Objective
Explored the mechanism of action of tanshinone IIA (TIIA) against atherosclerosis.
Methods
ApoE−/− mice were divided into two groups of 10: model and TIIA. A control group of 10 wild-type mice was created. ApoE−/− mice were fed a high-fat diet for 12 weeks. The TIIA group received TIIA once daily. Mice were anesthetized, blood collected by cardiac puncture, and the aortic sinus/arch collected for histology and molecular studies, respectively.
Results
Mice intima in the model group had large areas of plaque formation, serum levels of total cholesterol (TC), triglycerides, and low-density lipoprotein-cholesterol (LDL-C) increased significantly, and high-density lipoprotein-cholesterol (HDL-C) levels decreased significantly in the model group after 12 weeks. Staining [hematoxylin and eosin (H&E), Oil-Red-O] showed that the aorta had lesions, a higher degree of plaque formation, and considerable lipid deposition in model-group mice. After TIIA treatment, expression of HDL-C was increased significantly and that of TC, triglycerides and LDL-C decreased significantly, and plaque size and lipid deposition improved obviously. Analyses of protein phosphorylation in aortic tissue suggested that the transforming growth factor (TGF)-β/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/endothelial nitric oxide synthase (eNOS) pathway was activated in TIIA-treated mice.
Conclusion
TIIA can lower levels of serum lipids, stabilize atherosclerotic plaques, reduce endothelial injury, and inflammatory damage by activation of the TGF-β/PI3K/Akt/eNOS pathway.
Keywords: atherosclerosis, endothelial nitric oxide synthase, phosphatidylinositol 3-kinase/protein kinase B, tanshinone IIA, transforming growth factor β
Introduction
The incidence of cardiovascular/cerebrovascular diseases is increasing worldwide, and atherosclerosis is the main cause of cardiovascular/cerebrovascular diseases [1,2]. The study of the mechanism, prevention, and treatment of atherosclerosis is a hot topic in clinical medicine. Research suggests that atherosclerosis is a chronic inflammatory response. Hyperlipidemia, hypertension, high blood sugar levels, high levels of uric acid, obesity and other factors lead to injury to vascular endothelial cells, and thickening of vascular walls [3].
Tanshinone IIA (TIIA) is a component of Salvia miltiorrhiza, a perennial plant highly valued for its roots in traditional Chinese medicine. Previously, we showed that TIIA can lower lipid levels in serum, reduce lipid deposition in the liver, and regulate reverse cholesterol transport in hyperlipidemic rats and apolipoprotein E-deficient (ApoE−/−) mice to have a positive effect on atherosclerosis treatment, but its mechanism of action (MoA) is incompletely understood [4,5].
The present study is based on our previous research (effect of TIIA on aorta-related proteins in ApoE−/− mice using antibody chips), but we also explored the MoA of TIIA in atherosclerosis treatment.
Materials and methods
Ethical approval of the study protocol
The present study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, Maryland, USA). All protocols were approved by the Animal Ethics Committee of Liaoning University of Traditional Chinese Medicine (Liaoning, China).
Mice experiments
ApoE−/− mice and wildtype male mice with a C57BL/6 background were obtained from Beijing Vital Laboratory Animal Technology (Beijing, China).
Male ApoE−/− mice were divided randomly into two groups of 10: model and TIIA. A control group of 10 wildtype mice was also created. ApoE−/− mice were fed a high-fat diet [6,7] containing 21% butter fat and 0.15% cholesterol (Specialty Feeds, Memphis, Tennessee, USA) for 12 weeks commencing from 8 weeks of age. The TIIA group received 1.2 mL of sodium tanshinone IIA (10 mg/kg, i.p.; Shanghai Number One Biochemical Pharmaceuticals, Shanghai, China) once daily. Then, mice were anesthetized (5% isoflurane). Blood was collected by cardiac puncture, and the aortic sinus and arch collected for histology and molecular studies, respectively.
Plasma levels of cholesterol and triglycerides
Plasma levels of cholesterol and triglyceridess were determined by enzymatic means using a chemistry autoanalyzer (CX7; Beckman Coulter Diagnostics, Fullerton, California, USA). Plasma levels of triglycerides were measured with an Infinity Triglycerides kit (Thermo Scientific, Waltham, Massachusetts, USA). Levels of high-density lipoprotein-cholesterol (HDL-C) and low-density lipoprotein-cholesterol (LDL-C) were determined using homogeneous assay kits (Equal Diagnostics, Exton, Pennsylvania, USA).
Analyses of serum levels of tumor necrosis factor-α, interleukin-6, nitric oxide and endothelin-1 by ELISA
Blood from normal mice and atherosclerosis model mice was collected. The supernatant was harvested after centrifugation (2500 × g for 15 minutes at room temperature). Serum levels of tumor necrosis factor-α (TNF-α) (Dakewe, Beijing, China), interleukin-6 (IL-6) (Dakewe), nitric oxide (mlbio, Shanghai, China) and endothelin-1 (ET-1) (mlbio, Shanghai, China) were measured by ELISA. Experiments were undertaken according to manufacturer instructions. Values were averaged after repeating the experiments twice. In addition, the nitric oxide:ET-1 ratio was calculated.
Ultrasound of the aorta
Mice were anesthetized with isoflurane inhalation. They were fixed on a pad and inhaled 2% isoflurane continuously. Hair was removed from the xiphoid to the upper thighs to reduce ultrasound attenuation. A coupling agent was given at the exposed area. Detection of the aorta was done using an ultrahigh-resolution small-animal ultrasound imaging system (Vevo 2100, VisualSonics, Canada). Using a MS250 ultrasound probe, the probe frequency was set at 30 MHz and the gain to 10 dB. The total gain and sectional gain were adjusted to ensure that the echo at the front and back was uniform, and the dynamic range was more than 50 dB. The direction of the probe was 90°. The notch was toward the lower jaw of the mouse, and the probe was moved slowly to the right side of the sternum until a clear image was obtained.
Quantification of atherosclerotic lesions
The proximal aorta was dissected, embedded in optimum cutting temperature compound (Tissue-Tek; Sakura, Osaka, Japan) and frozen at −80°C. Sections (6 µm) of aortic tissues were analyzed for lesion size. The latter was defined as the cross-sectional surface area of an intimal lesion area according to staining (H&E, Oil-Red-O) within the aortic intima. Quantification of the extent and composition of the aortic lesions was done using methods described previously [4]. The mean size of a lesion for each mouse was calculated from measurement of cross sections in the ascending aorta [8,9]. The percentage area stained positively with Oil-Red-O was quantified employing computer-assisted image-analysis software (ImagePro, Silver Springs, Maryland, USA).
Phosphoprotein antibody microarray
Expression of phosphoproteins was detected and analyzed using a Phosphoprotein Antibody Microarray kit (Wayen Biotechnologies, Shanghai, China). Analyses were carried out using the protocol provided. Briefly, protein was extracted, lysates were purified and then the protein was labeled by biotinylation. Hybridized chips were scanned with GenePix 4000B (Molecular Devices, Silicon Valley, California, USA). GenePix Pro 6 (Molecular Devices) was used to read the original data and carry out one-step analysis [10,11].
Protein analyses by western blotting
The proximal aortas from ApoE−/− mice and control mice were lysed in 0.5 mL of 1% NP-40 lysis buffer [50 mM Tris (pH 7.2), 500 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM MgCl2 with 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 mM phenylmethane sulfonyl fluoride]. Protein lysates were quantified by the Bradford method. Lysates were resolved by SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose paper and blocked with a mixture of tris-buffered saline and Tween 20 (TBST) supplemented with 5% nonfat milk.
Blots were probed with appropriate primary antibodies: rabbit antitransforming growth factor (TGF)-β antibody (1:1000 dilution; Cell Signaling Technology, Danvers, Massachusetts, USA); rabbit antiphosphatidylinositol 3-kinase (PI3K) antibody (1:1000; Cell Signaling Technology); rabbit anti-protein kinase B (Akt) antibody (1:1000; Cell Signaling Technology); phospho-Akt (Ser473) rabbit mAb (1:1000; Cell Signaling Technology); rabbit antiendothelial nitric oxide synthase (eNOS) antibody (1:1000; Cell Signaling Technology); rabbit anti-eNOS (Phospho-Ser1179) antibody (1:1000; A Biochemicals, Shanghai, China); and rabbit anti-β-actin antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, California, USA). After washing, membranes were incubated with a peroxidase-conjugated secondary antibody (mouse antirabbit immunoglobulin-G; 1:3000 dilution; Cell Signaling Technology) for TGF-β, PI3K, Akt, p-Akt, eNOS, p-eNOS, and β-actin. Proteins were visualized using an electrochemiluminescence kit (Pierce, Rockford, Illinois, USA). Images were collected with a special system (5200; Tanon Science and technology, Beijing, China). Experiments were carried out thrice.
Analyses of gene expression by quantitative reverse transcription-PCR
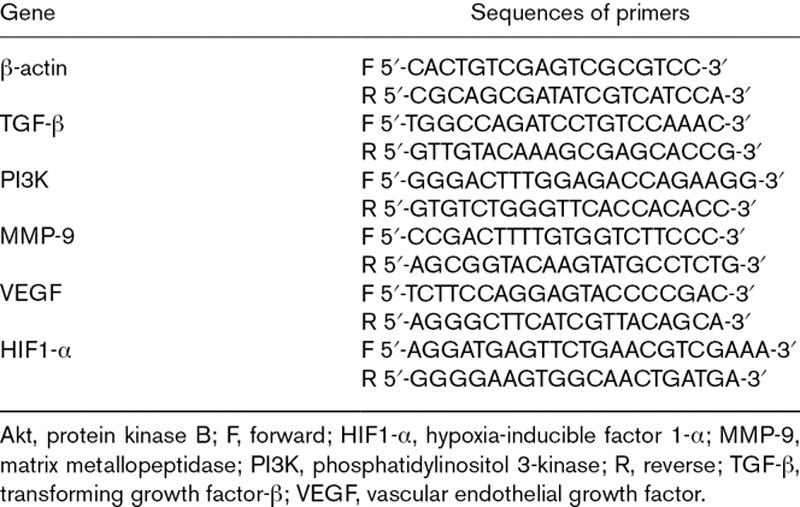
Total RNA was extracted from the proximal aortas of ApoE−/− and control mice by TRIzol Reagent according to manufacturer (Sigma–Aldrich, Saint Louis, Missouri, USA) instructions. Then, it was dissolved in sterile water and quantified by spectrophotometry at 260 nm, after which it was reverse-transcribed using TaqMan methodology (TaKaRa Biotechnology, Dalian, China). RT-qPCR was done on ABI Prism v2.04 (Applied Biosystems, Foster City, California, USA) using an ABI 7500 PCR instrument (Applied Biosystems) to determine the expression of specific genes using an SYBR Green PCR mix (TaKaRa Biotechnology). The PCR conditions were 30 seconds at 95°C followed by 45 cycles of 95°C for 5 seconds and 60°C for 34 seconds. The specific primers used to amplify genes are listed in Table 1. Relative amounts of mRNA for specific genes were calculated using 2−ΔΔCt values. Each sample was run in duplicate, the mean value of each set of duplicates normalized to that of mouse β-actin and used to calculate relative gene expression. Experiments were carried out thrice.
Table 1.
Sequences of primers used for quantitative reverse transcription-PCR
Statistical analyses
Data are the mean ± SD. Statistical analyses between two groups were done using the Student’s t-test. For multiple comparisons, the P value was determined by one-way ANOVA. Analyses were carried out with Prism 6 (GraphPad, San Diego, California, USA) and SPSS v20 (IBM, Armonk, New York, USA). P <0.05 was considered significant.
Results
Analyses of serum lipids by an automatic biochemical analyzer
After establishing an atherosclerosis model and TIIA treatment, there were significant changes in the levels of all serum lipids (Fig. 1). In atherosclerotic mice, levels of total cholesterol (TC), triglycerides, and LDL-C were increased significantly (P < 0.05) and that of HDL-C decreased significantly (P < 0.05) compared with those of the normal group. In TIIA-treated mice, the HDL-C level was increased significantly (P < 0.05) and levels of TC, triglycerides, and LD-C decreased significantly (P < 0.05) compared with those of the atherosclerosis model group.
Fig. 1.
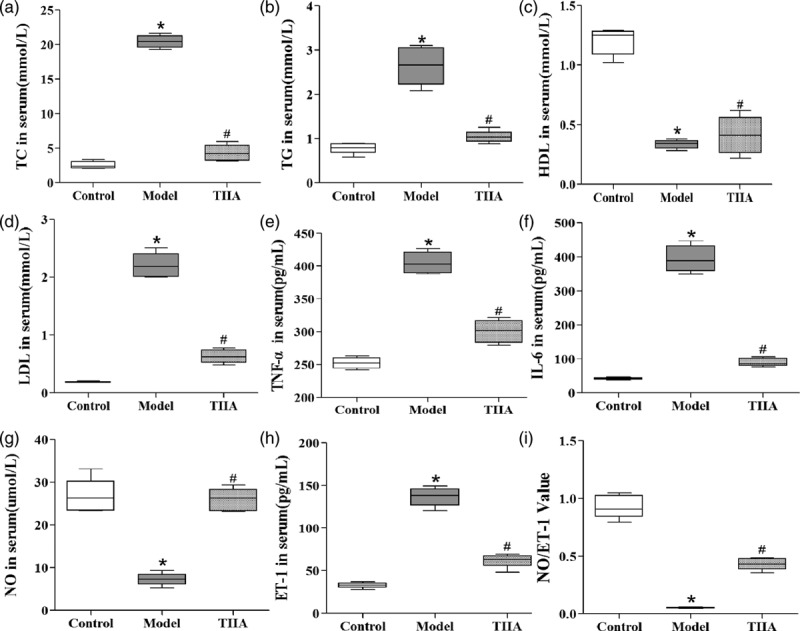
Changes in levels of lipid and compounds in serum by atherosclerosis and TIIA treatment. The level of (a) TC, (b) triglyceride, (c) HDL-C, (d) LDL-C, (e) TNF-α, (f) IL-6, (g) nitric oxide, (h) ET-1 and (i) the nitric oxide:ET-1 ratio. Values are the mean ± SD. *P < 0.05 compared with the normal group, #P < 0.05 compared with the atherosclerosis model group. N = 10 per group. ET-1, endothelin; HDL-C, high-density lipoprotein-cholesterol; IL-6, interleukin; LDL-C, low-density lipoprotein-cholesterol; TIIA, tanshinone IIA; TNF-α, tumor necrosis factor; TC, total cholesterol.
Analyses of inflammatory markers in serum by ELISA
After establishing an atherosclerosis model and TIIA treatment, there were significant changes in the serum levels of inflammatory markers (Fig. 1). In atherosclerotic mice, levels of TNF-α, IL-6 and ET-1 were increased significantly (P < 0.05), and the nitric oxide level and nitric oxide:ET-1 ratio decreased significantly (P < 0.05) compared with the normal group. In TIIA-treated mice, the nitric oxide level and nitric oxide:ET-1 ratio were increased significantly (P < 0.05) and the levels of TNF-α, IL-6 and ET-1 decreased significantly (P < 0.05) compared with the atherosclerosis model group.
Formation of atherosclerotic plaques in mouse aortas according to ultrasound
The aortic lumen was narrowed, and intima thickened in the model group (Fig. 2). The aortic lumen was widened and intima thinner in the TIIA-treated group.
Fig. 2.
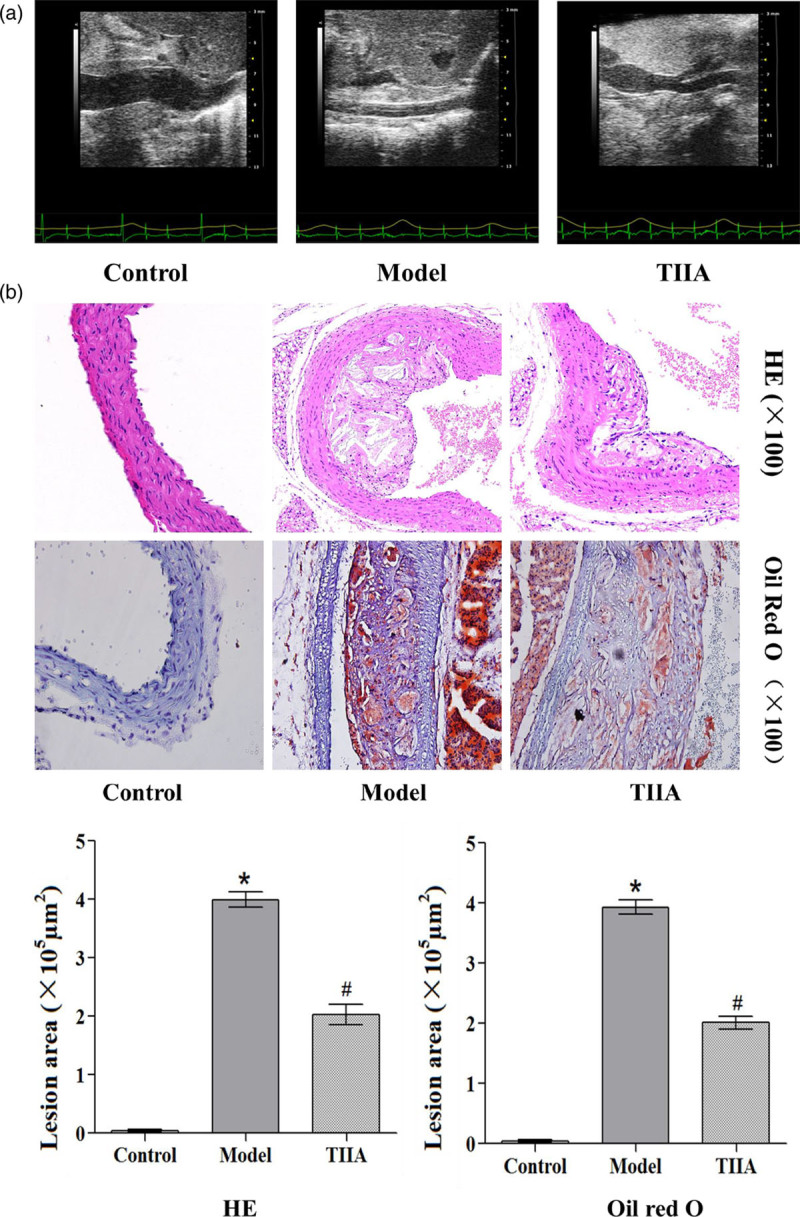
Formation of atherosclerotic plaques and lesions in aortas by hematoxylin and eosin, Oil-Red-O staining and ultrasound detection. *P < 0.05 compared with the normal group, #P < 0.05 compared with the atherosclerosis model group.
Pathologic and lipid-deposition changes observed by staining
Atherosclerotic lesions in the aortic root were assessed. Photomicrographs show the atherosclerotic lesions. Atherosclerosis assessment was done based on the intimal areas of atherosclerotic lesions (H&E) and lipid accumulation (Oil-Red-O) (Fig. 2). The intimal lesion areas shown by H&E staining were obviously larger in the model group and TIIA group than those in wildtype mice (P < 0.05), and the areas were nearly double in the atherosclerosis model group compared with those in the TIIA group. Lipid accumulation shown by Oil-Red-O staining was obvious in the atherosclerosis model group and TIIA group compared with wildtype mice (P < 0.01), especially in the atherosclerosis model group (P < 0.05 compared with the TIIA group) (Fig. 2).
Analyses of protein phosphorylation in aortic tissue by antibody microarrays
Protein phosphorylation was investigated using an antibody microarray system in the aortic tissue of atherosclerotic mice and after TIIA treatment. Results from six replicate samples were averaged (including the actin value). Analysis and prediction of the results by Ingenuity Pathway Analysis database shown that the TGF-β/PI3K/Akt/eNOS pathway may be activated in TIIA-treated mice.
Expression of transforming growth factor-β and phosphatidylinositol 3-kinase, endothelial nitric oxide synthase phosphorylation, and protein kinase B phosphorylation in aortic tissue by western blotting
We examined the expression of related proteins in aortic tissue by western blotting. Figure 3 shows the western blots of TGF-β, PI3K, eNOS phosphorylation, and Akt phosphorylation. In atherosclerotic mice, expression of TGF-β and PI3K was decreased significantly (P < 0.05) and, after TIIA treatment, was upregulated significantly (P < 0.05) (Fig. 3a and b). Also, levels of eNOS phosphorylation and Akt phosphorylation were decreased significantly (P < 0.05) (Fig. 3c and d), and they were upregulated significantly by TIIA (P < 0.05).
Fig. 3.
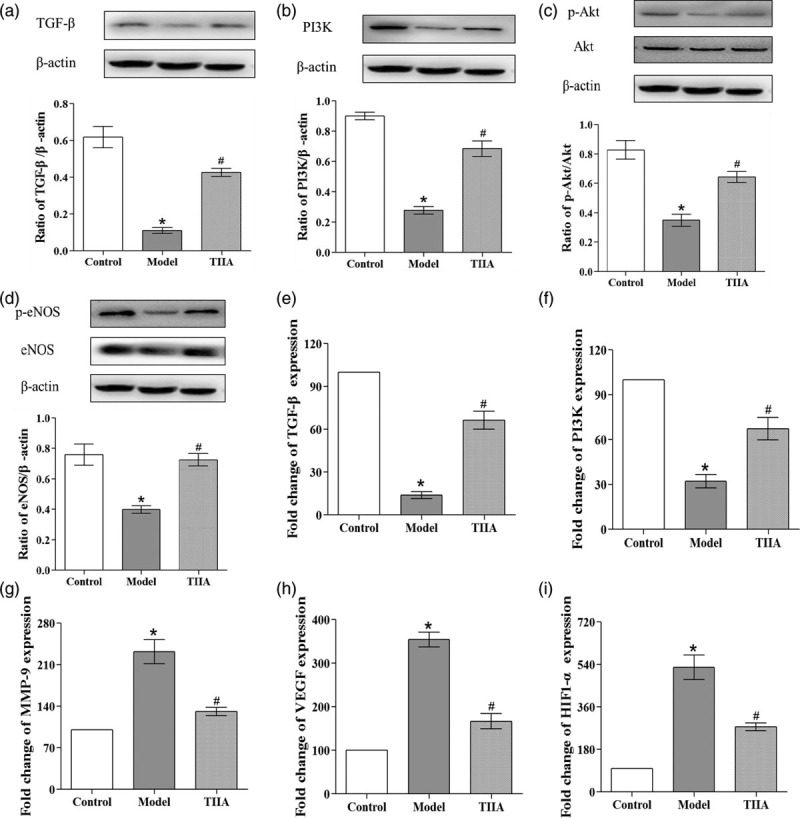
Analyses of protein and mRNA levels in the atherosclerotic lesions of aortic roots. Expression of (a) TGF-β, (b) PI3K, (c) p-Akt and (d) p-eNOS proteins in aortic tissue, and densitometric analyses relative to β-actin. Expression of (e) TGF-β, (f) PI3K, (g) MMP-9, (h) VEGF, and (i) HIF1-α mRNAs in aortic tissue. *P < 0.05 compared with the normal group, #P < 0.05 compared with the atherosclerosis model group, N = 3 for each group; values are the mean ± SD. Akt, protein kinase B; eNOS, endothelial nitric oxide synthase; HIF1-α, hypoxia-inducible factor 1-α; MMP-9, matrix metallopeptidase; PI3K, phosphatidylinositol 3-kinase; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
Analyses of gene expression in aortic tissue by quantitative reverse transcription-PCR
Expression of related genes in aortic tissue was detected by RT-qPCR. Figure 3 shows the mRNA levels of TGF-β, PI3K, matrix metallopeptidase (MMP)-9, vascular endothelial growth factor (VEGF), and hypoxia-inducible factor (HIF)1-α in aortic tissue. In atherosclerotic mice, the mRNA levels of TGF-β and PI3K were decreased significantly (P < 0.05) (Fig. 3d and e) and, after TIIA treatment, they were upregulated significantly (P < 0.05). Expression of the mRNA of MMP-9, VEGF, and HIF1-α was increased significantly in atherosclerotic mice (P < 0.05) (Fig. 3f–i), and was downregulated significantly after TIIA treatment (P < 0.05).
DISCUSSION
TIIA is one of the major components of Salvia miltiorrhiza, and exerts a number of beneficial health actions including antiatherosclerosis and antihyperlipidemia. In this study, we demonstrated the protective effect of TIIA in high-fat diet-induced atherosclerosis in Apoe−/− mouse model. Our data showed that TIIA could reduce the size of aortic plaque and decrease the serum lipid-related factors levels, including TC and triglyceride, alleviate inflammatory response, which is in agreement with the results of previous studies. Next our morphological data showed that treatment with TIIA could lower plaque formation and lipid deposition. In the present study, we also found that TGF-β/PI3K/Akt/eNOS pathway was activated in TIIA-treated mice. Taken together, our data showed that TIIA has its protective effects in atherosclerosis by reducing the size of plaque and other related serum factors may partly due to the activation of TGF-β/PI3K/Akt/eNOS pathway.
TGF-β is a cytokine involved in the growth, apoptosis, differentiation, and proliferation of cells, as well as the growth of tissues, organs and the immune response [12]. TGF-β has important roles in the injury to vascular endothelial cells, lipid deposition, and inflammatory response which cause atherosclerosis. Studies have suggested that TGF-β has bidirectional roles in atherosclerosis. In the early stage of atherosclerosis, TGF-β has anti-inflammatory and antioxidant effects, which can inhibit the proliferation of vascular smooth muscle cells (VSMCs); in the late stage of the disease, TGF-β can promote VSMC proliferation, promote vascular fibrosis, and accelerate plaque formation [13,14]. In recent years, studies have found that TGF-β has important roles in the stability of atherosclerotic plaques and can inhibit monocyte/macrophage chemotaxis, reduce VSMC proliferation, inhibit the absorption of oxidized low-density lipoprotein, reduce lipid absorption, and have anti-inflammatory and antioxidant effects [15,16].
PI3K/Akt is an important downstream pathway of TGF-β. TGF-β can activate expression of the PI3K/AKT signaling pathway [17]. The PI3K family is a type of kinase which is catalyzed specifically by phosphoryl inositol. It is used widely in various types of cells and is involved in various activities. PI3K is divided into types I, II, and III, and each type is divided into subtypes [18]. Akt (also known as protein kinase B) is a serine/threonine kinase, which is divided into types of 1, 2, and 3 (also called protein kinase-α, -β, and -γ, respectively). It is an important downstream target of PI3K. If Akt is activated by PI3K phosphorylation, it can act on the downstream target proteins Bad, caspase-9, glycogen synthase kinase-3β, mechanistic target of rapamycin and eNOS. Akt plays an important part in the proliferation, apoptosis and autophagy of cells [19,20].
Nitric oxide is an endogenous antiatherosclerosis factor. If the concentration and activity of nitric oxide decrease, then the intracellular concentration of free Ca+ will increase. This will cause vasoconstriction, alter cell permeability and cause the dysfunction of vascular endothelial cells to accelerate atherosclerosis. The nitric oxide level is an important indicator of endothelial-cell function [21]. Nitric oxide is produced mainly by endothelial cells, which is under the catalysis of the eNOS. Akt is activated by PI3K and phosphorylates eNOS to promote the synthesis and release of endogenous nitric oxide, which protects vascular endothelial cells [22,23]. TNF-α and IL-6 are crucial inflammatory factors in atherosclerosis. ET-1 is an important factor regulating cardiovascular function. ET-1 plays an important part in maintaining vascular tension and stability of the cardiovascular system. The nitric oxide:ET-1 ratio can reflect the degree of vascular injury.
We also detected genes closely related to angiogenesis in atherosclerosis: HIF-1α, VEGF, and MMP-9. Hypoxia accelerates atherosclerosis progression. Hypoxia can increase the number of reactive oxygen species of vascular endothelial cells, aggravate lipid oxidation, and lead to the degeneration of proteins and nucleic acids [24]. HIF-1α is synthesized by various types of macrophages and is a transcriptional factor that has an active role in hypoxia. HIF-1α can promote angiogenesis; infiltration of inflammatory cells; SMC proliferation/migration in atherosclerotic plaques; formation of macrophage foam cells. Simultaneously, a low concentration of HIF-1α can activate the transcription and expression of VEGF and epidermal growth factor receptors, thereby promoting angiogenesis further [25]. VEGF is a multifunctional cell factor that can function specifically in the vascular endothelium. It has important roles in the division, proliferation, and migration of endothelial cells, and promotes angiogenesis in atherosclerotic plaques. VEGF can aggravate atherosclerosis by altering vascular permeability and causing vascular-wall edema [26]. During the remodeling of atherosclerotic vessels, MMP-9 can degrade various protein components of the extracellular matrix and participate directly in the formation and rupture of atherosclerotic plaques [27]. Our results showed that the mRNA levels of MMP-9, VEGF, and HIF1-α in aortic tissue were increased significantly in atherosclerotic mice and were downregulated significantly after TIIA treatment.
Conclusion
TIIA can lower levels of serum lipids, stabilize atherosclerotic plaques, as well as reduce injury to the endothelium and inflammation. These phenomena may be achieved by activation of the TGF-β/PI3K/Akt/eNOS pathway.
Acknowledgements
We are grateful to Shanghai Wayen Biotechnologies Company for the antibody microarray technique.
J.W. and X.H. performed the majority of experiments and wrote the paper, W.C. helped in experimental design, L.Z., N.Z., and J.G. performed ELISA and RT-PCR, J.L. performed staining and western blotting, H.S. performed animal studies, and L.J. and G.Y. helped in experimental design and reviewed the paper.
The work was supported by the National Basic Research Program of China (2013CB513704), the National Natural Science Foundation of China (81300229 and 81874372), the open fund from Key Laboratory of Ministry of Education for TCM Viscera-State Theory and Applications, Liaoning University of Traditional Chinese Medicine (zyzx1503, zyzx1902), Young and Middle-aged Science and Technology Innovation Talent Support Plan of Shenyang, China (RC180069).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Dr. Junyan Wang and Xinyong He contributed equally to this article.
References
- 1.Sun Y, Jiang C, Jiang J, Qiu L. Dexmedetomidine protects mice against myocardium ischaemic/reperfusion injury by activating an AMPK/PI3K/akt/enos pathway. Clin Exp Pharmacol Physiol. 2017; 44:946–953 [DOI] [PubMed] [Google Scholar]
- 2.Maki KC, Davidson MH, Dicklin MR, Bell M, Witchger M, Feinstein SB. Predictors of anterior and posterior wall carotid intima media thickness progression in men and women at moderate risk of coronary heart disease. J Clin Lipidol. 2011; 5:141–151 [DOI] [PubMed] [Google Scholar]
- 3.Zmysłowski A, Szterk A. Current knowledge on the mechanism of atherosclerosis and pro-atherosclerotic properties of oxysterols. Lipids Health Dis. 2017; 16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang GL, Jia LQ, Wu J, Ma YX, Cao HM, Song N, Zhang N. Effect of tanshinone IIA on oxidative stress and apoptosis in a rat model of fatty liver. Exp Ther Med. 2017; 14:4639–4646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia LQ, Zhang N, Xu Y, Chen WN, Zhu ML, Song N, et al. Tanshinone IIA affects the HDL subfractions distribution not serum lipid levels: involving in intake and efflux of cholesterol. Arch Biochem Biophys. 2016; 592:50–59 [DOI] [PubMed] [Google Scholar]
- 6.Li Y, To K, Kanellakis P, Hosseini H, Deswaerte V, Tipping P, et al. CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin- and granzyme B-dependent cytotoxicity. Circ Res. 2015; 116:245–254 [DOI] [PubMed] [Google Scholar]
- 7.Koulis C, Chen YC, Hausding C, Ahrens I, Kyaw TS, Tay C, et al. Protective role for toll-like receptor-9 in the development of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2014; 34:516–525 [DOI] [PubMed] [Google Scholar]
- 8.Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, et al. CD40 ligand mediates inflammation independently of CD40 by interaction with mac-1. Circulation. 2007; 115:1571–1580 [DOI] [PubMed] [Google Scholar]
- 9.Ai D, Jiang H, Westerterp M, Murphy AJ, Wang M, Ganda A, et al. Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis. Circ Res. 2014; 114:1576–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taghavian O, Jain A, Joyner CJ, Ketchum S, Nakajima R, Jasinskas A, et al. Antibody profiling by proteome microarray with multiplex isotype detection reveals overlap between human and aotus nancymaae controlled malaria infections. Proteomics. 2018; 18:e1870115. [DOI] [PubMed] [Google Scholar]
- 11.Platonov AE, Toporkova MG, Kolyasnikova NM, Stukolova OA, Dolgova AS, Brodovikova AV, et al. Clinical presentation of ixodes tick-borne borreliosis caused by Borrelia miyamotoi in the context of an immune response to the pathogen. Ter Arkh. 2017; 89:35–43 [DOI] [PubMed] [Google Scholar]
- 12.Bobik A, Agrotis A, Kanellakis P, Dilley R, Krushinsky A, Smirnov V, et al. Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation. 1999; 99:2883–2891 [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa T, Lan HY, Zhu HJ, Kang DH, Schreiner GF, Johnson RJ. Differential regulation of VEGF by TGF-beta and hypoxia in rat proximal tubular cells. Am J Physiol Renal Physiol. 2004; 287:F658–F664 [DOI] [PubMed] [Google Scholar]
- 14.Shi X, DiRenzo D, Guo LW, Franco SR, Wang B, Seedial S, Kent KC. TGF-β/smad3 stimulates stem cell/developmental gene expression and vascular smooth muscle cell de-differentiation. Plos One. 2014; 9:e93995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castañares C, Redondo-Horcajo M, Magán-Marchal N, ten Dijke P, Lamas S, Rodríguez-Pascual F. Signaling by ALK5 mediates TGF-beta-induced ET-1 expression in endothelial cells: a role for migration and proliferation. J Cell Sci. 2007; 120:1256–1266 [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Li M, Wang Z, He S, Ma X, Li D. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J Lipid Res. 2010; 51:1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu WT, Huang KY, Lu MC, Huang HL, Chen CY, Cheng YL, et al. TGF-β upregulates the translation of USP15 via the PI3K/AKT pathway to promote p53 stability. Oncogene. 2017; 36:2715–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrealba N, Rodriguez-Berriguete G, Fraile B, Olmedilla G, Martínez-Onsurbe P, Sánchez-Chapado M, et al. PI3K pathway and bcl-2 family. Clinicopathological features in prostate cancer. Aging Male. 2018; 21:211–222 [DOI] [PubMed] [Google Scholar]
- 19.Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017; 9:E1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao KH, Zhang C, Bai Y, Li Y, Kang X, Chen JX, et al. Antiglioma effects of cytarabine on leptomeningeal metastasis of high-grade glioma by targeting the PI3K/akt/mtor pathway. Drug Des Devel Ther. 2017; 11:1905–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katakam PV, Wappler EA, Katz PS, Rutkai I, Institoris A, Domoki F, et al. Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2013; 33:752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CY, Wang LX, Dong SS, Hong Y, Zhou XH, Zheng WW, Zheng C. Phlorizin exerts direct protective effects on palmitic acid (PA)-induced endothelial dysfunction by activating the PI3K/AKT/enos signaling pathway and increasing the levels of nitric oxide (NO). Med Sci Monit Basic Res. 2018; 24:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hao HF, Liu LM, Pan CS, Wang CS, Gao YS, Fan JY, Han JY. Rhynchophylline ameliorates endothelial dysfunction via src-PI3K/akt-enos cascade in the cultured intrarenal arteries of spontaneous hypertensive rats. Front Physiol. 2017; 8:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain T, Nikolopoulou EA, Xu Q, Qu A. Hypoxia inducible factor as a therapeutic target for atherosclerosis. Pharmacol Ther. 2018; 183:22–33 [DOI] [PubMed] [Google Scholar]
- 25.Sigala F, Efentakis P, Karageorgiadi D, Filis K, Zampas P, Iliodromitis EK, et al. Reciprocal regulation of enos, H2S and CO-synthesizing enzymes in human atheroma: correlation with plaque stability and effects of simvastatin. Redox Biol. 2017; 12:70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Keller G. VEGF nuclear accumulation correlates with phenotypical changes in endothelial cells. J Cell Sci. 2000; 113Pt 91525–1534 [DOI] [PubMed] [Google Scholar]
- 27.Weekman EM, Wilcock DM. Matrix metalloproteinase in blood-brain barrier breakdown in dementia. J Alzheimers Dis. 2016; 49:893–903 [DOI] [PubMed] [Google Scholar]


