannot be shown, according to the guidelines of Statistics Denmark.
SnartForaeldre: Danish study, PRESTO: North American Pregnancy Study Online, MET: metabolic equivalents, IQR = interquartile range.
Alcohol consumption and fecundability
In pooled analyses, adjusted FRs for 1–5, 6–13 and ≥14 standard servings per week compared with no alcohol consumption were 1.02 (95% CI: 0.90–1.17), 1.10 (95% CI: 0.96–1.27) and 0.98 (95% CI: 0.81–1.18), respectively (Table II). In cohort-stratified analyses, FRs in SnartForaeldre for 1–5, 6–13 and ≥14 standard servings per week compared with no alcohol consumption were 0.97 (95% CI: 0.73–1.28), 0.81 (95% CI: 0.60–1.10) and 0.82 (95% CI: 0.51–1.30), respectively. In PRESTO, FRs for 1–5, 6–13 and ≥14 standard servings per week compared with no alcohol consumption were 1.02 (95% CI: 0.88–1.18), 1.20 (95% CI: 1.03–1.40) and 1.03 (95% CI: 0.84–1.26), respectively. Similarly, the restricted cubic spline curve indicated little association between lower levels of alcohol consumption and fecundability (Fig. 2). Starting at approximately 8.5 servings weekly, the spline curves show an inverse association between male alcohol consumption and fecundability in SnartForaeldre, but not in PRESTO.
Table II.
Couples’ fecundability by male alcohol consumption.
| Fecundability ratio (FR: 95% CI) | ||||
|---|---|---|---|---|
| Servings/week | Pregnancies | Cycles | Unadjusted FR | Adjusted FR * |
| SnartForaeldre and PRESTO, N = 2679 | ||||
| None | 292 | 1906 | (Reference) | (Reference) |
| 1–5 | 651 | 3975 | 1.03 (0.91–1.17) | 1.02 (0.90–1.17) |
| 6–13 | 578 | 3217 | 1.11 (0.97–1.26) | 1.10 (0.96–1.27) |
| ≥14 | 206 | 1346 | 0.95 (0.80–1.12) | 0.98 (0.81–1.18) |
| SnartForaeldre, N = 662 | ||||
| None | 51 | 256 | (reference) | (reference) |
| 1–5 | 216 | 1087 | 0.93 (0.71–1.22) | 0.97 (0.73–1.28) |
| 6–13 | 153 | 940 | 0.80 (0.60–1.07) | 0.81 (0.60–1.10) |
| ≥14 | 30 | 192 | 0.75 (0.49–1.14) | 0.82 (0.51–1.30) |
| PRESTO, N = 2017 | ||||
| None | 241 | 1650 | (Reference) | (Reference) |
| 1–5 | 435 | 2888 | 1.01 (0.87–1.17) | 1.02 (0.88–1.18) |
| 6–13 | 425 | 2277 | 1.19 (1.03–1.37) | 1.20 (1.03–1.40) |
| ≥14 | 176 | 1154 | 0.99 (0.83–1.18) | 1.03 (0.84–1.26) |
*Adjusted for male and female age, female alcohol intake (continuous), frequency of intercourse, previously fathered a child, education, BMI, smoking, consumption of sugar-sweetened beverages and caffeine. There was further adjustment for race/ethnicity in PRESTO and study cohort in pooled analysis.
Figure 2.
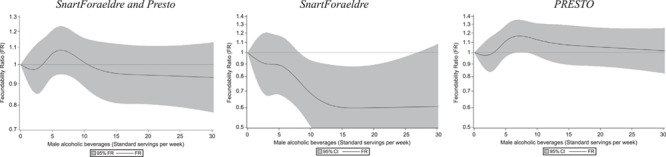
Association between male alcohol consumption and fecundability, examined using restricted cubic splines. Curves were adjusted for male and female age, female alcohol intake (continuous), frequency of intercourse, previously fathered a child, education, BMI, smoking, consumption of sugar-sweetened beverages and consumption of caffeine. PRESTO analyses were adjusted further for race/ethnicity. Five knots were located at 0, 3, 5, 10 and 18. CI: confidence interval.
In the pooled analysis (Table III), the relation between alcohol consumption and fecundability was similar across strata of attempt time at study entry, BMI and history of previously fathering a child. When we stratified by female age (<30 versus ≥30 years) and gravidity (yes versus no), alcohol consumption was slightly associated with decreased fecundability among older females and previous gravidity, though estimates were imprecise. In secondary analyses of the pooled data, in which we used a finer categorization of alcohol consumption, FRs for 14–20 and ≥21 standard servings per week compared with no alcohol consumption were 0.97 (95% CI: 0.79–1.21) and 0.99 (95% CI: 0.78–1.26), respectively.
Table III.
Male alcohol intake and couples’ fecundability, stratified by pregnancy attempt time at entry, male BMI, history of previously fathering a child, female age and gravidity (cohort = 2679).
| Alcohol intake, servings per week | ||||||||
|---|---|---|---|---|---|---|---|---|
| None | 1–5 | 6–13 | ≥14 | None | 1–5 | 6–13 | ≥14 | |
| ≤2 cycles of attempt at study entry | 3–6 cycles of attempt at study entry | |||||||
| Pregnancies, n | 207 | 492 | 429 | 153 | 85 | 159 | 149 | 53 |
| Cycles, n | 1212 | 2736 | 2352 | 1032 | 694 | 1239 | 864 | 314 |
| Adjusted FR (95% CI) | (Ref) | 1.06 (0.91–1.23) | 1.09 (0.92–1.28) | 0.97 (0.78–1.20) | (Ref) | 0.90 (0.70–1.16) | 1.14 (0.87–1.50) | 1.09 (0.76–1.57) |
| Male BMI <25kg/m 2 | Male BMI ≥25kg/m 2 | |||||||
| Pregnancies, n | 107 | 246 | 251 | 67 | 185 | 405 | 327 | 139 |
| Cycles, n | 647 | 1521 | 1346 | 454 | 1259 | 2454 | 1871 | 892 |
| Adjusted FR (95% CI) | (Ref) | 0.94 (0.76–1.17) | 1.03 (0.83–1.29) | 0.87 (0.64–1.19) | (Ref) | 1.07 (0.91–1.26) | 1.14 (0.95–1.37) | 1.04 (0.82–1.31) |
| Previously fathered a child | Did not previously father a child | |||||||
| Pregnancies, n | 157 | 285 | 249 | 90 | 135 | 366 | 329 | 116 |
| Cycles, n | 924 | 1519 | 1120 | 558 | 982 | 2456 | 2097 | 788 |
| Adjusted FR (95% CI) | (Ref) | 0.96 (0.81–1.15) | 1.10 (0.91–1.34) | 0.90 (0.69–1.17) | (Ref) | 1.08 (0.89–1.31) | 1.11 (0.91–1.36) | 1.08 (0.84–1.40) |
| Female age <30 years | Female age ≥30 years | |||||||
| Pregnancies, n | 160 | 364 | 281 | 103 | 132 | 287 | 297 | 103 |
| Cycles, n | 1092 | 2108 | 1515 | 647 | 814 | 1867 | 1702 | 699 |
| Adjusted FR (95% CI) | (Ref) | 1.15 (0.96–1.38) | 1.18 (0.97–1.43) | 1.06 (0.83–1.37) | (Ref) | 0.83 (0.69–1.00) | 0.96 (0.78–1.17) | 0.85 (0.65–1.12) |
| Gravidity ≥1 | Nulli-gravidity | |||||||
| Pregnancies, n | 160 | 285 | 247 | 83 | 132 | 366 | 331 | 123 |
| Cycles, n | 907 | 1571 | 1168 | 494 | 999 | 2404 | 2049 | 852 |
| Adjusted FR (95% CI) | (Ref) | 0.89 (0.74–1.07) | 0.99 (0.82–1.20) | 0.85 (0.65–1.12) | (Ref) | 1.16 (0.96–1.41) | 1.22 (0.99–1.51) | 1.12 (0.86–1.44) |
Discussion
Key findings
In these prospective preconception cohort analyses, we found little evidence of a consistent association between male alcohol consumption and fecundability in pooled analyses. In SnartForaeldre, consumption of 6–13 and ≥14 servings per week were associated with slightly reduced fecundability; however, the estimates were imprecise. Clinically, this finding indicates a slightly lower probability of conception in each menstrual cycle among couples in which the male partner consumed 6–13 or ≥14 servings per week compared with no alcohol intake. The restricted cubic spline analyses also indicated reduced fecundability with heavier alcohol consumption among men in SnartForaeldre, but not in PRESTO or both cohorts combined.
Although we used the same methods in SnartForaeldre and PRESTO overall, it is possible that minor differences, such as in the measure of drinks per week or unmeasured confounding by other behavioral factors, could have affected study-specific estimates. In pooled analyses, no appreciable association was observed for heavier alcohol consumption (14–20 and ≥21 standard servings per week) compared with no alcohol consumption.
Overall, there was limited variation in the amount of alcohol consumed, with most men consuming ≤14 drinks per week. Despite our relatively large study population compared with previous studies, results were imprecise in some stratified analyses.
Previous studies
The association between semen quality and fecundability has been shown to be approximately linear up to a concentration of 40 mill/Ml. Above this concentration elevated sperm counts do not markedly increase fecundability (Bonde et al., 1998). Therefore, the predictive value of semen quality for fecundability may be limited. This limitation may explain why some studies (Emanuele, 1998; Muthusami and Chinnaswamy, 2005; La Vignera et al., 2013; Jensen et al., 2014a; Borges, al., 2018) found an association between alcohol consumption and semen quality, whereas the association between alcohol consumption and fecundability was less evident.
Our findings are fairly consistent with previous studies that showed no or weak associations between male alcohol consumption and fecundability. A retrospective European multicenter study found that male alcohol consumption was slightly associated with prolonged TTP, when odds for subfecundity (TTP >9.5 months) for male alcohol consumption of 0–7 drinks per week were compared with ≥22 drinks per week (odds ratio (OR) = 1.3, 95% CI: 0.9–1.7) (Olsen et al., 1997). Another retrospective cohort study found little association between male alcohol consumption and TTP (FRs comparing 0.1–2, 2.1–6 and >6 ounces per week with no alcohol consumption were 1.05 (95% CI: 0.96–1.15) 1.02 (95% CI: 0.90–1.10) and 0.95 (95% CI: 0.83–1.09), respectively). However, heavier drinking of more than 10 glasses of beer or 6 glasses of liquor per week was associated with reduced fecundability (FR = 0.88 (95% CI: 0.75–1.02) and FR = 0.87 (95% CI: 0.71–1.06), respectively) (Curtis et al., 1997). Another prospective cohort study found that male alcohol consumption was positively associated with fecundability when ≥10 drinks per week were consumed compared with <5 drinks per week (OR = 1.6, 95% CI: 1.0–2.4) (Florack et al., 1994). In that study, 259 females with unrestricted pregnancy attempt time at study entry (21.6% had been trying to conceive for >1 year) were interviewed about their male partner’s alcohol intake. In contrast, men in our study reported their own alcohol intake and couples were enrolled in the preconception period, with 80.7% of couples enrolled within their first 3 cycles of pregnancy attempt.
Strengths and limitations
Our study population included the entire spectrum of fertility, from highly fertile to subfertile couples. However, we studied only pregnancy planners, which may overestimate TTP since unintended pregnancies are more likely to occur among highly fertile couples and alcohol consumers (Oulman et al., 2015). To address potential misclassification due to changes in alcohol consumption resulting from subfertility, we limited the study population to couples who had tried to conceive for ≤6 cycles at study entry.
Although the study population included self-selected couples recruited via the Internet, it seems unlikely that the association between male alcohol intake and fecundability differs between Internet users and nonusers. Participants in our study may be more health-conscious than non-participants (e.g. men are more likely to be non-smokers), but that should not influence the validity of the internal comparisons within the cohorts. Previous validation studies have shown that even when characteristics (such as age or smoking) differ between study participants and non-participants, well-established perinatal associations are not biased by self-selection (Nilsen et al., 2009; Hatch et al., 2016). Cohort retention was 88.8% after 12 months of follow-up, and overall we found similar baseline characteristics, including alcohol consumption, for couples with complete follow-up compared with couples lost to follow-up.
We collected detailed information on covariates and adjusted for potential confounders, but we cannot entirely rule out the possibility of residual confounding. In addition, we did not distinguish between regular and binge drinking (defined as at least five drinks per occasion) (National Institute on Alcohol Abuse and Alcoholism, 2004). Also, except for caffeine and sugar-sweetened beverages, we did not include information on male dietary habits or metabolic factors, e.g. lipid status, body fat or blood sugar level, which may have confounded or mediated the association between male alcohol intake and fecundability (Salas-Huetos et al., 2017; Sundaram et al., 2017; Maresch et al., 2018; Sedes et al., 2018). Male health and history of chronic disease, including cancer, are other potential confounders, which have been associated with low semen quality in some studies (Eisenberg et al., 2015; Ventimiglia et al., 2015). However, the prevalence of chronic disease among male participants was low and approximately equally distributed across levels of alcohol consumption in our cohorts.
Self-reported alcohol consumption was not validated. If alcohol intake was inaccurately reported, it was most likely underreported (Høyer et al., 1995; Ekholm, 2004). Although inaccuracies in self-reported alcohol intake would be independent of prospectively collected information on pregnancy status, such non-differential misclassification could explain the weak associations observed in our study. Finally, we examined alcohol intake at baseline only, which could have potentially biased our results if male alcohol intake changed with increasing pregnancy attempt time. However, studies of males and females in general populations have reported monthly stability in alcohol consumption over time for low to moderate drinkers, particularly when follow-up is short (Kerr et al., 2002; Knott et al., 2017).
In summary, we found little evidence in the combined cohorts of an association between male alcohol consumption and fecundability in couples. Data from only the Danish cohort indicate that consumption of six or more servings of alcohol per week may be weakly associated with reduced fecundability, but the estimates were imprecise.
Acknowledgements
We thank Tina Christensen for her help with data collection and media contacts and Kathryn A. McInerney, Sydney Willis, Tanran Wang and Michael Bairos for their assistance with data management. We are grateful to all the couples who participated.
Authors’ roles
E.E.H., K.J.R., L.A.W., H.T.S. and E.E.M. designed the study. S.H. took the lead on writing the manuscript. A.H.R. and A.K.W. conducted the statistical analyses. All authors interpreted the results and critically revised the article for publication. All authors approved the final manuscript.
Funding
This study was supported by the National Institutes of Health (R01-HD060680, R01-HD086742, R21-HD050264, R21-HD072326, R03-HD090315), the Novo Nordisk Foundation, Oticon Fonden, Politimester J.P.N. Colind og hustru Asmine Colinds mindelegat, and Erna og Peter Houtveds studielegat. PRESTO receives in-kind donations from FertilityFriend.com, Kindara.com, Swiss Precision Diagnostics, and Sandstone Diagnostics for the collection of data pertaining to fertility.
Conflict of interest
Dr Wise serves as a consultant on uterine leiomyomata for AbbVie.com. All other authors declare no conflict of interest.
References
- Ainsworth BE, Haskell WL et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:498–504. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Ernst E, Jensen TK et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet 1998;352:1172–1177. [DOI] [PubMed] [Google Scholar]
- Borges E Jr, Braga D, Provenza RR, Figueira RCS, Iaconelli A Jr, Setti AS. Paternal lifestyle factors in relation to semen quality and in vitro reproductive outcomes. Andrologia 2018; e13090. [DOI] [PubMed] [Google Scholar]
- Christensen T, Riis AH, Hatch EE, Wise LA, Nielsen MG, Rothman KJ, Toft Sorensen H, Mikkelsen EM. Costs and efficiency of online and offline recruitment methods: a web-based cohort study. J Med Internet Res 2017;19: e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JA, Burrows EA, Wilan AR. The prognosis for live birth among untreated infertile couples. Fertil Steril 1995;61:44–52. [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- Curtis KM, Savitz DA, Arbuckle TE. Effects of cigarette smoking, caffeine consumption, and alcohol intake on fecundability. Am J Epidemiol 1997;146:32–41. [DOI] [PubMed] [Google Scholar]
- Danish National Board of Health National Recommandation on alcohol consumption 2015 https://sundhedsstyrelsen.dk/da/sundhed-og-livsstil/alkohol . 2015.
- Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril 2015;103:66–71. [DOI] [PubMed] [Google Scholar]
- Ekholm O. Influence of the recall period on self-reported alcohol intake. Eur J Clin Nutr 2004;58:60–63. [DOI] [PubMed] [Google Scholar]
- Emanuele MAE. Alcohol's effects on male reproduction. Alcohol Health Res World 1998;22:195–201. [PMC free article] [PubMed] [Google Scholar]
- Florack EM, Zielhuis GA, Rolland R. Cigarette smoking, alcohol consumption, and caffeine intake and fecundability. Prev Med 1994;23:175–180. [DOI] [PubMed] [Google Scholar]
- Frank L. When an entire country is a cohort. Science 2000;287:2398–2399. [DOI] [PubMed] [Google Scholar]
- Hansen ML, Thulstrup AM, Bonde JP, Olsen J, Hakonsen LB, Ramlau-Hansen CH. Does last week's alcohol intake affect semen quality or reproductive hormones? A cross-sectional study among healthy young Danish men. Reprod Toxicol 2012;34:457–462. [DOI] [PubMed] [Google Scholar]
- Harlev A, Agarwal A, Gunes SO, Shetty A, Plessis SS. Smoking and male infertility: an evidence-based review. World J Mens Health 2015;33:143–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, Hahn KA et al. Evaluation of selection bias in an internet-based study og pregnancy planners. Epidemiology 2016;27:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch EE, Wesselink AK, Hahn KA, Michiel JJ, Mikkelsen EM, Sorensen HT, Rothman KJ, Wise LA. Intake of sugar-sweetened beverages and fecundability in a North American preconception cohort. Epidemiology 2018;29:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer G, Nilssen O, Brenn T, Schirmer H. The Svalbard study 1988-89: a unique setting for validation of self-reported alcohol consumption. Addiction 1995;90:539–544. [DOI] [PubMed] [Google Scholar]
- Irvine DS. Epidemiology and aetiology of male infertility. Hum Reprod 1998;13:33–44. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Gottschau M, Madsen JO, Andersson AM, Lassen TH, Skakkebaek NE, Swan SH, Priskorn L, Juul A, Jorgensen N. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open 2014a;4: e005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Swan S, Jorgensen N, Toppari J, Redmon B, Punab M, Drobnis EZ, Haugen TB, Zilaitiene B, Sparks AE et al. Alcohol and male reproductive health: a cross-sectional study of 8344 healthy men from Europe and the USA. Hum Reprod 2014b;29:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr WC, Filimore KM, Bostrom A. Stability of alcohol consumption over time: evidence from three longitudinal surveys from the United States. J Stud Alcohol 2002;63:325–333. [DOI] [PubMed] [Google Scholar]
- Knott CS, Bell S, Britton A. The stability of baseline-defined categories of alcohol consumption during the adult life-course: a 28-year prospective cohort study. Addiction 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupka MS, Ferraretti AP, Mouzon J, Erb K, D'Hooghe T, Castilla JA, Calhaz-Jorge C, De Geyter C, Goossens V. European Ivf-Monitoring Consortium ftESoHR et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHREdagger. Hum Reprod 2014;29:2099–2113.25069504 [Google Scholar]
- La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl 2013;15:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneesh M, Dutta S, Chakrabarti A, Vasudevan DM. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol 2006;50:291–296. [PubMed] [Google Scholar]
- Maresch CC, Stute DC, Alves MG, Oliveira PF, Kretser DM, Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Hum Reprod Update 2018;24:86–105. [DOI] [PubMed] [Google Scholar]
- Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort profile: the Danish web-based pregnancy planning study—‘Snart-Gravid’. Int J Epidemiol 2009;38:938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusami KR, Chinnaswamy P. Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril 2005;84:919–924. [DOI] [PubMed] [Google Scholar]
- National Food Institute Danish food composition tables, https://frida.fooddata.dk. Accessed 20-09-2018. Version 3, 2016.
- National Institute on Alcohol Abuse and Alcoholism https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking Accessed 09-07-2019. 2004.
- Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol 2009;23:597–608. [DOI] [PubMed] [Google Scholar]
- Olsen J, Bolumar F, Boldsen J, Bisanti L. Does moderate alcohol intake reduce fecundability? A European multicenter study on infertility and subfecundity. Alcohol Clin Exp Res 1997;21:206–212. [PubMed] [Google Scholar]
- Oulman E, Kim TH, Yunis K, Tamim H. Prevalence and predictors of unintended pregnancy among women: an analysis of the Canadian Maternity Experiences Survey. BMC Pregnancy Childbirth 2015;15:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci E, Noli S, Ferrari S, La Vecchia I, Cipriani S, De Cosmi V, Somigliana E, Parazzini F. Alcohol intake and semen variables: cross-sectional analysis of a prospective cohort study of men referring to an Italian Fertility Clinic. Andrology 2018;6:690–696. [DOI] [PubMed] [Google Scholar]
- Salas-Huetos A, Bullo M, Salas-Salvado J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update 2017;23:371–389. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol 2013;27:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedes L, Thirouard L, Maqdasy S, Garcia M, Caira F, Lobaccaro JA, Beaudoin C, Volle DH. Cholesterol: a gatekeeper of male fertility? Front Endocrinol (Lausanne) 2018;9:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Rohrmann S, Menke A, Selvin E, Crespo CJ, Rifai N, Dobs A, Feinleib M, Guallar E, Platz EA. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control 2009;20:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama R, Hansen OK, Ducot B, Bohet A, Sorensen D, Giorgis Allemand L, Eijkemans MJ, Rosetta L, Thalabard JC, Keiding N et al. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum Reprod 2012;27:1489–1498. [DOI] [PubMed] [Google Scholar]
- Soares SR, Melo MA. Cigarette smoking and reproductive function. Obstet Gynecol 2008;20:281–291. [DOI] [PubMed] [Google Scholar]
- Sterne JA, White IR, JRea C. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram R, Mumford SL, Buck Louis GM. Couples’ body composition and time-to-pregnancy. Hum Reprod 2017;32:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The U.S. Department of Health and Human Services and the U.S. Department of Agriculture Dietary Guidelines 2015-2020 http://health.gov/dietaryguidelines/2015/guidelines/ Accessed 06-04-2017. 2015.
- Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril 2013;99:1324–1331.e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventimiglia E, Capogrosso P, Boeri L, Serino A, Colicchia M, Ippolito S, Scano R, Papaleo E, Damiano R, Montorsi F et al. Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril 2015;104:48–55. [DOI] [PubMed] [Google Scholar]
- Wang YA, Healy D, Black D, Sullivan EA. Age-specific success rate for women undertaking their first assisted reproduction technology treatment using their own oocytes in Australia, 2002-2005. Hum Reprod 2008;23:1633–1638. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol 1989;129:1072–1078. [DOI] [PubMed] [Google Scholar]
- WHO Global status report on alcohol and health. http://apps.who.int/iris/bitstream/10665/112736/1/9789240692763_eng.pdf?ua=1. 2014.
- Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Hum Reprod 2010;25:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Rothman KJ, Mikkelsen EM, Stanford JB, Wesselink AK, McKinnon C, Gruschow SM, Horgan CE, Wiley AS, Hahn KA et al. Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol 2015;29:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Wesselink AK, Mikkelsen EM, Cueto H, Hahn KA, Rothman KJ, Tucker KL, Sorensen HT, Hatch EE. Dairy intake and fecundability in 2 preconception cohort studies. Am J Clin Nutr 2017;105:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]


