Abstract
INTRODUCTION:
Behavioral variant Frontotemporal Dementia (bvFTD) may present sporadically or due to an autosomal dominant mutation. Characterization of both forms will improve understanding of the generalizability of assessments and treatments.
METHODS:
135 sporadic (s-bvFTD; mean age 63.3 years; 34% female) and 99 familial (f-bvFTD; mean age 59.9; 48% female) bvFTD participants were identified. F-bvFTD cases included 43 with known or presumed C9orf72 expansions, 28 with known or presumed MAPT mutations, 14 with known GRN mutations, and 14 with a strong family history of FTD but no identified mutation.
RESULTS:
F-bvFTD were younger and had earlier age of onset. S-bvFTD had higher total NPI-Q scores due to more frequent endorsement of depression and irritability.
DISCUSSION:
Familial and sporadic bvFTD cases are clinically similar, suggesting the generalizability of novel biomarkers, therapies, and clinical tools developed in either form to the other.
Keywords: frontotemporal dementia, bvFTD, genetics, MAPT, GRN, C9orf72, clinical trials
1. Introduction
Behavioral variant Frontotemporal Dementia (bvFTD) is the most common dementia phenotype among frontotemporal lobar degeneration (FTLD) syndromes and may present sporadically (s-bvFTD) or as the result of an autosomal dominant genetic mutation (f-bvFTD). Approximately 30% of bvFTD cases are due to these autosomal dominant mutations [1]. Known FTLD-associated mutations include hexanucleotide expansion in chromosome 9 open reading frame 72 (C9orf72)[2, 3] gene and mutations in the microtubule associated protein tau (MAPT)[4] and progranulin (GRN)[5, 6] genes. Smaller numbers are due to alterations in other genes, such as TAR DNA binding protein (TARDBP), TANK binding kinase 1 (TBK1), and charged multivesicular body protein 2b (CHMP2B) genes [7–9]. Some f-bvFTD have a family history consistent with an autosomal dominant syndrome but do not have a known underlying mutation.
As a clinical syndrome, bvFTD is characterized by progressive changes in social behavior, personality, and cognition [10] due to degeneration of frontal and temporal cortical regions[11]. The underlying pathology can be heterogeneous and, in f-bvFTD, may be reflective of the causative genetic mutation [12, 13]. New therapeutic agents are under development for FTLD syndromes, including bvFTD. Most therapeutic strategies target specific underlying molecular pathologies, either tau or TDP-43 ( FTLD-tau and FTLD-TDP), which account for the pathology in most but not all bvFTD cases..[14]. In s-bvFTD, it is currently not possible to determine with confidence which underlying pathology is the cause of disease during life. In contrast, in f-bvFTD, the underlying pathology can be accurately predicted by genotype: FTLD-tau in MAPT mutation carriers, and FTLD-TDP in C9orf72 or GRN mutation carriers. Careful characterization and comparison of sporadic and familial forms will improve our understanding of whether treatments and assessments developed based on studies of f-bvFTD are generalizable to sporadic forms, and vice versa. A recent study compared sporadic and familial FTLD syndromes in Italy, but did not focus specifically on bvFTD [15]. Here, we compare clinical, behavioral, demographic, and motor features of a large cohort of bvFTD patients evaluated at sixteen sites across North America as part of the Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) and the Longitudinal Evaluation of Familial Frontotemporal Lobar Degeneration (LEFFTDS) consortia.
2. Methods
All participants were enrolled in ARTFL (Advancing Research and Treatment for Frontotemporal Lobar Degeneration; U54NS092089) or LEFFTDS (Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects; U01AG045390). Each participant was evaluated at one of the eighteen ARTFL/LEFFTDS sites in North America. Evaluation consisted of a neurological and physical exam, family history, neuropsychological testing, functional assessments, informant interviews, and a blood draw for biospecimen collection. Assessments included the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set 3.0 and FTLD modules (https://www.alz.washington.edu/NONMEMBER/NACCFormsAndDoc.html), the Schwab and England Activities of Daily Living (SEADL) functional scale[16], the Clinical Global Impression – Severity (CGIS) Rating[17], the Progressive Supranuclear Palsy Rating Scale (PSPRS)[18], and the Unified Parkinson’s Disease Rating Scale (UPDRS)[19]. We used the CDR® Dementia Staging Instrument [20] plus behavior and language domains from the NACC FTLD Module (CDR® plus NACC FTLD)[21]. We calculated a “sum of boxes” score by adding scores for all eight domains (CDR® plus NACC FTLD -SB). Some participants also underwent magnet resonance imaging (MRI); MRI was dependent on site and specific research project enrollment. Many participants had clinical MRIs for diagnostic purposes that were not comparable with the ARTFL/LEFFTDS imaging protocol; 53 participants in this report had ARTFL/LEFFTDS scans. All participants reported here were determined by ARTFL/LEFFTDS clinicians to meet criteria for probable bvFTD [10]. Participants with “FTD-ALS” phenotypes were not included in these analyses.
A participant was classified as having “sporadic” bvFTD if there was no autosomal dominant family history of an FTLD syndrome and there was no evidence for an underlying FTLD-associated genetic mutation. For enrollment in the familial FTLD projects in ARTFL and LEFFTDS, a participant had to a) have a strong autosomal dominant family history of FTLD (Goldman score of 1 (autosomal dominant) or 2 (family aggregation) [22]) or b) have a known FTLD-associated genetic mutation in the family. All ARTFL and LEFFTDS participants provide DNA for research genotyping of FTLD-associated genes (Ramos et al, personal communication); to date, research testing has confirmed 125 of the 135 “sporadic” bvFTD cohort do not carry known pathogenic mutations. If a pathogenic mutation was identified in research testing, the participant was included in the “familial” group, which also includes all participants who met the “familial” enrollment criteria. For initial comparisons, all familial participants were considered a single cohort; for more detailed comparisons, participants were divided into four groups (C9orf72, GRN, MAPT, and family history) according to the presence or expectation of a pathogenic mutation. Table 1 includes the numbers in each group where suspected mutation status has been verified (or is pending). Participants without an identified pathogenic mutation in the family but a clear autosomal dominant family history were classified in the “family history” group (n=14).
Table 1.
Demographic and Clinical Characteristics
| Sporadic | Familial (combined) | C9orf72 expansion | GRN mutation | MAPT mutation | Family history | |
|---|---|---|---|---|---|---|
| N | 135 | 99 | 43 | 14 | 28 | 14 |
| Sex (F:M) | 47:99 | 46:53 | 20:23 | 8:6 | 13:15 | 5:9 |
| Confirmed genetic status (pending) | 125 (10) | 96(3) | 41 (2) | 13 (1) | 28(0) | 14(0) |
| Age at visit [min max] | 63.6 (8.9)a [34 84] | 59.6 (9.6) [33 82] | 60.6 (9.6) [33 76] | 65.43 (8.2) [54 82] | 53.8 (8.7)d [37 70] | 62.0 (7.5) [45 70] |
| Age at onset [min max] | 57.8 (8.8)b [25 77] | 53.0 (10.2) [26 73] | 54.5 (10.5) [26 72] | 59.6 (8.2) [48 73] | 46.0 (8.4)e [30 59] | 55.9 (6.2) [42 66] |
| Year of Education | 15.7 (2.5) | 15.3 (2.6) | 15.6 (2.4) | 15.2 (3.8) | 14.6 (2.2) | 15.9 (2.1) |
| CDR-SB | 6.38 (3.3) | 7.28 (4.7) | 6.71 (3.5) | 10.29 (5.2) | 6.44 (5.2) | 7.68 (5.6) |
| CDR plus NACC FTLD SB | 8.65 (4.0) | 9.84 (6.0) | 9.12 (4.4) | 13.68 (6.6)f | 8.68 (6.6) | 10.57 (6.9) |
| CDR Language | 0.67 (0.7) | 0.92 (0.9) | 0.69 (0.7) | 1.39 (1.0) | 0.91 (1.0) | 1.18 (1.0) |
| CDR Comport | 1.60 (0.7) | 1.65 (0.7) | 1.72 (0.5) | 2 (0.8)i | 1.32 (0.7) | 1.71 (0.7) |
| FAQ | 18.8 (8.8) | 18.9 (8.9) | 18.2 (8.1) | 20.0 (11.4) | 18.9 (10.8) | 20.3 (6.9) |
| NPI-Q Total | 12.08 (7.5)c | 9.70 (6.2) | 10.05 (6.1) | 9.5 (5.2) | 8.68 (7.2) | 10.73 (5.8) |
| SEADL | 60.0 (21.8) | 56.8 (27.8) | 59.2 (26.1) | 40.7(25.9) | 61.1 (29.1) | 57.1 (29.5) |
| CGI-S | 3.7 (1.0) | 4 (1.2) | 3.8 (1.1) | 4.7 (1.0)j | 4 (1.4) | 3.8 (1.3) |
| GDS (15-item) | 3.3 (3.5) | 2.9 (3.0) | 2.1 (2.4) | 3.4 (4.1) | 3.5 (2.9) | 4.2 (4.0) |
| Social Behavior Observer Checklist Total | 12.3 (10.3) | 10.8 (8.9) | 11.8 (9.6) | 13/9 (12.1) | 8.4 (6.0) | 11.8 (8.0) |
For “Confirmed Genetic Status”, parentheses indicate number pending results. All other data are given as mean (sd). For age at visit and age at onset, the ranges are given in [].
Sporadic>Familial (combined); Mann-Whitney; p=0.0043
Sporadic> Familial (combined); p=0.0007.
Sporadic>Familial (combined); p=0.0183
MAPT < all other groups; p<0.01.. GRN > MAPT and GRN > Sporadic (p<0.023).
GRN >MAPT,p=0.0153.
GRN > Sporadic p=0.0095.
Statistical analyses were performed using t-tests (sporadic vs. familial) or ANOVA (familial cohorts) with post-hoc corrections for normally distributed variables (age, education) and non-parametric tests where indicated (e.g. CDR®, PSPRS, SEADL) with Holm corrections for multiple pairwise comparisons. Fisher’s exact test was used to compare binary variables such as sex and individual items of the NPI-Q. For core neuropsychological measures from the NACC battery, both raw scores and calculated Z-scores (Kornak et al., personal communication) were used for group comparisons. To determine whether non-significant results were due to a lack of statistical power, we conducted post hoc power analyses using GPower[23] on the basis of the mean, between-groups comparison effect size observed in the present study with power (1 - β) set at 0.80 and α =0.05, two-tailed [24].
MRI images collected through ARTFL/LEFFTDS were available in a subset of participants (31 f-bvFTD; 22 s-bvFTD). All T1-weighted images were visually inspected for quality control prior to pre-processing. Images with excessive motion or image artifact were excluded. T1-weighted images underwent bias field correction using N3 algorithm, and segmentation was performed using SPM12 (Wellcome Trust Center for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm) unified segmentation. A group template was generated from segmented gray and white matter tissues and cerebrospinal fluid by non-linear registration template generation. Subjects’ native space gray and white matter were normalized, modulated and smoothed in the group template. The applied smoothing used a Gaussian kernel with 10 mm full width half maximum. For statistical purposes, linear and non-linear transformations between the group template space and International Consortium of Brain Mapping (ICBM) were applied.
3. Results
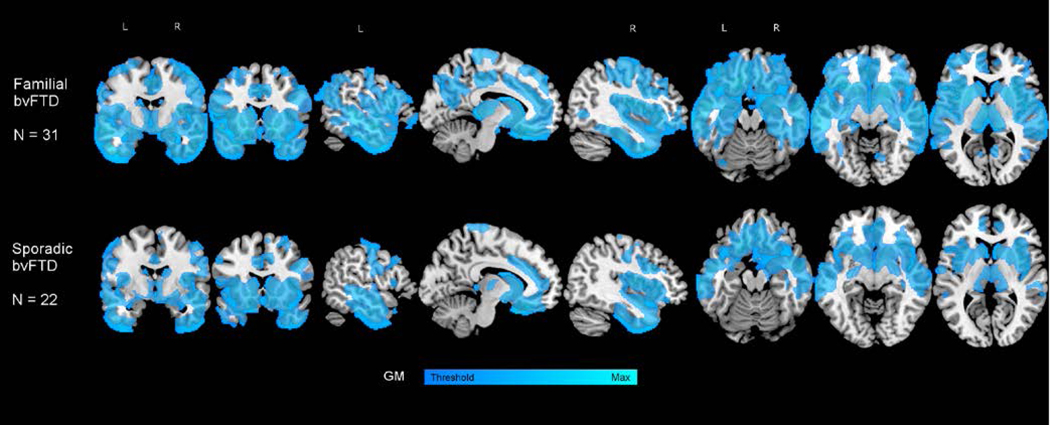
Of 237 participants with probable bvFTD, 135 were considered sporadic (s-bvFTD); 102 were determined to have a familial form (f-bvFTD). Three f-bvFTD were excluded from analyses as they had TARDBP mutations and the small group n did not allow for comparisons; thus 99 f-bvFTD were included, resulting in a total of 234 participants. In the subset of participants with available MRI imaging, both s-bvFTD (n=22) and f-bvFTD (n=31) showed typical patterns of cortical atrophy compared to age and gender matched controls (Figure 1). Demographic and clinical information is shown in Table 1. Sporadic patients were older (p=0.0012) and had later age of onset (p=0.0002) than f-bvFTD. The two groups were similar in education, race, sex distribution, and clinical measures of impairment, including the FAQ, SEADL, and CGI-S.
Figure 1.
Patterns of atrophy in familial (top; n=31 ) and sporadic (bottom; n=22) bvFTD. The figure represents the result of two-sample t tests compared with age and gender matched healthy controls (N=30). The models control for age, gender, and TIV. T- maps are thresholded at cluster level FWE-corrected p 0.05 (this corresponds to t = 3.24 and k = 1155 in familial, and t = 3.27 and k = 1004 in sporadic).
We also separated the familial cohort into four groups based on genetic mutation. The age differences were driven by the MAPT cohort; patients in the MAPT group were younger at their evaluating visit than all other groups except family history (p≤0.001 for all pairwise comparisons; ANOVA with Holm correction). The MAPT cohort were also younger at age of onset than any other cohort (p<0.001 for all pairwise comparisons). No other pairwise comparisons were significant for the age measurements, indicating that the age of onset and age at visit effects were due to the MAPT group. The C9orf72, GRN, and family history groups did not differ from each other or the sporadic cohorts.
With the four familial groups, we found that GRN mutation carriers showed slightly greater disease severity than MAPT carriers or s-bvFTD as measured by the CDR® plus NACC FTLD-SB (p=0.0145 and p=0.0226, respectively; Kruskal-Wallis with Holm correction). Similarly, there was a difference in the NACC FTLD comportment domain scores for GRN compared to MAPT (p=0.0153). These were moderate effect sizes (0.367, 0.243, and 0.411, respectively). The effect sizes for other pairwise comparisons were quite small (<0.2).
3.1. Behavioral measures.
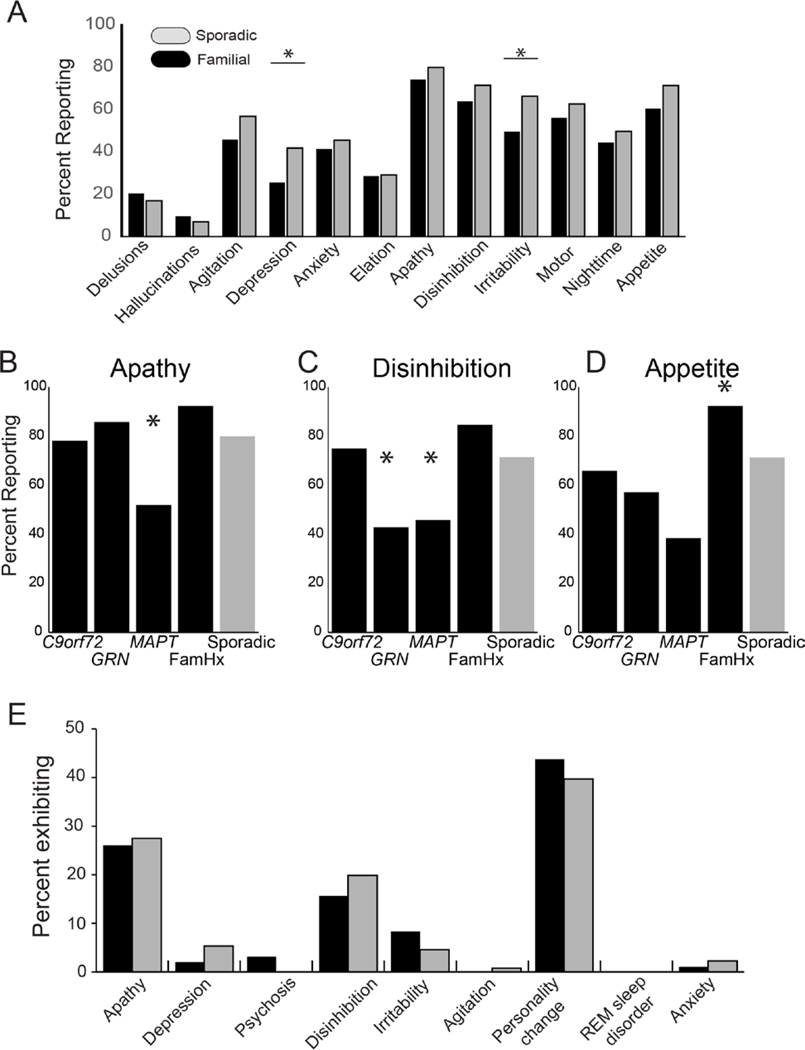
S-bvFTD had higher ratings on the NPI-Q (p=0.0183; chi-square) indicating that their informants more frequently reported neuropsychiatric features. When we examined individual items from the NPI-Q, we found that depression and irritability were more frequent in s-bvFTD (p=0.011 and 0.012 respectively, Fisher’s exact test; Figure 2A). No other items differed between the combined f-bvFTD cohort and s-bvFTD; however, within the f-bvFTD groups, differences were found for apathy (p=0.019; Fisher’s exact test), disinhibition (p=0.015), and appetite (p=0.005). MAPT carriers were less likely to exhibit apathy, disinhibition, and appetite changes. GRN carriers were less frequently reported to show disinhibition, while participants in the family history cohort were most likely to demonstrate changes in appetite (Figure 2B–D).
Figure 2.
A. Percentage of respondents endorsing each item of the Neuropsychiatric Inventory Questionnaire (NPI-Q). Bars indicate significant comparisons. B-D. Percentage of responders endorsing apathy (lB), disinhibition (C), and changes in appetite (D), by familial group. Asterisks indicate cohort driving effect. E. Distribution of predominant first behavioral feature (% total) reported for f-bvFTD and s-bvFTD. Initial features did not differ between groups.
To determine whether differences on the NPI-Q were due to differential reporting by the informant, we also examined the clinician’s judgement of symptoms (NACC UDS 3.0 Form B9). As on the NPI-Q, s-bvFTD tended to be judged as more irritable (p=0.054; chi-square), more depressed (p=0.07), and more agitated (p=0.055). This finding of increased irritability was also confirmed by the PSPRS irritability item (p=0.04). No other behavioral features from the NACC Form B9 differed between s-bvFTD and f-bvFTD. Both groups showed similar distributions of the predominant initial behavioral symptom (Figure 2E), with personality change, apathy, and disinhibition most common. Other behavioral assessments such as Social Behavior Observer Checklist, Behavior Inhibition Test, and Revised Self-Monitoring Scale did not differ between the groups.
3.2. Cognitive.
Scores for neuropsychological assessments are shown in Table 2. For measures from the NACC UDS neuropsychological battery, Z-scores were calculated adjusting for age and education (Kornak et al, personal comunication). Although impaired compared to “normal” performance, sporadic and familial bvFTD were similar across standard neuropsychological tests. There were small differences in executive function/working memory: s-bvFTD performed better than f-bvFTD on the “Number Span Forward” test, both for span length and number of correct trials (p=0.0113, and p=0.0073, respectively overall). This was primarily due to worse performance in the GRN and FamHx cohorts compared to sporadics (span length: p=0.0103 and p=0.016; correct trials: p=0.0089 for GRN). These were small effects (<0.3). No other core neuropsychological measures from the NACC UDS 3.0 battery differed. Since language features often occur in the context of bvFTD, we examined the language tests of the FTLD module; no language measures differed by across the cohorts.
Table 2.
Neuropsychological Scores
| Sporadic | Familial (combined) | C9orf72 expansion | GRN mutation | MAPT mutation | Family history | |
|---|---|---|---|---|---|---|
| MoCA | 18.40 (6.8) 126 | 17.34 (7.5) 88 | 18.8 (6.2) 40 | 12.33 (8.3) 12 | 18.17 (7.2) 23 | 16 (9.8) 13 |
| MoCA Z | −4.88 (4.0) | −5.68 (4.7) | −4.79 (3.6) | −7.68 (4.7) | −5.82 (4.7) | −6.31 (5.7) |
| MINT | 23.67 (8.8) 121 | 23.88 (7.1) 84 | 25.62 (5.7) 39 | 23.36 (8.8) 11 | 22.37 (7.1) 22 | 21.5 (9.1) 12 |
| MINT Z | −3.95 (5.2) | −3.87 (4.2) | −2.83 (3.2) | −4.04 (4.7) | −4.81 (4.5) | −5.35 (5.5) |
| Number Forward (correct trials) | 7.07 (2.6)a 127 | 5.92 (2.4) 88 | 6.13 (2.1) 39 | 4.36 (2.2)c 11 | 6.91 (2.3) 24 | 4.85 (2.8)e 14 |
| Number Forward (correct trials; Z) | −0.3 (1.1) a | −1.2 (1.0) | −1.07 (0.9) | −1.78 (1.0)c | −0.72 (1.0) | −1.62 (1.2)e |
| Number Forward (longest span) | 6.0 (1.4)a | 5.3 (1.5) | 5.5 (1.2) | 4.4 (1.8)d | 5.8 (1.3) | 4.6 (1.8) |
| Number Forward (longest span; Z) | −0.63 (1.0) a | −1.21 (1.2) | −1.07 (0.9) | −1.85 (1.4)d | −0.86 (1.0) | 1.71 (1.4) |
| Number Backward (correct trials) | 4.69 (2.8) 127 | 3.99 (2.6) 87 | 3.51 (1.9) 39 | 2.81 (3.0) 11 | 5.42 (2.4)f 24 | 3.77 (3.6) 13 |
| Number Backward (correct trials; Z) | −1.35 (1.3)b | −1.71 (1.2) | −1.94 (0.9) | −2.16 (1.4) | −1.07 (1.1)f | −1.81 (1.7) |
| Number Backward (longest span) | 3.59 (1.7) | 3.16 (1.8) | 2.97 (1.3) | 2.09 (2.2) | 4.08 (1.7) f | 2.92 (2.4) |
| Number Backward (longest span; Z) | −1.39 (1.4) | −1.75(1.4) | −1.91 (1.0) | −2.53 (1.7) | −1.03 (1.4) f | −1.95 (1.9) |
| Craft Story Immediate Recall | 10.80 (8.1) 121 | 12.53 (8.6) 85 | 13.62 (8.4) 39 | 11.10 (7.9) 10 | 12.96 (8.8) 23 | 9.6 (9.8) 13 |
| Craft Story Immediate Recall: Z | −1.98 (1.3) | −1.72 (1.4) | −1.55 (1.3) | −1.87 (1.4) | −1.66 (1.4) | −2.21 (1.6) |
| Craft Story Delay | 7.44 (7.8) | 9.73 (8.0) | 11.62 (8.2) | 6.57 (8.1) | 9.13 (8.5) | 7.6 (7.2) |
| Craft Story Delay: Z | −2.05 (1.2) | −1.73 (1.3) | −1.47 (1.2) | −1.84 (1.3) | −1.89 (1.2) | −2.14 (1.3) |
| Benson Copy | 14.2 (3.1) 127 | 13.8 (3.9) 87 | 14.6 (2.0) 41 | 10.5 (6.2) 11 | 15.0 (3.5)g 23 | 11.4 (4.9) 12 |
| Benson Copy: Z | −1.31 (2.6) | −1.74 ((3.4) | −1.00 (1.7) | −4.42 (5.3) | −0.68 (3.1) | −3.83 (4.4) |
| Category fluency: Animals | 10.59 (6.4) 124 | 12.50 (8.0) 88 | 13.10 (7.4) 41 | 8.5 (8.3) 11 | 13.25 (7.2) 24 | 12.58 (10/9) 12 |
| Category fluency: Animals: Z | −2.39 (1.2) | −2.07 (1.5) | −1.95 (1.4) | −2.63 (1.5) | −1.98 (1.5) | −2.12 (2.13) |
| Verbal Fluency (F&L words) | 14.8 (9.0) 117 | 13.1 (10.3) 85 | 12.5 (8.9) 39 | 8.8 (10.1) 11 | 16.2 (10.6) 23 | 12.8 (13.57) 12 |
| Trail Making Test A | 57.2 (34.0) 115 | 55.4 (36.6) 78 | 54.5 (31.06) 38 | 71.9 (47.6) 9 | 47.3 (36.0) 21 | 60.8 (44.7) 10 |
| Trail Making Test A: Z | −3.94 (4.7) | −4.04 (4.6) | −3.77 (3.8) | −4.91 (4.9) | −3.45 (5.5) | −4.7 (6.4) |
| Trail Making Test B | 144.5 (86.9) 90 | 140.0 (88.2) 56 | 156.8 (92.6) 24 | 180.2 (127.1) 6 | 100.6 (43.7) 19 | 154.9 (106.9) 7 |
| Trail Making Test B: Z | −3.63 (4.0) | −3.75 (3.9) | −4.64 (4.3) | −4.07 (4.8) | −2.41 (2.7) | −4.06 (4.6) |
| Word Reading Regular | 14.7 (0.9) 124 | 14.6 (1.5) 84 | 14.8 (1.0) 39 | 13.6 (3.6) 9 | 14.8 (0.5) 24 | 14.3 (1.5) 12 |
| Word Reading Irregular | 13.0 (3.2) 124 | 12.8 (2.5) 84 | 13.2 (2.1) 39 | 12.9 (3.3) 10 | 12.7 (2.2) 24 | 11.6 (3.5) 12 |
| Sentence Repetition | 4.2 (1.0) 122 | 3.8 (1.3) 86 | 3.9 (1.2) 39 | 3.1 (1.6) 11 | 4.0 (1.2) 24 | 3.8 (1.7) 12 |
| Sentence Reading | 4.3 (1.1) 120 | 4.3 (1.1) 83 | 4.4 (0.8) 39 | 4.1 (1.1) 9 | 4.2 (1.4) 24 | 4.1 (1.6) 11 |
All data presented as mean (sd).
Sporadic > Familial (combined); Mann-Whitney; p<0.012.
Sporadic>Familial, p=0.048.
c-e. All pairwise comparisons Kruskal-Wallis with Holm correction.
GRN < Sporadic; p<0.009
GRN <Sporadic; p<0.013.
.Family History<Sporadics; p<0.03.
MAPT>C9orf72; p<0.013.
MAPT>Family History and GRN; p<0.022
3.3. Motor.
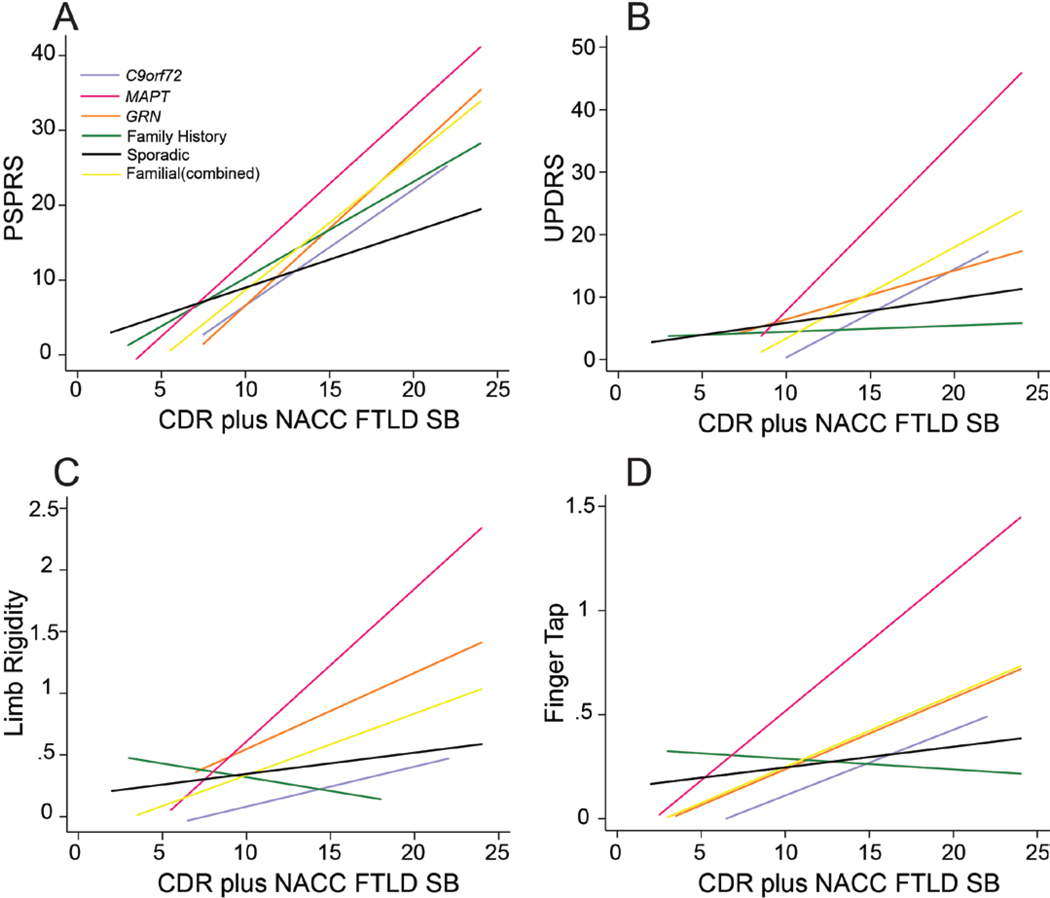
Participants underwent thorough neurological and physical exams, including motor assessments. There were no differences overall on the UPDRS and PSPRS scales, or on specific items such as gait, limb rigidity or dystonia, finger tap, tremor, and postural stability (Table 3). We hypothesized that groups within the f-bvFTD cohorts might differ in motor symptoms due to the differing underlying proteinopathies, but that these differences might not be observable until later in disease progression. To evaluate the effect of cohort on motor scores, we conducted linear regression analysis of motor scores as a function of the interaction between disease severity (CDR® plus NACC FTLD-SB) score and cohort (Figure 3). Compared to the sporadic cohort, f-bvFTD exhibit higher PSPRS scores (p=0.007) and higher UPDRS scores (p=0.017) with increased disease severity. Specifically, MAPT mutation carriers tended to have increased motor symptoms at worse disease severity, although these differed from s-bvFTD only for UPDRS (p=0.006).
Table 3.
Motor features
| Sporadic | Familial (combined) | C9orf72 expansion | GRN mutation | MAPT mutation | Family history | |
|---|---|---|---|---|---|---|
| PSPRS (Total) | 7.5 (7.7) | 7.2 (9.3) | 5.8 (6.9) | 6.7 (8.7) | 9.0 (12.3) | 8.8 (10.4) |
| UPDRS (Total) | 5.4 (8.5) | 5.8 (11.9) | 2.5 (4.7) | 9.0 (12.1) | 10.0 (18.3) | 4.5 (7.8) |
| Gait | 0.3 (0.8) | 0.3 (0.6) | 0.1 (0.6) | 0.5 (0.9) | 0.4 (0.7) | 0.1 (0.4) |
| Limb Rigidity | 0.3 (0.7) | 0.3 (0.7) | 0.1 (0.4) | 0.7 (1.0) | 0.6 (0.9) | 0.4 (0.7) |
| Dystonia | 0.02 (0.2) | 0.04 (0.3) | 0 | 0.3 (0.8) | 0 | 0 |
| Finger Tap | 0.23 (0.4) | 0.23 (0.5) | 0.08 (0.3) | 0.33 (0.7) | 0.40 (0.6) | 0.29 (0.5) |
| Tremor | 0.17 (0.4) | 0.23 (0.5) | 0.07 (0.3) | 0.36 (0.5) | 0.40 (0.6) | 0.29 (0.5) |
| Postural stability | 0.24 (0.7) | 0.16 (0.6) | 0.06 (0.3) | 0 | 0.40 (0.9) | 0.15 (0.6) |
Figure 3.
Regression models for motor scores as a function of CDR® plus NACC FTLD SB by cohort. A. PSPRS Total Score. B. UPDRS Total Score. C. Limb Rigidity Item from PSPRS. D. Finger Tap Score from PSPRS.
4. Discussion
Our sample of 235 s- and f-bvFTD patients were highly similar across behavioral, cognitive, and motor measures despite arising from different underlying pathologies and genetic factors. These similarities suggest that the Frontotemporal Dementia Consensus bvFTD criteria[10] capture a relatively homogenous clinical syndrome regardless of genetic or proteinopathy (tau vs TDP-43) origin. Differences in age at visit and age of onset between familial and sporadic bvFTD were due to earlier onset in MAPT mutation carriers, suggesting that genetic tau-mediated pathology may manifest earlier than the TDP-43 pathology found in C9orf72 and GRN mutation carriers. This might be explained by an ascertainment bias; families with known mutations or strong family history may seek diagnosis and treatment sooner due to anticipation of problems. However, while ascertainment bias could explain a younger age at visit, it would not alter the apparent age of onset. Additionally, the severity of most symptoms did not differ between s-bvFTD and f-bvFTD, suggesting it is unlikely that earlier diagnosis greatly affects our findings.
Previous reports have suggested that C9orf72 mutation carriers exhibit more features of psychosis, including hallucinations and delusions, than other FTD variants. [25, 26] Across our sample, rates of reported hallucinations (6.6–20%) and delusions (10–38%) were lower than previously reported and did not differ based on underlying genetic mutation. This may be partially due to the exclusion of FTD-ALS from the analyses, as well as the projects’ focus on patients with milder degrees of dementia severity than those reported previously.
In general, motor impairment was not severe in our bvFTD cohort; we excluded cases of FTD-ALS from the current analyses. We hypothesized that tau pathology might present more motor symptoms as found in other tauopathy forms of FTLD such as one of the progressive supranuclear palsy syndromes or corticobasal syndrome. We found that motor scores were higher with greater disease severity in f-bvFTD, especially for MAPT mutation carries. This may reflect greater involvement of the basal ganglia with tau disease pathology, as has been previously described for MAPT mutation carriers in post-mortem examination [27, 28]. Our s-bvFTD cohort presumably comprises both FTLD-tau and FTLD-TDP pathologies; we did not find indications of differential distribution of motor symptoms within the s-bvFTD group (not shown). It will be of great interest to follow the s-bvFTD patients to autopsy to determine whether s-bvFTD with tau pathology has similarly elevated parkinsonism, particularly in later stages of disease.
A variety of clinical trials of therapies targeting each of the common f-bvFTD genes are poised to begin in FTLD mutation carriers, including GRN (progranulin elevating drugs), MAPT (anti-tau monoclonal antibodies, antisense oligonucleotides), and C9orf72 (antisense oligonucleotides). While some treatments are aimed at prevention of disease onset, others may primarily intend to ameliorate symptoms and improve quality of life. Depending on mechanism, a number of these therapies also could be tested in s-bvFTD patients, especially if underlying pathology (Tau vs. TDP-43) could be established during life. As these treatments advance in clinical trials, new tools will be needed to determine whether they produce clinically meaningful benefits for patients with symptomatic disease. Given the rarity of f-bvFTD, it would be very difficult to develop clinical rating scales specific to these groups. Our analyses provide empirical support, based on cross-sectional data, that clinical rating scales developed from s-bvFTD may be generally applicable for f-bvFTD in future efficacy trials.
Despite the overall similarities of clinical features, our data suggest that the genetic etiology and associated pathologies may influence disease presentation and progression worth exploring further. Although we do not directly address rates of progression, we have found that MAPT carriers exhibit more motor features in conjunction with greater disease severity, suggesting that the disease trajectories may differ depending on etiology. Longitudinal assessments in larger cohorts will help determine whether disease progression patterns are similar, or whether disease presentations diverge more with increasing severity.
Several of our cohorts comprised small sample sizes, which may have limited our ability to detect differences between the familial cohorts. We examined the very small effect sizes of our non-significant results; for most analyses sample sizes would have to increase dramatically in order for group differences to reach statistical significance at the .05 level. For example, one of the largest observed non-significant effect sizes was between C9orf72 expansion carriers and MAPT mutation carriers for Number Span Backward Correct Trials. For the observed effect size (0.475), a sample size of 148 participants would be needed. For most comparisons, the sample sizes required are in the thousands. Thus, we believe it is unlikely that our negative findings can be attributed primarily to limited sample size.
Our findings of similar patterns of abnormalities on behavioral, clinical, and motor scales between familial and sporadic bvFTD in a large, multicenter, clinical trials-ready population suggest that therapeutics tested and developed for symptom alleviation may be generalizable across the clinical syndrome, independent of etiology. Our data suggest that the FTDC diagnostic criteria for probable bvFTD are a good starting point for FTLD diagnosis and treatment .These analyses strongly suggest that parallel longitudinal characterization of both sporadic and familial bvFTD will improve our understanding of the disease and guide trial design. Given the increasing numbers of planned clinical trials of agents targeting specific familial forms of bvFTD, clinical trial ready populations should also be genotyped for known FTLD causing mutations. Since there are no effective therapies at this time, we think it is premature to recommend routine genetic testing as part of a clinical evaluation for bvFTD.
Highlights.
Sporadic and familial forms of behavioral variant frontotemporal dementia (bvFTD) are similar and may inform treatments for each other.
The Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) and Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS) consortia provide a framework for comparing clinical phenotype across underlying pathologies.
Acknowledgements
We extend our appreciation to Drs. John Hsiao and Dallas Anderson from the National Institute on Aging, Drs. Marg Sutherland, Codrin Lungu, and Roderick Corriveau from the National Institute of Neurological Disorders and Stroke, the clinicians and coordinators at all ARTFL/LEFFTDS centers, and particularly to our patients and their families for their participation in this research.
Funding
This work is supported by the National Institutes of Health [grants U01 AG045390, U54 NS092089, U24 AG021886 and U01 AG016976].
Abbreviations
- bvFTD
behavioral variant Frontotemporal Dementia
- s-bvFTD
sporadic bvFTD
- f-bvFTD
familial bvFTD
- FTLD
Frontotemporal Lobar degeneration
- FTLD-tau
FTLD due to underlying tau pathology
- FTLD-TDP
FTLD due to underlying TDP-43 pathology
- C9orf72
Chromosome 9 open reading frame 72
- MAPT
Membrane associated protein tau gene
- GRN
progranulin gene
- TARDBP
transactive response DNA (TAR DNA) binding protein gene
- TBK1
TANK binding kinase1 gene (TANK: tumor necrosis factor receptor-associated factor family member-asociated NF-kappa-B-activator)
- CHMP2B
charged multivesicular body protein 2b gene
- ARTFL
Advancing Research and Treatment for Frontotemporal Lobar Degeneration
- LEFFTDS
Longitudinal Evaluation of Familial Frontotemporal Dementia Subjuects
- NACC
National Alzheimer’s Coordinating Center
- UDS
NACC Uniform Data Set 3.0
- SEADL
Schwab and England Activities of Daily Living scale
- CGIS
Clinical Global Impression – Severity
- PSPRS
Progressive Supranuclear Palsy Rating Scale
- UPDRS
Unified Parkinson’s Disease Rating Scale
- NPI-Q
Neuropsychiatric Inventory Questionnaire
- FAS
Functional Activity Scale
- CDR® plus NACC FTLD
CDR® Dementia Staging Instrument plus behavior and language domains from the NACC FTLD module
- MoCA
Montreal Cognitive Assessment
- MINT
Multi Item Naming Test
Disclosures
Heuer HW - nothing to disclose
Wang P – nothing to disclose
Rascovsky K – receives research support from NIH
Wolf A – nothing to disclose
Appleby B – receives research support from CDC
Bove J – nothing to disclose
Bordelon Y – has served as an investigator for clinical trials sponsored by AbbVie, Biogen, Bristol-Myers Squibb, and C2N Diagnostics
Brannelly P – employed by the Rainwater Charitable Foundation
Brushaber DE – nothing to disclose
Caso C – nothing to disclose
Coppola G – receives research support from the NIH, the Tau Consortium, the Adelson Medical Research Foundation, Takeda Pharmaceutical Company Ltd., the John Douglas French Alzheimer’s Foundation, the Friedreich’s Ataxia Research Alliance, the CHDI foundation, the Hillblom Foundation, the Eleanor Leslie Chair in Innovative Brain Research from the Brain Research Institute, and the Semel Institute for Neuroscience and Human Behavior at the University of California Los Angeles.
Dickerson BC – receives research support from NIH and royalties from Oxford University Press and Cambridge University; consults for Biogen, Merch, Lilly, Wave Lifesciences and Arkuda; and is paid by Elsevier for editorial activity.
Dickinson S – on staff at the Association for Frontotemporal Degeneration and a member of the National Institute for Neurological Disorders and Stroke Advisory Council.
Domoto-Reilly K – Has served as an investigator for clinical trials sponsored by Avid Radiopharmaceuticals, Biogen, Janssen Pharmaceuticals. Has served as Advisory Board consultant for Biogen. Receives research support from NIH.
Faber K – nothing to disclose
Fields J – receives research support from NIH
Fong J – nothing to disclose
Foroud T – receives research support from NIH
Forsberg L – nothing to disclose
Gearhart D – nothing to disclose
Ghazanfari B – nothing to disclose
Ghoshal N - has participated or is currently participating in clinical trials of anti-dementia drugs sponsored by the following companies: Bristol Myers Squibb, Eli Lilly/Avid Radiopharmaceuticals, Janssen Immunotherapy, Novartis, Pfizer, Wyeth, SNIFF (The Study of Nasal Insulin to Fight Forgetfulness) study, and A4 (The Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease) trial. She receives research support from Tau Consortium and Association for Frontotemporal Dementia and is funded by the NIH.
Goldman, JS – is serving as a consultant to the Novartis Alzheimer’s Prevention Advisory Board. She receives research support from NIH, HDSA, New York State Department of Health (RFA # 1510130358)
Graff-Radford J – receives research support from the NIH.
Graff-Radford N – receives royalties from UpToDate, has participated in multicenter therapy studies by sponsored by Biogen, TauRx, AbbVie, Novartis and Lilly. He receives research support from NIH.
Grant I – nothing to disclose
Grossman M - receives grant support from NIH, Avid and Piramal; participates in clinical trials sponsored by Biogen, TauRx, and Alector; serves as a consultant to Bracco and UCB; and serves on the Editorial Board of Neurology.
Haley D – nothing to disclose
Hsiung G-Y – has served as an investigator for clinical trials sponsored by AstraZeneca, Eli Lilly, and Roche / Genentech. He receives research support from Canadian Institutes of Health Research and the Alzheimer Society of British Columbia.
Huey E – Receives grant support from NIH, the Association for Frontotemporal Degeneration, and the Alzheimer’s Drug Discovery Foundation. He participates in clinical trials sponsored by NIH and the Lawson Health Research Institute
Irwin D – receives support from NIH, Brightfocus Foundation and Penn Institute on Aging.
Jones D- – receives research support from NIH and the Minnesota Partnership for Biotechnology and Medical Genomics
Kantarci K - served on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc.; data monitoring boards of Pfizer and Janssen Alzheimer Immunotherapy; research support from the Avid Radiopharmaceuticals, Eli Lilly, the Alzheimer’s Drug Discovery Foundation and NIH
Karydas A – nothing to disclose
Kaufer D - Has served as an investigator for clinical trials sponsored by Abbvie, Axovant, Janssen Research & Development, Navidea Biopharmaceuticals, and TauRx. He has consulted for Abbvie, Axovant, Janssen Research & Development, Takeda/Zinfandel. He serves on the Scientific Advisory Board of the Lewy Body Dementia Association. He receives research funding from the NIH, HRSA, and Bryan Family Foundation.
Kerwin, D has served on an Advisory Board for AbbVie and as site PI for studies funded by Roche/Genentech, AbbVie, Avid, Novartis, Eisai, Eli Lilly and UCSF.
Knopman D - serves on the DSMB of the DIAN-TU study, is a site PI for clinical trials sponsored by Biogen, Lilly and the University of Southern California, and is funded by NIH.
Kornak J - – has provided expert witness testimony for Teva Pharmaceuticals in Forest Laboratories Inc. et al. v. Teva Pharmaceuticals USA, Inc., Case Nos. 1:14-cv-00121 and 1:14-cv-00686 (D. Del. filed Jan. 31, 2014 and May 30, 2014) regarding the drug Memantine; for Apotex/HEC/Ezra in Novartis AG et al. v. Apotex Inc., No. 1:15-cv-975 (D. Del. filed Oct. 26, 2015, regarding the drug Fingolimod. He has also given testimony on behalf of Puma Biotechnology in Hsingching Hsu et al, vs. Puma Biotechnology, INC., et al. 2018 regarding the drug Neratinib. He receives research support from the NIH.
Kraft R – nothing to disclose
Kramer JH - receives research support from NIH and serves on an advisory board for Biogen.
Kremers WK - receives research funding from AstraZeneca, Biogen, Roche, DOD and NIH.
Kukull W – receives research support from NIH.
Lapid M – nothing to disclose
Litvan I– receives research support from NIH, Parkinson Study Group, Parkinson Foundation, Michael J Fox Foundation, AVID Pharmaceuticals, C2N Diagnostics/Abbvie and Bristol-Myers Squibb. She was a member of the Biogen and Bristol-Myers Squibb Advisory Boards, Biotie/Parkinson Study Group Medical Advisory Board and consultant for Toyama Pharmaceuticals. Receives salary from the University of California San Diego and as Editor in Frontiers in Neurology
Ljubenkov P – nothing to disclose
Mackenzie IR – receives research funding from Canadian Institutes of Health Research.
Maldonado M – nothing to disclose
Manoochehri M – nothing to disclose
McGinnis S – has served as an investigator for clinical trials sponsored by AbbVie, Allon Therapeutics, Biogen, Bristol-Myers Squibb, C2N Diagnostics, Eisai Inc., Eli Lilly and Co., Genentech, Janssen Pharmaceuticals, Medivation, Merck, Navidea Biopharmaceuticals, Novartis, Pfizer, and TauRx Therapeutics. He receives research support from NIH.
McKinley E – nothing to disclose
Mendez MF – supported by NIH (NIA) research grants and has received research support from Biogen
Miller BL – receives research support from NIH
Onyike C – receives research funding from the NIH, the CIHR, and Biogen, Inc. He is also supported by the Jane Tanger Black Fund for Young-Onset Dementias, the Nancy H. Hall Fund for Geriatric Psychiatry, and the gift from Joseph Trovato.
Pantelyat A – receives research support from the NIH and AbbVie, Inc. Participates in a research trial sponsored by Biogen, Inc. He has served as consultant for AbbVie, Inc.
Pearlman R – employed by The Bluefield Project to Cure FTD.
Petrucelli L – receives research support from the NIH
Potter M – nothing to disclose
Rademakers R – receives research funding from NIH and the Bluefield Project to Cure Frontotemporal dementia
Ramos EM – nothing to disclose
Rankin KP – receives research support from NIH, Quest Diagnostics, and the Rainwater Charitable Foundation
Roberson ED – receives research support from NIH, Bluefield Project to Cure Frontotemporal Dementia, Alzheimer’s Association, BrightFocus Foundation, Biogen, Alector, and owns intellectual property related to tau
Rogalski E – receives research support from NIH and the Association for Frontotemporal Dementia
Sengdy P – nothing to disclose
Shaw L – receives research support from NIH
Syrjanen J – nothing to disclose
Tartaglia MC – receives research funding from CIHR and NIH, and is an investigator on pharmaceutical studies with Biogen, Roche, Eli Lilly, and Boehringer
Tatton N – employed by the Association for Frontotemporal Degeneration
Taylor J – nothing to disclose
Toga A – receives research support from the NIH and the Alzheimer’s Association
Trojanowski J – may accrue revenue in the future on patents submitted by the University of Pennsylvania wherein he is coinventor and he received revenue from the sale of Avid to Eli Lily as coinventor on Aβ amyloid imaging–related patents submitted by the University of Pennsylvania. He receives research support from the NIH and several nonprofits.
Weintraub S – receives research support from the NIH
Wong B – receives research support from the NIH
Wszolek Z - supported by the NIH, Mayo Clinic Center for Regenerative Medicine, the gift from Carl Edward Bolch, Jr., and Susan Bass Bolch, The Sol Goldman Charitable Trust, and Donald G. and Jodi P. Heeringa. He has also received grant funding support from Allergan, Inc. (educational grant), and Abbvie (medication trials).
Boeve B – has served as an investigator for clinical trials sponsored by GE Healthcare and Axovant. He receives royalties from the publication of a book entitled Behavioral Neurology Of Dementia (Cambridge Medicine, 2009, 2017). He serves on the Scientific Advisory Board of the Tau Consortium. He receives research support from NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program and the Little Family Foundation.
Rosen H – has received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from NIH.
Boxer AL – receives research support from NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer’s Drug Discovery Foundation and the Alzheimer’s Association. He has served as a consultant for Aeton, Abbvie, Alector, Amgen, Arkuda, Arvinas, Ionis, Iperian, Janssen, Lundbeck, Merck, Novartis, Passage BIO, Pinteon, Samumed, Toyama and UCB, and received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche and TauRx.
References
- [1].Rohrer JD, Guerreiro R, Vandrovcova J, Uphill J, Reiman D, Beck J, et al. The heritability and genetics of frontotemporal lobar degeneration. Neurology. 2009;73:1451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Khan BK, Yokoyama JS, Takada LT, Sha SJ, Rutherford NJ, Fong JC, et al. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. Journal Of Neurology, Neurosurgery, And Psychiatry. 2012;83:358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hutton M. Missense and splice site mutations in tau associated with FTDP-17: multiple pathogenic mechanisms. Neurology. 2001;56:S21–5. [DOI] [PubMed] [Google Scholar]
- [5].Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. [DOI] [PubMed] [Google Scholar]
- [6].Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–4. [DOI] [PubMed] [Google Scholar]
- [7].Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Muller K, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci. 2015;18:631–6. [DOI] [PubMed] [Google Scholar]
- [8].Benajiba L, Le Ber I, Camuzat A, Lacoste M, Thomas-Anterion C, Couratier P, et al. TARDBP mutations in motoneuron disease with frontotemporal lobar degeneration. Ann Neurol. 2009;65:470–3. [DOI] [PubMed] [Google Scholar]
- [9].Isaacs AM, Johannsen P, Holm I, Nielsen JE, consortium FR. Frontotemporal dementia caused by CHMP2B mutations. Curr Alzheimer Res. 2011;8:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain : a journal of neurology. 2011;134:2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Perry DC, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, et al. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017;140:3329–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta neuropathologica. 2010;119:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rohrer JD, Ridgway GR, Modat M, Ourselin S, Mead S, Fox NC, et al. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage. 2010;53:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tsai RM, Lobach I, Bang J, Whitwell JL, Senjem ML, Jack CR Jr., et al. Clinical correlates of longitudinal brain atrophy in progressive supranuclear palsy. Parkinsonism & related disorders. 2016;28:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Capozzo R, Sassi C, Hammer MB, Arcuti S, Zecca C, Barulli MR, et al. Clinical and genetic analyses of familial and sporadic frontotemporal dementia patients in Southern Italy. Alzheimers Dement. 2017;13:858–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schwab R, England A. Projecton technique for evaluating surgery in Parkinson’s disease. In: Gillingham F, Donaldson M, editors. Third Symposium on Parkinson’s Disease Research Edinburgh, Scotland: ES Livingston; 1969. [Google Scholar]
- [17].Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- [18].Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain. 2007;130:1552–65. [DOI] [PubMed] [Google Scholar]
- [19].Fahn S, Elton RL, Committee motUD. Unified Parkinson’s Disease Rating Scale In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Health-care Information; 1987. p. 153–63. [Google Scholar]
- [20].Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. [DOI] [PubMed] [Google Scholar]
- [21].Knopman DS, Weintraub S, Pankratz VS. Language and behavior domains enhance the value of the clinical dementia rating scale. Alzheimers Dement. 2011;7:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Goldman JS, Rademakers R, Huey ED, Boxer AL, Mayeux R, Miller BL, et al. An algorithm for genetic testing of frontotemporal lobar degeneration. Neurology. 2011;76:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Erdfelder C, Faul F, Buchner A. GPower: A general power analysis program. Behav Res Methods. 1996;28:1–11. [DOI] [PubMed] [Google Scholar]
- [24].Cohen J Statistical Power Analysis for the Behaviorl Sciences. New York, NY: Routledge Academic Press; 1988. [Google Scholar]
- [25].Hall D, Finger EC. Psychotic symptoms in frontotemporal dementia. Curr Neurol Neurosci Rep. 2015;15:46. [DOI] [PubMed] [Google Scholar]
- [26].Snowden JS, Harris J, Richardson A, Rollinson S, Thompson JC, Neary D, et al. Frontotemporal dementia with amyotrophic lateral sclerosis: a clinical comparison of patients with and without repeat expansions in C9orf72. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:172–6. [DOI] [PubMed] [Google Scholar]
- [27].Ghetti B, Wszolek Z, Boeve BF, Spina S, Goedert M. Frontotemporal Dementia and Parkinsonism linked to Chromosome 17 In: Dickson D, Weller RO, editors. Neurodegeneration: the molecular pathology of dementia and movement disorders. West Sussex, UK: John Wiley & Sons; 2011. p. 110–34. [Google Scholar]
- [28].Forrest SL, Kril JJ, Stevens CH, Kwok JB, Hallupp M, Kim WS, et al. Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain. 2018;141:521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]