Abstract
Injuries to articular cartilage and menisci can lead to cartilage degeneration that ultimately results in arthritis. Different forms of arthritis affect ~50 million people in the USA alone, therefore it is crucial to identify methods that will halt or slow the progression to arthritis, starting with the initiating events of cartilage and meniscus defects. The surgical approaches in current use have a limited capacity for tissue regeneration and yield only short-term relief of symptoms. Tissue engineering approaches are emerging as alternatives to current surgical methods for cartilage and meniscus repair. Several cell-based and tissue-engineered products are currently in clinical trials for cartilage lesions and meniscal tears, opening new avenues for cartilage and meniscus regeneration. This Review provides a summary of surgical techniques, including tissue-engineered products, currently in clinical use, as well as a discussion of state-of-the-art tissue engineering strategies and technologies that are being developed for use in articular cartilage and meniscus repair and regeneration. The obstacles to clinical translation of these strategies are also included to inform the development of innovative tissue engineering approaches.
Introduction
Arthritis is a debilitating condition that affects >50 million adults in the USA; a prevalence that is projected to rise by ~60% in the next two decades1. Osteoarthritis (OA), the most common type of arthritis2, is associated with pain and loss of joint function. Although the aetiology of OA can be idiopathic, the disease is often characterized by cartilage degeneration in articulating joints as a result of ‘wear and tear’ or injury, including sports-related injuries. For example, in one study, individuals who sustained potentially cartilage-damaging knee injuries were 7.4 times more likely to develop OA than those who had not sustained knee injuries3. Meniscus and anterior cruciate ligament (ACL) tears can also contribute to the development of OA because damage to these structures alters joint loading4,5; OA occurs 10–20 years post-injury in ~50% of patients who sustain meniscal or ACL tears5. Globally, knee and hip cartilage degeneration is one of the leading causes that contribute to disability6. Rheumatoid arthritis (RA), the second most common type of arthritis, is a chronic autoimmune disease characterized by inflammation and deterioration of joints that results in loss of function, and affects 1.3 million adults in the USA7. Worldwide, arthritides such as OA and RA present a substantial burden to the healthcare system8,9.
Despite the pervasiveness of OA, most current treatments are palliative and do not prevent further joint degeneration10. Likewise, treatments for RA often reduce joint inflammation without treating cartilage damage11. Ultimately, many patients with arthritis will require total joint arthroplasty, an invasive end-stage treatment that uses implants that wear-out over time. Current surgical strategies for cartilage repair are designed to treat small cartilage defects and are not directly indicated for use in inflamed joints, such as occur in RA. However, using tissue engineering strategies, which focus on the complete regeneration of articular cartilage12,13 and menisci14,15, researchers can potentially create neotissue that has been fortified to withstand immune-mediated degeneration. Thus, in the future, tissue engineering strategies could offer new therapeutic avenues for patients with RA before total joint arthroplasty is indicated.
In this Review, we begin by discussing current surgical techniques, including tissue-engineered treatments, defined here as cell-based (scaffold-free and scaffold-based) therapies, for the repair of articular cartilage and meniscus lesions. We then discuss advances in tissue engineering research for articular cartilage and menisci repair, including novel scaffold-based and scaffold-free approaches, promising sources of cells for cell-based therapies and emerging data on biochemical and biomechanical stimuli. We also present data on cell-based tissue-engineered products for cartilage regeneration currently in development. Finally, we discuss scientific and regulatory obstacles to the clinical translation of tissue-engineered technologies, as well as future directions to encourage researchers in the field to overcome these challenges.
Current surgical strategies
Repairing articular cartilage defects
Articular cartilage is located at the ends of articulating bones and provides a low-friction surface to support joint movement. It is composed of predominantly type II collagen and proteoglycans (e.g., glycosaminoglycans), is avascular with low cellularity (Fig 1a), and, therefore, has a low healing capacity.
Figure 1. Articular cartilage structure and treatment methods.
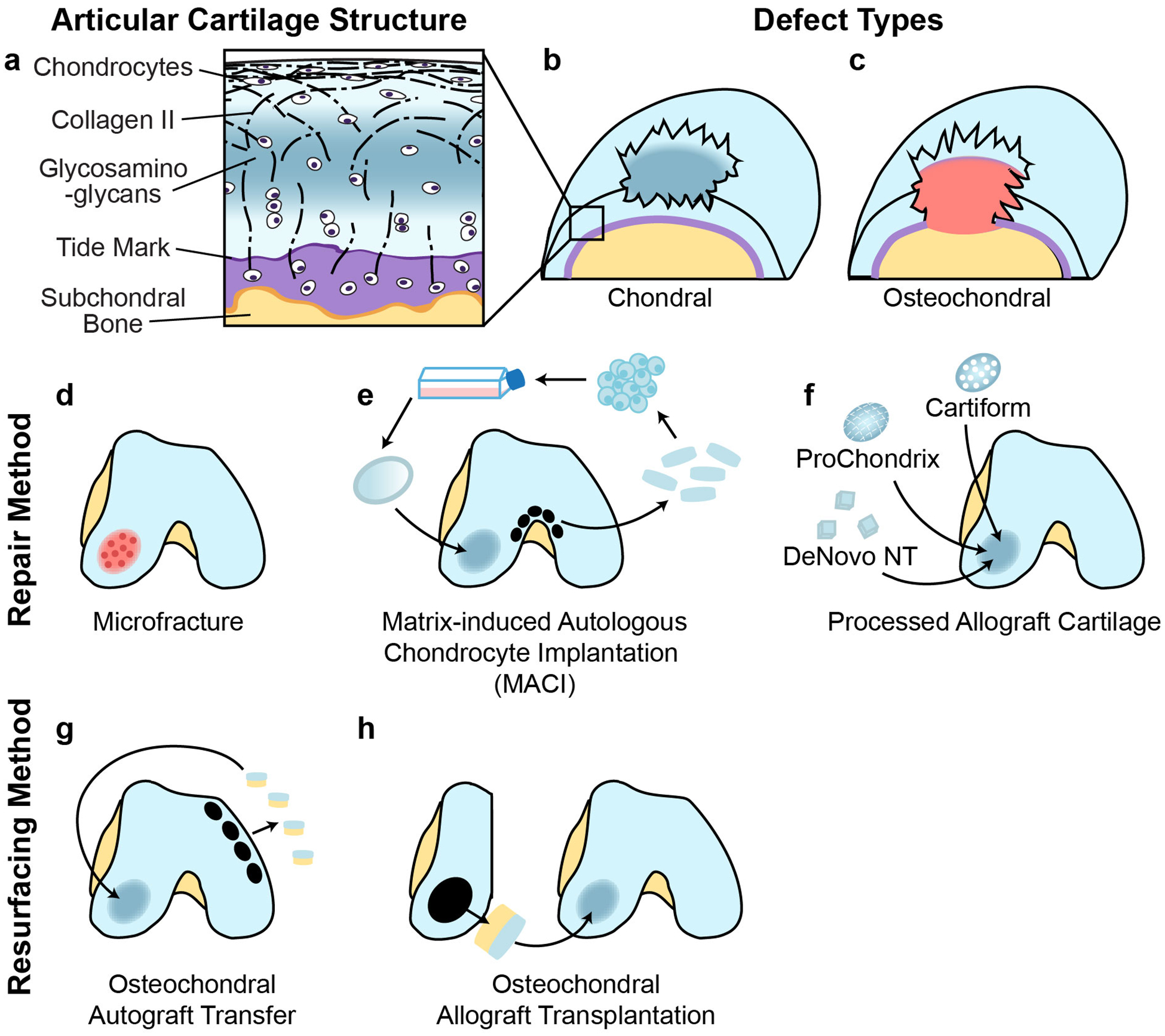
a| Articular cartilage consists of chondrocytes embedded in a defined structure of collagen fibres and glycosaminoglycans. Two main types of defects can occur; chondral defects, which only penetrate the cartilage and osteochondral defects, which also penetrate the subchondral bone. b| Currently used repair strategies for cartilage defects include microfracture, osteochondral autograft transfer, osteochondral allograft transplantation, implantation of processed allograft cartilage such as DeNovo NT, ProChondrix and Cartiform, and matrix-induced autologous chondrocyte implantation (MACI). The choice of treatment method depends on the size and type of the defect, the expertise and preferences of the surgeon and patient-specific factors such as age and activity level.
Clinicians encounter articular cartilage damage in more than half of knee arthroscopies performed as a result of injury or symptoms of cartilage damage16,17. Specifically, chondral (defects that do not penetrate into the subchondral bone) and osteochondral (defects that penetrate into the subchondral bone) lesions were found in 61% of patients surveyed (Fig 1a)12. Because cartilage defects are often asymptomatic18, careful assessment is required to determine whether the lesion is the source of pain in an individual. Current surgical strategies aim to repair small (<4cm2) cartilage defects to prevent further degeneration and progression toward OA (Fig. 1b). Cartilage repair strategies for the knee are well-established and produce improvements in clinical outcomes for patients19,20. However, repair of hip cartilage is less frequently performed than repair of knee cartilage. The use of bone marrow stimulation, grafting and cell-based techniques for articular cartilage repair are discussed in the following section.
Bone marrow stimulation and augmentation.
Bone marrow stimulation techniques for small (<4cm2), contained defects have evolved from open debridement of damaged cartilage and removal of subchondral bone to the Steadman microfracture technique21, in which the calcified cartilage is removed and an awl is used to create perforations in the subchondral plate. Bone marrow released into the defect forms a blood clot, which might ultimately lead to the formation of fibrocartilage. Unlike hyaline cartilage, fibrocartilage is rich in type I collagen and is of limited durability; individuals treated with microfracture showed initial clinical improvement after surgery, but had an accelerated decline in clinical outcome scores and a higher failure rate compared with those treated with osteochondral autograft treatment at long-term follow-up22,23. To overcome the shortcomings of microfracture, augmented bone marrow stimulation techniques were subsequently developed, including the concomitant injection of biologics (e.g., growth factors, such as bone morphogenetic protein 4 or 7), the use of acellular scaffolds (such as collagen membranes) or liquid hydrogels, and the use of micronized acellular cartilage extracellular matrix from allografts24. However, more high-quality studies are needed to demonstrate the superiority of augmented bone marrow stimulation techniques over other established procedures, such as microfracture or autologous chondrocyte implantation (ACI)25.
Autografts and allografts.
Osteochondral autograft transfer delivers viable, mature hyaline cartilage–bone units into chondral defects. These osteochondral grafts can bear load in the early post-operative period, enabling faster rehabilitation compared to other, currently available, cell-based cartilage repair strategies26. Osteochondral autograft transfer involves the harvesting of ‘plugs’ from regions of the distal femur that bear low loads (such as the intercondylar notch or medial or lateral trochlea) and, therefore, its use is reserved for small chondral defects (<2cm2) owing to limited graft availability27.
The avascular nature of cartilage renders it immune privileged28, thereby opening up the potential for allogenic approaches. Osteochondral allograft transplantation does not have the donor site limitations of osteochondral autograft transfer and can be used in revision surgeries of failed cartilage repair, making osteochondral allografting an appealing technique, although the availability of allograft tissue limits the use of this technique. Matching allografts to the shape and contours of the native knee architecture can also be difficult to achieve, potentially creating biomechanical loading imbalances and resulting in degenerative joint changes29,30. Techniques to improve the viability of chondrocytes in fresh osteochondral allografts and to accelerate the remodelling of graft tissue into host tissue are continually being investigated because both factors seem to be important for the longevity of the transplanted allograft31,32.
Both osteochondral autograft transfer and osteochondral allograft transplantation have produced high rates of long-term graft survival, as well as high degrees of reported patient satisfaction and return-to-play among athletes26,33–35. For example, a 2016 systematic review found that ~90% of patients who underwent osteochondral autograft transfer had good or excellent outcomes <10 years post-surgery19. Another study showed that the survival of fresh osteochondral allografts was 82% at 10 years post-transplantation and 66% at 20 years post-transplantation33. Cryopreserved osteochondral allografts (Cartiform), fresh osteochondral allografts (ProChondrix) and particulated juvenile allograft cartilage (DeNovo NT), processed by laser cutting or mincing, have also been used to treat articular cartilage defects36; however, short-term and long-term data need to be collected to determine the clinical success of these products.
Cell-based techniques.
Current cell-based cartilage repair techniques enable the implant to be contoured to the recipient defect, making these techniques attractive for treating large (>3–4cm2) chondral lesions in areas with variable topographies, such as the patellofemoral joint or acetabulum. Autologous chondrocyte implantation (ACI) requires two surgeries; chondrocytes are harvested from healthy articular cartilage in one operation and are then re-implanted into the chondral defect in a second surgery after expansion in culture. A newer iteration of this technique known as matrix-induced ACI (MACI) incorporates seeding of the chondrocytes onto a scaffold before implantation37. Patients treated with MACI have reported substantial long-term improvements in knee function and high rates of patient satisfaction38,39. In one study, at 5 years post-surgery, 93% of patients expressed satisfaction with their postoperative pain relief, 90% of patients had an improved ability to perform daily activities and 80% of patients were able to participate more in sports compared with before the operation38. However, procedures that require only one surgery are currently more appealing for clinicians than ACI or MACI.
Repairing meniscus defects
Two semicircular, wedge-shaped menisci are located between the distal femur and the tibial plateau and serve to distribute loads and protect articular cartilage. Each meniscus has two distinct regions (Fig 2a): 1) the outer, vascular, neural region (red-red zone) which contains elongated fibroblast-like cells and predominantly type I collagen and 2) the inner, avascular, aneural zone (white-white zone) which contains rounded chondrocyte-like cells (fibrochondrocytes) and predominantly type II collagen. These two zones are separated by the ‘red-white’ zone which has characteristics of both the ‘red-red’ zone and ‘white-white’ zone. The meniscus functions by distributing load through its circumferentially aligned collagen fibers (Fig 2a). Meniscus tears disrupt this function; however, only a small portion of tears are considered repairable on the basis of tissue vascularity, tear pattern, anatomic location and tear acuity (Fig 2b). For example, vertical longitudinal tears residing within the ‘red-red’ or ‘red-white’ zones of the meniscus are often amenable to repair40. Horizontal and radial tears are thought to rarely heal owing to incursion into the avascular ‘white-white’ zone. Furthermore, radial tears disrupt the circumferential collagen fibers that are critical for maintaining hoop stresses, whereas circumferential vertical or horizontal tears can leave the meniscus with the potential for residual functionality because these tears follow the circumferential collagen fibers. The length, depth and size of tear, as well as joint stability and other patient-related factors such as age and symptoms also affect healing41,42. Despite our understanding of the crucial function of the meniscus in knee biomechanics, partial meniscectomy to remove unstable, damaged portions of the tear remains the gold standard for surgical treatment of meniscus tears and accounts for half of the knee arthroscopic procedures performed in the USA43. However, both partial and total meniscectomy are linked to the development of knee OA44, a fact that provides motivation for the development of novel interventions such as cell-based regenerative therapies.
Figure 2. Meniscus structure and treatment methods.
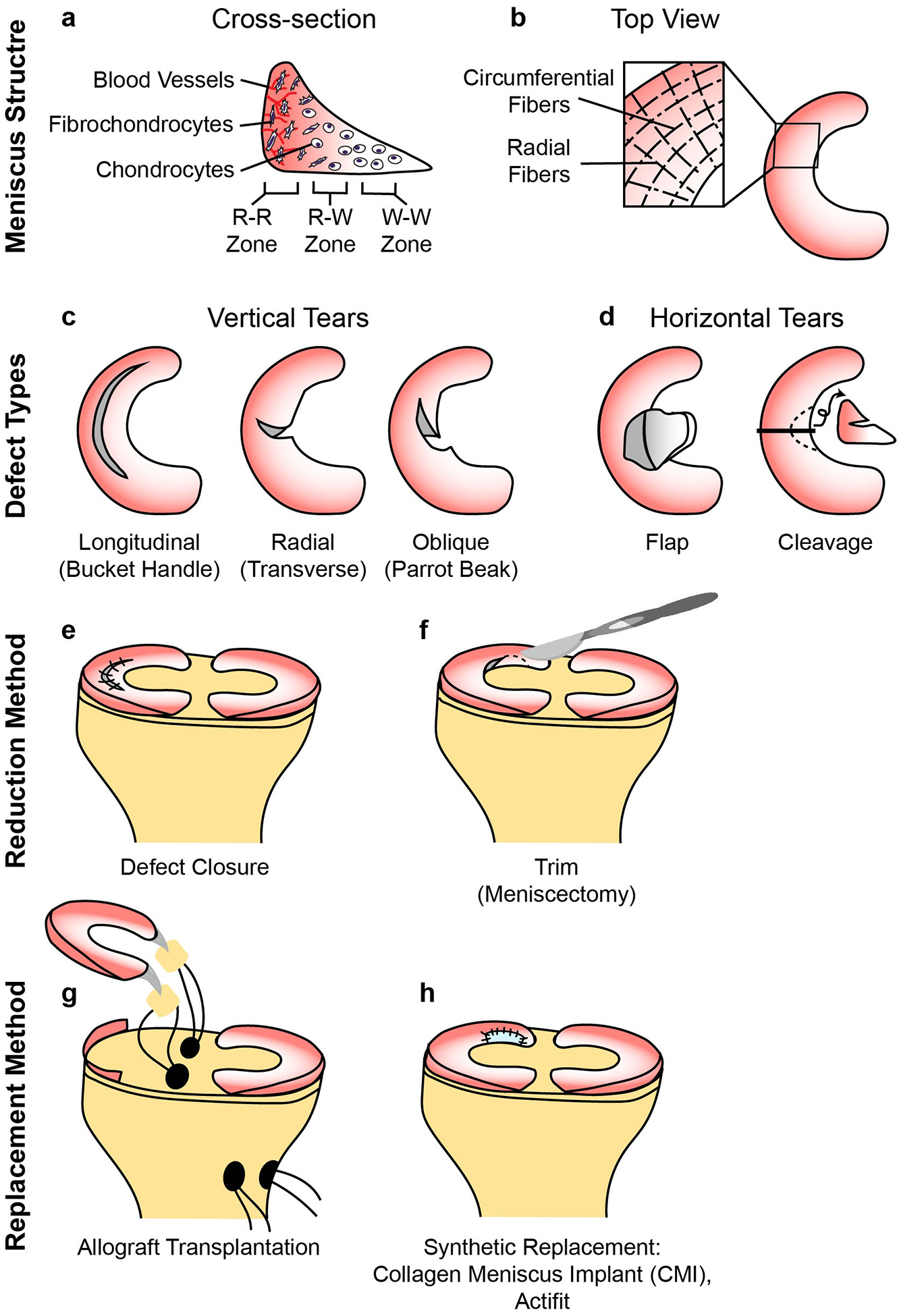
a| The meniscus consists of three main zones; red-red (R-R), red-white (R-W) and white-white (W-W). The R-R zone is fully vascularized and the W-W zone is avascular. b| A variety of different types of defects can occur in the meniscus, some of which are easier to repair than others owing to their intrusion into vascular or avascular zones. c| Reduction strategies in current use include defect closure with sutures or anchors and the trimming of torn pieces (partial or total meniscectomy). d| Replacement strategies in current use include allograft transplantation and the use of synthetic implants. As with articular cartilage, the size and type of defect, the expertise and preferences of the surgeon and patient-specific factors such as age and activity level affect the choice of treatment method.
Reduction of meniscal tears.
Lesions in the meniscus that are mechanically unstable, complex or of a degenerative nature are conventionally treated with partial meniscectomy; however, attempts to reduce meniscal tears instead of perform partial meniscectomy have become more common within last 15 years45 (Fig 2c). Meniscus defect reduction (often described by clinicians as meniscus repair) is usually accomplished by closure of the tear with sutures and/or anchors. For example, suturing of defects in the red-red and red-white zones led to satisfactory clinical healing in 76% of patients with meniscal tears46. Tear reduction also resulted in meniscus preservation without degeneration in young patients aged between 16 and 52 years47,48. Meniscal tear reductions that take place with concurrent ACL reconstructions have superior healing rates than meniscal tear reductions alone49, potentially owing to the intra-articular release of cells and growth factors from the bone marrow that occurs when drilling a bone tunnel during ACL reconstruction50. arameters affecting meniscus repair are probably multifactorial, but biological augmentation techniques, such as mechanical stimulation of the adjacent synovium or meniscus by rasping or radial trephination51,52, the addition of an exogenous fibrin clot53 or the introduction of bone marrow stem cells by marrow venting54, are thought to promote healing.
Allografts.
Meniscus allograft transplantation is the only option for total meniscus replacement, and is widely performed following total or near total meniscectomy (Fig 2d). Allograft transplantation is indicated in patients who have a stable, correctly aligned joint and, at most, early knee OA55. Meniscus allografts can be inserted with several forms of attached bone, such as bone plugs, common bone bridge or a hemi-plateau, or without attached bone56. In particular, meniscus fixation using bone plug demonstrates superior load transmission compared with not using bone plugs.56 Appropriate allograft sizing to the recipient knee56 is also an important factor for tissue healing57 and for the preservation of knee biomechanics58. Allograft recipients have good rates of clinical improvement; in a long-term follow up study (mean 152 months) of 30 patients who received meniscal allografts, all patients showed significant improvement in function, as measured by Lysholm and Short Form-36 (SF-36) scores, as well as the Knee Injury and Osteoarthritis Outcome Score (KOOS), and 90% were satisfied with the outcome of the surgery59. However, meniscus replacement does not prevent joint space narrowing60.
Synthetic implants.
Partial meniscus replacements, such as collagen meniscus implants (CMI, available in the USA) and polyurethane polymeric implants (Actifit, available in Europe), can be used for patients with segmental meniscus defects, an intact peripheral rim and limited articular cartilage damage61. CMI provided substantial pain relief and functional improvement and had a low rate of implant failure at follow-up (mean 9.6 years) in patients receiving implants following partial meniscectomy62. Similarly, polyurethane polymeric implants improved clinical outcomes in patients following partial meniscectomy up to 4 years after implantation63. For replacement of the entire meniscus, a polyethylene reinforced polycarbonate urethane prosthetic (NuSurface) is currently in FDA clinical trials64. Although synthetic meniscus implants can improve clinical outcomes, their use is limited by several shortcomings and technical difficulties: synthetic implants do not result in meniscus regeneration; the ability of synthetic implants to stop the progression of OA is unproven; synthetic implants are difficult to place properly within the defect using an arthroscopic approach; and they are challenging to handle and suture65. Therefore, a great need exists for cell-based approaches that can regenerate damaged meniscus.
Age-related differences in outcomes
Parameters that affect the outcomes of articular cartilage and meniscus repair are multifactorial, but generally, increased patient age has a negative correlation with good outcomes, in particular after bone marrow stimulation techniques. Treatments that are acceptable for use in pediatric and adolescent patients might not be viable in adults, who are expected to have degenerative, rather than acute traumatic lesions. Two main principles exist for treating pediatric articular cartilage or meniscus defects: techniques must be effective to help prevent the risk of developing OA at a young age; and joint anatomy and functionality must be restored to ensure symptomatic relief and resumption of pre-injury levels of physical activity66. Given the increase in pediatric joint injuries67,68, potentially as a result of increased participation in sports, the development of therapies that will withstand the test of time is greatly needed.
Treatment of articular cartilage defects in young patients.
Although many of the same techniques are used to treat cartilage lesions in children and adolescents as in adults, outcomes can differ. For microfracture, patients older than 40 years had worse outcomes than younger patients (<30–40 years of age) in many studies69–72, potentially because older patients have fewer bone marrow progenitor cells and diminished regenerative capacity than younger patients. A similar trend occurs with osteochondral autograft transfer, for which better outcomes are reported in young patients (<30 years of age)73. By contrast, 88% of pediatric and adolescent patients had successful outcomes following osteochondral allograft transplantation after a median of 2.7 years74, similar to success rates reported for adults75. ACI in young patients (≤18 years of age) produced an improvement in postoperative outcomes for 84–96% of patients at 2–4 years follow-up76,77, which was higher than the rate of improvement in adults for the same follow-up period (78–83%)78,79. Overall, for younger patients (≤40 years of age) (many of whom are athletes), osteochondral autograft transfer22,80 and ACI/MACI81 might result in better long-term outcomes and higher rates of return-to-play than microfracture.
Treatment of meniscus defects in young patients.
As with articular cartilage, outcomes associated with treating meniscus pathologies differ as a result of multiple factors, including age and tear type. In general, meniscus allograft transplantation is indicated for young patients (<50 years of age) with meniscal deficiency and is contraindicated in patients with evidence of advanced OA82. In patients under the age of 16, an improved Lysholm score and a revision rate of 22% have been reported after a mean follow-up of 7.2 years following meniscus allograft transplantation83. For meniscal tear reduction, most studies in a meta-analysis reported little difference between failure rates in patients under the age of 40 and those over the age of 4084,85. Another meta-analysis on meniscus repair that included 13 studies in adults, found a healing rate of 62–79% and a pooled re-tear rate of 23% after >5 years86. Comparisons between surgical outcomes in pediatric and adolescent versus adult patients need to take into consideration the types of tears that are being reduced. In pediatric and adolescent patients, meniscus defect reduction can be attempted for most meniscal tears regardless of zone, size and patient-specific factors, as the priority is to preserve the knee. By contrast, in adults, meniscus defect reduction is usually only performed for tears that have a high potential to heal, such as peripheral tears. Thus, despite the beneficial healing environment in pediatric and adolescent patients that results from a high degree of vascularization and increased cellular metabolism87,88, healing rates in pediatric and adolescent patients compared to adult patients can seem similar because of the types of tears that are treated.
Tissue engineering strategies
Current surgical approaches do not provide long-term solutions for articular cartilage and meniscus regeneration, but tissue engineering techniques could provide alternative treatment strategies. Scaffolds, cells and biochemical and biomechanical stimuli, the main tools used to create engineered tissues (Fig. 3), are discussed in this section, as well as advances in cartilage engineering and the results of preclinical and clinical studies using engineered articular cartilage and meniscus products.
Figure 3. Advances in tissue engineering strategies for articular cartilage and meniscus.
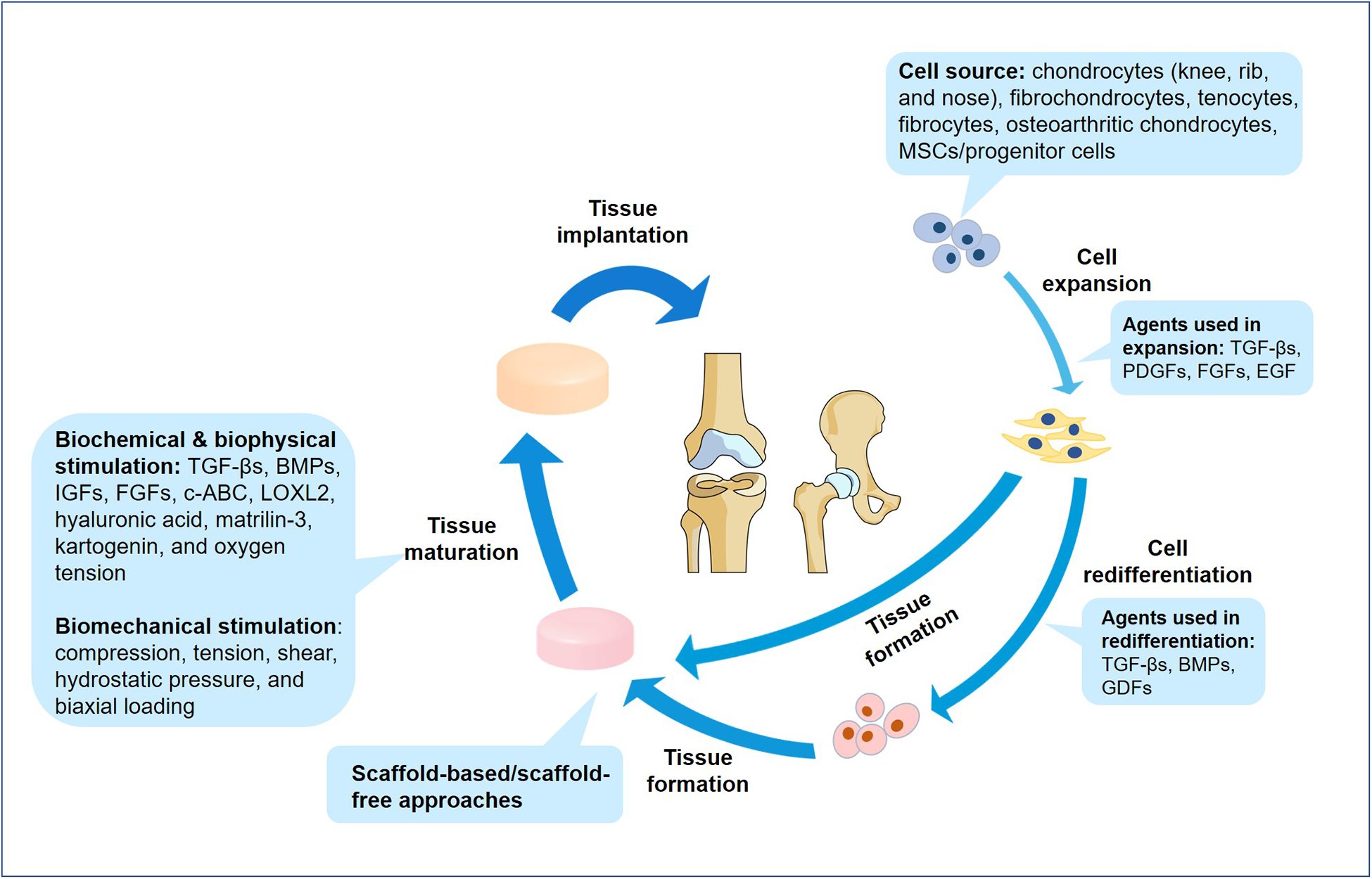
Engineered implants go through several stages of development that can be modified or enhanced by the addition of appropriate stimuli. The source of cells is important as many cells dedifferentiate in culture; alternative cell sources currently being trialed include non-articular chondrocytes, tenocytes, fibrocytes, osteoarthritic chondrocytes and stem cells or progenitor cells. Growth factors such as TGFβs, PDGFs, FGFs, EGF, BMPs and GDFs are used to effectively expand and help to redifferentiate cells prior to neotissue formation. Scaffold-based and scaffold-free methods can be used to engineer articular cartilage and menisci, and biochemical and biophysical factors such as TGFβs, BMPs, IGFs, FGFs, chondroitinase ABC (c-ABC), lysyl-oxidase-like 2 (LOXL2), hyaluronic acid, matrilin 3, kartogenin and variations in oxygen tension are used to promote the maturation of engineered tissues. Similarly, biomechanical stimulation such as compression, tension, shear, hydrostatic pressure and biaxial loading can be used to improve the functional properties of the neotissue.
Scaffold and scaffold-free approaches
A variety of synthetic or natural materials, including polylactides, polyglycolides and silk, have been investigated for use as scaffolds for engineered articular cartilage89 and meniscus88. Decellularized cartilage-derived matrix has also been investigated for use as a scaffold in cartilage regeneration90,91. For example, decellularized cartilage-derived matrix scaffolds inhibited the hypertrophic differentiation of embedded mesenchymal stem cells (MSCs) and promoted the synthesis of cartilage matrix by these cells90. Decellularized extracellular matrix scaffolds derived from inner and outer regions of the meniscus supported differentiation of MSCs toward fibrochondrocyte and elongated fibroblastic phenotypes, respectively91. Various other types of scaffolds, including hydrogels and porous polymeric structures, are also under investigation for use in articular cartilage and meniscus tissue-engineering. For example, injectable hydrogels, which can form into irregular shapes to better fill defects, enable the use of minimally invasive implantation methods92. Within 20 years, both natural materials (for example alginate and hyaluronan) and synthetic materials (for example polycaprolactone and polylactic acid) have been used in 3D printers to create anatomically shaped scaffolds for articular cartilage and menisci93,94. The advantages of using scaffolds for cartilage engineering include the ability to incorporate growth factors into the scaffold and the initial mechanical stability that they provide95.
Despite the advantages scaffolds provide, scaffold use can also result in degradation-associated toxicity, stress shielding, altered cell phenotypes and hindrances to remodelling95, facts that have provided motivation for investigations into scaffold-free techniques to engineer cartilage96 and menisci97. In particular, the scaffold-free self-assembling process, which facilitates cell-to-cell interactions by minimizing free energy, recapitulates the conditions of cartilage development, which result in changes of the ratios of chondroitin-6-sulfate to chondroitin-4-sulfate and type VI collagen to type II collagen within the engineered neocartilage as it develops98. Through the use of biochemical and biomechanical stimuli, cartilage engineered using a scaffold-free approach has attained functional properties on par with native tissue99. For example, engineered articular cartilage has achieved compressive and tensile moduli of ~0.32MPa100 and ~8MPa99, respectively, which are in the range of native compressive (0.1–2MPa) and tensile (5–25MPa) values101. Similarly, scaffold-free engineered menisci have compressive and tensile moduli of ~0.12MPa102 and ~5MPa103, respectively, compared with native tissue compressive and tensile moduli of 0.1–0.15MPa and 10–30MPa, respectively88. Thus, scaffold-free methods have the potential to circumvent challenges associated with scaffolds and to produce biomechanically functional implants.
Advances in scaffold-based and scaffold-free approaches have also focused on the recapitulation of native tissue architecture104–107. For example, stiffness gradient hydrogels (0.005–0.06MPa) derived from poly(ethylene glycol) and chondroitin sulfate yielded constructs with stiffness-dependent glycosaminoglycan gradients that mimicked the glycosaminoglycan gradient found in articular cartilage between the superficial and deep zones104. In another study, bi-layered poly-(ε-caprolactone) scaffolds with porous layers and aligned fibrous layers supported the development of zonal arrangement of engineered cartilage105. Collagen density and the alignment of porous collagen scaffolds can also be tailored via biaxial compression106, which might be useful for engineering anisotropy in the meniscus. Scaffold-free approaches have also been used to generate zonal tissue and anisotropy; for example, anisotropic menisci with zonal variations have been produced using the self-assembling process107. These studies104–107 suggest that recapitulating zonal and anisotropic properties of cartilage and menisci might be necessary to impart native functional properties to a tissue-engineered product.
Engineering articular cartilage
Cell sources.
Although chondrocytes are the obvious choice for use when engineering articular cartilage, chondrocyte scarcity necessitates cell expansion in vitro, which results in rapid dedifferentiation108. Although, to date, no evidence exists to prove that dedifferentiated cells can be redifferentiated in vivo, the results of some studies have suggested that in vitro redifferentiation can be accomplished109,110. For example, culturing either in vitro expanded chondrocytes or MSCs in a 3D culture condition supplemented with transforming growth factor-β1 (TGFβ1), growth and differentiation factor 5 (GDF5) and bone morphogenetic protein 2 (BMP2), collectively termed aggregate redifferentiation, resulted in increased expression of the chondrogenic genes SOX9, ACAN and COL2A1 compared with untreated cells111. Alternative cell sources include chondrocytes from non-articular cartilages; for example, costal (rib) chondrocyte-derived neocartilage has compressive properties on par with those of native articular cartilage109. HOX-negative nasal chondrocytes are thought to possess greater self-renewal capacity than articular chondrocytes112 and a nasal-chondrocyte-based articular cartilage product (N-TEC) is currently in clinical trials for articular cartilage repair in Europe113. In addition, constructs engineered using osteoarthritic chondrocytes have yielded neocartilage containing type II collagen and lubricin, but not type I or type X collagen, which are indicative of chondrocyte dedifferentiation and hypertrophy114. Thus, non-articular and osteoarthritic cartilage might yield viable cells for use in articular cartilage repair.
Adult MSCs derived from adipose tissue, bone marrow, synovium or skin have been extensively investigated for use in cartilage tissue-engineering. Bone marrow-derived MSCs and umbilical cord blood-derived MSCs are already used to create engineered cartilage repair products, and dermis-derived MSCs and precursor cells have chondrogenic differentiation potential115,116. Other types of MSCs and progenitor cells are emerging as candidates for use in tissue engineering. For example, peripheral blood-derived MSCs and endothelial progenitor cells have both been used to fill osteochondral defects in rabbits117,118. In a non-controlled, clinical pilot study with 15 participants, adult CD146+ cartilage progenitor cells formed hyaline-like cartilage when implanted into knee articular cartilage defects119. After 12 months, a 52% improvement was achieved in the International Knee Documentation Committee (IKDC) score and a 71% improvement was achieved in the Lysholm score compared with pre-operative scores119. Notably, hypertrophy frequently occurs in MSCs during in vitro chondrogenic differentiation120, indicating the possibility that MSC-derived neocartilage might progress toward endochondral ossification121, resulting in neotissue that is not suitable for cartilage repair and regeneration. Thus, despite promising early data, the long-term (>1 year) durability of MSC-derived tissues remains to be investigated.
Biochemical stimuli.
Growth factors have long been recognized as important factors in neocartilage formation122, but other molecules are emerging as potential modulators of engineered cartilage. In the past few years, hyaluronic acid has been shown to stimulate chondrogenesis and reduce hypertrophy in bone marrow-derived MSCs123 and in a co-culture of adipose-derived MSCs and chondrocytes124. Similar effects have also been shown for the addition of matrilin 3 to cultures of bone marrow-derived MSCs125. The addition of kartogenin induced chondrogenic differentiation in MSCs and reduced type II collagen breakdown by 1.8-fold in a mouse model of OA126; however, the therapeutic dose and long-term in vivo efficacy of kartogenin have to be yet to be determined, limiting its use127. Biophysical stimuli such as glycosaminoglycan-depleting enzymes (such as chondroitinase ABC) or crosslinking agents (such as lysyl oxidase-like 2 (LOXL2)) have also been used to increase collagen content and to form collagen crosslinks, leading to improved tensile properties in neocartilage128–130. In fact, a regimen of TGFβ1, chondroitinase ABC and LOXL2 applied after aggregate redifferentiation generated neocartilage with tensile modulus and ultimate tensile strength values approximately twice those of untreated neocartilage99. Oxygen tension also has an important role in chondrogenesis and in improving neotissue functional properties. In one study, hypoxia upregulated LOX expression in chondrocytes by 18-fold, leading to an increase in tensile stiffness of neocartilage by ~80% compared with neocartilage formed under normoxic conditions131. Overall, these studies suggest that novel biochemical and biophysical stimuli should be used for effective neocartilage formation.
Biomechanical stimuli.
Biomechanical stimuli such as compression, shear and hydrostatic pressure are important for cartilage homeostasis and are already used to improve the properties of engineered cartilage132. One advance in the use of biomechanical stimuli in tissue engineering has been the application of these stimuli to non-articular chondrocytes. Passive axial compression applied to costal chondrocytes increased the instantaneous modulus of the engineered constructs by <92% compared with unstimulated neocartilage constructs133. Tension has also been trialed as an additional stimulus to improve the biomechanical properties of neocartilage. Tension stimulation of scaffold-free neocartilage treated with TGFβ1, chondroitinase ABC and LOXL2 resulted in an almost 6-fold increase in tensile modulus and strength99. After in vivo implantation, these constructs contained 90% of the collagen content and had <94% of the tensile properties of native tissue99. A combination of compression and shear has also been tested, which resulted in a substantial increase in type II collagen production by chondrocytes within engineered neocartilage134. The results of these studies suggest that biomechanical stimulation has a pivotal role in engineering functional cartilage tissue in vitro. Understanding biomechanical stresses in the native environment of the joint, as well as their effects on both the generation of robust neotissue in vitro and on the generated tissue in vivo, is important for achieving clinical translation of engineered cartilage.
Engineering menisci
Cell sources.
Although meniscal fibrochondrocytes might seem to be an obvious choice for engineering the meniscus, co-culturing these cells with others might be required to achieve the best results. Similar to chondrocytes, meniscal fibrochondrocytes dedifferentiate when expanded135, a fact that has led to the investigation of MSCs from the bone marrow136, synovium137 and adipose tissue as alternative cell sources138. In a 2017 study, COL1, COL2, ACAN and SOX9 were induced in tonsil-derived MSCs, providing evidence for the feasibility of using these cells to repair meniscus defects in rabbits139. Co-culture of synovium-derived stem cells and meniscus cells at a ratio of 1:3 increased glycosaminoglycan production by ~82% compared with stem cell monoculture and by ~33% compared with meniscus cell monoculture140. These findings echo those from co-culture studies of chondrocytes with differentiated cells141 (such as tenocytes, ligament fibrocytes or meniscus fibrochondrocytes) for forming neomenisci. For example, neomenisci formed using 50% articular chondrocytes and 50% meniscal fibrochondrocytes contain 700% more glycosaminoglycan and 90% more collagen than neomenisci formed using fibrochondrocytes alone97. The identification of new cell sources, as well as the optimization of co-culture systems, will both be important for overcoming the hurdles of cell culture for meniscus tissue-engineering.
Biochemical stimuli.
Growth factors such as TGFβ family members, fibroblast growth factors (FGFs), platelet-derived growth factors (PDGFs) and epidermal growth factor (EGF) have shown efficacy in improving extracellular matrix production in engineered meniscus88. The addition of TGFβ1 and FGF2 stimulated collagen synthesis in meniscus constructs by 144% and 60%, respectively, compared with untreated constructs, although only TGFβ1 was effective in stimulating glycosaminoglycan production142. Growth factors have also been used to induce lubrication in engineered menisci; the use of IGFI localized lubricin to the neotissue surface and resulted in a coefficient of friction of ~0.2143. Zonal development can also be engineered using growth factors. Modulating the release of TGFβ3 and connective tissue growth factor using 3D printed scaffolds resulted in MSC-derived menisci with zone-specific COL1 and COL2 expression, as well as zone-specific type I and type II collagen production144. Other biochemical stimuli can also aid the production of engineered menisci with improved functional properties. Treatment of neofibrocartilage implants with a combination of TGFβ1, LOXL2 and chondroitinase ABC increased collagen crosslink formation by 3.8-fold compared with untreated implants103. Upon implantation, the tensile strength of the interface of native meniscus and treated neofibrocartilage increased by 745% compared with the in vitro properties of untreated implants103. By contrast, changes in oxygen tension have yielded mixed results for engineering menisci. A 2017 study showed increased ACAN and COL2 expression, as well as proteoglycan and type II collagen production by expanded human meniscus fibrochondrocytes in hypoxic conditions145, whereas a 2013 study showed that normoxic conditions resulted in increased expression of COL2 and ACAN, as well as the production of type II collagen and aggrecan by expanded human fibrochondrocytes compared with hypoxic conditions146. Therefore, modulation of oxygen tension as a biochemical stimulus might hold promise for meniscus engineering130, but further investigations are needed to identify optimal culture conditions.
Biomechanical stimuli.
The meniscus functions under compression, which results in the development of tensile hoop stress, therefore both of these mechanical forces are important for meniscus engineering. For example, using a compressive regimen of 10% strain at 1Hz (which also results in tension), the collagen content, circumferential tensile modulus and radial tensile modulus of neomeniscus constructs can be increased compared with unstimulated constucts147. Over the past few years, studies into the development of biomechanical stimuli for meniscus engineering have focused on replicating native zonal arrangement and matrix-level organization. For example, application of sinusoidal hydrostatic pressure between 0.55–5.03MPa at 1Hz for 4 hours per day to aggregates of human fibrochondrocytes resulted in a substantial difference in type II collagen production between inner and outer zone meniscus fibrochondrocytes148, providing support for using this stimulus to help recapitulate zonal architecture. A bioreactor applying 5–10% compressive strain was used to produce neomenisci with a fibrous collagen matrix in the outer zone that was similar in alignment to native tissue149. Investigations into how biomechanical stimuli can induce anisotropy in other engineered fibrocartilages have also been informative for meniscus engineering. For example, the application of passive axial compression during culture promoted anisotropic collagen organization similar to what is seen in native tissue in tissue-engineered temporomandibular joint discs150. In addition to recapitulating native tissue biochemical and biomechanical properties, it is important to mimic other native features such as anisotropy and zonal organization because these structural features are necessary for meniscus function.
Clinical studies
The technologies used to produce cell-based repair products for articular cartilage repair have been reviewed elsewhere151. This section focuses on the clinical applications of articular cartilage and meniscus repair products in development (Table 1) and promising results from clinical trials of these products (Table 2). Acellular, scaffold-based products are not discussed. Additional clinical studies that have been performed under Institutional Review Board approval and under the principles of the Declaration of Helsinki, but not as part of registered clinical trials, are listed in Supplemental Table S1.
Table 1.
Cell-based tissue-engineered products for articular cartilage and meniscus repair.
| Product name (company) | Cell or tissue source | Seeding density | Biomaterial or scaffold | Stimuli | Time between surgeries (time in culture) | No. of patient surgeries | Ref(s) |
|---|---|---|---|---|---|---|---|
| Articular cartilage | |||||||
| BioCart II (ProChon Biotech) | Autologous chondrocytes (passage number unknown) | 0.4×106 cells plus 0.1×106 cells/cm2 of scaffold | Freeze-dried fibrin-hyaluronan | Autologous serum and FGF2 | 3–4 weeks (3–4 days in 3D culture) | 2 | 175,176 |
| Bioseed-C (BioTissue SA) | Expanded autologous chondrocytes (passage number unknown) | 20×106 cells per scaffold | Fibrin, polyglycolic acid, polylactic acid and polydioxanone | Autologous serum | 4–5 weeks | 2 | 177–179 |
| BST-CarGel (Piramal Healthcare Canada) | Autologous whole peripheral blood | 3:1 ratio of autologous whole peripheral blood to biomaterial | Dissolved chitosan in glycerophosphate buffer | Unknown | n/a | 1 | 180 |
| CaReS (Arthro Kinetics Biotechnology) | Primary autologous chondrocytes | Unknown | Type I collagen hydrogel | Autologous serum | 2 weeks (10–13 days in 3D culture) | 2 | 181 |
| Cartilage autograft implantation system (CAIS) (DePuy Mitek) | Autologous cartilage fragments | 1–2 mm minced cartilage dispersed onto scaffold | Absorbable co-polymer of 35% polycaprolactone and 65% polyglycolic acid with a polydioxanone mesh | Unknown | n/a | 1 | 182 |
| Cartipatch (TBF Genie Tissulaire) | Expanded autologous chondrocytes (passage 3) | 10×106 cells/ml of hydrogel | Agarose–alginate | Autologous serum | 6–7 weeks | 2 | 183,184 |
| Cartistem (Medipost) | Expanded, allogeneic, umbilical cord blood-derived MSCs (passage number unknown) | 500 μl of hydrogel per cm2 of defect area, 5×106 cells/ml of hydrogel | Hyaluronic acid hydrogel | Fetal bovine serum | n/a | 1 | 153 |
| co.don chondrosphere (co.don AG) | Expanded, autologous chondrocytes (passage number unknown) | 10–70 spheroids/cm2 of defect area or ~3×106 cells/cm2 of defect area | Scaffold-free | Autologous serum | ~5–10 weeks | 2 | 185,186 |
| Hyalofast (Anika Therapeutics) | Autologous bone marrow aspirate concentrate (BMAC) | 2 mL BMAC per scaffold | Benzyl ester of hyaluronic acid (HYAFF-11) | Unknown | n/a | 1 | 187 |
| Hyalograft C (Anika Therapeutics) | Expanded autologous chondrocytes (passage 1 or passage 2) | 1.5–4×106 cells per scaffold | Benzyl ester of hyaluronic acid (HYAFF-11) | Autologous serum and TGFβ1 | 4 weeks (2 weeks in 3D culture) | 2 | 188–191 |
| INSTRUCT (CellCoTec B.V.) | Autologous, primary articular chondrocytes and bone marrow-derived cells | Unknown | Poly ((ethylene oxide) terephthalate-co-poly(butylene) terephthalate) | Unknown | n/a | 1 | 192 |
| Novocart 3D (Aesculap Biologics) | Expanded autologous chondrocytes (passage 1) | 0.5–3×106 cells/cm2 of scaffold | Type I collagen and chondroitin sulfate | Autologous serum | 3 weeks (2 days in 3D culture) | 2 | 193 |
| Novocart Inject (TETEC AG) | Expanded autologous chondrocytes (passage number unknown) | Unknown | In situ polymerized injectable albumin–hyaluronic acid hydrogel | Autologous serum, BMP2 and insulin | Unknown (3–4 weeks in 2D culture) | 2 | 157 |
| Neocart (Histogenics) | Expanded autologous chondrocytes (passage number unknown) | 12×106 cells/ml collagen solution | Bovine type I collagen | Hypoxia and hydrostatic pressure | 6–12 weeks | 2 | 194–196 |
| N-TEC (BIO-CHIP) | Expanded autologous nasal chondrocytes (passage number unknown) | 50×106 cells per membrane | Type I and type III collagen membrane (Chondro-Gide) | Autologous serum, FGF2, and TGFβ1 (expansion) Autologous serum, insulin and ascorbic acid 2-phosphate (3D culture) |
≥7 weeks (2 weeks in 2D culture and 2 weeks in 3D culture) | 2 | 197 |
| RevaFlex (ISTO Technologies) | Expanded allogeneic juvenile chondrocytes (passage number unknown) | Unknown | Scaffold-free | Unknown | n/a | 1 | 198 |
| Meniscus | |||||||
| Chondrogen (Mesoblast) | Expanded allogeneic adult bone marrow-derived MSCs (passage 2) | 25×106 or 75×106 cells/ml of sodium hyaluronate | Sodium hyaluronate | Fetal bovine serum (expansion) | Not applicable | 1 | 159 |
| Cell Bandage (Azellon) | Expanded autologous bone marrow-derived MSCs (passage 1) | 1×106 cells/cm2 of scaffold | Collagen sponge from bovine corium | Fetal bovine serum and FGF (expansion) | >2 weeks (6 hours in 3D culture) | 2 | 158 |
Acellular, scaffold-based products are not included. The term ‘chondrocytes’ refers to articular chondrocytes unless otherwise specified. The sponsors and products listed here might since have been acquired by other companies.
Table 2.
Clinical trials of cell-based tissue-engineered products for cartilage and meniscus repair.
| Product (company) | Clinical status | Study location | No. patients | Clinical indication | Comparator | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Articular cartilage | |||||||
| BioCart II (ProChon Biotech) | Phase II (status unknown) | USA and Israel | 40 | Single contained cartilage defect on the femoral condyle of the knee (1.5–7.5 cm2, depth up to 6 mm) | Microfracture | Results not published | 199 |
| Bioseed-C (BioTissue SA) | Phase III (ongoing) | Germany | 80 | Focal contained, full-thickness cartilage defect on the lateral and medial condyles of the knee (Outerbridge grade III-IV) | Chondrotissue (BioTissue SA) | Results not published | 200 |
| Non-interventional study (completed 2016) | Germany | 76 (target) | Focal cartilage defects on the femoral condyles, trochlea and patella of the knee (>2×2 cm and Outerbridge grade III-IV) that have been previously treated with BioSeed-C | None | Results not published | 201 | |
| BST-CarGel (Piramal Healthcare Canada) | Phase IV (terminated) | Canada and Europe | 5 | Single, focal, full-thickness cartilage defect on the femoral condyle of the knee (1.5–3 cm2 and ICRS grade III-IV) | Microfracture | Results not published | 202 |
| Phase III (status unknown) | Unknown | 50 (estimated) | Focal chondral defects of the hip (>2 cm2) | Microfracture | Results not published | 203 | |
| RCT (completed 2011) | Canada, South Korea and Spain | 80 | Focal cartilage defect on the medial femoral condyle of the knee (grade III-IV, unknown scoring system) | Microfracture | Improved lesion filling and superior quality of repair tissue than microfracture alone at 12 months Equivalent WOMAC scores and comparable safety outcomes between groups at 12 months |
180 | |
| Observational study (completed 2014) | Canada and Spain | 67 | Focal cartilage defects on the femoral condyle of the knee (ICRS grade III-IV or Outerbridge grade III-IV) | Microfracture | Improved lesion filling and superior quality of repair tissue than microfracture alone at 5 years No difference in WOMAC scores and comparable safety outcomes between groups at 5 years |
204 | |
| Cartilage autograft implantation system (CAIS) (DePuy Mitek) | Phase III (status unknown) | Singapore | 36 (estimated) | Full-thickness cartilage defect on the femoral condyle or trochlea of the knee (2–10 cm2) | Microfracture | Results not published | 205 |
| Clinical trial (terminated) | USA and Canada | 75 | One or two focal chondral defects (1–10 cm2, depth up to 6 mm) or a non- osteochondritis dissecans lesion between grades I and III or an osteochondritis dissecans lesion between grades I and IV | Microfracture | Results not published | 206 | |
| Cartipatch (TBF Genie Tissulaire) | Phase III (terminated) | Belgium | 40 | Isolated femoral osteochondral defect (2.5–7.0 cm2, maximum depth of 10 mm, ICRS grade III-IV) | Microfracture | Results not published | 207 |
| Phase III (completed 2013) | Belgium | 64 | Single femoral osteochondral defect (2.5–7.0 cm2, maximum depth of 10 mm, ICRS grade III-IV) | Microfracture | Results not published | 208 | |
| Phase III (completed 2013) | France | 55 | Isolated femoral osteochondral defect (2.5–7.5 cm2, ICRS grade III-IV) | Mosaicplasty | Decreased IKDC score compared with mosaicplasty at 24 months Decreased O’Driscoll score compared with mosaicplasty at 24 months |
184 | |
| Phase II (completed 2006) | France | 17 | Isolated chondral or osteochondral defect on the femoral condyles of the knee (1–5 cm2, ICRS grade III-IV) | None | Increased IKDC score at 24 months compared with baseline 81% defect fill observed by MRI at 24 months | 183 | |
| Cartistem (Medipost) | Phase I/II (completed 2017) | USA | 12 | Single, focal, full-thickness cartilage defect of the knee (≥2 cm2, ICRS grade III-IV) | None | Results not published | 154 |
| Phase III (completed 2015) | South Korea | 103 | Cartilage defect of the knee (2–9 cm2, ICRS grade IV) | Microfracture | Results not published | 209 | |
| Phase III (completed 2011) | South Korea | 104 | Cartilage defect of the knee (2–9 cm2, ICRS grade IV) | Microfracture | Results not published | 210 | |
| Phase I/II (completed, date unknown) | South Korea | 7 | Full-thickness cartilage defects of the knee (>2 cm2, Kellgren-Lawrence grade III & ICRS grade IV) | None | Maturing repair tissue by arthroscopy reported at 12 weeks Improved VAS pain score and IKDC score at 24 months compared with pre-transplantation scores Regenerated cartilage detected by MRI at 36 months Improved outcomes stable and no signs of osteogenesis and tumorigenesis at 7 years |
153 | |
| co.don chondrosphere (co.don AG) | Phase III (active, not recruiting) | Germany and Poland | 102 | Isolated, single chondral defect on the femoral condyle of the knee (1–4 cm2, depth up to 6 mm, ICRS grade III-IV) | Microfracture | Results not published | 211 |
| Phase II (completed 2018) | Germany | 75 | Isolated, single, chondral defect or osteochondritis dissecans lesion on the femoral condyle, trochlea, tibia or retropatella (4–10 cm2, depth up to 6 mm, ICRS grade III-IV) | Different doses of co.don chondrosphere | No substantial differences in the incidence of adverse events reported between the different doses | 212 | |
| Hyalofast (Anika Therapeutics) | Prospective study (recruiting) | USA and Europe | 200 (estimated) | Cartilage defect on the femoral condyle or trochlea (1–6 cm2, ICRS grade III-IV) | Microfracture | Results not published | 213 |
| INSTRUCT (CellCoTec B.V.) | Prospective study (completed 2014) | Europe | 40 | Cartilage defect on the femoral condyle and trochlea of the knee (modified Outerbridge grade III-IV) | None | Graft delamination reported in 2 patients leading to a treatment failure in 1 patient ~90–100% defect filling at 24 months Improved VAS pain score and IKDC score at 24 months compared with baseline Improved KOOS at 12 months compared with baseline Histological presence of hyaline cartilage in 72% of tissue samples and fibrocartilage and hyaline cartilage in 97% of tissue samples Presence of repair tissue detected by MRI at 12 months |
192 |
| Novocart 3D and Novocart 3D Plus (Aesculap Biologics, TETEC AG) | Phase III (recruiting); Novocart 3D | USA | 30 (estimated) | Patients for whom microfracture failed in a previous trial | None | Results not published | 214 |
| Observational study (active, not recruiting); Novocart 3D | Germany | 82 | Localized, full-thickness cartilage defect of the knee (2.5–10 cm2, ICRS grade III-IV) | None | Results not published | 215 | |
| Phase III (recruiting); Novocart 3D | USA and Canada | 233 (estimated) | Isolated cartilage defects on the femoral condyle of the knee (2–6 cm2) | Microfracture | Results not published | 216 | |
| Phase III (active, not recruiting); Novocart 3D Plus | Europe | 263 | One or two cartilage defects on the femoral condyle and/or the trochlea of the knee (2–6 cm2, ICRS grade III-IV) | Microfracture | Results not published | 217 | |
| Novocart Inject and Novocart Inject Plus (TETEC AG) | Phase III (recruiting); Novocart Inject Plus | Europe | 96 (estimated) | One or two focal cartilage defects on the femoral condyle, trochlea, patella or tibial plateau of the knee (4–12 cm2, ICRS grade III-IV) | None | Results not published | 218 |
| Non-interventional study (recruiting); Novocart Inject | Germany | 125 (estimated) | ‘Insulated’ full-thickness cartilage defects of the knee (2.5–10 cm2, ICRS grade III-IV) | None | Results not published | 219 | |
| Observational study (active, not recruiting); Novocart Inject | Germany | 21 | ‘Insulated’ full-thickness cartilage defects of the hip (1.5–10 cm2, ICRS grade III) | None | Results not published | 220 | |
| Neocart (Histogenics) | Phase III (active, not recruiting) | USA | 245 | Cartilage defect of femur and/or trochlea of the knee | Microfracture | Results not published | 221 |
| Phase II (completed 2014) | USA | 30 | Cartilage defect on the femoral condyle of the knee (ICRS grade III) | Microfracture | No difference in adverse event rates between groups Greater improvement in KOOS, IKDC and VAS pain scores at 6, 12, and 24 months compared with microfracture Improved MOCART scores at 24 months compared with scores at 3 months Improved KOOS, SF-36 and IKDC scores at 5 years compared with baseline Decreased VAS pain score and improved range of motion at 5 years compared with baseline |
194,222 | |
| Phase I (completed, date unknown) | USA | 8 | Full-thickness cartilage defect on the femoral condyle of the knee (grade III, unknown scoring system) | None | Improved VAS pain score at 12 months compared with baseline Improved IKDC score and range of motion at 24 months compared with baseline 6 patients with 67–100% defect filling, 1 patient with 33–66% defect filling, and 1 patient with <33% defect filling as determined by MRI No arthrofibrosis or implant hypertrophy found |
195 | |
| N-TEC (BIO-CHIP) | Phase II (recruiting) | Europe | 108 (estimated) | One or two localized cartilage defects on the femoral condyle and/or trochlea of the knee (2–8 cm2, ICRS grade III-IV) | N-CAM (BIO-CHIP) | Results not published | 113 |
| Phase I (completed 2018) | Switzerland | 18 | One or two cartilage defects on the femoral condyle and/or trochlea of the knee (2–8 cm2, ICRS grade III-IV) | None | No adverse events Defect filling with repair tissue variable Improved KOOS and IKDC scores at 24 months compared with pre-operative values Approaching “ideal level” of glycosaminoglycan content determined by ΔR (R=1/T1) and water and collagen contents “similar to those in native tissue” at 24 months |
197 | |
| RevaFlex (ISTO Technologies) | Phase III (terminated) | USA | 14 | One or two cartilage defects on the femur of the knee (≤5 cm2) | Microfracture | Results not published | 223 |
| Phase I/II (completed, date unknown) | USA | 12 | Up to two cartilage defects on the femoral condyle or trochlea of the knee (1–5cm2, ICRS grade III-IV) | None | Improved patient-reported outcome measures at 12 months Cartilage repair graded as grossly normal/near normal in 66.7% of patients at 12 months Maturation of the implant (determined by defect filling and quality of repair tissue) observed by MRI at 12 months |
152 | |
| Meniscus | |||||||
| Chondrogen (Mesoblast) | Phase I/II (completed 2011) | USA | 55 | Following meniscectomy | Placebo (Hyaluronan) | Results not published | 224 |
| Phase I/II (completed 2008) | USA | 55 | Following meniscectomy | Placebo (Hyaluronan) | Three patients with >15% increase in meniscus volume in 50×106 cells group, 0 in the control group and 0 in the 150×106 cell group at 24 months Decreased VAS pain score and increased Lysholm score for all treatment groups at 24 months compared with baseline |
159 | |
| Cell Bandage (Azellon) | Phase I (ongoing) | Europe | 10 | Meniscus tear that would otherwise be treated by meniscectomy (white-white zone) | None | Results not published | 225 |
Acellular scaffold-based products are not included. The term ‘Europe’ refers to trials that took place in three or more European countries; if a trial took place in fewer than three European countries, all countries are listed. The sponsors and products listed here might since have been acquired by other companies.
The majority of engineered cartilage products in the clinical pipeline, such as Novocart 3D and Neocart, are manufactured using expanded autologous chondrocytes (Table 1). Because chondrocytes dedifferentiate upon in vitro expansion, products derived from expanded chondrocytes are likely to have inferior biomechanical properties to native tissue. Strategies such as the application of hydrostatic pressure have been developed to recover the chondrogenic phenotype. These strategies have resulted in articular cartilage repair implants that showed early-stage clinical improvements, but the long-term success and durability of these implants remains to be seen.
RevaFlex and Cartistem are both manufactured using allogenic cells (Table 1). In a phase I/II study, chondral defects treated with RevaFlex had grossly ‘normal or nearly normal’ cartilage repair (as measured by the International Cartilage Repair Society (ICRS)-Visual Histological Assessment Scale) with no signs of immunologic response after 1 year in 66.7% of patients treated152. In South Korea, treatment of chondral lesions with Cartistem improved clinical outcomes compared to pre-operative scores and showed no signs of bone or tumor growth up to 7 years post-surgery153. Cartistem has completed a phase I/IIa study in the USA154. The successful clinical outcomes of allogeneic therapies to date open up a new avenue for eliminating donor site morbidity and the extra surgical step of tissue harvest when treating cartilage lesions.
Although engineered cartilage products in the clinical pipeline are primarily indicated for knee defects, several products have also been used in the hip (Table 2). Treatment of acetabular chondral defects with BST-CarGel improved International Hip Outcome Tool (iHOT) scores by 46% in a retrospective case series of 37 patients155. In a prospective study of 13 patients, BST-CarGel treatment of acetabular chondral delamination (average defect size 3.7cm2) resulted in over 90% filling by volume of each chondral defect after 2 years156. In another study, the application of either Novocart 3D Inject or co.don chondrosphere to acetabular cartilage defects (average size 2.21cm2) produced substantial improvements in activity and quality of life and reduced pain after a mean of 19 months157.
Compared with articular cartilage, few clinical trials have been carried out with engineered meniscus products (Table 2). Cell Bandage, which is composed of autologous bone marrow-derived MSCs embedded in a collagen sponge, is placed between the torn edges of the meniscus and the defect is sutured closed. It is thought that the MSCs embedded in Cell Bandage release growth factors that promote defect repair158. In the first in human study, Cell Bandage improved IKDC scores by ~40 points, Tegner-Lysholm score by ~40 points and range of motion (ROM) score by ~10 degrees at 12 months post-surgery, results that were maintained at 24 months158. In another study, Chondrogen injections of 50-million or 150-million allogeneic bone marrow-derived MSCs also substantially decreased patient-reported Visual Analogue Scale (VAS) pain scores for up to 24 months159. Although meniscus repair products are not as numerous as articular cartilage products and fewer clinical trials have been performed, preliminary clinical data suggest positive outcomes for cell-based therapies.
Challenges to clinical translation
Cell sourcing
Obtaining sufficient numbers of autologous cells remains a major limiting factor to the translation of engineered articular cartilage and meniscus products (Fig. 4a). As previously noted, sourcing cells from non-articular cartilages, such as costal cartilage, might be a solution to the lack of autologous chondrocytes, although passaging might still be necessary with these cells. Expression of COL1 and COL2 by these cells decreases after just one passage160, but although this collagen expression profile is undesirable for engineering articular cartilage, passaged cells that express COL1 might still be useful in meniscus tissue engineering because native meniscus contains ~80% type I collagen in the red-red zone88. Furthermore, a spectrum of engineered cartilages from hyaline to fibrous can be engineered from costal chondrocytes by modulating their redifferentiation after passaging161. Innovative use of cells and non-articular cartilage cell sources has the potential to greatly alleviate the scarcity of cells for autologous articular cartilage and meniscus therapies.
Figure 4. Challenges to the clinical translation of engineered cartilage and meniscus products.
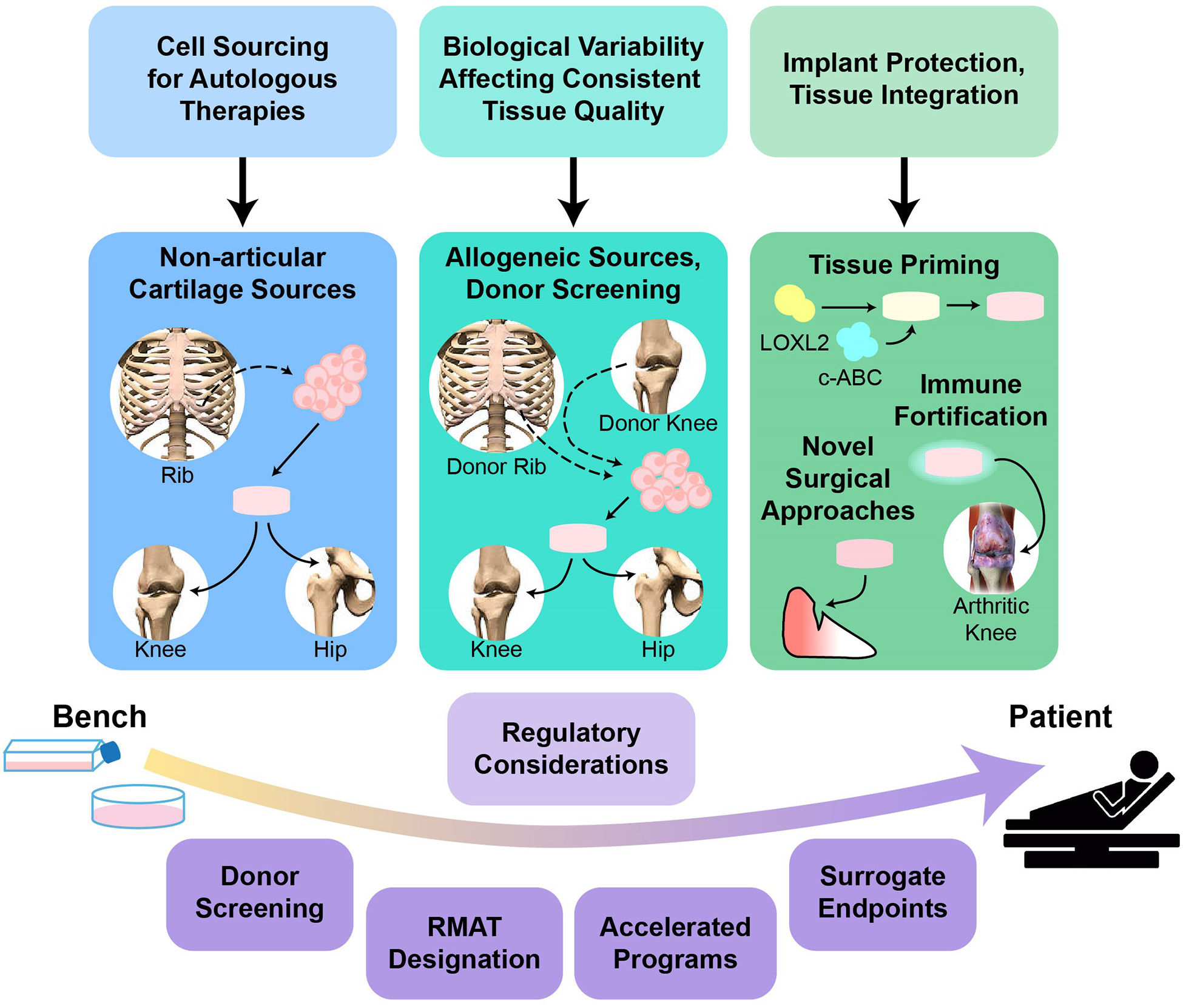
a| The main technical challenges to clinical translation include obtaining sufficient numbers of autologous cells, the effects of biological variability on the consistent production of high-quality engineered tissues and integration of the engineered tissues once implanted in vivo. Potential solutions and avenues of further investigation include; cells sourced from non-articulating cartilages (such as costal cartilage); allogeneic approaches, including extensive screening to identify appropriate donors; the fortification of engineered tissues to withstand immune-mediated degeneration within an inflamed joint; the priming of engineered tissues with chondroitinase ABC (c-ABC) and lysyl-oxidase-like 2 (LOXL2) for enhanced integration; and novel in vivo implantation methods that protect tissue-engineered implants. b| Regulatory challenges to clinical translation include the long time-frames and high costs associated with clinical trials. Solutions such as the Regenerative Medicine Advanced Therapy (RMAT) designation, other FDA programs that enable accelerated review and approval of applications and the use of surrogate endpoints are hoped to help overcome these challenges.
Biological variability
Biologic variability between donors makes the consistent production of high quality autologous neotissue difficult to achieve (Fig. 4a). Not all donors possess cells capable of forming robust neotissue. For example, chondrocytes sourced from 64–80-year-old donors, exhibited variable expression of chondrogenic genes at passage two162. In cells from one group of donors, COL2A1 expression increased when cultured as a microtissue compared with monolayer culture, whereas in cells from another group of donors, COL2A1 expression did not increase upon microtissue culture162. Using allogeneic cells would reduce problems related to donor variability during manufacturing, but the allogeneic implants would need to be well-tolerated by the recipient. Several cartilage repair products already include allogeneic cells or tissues (Table 1). Lending further credence to this approach, the healing of temporomandibular joint disc defects using allogeneic neocartilage has been achieved in mini-pigs163. In this study, costal chondrocyte-generated neocartilage implants were well-tolerated immunologically and resulted in a decrease in OA163. Although allogeneic approaches come with increased concern about disease transmission, tissue banks already provide allogeneic cells and tissues for transplantation in accordance with FDA guidance on donor screening and testing164. Thus, the use of well-characterized allogeneic cells might obviate disease transmission while mitigating the intractable problem of biologic variability.
Achieving biomimicry
Insofar as the functions of articular cartilage and the meniscus are to distribute loads and enable frictionless joint movement, tissue engineering efforts should reflect these functions. Advances have been made in improving the robustness of engineered cartilage towards native tissue values; however, considerable efforts are still required to engineer tribological properties and durability into neocartilage and neomenisci to achieve biomimicry. The use of a functionality index (FI) has been well-documented to provide a comparison of the quality of engineered tissues relative to healthy native tissues150,165,166. However, to be more powerful, the FI should be modified to reflect the relevant salient properties of each target tissue, such as including the coefficient of friction for articular cartilage or an anisotropy index for the meniscus. Although complete biomimicry (FI = 1) in engineered cartilage has traditionally been the goal of tissue engineering approaches, a 2018 study163 in which the implantation of engineered cartilage with an FI of 0.42 resulted in the complete healing of temporomandibular joint disc defects raises questions regarding the degree of biomimicry necessary to achieve regeneration. It remains to be seen if the achievement of biomimicry, especially with respect to biomechanical properties, imparts long-term durability to neotissue in vivo. Furthermore, no data exist to definitively show that the repair of articular cartilage and meniscus damage delays or halts the progression of OA. The ability of small defect repairs to stop OA progression would be difficult to assess in a well-controlled, randomized clinical trial owing to the need to include a no-treatment study arm and the long timeframes involved. Although evidence exists that neotissue with an FI of less than 1 elicits successful healing and that complete biomimicry might not be necessary163, data on the long-term outcomes of using such an approach are lacking. Thus, it will be instructive to continue examining the degree of biomimicry necessary to ensure satisfactory long-term healing outcomes.
Implant integration and protection
The clinical translation of tissue-engineered products requires many factors to be taken into consideration beyond the manufacture of robust neotissue. Articular cartilage and the white-white zone of the meniscus are avascular, which makes integration of implants into existing native tissue difficult (Fig. 4a). The removal of anti-adhesive glycosaminoglycans167 and the priming of engineered tissue with collagen crosslinking agents168 are promising strategies that have shown preliminary success towards improving implant integration. Implant integration can also be affected by post-operative recovery regimens. Unlike humans, animals operated on in preclinical studies will not obey strict rehabilitation regimens and might disrupt implant integration by engaging in impulsive physical activity immediately after surgery. Thus, for both animals and humans, the use of novel tissue-engineered implants might require novel surgical procedures that protect engineered implants and prevent implant displacement. For example, a reproducible intralaminar fenestration technique has been developed that enables engineered neocartilage to be secured into native tissue without directly suturing the implant163. Because implant integration, surgical techniques and rehabilitation all contribute to the efficacy of cartilage regeneration, developing appropriate protocols to address these factors should be as much of a priority for researchers as developing the implants themselves.
Inflammation and immunogenicity
Upon implantation, engineered neotissue must also withstand the pro-inflammatory environment of the injured or diseased joint. Chronic joint inflammation, as can be present in OA and RA, can be destructive to tissue-engineered implants and impede their integration and performance. Many studies have examined ways to ameliorate the immune response to ensure the survival of tissue-engineered implants in inflammatory environments, such as OA and RA joints. Macrophage phenotypes can be modulated in vitro to promote healing and to potentially reduce inflammation in OA169. Other strategies to reduce inflammation, such as the use of adipose-derived MSCs to reduce MMP3 and MMP13 expression, also hold promise170. The rejection of allogeneic engineered cartilage and menisci is also a concern. Although articular cartilage is considered to be immune privileged, and fresh allografts (such as osteochondral allografts, DeNovo NT and meniscus allografts) are in current clinical use, the degree of immune privilege an implant has depends on its location within the knee joint and its proximity to the synovium28. Meniscus allografts are well-tolerated, but it remains to be seen if allogeneic neomenisci implanted into the vascular red-red zone of the meniscus would elicit an immune response. Osteochondral allografts are frequently used in articular cartilage repair and are well-tolerated33 despite the fact the subchondral bone is vascularized, lending some support to the idea that red-red zone allografts might be tolerated. However, most irreparable meniscus defects that would require engineered meniscus grafts occur in the white-white zone, which does not contain vasculature. Thus, this area might also possess a degree of immune privilege, similar to articular cartilage, although the exact immune privilege status of the meniscus still needs further study. Efforts to minimize the immunogenicity of allogeneic and xenogeneic articular cartilage and menisci include decellularization and antigen removal171–173, but these methods typically create disrupted matrix and non-viable cells, depriving the neotissue of the capacity for homeostasis, remodeling and integration. A variety of immunological challenges associated with cartilage and meniscus tissue engineering, such as the pro-inflammatory environment of arthritic joints and the antigenicity of allogeneic cells and matrix components, indicate that neotissue should be modified to be able withstand or modulate the immune response to ensure graft survival and integration.
Regulatory concerns
Several regulatory hurdles surround the translation of engineered cartilage and meniscus products into patients (Fig. 4b). Clinical trials to examine the safety and efficacy of engineered cartilage and meniscus products in large patient populations are costly and time-consuming. Recognizing this, the FDA has announced a new policy framework to expedite the approval of new therapies while preserving public health via a risk-based approach. Special designations, such as the Regenerative Medicine Advanced Therapy (RMAT) designation, have been created to expedite the approval process174. Advantages of the RMAT designation include FDA assistance as early as at phase I trials, the discussion of potential surrogate or intermediate endpoints to accelerate approval and eligibility for priority review of marketing applications. The use of surrogate endpoints might accelerate time to market by shifting some of the burden of proof to post-market follow-up studies. The RMAT designation, as well as other special designations and accelerated programs174, might be solutions to reduce the cost and time required to gain marketing approval for engineered articular cartilage and meniscus products.
Conclusions
Current surgical repair techniques for articular cartilage and meniscus pathologies are insufficient to halt the development and progression of OA, which has accelerated the development of alternative tissue engineering strategies. Many advances have been made in cell sourcing and the use of stimuli to engineer neotissue akin to native articular cartilage and menisci, which can potentially provide long-term solutions for cartilage and meniscus healing. For example, the use of cells from allogeneic, non-articulating and/or diseased cartilage might counter the lack of native autologous cells. Although the goal of tissue engineering is to achieve biomimicry, tissue engineering approaches must also aim to create neotissue that withstands joint inflammation, readily integrates into surrounding native tissues and ensures positive outcomes regardless of biological variability and the age of the patient. The progression towards the use of cell-based tissue-engineered therapies in the clinic can be seen in the numerous clinical trials and IRB-approved studies that are currently underway. Although most products are primarily indicated for use in the knee, many of the same engineering principles can be translated to develop products for other joints such as the hip. The establishment of the RMAT designation should accelerate the regulatory process for these products. Rapidly emerging tissue engineering technologies could lead to the development of long-lasting products that are readily available off-the-shelf for articular cartilage and meniscus regeneration in the not-so-distant future.
Supplementary Material
Key points.
Current cartilage repair techniques include surgery and cell-based therapies for articular cartilage and surgery for meniscus repair; however, such treatments have limited capacity to induce regeneration.
Tissue engineering strategies to create cartilage using a variety of cell sources and exogenous stimuli have made advances towards replicating the native architecture and functional properties of cartilage.
Most cell-based tissue engineering products currently in clinical trials are indicated for knee articular cartilage, with very few indicated for hip cartilage or the meniscus.
Allogeneic and non-articulating cartilage might serve as additional cell sources for engineered articular cartilage and meniscus products.
The pro-inflammatory environment of arthritic joints and issues surrounding neotissue integration need to be addressed to maximize the clinical translation of new tissue-engineered products.
Acknowledgements
This work was supported by the National Institutes of Health R01AR067821 and R01AR071457, as well as funds provided by the Henry Samueli Chair in Engineering.
Glossary
- Debridement
Removal of damaged tissue/torn fragments from a defect
- Hoop stresses
Compressive forces experienced by the meniscus in the circumferential direction
- Rasping
Mechanical scraping to expose fresh/bleeding tissue
- Radial trephination
Puncturing small holes into the joint lining/synovium and into the tissue to stimulate healing
- Bone plugs
Created/fashioned bone cylinders containing the enthesis of the meniscal roots
- Common bone bridge
Excised bone containing and preserving the anatomic relationship between the anterior and posterior meniscal horns (also known as “slot”)
- Hemi-plateau
Half of the tibial plateau containing articular surface, subchondral bone, and meniscus with root attachments
- Lysholm score
A scoring system used to measure changes in limping, support, locking, instability, pain, swelling, stair climbing and squatting (originally developed to evaluate outcomes of knee ligament surgery)
- Stress shielding
Protection of tissue from normal mechanical stresses by the presence of a much stiffer implant, often resulting in tissue loss
- Self-assembling process
A scaffold-free technology that produces tissues that demonstrate spontaneous organization without external forces via the minimization of free energy through cell-to-cell interactions
- Anisotropy
Having directionally dependent properties
- International Knee Documentation Committee (IKDC) score
A scoring system used to measure symptoms, sports and daily activities, current knee function and function prior to injury.
- International Cartilage Repair Society (ICRS)-Visual Histological Assessment Scale
A tool used to histologically evaluate the quality of cartilage repair tissue.
- International Hip Outcome Tool
A tool used to measure symptoms, functional limitation, work-related concerns, sports and recreational activities, and social, emotional and lifestyle concerns using a visual analog scale.
- Tegner-Lysholm score
A patient-reported score of the impact of knee pain and stability on daily life
- Range of motion (ROM) score
A measurement of the range of flexion and extension of a joint
- Tribological properties
Functional properties relating to friction and lubrication of tissues
Footnotes
Competing interests
W.E.B. declares she is the Director of Outreach and a social media contributor for Science Cheerleaders, Incorporated. C.A.L. declares she is on the advisory board of Vericel. N.P. declares he is an associate editor of the Arthroscopy Journal. K.A.A. declares he is on the scientific advisory board of Histogenics. K.A.A., J.C.H., H.K. and W.E.B. declare they are listed as co-authors of submitted US patent applications (16/136,894 and 16/137,120). D.A. declares no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention. Arthritis-related statistics, <https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm> (2018).
- 2.Centers for Disease Control and Prevention. Osteoarthritis (OA), <https://www.cdc.gov/arthritis/basics/osteoarthritis.htm> (2019).
- 3.Wilder FV, Hall BJ, Barrett JP Jr. & Lemrow NB History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment. The Clearwater Osteoarthritis Study. Osteoarthritis Cartilage 10, 611–616 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Wellsandt E et al. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. Am J Sports Med 44, 143–151, doi: 10.1177/0363546515608475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohmander LS, Englund PM, Dahl LL & Roos EM The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 35, 1756–1769, doi: 10.1177/0363546507307396 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Cross M et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 73, 1323–1330, doi: 10.1136/annrheumdis-2013-204763 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Helmick CG et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 58, 15–25, doi: 10.1002/art.23177 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Losina E et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 67, 203–215, doi: 10.1002/acr.22412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnbaum H et al. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin 26, 77–90, doi: 10.1185/03007990903422307 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Hochberg MC et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrit Care Res 64, 465–474, doi: 10.1002/acr.21596 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Singh JA et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 68, 1–25, doi: 10.1002/acr.22783 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Makris EA, Gomoll AH, Malizos KN, Hu JC & Athanasiou KA Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 11, 21–34, doi: 10.1038/nrrheum.2014.157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Athanasiou KA, Darling EM, Hu JC, DuRaine GD & Reddi AH Ch. 4, 257–389 (Taylor & Francis, 2016).
- 14.Moran CJ, Busilacchi A, Lee CA, Athanasiou KA & Verdonk PC Biological augmentation and tissue engineering approaches in meniscus surgery. Arthroscopy 31, 944–955, doi: 10.1016/j.arthro.2014.11.044 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Athanasiou KA & Sanchez-Adams J Ch. 3, 35–53 (Morgan & Claypool, 2009).
- 16.Curl WW et al. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 13, 456–460 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Hjelle K, Solheim E, Strand T, Muri R & Brittberg M Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 18, 730–734 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Flanigan DC, Harris JD, Trinh TQ, Siston RA & Brophy RH Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc 42, 1795–1801, doi: 10.1249/MSS.0b013e3181d9eea0 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Richter DL, Schenck RC Jr., Wascher DC & Treme G Knee Articular Cartilage Repair and Restoration Techniques: A Review of the Literature. Sports Health 8, 153–160, doi: 10.1177/1941738115611350 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedi A, Feeley BT & Williams RJ 3rd. Management of articular cartilage defects of the knee. J Bone Joint Surg Am 92, 994–1009, doi: 10.2106/JBJS.I.00895 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Steadman JR, Rodkey WG & Rodrigo JJ Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res, S362–369 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Gudas R et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med 40, 2499–2508, doi: 10.1177/0363546512458763 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Solheim E, Hegna J, Strand T, Harlem T & Inderhaug E Randomized Study of Long-term (15–17 Years) Outcome After Microfracture Versus Mosaicplasty in Knee Articular Cartilage Defects. Am J Sports Med 46, 826–831, doi: 10.1177/0363546517745281 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Albright JC & Daoud AK Microfracture and Microfracture Plus. Clin Sports Med 36, 501–507, doi: 10.1016/j.csm.2017.02.012 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Gao L, Orth P, Cucchiarini M & Madry H Autologous Matrix-Induced Chondrogenesis: A Systematic Review of the Clinical Evidence. Am J Sports Med, 363546517740575, doi: 10.1177/0363546517740575 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Krych AJ et al. Return to sport after the surgical management of articular cartilage lesions in the knee: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 25, 3186–3196, doi: 10.1007/s00167-016-4262-3 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Hangody L et al. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am 86-A Suppl 1, 65–72 (2004). [PubMed] [Google Scholar]
- 28.Arzi B et al. Cartilage immunoprivilege depends on donor source and lesion location. Acta Biomater 23, 72–81, doi: 10.1016/j.actbio.2015.05.025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh JL, Kowalski A & Lautenschlager E The effect of angled osteochondral grafting on contact pressure: a biomechanical study. Am J Sports Med 34, 116–119, doi: 10.1177/0363546505281236 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Koh JL, Wirsing K, Lautenschlager E & Zhang LO The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med 32, 317–320, doi: 10.1177/0363546503261730 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Cook JL et al. Importance of Donor Chondrocyte Viability for Osteochondral Allografts. Am J Sports Med 44, 1260–1268, doi: 10.1177/0363546516629434 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Williams RJ 3rd, Ranawat AS, Potter HG, Carter T & Warren RF Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am 89, 718–726, doi: 10.2106/JBJS.F.00625 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Levy YD, Gortz S, Pulido PA, McCauley JC & Bugbee WD Do Fresh Osteochondral Allografts Successfully Treat Femoral Condyle Lesions? Clinical orthopaedics and related research 471, 231–237, doi: 10.1007/s11999-012-2556-4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hangody L & Fules P Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am 85-A Suppl 2, 25–32 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Balazs GC et al. Return to Play Among Elite Basketball Players After Osteochondral Allograft Transplantation of Full-Thickness Cartilage Lesions. Orthop J Sports Med 6, 2325967118786941, doi: 10.1177/2325967118786941 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinckel BB, Gomoll AH & Farr J 2nd. Cartilage Restoration in the Patellofemoral Joint. Am J Orthop (Belle Mead NJ) 46, 217–222 (2017). [PubMed] [Google Scholar]
- 37.Dunkin BS & Lattermann C New and Emerging Techniques in Cartilage Repair: MACI. Oper Tech Sports Med 21, 100–107, doi: 10.1053/j.otsm.2013.03.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebert JR, Fallon M, Wood DJ & Janes GC A Prospective Clinical and Radiological Evaluation at 5 Years After Arthroscopic Matrix-Induced Autologous Chondrocyte Implantation. Am J Sports Med 45, 59–69, doi: 10.1177/0363546516663493 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Ebert JR, Fallon M, Smith A, Janes GC & Wood DJ Prospective clinical and radiologic evaluation of patellofemoral matrix-induced autologous chondrocyte implantation. Am J Sports Med 43, 1362–1372, doi: 10.1177/0363546515574063 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Fillingham YA, Riboh JC, Erickson BJ, Bach BR Jr. & Yanke AB Inside-Out Versus All-Inside Repair of Isolated Meniscal Tears: An Updated Systematic Review. Am J Sports Med 45, 234–242, doi: 10.1177/0363546516632504 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Barrett GR, Field MH, Treacy SH & Ruff CG Clinical results of meniscus repair in patients 40 years and older. Arthroscopy 14, 824–829 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Eggli S, Wegmuller H, Kosina J, Huckell C & Jakob RP Long-term results of arthroscopic meniscal repair. An analysis of isolated tears. Am J Sports Med 23, 715–720, doi: 10.1177/036354659502300614 (1995). [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Bosque J, Meehan JP, Jamali A & Marder R Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am 93, 994–1000, doi: 10.2106/JBJS.I.01618 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Papalia R, Del Buono A, Osti L, Denaro V & Maffulli N Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull 99, 89–106, doi: 10.1093/bmb/ldq043 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Abrams GD et al. Trends in meniscus repair and meniscectomy in the United States, 2005–2011. Am J Sports Med 41, 2333–2339, doi: 10.1177/0363546513495641 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Johnson MJ, Lucas GL, Dusek JK & Henning CE Isolated arthroscopic meniscal repair: a long-term outcome study (more than 10 years). Am J Sports Med 27, 44–49, doi: 10.1177/03635465990270011501 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Pujol N, Bohu Y, Boisrenoult P, Macdes A & Beaufils P Clinical outcomes of open meniscal repair of horizontal meniscal tears in young patients. Knee Surg Sports Traumatol Arthrosc 21, 1530–1533, doi: 10.1007/s00167-012-2099-y (2013). [DOI] [PubMed] [Google Scholar]
- 48.Choi NH, Kim TH, Son KM & Victoroff BN Meniscal repair for radial tears of the midbody of the lateral meniscus. Am J Sports Med 38, 2472–2476, doi: 10.1177/0363546510376736 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Wasserstein D et al. A matched-cohort population study of reoperation after meniscal repair with and without concomitant anterior cruciate ligament reconstruction. Am J Sports Med 41, 349–355, doi: 10.1177/0363546512471134 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Hutchinson ID, Moran CJ, Potter HG, Warren RF & Rodeo SA Restoration of the meniscus: form and function. Am J Sports Med 42, 987–998, doi: 10.1177/0363546513498503 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z, Arnold JA, Williams T & McCann B Repairs by trephination and suturing of longitudinal injuries in the avascular area of the meniscus in goats. Am J Sports Med 23, 35–41, doi: 10.1177/036354659502300106 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Taylor SA & Rodeo SA Augmentation techniques for isolated meniscal tears. Curr Rev Musculoskelet Med 6, 95–101, doi: 10.1007/s12178-013-9165-z (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henning CE et al. Arthroscopic meniscal repair using an exogenous fibrin clot. Clin Orthop Relat Res, 64–72 (1990). [PubMed] [Google Scholar]
- 54.Dean CS, Chahla J, Matheny LM, Mitchell JJ & LaPrade RF Outcomes After Biologically Augmented Isolated Meniscal Repair With Marrow Venting Are Comparable With Those After Meniscal Repair With Concomitant Anterior Cruciate Ligament Reconstruction. Am J Sports Med 45, 1341–1348, doi: 10.1177/0363546516686968 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Verdonk R et al. Indications and limits of meniscal allografts. Injury 44 Suppl 1, S21–27, doi: 10.1016/S0020-1383(13)70006-8 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Rodeo SA Meniscal allografts--where do we stand? Am J Sports Med 29, 246–261, doi: 10.1177/03635465010290022401 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Lee SR, Kim JG & Nam SW The tips and pitfalls of meniscus allograft transplantation. Knee Surg Relat Res 24, 137–145, doi: 10.5792/ksrr.2012.24.3.137 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dienst M, Greis PE, Ellis BJ, Bachus KN & Burks RT Effect of lateral meniscal allograft sizing on contact mechanics of the lateral tibial plateau: an experimental study in human cadaveric knee joints. Am J Sports Med 35, 34–42, doi: 10.1177/0363546506291404 (2007). [DOI] [PubMed] [Google Scholar]
- 59.Vundelinckx B, Vanlauwe J & Bellemans J Long-term Subjective, Clinical, and Radiographic Outcome Evaluation of Meniscal Allograft Transplantation in the Knee. Am J Sports Med 42, 1592–1599, doi: 10.1177/0363546514530092 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Lee SM et al. Long-term Outcomes of Meniscal Allograft Transplantation With and Without Extrusion: Mean 12.3-Year Follow-up Study. Am J Sports Med, 363546518825251, doi: 10.1177/0363546518825251 (2019). [DOI] [PubMed] [Google Scholar]
- 61.van Tienen TG, Hannink G & Buma P Meniscus Replacement Using Synthetic Materials. Clin Sport Med 28, 143–+, doi: 10.1016/j.csm.2008.08.003 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Bulgheroni E et al. Long-term outcomes of medial CMI implant versus partial medial meniscectomy in patients with concomitant ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 23, 3221–3227, doi: 10.1007/s00167-014-3136-9 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Schuttler KF et al. Midterm follow-up after implantation of a polyurethane meniscal scaffold for segmental medial meniscus loss: maintenance of good clinical and MRI outcome. Knee Surg Sports Traumatol Arthrosc 24, 1478–1484, doi: 10.1007/s00167-015-3759-5 (2016). [DOI] [PubMed] [Google Scholar]
- 64.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02136901 (2014).
- 65.Marinescu R & Antoniac I in Handbook of Bioceramics and Biocomposite (ed Vasile Antoniac Iulian) 1–31 (Springer, Cham, 2015). [Google Scholar]
- 66.Kramer DE & Micheli LJ Meniscal tears and discoid meniscus in children: diagnosis and treatment. J Am Acad Orthop Surg 17, 698–707 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Adirim TA & Cheng TL Overview of injuries in the young athlete. Sports Med 33, 75–81, doi: 10.2165/00007256-200333010-00006 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Beck NA, Lawrence JTR, Nordin JD, DeFor TA & Tompkins M ACL Tears in School-Aged Children and Adolescents Over 20 Years. Pediatrics 139, doi: 10.1542/peds.2016-1877 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Kreuz PC et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy 22, 1180–1186, doi: 10.1016/j.arthro.2006.06.020 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Asik M, Ciftci F, Sen C, Erdil M & Atalar A The microfracture technique for the treatment of full-thickness articular cartilage lesions of the knee: midterm results. Arthroscopy 24, 1214–1220, doi: 10.1016/j.arthro.2008.06.015 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Mithoefer K, McAdams T, Williams RJ, Kreuz PC & Mandelbaum BR Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 37, 2053–2063, doi: 10.1177/0363546508328414 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Mithoefer K et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am 87, 1911–1920, doi: 10.2106/JBJS.D.02846 (2005). [DOI] [PubMed] [Google Scholar]
- 73.Gudas R et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy 21, 1066–1075, doi: 10.1016/j.arthro.2005.06.018 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Murphy RT, Pennock AT & Bugbee WD Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med 42, 635–640, doi: 10.1177/0363546513516747 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Frank RM et al. Osteochondral Allograft Transplantation of the Knee: Analysis of Failures at 5 Years. Am J Sports Med 45, 864–874, doi: 10.1177/0363546516676072 (2017). [DOI] [PubMed] [Google Scholar]
- 76.Micheli LJ et al. Articular cartilage defects of the distal femur in children and adolescents: treatment with autologous chondrocyte implantation. J Pediatr Orthop 26, 455–460, doi: 10.1097/01.bpo.0000224565.72762.eb (2006). [DOI] [PubMed] [Google Scholar]
- 77.Mithofer K, Minas T, Peterson L, Yeon H & Micheli LJ Functional outcome of knee articular cartilage repair in adolescent athletes. Am J Sports Med 33, 1147–1153, doi: 10.1177/0363546504274146 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Knutsen G et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86-A, 455–464 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Saris DB et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med 37 Suppl 1, 10S–19S, doi: 10.1177/0363546509350694 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Gudas R, Stankevicius E, Monastyreckiene E, Pranys D & Kalesinskas RJ Osteochondral autologous transplantation versus microfracture for the treatment of articular cartilage defects in the knee joint in athletes. Knee Surg Sports Traumatol Arthrosc 14, 834–842, doi: 10.1007/s00167-006-0067-0 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Kon E et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med 37, 33–41, doi: 10.1177/0363546508323256 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Noyes FR & Barber-Westin SD Meniscus transplantation: indications, techniques, clinical outcomes. Instr Course Lect 54, 341–353 (2005). [PubMed] [Google Scholar]
- 83.Riboh JC, Tilton AK, Cvetanovich GL, Campbell KA & Cole BJ Meniscal Allograft Transplantation in the Adolescent Population. Arthroscopy 32, 1133–1140 e1131, doi: 10.1016/j.arthro.2015.11.041 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Steadman JR et al. Meniscus suture repair: minimum 10-year outcomes in patients younger than 40 years compared with patients 40 and older. Am J Sports Med 43, 2222–2227, doi: 10.1177/0363546515591260 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Rothermel SD, Smuin D & Dhawan A Are Outcomes After Meniscal Repair Age Dependent? A Systematic Review. Arthroscopy 34, 979–987, doi: 10.1016/j.arthro.2017.08.287 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Nepple JJ, Dunn WR & Wright RW Meniscal repair outcomes at greater than five years: a systematic literature review and meta-analysis. J Bone Joint Surg Am 94, 2222–2227, doi: 10.2106/JBJS.K.01584 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shyh-Chang N et al. Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 155, 778–792, doi: 10.1016/j.cell.2013.09.059 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Makris EA, Hadidi P & Athanasiou KA The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 32, 7411–7431, doi: 10.1016/j.biomaterials.2011.06.037 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhattacharjee M et al. Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv Drug Deliv Rev 84, 107–122, doi: 10.1016/j.addr.2014.08.010 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Rowland CR, Colucci LA & Guilak F Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials 91, 57–72, doi: 10.1016/j.biomaterials.2016.03.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shimomura K, Rothrauff BB & Tuan RS Region-Specific Effect of the Decellularized Meniscus Extracellular Matrix on Mesenchymal Stem Cell-Based Meniscus Tissue Engineering. Am J Sports Med 45, 604–611, doi: 10.1177/0363546516674184 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Liu M et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res 5, 17014, doi: 10.1038/boneres.2017.14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo T, Lembong J, Zhang LG & Fisher JP Three-Dimensional Printing Articular Cartilage: Recapitulating the Complexity of Native Tissue<sup/>. Tissue Eng Part B Rev 23, 225–236, doi: 10.1089/ten.TEB.2016.0316 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Shen S et al. 3D printing-Based strategies for functional cartilage regeneration. Tissue Eng Part B Rev, doi: 10.1089/ten.TEB.2018.0248 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Athanasiou KA, Eswaramoorthy R, Hadidi P & Hu JC Self-organization and the self-assembling process in tissue engineering. Annu Rev Biomed Eng 15, 115–136, doi: 10.1146/annurev-bioeng-071812-152423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu JC & Athanasiou KA A self-assembling process in articular cartilage tissue engineering. Tissue Eng 12, 969–979, doi: 10.1089/ten.2006.12.969 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Hoben GM, Hu JC, James RA & Athanasiou KA Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue engineering 13, 939–946, doi: 10.1089/ten.2006.0116 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Ofek G et al. Matrix development in self-assembly of articular cartilage. PLoS One 3, e2795, doi: 10.1371/journal.pone.0002795 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee JK et al. Tension stimulation drives tissue formation in scaffold-free systems. Nat Mater 16, 864–873, doi: 10.1038/nmat4917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elder BD & Athanasiou KA Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage 17, 114–123, doi: 10.1016/j.joca.2008.05.006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Little CJ, Bawolin NK & Chen X Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng Part B Rev 17, 213–227, doi: 10.1089/ten.TEB.2010.0572 (2011). [DOI] [PubMed] [Google Scholar]
- 102.Gunja NJ, Huey DJ, James RA & Athanasiou KA Effects of agarose mould compliance and surface roughness on self-assembled meniscus-shaped constructs. J Tissue Eng Regen Med 3, 521–530, doi: 10.1002/term.191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Makris EA, MacBarb RF, Paschos NK, Hu JC & Athanasiou KA Combined use of chondroitinase-ABC, TGF-beta1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair. Biomaterials 35, 6787–6796, doi: 10.1016/j.biomaterials.2014.04.083 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu D, Tong X, Trinh P & Yang F Mimicking Cartilage Tissue Zonal Organization by Engineering Tissue-Scale Gradient Hydrogels as 3D Cell Niche. Tissue Eng Part A 24, 1–10, doi: 10.1089/ten.TEA.2016.0453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steele JA et al. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater 10, 2065–2075, doi: 10.1016/j.actbio.2013.12.030 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zitnay JL et al. Fabrication of dense anisotropic collagen scaffolds using biaxial compression. Acta Biomater 65, 76–87, doi: 10.1016/j.actbio.2017.11.017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Higashioka MM, Chen JA, Hu JC & Athanasiou KA Building an anisotropic meniscus with zonal variations. Tissue Eng Part A 20, 294–302, doi: 10.1089/ten.TEA.2013.0098 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Darling EM & Athanasiou KA Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res 23, 425–432, doi: 10.1016/j.orthres.2004.08.008 (2005). [DOI] [PubMed] [Google Scholar]
- 109.Huwe LW, Brown WE, Hu JC & Athanasiou KA Characterization of costal cartilage and its suitability as a cell source for articular cartilage tissue engineering. J Tissue Eng Regen Med 12, 1163–1176, doi: 10.1002/term.2630 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kwon H, O’Leary SA, Hu JC & Athanasiou KA Translating the application of transforming growth factor-beta1, chondroitinase-ABC, and lysyl oxidase-like 2 for mechanically robust tissue-engineered human neocartilage. J Tissue Eng Regen Med 13, 283–294, doi: 10.1002/term.2791 (2019). [DOI] [PubMed] [Google Scholar]
- 111.Murphy MK, Huey DJ, Hu JC & Athanasiou KA TGF-beta1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 33, 762–773, doi: 10.1002/stem.1890 (2015). [DOI] [PubMed] [Google Scholar]
- 112.Pelttari K et al. Adult human neural crest-derived cells for articular cartilage repair. Sci Transl Med 6, 251ra119, doi: 10.1126/scitranslmed.3009688 (2014). [DOI] [PubMed] [Google Scholar]
- 113.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02673905 (2016).
- 114.Bianchi VJ, Weber JF, Waldman SD, Backstein D & Kandel RA Formation of Hyaline Cartilage Tissue by Passaged Human Osteoarthritic Chondrocytes. Tissue Eng Part A 23, 156–165, doi: 10.1089/ten.TEA.2016.0262 (2017). [DOI] [PubMed] [Google Scholar]
- 115.Kwon H et al. Tissue engineering potential of human dermis-isolated adult stem cells from multiple anatomical locations. PLoS One 12, e0182531, doi: 10.1371/journal.pone.0182531 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lavoie JF et al. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem Cells Dev 18, 893–906, doi: 10.1089/scd.2008.0260 (2009). [DOI] [PubMed] [Google Scholar]
- 117.Fu WL, Zhou CY & Yu JK A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model. Am J Sports Med 42, 592–601, doi: 10.1177/0363546513512778 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Chang NJ et al. Transplantation of autologous endothelial progenitor cells in porous PLGA scaffolds create a microenvironment for the regeneration of hyaline cartilage in rabbits. Osteoarthritis Cartilage 21, 1613–1622, doi: 10.1016/j.joca.2013.07.016 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Jiang Y et al. Human Cartilage-Derived Progenitor Cells From Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl Med 5, 733–744, doi: 10.5966/sctm.2015-0192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Somoza RA, Welter JF, Correa D & Caplan AI Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev 20, 596–608, doi: 10.1089/ten.TEB.2013.0771 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen S, Fu P, Cong R, Wu H & Pei M Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis 2, 76–95, doi: 10.1016/j.gendis.2014.12.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwon H, Paschos NK, Hu JC & Athanasiou K Articular cartilage tissue engineering: the role of signaling molecules. Cell Mol Life Sci 73, 1173–1194, doi: 10.1007/s00018-015-2115-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng Q et al. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater 53, 329–342, doi: 10.1016/j.actbio.2017.02.015 (2017). [DOI] [PubMed] [Google Scholar]
- 124.Amann E, Wolff P, Breel E, van Griensven M & Balmayor ER Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater 52, 130–144, doi: 10.1016/j.actbio.2017.01.064 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Liu Q et al. Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3. Acta Biomater 76, 29–38, doi: 10.1016/j.actbio.2018.06.027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson K et al. A stem cell-based approach to cartilage repair. Science 336, 717–721, doi: 10.1126/science.1215157 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Cai G et al. Recent advances in kartogenin for cartilage regeneration. J Drug Target 27, 28–32, doi: 10.1080/1061186X.2018.1464011 (2019). [DOI] [PubMed] [Google Scholar]
- 128.Bian L et al. Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A 15, 2065–2072, doi: 10.1089/ten.tea.2008.0495 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Natoli RM, Revell CM & Athanasiou KA Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A 15, 3119–3128, doi: 10.1089/ten.TEA.2008.0478 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Makris EA, Responte DJ, Paschos NK, Hu JC & Athanasiou KA Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci U S A 111, E4832–4841, doi: 10.1073/pnas.1414271111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Makris EA, Hu JC & Athanasiou KA Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage 21, 634–641, doi: 10.1016/j.joca.2013.01.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Athanasiou KA, Darling EM, Hu JC, DuRaine GD & Reddi AH Ch. 1, 18–25 (Taylor & Francis, 2016).
- 133.Huwe LW, Sullan GK, Hu JC & Athanasiou KA Using Costal Chondrocytes to Engineer Articular Cartilage with Applications of Passive Axial Compression and Bioactive Stimuli. Tissue engineering. Part A 24, 516–526, doi: 10.1089/ten.TEA.2017.0136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Meinert C, Schrobback K, Hutmacher DW & Klein TJ A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci Rep 7, 16997, doi: 10.1038/s41598-017-16523-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Son MS & Levenston ME Quantitative tracking of passage and 3D culture effects on chondrocyte and fibrochondrocyte gene expression. J Tissue Eng Regen Med 11, 1185–1194, doi: 10.1002/term.2022 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Zellner J et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res A 94, 1150–1161, doi: 10.1002/jbm.a.32796 (2010). [DOI] [PubMed] [Google Scholar]
- 137.Moriguchi Y et al. Repair of meniscal lesions using a scaffold-free tissue-engineered construct derived from allogenic synovial MSCs in a miniature swine model. Biomaterials 34, 2185–2193, doi: 10.1016/j.biomaterials.2012.11.039 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Sasaki H et al. In Vitro Repair of Meniscal Radial Tear With Hydrogels Seeded With Adipose Stem Cells and TGF-beta3. Am J Sports Med 46, 2402–2413, doi: 10.1177/0363546518782973 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Koh RH, Jin YJ, Kang BJ & Hwang NS Chondrogenically primed tonsil-derived mesenchymal stem cells encapsulated in riboflavin-induced photocrosslinking collagen-hyaluronic acid hydrogel for meniscus tissue repairs. Acta Biomaterialia 53, 318–328, doi: 10.1016/j.actbio.2017.01.081 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Xie X et al. A co-culture system of rat synovial stem cells and meniscus cells promotes cell proliferation and differentiation as compared to mono-culture. Sci Rep 8, 7693, doi: 10.1038/s41598-018-25709-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hadidi P et al. Tendon and ligament as novel cell sources for engineering the knee meniscus. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society 24, 2126–2134, doi: 10.1016/j.joca.2016.07.006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pangborn CA & Athanasiou KA Growth factors and fibrochondrocytes in scaffolds. J Orthop Res 23, 1184–1190, doi: 10.1016/j.orthres.2005.01.019 (2005). [DOI] [PubMed] [Google Scholar]
- 143.Bonnevie ED, Puetzer JL & Bonassar LJ Enhanced boundary lubrication properties of engineered menisci by lubricin localization with insulin-like growth factor I treatment. J Biomech 47, 2183–2188, doi: 10.1016/j.jbiomech.2013.10.028 (2014). [DOI] [PubMed] [Google Scholar]
- 144.Lee CH et al. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Sci Transl Med 6, 266ra171, doi: 10.1126/scitranslmed.3009696 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liang Y et al. Plasticity of Human Meniscus Fibrochondrocytes: A Study on Effects of Mitotic Divisions and Oxygen Tension. Sci Rep 7, 12148, doi: 10.1038/s41598-017-12096-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Croutze R, Jomha N, Uludag H & Adesida A Matrix forming characteristics of inner and outer human meniscus cells on 3D collagen scaffolds under normal and low oxygen tensions. BMC Musculoskelet Disord 14, 353, doi: 10.1186/1471-2474-14-353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Huey DJ & Athanasiou KA Tension-compression loading with chemical stimulation results in additive increases to functional properties of anatomic meniscal constructs. PLoS One 6, e27857, doi: 10.1371/journal.pone.0027857 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zellner J et al. Dynamic hydrostatic pressure enhances differentially the chondrogenesis of meniscal cells from the inner and outer zone. J Biomech 48, 1479–1484, doi: 10.1016/j.jbiomech.2015.02.003 (2015). [DOI] [PubMed] [Google Scholar]
- 149.Puetzer JL & Bonassar LJ Physiologically Distributed Loading Patterns Drive the Formation of Zonally Organized Collagen Structures in Tissue-Engineered Meniscus. Tissue Eng Part A 22, 907–916, doi: 10.1089/ten.TEA.2015.0519 (2016). [DOI] [PubMed] [Google Scholar]
- 150.MacBarb RF, Chen AL, Hu JC & Athanasiou KA Engineering functional anisotropy in fibrocartilage neotissues. Biomaterials 34, 9980–9989, doi: 10.1016/j.biomaterials.2013.09.026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang BJ, Hu JC & Athanasiou KA Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 98, 1–22, doi: 10.1016/j.biomaterials.2016.04.018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McCormick F et al. Treatment of Focal Cartilage Defects With a Juvenile Allogeneic 3-Dimensional Articular Cartilage Graft. Oper Techn Sport Med 21, 95–99, doi: 10.1053/j.otsm.2013.03.007 (2013). [DOI] [Google Scholar]
- 153.Park YB, Ha CW, Lee CH, Yoon YC & Park YG Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl Med 6, 613–621, doi: 10.5966/sctm.2016-0157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01733186 (2012).
- 155.Rhee C, Amar E, Glazebrook M, Coday C & Wong IH Safety Profile and Short-term Outcomes of BST-CarGel as an Adjunct to Microfracture for the Treatment of Chondral Lesions of the Hip. Orthop J Sports Med 6, 2325967118789871, doi: 10.1177/2325967118789871 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tahoun M et al. Results of arthroscopic treatment of chondral delamination in femoroacetabular impingement with bone marrow stimulation and BST-CarGel((R)). SICOT J 3, 51, doi: 10.1051/sicotj/2017031 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thier S, Weiss C & Fickert S Arthroscopic autologous chondrocyte implantation in the hip for the treatment of full-thickness cartilage defects - A case series of 29 patients and review of the literature. SICOT J 3, 72, doi: 10.1051/sicotj/2017037 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Whitehouse MR et al. Repair of Torn Avascular Meniscal Cartilage Using Undifferentiated Autologous Mesenchymal Stem Cells: From In Vitro Optimization to a First-in-Human Study. Stem Cells Transl Med 6, 1237–1248, doi: 10.1002/sctm.16-0199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vangsness CT Jr. et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. The Journal of bone and joint surgery. American volume 96, 90–98, doi: 10.2106/JBJS.M.00058 (2014). [DOI] [PubMed] [Google Scholar]
- 160.Darling EM & Athanasiou KA Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res 23, 425–432, doi:DOI 10.1016/j.orthres.2004.08.008 (2005). [DOI] [PubMed] [Google Scholar]
- 161.Murphy MK, Masters TE, Hu JC & Athanasiou KA Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation. Cell transplantation 24, 235–245, doi: 10.3727/096368913X676204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martin F, Lehmann M, Sack U & Anderer U Featured Article: In vitro development of personalized cartilage microtissues uncovers an individualized differentiation capacity of human chondrocytes. Exp Biol Med (Maywood) 242, 1746–1756, doi: 10.1177/1535370217728498 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vapniarsky N et al. Tissue engineering toward temporomandibular joint disc regeneration. Sci Transl Med 10, doi: 10.1126/scitranslmed.aaq1802 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.US Food and Drug Administration. Guidance for Industry: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps), <https://www.fda.gov/media/73072/download> (2015). [PubMed]
- 165.Huwe LW, Brown WE, Hu JC & Athanasiou KA Characterization of costal cartilage and its suitability as a cell source for articular cartilage tissue engineering. J Tissue Eng Regen Med, doi: 10.1002/term.2630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Elder BD & Athanasiou KA Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arvayo AL, Wong IJ, Dragoo JL & Levenston ME Enhancing integration of articular cartilage grafts via photochemical bonding. J Orthop Res 36, 2406–2415, doi: 10.1002/jor.23898 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Athens AA, Makris EA & Hu JC Induced collagen cross-links enhance cartilage integration. PloS one 8, e60719, doi: 10.1371/journal.pone.0060719 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Utomo L, van Osch GJ, Bayon Y, Verhaar JA & Bastiaansen-Jenniskens YM Guiding synovial inflammation by macrophage phenotype modulation: an in vitro study towards a therapy for osteoarthritis. Osteoarthritis Cartilage 24, 1629–1638, doi: 10.1016/j.joca.2016.04.013 (2016). [DOI] [PubMed] [Google Scholar]
- 170.Lai JH et al. Interaction Between Osteoarthritic Chondrocytes and Adipose-Derived Stem Cells Is Dependent on Cell Distribution in Three-Dimension and Transforming Growth Factor-beta 3 Induction. Tissue Eng Pt A 21, 992–1002, doi: 10.1089/ten.tea.2014.0244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Elder BD, Eleswarapu SV & Athanasiou KA Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials 30, 3749–3756, doi:DOI 10.1016/j.biomaterials.2009.03.050 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cissell DD, Hu JC, Griffiths LG & Athanasiou KA Antigen removal for the production of biomechanically functional, xenogeneic tissue grafts. J Biomech 47, 1987–1996, doi: 10.1016/j.jbiomech.2013.10.041 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McNickle AG, Wang VM, Shewman EF, Cole BJ & Williams JM Performance of a sterile meniscal allograft in an ovine model. Clinical orthopaedics and related research 467, 1868–1876, doi: 10.1007/s11999-008-0567-y (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.US Food and Drug Administration. Guidance for Industry: Expedited Programs for Regenerative Medicine Therapies for Serious Conditions, <https://www.fda.gov/media/120267/download> (2017).
- 175.Nehrer S, Chiari C, Domayer S, Barkay H & Yayon A Results of chondrocyte implantation with a fibrin-hyaluronan matrix: a preliminary study. Clinical orthopaedics and related research 466, 1849–1855, doi: 10.1007/s11999-008-0322-4 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Domayer SE et al. T2 mapping and dGEMRIC after autologous chondrocyte implantation with a fibrin-based scaffold in the knee: preliminary results. Eur J Radiol 73, 636–642, doi: 10.1016/j.ejrad.2008.12.006 (2010). [DOI] [PubMed] [Google Scholar]
- 177.Fontana A, Bistolfi A, Crova M, Rosso F & Massazza G Arthroscopic treatment of hip chondral defects: autologous chondrocyte transplantation versus simple debridement--a pilot study. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 28, 322–329, doi: 10.1016/j.arthro.2011.08.304 (2012). [DOI] [PubMed] [Google Scholar]
- 178.Ossendorf C et al. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis research & therapy 9, R41, doi: 10.1186/ar2180 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kreuz PC, Muller S, Ossendorf C, Kaps C & Erggelet C Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis research & therapy 11, R33, doi: 10.1186/ar2638 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stanish WD et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J Bone Joint Surg Am 95, 1640–1650, doi: 10.2106/JBJS.L.01345 (2013). [DOI] [PubMed] [Google Scholar]
- 181.Schneider U et al. A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med 39, 2558–2565, doi: 10.1177/0363546511423369 (2011). [DOI] [PubMed] [Google Scholar]
- 182.Cole BJ et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med 39, 1170–1179, doi: 10.1177/0363546511399382 (2011). [DOI] [PubMed] [Google Scholar]
- 183.Selmi TA et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br 90, 597–604, doi: 10.1302/0301-620X.90B5.20360 (2008). [DOI] [PubMed] [Google Scholar]
- 184.Clave A et al. Third-generation autologous chondrocyte implantation versus mosaicplasty for knee cartilage injury: 2-year randomized trial. J Orthop Res 34, 658–665, doi: 10.1002/jor.23152 (2016). [DOI] [PubMed] [Google Scholar]
- 185.Fickert S et al. One-Year Clinical and Radiological Results of a Prospective, Investigator-Initiated Trial Examining a Novel, Purely Autologous 3-Dimensional Autologous Chondrocyte Transplantation Product in the Knee. Cartilage 3, 27–42, doi: 10.1177/1947603511417616 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Meyer U Fundamentals of tissue engineering and regenerative medicine. (Springer, 2009). [Google Scholar]
- 187.Gobbi A et al. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc 25, 2494–2501, doi: 10.1007/s00167-016-3984-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marcacci M et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clinical orthopaedics and related research, 96–105 (2005). [DOI] [PubMed] [Google Scholar]
- 189.Nehrer S et al. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol 57, 3–8, doi: 10.1016/j.ejrad.2005.08.005 (2006). [DOI] [PubMed] [Google Scholar]
- 190.Tognana E, Borrione A, De Luca C & Pavesio A Hyalograft C: hyaluronan-based scaffolds in tissue-engineered cartilage. Cells, tissues, organs 186, 97–103, doi: 10.1159/000102539 (2007). [DOI] [PubMed] [Google Scholar]
- 191.Brittberg M in Techniques in Cartilage Repair Surgery (eds Shetty Asode Ananthram, Nakamura Norimasa, Kim Seok-Jung, & Brittberg Mats) (Springer, 2014). [Google Scholar]
- 192.Hendriks J et al. First clinical experience with INSTRUCT–a single surgery, autologous cell based technology for cartilage repair. European Society of Sports Traumatology, Knee Surgery and Arthroscopy Congress, 14–17 (2013). <www.cellcotec.com/wp-content/uploads/2017/11/P187-first-clinical-experience-with-INSTRUCT-final2.pdf>. [Google Scholar]
- 193.Zak L et al. Results 2 Years After Matrix-Associated Autologous Chondrocyte Transplantation Using the Novocart 3D Scaffold: An Analysis of Clinical and Radiological Data. Am J Sports Med 42, 1618–1627, doi: 10.1177/0363546514532337 (2014). [DOI] [PubMed] [Google Scholar]
- 194.Crawford DC, DeBerardino TM & Williams RJ 3rd. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am 94, 979–989, doi: 10.2106/JBJS.K.00533 (2012). [DOI] [PubMed] [Google Scholar]
- 195.Crawford DC, Heveran CM, Cannon WD Jr., Foo LF & Potter HG An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med 37, 1334–1343, doi: 10.1177/0363546509333011 (2009). [DOI] [PubMed] [Google Scholar]
- 196.Mizuno S, Kusanagi A, Tarrant LJB, Tokuno T & Smith RL Systems for cartilage repair. United States patent (2013). [Google Scholar]
- 197.Mumme M et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet 388, 1985–1994, doi: 10.1016/S0140-6736(16)31658-0 (2016). [DOI] [PubMed] [Google Scholar]
- 198.McCormick F et al. Treatment of focal cartilage defects with a juvenile allogeneic 3-dimensional articular cartilage graft. Oper Techn Sport Med 21, 95–99 (2013). [Google Scholar]
- 199.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00729716 (2008). [DOI] [PubMed]
- 200.European Medicines Agency. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-003594-28/DE (2011).
- 201.German Institute of Medical Documentation and Information. German Clinical Trials Register http://www.drks.de/DRKS00010658 (2016).
- 202.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02981355 (2016).
- 203.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02540200 (2015).
- 204.Shive MS et al. BST-CarGel(R) Treatment Maintains Cartilage Repair Superiority over Microfracture at 5 Years in a Multicenter Randomized Controlled Trial. Cartilage 6, 62–72, doi: 10.1177/1947603514562064 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01498029 (2011).
- 206.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00881023 (2009).
- 207.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00945399 (2009).
- 208.European Medicines Agency. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2007-003481-18/BE (2008).
- 209.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01626677 (2012).
- 210.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01041001 (2009).
- 211.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01222559 (2010).
- 212.Becher C et al. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J Orthop Surg Res 12, 71, doi: 10.1186/s13018-017-0570-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02659215 (2016).
- 214.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03219307 (2017).
- 215.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02348697 (2015).
- 216.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01957722 (2013).
- 217.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01656902 (2012).
- 218.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03319797 (2017).
- 219.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02941120 (2016).
- 220.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02179346 (2014).
- 221.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01066702 (2010).
- 222.Anderson DE et al. Magnetic Resonance Imaging Characterization and Clinical Outcomes After NeoCart Surgical Therapy as a Primary Reparative Treatment for Knee Cartilage Injuries. Am J Sports Med 45, 875–883, doi: 10.1177/0363546516677255 (2017). [DOI] [PubMed] [Google Scholar]
- 223.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01400607 (2011).
- 224.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00702741 (2008). [DOI] [PubMed]
- 225.European Medicines Agency. EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-024162-22/GB (2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


