Abstract
Background
Cholesterol efflux capacity is a tissue culture assay for HDL function that is not amenable for high-throughput monitoring of risk assessment.
Methods
We devised a cell-free HDL function assay to measure the exchange rate of exogenous apoA1 into serum HDL using NBD/Alexa647 double-labeled apoA1, whose NBD/Alexa647 emission ratio increased upon exchange into HDL. ApoA1 exchange rate (AER) was assayed by incubating labeled apoA1 with human serum, and the rate of the increase of the NBD/Alexa647 ratio over time was calculated as AER.
Results
Fast protein liquid chromatography analysis of serum confirmed that the labeled apoA1 selectively exchanged into the HDL lipoprotein fraction. Characterization studies demonstrated that the AER assay had excellent intra- and inter-day reproducibility, was stable over 3 freeze-thaw cycles, and yielded similar results with serum or plasma. We quantified AER in serum from randomly selected stable subjects undergoing elective diagnostic coronary angiography (n = 997). AER was correlated with HDL-cholesterol (r = 0.58, P < 0.0001) and apoA1 levels (r = 0.56, P < 0.0001). Kaplan-Meier survival plot showed subjects in the lowest quartile of AER experienced a significantly higher rate of incident major adverse cardiovascular events (MACE = myocardial infarction, stroke, or death) (P < 0.0069 log rank). Moreover, compared to subjects in the lowest AER quartile, the remaining subjects showed significantly lower incident (3 year) risk for MACE, even after adjustment for traditional risk factors and apoA1 (HR 0.58; 95% CI 0.40–0.85; P = 0.005).
Conclusions
In a prospective cohort of stable subjects undergoing elective diagnostic cardiac evaluations, low AER was associated with increased incident risk of MACE.
Keywords: ApoA1 exchange rate, HDL, major adverse cardiovascular events
Introduction
Low levels of high density lipoprotein-cholesterol (HDL-C) are associated with increased incidence of cardiovascular disease (CVD) in epidemiological studies (1, 2). However, recent niacin and cholesteryl ester transfer protein inhibitor drug trials to increase HDL-C, as well as a genetic Mendelian randomization study failed to show that HDL-C levels are causally linked to CVD risk (3–7). As HDL is a major player in reverse cholesterol transport (RCT), the process of removing peripheral cholesterol to the liver for excretion into the bowel, the concept has emerged that low HDL function, rather than low HDL-C, is mechanistically linked with CVD. There is a growing literature that an HDL function assay, cholesterol efflux capacity (CEC) of apoB-depleted serum, is inversely associated with prevalent CVD; and, in some, but not all, studies, CEC is inversely associated with incident major adverse cardiovascular events (MACE) (8–11). The CEC assay requires cell culture and cannot be performed as a rapid, cost-effective clinical risk assessment assay. Therefore, developing a clinically applicable HDL function assay is of great interest. Cell-free assays for HDL structure and activity have been described previously in attempt to find simpler ways to assess HDL function (12–17). An HDL-apoA1-exchange end point assay, using spin-labeled lipid-free apoA1, has shown that apoA1 exchange into apoB-depleted plasma is correlated with CEC in two small human studies; however, this assay has not been evaluated in a prospective CVD cohort (12, 13). ApoA1 is freely exchangeable between lipid-free and lipid-bound state (14, 18), and lipid-free apoA1 is the major ABCA1-dependent acceptor to initiate the RCT pathway and mobilize cholesterol from peripheral tissues. ApoA1 exchange into HDL is an indication of HDL remodeling, which might be an indicator of HDL’s cholesterol efflux capacity via ABCA1-dependent or ABCA1-independent pathways, or its altered protein or lipid composition that may influence HDL’s antiatherogenic properties.
We previously reported a dual fluorescent labeled apoA1 lipidation indicator that measures apoA1 lipidation after incubation with liposomes or cultured cells (19). This indicator was labeled with two fluorophores – one that is lipid sensitive (7-nitrobenz-2-oxa-1, 3-diazole, NBD) and another that is insensitive to lipidation (Alexa647). Using this dual fluorophore labeled reporter, we predicted that the ratio of NBD/Alexa647 fluorescence emission could serve as an indicator of apoA1 lipidation, independent of apoA1 concentration. In the current study, we develop and characterize an apoA1 exchange rate (AER) assay utilizing the apoA1 lipidation indicator as a probe to measure the rate of apoA1 exchange into HDL. We then demonstrate in a large prospective cohort that the subjects in the lowest quartile of AER have increased incident MACE, even after adjustment for traditional risk factors including HDL-C and apoA1 levels.
Materials and Methods
Human Samples
To develop our assay we obtained fasting blood from healthy volunteers not taking lipid lowering medication under a Cleveland Clinic Institutional Review Board approved protocol. All subjects provided written informed consent. Serum samples were collected using red top clot activator Vacutainer tubes (BD #367820) or gold top serum separator SST Vacutainer tubes (BD #367986). Plasma samples were collected using EDTA Vacutainer tubes (BD #366643), lithium heparin Vacutainer tubes (BD # 5014830), or sodium citrate Vacutainer tubes (BD # 369714).
Human serum samples and associated clinical data were collected from a Cleveland Clinic outpatient cohort (BioBank) and an angiographic cohort (GeneBank) (11). All subjects studied provided written informed consent under protocols approved by the Cleveland Clinic Institutional Review Board. The outpatient BioBank cohort (n = 258) was comprised of sequential consenting subjects undergoing cardiac risk factor evaluation/modification in an outpatient preventive cardiology clinic at the Cleveland Clinic, as well as sequential consenting volunteers undergoing health screenings. Non-CVD control subjects represented 42.4% of the Biobank cohort. The angiographic cohort (n = 997) was comprised of randomly chosen GeneBank subset of stable subjects without evidence of acute coronary syndrome (troponin I < 0.03 ng/mL) who underwent elective diagnostic coronary angiography (cardiac catheterization or coronary computed tomography) for evaluation of coronary artery disease. All subjects in the angiographic cohort had extensive clinical and longitudinal outcomes data monitored, including adjudicated outcomes MACE and all cause of death over the ensuing 3 years after enrollment. MACE was defined as death, nonfatal myocardial infarction (MI), or nonfatal cerebrovascular accident (stroke) following enrollment. Subjects in the outpatient Biobank were not followed for longitudinal outcomes.
ApoA1 Exchange Indicator
Human apoA1 was purified from pooled plasma HDL (20), was labeled with NBD and Alexa Fluor 647, and characterized by fast protein liquid chromatography (FPLC) as described in the Supplemental methods (Supplemental Fig. 1).
ApoA1 Exchange Rate Assay
The standard protocol of the AER assay was performed by adding 5 µg of the apoA1 exchange indicator (10 µL) to 85 µL of phosphate buffered saline (PBS) in a 96 well plate, then 5 µL of human serum or plasma samples was added to each well. The plate was put into a 96 well fluorescent plate reader set at 37°C, and mixed for 15 s. The NBD (460 nm excitation, 540 nm emission) and Alexa 647 (640 nm excitation, 670 nm emission) fluorescence was read at 1 min intervals for 1 h. AER was calculated as the slope of linear regression of the NBD/Alexa647 ratio (excluding the nonlinear first 10 min). In order to account for inter-assay variation, sample results were normalized to the mean of AER of triplicate readings from a standard human serum pool run with every assay.
Statistical Analysis
Statistical tests are described in the supplemental material.
Results
ApoA1 Exchange Indicator Selectively Exchanges into HDL in Human Serum
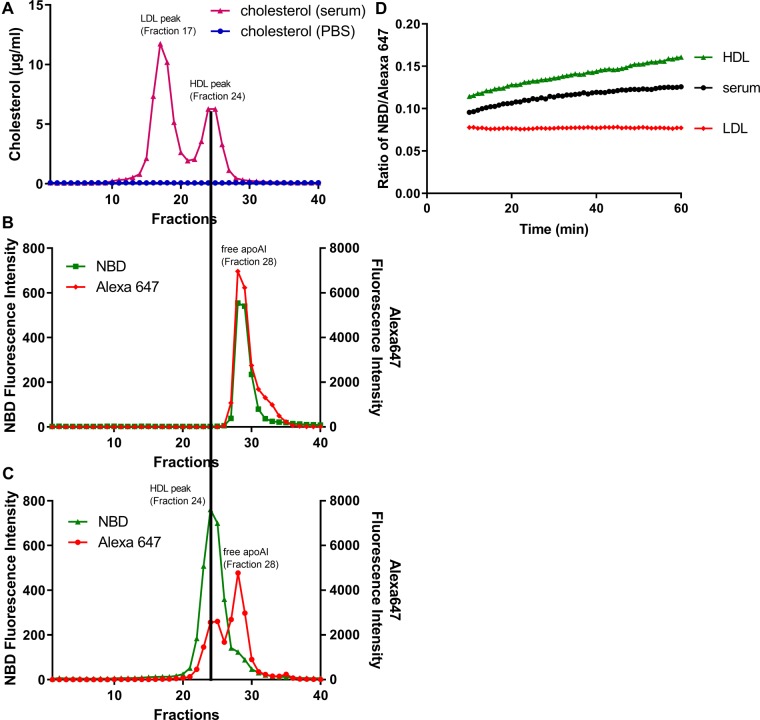
The apoA1 lipidation indicator was incubated with PBS or human serum for 1 h at 37°C and run on a size-exclusion FPLC Superose 6 (10/300 GL) column. The cholesterol concentration in each fraction showed that the LDL-C and HDL-C peaks were in fractions 17 and 24, respectively (Fig. 1A). For the apoA1 lipidation indicator in PBS, the NBD and Alexa 647 peaks co-localized in fraction 28 where lipid-free apoA1 migrates (Fig. 1B). However, for the apoA1 lipidation indicator incubated with human serum, the NBD peak was shifted to fraction 24, co-migrating with the HDL-C peak, and there was no peak co-migrating with LDL-C in fraction 17 (Fig. 1C). There were two Alexa 647 peaks, one that co-localized with the HDL-C fraction, and one that co-localized with lipid-poor apoA1. To confirm that the apoA1 lipidation indicator only exchanged into HDL and not LDL, we incubated apoA1 indicator with serum, human LDL (5 µg cholesterol), or human HDL (2.5 µg cholesterol) using our standard assay conditions. There was no increase of the NBD/Alexa 647 ratio with human LDL; however, this ratio increased with human serum or HDL (Fig. 1D). Therefore, the apoA1 exchange indicator selectively exchanged with HDL particles in human serum.
Fig. 1.
ApoA1 exchange indicator exclusively exchanges into HDL fraction in human serum. 100 µg apoA1 exchange indicator was incubated with 100 µL PBS (blue symbols) or normal human serum (pink symbols) in a total volume of 300 µL at 37°C for 1 h, and 100 µL of the product was size fractioned by FPLC using a Superose 6 (10/300 GL) column and 0.5 ml fractions were collected. The cholesterol concentration was measured in each fraction (A), with the LDL-C peak at fraction 17 and HDL-C peak at fraction 24. The fluorescent intensities of NBD (green symbols) and Alexa647 (red symbols) were measured in each fraction for the apoA1 exchange indicator incubated with PBS only (B), or incubated with human serum (C). D. 5 µg apoA1 exchange indicator was incubated with serum (black symbols), human LDL (5 µg LDL-C, red symbols), or human HDL (2.5 µg HDL-C, green symbols) at 37°C in a 96 well dish the NBD/Alexa647 fluorescence ratio determined at 1 min intervals over 1 h (excluding the nonlinear first 10 min), demonstrating the method used to calculate AER.
Impact Statement
Our studies demonstrated that the apoA1 exchange rate, an indicator of HDL remodeling and function, may be useful for patients at risk for a heart attack or stroke. Those subjects in the lowest quartile of this indicator were at increased risk for having a subsequent event, even after correcting for HDL-cholesterol and apoA1 levels. This is an advancement in this area as the described assay is a cell-free fluorescent ratiometric assay compared to other assays of HDL function that either require specialized equipment or rely on cell lines and are not amenable for high throughput clinical diagnostics.
AER Assay Characterization
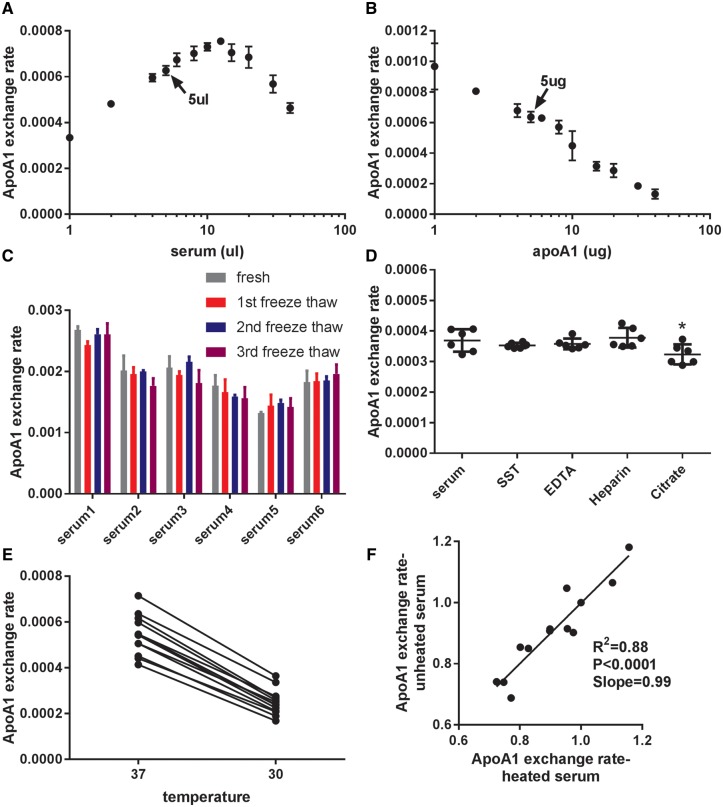
When apoA1 indicator was kept at 5 µg and serum varied between 1 and 40 µL, we observed a biphasic reaction with the peak rate obtained at 12.5 µL serum (Fig. 2A). The slower rate at higher serum doses may be due to competition of the exogenous indicator with lipid-free apoA1 in the serum. Therefore, we chose to use 5 µL of serum, which is in the middle of the rising linear part of the reaction in our AER assay. When serum was kept at 5 µL and apoA1 lipidation indicator varied between 1 and 40 µg, higher rates were observed with less apoA1 indicator added (Fig. 2B). The slower AER at higher levels of the indicator is probably due to a larger percentage of the indicator staying in the lipid-free form as the HDL acceptor is limiting, yielding a lower ratio of lipidated to nonlipidated indicator. To avoid the background noise at very low doses of apoA1, we used 5 µg apoA1 lipidation indicator in our standard assay.
Fig. 2.
Characteristics of apoA1 exchange assay. (A) Varying doses of human serum in the AER assay were incubated with 5 µg apoA1 exchange indicator (N = 3; mean±SD). (B) Varying doses of the apoA1 exchange indicator in the AER assay were incubated with 5 µL human serum (N = 3; mean±SD). (C) AER assay was performed with 6 control serum samples obtained freshly, and with the same samples subjected to 1–3 freeze-thaw cycles (N = 3; mean ± SD; P > 0.05 by ANOVA posttests). (D) AER assay was performed using one donor with different blood collection tubes: serum (clot activator tube), SST (serum separator tube), EDTA plasma, heparin plasma, or sodium citrate plasma (N = 6; mean ± SD; *P < 0.05 by ANOVA with Dunnett’s posttest). (E) AER assay was performed with 12 control serum samples at 37°C and 30°C. F. 12 control serum samples with or without heat pretreatment at 56°C for 1 h were assayed for AER.
To determine the freeze-thaw stability of serum in the AER assay, we compared 6 control freshly obtained serum samples from healthy donors with the same samples subjected to 1–3 cycles of freeze-thaw (dry ice to 37°C water bath). There was no loss of AER upon freeze-thaw cycles (Fig. 2C). We compared two preparations of serum and three plasma preparations (lithium heparin, EDTA, and sodium citrate) from one healthy donor in the AER assay. Compared to the standard serum red top tube, all of the preparations gave similar AER, except for the sodium citrate plasma, which yielded modest (12.4 ± 1.5%) but statistically significantly lower AER (Fig. 2D, P < 0.05 by ANOVA with Dunnett’s posttest). To demonstrate if AER was temperature dependent, we performed the assay at 37°C and 30°C with 12 control serum samples, and we observed faster AER at 37°C (Fig. 2E). To determine if the apoA1 exchange reaction was mediated by blood heat-labile enzymes, we compared 12 untreated control serum samples with the same samples heat-treated at 56°C for 1 h, a condition previously reported to inactivate serum lipases and lipid transfer proteins (21). There was no effect of serum heat treatment on AER, suggesting that the exchange of the indicator was not dependent on heat-labile plasma enzymes (Fig. 2F, R2 = 0.88, slope = 0.99).
To assess the precision of the AER assay, we performed AER with 6 replicates for each of 12 control serum samples sample on 12 different days. The AER intra-day coefficient of variation for each sample was 6.03% ± 3.17% (n = 144) (Supplemental Table 1), and inter-day coefficient of variation for each sample was 7.52% ± 2.27% (n = 12) (Supplemental Table 2).
AER is Correlated with ABCA1-Independent Cholesterol Efflux Capacity
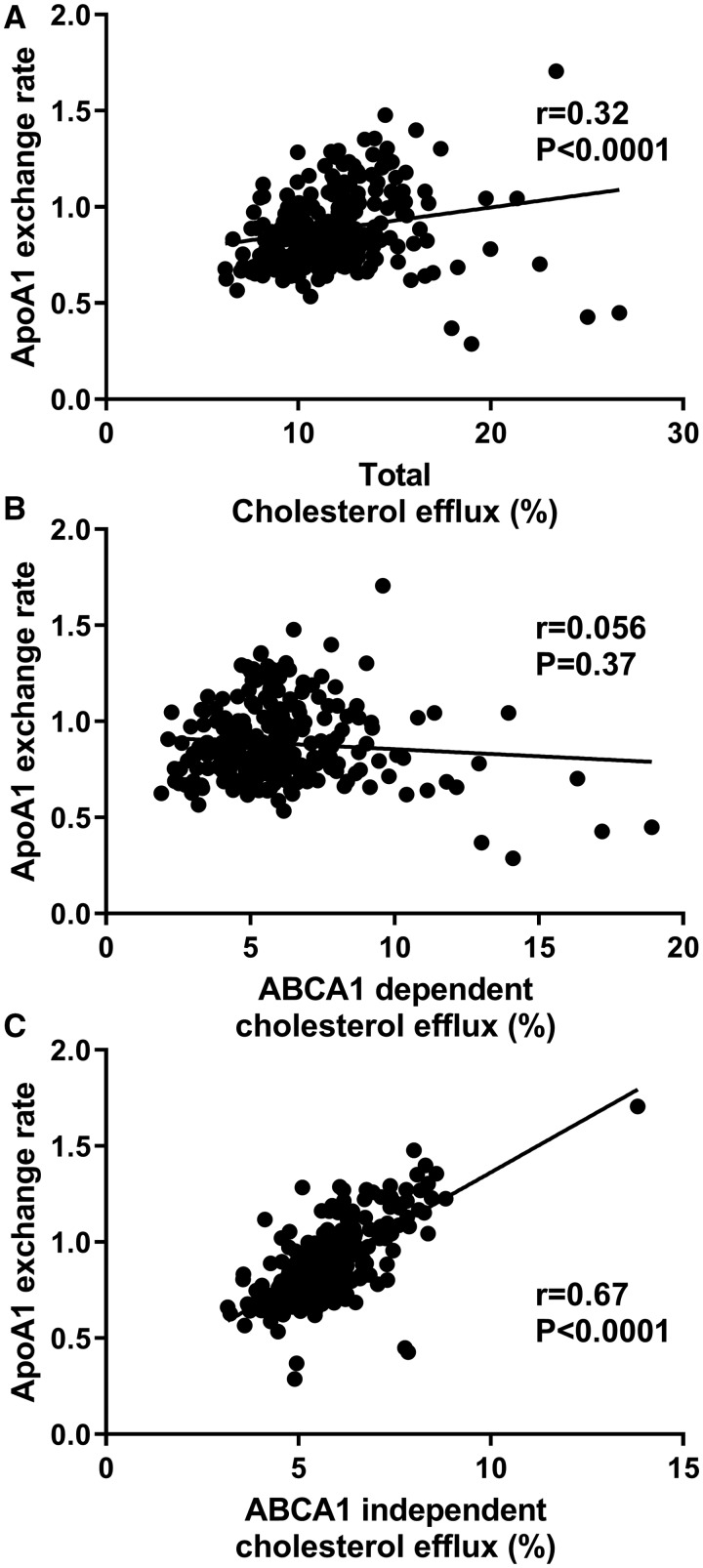
We obtained 258 human serum samples from an outpatient clinic and subjects with CVD had significantly higher age, more male, diabetes and hypertension (Supplemental Table 3). We assessed cholesterol efflux capacity using cholesterol labeled RAW264.7 macrophages, as previously described (11) (Supplemental Methods). We measured AER in these samples, and calculated the Spearman’s correlation coefficient r values with total efflux capacity (r = 0.32, P < 0.0001), ABCA1-dependent efflux capacity (r = 0.056, P = 0.37), and ABCA1-independent efflux capacity (r = 0.67, P < 0.0001), respectively (Fig. 3). Thus, AER was best correlated with the ABCA1-independent cholesterol efflux capacity.
Fig. 3.
Spearman’s correlation of apoA1 exchange rate to cholesterol efflux capacity. AER and total cholesterol efflux capacity (2% apoB-depleted serum, v/v with ABCA1 induction) were assayed in 258 human serum samples from the outpatient cohort (A). The ABCA1 dependent cholesterol efflux capacity (B) was calculated as the difference between total efflux capacity (A) and cells without ABCA1 induction (C). The lines were calculated by linear regression.
Low AER Is Associated with Increased Incident MACE
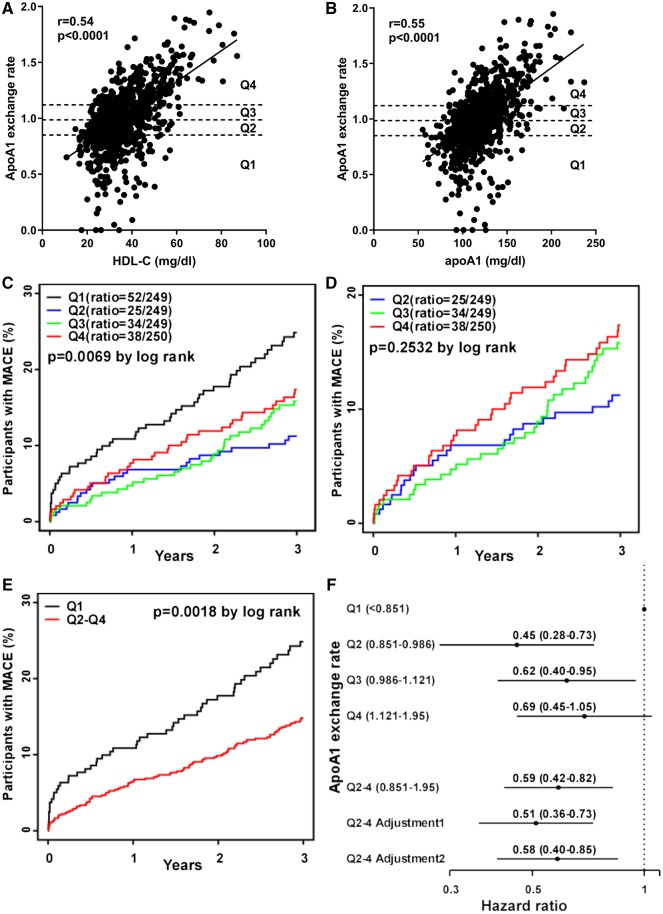
We next examined the relationship between AER and incident MACE in an independent angiographic cohort with longitudinal follow-up data, as described in the Methods. Patient demographics and clinical characteristics at time of enrollment are shown in Table 1. Over the subsequent 3 years following enrollment, 149 (14.9%) of these subjects had incident MACE (death, nonfatal MI, or nonfatal stroke). Subjects with subsequent MACE had significantly higher age, diabetes, C-reactive protein, baseline coronary artery disease (CAD), and baseline CVD, but lower apoA1 levels (Table 1). We observed significant positive correlations of AER with HDL-C (Fig. 4A) and apoA1 (Fig. 4B), to a lesser extent with total cholesterol, LDL-C and Lp(a), and an inverse correlation with triglycerides (Supplemental Table 4).
Table 1.
Baseline characteristics of the angiographic cohort.
| MACE (3 year) |
AER quartiles |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | All Subjects (N = 997) | No (N = 848) | Yes (N = 149) | P value | Q1 (n = 249) | Q2 (n = 249) | Q3 (n = 249) | Q4 (n = 250) | P value |
| Age (yr) | 62.7±10.6 | 61.9±10.5 | 67.3±9.9 | <0.0001 | 61.2±10.2 | 62.4±9.6 | 63.5±10.9 | 63.6±11.4 | 0.0491 |
| Male (%) | 65.6 | 65.8 | 64.4 | 0.82 | 73.9 | 72.7 | 63.5 | 52.4 | <0.0001 |
| Diabetes (%) | 36.1 | 34.3 | 46.3 | 0.0066 | 40.0 | 36.1 | 38.6 | 30.0 | 0.1056 |
| Hypertension (%) | 72.7 | 71.6 | 78.9 | 0.08 | 74.6 | 71.3 | 73.5 | 71.5 | 0.8146 |
| Smoking (%) | 66.6 | 66.0 | 69.8 | 0.42 | 69.1 | 71.9 | 65.1 | 60.4 | 0.0384 |
| LDL-C (mg/dL) | 96.0 (76.0– 118.0) | 96.0 (77.0– 118.0) | 92.0 (73.0– 116.0) | 0.17 | 91.0 (73.0– 115.0) | 96.0 (76.0– 122.0) | 97.0 (81.0– 114.0) | 97.5 (77.0– 119.0) | 0.0970 |
| HDL-C (mg/dL) | 33.8 (28.1– 41.2) | 34.1 (28.2– 41.5) | 32.5 (26.9– 39.3) | 0.07 | 29.1 (24.6– 34.3) | 30.5 (26.7– 36.1) | 34.3 (30.2– 40.4) | 43.5 (36.1– 53.0) | <0.0001 |
| Lp(a) (mg/dL) | 16.4 (6.6– 60.8) | 15.9 (6.6– 57.6) | 20.4 (6.7– 66.5) | 0.32 | 12.3 (6.6– 48.7) | 18.8 (6.4– 61.3) | 16.4 (7.2– 55.7) | 17.7 (7.6– 65.2) | 0.1876 |
| Total cholesterol (mg/dL) | 161.2 (140.5– 188.8) | 161.4 (141.3– 189.2) | 160.0 (135.0– 186.0) | 0.31 | 154.2 (132.0– 188.9) | 159.6 (136.7– 187.7) | 161.2 (142.6– 181.3) | 171.8 (148.2– 193.9) | 0.0006 |
| Triglycerides (mg/dL) | 116.0 (84.0– 172.0) | 114.5 (84.0– 172.2) | 127.0 (85.0– 170.0) | 0.47 | 141.0 (91.0– 221.0) | 132.0 (88.0– 187.0) | 112.0 (88.0– 153.0) | 97.0 (75.0– 132.8) | <0.0001 |
| apoA1 (mg/dL) | 115.0 (103.0– 131.0) | 116.0 (103.0– 132.0) | 113.0 (100.0– 124.0) | 0.0164 | 104.0 (93.0– 115.0) | 110.0 (101.0– 120.0) | 118.5 (107.0– 130.0) | 136.0 (122.0– 155.0) | <0.0001 |
| hsCRP (mg/L)a | 2.47 (1.09– 5.99) | 2.27 (0.99– 5.47) | 3.65 (1.89– 8.51) | <0.0001 | 3.12 (1.28– 8.16) | 2.24 (1.03– 5.32) | 2.21 (0.97– 4.67) | 2.56 (1.13– 5.78) | 0.0116 |
| Statin usage (%) | 58.8 | 59.3 | 55.7 | 0.46 | 63.9 | 63.5 | 55.4 | 52.4 | 0.0166 |
| Aspirin (%) | 71.6 | 72.2 | 68.5 | 0.41 | 76.3 | 67.1 | 73.1 | 70.0 | 0.1210 |
| CAD (%)b | 78.5 | 76.6 | 89.3 | 0.0008 | 88.3 | 83.5 | 75.8 | 66.3 | <0.0001 |
| PAD (%)c | 24.0 | 21.9 | 35.6 | <0.0001 | 24.1 | 26.5 | 26.1 | 19.2 | 0.2019 |
| CVD (%)d | 81.1 | 79.4 | 90.6 | 0.002 | 89.6 | 86.3 | 78.3 | 70.0 | <0.0001 |
Values are mean ± SD, %, or median (interquartile range).
P values were calculated by Wilcoxon rank-sum test for continuous data and Pearson’s chi-square test for categorical factors.
High-sensitivity C-reactive protein.
Coronary artery disease: myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft, angiography ≥50% stenosis in one or more major coronary vessels.
Peripheral artery disease: noncoronary arterial disease including carotid artery stenosis, upper extremity artery stenosis, mesenteric artery stenosis, renal artery stenosis, and lower extremity peripheral artery disease.
Cardiovascular disease: CAD and/or peripheral vascular disease.
Fig. 4.
Low apoA1 exchange rate is associated with increased incident MACE. AER was assayed in 997 serum samples obtained from a Cleveland Clinic angiographic cohort with 3 years follow-up. The Spearman’s correlation of AER with levels of HDL-C (A) and apoA1 (B) were plotted (r = 0.54 and 0.55, respectively); the lines were calculated by linear regression. (C) A Kaplan-Meier curve for incident MACE over 3 years in the AER Q1-Q4 (black, blue, green, red lines, respectively) yielded a significant log rank order for time to MACE (P = 0.0069). The number of deaths after 3-year follow up: Q1 = 32; Q2 = 12; Q3 = 17; and Q4 = 25. (D) A Kaplan-Meier curve for incident MACE over 3 years in the AER Q2-Q4 (blue, green, red lines, respectively) yielded no significant log rank order for time to MACE (P = 0.25). (E) A Kaplan-Meier curve for incident MACE over 3 years for AER Q1 (black line) and Q2–4 (red line) yielded a significant log rank order for time to MACE (P = 0.0018). (F) Forrest plot of the HR between AER and MACE plotted from Q1 to Q4. Q2–4 were pooled for subsequent analysis, yielding unadjusted HR (95% CI) = 0.59 (0.42–0.82). Multivariate adjustment 1 included age, sex, BMI, smoking, diabetes mellitus, hypertension, CVD, myeloperoxidase, LDL-C, triglycerides, statin, and aspirin with HR = 0.51 (0.36–0.73). Multivariate adjustment 2 includes adjustment 1 plus HDL-C and apoA1 levels with HR = 0.58 (0.40–0.85).
We divided AER values into four quartiles, and patient demographics and clinical characteristics of AER quartiles are shown in Table 1. Among the significant findings, AER Q1 had younger age, higher % male, lower total cholesterol, higher C-reactive protein, higher % statin usage, and higher prevalent CAD and CVD. We compared the time to incident MACE in a Kaplan-Meier plot that showed a significant log-rank effect (P = 0.0069) with the lowest AER quartile (Q1) having the fastest time to event, but without the expected step-wise time to event rank order of Q2>Q3>Q4 (Fig. 4C). Chi-square analysis (P = 0.0075) recapitulates the Kaplan-Meier findings; however, the chi-square test for linear trend was not significant (P = 0.11). Cubic Spline regression of AER and HR showed the trend of HR is decreasing from Q1 to Q2, with a slight rise from Q2 to Q4 (Supplemental Fig. 2A). Next we performed a separate Kaplan-Meier analysis for Q2, Q3, and Q4 and determined that the log-rank time to event was not statistically significant (P = 0.25, Fig. 4D). Thus, we combined Q2-Q4 and compared incident MACE vs. Q1 and found significantly faster progression to MACE for Q1, with the curves separating at the earliest time points (P = 0.0018, Fig. 4E). These results indicate that this assay may provide a prediction of short-term risk assessment beyond currently used measures. We performed 3-year endpoint analyses for incident MACE compared to Q1. Q2 (HR = 0.45, 95% CI 0.28–0.73) and Q3 (HR = 0.62, 95% CI 0.40–0.95) had significantly lower HR, while Q4 only trended in the same direction (HR = 0.69, 95% CI 0.45–1.05) (Fig. 4F). We further compared incident MACE of Q3 and Q4 vs. Q2, and there was no significant difference, justifying combining Q2–Q4 (Supplemental Fig. 2B, Q3 vs. Q2 P = 0.24, Q3 vs. Q2 P = 0.11).
Without further adjustment, Q2–4 had a HR for MACE of 0.59 (95% CI 0.42–0.82; P = 0.002). In the crude model adjusting just for age and sex (Supplemental Fig. 2C), Q2–4 had a lower HR for MACE (HR = 0.53, 95% CI 0.37–0.74) compared to unadjusted. We further performed multivariable adjustments for baseline demographic values and risk factors. Adjustment 1 included age, sex, BMI, smoking, diabetes, hypertension, CVD, myeloperoxidase (which selectively targets apoA1 for modification leading to its dysfunction), LDL-C, triglycerides, statin, and aspirin; and the Q2–4 HR for MACE was 0.51 (95% CI 0.36–0.73; P = 0.00021). Adjustment 2 included the above factors plus HDL-C and apoA1; and the Q2–4 HR for MACE was 0.58 (95% CI 0.40–0.85; P = 0.005). We examined what was different in the Q1 samples, and we observed that the AER levels fell below the correlation lines for both HDL-C and apoA1 (Fig. 4 A and B). We separately correlated AER with HDL-C for the samples in Q1–4, and we found that the AER levels in Q1 were not associated with HDL-C (Spearman’s r = -0.055, P = 0.39, Supplemental Fig. 3A). However, the AER levels in Q2, Q3, and Q4 samples were all positively and significantly correlated with HDL-C (Supplemental Fig. 3B–D). Similar findings were made with the separate correlations between Q1–4 AER with apoA1 levels, where only Q1 showed no significant correlation (Supplemental Fig. 4). We performed Kaplan-Meier and HR analyses for known risk factors HDL-C and apoA1 to compare risk assessment vs. AER. There was a dose response with the lower risk for higher quartiles of HDL and apoA1 levels. However, the Kaplan-Meier P-value for events using all four quartiles showed that the AER assay was a better predictor of MACE (P = 0.0069) than either HDL or apoA1 levels (P = 0.28 and 0.0363, respectively, Supplemental Results, Supplemental Figs 5 and 6).
Discussion
A consensus is building that HDL function may be a better target for therapeutic intervention than HDL-C levels. The most used assay for HDL function is the CEC assay; however, it is not amenable for routine risk assessment as it is a labor intensive cell-based assay usually performed with radioactive cholesterol. Here, we report a simple and fast HDL function assay, the AER assay, which is not cell based and works with frozen serum or plasma. We incubated NBD/Alexa647 double-labeled apoA1 with human serum/plasma, and the NBD/Alexa647 emission ratio increased upon lipid-free apoA1 exchange into HDL; therefore, we were able to quantify the rate of exchange of NBD/Alexa647 apoA1 by following the fluorescence ratio over time (defined as AER), as an indicator of HDL remodeling and function. In a large prospective cohort, we demonstrated that the subjects in the lowest quartile of AER have increased incident MACE after the adjustment for traditional risk factors including HDL-C and apoA1 levels. Therefore, AER assay could be a useful tool to identify patients at high risk for MI and stroke.
The exchangeability of apoA1 in HDL was described by Cavigiolio et al. (18). Upon addition of excess lipid-free apoA1 to reconstituted HDL (rHDL), they observed that virtually all of the apoA1 on the rHDL was replaced with the donor lipid-free apoA1 (18). This exchangeability was confirmed by a separate group using similar methods (14). An apoA1 exchange end-point assay was developed by incubating spin-labeled lipid-free apoA1 with apoB-depleted plasma at 37°C for 15 min, using electron paramagnetic resonance (EPR) spectrometry to quantify the lipidation of the apoA1 probe (12, 13). In a small human study, HDL-apoA1-exchange was found to be lower in apoB-depleted plasma from acute coronary syndrome patients (n = 16) compared to healthy control donors (n = 9) (12). HDL-apoA1-exchange was correlated with the CEC of these apoB-depleted plasma samples, as well as in a separate set of 77 plasma samples (12, 13).
There are several differences between the current AER assay and the prior EPR assay. First, we used ∼ equal amounts of the apoA1 exchange indicator compared to the apoA1 in the tested serum/plasma samples, while the EPR assay uses ∼3-fold excess of spin-labeled apoA1 vs. the plasma apoA1. Second, we observed an increase in the lipidation of the apoA1 exchange indicator over a 1 h incubation without reaching a plateau, while the EPR assay reached an endpoint after 15 min. Third, our assay used a fluorescence plate reader compared to the specialized EPR spectrometer. Finally, our study was performed using a much larger clinical cohort (n = 997), and demonstrated an association with incident CVD outcomes, even after adjustment for HDL-C and apoA1 levels, while the EPR assay was only evaluated for prevalent CVD in a small case-control cohort. Thus, we propose that our assay can be used as cell-free alternative to the CEC assay in the assessment of CVD risk.
Developing simpler HDL function assays has also been carried out by other groups. One developed a cell-free cholesterol exchange assay using immobilized liposomes containing Bodipy-labeled cholesterol that were incubated with apoB-depleted serum (16). Another developed a ‘cholesterol uptake capacity’ assay, incubating apoB-depleted serum with a trace amount of Bodipy-labeled cholesterol in aqueous solution, and then precipitating the apoA1/HDL to quantify uptake of the label (17). One of these two assays requires the use a larger serum sample (150 µL) (16), while the second assay requires a complex methodology using an apoA1 antibody (17). Although both of these assays showed good correlation with total CEC, they were performed using much smaller sample sizes (n = 16 and 30, respectively). Recently, an alternative assay to CEC was developed by measuring the apoA1-assosciated lipoprotein proteome panel using mass spectrometry (15). The proteome panel shows good correlation with CEC, but it was only evaluated for CAD in case-control studies.
HDL is very heterogeneous, and ABCA1 efficiently effluxes cellular lipids to lipid-poor apoA1, while ABCA1 has less efflux activity to large, lipid-rich HDL (22, 23). ABCA1-independent efflux requires lipidated apoA1/HDL and is mediated by ABCG1, SRB1, and passive diffusion (24, 25). We expected that AER might reflect HDL remodeling leading to increased lipid-free apoA1 and thus correlate well with ABCA1-mediated efflux. Instead, we observed that the AER was well correlated with ABCA1-independent cholesterol efflux, but only weakly correlated with ABCA1-dependent efflux. Thus, it appears that apoA1 exchange occurs preferentially with the large, lipid-rich HDL particles that are excellent ABCA1-independent acceptors. We saw excellent correlation of AER with HDL-C (Fig. 4A), and in a small pilot with only 6 serum samples, we saw even stronger correlation of AER with HDL-phospholipids. In serum, there is much more circulating lipid-bound apoA1 in HDL than lipid-free apoA1, and thus there is more capacity for efflux via non-ABCA1-mediated efflux pathways. This ABCA1-independent acceptor activity of HDL may be its most critical function in regard to promoting RCT and protecting against CVD.
HDL can be remodeled by plasma lipid transfer proteins or lipases. However, we found that heat treatment of the serum did not alter its AER, suggesting that apoA1 exchange may be independent of heat-labile enzymes. Similarly, the rHDL compound CSL112 when injected into human subjects or added ex vivo to human plasma leads to dramatic HDL remodeling, by fusion to HDL and subsequent release of lipid-free apoA1, which was not reduced by heat treatment (21).
Subjects in the lowest quartile (Q1) of AER had an increased HR for incident MACE compared to those in Q2–4; however, we did not observe a stepwise decrease in the HR going from Q1 to Q4. One possible explanation is that there is a threshold effect instead of a linear relationship between AER and incident MACE. Alternatively, there may be inherent differences in the Q1 samples vs. those in Q2–4. When we examined the correlation of HDL-C/apoA1 with AER, we observed that those in the AER Q1 fell below the correlation lines; and only Q1 samples did not display significant correlations with HDL-C/apoA1 levels (Fig. 4 A and B, Supplemental Figs 3, 4). Thus, AER in the Q1 samples were not as high as predicted based on their HDL-C or apoA1 levels, an indication of dysfunctional HDL in Q1 samples. Another possible explanation for the nonlinear relationship between AER and incident MACE is that too much AER may not be beneficial. In fact, several recent studies have shown a U-shaped relationship between HDL-C with all-cause mortality, CVD mortality, risk of infectious disease, and progression of chronic kidney disease, such that those at most risk were in the lowest and highest HDL-C groups (26–29).
We looked at two large cohort CEC studies to compare the relative CVD risk with the current study. In the prospective Dallas Heart Study, total CEC was shown to predict incident cardiovascular disease, and the HR was 0.33 (CI 0.19 to 0.55) comparing the top to lowest quartile after full adjustment (9). A nested case-control study from the Epic-Norfolk Study demonstrated that CEC was inversely associated with incident cardiovascular disease events, with the odds ratios 0.64 (CI 0.51 to 0.80) comparing the top to lowest tertile after full adjustment (10). In the current study, HR of Q1 vs. Q2–4 in our fully adjusted model was 0.58. AER predicts risk for future cardiovascular events similarly to CEC, but it is hard to compare the current study to the prospective Dallas Heart Study due to different study design, populations, sample size, and method used to measure HDL function. The Kaplan-Meier plot showed that the Q1 AER subjects had increased MACE with the most dramatic separation occurring in the first months. Thus, this simple assay using small volumes of serum may be useful for risk assessment and in selecting subjects for drug trials. More work is needed to confirm that various measures of HDL function are good indicators of risk assessment, and whether these functional indicators are good targets for drug development. Limitations of the current study include the limited time of follow up (3 years), the low incidence of MACE (∼15%), and lack of replication in an independent cohort.
Supplementary Material
Abbreviations
- AER
apoA1 exchange rate;
- HDL-C
high density lipoprotein-cholesterol;
- CVD
cardiovascular disease;
- MACE
major adverse cardiovascular events;
- RCT
reverse cholesterol efflux;
- CEC
cholesterol efflux capacity;
- NBD
7-nitrobenz-2-oxa-1, 3-diazole;
- MI
myocardial infarction;
- PBS
phosphate buffered saline;
- FPLC
fast protein liquid chromatography;
- HR
hazard ratio;
- CI
confidence interval;
- BMI
body mass index;
- CAD
coronary artery disease;
- rHDL
reconstituted HDL;
- LDL-C
low density lipoprotein-cholesterol
Previous presentation
Gordon Research Conference Lipoprotein Metabolism. (June 10–15, 2018). Poster presentation: Low ApoA1 Exchange Rate Associated with Increased Incident Cardiovascular Events.
Nonstandard Abbreviations
AER, apoA1 exchange rate; HDL-C, high density lipoprotein-cholesterol; CVD, cardiovascular disease; MACE, major adverse cardiovascular events; RCT, reverse cholesterol efflux; CEC, cholesterol efflux capacity; NBD, 7-nitrobenz-2-oxa-1, 3-diazole; MI, myocardial infarction; PBS, phosphate buffered saline; FPLC, fast protein liquid chromatography; HR, hazard ratio; CI, confidence interval; BMI, body mass index; CAD, coronary artery disease; rHDL, reconstituted HDL; LDL-C, low density lipoprotein-cholesterol.
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
J.D. Smith, financial support, statistical analysis, administrative support.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest: Employment or Leadership: S.W. Lorkowski, Cleveland Clinic; J.D. Smith, Cleveland Clinic. Consultant or Advisory Role: None declared. Stock Ownership: None declared. Honoraria: None declared. Research Funding: S.L. Hazen, Transatlantic Networks of Excellence: Foundation Leducq, R01 HL 128300 and P01 HL076491; J.D. Smith, NIH Grant P01 HL029582, NIH Grant R01 HL111314, American Heart Association Grant 18SFRN34110067 to institution. Expert Testimony: None declared. Patents: S.W. Lorkowski, US Provisional Patent serial # 62\682-628; S.L. Hazen, 62\682-628; J.D. Smith, US Provisional Patent serial # 62\682-628.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
REFERENCES
- 1. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR.. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977;62:707–14. [DOI] [PubMed] [Google Scholar]
- 2. Assmann G, Schulte H, von Eckardstein A, Huang Y.. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996;124:S11–20. [DOI] [PubMed] [Google Scholar]
- 3.Investigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 2012;367:2089–99. [DOI] [PubMed] [Google Scholar]
- 5. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–22. [DOI] [PubMed] [Google Scholar]
- 6. Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med 2017;376:1933–42. [DOI] [PubMed] [Google Scholar]
- 7. Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 2012;380:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371:2383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 2015;3:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol 2013;33:1696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borja MS, Zhao L, Hammerson B, Tang C, Yang R, Carson N, et al. HDL-apoA-I exchange: rapid detection and association with atherosclerosis. PLoS One 2013;8:e71541.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borja MS, Ng KF, Irwin A, Hong J, Wu X, Isquith D, et al. HDL-apolipoprotein A-I exchange is independently associated with cholesterol efflux capacity. J Lipid Res 2015;56:2002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Handa D, Kimura H, Oka T, Takechi Y, Okuhira K, Phillips MC, et al. Kinetic and thermodynamic analyses of spontaneous exchange between high-density lipoprotein-bound and lipid-free apolipoprotein A-I. Biochemistry 2015;54:1123–31. [DOI] [PubMed] [Google Scholar]
- 15. Jin Z, Collier TS, Dai DLY, Chen V, Hollander Z, Ng RT, et al. Development and validation of apolipoprotein AI-associated lipoprotein proteome panel for the prediction of cholesterol efflux capacity and coronary artery disease. Clin Chem 2019;65:282–90. [DOI] [PubMed] [Google Scholar]
- 16. Horiuchi Y, Lai SJ, Yamazaki A, Nakamura A, Ohkawa R, Yano K, et al. Validation and application of a novel cholesterol efflux assay using immobilized liposomes as a substitute for cultured cells. Biosci Rep 2018;38:BSR20180144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harada A, Toh R, Murakami K, Kiriyama M, Yoshikawa K, Kubo T, et al. Cholesterol uptake capacity, a novel measure of high density lipoprotein functionality for coronary risk stratification. Circulation 2016;134:A15652. [Google Scholar]
- 18. Cavigiolio G, Geier EG, Shao B, Heinecke JW, Oda MN.. Exchange of apolipoprotein A-I between lipid-associated and lipid-free states: a potential target for oxidative generation of dysfunctional high density lipoproteins. J Biol Chem 2010;285:18847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang S, Gulshan K, Brubaker G, Hazen SL, Smith JD.. ABCA1 mediates unfolding of apolipoprotein AI N terminus on the cell surface before lipidation and release of nascent high-density lipoprotein. Arterioscler Thromb Vasc Biol 2013;33:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Z, Gogonea V, Lee X, Wagner MA, Li XM, Huang Y, et al. Double superhelix model of high density lipoprotein. J Biol Chem 2009;284:36605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Didichenko SA, Navdaev AV, Cukier AM, Gille A, Schuetz P, Spycher MO, et al. Enhanced HDL functionality in small HDL species produced upon remodeling of HDL by reconstituted HDL, CSL112: effects on cholesterol efflux, anti-inflammatory and antioxidative activity. Circ Res 2016;119:751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du XM, Kim MJ, Hou L, Le Goff W, Chapman MJ, Van Eck M, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res 2015;116:1133–42. [DOI] [PubMed] [Google Scholar]
- 23. Camont L, Lhomme M, Rached F, Le Goff W, Negre-Salvayre A, Salvayre R, et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol 2013;33:2715–23. [DOI] [PubMed] [Google Scholar]
- 24. Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, et al. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res 2007;48:2453–62. [DOI] [PubMed] [Google Scholar]
- 25. Larrede S, Quinn CM, Jessup W, Frisdal E, Olivier M, Hsieh V, et al. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. ATVB 2009;29:1930–6. [DOI] [PubMed] [Google Scholar]
- 26. Li ZH, Lv YB, Zhong WF, Gao X, Kraus VB, Zou MC, et al. High-density lipoprotein cholesterol and all-cause and cause-specific mortality among the elderly. J Clin Endocrinol Metab 2019;doi:10.1210/jc.2018-02511. [DOI] [PubMed] [Google Scholar]
- 27. Oh IH, Hur JK, Ryoo JH, Jung JY, Park SK, Yang HJ, et al. Very high high-density lipoprotein cholesterol is associated with increased all-cause mortality in South Koreans. Atherosclerosis 2019;283:43–51. [DOI] [PubMed] [Google Scholar]
- 28. Madsen CM, Varbo A, Tybjærg-Hansen A, Frikke-Schmidt R, Nordestgaard BG.. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J 2018;39:1181–90. [DOI] [PubMed] [Google Scholar]
- 29. Nam KH, Chang TI, Joo YS, Kim J, Lee S, Lee C, et al. Association between serum high-density lipoprotein cholesterol levels and progression of chronic kidney disease: results from the KNOW-CKD. J Am Heart Assoc 2019;8:e011162.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.