Abstract
Antibody-mediated rejection (AbMR) adversely affects long-term graft survival in kidney transplantation. Currently, the diagnosis of AbMR requires a kidney biopsy, and detection of complement C4d deposition in the allograft is one of the diagnostic criteria. Complement activation also generates several soluble fragments which could potentially provide non-invasive biomarkers of the process. Furthermore, microvesicles released into the plasma from injured cells can serve as biomarkers of vascular injury. To explore whether soluble complement fragments or complement fragments bound to endothelial microvesicles can be used to non-invasively detect AbMR, we developed an in vitro model in which human endothelial cells were exposed to anti-HLA antibodies and complement sufficient serum. We found that complement fragments C4a and sC5b-9 were increased in the supernatants of cells exposed to complement-sufficient serum compared to cells treated complement-deficient serum. Furthermore, complement activation on the cell surface was associated with the release of microvesicles bearing C4 and C3 fragments. We next measured these analytes in plasma from kidney transplant recipients with biopsy-proven acute AbMR (n=9) and compared the results with those from transplant recipients who also had impaired allograft function but who did not have AbMR (n=30). Consistent with the in vitro results, complement fragments C4a and Ba were increased in plasma from patients with AbMR compared to control subjects (P<0.001 and P<0.01, respectively). Endothelial microvesicle counts were not increased in patients with AbMR, however, and the number of microvesicles with C4 and C3 bound to the surface was actually lower compared to control subjects (both P<0.05). Our results suggest that plasma complement activation fragments may be useful as non-invasive biomarkers of antibody-mediated complement activation within the allograft. Complement-opsonized endothelial microvesicles are decreased in patients with AbMR, possibly due to enhanced clearance of microvesicles opsonized with C3 and C4 fragments.
Keywords: complement, antibody mediated rejection, biomarker, donor specific antibody
1. Introduction
Kidney transplantation is the optimal treatment for patients with end stage renal disease (ESRD) (Wolfe et al., 1999). However, acute and/or chronic immunologic injury mediated by donor-specific antibodies (DSAs) against donor human leukocyte antigens (HLA) is a major cause of kidney graft loss and is a significant barrier to long-term graft survival (Sellares et al., 2012). AbMR is characterized pathologically by microvascular injury and inflammation. Even in the absence of graft dysfunction, these pathologic changes on surveillance biopsies (subclinical AbMR) are associated with inferior graft outcomes (Loupy et al., 2015; Orandi et al., 2015). The diagnosis of AbMR also requires evidence of DSA interaction with the endothelium, usually detected as C4d deposition in the peritubular capillaries (Haas, 2014). During antibody-mediated classical pathway activation C4 is covalently attached to target tissues, providing a durable marker of this process. Although there are cases of C4d-negative AbMR, complement activation is associated with a poorer prognosis and is generally considered an important contributor to graft injury in AbMR (Feucht et al., 1993; Kikic et al., 2015).
Because of the impact of AbMR, with or without concurrent graft dysfunction, on short- and long-term graft outcomes, better methods for diagnosing and monitoring its presence are needed. Currently, a kidney biopsy is required for diagnosis of AbMR, and the only tools available for surveillance are protocol biopsy and serially monitoring for DSA. While DSA monitoring is an attractive option because it is noninvasive and widely available, it has low specificity for subclinical AbMR and frequently does not reflect active inflammation and injury (Parajuli et al., 2017; Schinstock et al., 2017). In addition to a potential pathogenic role in allograft injury, complement activation also generates several soluble fragments that can serve as clinical biomarkers of inflammation (Frazer-Abel et al., 2016). For example, every molecule of C4b that is attached to target cells is accompanied by release of a C4a fragment. Classical pathway activation within the allograft may, therefore, increase C4a levels in the plasma.
Damaged endothelial cells also release microvesicles. Microvesicles are submicrometer-sized membrane-bound vesicles (0.05–1 μm) shed from a variety of cells, including endothelial cells, both constitutively and in response to activation, injury, and apoptosis (Anderson et al., 2010; Beyer and Pisetsky, 2010). Studies have demonstrated that endothelial microvesicles increase in diseases associated with endothelial injury, and they can be isolated from peripheral blood as circulating markers of events at the cellular level (Boulanger et al., 2007; Chironi et al., 2009; Hsu et al., 2013). It was also reported that AbMR increases the number of C4-opsonized endothelial microvesicles, possibly reflecting complement-mediated endothelial injury (Tower et al., 2016).
We hypothesized that antibody-mediated complement activation would lead to increased generation of soluble complement activation fragments and release of C3 or C4-bearing endothelial microvesicles that could serve as plasma-derived biomarkers of AbMR in renal transplant recipients. To test this hypothesis, we developed and characterized an in vitro model of AbMR using immortalized human endothelial cells and collected pilot data with samples from renal transplant recipients with impaired graft function in the absence of AbMR and from patients with biopsy-proven AbMR.
2. Materials and Methods
2.1. Complement activation, deposition, and clearance on endothelial cells
Confluent HMEC-1 cells grown in 6-well plates were incubated with a saturating concentration of mAb W6/32 (3μg/mL) for 30 minutes at 37°C (see supplementary Figure 1). Cells were subsequently washed with PBS and incubated with culture medium with or without 10% normal human serum (NHS; Quidel, San Diego, CA) at 37°C. Higher NHS concentrations were examined but did not significantly alter the results using this model. The degree of C4 deposition per cell was greater when subconfluent cells were tested (supplementary Figure 1c), possibly because of a greater quantity of C4 per cell in the reaction. Confluent cells were used for all experiments in which the degree of complement activation was compared. At specified time points, cells were again washed with PBS and detached with Accutase. We examined whether treatment with Accutase could affect the results by also testing removal of the cells by either cell scraping or treating the cells with trypsin. The specific method of detachment does influence cell number and morphology (data not shown). The use of Accutase may, therefore, have affected the results, although the same treatment protocol was used for all of the cell culture experiments. FITC-conjugated anti-human C4c antibody (Agilent, Santa Clara, CA) and anti-human C4d (American Research Products, Inc, Waltham, MA) with APC-conjugated anti-rabbit secondary antibody (ThermoFisher Scientific, Waltham, MA) were used to detect C4 fragments. FITC-conjugated anti-human C3b/iC3b mAb 3E7 and Al647-conjugated anti-human C3b/iC3b/C3dg mAb 1H8 [generated as previously described (DiLillo et al., 2006; M. A. Lindorfer et al., 2003)] were used to detect C3 fragment deposition. All antibodies were used at a concentration of 0.5 μg/100 μL. Complement regulatory proteins were detected with a FITC-labeled antibody to CD46 and an isotype control (both from BD Biosciences, San Jose, CA), a biotin-labeled antibody to CD55 detected with APC labeled streptavidin (both from BioLegend, San Diego, CA), and an anti-CD59 antibody detected with a Cy-3 labeled secondary antibody (both from Abcam, Cambridge, MA). Fluorescence was measured by flow cytometry on a FACSCalibur flow cytometer (BD Biosciences).
HMEC-1 cells were grown on coverslips, exposed to W6/32 and NHS, and blocked using the methods discussed in supplementary material. Cells were then incubated overnight at 4°C with DyLight-647-conjugated C3d29, a murine mAb that only recognizes the iC3b and C3d fragments of C3 (Thurman et al., 2013) or unconjugated anti-human C4d followed by a one-hour incubation with the APC-conjugated anti-rabbit secondary, diluted 1:200. Both primary antibodies were diluted 1:100. Cells were washed, DAPIstained, and imaged as discussed in supplementary material.
Endothelial cells were incubated with anti-HLA antibody W6/32 and NHS as described above then washed with PBS and given fresh medium without human serum for another 0, 24, or 48 hours. We did not add additional serum during this period in order to observe the persistence of anti-HLA antibody and complement fragments on cells in culture in the absence of additional complement deposition. Anti-HLA antibody, C4 fragments, and C3 fragments were detected with the antibodies described previously and fluorescence was measured on a FACSCalibur flow cytometer.
2.2. Complement deposition with complement depleted human sera or complement inhibitors
HMEC-1 cells were cultured and incubated with a mAb W6/32. Cells were subsequently washed with PBS and incubated with culture medium with or without 10% NHS, C1q-depleted human serum (Quidel), C4-depleted human serum (Quidel), or NHS with addition of an inhibitor of factor B (fB, mAb 1379 described previously in (Thurman et al., 2005)) or an inhibitor of factor I (fI, mAb A247, Quidel) at 37°C for one hour.
Complement inhibited serum was prepared by adding either mAb 1379 (40μg/mL) or mAb A247 (11μg/mL) to NHS. Preparations were incubated on ice for 30 minutes prior to incubation with cultured cells (Margaret A Lindorfer et al., 2013; Thurman et al., 2005). For these experiments, control NHS was also incubated on ice for 30 minutes prior to incubation with cultured cells to control for complement turnover in solution. Complement fragments were detected with the antibodies described above and fluorescence was measured on a FACSCalibur flow cytometer.
2.3. In vitro complement fragment analysis
Fifty μL aliquots of the medium collected for microvesicle analysis were separated for complement fragment analysis prior to microvesicle processing described above. Ethylenediaminetetraacetic acid (EDTA) was immediately added to each sample at a concentration of 10mM and samples were stored at −80°C until use. Complement activation fragments (Ba, C4a, sC5b-9) were measured using commercial enzyme-linked immunosorbent assays (ELISAs) according to the manufacturer’s instructions (Quidel). The samples were diluted 1:1000 for Ba, 1:10 for sC5b-9, and 1:40 for C4a.
2.4. Endothelial cell microvesicle analysis
HMEC-1 cells were labelled with PKH26 fluorescent cell linker (Sigma-Aldrich, St Louis, MO) per the manufacturer’s instructions. Labelled cells were subsequently incubated for 16 hours with saturating concentrations of mAb W6/32 followed by medium with or without 10% NHS, C1q-depleted human serum, or C4-depleted human serum. The medium was then collected and centrifuged at 400 × g for 15 minutes at 4°C. The supernatants were collected and then centrifuged at 20,000 × g for 2.5 hours. The pellets were resuspended in microvesicle buffer (Hank’s buffered saline solution containing 20mM HEPES and 5mM glucose) to a volume of 80μL. After microvesicles were isolated and re-suspended they were stained using the antibodies to IgG, C4d, and C3b/iC3b/C3dg at a concentration of 0.1μg/80μL. Antibody labels were detected using a FACSCalibur flow cytometer. Equivalent numbers of counting beads (CountBright beads from Life Technologies, Grand Valley, NY) were added to the suspensions prior to antibody staining to determine the number of microvesicles.
2.5. Study subjects
EDTA plasma was obtained from 30 renal transplant recipients with chronic kidney disease stage 3–4 [results for these patients have been previously published (Jalal et al., 2018)]. These patients were clinically stable, even though their kidney function was impaired. We also collected plasma samples from six renal transplant recipients with impaired kidney function and biopsy-proven acute AbMR at the University of Colorado Hospital within 48 hours of diagnosis of AbMR. An additional three plasma samples from subjects with biopsy-proven AbMR were obtained from transplant recipients in France within three months of diagnosis of AbMR. An institutional review board approved the use of patient samples, and informed consent was obtained from patients in accordance with the Declaration of Helsinki.
2.6. DSA measurements
All HLA testing and antibody analyses were performed by American Board of Histocompatibility and Immunogenetics (ABHI) board-certified technologists and reviewed by ABHI board-certified HLA specialists in an ASHI- and College of American Pathologists (CAP)-accredited laboratory (ClinImmune Labs, Aurora, CO) for subjects from the University of Colorado. Patient sera was screened for both class I and class II antibodies using LABscreen Mixed beads (One Lambda). DSA was defined as an MFI greater than 500 units, a cutoff that is commonly used clinically.
2.7. Human subject microvesicle analysis
Plasma was collected by drawing patient blood into EDTA containing tubes. The tubes were inverted several times and then placed immediately on ice. Within 10 minutes the samples were centrifuged at 1000 g at 4°C, and the layer of plasma was then removed with a Pasteur pipette. Samples were stored at −80°C until use.
To isolate microvesicles from patient plasma, the samples were thawed in a 37°C water bath then centrifuged as previously described. The pellets were resuspended in microvesicle buffer at a volume of 40% of the initial plasma volume. After microvesicles were isolated and re-suspended they were stained using the antibodies to CD105, CD41a, IgG, C4d, and C3b/iC3b/C3dg. CD41a is a marker of platelet-derived microvesicles, and CD105 is a marker of endothelial-derived microvesicles (Boulanger et al., 2007; Jalal et al., 2018). Countbright counting beads were used to determine the number of microvesicles. Antibody labels were detected using an Astrios flow cytometer (Beckman Coulter Life Sciences, Indianapolis, IN).
2.8. Human subject complement fragment analysis
Complement activation fragments (Ba, C4a, sC5b-9) in plasma were measured using commercial ELISAs according to the manufacturer’s instructions (Quidel). The plasma samples were diluted 1:1000 for Ba, 1:10 for sC5b-9, and 1:40 for C4a.
2.9. Statistical analysis
Descriptive statistics are reported according to study group as n (%) for categorical variables and mean (SD) for continuous variables. Fischer’s exact test was used for categorical variables, t-test was used for continuous variables with equal variances, and Satterthwaite approximation for continuous variables with unequal variances. Comparisons between multiple groups were performed using one-way analysis of variance with a Bonferroni multiple comparison test using GraphPad Prism software.
3. Results
3.1. Complement activation on endothelial cells in culture
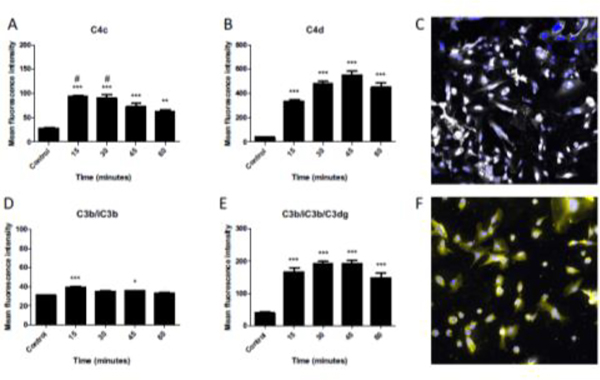
HMEC-1 cells were incubated with a saturating concentration of anti-HLA class I antibody (W6/32) (Supplemental Fig 1) and subsequently exposed to NHS to elicit complement activation and deposition on the cell surface (Fig 1). Early after serum exposure, active C4 in the form of C4b (recognized by an anti-C4c detection antibody) is abundant but subsequently declines at later time points (Fig 1A). This decline is presumably due to conversion to the inactive form C4d that is detected early after exposure and does not decline as rapidly (Fig 1B). Interestingly, active C3b is detected on the cell surface only at the earliest time point of 15 minutes (Fig 1D), and only the inactive form C3dg remains thereafter (Fig 1E). This is likely due to rapid conversion of C3b to the inactive form, though some degree of shedding or internalization is also possible. These results indicate anti-HLA activates the classical pathway of complement on endothelial cells, and but the cells can rapidly eliminate deposited C3b.
FIGURE 1. C4 and C3 fragments are deposited on HMEC-1 cells after exposure to anti-HLA antibody and normal human serum.
HMEC cells were treated with W6/32 antibody and then exposed to normal human serum. Complement activation on the cells was then analyzed by flow cytometry after various periods of time. Data are plotted as mean fluorescence intensity (MFI) ± SEM. A) C4c was rapidly deposited on the cell surface (**P<0.01, ***P<0.001 vs control) but was significantly decreased by 60 minutes (#P<0.05 vs 60 minutes). B) C4d was also quickly deposited but remained on the cell surface with no significant change from 30 to 60 minutes (***P<0.001 vs control). C) Immunofluorescence microscopy confirmed deposition of C4d on the cell surface. Nuclei are stained with Dapi (blue). Original magnification x600. D) C3b/iC3b was detected on the cell surface at 15 minutes but was not statistically different than control by 60 minutes (*P<0.05, ***P<0.001 vs control). E) C3b/iC3b/C3dg was rapidly deposited and did not change significantly from 15 to 60 minutes (***P<0.001 vs control). C) Immunofluorescence microscopy confirmed deposition of C3d on the cell surface. Nuclei are stained with Dapi (blue). Original magnification x600.
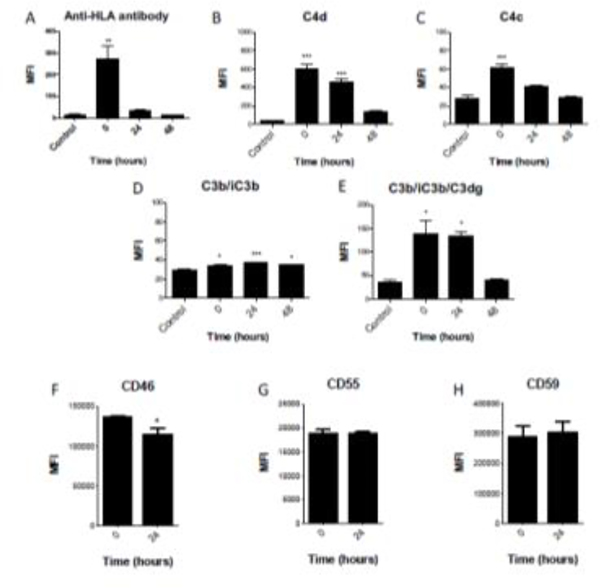
To analyze the persistence of complement fragments and anti-HLA antibody on the cell surface, HMEC-1 cells were incubated with anti-HLA antibody and NHS, then washed and given fresh medium for an additional zero to 48 hours. We did not add additional serum to the cells so that we could monitor the persistence of antibody and complement fragments on the cell surface. The anti-HLA antibody and fragments of C4 and C3 are all detected at time zero after initial exposure, but the anti-HLA antibody is no longer detected 24 hours later (Fig 2A) and the C4 and C3 fragments are not detectable above control by 48 hours (Fig 2B–2E). The relatively transient presence of anti-HLA antibodies on the cell surface is consistent with the observation that immunoglobulin is inconsistently detected in renal allograft biopsies during AbMR (Halloran et al., 1992; Trpkov K, 1996). Interestingly, C4d and C3dg are also reduced to undetectable levels by 48 hours. Because these fragments are covalently attached to target surfaces, these results suggest that endothelial cells can remove these molecules and their presence on capillaries indicates ongoing or recent complement activation.
FIGURE 2. Anti-HLA antibody, C4 fragments, and C3 fragments are cleared from the surface of HMEC-1 cells within 48 hours.
HMEC cells were treated with W6/32 antibody followed and then incubated with 10% normal human serum in culture medium for 2 hours to allow activation complement on the cell surface. They were then washed with PBS and incubated with normal culture medium for another 0 to 48 hours. Complement fragments were then analyzed by flow cytometry. Data are plotted as mean fluorescence intensity (MFI) ± SEM). Anti-HLA antibody (A), C4d (B), C4c (C), C3b/iC3b (D), and C3b/iC3b/C3dg (E) were detected on the cells at time zero, but by 48 hours the levels of these proteins were not significantly different than untreated cells. Endothelial cells also express the complement regulators CD46 (F), CD55 (G), and CD59 (H). Expression of CD55 and CD59 was stable, but levels of CD46 were reduced 24 hours after complement activation on the cells. (*P<0.05, **P<0.01, ***P<0.001 vs control).
Endothelial cells express the complement regulators CD46, CD55, and CD59 (Fig 2F–H). The expression of CD46 by the cells was reduced 24 hours after exposure to anti-HLA antibody and NHS, suggesting the complement activation on the cells may impair complement regulation. This may render the cells even more susceptible to complement-mediated injury.
3.2. Classical and alternative pathways are activated on endothelial cells in vitro
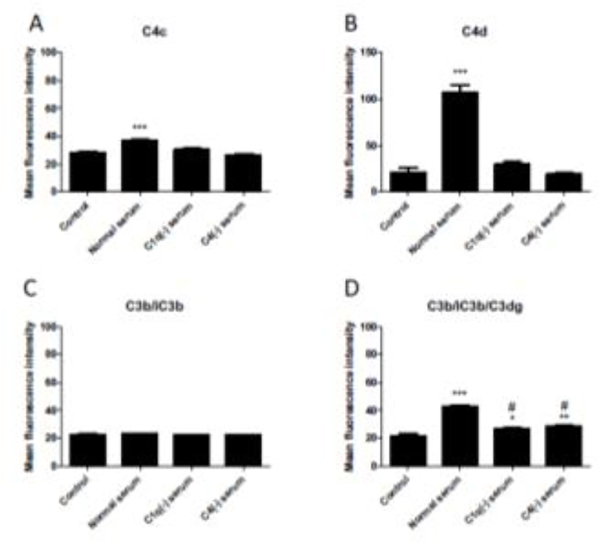
To identify the complement pathways involved in activation on the endothelial cell surface, we tested the model using human sera depleted of C1q (necessary for the classical pathway) or C4 (necessary for the classical and lectin pathways) and monoclonal antibodies that inhibit complement fB (a necessary component of the alternative pathway) or complement fI (a circulating protease involved in cleavage of C4b and C3b to inactive forms). As expected, sera depleted of either C1q or C4 eliminated the deposition of C4 fragments on the endothelial cell surface (Fig 3A and 3B). However, C3 deposition was reduced but not eliminated with sera depleted of classical pathway components (Fig 3D), likely due to persistent low-level alternative pathway activation. As shown above, C4b and C3b are rapidly inactivated on the endothelial cell surface. Inactivation of these proteins is usually mediated by fI, and an inhibitory antibody to fI delays the cleavage of C4b and C3b (Margaret A Lindorfer et al., 2013). In our model system this inhibitor did not prevent the conversion of C4b and C3b to C4d and C3dg, respectively (Fig 4). This suggests that endothelial cells may have an alternative mechanism of inactivating C4b and C3b that is independent of fI. Other proteases not traditionally thought of as being involved in the complement cascade have cleavage activity. For example, cathepsins L and D are able to activate C3 and C5, respectively (Freeley et al., 2016).
FIGURE 3. C1q or C4 deficiency reduces C4 and C3 deposition on HMEC-1 cells.
HMEC-1 cells were exposed to W6/32 antibody followed by medium with 10% normal human serum, 10% C1q-depleted (C1(−)) serum, or 10% C4-depleted (C4(−)) serum for one hour. Complement fragments were then analyzed by flow cytometry. Data are plotted as mean fluorescence intensity (MFI) ± SEM. A and B) No C4c or C4d was deposited on cells exposed to C1q(−) serum or C4(−) serum (P<0.001 vs control). C) C3b/iC3b was not detected in any condition. D) C3b/iC3b/C3dg was deposited with all serum conditions (*P<0.05, **P<0.01, ***P<0.001 vs control) but significantly reduced with C1q(−) serum and C4(−) serum compared to normal serum (# P<0.001).
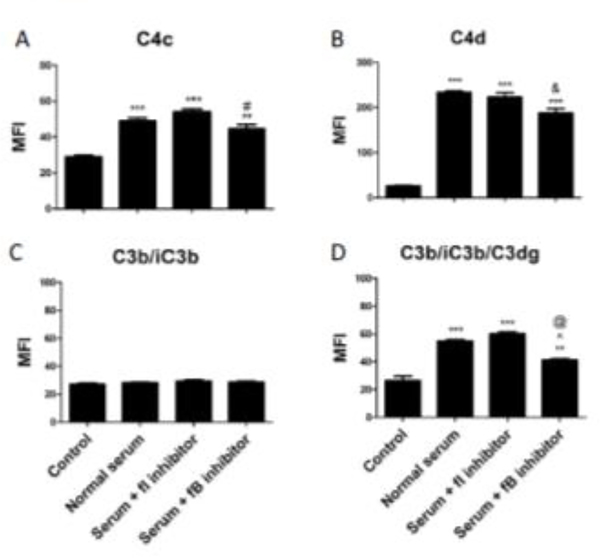
FIGURE 4. The roles of factor I and factor B in regulating complement activation on endothelial cells.
HMEC-1 cells were exposed to W6/32 antibody followed by medium with 10% normal human serum for one hour. A monoclonal antibody that inhibits complement factor I (fI) was added to some of the cells to block factor I mediated cleavage of C3b and C4b. A monoclonal antibody that inhibits complement factor B (fB) was added to some of the cells to block alternative pathway activation. C3 and C4 fragments were analyzed by flow cytometry. Data are plotted as mean fluorescence intensity (MFI) ± SEM (*P<0.05, **P<0.01, ***P<0.001 vs control). No differences between normal serum and serum + fI inhibitor antibody were observed. Serum + fB inhibitor was associated with decreased deposition of C4c compared to fI inhibitor (# P<0.05), decreased C4d deposition compared to normal serum alone (& P<0.05), and decreased C3dg deposition compared to normal serum (@ P<0.01) or fI inhibitor (^ P<0.001).
The inhibition of the alternative pathway with the mAb 1379 decreased deposition of C3 fragments, indicating that C3 deposition on the cells is dependent on both the classical pathway and the alternative pathway in our model (Fig 4D). Interestingly, C4 deposition was also slightly decreased with inhibition of fB (Fig 4A and 4B).
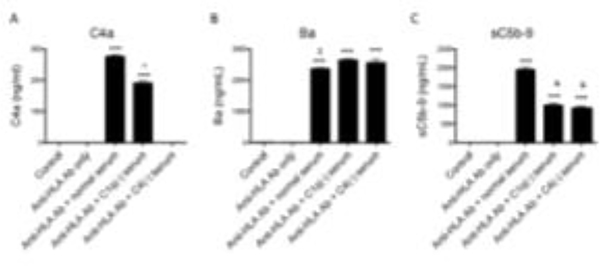
3.3. Serum exposure is associated with increased complement fragment generation in vitro
Culture medium from HMEC-1 cells exposed to anti-HLA antibody and NHS had increased levels of C4a and sC5b-9 compared to controls (Figure 5). This suggests that complement activation on the cells proceeds to formation of the membrane attack complex (MAC, or C5b-9). Ba is a marker of alternative pathway activation. Ba levels were also elevated in medium from cells exposed to the different types of serum, indicating that alternative pathway activation in this model does not require initiation through the classical pathway.
FIGURE 5. Antibody mediated complement activation on HMEC-1 cells generates soluble complement fragments.
A) C4a was generated in the supernatants of HMEC-1 cells treated with W6/32 antibody and then exposed to normal serum. C4a was also generated when C1q-deficient (C1q(−)) serum was used (***P<0.001 vs control), although the levels were lower than those seen using normal serum (^P<0.001). B) Ba was generated after treatment of HMEC-1 cells with W6/32 antibody followed by exposure to normal serum (***P<0.001 vs control). Ba levels were slightly lower with normal serum vs C1q(−) serum ($P<0.05). C) sC5b-9 was also generated after treatment of HMEC-1 cells with W6/32 antibody followed by exposure to normal serum. sC5b-9 levels were increased in cells exposed to sera deficient in C1q or C4 (***P<0.001 vs control), although the levels were significantly lower than for cells exposed to normal serum (&P<0.001 vs normal serum).
3.4. Serum exposure is associated with increased endothelial microvesicle release in vitro
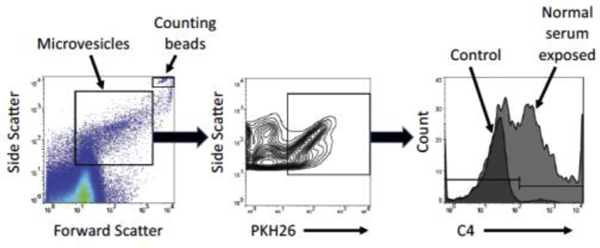
We used PKH26 to fluorescently label the membranes of HMEC-1 cells and then exposed cells to anti-HLA antibody with or without human serum. The PKH26 label allowed for isolation of HMEC-1-derived microvesicles from those present in the human serum. Microvesicles were also probed for C4, C3 fragments and IgG bound to the surface. The FACS gating strategy is demonstrated in Fig 6.
FIGURE 6. Representative example of gating used to detect HMEC-1 microvesicles by flow cytometry.
Microvesicles were initially gated by size using forward and side scatter, then by fluorescence of PKH26 to identify microvesicles originating from the labelled HMEC-1 cells.
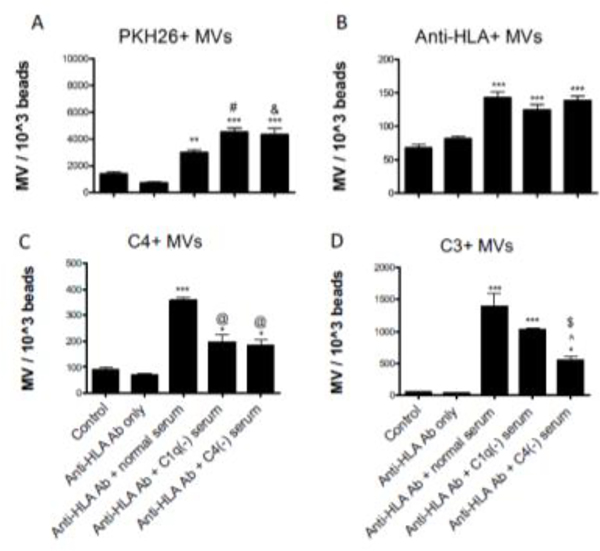
Exposure to anti-HLA antibody alone did not elicit an increase in microvesicle release, but serum exposure was associated with increased release (Fig 7A). The serum-induced release of microvesicles is not dependent on activation of the classical complement cascade, and, in fact, sera depleted of classical complement proteins C1q and C4 were associated with a greater release of microvesicles compared to serum with normal complement protein levels. The number of microvesicles bound with anti-HLA antibody was similar in cells treated with complement-depleted sera compared to cells treated with NHS. However, exposure to NHS was associated with the greatest release of microvesicles with C4 bound (P<0.001 vs complement depleted sera). In Figure 7D, comparison of the results using C1q-deficient and C4-deficient serum also indicates that some of the complement activation on the endothelial cells may be due to activation of the mannose binding lectin pathway. And comparison of the results using C4-deficient serum to the controls suggests that there is activation of the alternative pathway.
FIGURE 7. Anti-HLA antibodies and complement fragments on endothelial microvesicles (MV) generated in vitro.
HMEC-1 cells were labelled with PKH26 fluorescent dye and exposed to W6/32 antibody. Cells were then incubated with medium containing 10% normal human serum or serum deficient in C1q or C4. Microparticles in the supernatants were purified by ultracentrifugation and analyzed by flow cytometry. The number of MVs was standardized using fluorescent counting beads and are presented as MV count per 1000 counting beads ± SEM (*P<0.05, **P<0.01, ***P<0.001). A) MV release was not elicited by exposure to anti-HLA antibody alone. All serum conditions were associated with an increased release of PKH-26 MVs vs control (**P<0.01, ***P<0.001), but complement deficient sera were associated with an increased release of PKH26-positive MVs compared to normal serum (# P<0.01 for C1q(−) serum and & P<0.05 for C4(−) serum). B) MVs with anti-HLA antibody bound were detected in similar quantities after exposure to the various sera (***P<0.001). C) MVs with C4 bound were detected with all serum conditions (*P<0.05, ***P<0.001 vs control), but significantly more were detected after exposure to normal serum than the complement deficient sera (@P<0.001). D) MVs with C3 were also detected with all sera exposures (*P<0.05, ***P<0.001 vs control), but C4(−) serum elicited reduced release of MVs bound with C3 compared to normal serum ($ P<0.001) or C1(−) serum (^ P<0.05).
3.5. Complement fragments are increased in plasma from patients with AbMR
To test whether complement activation fragments and endothelial microvesicles are biomarkers of AbMR in human patients, we collected plasma samples from patients with biopsy-proven acute C4d-positive AbMR and from patients with stable kidney graft function. Compared to patients with biopsy-provenAbMR, stable transplant control subjects were older, further removed from transplant, and less likely to have DSAs (Table 1). Serum creatinine tended to be higher in AbMR patients, but this difference was not statistically significant. However, by definition, all patients with AbMR had graft dysfunction (kidney injury) as opposed to patients in the control group, who had impaired kidney function but were otherwise clinically stable. There were no significant differences in baseline maintenance immunosuppression between the AbMR patients and the control group.
Table 1.
Clinical characteristics of human subjects
| Control transplant patients (n=30) | AbMR Patients (n=9) | P | ||
|---|---|---|---|---|
| Age, mean (SD), y | 51.8 (14.4) | 38.9 (14.9) | 0.025 | |
| Male, n (%) | 17 (56.7) | 3 (33.3) | 0.15 | |
| Years since transplant, mean (SD) | 8.2 (7.0) | 2.15 (2.0) | 0.0002 | |
| Any DSA MFI >500, n (%) | 9 (30) | 9 (100) | 0.0002 | |
| C1q-binding DSA, n (%)*** | 5 (16.7) | 4 (66.7) | 0.018 | |
| Serum Creatinine, mean (SD), mg/dL | 1.6 (0.4) | 3.24 (2.9) | 0.13 | |
| Immunosuppression | ||||
| CNI, n (%) | 30 (100) | 9 (100) | 1.0 | |
| mTORi, n (%) | 8 (26.7) | 1 (16.7) | 0.25 | |
| Mycophenolate, n (%) | 16 (53.3) | 8 (83.3) | 0.25 | |
| Prednisone, n (%) | 27 (90) | 8 (83.3) | 0.44 | |
Samples missing for 3 AbMR subjects for C1q-binding assay
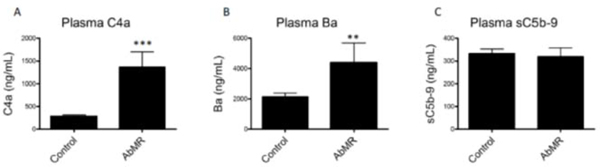
We measured levels of C4a, Ba, and sC5b-9 in in the patient plasma samples. C4a and Ba are elevated in the plasma of kidney transplant recipients with biopsy-provenAbMR compared to controls indicating activation of both the classical and alternative pathways of complement (Figure 8), similar to what we found in vitro. Plasma sC5b-9 was not different between groups.
FIGURE 8. Antibody-mediated rejection (AbMR) is associated with elevation of plasma complement activation fragments.
Plasma C4a and plasma Ba were both elevated in patients with biopsy-proven AbMR compared to clinically stable renal transplant recipients (**P<0.01, ***P<0.001). These results indicate that classical and alternative pathways are activated in these patients. Plasma sC5b-9 levels were not different between groups.
3.6. Circulating endothelial microvesicles are decreased in patients with AbMR
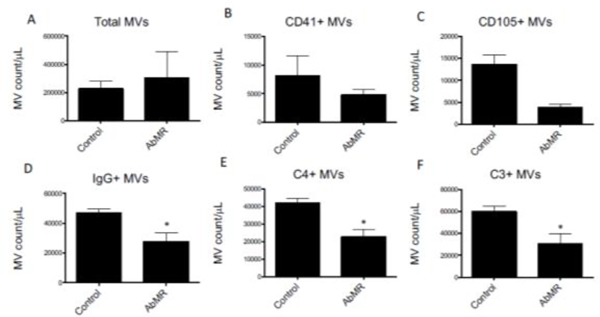
Microvesicles in patient plasma samples were analyzed by flow cytometry. An example of the gating strategy used is depicted in Figure 9. There was no difference in number of platelet-derived (CD41+), or endothelial-derived (CD105+) microvesicles between groups (Figures 10A–10C). The number of microvesicles with surface-bound IgG, C4, and C3 were decreased in plasma from patients with AbMR compared to controls (Figures 10D–10F). This indicates that fewer or the microvesicles are generated in patients with AbMR, or that the opsonized microvesicles are cleared in vivo.
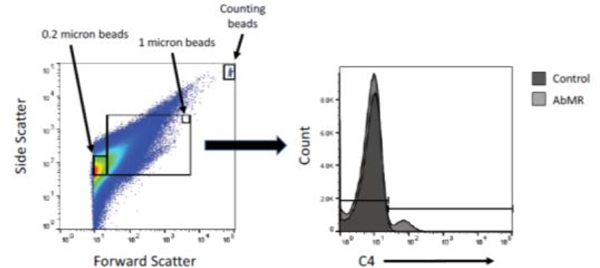
FIGURE 9. Representative example of gating used to detect plasma microvesicles by flow cytometry.
Microvesicles were initially gated by size using forward and side scatter. They were next gated by fluorescence of the detection antibody of interest, such as C4 as is shown here.
FIGURE 10. Anti-HLA antibodies and complement fragments on endothelial microvesicles (MV) in plasma of patients with antibody-mediated rejection (AbMR).
Microvesicles were purified from the plasma of patients with stable kidney transplants or biopsy-proven antibody-mediated rejection. The microparticles were then analyzed by flow cytometry. MV counts are standardized using fluorescent counting beads and presented as MV count per microliter of plasma ± SEM. Total MV and CD41+ MV counts were similar between groups, but MVs with surface-bound IgG, C4, and C3 were significantly lower in the AbMR group compared to control subjects (*P<0.05, **P<0.01, ***P<0.001).
4. Discussion
In this study we developed an in vitro model of AbMR using immortalized human microvascular endothelial cells and a monoclonal anti-class I HLA antibody to test whether complement activation fragments and endothelial microvesicles could serve as biomarkers of AbMR. Our model approximates the pathophysiology of AbMR in transplant recipients as demonstrated by the deposition of complement fragments on the cell surface, analogous to C4d fixation on endothelial cells in transplant biopsies with AbMR.
Active C4 on endothelial cells, in the form of C4b, is steadily cleaved to the inactive form C4d. Interestingly, C3b on the cells is cleaved to its inactive form so rapidly that little C3b is detected, even at the earliest time point we evaluated. The low levels of C3b indicate that complement regulation is very efficient on endothelial cells. Furthermore, the cleavage and inactivation of C4 and C3 was unaffected by the addition of an inhibitor of fI, which is a key mediator of degradation of C4 and C3 (Merle et al., 2015). This suggests that endothelial cells possess an alternative means of inactivating complement that is independent of fI. This cleavage could be mediated by proteases such as cathepsins that have been shown to have activity on complement proteins. It is also possible that endothelial cells eliminate some of the C3b through internalization and/or shedding of the proteins.
Some studies have suggested that C3 fragments in the renal allograft are a more specific but less sensitive marker of AbMR than the presence of C4 fragments and may be associated with poorer graft outcomes (Haas et al., 2006; Kuypers et al., 2003; Sund et al., 2003). Despite these associations, the utility of C3 detection is unclear and thus not included in the diagnostic criteria for AbMR (Haas et al., 2018; Herman et al., 2005). Our data suggests that the presence of C3dg is as durable on the cell surface as C4d, and is a better marker of complement activation on the cells than is C3b. However, many clinical labs use antibodies to C3c for biopsy tissue staining. These antibodies detect intact C3 and C3b, but not C3dg. As a result, this method of tissue staining may not detect C3 deposition in AbMR biopsies, and tissue staining with antibodies that detect C3d may be a more sensitive method of detecting complement activation on the endothelium.
The presence of C4d in the renal allograft is an important marker of AbMR (Racusen LC, 2003). However, the persistence of C4d bound to the endothelium is rarely described. Minami et al reported that C4d remained detectable in a rat cardiac transplant model for only five days, and Eskandary et al demonstrated with C1s inhibition can lead to reduction or elimination of C4d deposition in human kidney transplant recipients (Eskandary et al., 2018; Minami et al., 2006). We also demonstrate that C4, C3, and IgG are all transient on the cell surface and are undetectable above baseline in 1–2 days. The presence of IgG on the endothelium of renal allografts with AbMR has long been recognized to be inconsistent, but in our model, complement fragments are only modestly more stable and may be more limited as a marker of AbMR than traditionally perceived. This observation poses the question whether the presence of C4d in AbMR may be indicative of a more active process with ongoing complement activation and deposition, and its absence, in C4d-negative AbMR, indicative of a period of relatively decreased activity rather than representing distinct pathologies.
Invitro, classical pathway complement activation on the surface of endothelial cells leads to generation of soluble C4a and C5b-9, which can be measured by ELISA. Ba is also elevated, indicating involvement of the alternative pathway. Similar to the in vitro results, patients with AbMR have significantly higher plasma levels of C4a and Ba. Based on these results, increased levels of C4a and Ba in plasma may prove to be useful biomarkers for the detection of AbMR. C5b-9 levels, however, are not increased in plasma from patients with AbMR. The absence of C5b-9 in patient samples could be due to limited formation of MAC, or to more rapid clearance of C5b-9 from plasma in vivo. Treatment with an inhibitory antibody to C5 has been considered in AbMR, and the absence of a detectable increase in C5b-9 indicates that this would not be a useful guide to treatment. It is possible that anti-C5 treatment could reduce injury and thereby reduce C3 and C4 fragment generation, even though these proteins are cleaved earlier in the complement cascade.
Prior studies have shown that complement activation on the cell surface can elicit release of microvesicles from the cell (Moskovich, 2007). We hypothesized that detection of complement-opsonized microvesicles in plasma could provide a non-invasive marker of complement-mediated endothelial injury in renal allografts. We found that endothelial cell release of microvesicles is induced by exposure to serum in vitro, but this is not dependent on anti-HLA antibody binding or activation of the classical pathway of complement (Fig 7).
Complement fragments and IgG bound to endothelial microvesicles may also serve as a marker of classical pathway activation on the cell of origin. However, plasma from patients with biopsy-proven AbMR had decreased numbers of microvesicles with IgG, C4, and C3 bound to their surface compared to patients with stable renal allograft function, despite similar numbers of total microvesicles, platelet-derived microvesicles, and endothelial microvesicles. This is in contrast to data from Tower et al showing a significant increase in plasma-derived endothelial microvesicles with C4d in subjects with AbMR (Tower et al., 2017). A number of methodologic differences exist between these two studies that could be responsible for the discrepant results, such as plasma sample processing and detection antibodies. The data from Tower et al also included a larger sample size and controls with acute graft dysfunction due to etiologies other than AbMR. Furthermore, our control subjects did not have biopsies to confirm the absence of subclinical pathology, and this deserves further investigation.
Limitations of our study include the small sample size of patients with biopsy-proven AbMR and the cross-sectional study design. A subset of control patients had known DSA but did not have biopsies to confirm the absence of subclinical injury, which could confound results. Future studies should enroll more patients including controls with graft dysfunction from other etiologies such as tubular injury, cellular rejection, and BK nephropathy and include subsequent time points to evaluate responses to treatment.
5. Conclusion
Our in vitro model and pilot clinical data show that soluble complement fragments generated by antibody-mediated complement activation on the endothelium can be detected and give insight to events at the cellular level. Our data also suggests that circulating microvesicles are not a reliable biomarker of AbMR in contrast to a previous report. Current methods for surveillance and diagnosis of AbMR rely on detection of DSA and analysis of various antibody characteristics (i.e. titer, sub-class, or ex vivo complement fixation) or an invasive procedure (allograft biopsy). Our data also suggest that the precision of allograft biopsy could also be limited by the time course of disease with the relatively transient presence of detectable complement bound to the endothelium, particularly in the case of active C3 fragments. Plasma complement fragments have the advantage of being easily measured with a non-invasive method and reflecting active inflammation at the cellular level and further investigations are warranted to explore their utility in larger clinical study.
Supplementary Material
Highlights.
Complement activation on endothelial cells generates complement fragments
Complement activation on endothelial cells generates microvesicles
Complement fragments are increased in antibody-mediated rejection
Plasma endothelial microparticles decreased in antibody-mediated rejection
Acknowledgements
This work was supported by National Institutes of Health T32 DK007135 (ES), DK113586 and DK076690 (JMT). Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by NIH/NCATS Colorado CTSI Grant Number UL1 TR001082. We would like to acknowledge the assistance of the University of Colorado Flow Cytometry Shared Resource, as well as Ronald P. Taylor and Margaret A. Lindorfer who generously provided the 3E7 and 1H8 antibodies.
Abbreviations
- AbMR
Antibody-mediated rejection
- ESRD
end stage renal disease
- DSA
donor-specific antibody
- HLA
human leukocyte antigen
- NHS
normal human serum
- fB
factor B
- fI
factor
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- MAC
membrane attack complex
Footnotes
Conflict of interest
JMT receives royalties from Alexion Pharmaceuticals, Inc., and is a consultant for AdMIRx, Inc., a company developing complement inhibitors. He also holds stock and will receive royalty income from AdMIRx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson HC, Mulhall D, Garimella R, 2010. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Laboratory Investigation 90, 1549–1557. [DOI] [PubMed] [Google Scholar]
- Beyer C, Pisetsky DS, 2010. The role of microparticles in the pathogenesis of rheumatic diseases. Nature reviews. Rheumatology 6, 21–29. [DOI] [PubMed] [Google Scholar]
- Boulanger CM, Amabile N, Guerin AP, Pannier B, Leroyer AS, Mallat CN, Tedgui A, London GM, 2007. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension 49, 902–908. [DOI] [PubMed] [Google Scholar]
- Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A, 2009. Endothelial microparticles in diseases. Cell Tissue Res 335, 143–151. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Pawluczkowycz AW, Peng W, Kennedy AD, Beum PV, Lindorfer MA, Taylor RP, 2006. Selective and efficient inhibition of the alternative pathway of complement by a mAb that recognizes C3b/iC3b. Mol Immunol 43, 1010–1019. [DOI] [PubMed] [Google Scholar]
- Eskandary F, Jilma B, Muhlbacher J, Wahrmann M, Regele H, Kozakowski N, Firbas C, Panicker S, Parry GC, Gilbert JC, Halloran PF, Bohmig GA, 2018. Anti-C1s monoclonal antibody BIVV009 in late antibody-mediated kidney allograft rejection-results from a first-in-patient phase 1 trial. Am J Transplant 18, 916–926. [DOI] [PubMed] [Google Scholar]
- Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, Land W, Albert E, 1993. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int 43, 1333–1338. [DOI] [PubMed] [Google Scholar]
- Frazer-Abel A, Sepiashvili L, Mbughuni MM, Willrich MA, 2016. Overview of Laboratory Testing and Clinical Presentations of Complement Deficiencies and Dysregulation. Adv Clin Chem 77, 1–75. [DOI] [PubMed] [Google Scholar]
- Freeley S, Kemper C, Le Friec G, 2016. The “ins and outs” of complement-driven immune responses. Immunol Rev 274, 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, 2014. An updated Banff schema for diagnosis of antibody-mediated rejection in renal allografts. CurrOpin Organ Transplant 19, 315–322. [DOI] [PubMed] [Google Scholar]
- Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, van Huyen JPD, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, Mengel M, 2018. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18, 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Rahman MH, Racusen LC, Kraus ES, Bagnasco SM, Segev DL, Simpkins CE, Warren DS, King KE, Zachary AA, Montgomery RA, 2006. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant 6, 1829–1840. [DOI] [PubMed] [Google Scholar]
- Halloran PF, Schlaut J, Solez K, Srinivasa NS, 1992. The significance of the anti-class I response. II. Clinical and pathologic features of renal transplants with anti-class I-like antibody. Transplantation 53, 550–555. [PubMed] [Google Scholar]
- Herman J, Lerut E, Van Damme-Lombaerts R, Emonds MP, Van Damme B, 2005. Capillary deposition of complement C4d and C3d in pediatric renal allograft biopsies. Transplantation 79, 1435–1440. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Huang PH, Chiang CH, Leu HB, Huang CC, Chen JW, Lin SJ, 2013. Increased circulating endothelial apoptotic microparticle to endothelial progenitor cell ratio is associated with subsequent decline in glomerular filtration rate in hypertensive patients. PloS one 8, e68644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal D, Renner B, Laskowski J, Stites E, Cooper J, Valente K, You Z, Perrenoud L, Le Quintrec M, Muhamed I, Christians U, Klawitter J, Lindorfer MA, Taylor RP, Holers VM, Thurman JM, 2018. Endothelial Microparticles and Systemic Complement Activation in Patients With Chronic Kidney Disease. J Am Heart Assoc 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikic Z, Kainz A, Kozakowski N, Oberbauer R, Regele H, Bond G, Bohmig GA, 2015. Capillary C4d and Kidney Allograft Outcome in Relation to Morphologic Lesions Suggestive of Antibody-Mediated Rejection. Clin J Am Soc Nephrol 10, 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers DR, Lerut E, Evenepoel P, Maes B, Vanrenterghem Y, Van Damme B, 2003. C3D deposition in peritubular capillaries indicates a variant of acute renal allograft rejection characterized by a worse clinical outcome. Transplantation 76, 102–108. [DOI] [PubMed] [Google Scholar]
- Lindorfer MA, Beum PV, Taylor RP, 2013. CD20 mAb-mediated complement dependent cytotoxicity of tumor cells is enhanced by blocking the action of factor I. Antibodies 2, 598–616. [Google Scholar]
- Lindorfer MA, Jinivizian HB, Foley PL, Kennedy AD, Solga MD, Taylor RP, 2003. B cell complement receptor 2 transfer reaction. J Immunol 170, 3671–3678. [DOI] [PubMed] [Google Scholar]
- Loupy A, Vernerey D, Tinel C, Aubert O, Duong van Huyen JP, Rabant M, Verine J, Nochy D, Empana JP, Martinez F, Glotz D, Jouven X, Legendre C, Lefaucheur C, 2015. Subclinical Rejection Phenotypes at 1 Year Post-Transplant and Outcome of Kidney Allografts. J Am Soc Nephrol 26, 1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT, 2015. Complement System Part I - Molecular Mechanisms of Activation and Regulation. Frontiers in immunology 6, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami K, Murata K, Lee CY, Fox-Talbot K, Wasowska BA, Pescovitz MD, Baldwin WM 3rd, 2006. C4d deposition and clearance in cardiac transplants correlates with alloantibody levels and rejection in rats. Am J Transplant 6, 923–932. [DOI] [PubMed] [Google Scholar]
- Moskovich O, and Fishelson Z, 2007. Live cell imaging of outward and inward vesiculation induced by the complement C5b-9 complex. J Biol Chem 282, 29977–29986. [DOI] [PubMed] [Google Scholar]
- Orandi BJ, Chow EH, Hsu A, Gupta N, Van Arendonk KJ, Garonzik-Wang JM, Montgomery JR, Wickliffe C, Lonze BE, Bagnasco SM, Alachkar N, Kraus ES, Jackson AM, Montgomery RA, Segev DL, 2015. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant 15, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli S, Reville PK, Ellis TM, Djamali A, Mandelbrot DA, 2017. Utility of protocol kidney biopsies for de novo donor-specific antibodies. Am J Transplant 17, 3210–3218. [DOI] [PubMed] [Google Scholar]
- Racusen LC, C.R., Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K, 2003. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3, 708–714. [DOI] [PubMed] [Google Scholar]
- Schinstock CA, Cosio F, Cheungpasitporn W, Dadhania DM, Everly MJ, Samaniego-Picota MD, Cornell L, Stegall MD, 2017. The Value of Protocol Biopsies to Identify Patients With De Novo Donor-Specific Antibody at High Risk for Allograft Loss. Am J Transplant 17, 1574–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF, 2012. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12, 388–399. [DOI] [PubMed] [Google Scholar]
- Sund S, Hovig T, Reisaeter AV, Scott H, Bentdal O, Mollnes TE, 2003. Complement activation in early protocol kidney graft biopsies after living-donor transplantation. Transplantation 75, 1204–1213. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, Holers VM, 2005. A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol 42, 87–97. [DOI] [PubMed] [Google Scholar]
- Thurman JM, Kulik L, Orth H, Wong M, Renner B, Sargsyan SA, Mitchell LM, Hourcade DE, Hannan JP, Kovacs JM, Coughlin B, Woodell AS, Pickering MC, Rohrer B, Holers VM, 2013. Detection of complement activation using monoclonal antibodies against C3d. J Clin Invest 123, 2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower CM, Reyes M, Nelson K, Leca N, Kieran N, Muczynski K, Jefferson JA, Blosser C, Kukla A, Maurer D, Chandler W, Najafian B, 2016. Plasma C4d+ Endothelial Microvesicles Increase in Acute Antibody-mediated Rejection. Transplantation. [DOI] [PubMed] [Google Scholar]
- Tower CM, Reyes M, Nelson K, Leca N, Kieran N, Muczynski K, Jefferson JA, Blosser C, Kukla A, Maurer D, Chandler W, Najafian B, 2017. Plasma C4d+ Endothelial Microvesicles Increase in Acute Antibody-Mediated Rejection. Transplantation 101, 2235–2243. [DOI] [PubMed] [Google Scholar]
- Trpkov K CP, Pazderka F, Cockfield S, Solez K, Halloran PF, 1996. Pathologic features of acute renal allograft rejection associated with donor-specific antibody, Analysis using the Banff grading schema. Transplantation 61, 1586–1592. [DOI] [PubMed] [Google Scholar]
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK, 1999. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341, 1725–1730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.